Abstract
The transcription factor, nuclear factor (NF), erythroid-derived 2–related factor 2 (NRF2), was discovered nearly 2 decades ago. Since then, over 4,000 papers have been published on NRF2 function in diverse biological systems, and it has been found to be a critical regulator of antioxidant and defense genes with antioxidant response elements in their promoters. NRF2 is particularly important in protecting cells and tissues under highly oxidative microenvironments, including the airways that interface with the external environment and are exposed to pollutants and other oxidant stressors. Using mice with targeted deletion of Nrf2, a protective role for this transcription factor has been determined in many model diseases, including acute lung injury, emphysema, allergy and asthma, pulmonary fibrosis, and respiratory syncytial virus disease. Recent studies have also found that murine Nrf2 is important in lung development and protection against neonatal lung injury. Moreover, functional polymorphisms in human NRF2 have been known to associate with disease severity, indicating a potentially important protective function. However, there is also a “dark side” to NRF2 function, as it has been found to enhance advanced stages of carcinogenesis in the lung and some other tissues. NRF2 inducers such as phytochemical isothyocyanates and synthetic triterpenoids, have been discovered and used in model systems of oxidant-induced lung diseases, and data suggest a potential for clinical interventions. Future investigations of NRF2 should yield further insight into its contribution to normal and pathophysiological conditions in the airways, and alternative treatment strategies to protect against oxidative respiratory disease.
Keywords: acute lung injury, tumorigenesis, transcription factor
Nuclear factor (NF), erythroid-derived 2 (E2), like 2 (NFE2L2; or Nfe2l2 for rodents) or NF-E2–related factor 2 (NRF2; or Nrf2 for rodents) was discovered about 2 decades ago as a novel transcription factor for cytoprotective genes bearing cis-acting antioxidant response element (ARE; also called electrophile response element) in their promoters (1, 2). Research on NRF2 has accelerated in parallel with evolving interest in reactive oxygen species and oxidative stress that are implicated in the pathogenesis of many pulmonary diseases. A broad spectrum of NRF2 functions has been compiled from animal studies as well as in clinical settings, and NRF2 fulfills host defense not only by maintaining cellular redox balance, but also by controlling gene networks for cell cycle and death, metabolism, immunity, selective protein degradation, development, and carcinogenesis. Considerable effort has also been directed to understanding the role of Kelch-like erythroid-cell-derived protein with CNC homology–associated protein 1 (KEAP1; INrf2 for rodents), a cytoplasmic suppressor of NRF2. The “hinge and latch”–like NRF2–KEAP1 affinity binding model was described to explain homeostasis and transactivation of NRF2 in response to housekeeping proteolytic demands or cellular stimuli, including oxidants, xenobiotics, carcinogens, antioxidants, and chemopreventive agents (3).
NRF2 is ubiquitous and relatively abundant in tissues such as liver, kidney, and lung, where routine detoxification processes occur. The airways are particularly vulnerable to oxidant injury, as they are continuously exposed to environmental airborne toxicants, and thus redox balance needs to be tightly controlled. To date, mice with targeted deletion of Nrf2 (Nrf2−/−) in three different genetic backgrounds (ICR, C57BL6/J, Balbc/J) as well as lung-specific conditional knockout mice have been developed (1, 4–6) and widely applied to determine the role of Nrf2 and its therapeutic potential in respiratory disorders (Figure 1). Although the genetic sequence is evolutionally conserved with high sequence homology among vertebrate species, NRF2 or Nrf2 is highly mutable, and diverse genetic variants have been reported in human ethnic groups as well as inbred mouse strains (7). Importantly, single-nucleotide polymorphisms (SNPs) or haplotypes as well as somatic mutations of NRF2 have been determined to be related to functional alterations and as “at-risk” alleles in various cohorts (see review in Ref. 7).
Figure 1.
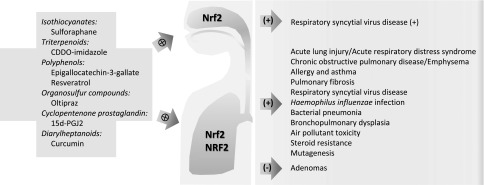
Functional roles of nuclear factor (NF), erythroid-derived 2–related factor 2 (NRF2) in upper and lower airway disorders learned from model studies using mice genetically deficient in Nrf2 or from human translational studies and exogenous NRF2 agonists for potential therapeutic intervention. Plus signs indicate protective or beneficial effects; minus sign indicates aberrant roles; crosses in circle indicate Nrf2 induction.
Acute Lung Injury, Inflammation, and Infectious Diseases
Acute lung injury (ALI) and the more severe form, acute respiratory distress syndrome, are the major acute airway diseases in adults, with approximately 50–80% mortality. ALI is caused by various clinical conditions, including pneumonia, sepsis, and major trauma. We initially became interested in Nrf2 when we performed a genome-wide linkage analysis in a murine model of hyperoxia-induced ALI (8). Nrf2 was located in a quantitative trait locus and chosen as a candidate susceptibility gene for the model, because 2 years earlier it was demonstrated to bind AREs of a number of antioxidant and phase 2 detoxification enzyme genes (4). Potentially functional Nrf2 SNPs were found in hyperoxia-susceptible C57BL/6J (B6) and -resistant C3H/HeJ (C3) strains of mice (7, 8), and cosegregation of a promoter SNP with hyperoxia susceptibility in B6C3F2 progeny supported Nrf2 as a genetic determinant of the ALI model (8). Functional relevance of Nrf2 in ALI was further determined using Nrf2−/− mice, which were significantly more susceptible to development of ALI-like phenotypes (e.g., edema, inflammation, lethality) caused by hyperoxia or butylated hydroxytoluene than similarly treated wild-type mice (9, 10). Importantly, a significant increase in the risk of ALI after major trauma was found in European and African American populations bearing a functional NRF2 SNP, which supported NRF2 as a signature transcription factor associated with human ALI risk (11).
Roles for murine Nrf2 in other pulmonary diseases were subsequently discovered (see review in Ref. 12 for early studies). For example, Nrf2−/− mice had enhanced pulmonary fibrosis and lethality after bleomycin or thoracic radiation treatment relative to wild-type controls (12, 13). Airway toxicity caused by air pollutant ultrafine particles and ozone, as well as allergic airway inflammation and hyperresponsiveness caused by allergens, were also further augmented in Nrf2−/− mice compared with Nrf2+/+ mice (12, 14, 15). Antiviral and antibacterial activity of murine Nrf2 were found in respiratory syncytial virus disease of the lung and nose, Haemophilus influenzae–induced lung inflammation, and Staphylococcus aureus–induced pneumonia (16–18). Functions of the Nrf2 pathway have been studied extensively in models of chronic obstructive airway disease (COPD) and emphysema, where emphysema-like phenotypes (e.g., enlargement of airspaces and alveolar wall destruction) caused by elastase or prolonged main-stream cigarette smoke exposure were exacerbated in Nrf2−/− mice compared with those in Nrf2+/+ mice (see review in Ref. 12). Furthermore, epidemiology and association studies have revealed significant effects of NRF2 sequence variations on risk of cigarette smoke–induced COPD and asthma in diverse ethnic groups, and interaction with environmental pollution (e.g., particulate matter up to 10 μm) was reported (7).
Lung Tumorigenesis
Although a protective effect of NRF2 has been found for many pulmonary diseases, as described previously here, the role of NRF2 in cancer pathogenesis is less clear. Experimental tumorigenesis models of nonpulmonary tissues (e.g., gallbladder, liver, stomach, colon, esophagus, skin, head/neck, prostate, bladder, mammary) have demonstrated that tumor incidence and/or size was increased in Nrf2−/− mice relative to Nrf2+/+ mice, supporting the hypothesis that NRF2-mediated induction of phase 2 detoxification enzymes are pivotal in opposing mutagenesis and carcinogenesis. In lung, DNA mutation and adduct formation were enhanced in Nrf2−/− mice relative to wild-type mice after acute exposure to environmental carcinogens, diesel exhaust particles and benzo(a)pyrene (19). However, a pulmonary carcinogenesis study with chronic urethane treatment in mice showed results that diverged from those of other tissues as detrimental functions of Nrf2 were demonstrated. That is, lack of Nrf2-ARE responsiveness enhanced lung inflammation, and injury during the early, preneoplastic stages significantly reduced lung tumor development (20, 21). These findings suggested that Nrf2-mediated cellular defense processes are essential in protection against tumor initiation, whereas, in advanced stages of carcinogenesis, enhanced Nrf2-ARE activity may create a favorable intracellular environment for cancer cells to grow and survive. This concept has been supported by many clinical investigations of Asian populations in which aberrant activation of NRF2 by its somatic (missense) mutation was associated with increased risk of non–small cell lung carcinoma cases (squamous cell lung carcinoma, large cell carcinoma, adenocarcinoma). Those studies demonstrated that multiple somatic mutations clustered in KEAP1 recognition domain of NRF2 in cancer cells caused persistent, uncontrolled transactivation of NRF2; as a result, overexpression of cytoprotective genes, including drug efflux pumps, are thought to give selective growth advantage and chemoresistance of the metastatic cells (see review in Ref. 7). Importantly, smoking history was correlated with mutation occurrence in all the cases. From these translational observations in oncology, investigators suggest that KEAP1 may be a potential “tumor suppressor” gene, whereas NRF2 may conversely be “oncogenic” in chemotherapy-resistant cancers by its excess “gain of function.”
NRF2 in Neonatal Lung Development and ALI
Relevance of Nrf2 in immature lung development and injury has also been recently investigated. Nrf2 deficiency did not affect saccular-to-alveolar transition of lung development, but augmented pulmonary injury and arrest of alveolarization when saccular phase of murine lung was exposed to hyperoxia (22, 23). Therapeutically administered oxygen (hyperoxia) has been known to contribute paradoxically to the development or exacerbation of bronchopulmonary dysplasia, a chronic lung disease and common outcome of susceptible preterm infants. The findings in murine models thus suggest a therapeutic potential for NRF2 inducers in prevention of bronchopulmonary dysplasia in preterm infants. Evidence has also indicated an effect of prenatal stimuli to postnatal lung symptoms in NRF2 variants. That is, early gestational acetaminophen exposure significantly enhanced the risk of asthma and wheezing when maternal copies of a promoter SNP enhanced the association at age 7 years (see review in Ref. 7).
Conclusions
Where is NRF2 and pulmonary biology research heading in the next decade? In lung cancer biology, as gain-of-function mutations in NRF2 are suggested to be predictive markers for poor responsiveness to chemotherapy and radiation therapy, further studies on effective personalized medicine may be warranted in patients with lung cancer with NRF2 mutations. In the field of preventive medicine, current applications of potent NRF2 agonists, such as phytochemical isothyocyanates and synthetic triterpenoids (e.g., 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl] [COOD]) to experimental disease systems (Figure 1), have supported their efficacy on certain phenotypes of respiratory syncytial virus disease, COPD, and ALI in mice (16, 24–26). Continued growth in knowledge and understanding of NRF2-mediated molecular and cellular events should add novel insights into therapeutic intervention strategies of NRF2 agonists or antagonists in critical respiratory disorders.
Acknowledgments
Acknowledgments
The authors thank Drs. Michael Fessler and Donald Cook for thoughtful comments on the manuscript.
Footnotes
This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences, Department of Health and Human Services.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci USA. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M. Cloning and characterization of a novel erythroid cell–derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol. 1995;15:4184–4193. doi: 10.1128/mcb.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong KI, Kobayashi A, Katsuoka F, Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006;387:1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 4.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 5.Martin F, van Deursen JM, Shivdasani RA, Jackson CW, Troutman AG, Ney PA. Erythroid maturation and globin gene expression in mice with combined deficiency of NF-E2 and nrf-2. Blood. 1998;91:3459–3466. [PubMed] [Google Scholar]
- 6.Reddy NM, Potteti HR, Mariani TJ, Biswal S, Reddy SP. Conditional deletion of Nrf2 in airway epithelium exacerbates acute lung injury and impairs the resolution of inflammation. Am J Respir Cell Mol Biol. 2011;45:1161–1168. doi: 10.1165/rcmb.2011-0144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho HY. Genomic structure and variation of nuclear factor (erythroid-derived 2)–like 2. Oxid Med Cell Longev. 2013;2013:286524. doi: 10.1155/2013/286524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho HY, Jedlicka AE, Reddy SP, Zhang LY, Kensler TW, Kleeberger SR. Linkage analysis of susceptibility to hyperoxia: Nrf2 is a candidate gene. Am J Respir Cell Mol Biol. 2002;26:42–51. doi: 10.1165/ajrcmb.26.1.4536. [DOI] [PubMed] [Google Scholar]
- 9.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 10.Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci USA. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, Aplenc R, Yamamoto T, Yamamoto M, Cho HY, et al. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007;21:2237–2246. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 12.Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol Appl Pharmacol. 2010;244:43–56. doi: 10.1016/j.taap.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Travis EL, Rachakonda G, Zhou X, Korhonen K, Sekhar KR, Biswas S, Freeman ML. NRF2 deficiency reduces life span of mice administered thoracic irradiation. Free Radic Biol Med. 2011;51:1175–1183. doi: 10.1016/j.freeradbiomed.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho HY, Gladwell W, Yamamoto M, Kleeberger SR. Exacerbated airway toxicity of environmental oxidant ozone in mice deficient in Nrf2. Oxid Med Cell Longev. 2013;2013:254069. doi: 10.1155/2013/254069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Wang M, Barajas B, Sioutas C, Williams MA, Nel AE. Nrf2 deficiency in dendritic cells enhances the adjuvant effect of ambient ultrafine particles on allergic sensitization. J Innate Immun. 2013;5:543–554. doi: 10.1159/000347060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho HY, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, Polack FP, Kleeberger SR. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am J Respir Crit Care Med. 2009;179:138–150. doi: 10.1164/rccm.200804-535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lugade AA, Vethanayagam RR, Nasirikenari M, Bogner PN, Segal BH, Thanavala Y. Nrf2 regulates chronic lung inflammation and B-cell responses to nontypeable Haemophilus influenzae. Am J Respir Cell Mol Biol. 2011;45:557–565. doi: 10.1165/rcmb.2010-0321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Athale J, Ulrich A, Chou Macgarvey N, Bartz RR, Welty-Wolf KE, Suliman HB, Piantadosi CA. Nrf2 promotes alveolar mitochondrial biogenesis and resolution of lung injury in Staphylococcus aureus pneumonia in mice. Free Radic Biol Med. 2012;53:1584–1594. doi: 10.1016/j.freeradbiomed.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoki Y, Hashimoto AH, Amanuma K, Matsumoto M, Hiyoshi K, Takano H, Masumura K, Itoh K, Nohmi T, Yamamoto M. Enhanced spontaneous and benzo(a)pyrene-induced mutations in the lung of Nrf2-deficient gpt delta mice. Cancer Res. 2007;67:5643–5648. doi: 10.1158/0008-5472.CAN-06-3355. [DOI] [PubMed] [Google Scholar]
- 20.Bauer AK, Cho HY, Miller-Degraff L, Walker C, Helms K, Fostel J, Yamamoto M, Kleeberger SR. Targeted deletion of Nrf2 reduces urethane-induced lung tumor development in mice. PLoS ONE. 2011;6:e26590. doi: 10.1371/journal.pone.0026590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satoh H, Moriguchi T, Takai J, Ebina M, Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73:4158–4168. doi: 10.1158/0008-5472.CAN-12-4499. [DOI] [PubMed] [Google Scholar]
- 22.Cho HY, van Houten B, Wang X, Miller-DeGraff L, Fostel J, Gladwell W, Perrow L, Panduri V, Kobzik L, Yamamoto M, et al. Targeted deletion of nrf2 impairs lung development and oxidant injury in neonatal mice. Antioxid Redox Signal. 2012;17:1066–1082. doi: 10.1089/ars.2011.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGrath-Morrow S, Lauer T, Yee M, Neptune E, Podowski M, Thimmulappa RK, O’Reilly M, Biswal S. Nrf2 increases survival and attenuates alveolar growth inhibition in neonatal mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2009;296:L565–L573. doi: 10.1152/ajplung.90487.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, et al. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke–induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci USA. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, Feller-Kopman D, Wise R, Biswal S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci Transl Med. 2011;3:78ra32. doi: 10.1126/scitranslmed.3002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy NM, Suryanaraya V, Yates MS, Kleeberger SR, Hassoun PM, Yamamoto M, Liby KT, Sporn MB, Kensler TW, Reddy SP. The triterpenoid CDDO-imidazolide confers potent protection against hyperoxic acute lung injury in mice. Am J Respir Crit Care Med. 2009;180:867–874. doi: 10.1164/rccm.200905-0670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


