Abstract
This review/research paper summarizes data on development of the external genitalia of the spotted hyena, a fascinating mammal noted for extreme masculinization of the female external genitalia. The female spotted hyena is the only extant mammal that mates and gives birth through a pendulous penis-like clitoris. Our studies indicate that early formation of the phallus in both males and females is independent of androgens; indeed the phallus forms before the fetal testes or ovaries are capable of synthesizing androgens. Likewise, pre- and postnatal growth in length of the penis and clitoris is minimally affected by “androgen status”. Nonetheless, several internal morphologies, as well as external surface features of the phallus, are androgen-dependent and thus account for dimorphism between the penis and clitoris. Finally, estrogens play a critical role in penile and clitoral development, specifying the position of the urethral orifice, determining elasticity of the urethral meatus, and facilitating epithelial-epithelial fusion events required for proper formation of the distal urethra/urogenital sinus and prepuce. Accordingly, prenatal inhibition of estrogen synthesis via administration of letrozole (an aromatase inhibitor) leads to malformations of the glans as well as the prepuce (hypospadias). The effects of prenatal androgens, anti-androgens and impaired estrogen synthesis correlated with the tissue expression of androgen and estrogen receptors.
Keywords: Penis, clitoris, urogenital sinus, hyena, androgens, estrogens
Introduction
Contemporary understanding of mammalian sexual differentiation of internal and external genitalia (ExG) is based upon the pioneering studies of Alfred Jost many decades ago (Jost, 1953). Namely, secretion of androgens by the fetal testes is required for fusion of the genital swellings to form a scrotum and development of the penis from the ambisexual genital tubercle (GT), while absence of androgens during development leads to the female pattern of ExG. Actually, the first action of androgen on the ambisexual genital tubercle is specification of penile identity (Rodriguez et al., 2012), while subsequently androgens elicit morphogenesis of penile features. As detailed in the next section of this manuscript, female spotted hyenas (Crocuta crocuta) display the most “male-typical” ExG of any extant female mammal including other members of the family Hyaenidae.
There are four living species in the family Hyaenidae: spotted hyenas (Crocuta crocuta), striped hyenas (Hyaena hyaena), brown hyenas (Parahyaena brunnea), and aardwolves (Proteles cristatus). However, only female spotted hyenas display the full assortment of “masculine” characters in their ExG, as described below. Female spotted hyenas have normal internal genitalia composed of ovaries, oviducts, and two uterine horns, which merge proximally into a common uterine body. The cervix is indistinct anatomically, but has a distinctive histology (Cunha et al., 2003) and merges caudally into the vagina located internally within the abdominal-pelvic cavity (Fig. 1) (Cunha et al., 2003). Immediately caudal to the vaginal segment, the urethra joins the reproductive tract to form a urogenital sinus (UGS), which upon traversing the pelvic outlet turns almost 180 degrees and continues as the central canal within a large pendulous penis-like clitoris, which is the approximate size of the penis in the flaccid state (Fig. 1) (Cunha et al., 2003). Although a number of other female mammals also possess a large clitoris penetrated by a urethra, e.g., moles (Matthews, 1937; Rubenstein et al., 2003) and lemurs (Drea and Weil, 2008; Hill, 1953), female spotted hyenas are the only extant mammals that mate and give birth through a clitoris (Fig. 1) (Drea et al., 1999; Frank and Glickman, 1994; Schneider, 1926; Drea et al., 2002). Female spotted hyenas also have a pseudo-scrotum but no external vaginal opening (Figs. 1-2). The pseudo-scrotum of the female spotted hyena resembles that of the male in gross appearance and position, but of course does not contain testes.
Figure 1.
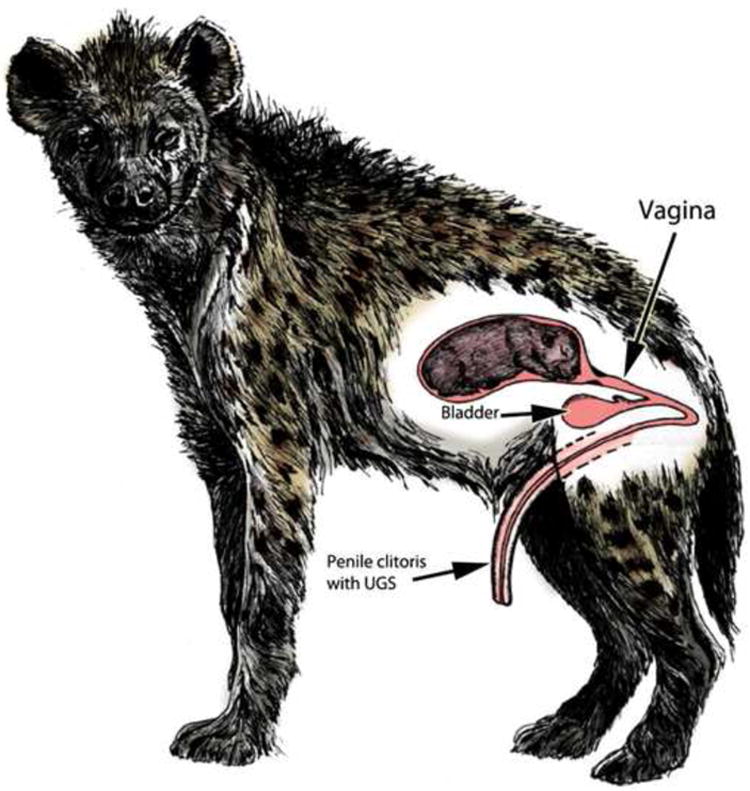
Drawing of a pregnant female spotted hyena with a fetus in a uterine horn. A segment of the reproductive tract caudal to the uterus exhibits vagina histology. The urethra joins the caudal end of the vaginal segment, and the common urogenital sinus (UGS) thus formed extends through the pelvic outlet and makes a ∼180-degree turn to traverse to the exterior through the penis-like clitoris. Note the absence of external vaginal orifice. (Adapted from Drea with permission).
Figure 2.

(A) Drawing of the adult spotted hyena clitoris by Gordon with permission (Funk, 2012). (B) Photo of the adult spotted hyena clitoris. (C) Photo of the adult spotted hyena penis.
Given the presence of a pseudo-scrotum, the absence of an external vaginal opening, and a penile-like clitoris traversed by a UGS, it is not surprising that the distinction between ExG of female and male spotted hyenas eluded writers from the time of Aristotle (Barnes, 1985; Glickman, 1995) until the 18th century. In 1777, a Dutch army officer and amateur zoologist, Robert Jacob Gordon, while deployed in South Africa, made some remarkably accurate drawings of the ExG of the female spotted hyena (Fig. 2A), and provided an equally accurate interpretation of their significance. These drawings were recently discovered in the Rijksmuseum in Amsterdam (Funk, 2012) and were unknown to Morrison Watson, who believed that he had published the first scientific description of the genitalia of female and male spotted hyenas in 1877 and 1878 (Watson, 1877; Watson, 1878). Watson portrayed for the first time the internal urogenital systems of male and female spotted hyenas.
In the years that followed, other investigators were drawn to the unusual reproductive systems of this unique animal (Chapman, 1888; Davis and Story, 1949; Grimpe, 1916; Neaves et al., 1980; Matthews, 1939), with Matthews' (1939) monograph providing the most detailed description, and Neaves et al recognizing the functional significance of sex differences in the internal morphology of the penis and the clitoris (Neaves et al., 1980). These anatomical studies were extended with additional observations and functional correlates by our studies (Cunha et al., 2003). Perhaps anticipating Jost, Matthews noted the unusual structure of the hyena ovary, with its abundance of interstitial tissue, and speculated that androgens secreted by this organ were responsible for the “masculine” phenotype of female spotted hyenas.
This report is a hybrid review/research paper that focuses on the ExG of the spotted hyena (Crocuta crocuta) to reveal novel mechanisms of sexual differentiation and to direct attention to developmental processes that are often neglected in more traditional mammals, including humans (Glickman et al., 2005; Glickman et al., 2006). In this paper we will integrate new findings with previous reports to summarize our anatomical and endocrine studies on this fascinating animal.
Materials and Methods
Subjects
Data presented in this paper are based upon analysis of 116 spotted hyenas ranging in age from 25 days of gestation to adulthood (≥2 years). Ages of untreated specimens were as follows: 37 fetuses from 25-103 days of gestation, 16 neonates from birth (or stillbirth) to 15 days postpartum, and 19 adults (≥2 years of age), with roughly equal numbers of males and females in each group (see supplementary Table 1). Details of the 38 hormonally treated animals are given in Table 1.
Table 1. Spotted hyenas treated with hormonally active agents or hormone-modulating procedures.
| Sex | Age at harvest | Treatment |
|---|---|---|
| Male | 45 day | Letrozole |
| Female | 62 day | Letrozole |
| Male | 68 day | Letrozole |
| Male | 68 day | Letrozole |
| Male | Stillborn | Letrozole |
| Male | 2 day | Letrozole |
| Male | Adult | Letrozole |
| Female | Adult | Letrozole |
| Male | 94 day | Flutamide + finasteride |
| Female | 94-day | Flutamide + finasteride |
| Male | 105 day | Flutamide + finasteride |
| Female | 105 day | Flutamide + finasteride |
| Male (3) | Stillborn | Flutamide + finasteride |
| Male (4) | Stillborn | Flutamide |
| Female (4) | Stillborn | Flutamide + finasteride |
| Male | Stillborn | Casodex+ finasteride |
| Female | Stillborn | Casodex+ finasteride |
| Female | 1 day | Flutamide + finasteride |
| Male | 2 day | Flutamide + finasteride |
| Male (2) | Adult | Flutamide |
| Male (2) | Adult | Flutamide + finasteride |
| Female (5) | Adult | Flutamide + finasteride |
| Female | Stillborn | Mibolerone |
| Female (4) | Adult | Ovariectomy |
| Male (4) | Adult | Castration |
Numbers in ( ) indicate the numbers of animals per group.
Some pregnant hyenas were treated orally with anti-androgens (flutamide [18-50mg/kg/day], casodex [50 mg/day], alone or in combination with the 5α-reductase inhibitor finasteride (0.66-2mg/kg/day) for various periods of gestation. These doses of flutamide and casodex are known to achieve androgen receptor blockade (Imperato-McGinley et al., 1992; Cunha et al., 2005). Another group of pregnant hyenas was treated orally for various periods with the aromatase inhibitor, letrozole, at a dosage of 12.5mg/day. Finally, one pregnant hyena was treated with the synthetic androgen, mibolerone (6.60μg/kg/day). All of these drugs were administered as capsules introduced into food. Periods of drug treatment are specified below.
Embryos/fetuses were derived via Caesarian section of pregnant female hyenas in the UC Berkeley colony. In the optimal case, estimates of gestational age were derived from the dam's observed matings. In the absence of observed mating, timing of gestation was inferred from crown-rump and/or femur lengths obtained via ultrasound (Place et al., 2002) or by using the midpoint of a period during which animals were paired (Licht et al., 1998). Sterile surgical fetectomies were performed on pregnant hyenas immobilized with intramuscular injection of ketamine (4-6 mg/kg) and xylazine (1mg/kg) administered by blow darts and then anaesthetized with isoflurane. Euthanasia was performed via pento-barbitol over-dose. Following stillbirths, neonatal carcasses were retrieved shortly after birth and placed on ice. Urogenital tracts were dissected and preserved in 10% formaldehyde. All procedures were approved by the Animal Care and Use Committee of the University of California, Berkeley.
Morphological methods and determination of sex
Hyena embryos were photographed intact to achieve unobstructed lateral and frontal views of the ExG. The entire urogenital tract was then removed from the carcass by sharp dissection. Photographic documentation was made of the dissection process and the intact isolated urogenital tracts. The isolated penis and clitoris were in turn cut into 2 to 5 segments along the proximal-distal axis. The proximal surface of each segment was scanned on an Epson scanner to visualize gross cross-sectional structures (corporal body, urethra/urogenital sinus, and retractor muscles). Each segment was then embedded in paraffin and sectioned at 7μm. Sections were mounted on glass slides, de-paraffinized and stained with hematoxylin and eosin or immunostained.
Sex of the fetuses was determined by visual observation of dissected gonads and confirmed by histology. This method of sexing was not possible in the youngest group of fetuses aged 25 to 38 days of gestation, which is before gonadal sex differentiation. For this group, sex was determined by PCR analysis of a Y chromosomal gene, SRY, using primers designed from the pig SRY gene as reported previously (Licht et al., 1998). Alternatively, a portion of the undifferentiated gonad was transplanted under the renal capsule of castrated male athymic mice and grown for 1 month during which time gonadal sex differentiation occurred (Cunha et al., 2005). Histology of the grafts confirmed testicular or ovarian differentiation. In some cases, sex of specimens was assessed by both methods.
In Vivo Measures of the External Genitalia
We have developed a standardized technique for measuring four crucial aspects of adult hyena phallic morphology in vivo. These include (a) length of the penis/clitoris, when stretched with a constant force of 500 grams, (b) maximal diameter of the shaft and glans penis or glans clitoris in the flaccid state, and (c) diameter of the urogenital meatus in a “relaxed” state, or when gently distended with a constant force applied using a specially designed instrument. These procedures were described and illustrated previously (Drea et al., 1998). In normal adult hyenas there are significant sex differences in all of these measurements. The penis is slightly longer than the clitoris. The rounded clitoral glans has a greater diameter than that of the glans penis. The meatus of the clitoris is much larger and more elastic than the penile urethral meatus (Drea et al., 1998; Drea et al., 2002; Glickman et al., 1998; Glickman et al., 1992).
Immunohistochemistry
Methods for androgen receptor immunohistochemistry (IHC) have been described (Cunha et al., 2005). Tissue sections were de-paraffinized in Histoclear (National Diagnostic, Atlanta, GA) and hydrated in graded alcohols and phosphate-buffered saline (PBS). Endogenous peroxidase activity was blocked with 0.5% hydrogen peroxide in methanol for 30 minutes. Normal goat serum was applied to the sections for 30 minutes to bind nonspecific sites. The sections were then incubated with the primary antibodies overnight at 4°C or with non-immune serum or mouse IgG. The following antibodies were used: a rabbit polyclonal anti-androgen receptor antibody (Affinity BioReagents, Golden, CO); anti-ERα (mouse monoclonal clone 1D5, diluted 1:30, Dako, Carpinteria, CA, USA); and anti-ERβ (mouse monoclonal clone EMR02, diluted 1:200, Leica Microsystems, Newcastle Upon Tyne, UK). Signal detection was achieved using the Vector ABC System (Vector Laboratories, Foster City, CA, USA) followed by exposure to diaminobenzidine (Sigma, St. Louis, MO). Sections exposed to all steps except the application of the primary antibodies were used as negative controls.
Three-Dimensional Reconstruction
Three-dimensional computer reconstructions were created from serial transverse sections utilizing SURF driver 3.5 (SURF driver, University of Hawaii and University of Alberta) or Maya (Autodesk, Inc., San Rafael, CA) software. Sections were digitized to achieve linear tracings of relevant structures. Digital linear tracings were oriented using Photoshop software (Adobe, Inc. San Rafael, CA 94903) and then were exported into SURF driver or Maya software for three-dimensional reconstruction.
Serum Gonadal Steroid Analysis
For the letrozole studies serum samples from untreated control and letrozole-treated pregnant hyenas were shipped to Esoterix Endocrinology (Calabasas Hills, CA) for measurement of estrone (E1) and estradiol (E2) concentrations by highly sensitive radioimmunoassays (RIA), which followed a pre-assay organic extraction and chromatographic separation of E1 and E2 on Sephadex LH-20 columns (Place et al. 2002). A subset of samples were shipped to the Endocrinology Laboratory, Animal Health Diagnostic Center, Cornell University for measurement of total and free testosterone (T) concentrations by RIA (Diagnostic Products Corp, Los Angeles, CA); the total T RIA was previously validated for use on spotted hyenas (Holekamp and Smale, 1998).
Results
Morphology of the phallus in adult male and female spotted hyenas
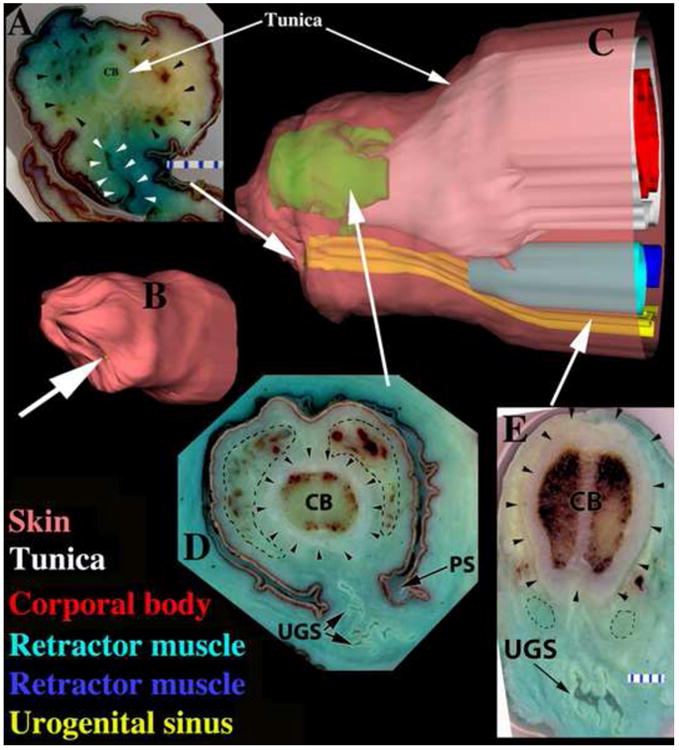
While studies on development of ExG should appropriately focus on genital organs during actual pre- and early postnatal periods when developmental processes are occurring, development of ExG can also be inferred through examination of adult morphology, which after all is the endpoint of development. Such studies have revealed profound sexual dimorphism of internal penile and clitoral morphology. The hyena penis and clitoris are similar in size in the flaccid state. Profound differences in phallic morphology are seen in the fully erect condition. The penis is larger and more flexible than the clitoris, as required of the male for successful intromission during mating (Drea et al., 1999). Moreover, following erection shapes of the glans penis and glans clitoris are quite different in both neonates and adults (Fig. 3). The tip of the penis is chisel-shaped, whereas the tip of the clitoris is blunt (Figs. 3A & B). Differences in morphology of the glans penis and glans clitoris are also apparent in the flaccid condition (Fig. 2). We believe that this dramatic difference in glans shape evident upon erection (Fig. 3), is accomplished through a hemo-dynamic mechanism involving a pair of bilateral distal glanular erectile bodies (GEB) whose shape and position in the adult glans are sexually dimorphic, implying sex differences in the developmental process. These GEBs were revealed by examination of serial 2mm thick sections of the adult glans penis and glans clitoris that in turn were used to create three-dimensional reconstructions (3DR) of ExG of adult male and female hyenas (Figs. 4, 5, 7). The 3DRs of the adult hyena penis and clitoris reveal shape and position differences in the GEBs consistent with sexual dimorphism of the glans penis and clitoris seen in fully erect adult organs (Figs. 3, 4, 5, 7). In the adult penis the bilateral GEBs have a distinct chisel shape, are larger than those of the clitoris, and completely surround the urethra (Fig. 7). In contrast, in the adult clitoris the GEBs are smaller, lie dorsal to the urethra and are blunt distally (Fig. 5 & 7). As a caveat, we had previously discovered the adult clitoral GEB through examination of a limited number of thin (7μm) para-sagittal sections, and we previously had named it the hyena urogenital glanular extension (HUGE) (Cunha et al., 2003). Examination of serial 2mm thick transverse sections through the entire glans has now revealed the true nature of these glanular erectile bodies.
Figure 3.
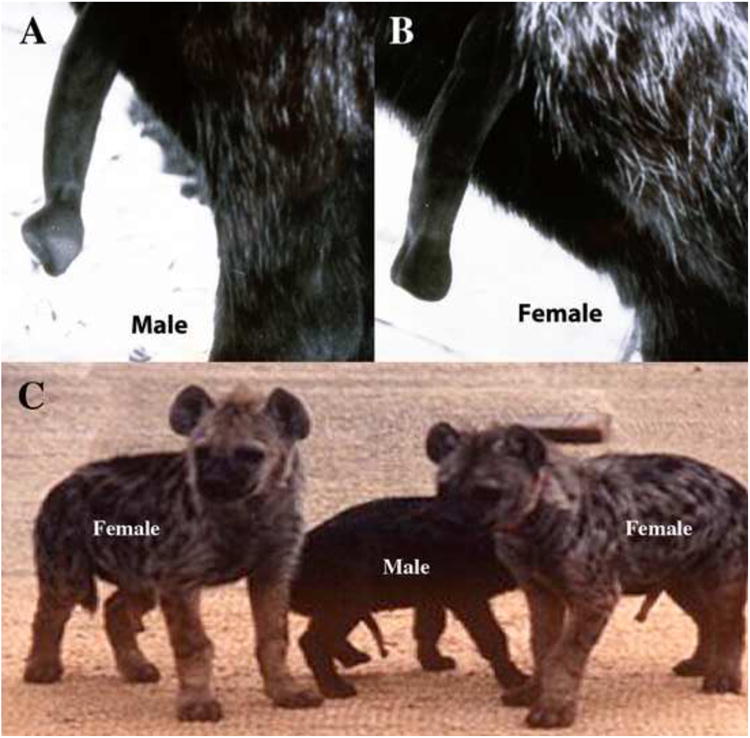
The adult erect hyena penis (A) and clitoris (B). Note the distinctive shapes of the glans. (A) and (B) adapted from (Place and Glickman, 2004) with permission). (C) Three infant spotted hyenas (3-4 months old) in full erection (From Glickman et al 2005) with permission. Based upon glans shape, these infants can be accurately sexed as indicated.
Figure 4.

Three-dimensional reconstructions (3DR) (B-E) as well as sections (A, F-H) of the penis of an untreated adult male hyena. (A) Distal section, note urethral orifice (black arrowheads) opening onto the dorsal penile surface. Orange arrows pointing to 3DRs indicate positions of sections (A, F-H)). (B) 3DR, ¾ view of the distal end of the adult hyena penis. Skin is flesh color, urethral orifice (yellow) opens on the dorsal surface of the penis. (C) 3DR, side view with skin rendered semi-transparent to reveal the GEB (green) surrounding the urethra (yellow) and the corporal body (red). (D) 3DR of the mid-shaft of the penis. Note slit-like urethra (yellow arrow) surrounded by the purple corpus spongiosum. The tunica albuginea (white) surrounds both the corporal body (red), urethra and corpus spongiosum. Retractor muscles (blue) lie ventral to the penile urethra. (E) 3DR, side view with penile skin rendered invisible and the tunica (white) rendered semi-transparent to reveal internal structures. (F) Proximal section showing corporal body (CB), and corpus spongiosum (CS) surrounding the slit-like urethra (Ur). Retractor muscles (dotted lines) are ventral to the urethra. Orange arrow indicates the position in 3DR (E) from which section (F) was taken. (G) Section through to the glanular erectile body (GEB); the orange arrow indicates the position in 3DR (D) from which section (G) was taken. The tunica albuginea surrounds both the corporal body (CB) and urethra (Ur). (H) Section just proximal to the glanular erectile body (GEB); the orange arrow indicates the position in 3DR (E) from which section (H) was taken. The tunica surrounds the corporal body (CB), slit-like urethra (Ur) and corpus spongiosum (CS).
Figure 5.
Three-dimensional reconstructions (3DR) of the clitoris (B-C) as well as sections (A, D, E) of an untreated adult female hyena. (A) Distal section, note corporal body (CB), glanular erectile body (black arrowheads), and UGS (white arrowheads) opening ventrally. Large white arrow pointing to 3DR (B) indicates the position of section (A) in the clitoris. (B) 3DR, ¾ view of the distal end of the clitoris. White arrow indicates urethral orifice on the ventral surface of the clitoris. (C) 3DR, side view with skin semi-transparent to reveal internal structures. Note color-coding of labels and that the retractor muscles are displayed in two different blue hues. (D) Section through the glanular erectile body (dotted lines) as indicated by the large white arrow pointing to a position in 3DR (C). Black arrowheads demarcate the tunica, and dotted lines denote the GEB. Note pleated UGS in ventral position. (E) Proximal section showing corporal body (CB), pleated UGS, tunica (arrowheads) surrounding corporal body only, and retractor muscles (dotted lines) dorsal to the UGS.
Figure 7.
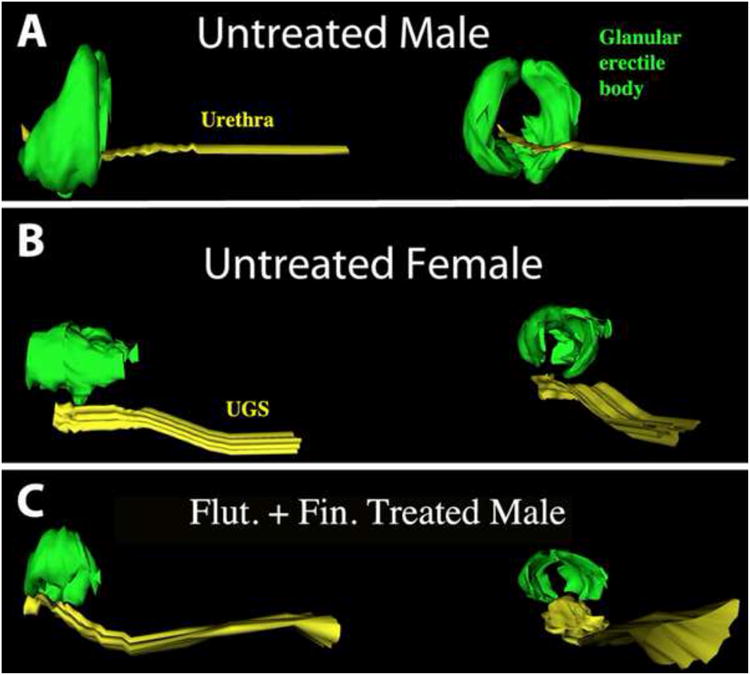
3DRs of the urethra/UGS (yellow) and glanular erectile body (green) of adult phalluses of (A) an untreated male, (B) an untreated female, and (C) a male treated with flutamide (25mg/kg/day) from 32 to 110 days of gestation. Side views (left) and proximal to distal views (right). Note male/female sexual dimorphism and that the prenatally flutamide/finasteride-treated male exhibits the adult female pattern.
In this report we confirm and extend previous observations of sexual dimorphism within adult hyena ExG (Cunha et al., 2003; Matthews, 1939; Neaves et al., 1980; Watson, 1877; Watson, 1878) through examination of complete sets of 2mm serial transverse sections of the adult penis and clitoris. These sections begin at the distal tip and continue well into mid-shaft. Accordingly, figures 4, 5 and 7 reveal 3-dimensional morphology, anatomical relationships, and 2-dimensional morphology in relevant tissue sections. In the penis, the urethral orifice is located on the dorsal (surface farthest from the anus) aspect of the glans, and the distal aspect of the male urethra is surrounded by the GEBs (Fig. 4 and 7). Proximal to the glans, the penile urethra has a narrow dorso-ventrally flattened lumen and is surrounded by the corpus spongiosum. The corporal body lies dorsal to the urethra and corpus spongiosum and is demarcated by a thick (2-3mm) tunica albuginea, which surrounds the corporal body, penile urethra and corpus spongiosum. Male retractor muscles composed of smooth muscle (Fig. 4) lie ventral to the penile urethra.
In contrast, the orifice of the urogenital sinus (UGS) in females is positioned on the ventral aspect (surface closest to the anus) of the glans clitoris (Fig. 5). The term UGS is used to describe the central canal through the clitoris because this canal passes urine from the bladder, transmits seminal fluid to the uterine horns, and conducts the fetus through the urogenital meatus at parturition. Because the fetus must traverse the clitoral UGS during birth, this canal has a voluminous lumen with a pleated, highly redundant mucosa (Fig. 5). These 3 features (UGS shape, corpus spongiosum and tunica albuginea) are thus dramatically different in ExG of male versus female spotted hyenas. Finally, the retractor muscles in the clitoris are dorsal to the UGS (Fig. 5), as opposed to ventral to the penile urethra (Fig. 4). As observed previously (Neaves et al., 1980), the UGS of the female spotted hyena is not surrounded by a corpus spongiosum or the GEBs or constrained by a thick tunica albuginea. Thus, expansion (stretching) of the female UGS is enabled during mating and parturition. Sexual dimorphism in the glans and at mid-shaft, is summarized in Table 2. All of these sexually dimorphic features are functionally correlated with the different roles assumed by the clitoris and penis during mating and parturition.
Table 2. Sexually Dimorphic Androgen-Regulated Features in External Genitalia of Spotted Hyenas.
| Feature | Penis | Clitoris | Male features inhibited by anti-androgens | Female features induced by androgen |
|---|---|---|---|---|
| Position of urethral meatus | Dorsal | Ventral | yes | ND |
| Size and elasticity of urethral meatus | Small and Non-Elastic | Large and Highly Elastic | yes | ND |
| Glans shape/GEB shape | Chisel-shaped | Blunt | yes | ND |
| GEB position re urethra | Surrounds urethra | Dorsal to urethra | yes | ND |
| Corpus spongiosum | Present | Absent | yes | yes |
| Urethra/UGS lumen | Slit-like | Pleated/redundant | yes | yes |
| Position of retractor muscles | Ventral | Dorsal | yes | yes |
| Tunica albuginea | Surrounds corporal body and urethra | Surrounds corporal body only | yes | yes |
ND= not done
Normal embryonic development of the external genitalia of spotted hyenas: Formation of the penis/clitoris and scrotum/pseudo-scrotum prior to differentiation of the gonads and subsequent sexual differentiation of the phallus under the influence of androgen
Gestation length in spotted hyenas is 111 days based upon observed captive matings (n=6; Weldele, unpublished) and observations on captive spotted hyenas (Schneider, 1926), making it possible to surgically collect fetuses at well-defined ages. The two earliest specimens that we examined were 25 and 28 days of gestation (mating by the dam was observed visually), when hyena embryos can be characterized as preambisexual or early ambisexual as the ExG are barely visible and internal urogenital tracts are extremely primitive (Fig. 8).
Figure 8.
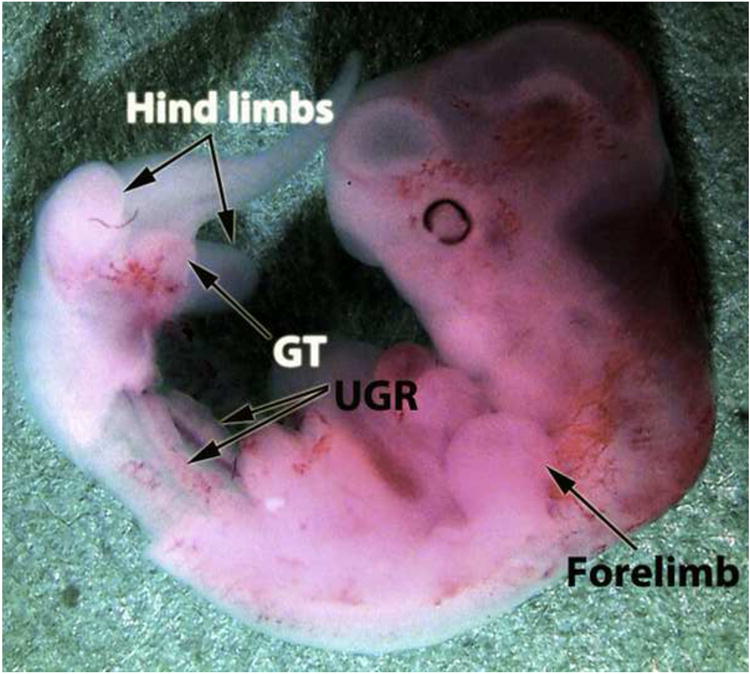
Spotted hyena embryo at 27 days of gestation. The abdominal wall and abdominal viscera have been removed to reveal the urogenital ridges (UGR). The genital tubercle (GT) is already a prominent projection from the ventral body flanked by the hind limb buds.
By 30 to 32 days of gestation, the genital tubercles (GT) of both male and female fetuses were well-formed, prominent, similar in size, and in both sexes contained a solid urethral plate, the precursor of the urethra (Fig. 9) (Cunha et al., 2005). Despite the advanced state of phallic development at this age, the gonads of both male and female fetuses (sexed by RT-PCR) were completely undifferentiated morphologically with no evidence of seminiferous cords, ovarian follicles (Fig. 10A), interstitial cells or androgen production (Cunha et al., 2005; Licht et al., 1998; Browne et al., 2006). At ∼30 days of gestation Wolffian and Müllerian duct morphologies were consistent with early ambisexual stage in so far as the Wolffian ducts extended the full length of the urogenital ridge, whereas the Müllerian ducts extended only about 75% of the length of the urogenital ridge (Cunha et al., 2005; Licht et al., 1998). In spite of the primitive state of the testes at 30 days of gestation, low levels of anti-Müllerian hormone (AMH) were detected in presumptive Sertoli cells, and low levels of 17α -hydroxylase/17, 20-lyase cytochrome P450 (P450c17) (the enzyme that catalyzes the synthesis of 19-carbon steroids such as androgens) were detected in presumptive Leydig cells (Browne et al., 2006). By comparison, the 30-day ovaries were unorganized and devoid of steroidogenic enzymes (Browne et al., 2006; Licht et al., 1998).
Figure 9.
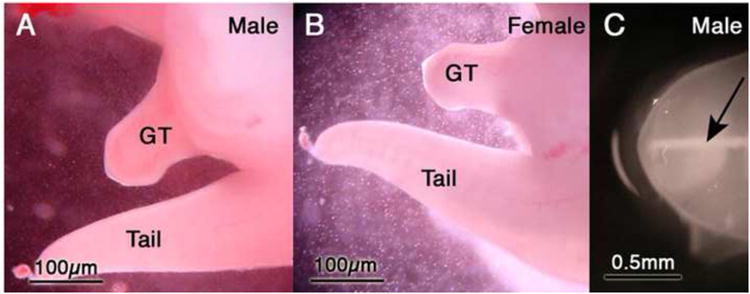
(A and B) Male and female genital tubercles (GT) at 30 days of gestation. Note similarity in size of male and females GTs. (C) Genital tubercle dissected from a 30-day-old male spotted hyena fetus and photographed with oblique lighting. Note the whitish midline structure (arrow), which represents the solid urethral plate. Adapted from (Cunha et al., 2005) with permission.
Figure 10.
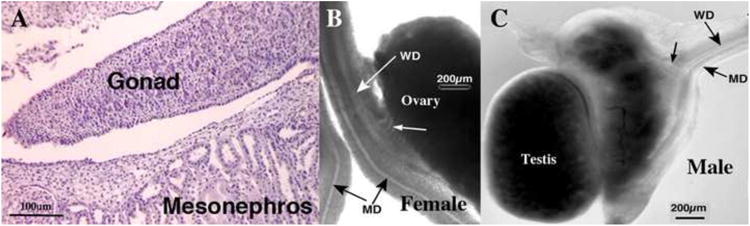
(A) Histological section of an undifferentiated gonad from a 31-day-old fetus sexed by PCR as male. Note absence of seminiferous cords/tubules. (B & C) Wholemount images of spotted hyena genital tracts. (B) Wholemount of a 48-day-old female specimen. Note presence of Mullerian (MD) and Wolffian (WD) ducts and mesonephric tubules (small white arrow). (C) Wholemount of a 50-day-old male specimen. Note presence of Mullerian (MD) and Wolffian (WD) ducts and mesonephric tubules (small black arrow). Both of these specimens exhibit the ambisexual stage based upon differentiation status of the gonads, Wolffian and Mullerian ducts. Subtle texture at the periphery of the testis is indicative of seminiferous tubules, which was confirmed in tissue sections (not illustrated). Adapted from (Cunha et al., 2005) with permission.
By gestation day 45, testicular development was advanced with well-developed seminiferous tubules expressing AMH as well as interstitial cells expressing P450c17 as well as other enzymes in the androgen synthetic cascade. In contrast, the ovaries were only beginning to organize morphologically by gestation day 45, and while steroidogenic enzyme expression was evident in the developing ovarian cortex, androstenedione was not detectable in fetal female plasma (Browne et al., 2006). Several specimens were examined in the 45 to 58 day gestational age range (see supplemental Table 1). The salient feature for this age range is morphologic differentiation of the ovaries and testes, which was evident as overall gross shape of the gonads. Fetal testes of the 45 to 58 day specimens have an elliptical shape and contained seminiferous cords (Fig. 10C). In contrast, the gonads of female specimens are more elongated (Fig. 10B), and exhibited a nondescript undifferentiated histology devoid of ovarian follicles (not illustrated). In this age group Wolffian and Müllerian ducts were observed throughout the full length of the urogenital ridge of both male and female fetuses indicative of the ambisexual stage (Fig. 10B & C) (Cunha et al., 2005). The developing clitoris and penis were prominent and similar in size and gross morphology (Fig. 9) in both male and female fetuses of this age range (Cunha et al., 2005) even though capacity for androgen synthesis was limited in fetal testes and absent in fetal ovaries (Browne et al., 2006). Comparable male and female phallic development during prenatal stages (before, during and after the ambisexual stage) when androgen levels are low to non-existent is consistent with the idea that formation and growth in length of the penis-like clitoris and the penis are largely androgen-independent.
By gestation day 65, the ovary was well organized into cortex and medulla; relative to the testis, the ovary had comparable or even greater expression of the enzymes required for androgen synthesis (Browne et al., 2006). Androstenedione concentrations in plasma of male and female fetuses were also similar by this stage (Browne et al., 2006; Licht et al., 1992; Lindeque and Skinner, 1982). Therefore, the fetal ovaries develop the capacity for androgen synthesis equal to that of the fetal testes, but only in the second half of gestation after differentiation of the ExG is well advanced. This timing provides “further support for the hypothesis that formation of the penis-like clitoris in spotted hyenas by 30 days of gestation occurs without appreciable influence of androgens from fetal gonads” (Browne et al., 2006). It is worth noting that androgens of ovarian or placental origin are not sufficient to maintain Wolffian ducts in female spotted hyenas (Cunha et al., 2005).
Role of androgens in development of hyena external genitalia Prenatal treatment with anti-androgens
Because of the extreme masculinization of ExG of female spotted hyenas, several decades ago we initiated search for a source of naturally circulating prenatal androgens during development and found that the hyena placenta produces testosterone from an early stage of gestation through metabolic conversion of maternal androstenedione (Licht et al., 1992; Yalcinkaya et al., 1993). To determine whether androgens of placental or gonadal origin were actually responsible for masculinization of male and female ExG, we administrated androgen receptor blockers (anti-androgens, flutamide or casodex), alone or in combination with the 5α-reductase inhibitor, finasteride (which inhibits conversion of testosterone to the more active androgen, 5α-dihydrotestosterone) to pregnant spotted hyenas over extended periods encompassing the ambisexual stage and beyond and examined effects in adulthood. Treatment of pregnant hyenas with anti-androgens resulted in male and female offspring that displayed a somewhat shorter, thicker phallus, with a much larger and more elastic urogenital meatus when examined in infancy and in adulthood (Drea et al., 1998; Drea et al., 2002). It might also be noted that the modest reduction (15 – 20%) in adult penile and clitoral length following prenatal exposure to anti-androgens in utero produced a penis that was the size of a normal adult hyena clitoris (Drea et al., 2002). This is a more substantial effect than that produced by pre-pubertal castration as described below, and suggests that a growth trajectory for adult penile length is set by androgen exposure in utero. However, such in utero anti-androgen exposure failed to block formation of the penis-like clitoris or penis, as well as the development of a pseudo-scrotum. The persistence of these structures contrasts with the situation in other mammals including rats and dogs (Imperato-McGinley et al., 1992; Neri, 1977). When rats and dogs were exposed prenatally to anti-androgens, the ExG of male offspring were feminized. Such “feminization” included the development of a small penis no longer traversed by a urethra and a blind “vaginal” pouch in the scrotal area.
The relatively muted changes expressed in adult spotted hyenas by prenatal anti-androgen exposure are nevertheless of major biological significance for reproduction of spotted hyenas (Drea et al., 2002). First births of cubs through the clitoris of untreated females are very difficult for adult female hyenas and result in 60% stillbirths in primiparous mothers (Frank et al., 1995). In contrast, females exposed prenatally to anti-androgens consistently give birth without difficulty to live cubs at end of their first pregnancy (Drea et al., 2002). However, the same prenatal anti-androgen exposure (that facilitated reproduction in females) prevents successful mating by adult male spotted hyenas due to reduction in length of the erect penis and a blunt (i.e., “feminine”) contour of the glans penis that precludes intromission (Drea et al., 2002).
These morphologically and physiologically important effects in sexually mature hyenas prenatally anti-androgens, are also reflected by observations in hyena fetuses treated with anti-androgens. In this regard, hyena ExG were examined prenatally, at birth and in adulthood following treatment with anti-androgens (flutamide, casodex alone or in combination with the 5α-reductase inhibitor, finasteride). In all cases, anti-androgens induced “feminization” of male internal phallic structures (Figs. 6, 11). The effectiveness of the anti-androgen treatment was manifest as complete absence of fetal prostate (Cunha et al., 2005). In untreated male fetuses/neonates, the retractor muscles were positioned ventral to the penile urethra, and the tunica albuginea surrounded both the slit-like urethra and corpus spongiosum (Fig. 11A). In contrast, untreated female fetuses/neonates had a large pleated UGS, lacked a corpus spongiosum, and had retractor muscles positioned dorsal to the UGS. The tunica albuginea surrounded the corporal body only in untreated female fetuses (Fig. 11B). Anti-androgen-treated (flutamide or casodex ± finasteride) males exhibited the female pattern, i.e., having a large pleated UGS, dorsally positioned retractor muscles, without a corpus spongiosum, and had a tunica albuginea surrounding only the corporal body (Figs. 6 & 11C). In addition to the preceding effects on penile morphology, anti-androgen treatment in utero “feminized” the normal male-biased dimorphism in the bulbocavernosus muscle and the number of motor neurons in Onufs nucleus in the spinal cord, the region where neural control of penile muscles originates (Forger et al., 1996). Finally, the one pregnant hyena treated with the synthetic androgen, mibolerone, from days 37 to 114 of gestation produced a daughter that at 2 days postnatal life exhibited profound masculinization of internal phallic anatomy without any obvious gross enlargement of the phallus. The retractor muscles in this androgen-treated female were observed in the ventral (male) position, the corpus spongiosum was present, the tunica albuginea surrounded both the corporal body and the urethra, and the normally pleated highly redundant female-like UGS had developed into a more compact urethra, all indicative of the male pattern (Fig. 11D).
Figure 6.

Three-dimensional reconstructions (3DR) (A, E & F) as well as sections (BD) of an adult male hyena penis exposed prenatally to flutamide (25mg/kg/day) from 32 to 110 days of gestation. (A) Side view with the skin (tan) semi-transparent to reveal internal structures. Note color-coding of labels throughout the figure. Prenatal flutamide has elicited the female pattern of the urethral orifice opening onto the surface ventral to the tip of the penis. (B) Distal section demonstrating the urethra (black arrowheads) opening ventrally. Large white arrow pointing to 3DR (B) indicates the position of section (A). (C) Section through the glanular erectile body (green in A & E) as indicated by the tip of the large white arrow pointing to 3DR (A). Note glanular erectile body (dotted lines), tunica (black arrowheads) only surrounding the corporal body (CB). The urethra (Ur) is large, pleated and ventrally positioned. (D) Proximal section through the retractor muscles (blue in A & F) as indicated by the tip of the large white arrow pointing to 3DR (A). Retractor muscles (dotted lines) are dorsal to the urethra (UR). The tunica (arrowheads) surrounds the corporal body (CB) only. (E) ¾ side view of proximal end of penis of a prenatally flutamide-treated adult male hyena. The arrangement of structures of this male specimen exhibits the female pattern (compare with Fig. 5C).
Figure 11.
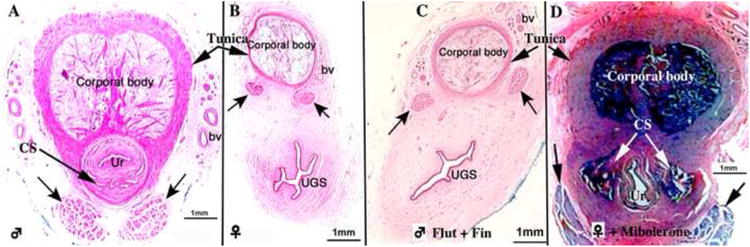
Sections though mid-phallus of (A) untreated male 95 days of gestation, (B) newborn untreated female, (C) a stillborn male spotted hyena whose mother was treated from day 21 to 94 of gestation with flutamide (50mg/kg/day) plus finasteride (2 mg/kg/day) (Flut + Fin), and (D) a newborn female spotted hyena whose mother was treated with mibolerone from 37-114 days of gestation (6.6/μg/kg/day). These four specimens are of roughly the same age (94 to 114 days of gestation). Note that sexual dimorphism (A & B) at this age corresponds exactly to that seen in adulthood (compare with Figures 4 & 5). Note that the pattern seen in the flutamide/finasteride-treated male (C) is virtually identical to that of the untreated female (B) in regard to (a) position of the retractor muscles, (b) size and shape of the urethra (Ur)/urogenital sinus (UGS), (c) presence or absence of the corpus spongiosum, and (d) extent of the tunica. (D) The mibolerone-treated female exhibits the male pattern. Black arrows in (A-D) denote the retractor muscles. Adapted from (Cunha et al., 2005) with permission. Intact male= ♂, Castrated male=
 , Intact female= ♀, Ovariectomized female=
, Intact female= ♀, Ovariectomized female=
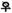
Role of postnatal androgens in phallic growth
Absence of a pubertal growth spurt
In the Berkeley spotted hyena colony, puberty occurs at 18-24 months of age in males and at 24-30 months of age in females. Maximal growth of the clitoris/penis paralleled skeletal growth (i.e., body length), occurring between 3-13 months of age, when androgen concentrations are low and well prior to the onset of puberty. In males 77% of skeletal growth and 82% of growth in phallic length occurred during a juvenile period (between 3 and 13 months of age). In females, 82% of skeletal growth, and 66% of clitoral growth occurred during this period. Full adult size, in terms of body length and clitoral/penile length, occurred between 14 to 30 months of age in both sexes (Glickman et al., 1998). Surprisingly, this interval was marked by deceleration of both phallic and skeletal growth despite the elevation in androgens and estrogens accompanying puberty in male and female spotted hyenas (Glickman et al., 1998). As noted elsewhere, the pubertal growth spurt in penile length/volume is characteristic of humans (Schoenfeld, 1943; Tanner, 1978), but not all mammals (Tanner, 1978). Obviously, spotted hyenas fall into the category of mammals that do not display accelerated phallic length during puberty.
Effects of prepubertal gonadectomy on penile and clitoral length
A limited role for postnatal androgens in determining stretched length of the adult hyena phallus is emphasized in studies in which pre-pubertal hyena infants/juveniles were castrated or ovariectomized at 1-7 months of age and compared with control groups of intact adult male and female hyenas after puberty (≥ 30 months). In this comprehensive evaluation of the effects of prepubertal gonadectomy, there were no statistical differences between stretched penile lengths in intact versus prepubertally castrated males when analyzed after puberty (≥ 30 months). Stretched clitoral length was modestly reduced in prepubertally ovariectomized versus intact females, and the effect was statistically significant based upon ANOVA analysis (Figure 12). Stretched phallic length was statistically greater in post-pubertal males versus females even though the differences were slight. Thus, gonadal hormones minimally affected postnatal growth in length of the penis and clitoris.
Figure 12.

Phallic length in millimeters of adult (≥ 30 months) intact males and females versus adult males and females gonadectomized prepubertally. Analysis of variance revealed that there were highly significant differences among the four groups (F = 21.74, df = 3, p<.0001). Post-hoc tests indicated that there was a significant sex difference between intact males and intact females in phallic length (Fisher PLSD, p<.0001), and between Intact Females and Ovariectomized Females in clitoral length (Fisher PLSD, p<.01). Prepubertal castration had no significant effect on penile length (Fisher PLSD, p=.36) Numbers indicate numbers of specimens per group. ♂ = Male ♀= Female
The role of estrogen in phallic development
An intriguing sexual dimorphism within the phallus was observed in specimens at 45 days of gestation in regard to the solid urethral plate, the precursor of the urethra. In males the solid urethral plate is attached to the dorsal ectoderm of the genital tubercle (Fig. 13A), while in females it is attached to the ventral ectoderm (Fig. 13B). This pattern corresponds to adult sexual dimorphism in the urethral/UGS meatus as described above (Figs. 4-5). Additional sexual dimorphisms at 45 days of gestation involving the corporal body rudiment and the tubular urethra were revealed through analysis of histologic sections and 3DRs (Fig. 13D). The corporal body rudiment dorsally overlapped the ventrally placed solid urethral plate in females but not in males. The tubular urethra extended more distally in males versus females (Fig. 13D). Surprisingly, the male pattern of sexual dimorphism was completely reversed in a 45-day male embryo whose mother was exposed continuously to letrozole (12.5mg/day) from 25 to 44 days of gestation (Fig. 13C).
Figure 13.
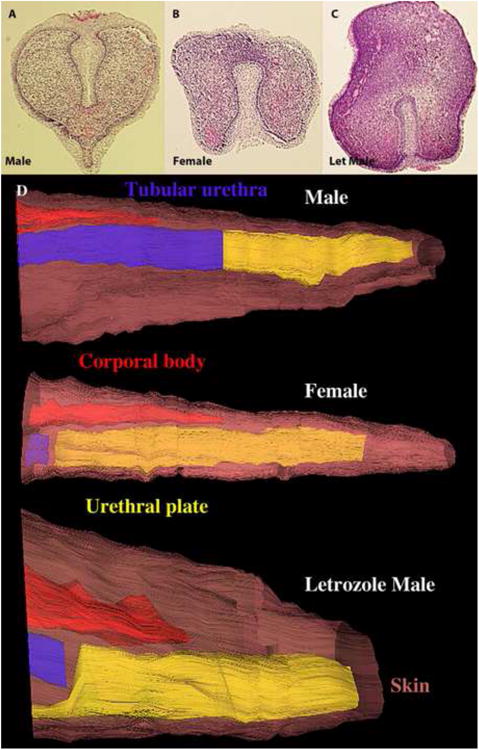
Transverse sections through the distal aspect of the phallus of an untreated 45-day male (A), an untreated female (B) and a hyena fetus treated from 25 to 45 days with letrozole (12.5mg/day to the pregnant mother). Note the attachment of the urethral plate, which is dorsal in the male (A), ventral in the female (B), and ventral in the letrozole-treated male (C). (D) Three-dimensional reconstructions of the three specimens above showing additional sexual dimorphism in regard to the relationship between the corporal body (CB, red), tubular urethra (Ur, blue) and solid urethral plate (UP, yellow). The brown skin has been rendered semi-transparent.
Letrozole is a drug that inhibits the enzyme aromatase, which converts testosterone to estradiol and other estrogens. We verified the effectiveness of this drug in our experiment via assays of sex steroids in maternal (pregnant) serum. Notably, letrozole treatment of dams markedly suppressed estrone and estradiol concentrations in maternal serum, as compared with control pregnancies at comparable gestational ages (Table 3). As expected, letrozole treatment was also associated with higher serum concentrations of total and free testosterone, when measured in near-term dams. The male-to-female shift in GT pattern observed at 45 days of gestation elicited by prenatal letrozole exposure consisted of the following: (a) the male urethral plate was attached ventrally (instead of dorsally), (b) the corporal body rudiment dorsally overlapped the solid urethral plate, and (c) the tubular urethra conformed to the female pattern (Fig. 13D). This male to female shift in patterning elicited by letrozole treatment, especially in the urethral plate, suggests a role for estrogen in development of the distal urethra/UGS within the glans. Because letrozole inhibits synthesis of estrogens, which may be important in pregnancy, this drug was administered either early or late in gestation, but not continuously throughout gestation. Accordingly, most hyena fetuses derived from letrozole-treated pregnancies were exposed during different time frames and consequently have a spectrum of malformations presumably dependent on the timing of letrozole exposure.
Table 3.
Serum sex steroid concentrations in the maternal circulation of spotted hyenas during control and letrozole-treated pregnancies.
| Subject | Condition | Gestational age at sample collection | Estrone (pg/ml) | Estradiol (pg/ml) | Total Testosterone (ng/ml) | Free Testosterone (pg/ml) |
|---|---|---|---|---|---|---|
| 56a | Control | 91 days | 6090 | 670 | 0.49 | <0.15 |
| 56b | Letrozole | 98 days | 220 | <5 | 2.88 | 1.34 |
| 49a | Control | 100 days | 4180 | 450 | 0.61 | <0.15 |
| 49b | Letrozole | 99 days | 52 | <5 | 1.38 | 2.51 |
One particularly interesting specimen is the daughter of a spotted hyena treated from 58 to 108 days of gestation with letrozole. This letrozole-treated female has profound hypospadias of the prepuce (Fig. 14D), but mated and gave birth despite her clitoral malformation. Two male fetuses, harvested at 68 days from mothers treated with letrozole from ∼23 to 67 days of gestation, showed disturbance in the midline raphe on the ventral surface of the penis and scrotum (compare Figs. 14A-C), indicative of incomplete midline fusion of the bilateral halves of the prepuce and scrotum. This abnormality seen at 68 days of gestation would be expected to generate a malformation similar to that seen in the prenatally letrozole-exposed adult female (Fig. 14D). Impaired midline fusion in specimens exposed prenatally to letrozole is also manifest as deep ventral grooves in the ExG seen at birth (not illustrated). Another male fetus was exposed to letrozole during the last 30 days of gestation and euthanized in adulthood. This male hyena displayed a hypoplastic prepuce, which failed to cover the glans penis (Fig. 14F), and a hypospadic urethral orifice on the ventral surface of the penis (Fig. 14G) (female pattern) (Place and Glickman, 2004), consistent with the effect of letrozole seen in the 45-day specimen described above (Fig. 13). Formation of the penile (or clitoral) urethra/UGS and prepuce is thought to involve midline fusion of bilateral urethral and preputial folds (Fig. 15). Our observations of prenatally letrozole-exposed hyenas implicate estrogen action in the epithelial-epithelial fusion events required for proper development of the prepuce and urethra/UGS.
Figure 14.
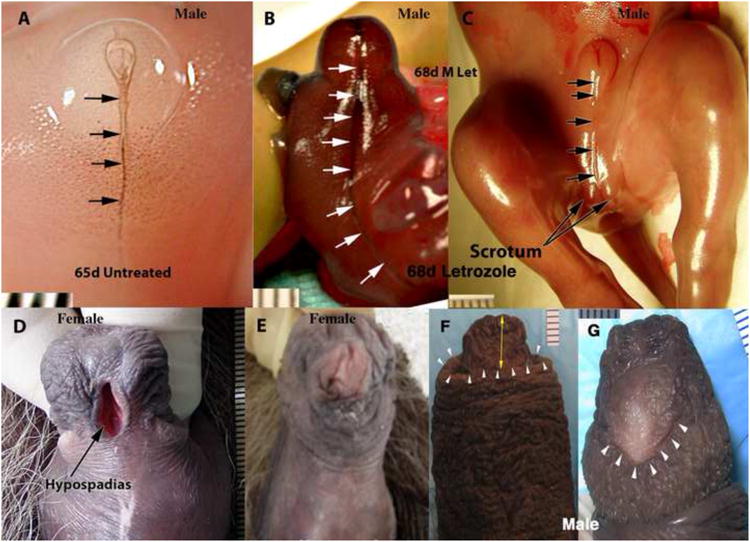
External genitalia of untreated (A and E) and letrozole-treated (B-D, F, G) hyenas. (A) ExG of a 68-day untreated male hyena fetus. Note sharply defined ventral raphe (arrows). (B) ExG of a 67-day male hyena fetus treated from 23 to 67 with letrozole (12.5mg/day to the pregnant mother). Note the deeply furrowed ventral raphe (white arrows). (C) ExG of a 68-day male hyena fetus treated from 23 to 68 with letrozole (12.5mg/day to the pregnant mother). Note the deeply furrowed ventral raphe and bifid scrotum. (D) Flaccid adult clitoris of a female hyena treated from 58 to 108 days of gestation with letrozole. Note prominent preputial hypospadias. (E) Flaccid adult clitoris of an untreated adult female hyena. (F) Dorsal view of the penis of an adult male hyena treated from days of gestation 82 to 109 with letrozole (12.5mg/day to the pregnant mother). Note that the tip of the penis (yellow double arrow) extends over 1cm beyond the distal edge of the prepuce (white arrowheads). Normally the prepuce completely covers the glans penis. (G) Ventral view of the letrozole-treated penis in (F). Note the inordinately large urethral orifice (hypospadias, white arrowheads) situated on the ventral aspect of the glans penis. Normally the urethra opens on the dorsal aspect of the glans penis. (D and E) from (Frank et al., 1990) with permission.
Figure 15.

Drawings of morphogenesis of the human penile urethra. The solid urethral plate canalizes to form the urethral groove, whose edges fuse in the midline to form the tubular penile urethra. Small opposing arrows indicate future fusion of adjacent epithelial surfaces. This developmental process has been observed in all species examined to date and appears to also apply to the spotted hyena. (adapted from Yamada, Satoh, Baskin, L. and Cunha, 2003 with permission)
Of course, it is important to recognize that as letrozole blocks aromatase and thus lowers serum and tissue levels of estrogen, there is a corresponding elevation in testosterone concentration and an alteration in the testosterone/estrogen ratio. However, given that the effects of prenatal letrozole exposure involved a variety of malformations in the developing offspring, including varying degrees of “feminization,” it seems unlikely that elevation in maternal/fetal androgens account for the observed malformations, especially since comparable effects were not seen in the mibolerone-treated specimen. It should also be noted that letrozole treatment during the latter stages of gestation produced no effects on fetal clitoral or penile length. In the two prenatally letrozole-exposed hyenas that displayed varying degrees of hypospadias, the adult male penile length of 172.5 mm and the adult female clitoral length of 136 mm placed them within the normal distributions for their sex.
Steroid receptor localization
Administration of anti-androgens, androgens or letrozole (which alters estrogen levels) can modify expression and signaling androgen (AR) and estrogen receptors (ER). Accordingly, immunohistochemical studies of the tissue distribution of AR, ERα and ERβ were undertaken. Table 4 summarizes these data and indicates a correlation between response to hormonal conditions (androgens, anti-androgens, as well as letrozole-altered serum androgen/estrogen levels) and the histo-localization of AR, ERα and ERβ˜ AR was detected in hyena ExG in the following structures: the corporal body, preputial lamina and associated mesenchyme, preputial skin and associated mesenchyme, retractor muscles (Cunha et al., 2005), glanular erectile bodies (GEB) and mesenchyme surrounding the urethra/UGS, but not in the epithelium of the urethra/UGS (Figs. 16-18). The two ER subtypes exhibited differential expression in so far as ERα was detected only in the corporal body and GEB (Figs. 16-18), while ERβ was more widespread and was detected in the corporal body, epithelia of the preputial lamina, preputial skin and urethra/UGS, but not in the mesenchyme associated with these epithelia (Figs. 16-18). ERα, ERβ and AR were co-expressed in the corporal body. Sections in which the primary antibodies were deleted exhibited no staining.
Table 4.
Immunohistochemical detection of androgen receptor (AR), estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) in male and female* fetal spotted hyena external genitalia.
| Structure | AR | ERα | ERPβ |
|---|---|---|---|
| Preputial lamina epithelium | + | - | + |
| Preputial lamina mesenchyme | + | - | - |
| Skin epithelium | + | - | + |
| Skin mesenchyme | + | - | - |
| Location of urethral orifice | + | - | + |
| Corporal body | + | + | + |
| GEB | + | + | ? |
| Urethral/UGE epithelium | - | - | + |
| Urethral mesenchyme | + | - | - |
| Retractor muscles | + | ND | ND |
Homologous male and female structures exhibited comparable steroid receptor expression. ND= not done.
Fig. 16.
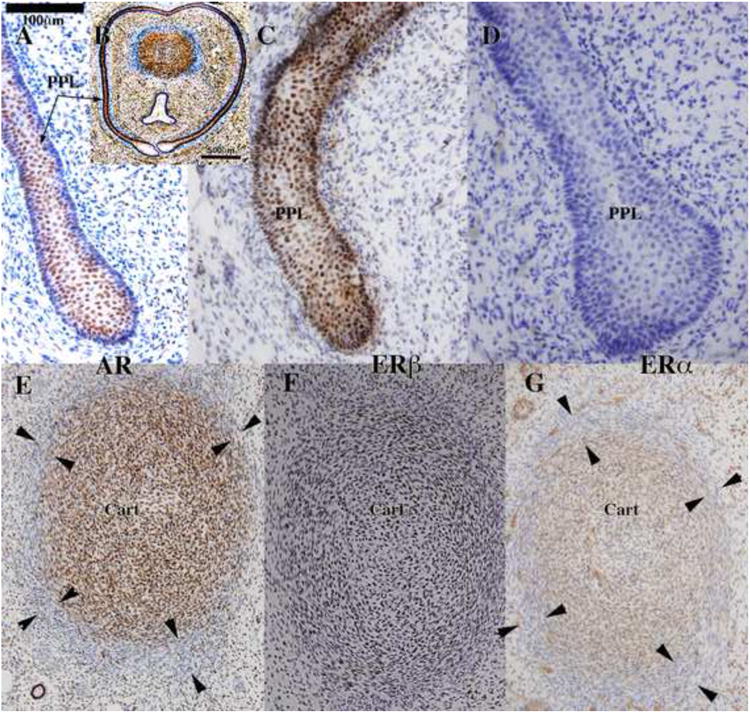
Androgen receptor (AR) (A, B, E), estrogen receptor beta (ERβ) (C and F) and estrogen receptor alpha (ERD) (D and G) immunohistochemistry of 62-day hyena clitoris. In (B) the preputial lamina circumscribes the clitoris, which contains a corporal body dorsally, and a UGS ventrally. The preputial lamina (PPL) is AR-positive (A), ERβ-positive (C), and ERα-negative (D). AR, ERα, and ERα were detected in cells of the corporal body (E-G). The tunica albuginea, which is apparent by virtue of its blue color in (B) and indicated by arrowheads in (E and G), exhibits reduced or negative staining for AR and ERα.
Fig. 18.

Androgen receptor (AR) immunohistochemistry of 68-day hyena penis. (A) Low magnification overview showing preputial lamina, tunica, urethra (Ur), corporal body (CB), and glanular erectile body (dotted lines). (B) Higher magnification of left box. Almost all cells within the corporal body (CB) are AR-positive. The tunica (between the two opposing arrows) consists of a mixture of AR-positive and AR-negative cells. (C) Higher magnification of right box in (A). The glanular erectile body contains a high percentage of AR-positive cells. (D) The urethral epithelium is mostly AR-negative, but is surrounded by AR-positive mesenchymal cells.
Discussion
Our current understanding of mammalian sex differentiation of the ExG is derived from the pioneering studies of Alfred Jost, who emphasized the key role of fetal testicular androgens. In the presence of androgens, ExG develop according to the male pattern, and in their absence the female pattern develops (Jost, 1953). While this concept has stood the test of time, other developmental mechanisms (which may be operative, but unrecognized in traditional animals) are revealed by analysis of exotic animals such as the spotted hyena (Cunha et al., 2005; Renfree and Short, 1988; Wilson et al., 2002; Glickman et al., 2005). Accordingly, data presented in this report/review support and extend our previous conclusions, i.e., (a) that androgens are responsible for some sex differences in external and internal phallic morphology in male and female spotted hyenas, but not for formation of the male or female hyena phallus (Drea et al, 1998; Cunha et al, 2005), (b) that patterns of normal clitoral and/or penile growth (i.e., length) are minimally affected by exogenous androgens. Accordingly, modest effects of prepubertal gonadectomy on phallic growth implicate a substantial role for non-androgenic mechanisms in regulating the length of the male and female hyena phallus (Cunha et al., 2005; Drea et al., 1998; Glickman et al., 1998). In addition, letrozole studies indicate that prenatal estrogens play an important role in formation and development of hyena ExG. Finally, our ovariectomy and estrogen replacement studies (Glickman et al., 1998) suggest that postnatal estrogens play a crucial role in facilitating such reproductively crucial events as elasticity of the urogenital meatus, which permits accommodation of the male during mating and birth through the clitoris (Drea et al., 2002; Frank et al., 1995).
These preceding conclusions are based on evidence from a number of sources, including data presented for the first time herein. We review these issues in turn, and then consider the implications of our findings suggesting that endogenous estrogens play an important role in development of ExG in spotted hyenas, with estrogens assuming at least some roles traditionally assigned in other species to androgens. Finally, we speculate that novel mechanisms, possibly involving ligand-independent activation of the spotted hyena androgen receptor, are responsible for formation and development of ExG in spotted hyenas. Since it is clear that androgens account for many sex differences in ExG of the spotted hyena, this hypothesis would require the simultaneous presence of both ligand-dependent and ligand-independent AR, albeit with tissue- and age-specific expression.
There is wide variation in ExG morphology in male and female mammals (Dixson, 1998; Simmons and Jones, 2007) with several species exhibiting clitoral “hypertrophy,” with or without a “clitoral urethra” (Dixson, 1998; Drea and Weil, 2008; Matthews, 1937; Petter-Rousseaux, 1964; Rubenstein et al., 2003). Existence of species variation in male and female ExG emphasizes the importance of maintaining a comparative perspective, and viewing the spotted hyena as providing information about novel developmental mechanisms operating in some species. While it is possible that penis-like clitoral development is androgen-regulated, other (androgen-independent) mechanisms certainly apply in some circumstances, as indicated by our anti-androgen studies of the spotted hyena and studies of certain North American moles (Rubenstein et al., 2003).
Androgens and Sex Differences in External Genitalia of Spotted Hyenas
First, as emphasized by Neaves et al and confirmed in our previous reports (Neaves et al., 1980; Cunha et al., 2005; Drea et al., 2002), sex differences in internal and external structure of the penis and clitoris of the spotted hyena are essential for successful reproduction. In particular, in males the penile urethra is small, inelastic, and surrounded by both corpus spongiosum and tunica albuginea, an arrangement that places severe limits on expansion of the urethral lumen. In contrast, in females the clitoral meatus is larger and more elastic, and the female UGS is pleated and not constrained by either a surrounding corpus spongiosum or tunica albuginea. Thus, expansion of the female UGS permits receipt of the penis during mating and passage of a 1.5 – 2 kg fetus at the time of parturition. Elasticity of the clitoral meatus is facilitated by action of estrogens (Glickman et al., 1998) and relaxin, whose concentration peaks just prior to birth (Steinetz et al., 1997). Delivery through the clitoris is very difficult in first pregnancies, and stillbirths occur approximately 60% of the time in primiparous female spotted hyenas (Frank et al., 1995). Such difficulties, presumably due to the anatomy of the female penile UGS, appear to be eliminated in adult female spotted hyenas exposed prenatally to anti-androgens (Drea et al., 2002). Changes in clitoral morphology, subsequent to prenatal anti-androgen treatment, enable successful passage of the fetus through the clitoris of the primipara female. While the consequence of natural prenatal androgens impairs adult female reproductive success (primiparous stillbirths), natural prenatal androgen exposure is essential for execution of successful adult male mating. Note that while substantial penile length persists in prenatally anti-androgen-treated adult males, intromission is much more difficult (if not impossible) for such males because of a slight reduction in penile length and, perhaps more importantly, glans shape is round (instead of chisel-shaped) (Drea et al., 1998; Drea et al., 1999; Drea et al., 2002).
Anti-androgen treatment, from the earliest stages of fetal life, fail to prevent formation of the penis/clitoris or scrotum/pseudo-scrotum of the spotted hyena, as all anti-androgen-treated offspring were born with a penis or clitoris containing a urethra or UGS exiting at the tip and a scrotum/pseudo-scrotum as reported previously (Drea et al., 1998) and presented herein. Advanced development of male and female GT prior to acquisition of steroidogenic activity of the gonads provides additional support for the idea that formation of the penis, clitoris, scrotum/pseudo-scrotum in the spotted hyena is independent of androgen action. In contrast, in rodents and dogs treatment with anti-androgens at doses comparable to that used herein (i.e., flutamide at 25 mg/kg) during critical stages of development, resulted in males with small penises no longer traversed by a urethra exiting at the tip as well as a blind vaginal pouch (Imperato-McGinley et al., 1992; Neumann et al., 1970; Neri et al., 1972).
While formation of the hyena penis and clitoris appears to be androgen-independent, certain male features in hyena ExG (both external and internal morphology of the penis [see Table 1]) are eliminated or “feminized” by prenatal anti-androgens (flutamide or casodex alone or in combination with finasteride) with persistence of anti-androgen effects into adulthood as reported earlier (Cunha et al., 2005; Drea et al., 2002) and confirmed herein (Figs. 6, 7, 11). Furthermore, the importance of androgens in penile development was corroborated in a stillborn female exposed in utero to mibolerone (a synthetic androgen), which displayed “masculine” structural characteristics in her ExG (Fig. 11).
Female fetuses exposed in utero to anti-androgens maintained their typical female sex-specific features into adulthood and additionally exhibited enhanced diameter of the glans and a much larger and more elastic urogenital meatus, which as stated above facilitated reproduction (Drea et al., 1998; Drea et al., 2002). In sum, the results of our experiments with anti-androgens, as well as treatment with a synthetic androgen, indicate that biologically crucial sex differences in clitoral and penile morphology are dependent upon androgen action at critical stages of development. It is likely that for each male/female feature, a precise temporal “window of sensitivity” may exist for eliciting either the male or female pattern as demonstrated for internal and external genitalia in rats and mice (Rodriguez et al., 2012; Welsh et al., 2010; Welsh et al., 2008).
Response of developing hyena ExG to anti-androgens and androgen (mibolerone) implies the presence of androgen receptors (AR) in the developing ExG. Dorsal versus ventral position of the retractor muscles is sexually dimorphic, is sensitive to both anti-androgens and mibolerone, and is correlated with expression of AR in the developing retractor muscles (Cunha et al., 2005). Likewise, shape and positioning of the GEB (erectile bodies that determine shape of the glans) are sensitive to anti-androgens, and these structures expressed AR (as well as ERα, implying dual regulation of distal GEB development. The corpus spongiosum (which is derived from AR-positive peri-urethral mesenchyme) is present only in males, is inhibited in males with anti-androgens and induced in females by the synthetic androgen, mibolerone. Shape of the male urethra/female UGS is sexually dimorphic and affected by exogenous androgen as well as anti-androgens, and thus could be regulated through AR expressed in urethral mesenchyme via paracrine mesenchymal-epithelial signaling, an important developmental mechanism in both internal and external genitalia (Cunha et al., 2004; Murakami, 1987). The epidermis and dermis of developing hyena ExG are destined to form the outer surface of the male and female prepuce, while epithelium of the preputial lamina and associated mesenchyme form the inner surface of the prepuce and the outer surface of the penis/clitoris. Both of these epithelia and associated mesenchyma express AR, thus providing a plausible mechanism for explaining the effects of anti-androgens on these structures. As described above, the glans penis is chisel-shaped with a dorsal urethral orifice, while the glans clitoris is blunt with a ventral UGS orifice. Both androgens (and estrogens, as shown by administration of letrozole) affect the position of the urethral orifice, and AR, ERα and ERβ are present in these developing tissues/structures. Finally, the tunica albuginea is a sexually dimorphic androgen-dependent/sensitive structure that surrounds the corporal body and urethra in males but surrounds only the corporal body in females. Cells forming the periphery of the corporal body and thus destined to form the tunica albuginea are AR-positive. Thus, virtually all of the effects of androgen and anti-androgen are corroborated by the presence of AR in the cells/tissues/rudiments responding to such hormonal treatments. Expression of AR in the developing male and female hyena ExG corresponds closely to that seen in other mammals including human as reported previously [see Table 2 in (Rodriguez et al., 2012)], thus corroborating the idea that hormonal response in the developing ExG is mediated by the appropriate steroid receptors in the specific responsive cells/tissues. Given the more extensive distribution of ERβ versus ERα, it is evident that ERβ is the dominant ER subtype in developing hyena ExG, an observation similar to findings in the mouse (Blaschko et al., 2013; Rodriguez et al., 2012). While signaling through ERβ may be particularly important by virtue of it wide distribution, it is possible that ERα signaling, or lack thereof, is equally significant.
Gonadal and placental androgens and formation of the genital tubercle
There are three major sources of androgens in the developing hyena fetus: the mother, fetal gonads and the placenta. The genital tubercle of the spotted hyena appears between day 28 and 30 days of gestation as reported previously (Cunha et al., 2005) and in Figures 8-9 (this paper). As demonstrated previously, a well-formed GT containing a urethral plate is already in place by 30 to 32 days of gestation in both female and male spotted hyenas (Fig. 9) (Cunha et al., 2005; Licht et al., 1998) when steroidogenic activity of the undifferentiated testes is low at best and completely absent in fetal ovaries (Browne et al., 2006). The first signs of ovarian androgen synthetic capacity were detected at day 45 and increased thereafter to high levels. Thus, initial male and female GT development cannot be attributed to androgens secreted by the fetal gonads, as advanced GT development is present well before testicular and ovarian morphology is established and well before gonads are capable of synthesizing steroid hormones (Browne et al., 2006).
The placenta of the spotted hyena is another source of androgen for the spotted hyena fetus. Previously, we determined that androstenedione in the maternal circulation is converted to testosterone by the placenta, which is transferred to the developing fetus via the umbilical vein (Licht et al., 1992; Yalcinkaya et al., 1993). However, aromatase, also present at high levels in placenta, would be expected to convert testosterone to estradiol at the time when sex differences are organized by androgens (Conley et al., 2007), thus protecting the female spotted hyena fetus from maternal androgens, much as it does in humans (Siiteri and Thompson, 1975).
Absence of a pubertal growth spurt and minimal effects of prepubertal gonadectomy on penile/clitoral length
A pubertal growth spurt is characteristic of the human penis (Tanner, 1978), whose length increases 75% between 11.5 and 17 years of age, presumably because of the stimulating effects of testosterone associated with adolescence (Schoenfeld, 1943). The most rapid acceleration of ExG growth in both male and female spotted hyenas occurs between 3 and 13 months of age, when circulating concentrations of gonadal steroids are particularly low (Glickman et al., 1998). The rapid growth of the male and female phallus during such early periods is critical in the meeting ceremonies of spotted hyenas and has broad implications. As described by Hans Kruuk when engaged in such ceremonies, two spotted hyenas stand adjacent to one another, head-to-tail, and commonly inspect the erect penis or clitoris of their partner. Hyena etiquette demands that the subordinate hyena offer its erect organ to the dominant hyena, who may, or may not, reciprocate (Kruuk, 1972; East et al., 1993). Kruuk observes that meeting ceremonies commonly involve cubs, and that: “…the morphological structures associated with the meeting ceremony are very well developed, relatively even more so than in adults; the penis or clitoris of a cub is almost as large in an absolute sense as that of an adult” (Kruuk, 1972). Since incorporation of juvenile hyenas into the social group begins well before puberty (Holekamp and Smale, 1998), it is not surprising that development of the penis or clitoris (with concomitant erectile capability) occurs before androgens are secreted in volume from the adolescent/adult gonads (Fig. 3C). Accordingly, in a group of captive spotted hyenas, phallic growth paralleled bodily growth, with maximum growth acceleration taking place between 3 - 13 months of age, well before the pubertal rise in androgens (Glickman et al., 1998).
The relative independence of penile/clitoral growth from gonadal androgens is also supported by the relatively normal growth of these organs in male and female hyenas following prepubertal gonadectomy (Fig. 12) (Glickman et al., 1992; Glickman et al., 1998). Prepubertal castration results in a marked failure in penile growth in all mammals studied to date (Beach et al., 1983; Beach and Levinson, 1950). In contrast, in spotted hyenas prepubertal gonadectomy had no effect on growth in length of the penis and only modest reduction in length of the clitoris when examined at ≥2 years of age when adult phallic size is normally achieved (Fig. 12). Thus, several lines of evidence corroborate the view that initial formation of the hyena GT and subsequent increase in phallic length are androgen-independent, which is in stark contrast with almost all other mammals. Conversely, development of ExG sex-specific external and internal morphology is androgen-dependent in hyenas.
Endogenous Estrogens and Formation, Differentiation and Development of External Genitalia
As previously noted, we had gathered data on the effects of prepubertal ovariectomy on clitoral growth. Removal of the ovaries in 4 to 7 month old hyenas had minimal effects on clitoral length, but did reduce size and elasticity of the urogenital meatus in adulthood. These changes elicited by ovariectomy were predictably accompanied by a marked reduction in circulating estrogens, whose effects were reversed by administration of oral estrogens (Glickman et al., 1998). Given these postnatal effects of estrogens on ExG of female spotted hyenas, we examined the effects of prenatal estrogen deprivation, which was accomplished by administration of an aromatase inhibitor (letrozole) to pregnant female spotted hyenas. Aromatase inhibitors prevent the formation of estrogens from androgenic pro-hormones. Since estrogens may be required for vasodilation and appropriate uterine blood flow to and from the fetus, we restricted treatment to either early or late stages of gestation. The effectiveness of letrozole treatment was verified by a major decrease in circulating estrogens in the maternal circulation (Table 3).
Letrozole administered to pregnant female spotted hyenas had surprising and unpredicted effects on ExG development. Position of the urethral orifice (dorsal in males and ventral in females) is hormonally regulated in so far as this feature is (a) sexually dimorphic (Figs. 4-5), and (b) a male to female shift in the urethral orifice is elicited by anti-androgen (Figs. 5 &7) as well as (c) by letrozole (Fig. 14). Sexual dimorphism of urethral orifice position in adulthood appears to be a consequence of the dorsal (male) versus ventral (female) attachment of the urethral plate in the fetal phallus (Fig. 13). A male fetus, treated with letrozole from 25 to 45 days of gestation and examined at day 45 of gestation, exhibited the female (ventral) urethral plate pattern (Fig. 13), which also led to ventral positioning of the urethral orifice in an adult male treated prenatally with letrozole (Fig. 15). Likewise, letrozole treatment of a pregnant hyena, between days 23 and 68 of gestation produced a male fetus with an exceptionally deep “groove” on the ventral surface of the genital tubercle and the scrotum (Fig. 14). Treatment of pregnant females during later stages of gestation, when sex differences are “consolidated” and sex-typical growth occurs, resulted in male and female offspring that displayed preputial and urethral hypospadias as reported previously (Place and Glickman, 2004) and in this paper (Figs. 14). Such hypospadias was more dramatic in a female treated from mid-gestation to birth (Fig. 14D), than in a male, whose treatment was limited to the last 30 days of gestation (Fig. 14G).
In a general sense letrozole (which substantially reduced estrogen levels) impaired development of the prepuce and the distal aspect of the urethra via: (a) Specification of the position of the urethral plate and thus the final position of the urethral orifice (either on the dorsal [male] or ventral [female] aspect of the glans penis/clitoris). (b) Impairment of epithelial-epithelial fusion processes leading to preputial or urethral hypospadias. Persistence of deep grooves on the ventral aspect of the phallus, scrotum/pseudo-scrotum (Fig. 14) also provide clear evidence of perturbation of formation of the normal midline raphe, and speak to perturbation of a common mechanism, namely impaired midline epithelial-epithelial fusion, the known mechanism for formation of the penile urethra (presumably also the clitoral UGS), prepuce and scrotum (pseudo-scrotum) (Moore and Persaud, 2003; Yamada et al., 2003).
The question arises as to whether the effect of letrozole on ExG development is due to reduction in estrogens, elevation in testosterone or alteration in the balance of estrogen/androgen signaling. While definitive answer to this question is beyond the scope of the present investigation, elevation in testosterone is incompatible with the finding that anti-androgens (flutamide and casodex) elicit this same effect. The more likely scenario is that estrogen plays an important role in normal male/female positioning of the urethral orifice, and thus reduced estrogen synthesis elicited by letrozole is the likely cause of the male to female shift in position of the urethral orifice. Given traditional assumptions about androgenic effects on penile development (Jost, 1953; Wilson et al., 1981), a naïve observer would have suspected interference with androgenic effects during gestation. However, our observations raise the distinct possibility that estrogens have assumed the role of androgens in regulating and promoting epithelial fusion events in spotted hyenas. Finally, it is worth noting that while reduction in estrogen levels impairs epithelial fusion events in spotted hyenas, conversely in mice elevation in estrogen levels during ExG development is associated with impaired epithelial fusion events and hypospadias (Blaschko et al., 2013). Of course, in both species the balance of estrogen/androgen signaling was perturbed experimentally.
If Not Androgens, What Mechanisms are Responsible for Formation of the Phallus/Scrotum of the Spotted Hyena?
In 1992 Yalcinkaya, Cunha and Siiteri reported some puzzling failures of binding of androgens to the androgen receptor (AR) in fibroblast cultures derived from the genital skin of spotted hyenas, despite detection of AR in the cultures by immunohistochemistry (Yalcinkaya et al., 1992). This unexplained disparity also occurs in cells from more common laboratory animals (unpublished observations). However, this observation led Yalcinkaya et al to “…suggest that the (spotted hyena) AR may have lost its ligand-binding ability and become constitutively active” (Yalcinkaya et al., 1993). These provocative findings led Michael McPhaul and colleagues to explore the molecular structure of the hyena AR. As described previously, “Although a limited number of amino acid substitutions are present within the ligand binding and DNA binding domains of the spotted hyena androgen receptor, functional studies in heterologous cells demonstrate no qualitative differences between spotted hyena and human androgen receptors under basal conditions or in response to ligand (Catalano et al., 2002)”
The existence of a “complete” ligand-dependent AR in the spotted hyena is in accord with the numerous effects of anti-androgen and androgen treatments on the differentiation and development of the ExG of male and female spotted hyenas, as reported previously (Cunha et al., 2005; Drea et al., 1998; Drea et al., 2002) and herein (Figs. 6-7), as well as elimination of male mating behavior following castration (unpublished observations). However, if both ligand-dependent and ligand-independent ARs were present and differentially expressed in hyena genital tissues, the puzzling array of androgen-dependent and androgen-independent effects could be explained, including (a) the near-normal growth of the penis/clitoris following prepubertal gonadectomy and (b) the very limited effects of prenatal anti-androgen treatment on formation and development of the penis/clitoris and scrotum/pseudo-scrotum.
In recent years, numerous mutations in the human AR have been identified and linked with “castration-resistant” or “hormone-refractory” prostate cancer (Bergerat and Ceraline, 2009; Hu et al., 2009; McPhaul, 2008). This is a very complex literature, but constitutive androgenic activity has been observed in the AR-V7 variant (in which the ligand-binding domain of the AR is deleted), providing a complete AR is also present in the cells under study (Watson et al., 2010). Such constitutively active ARs lacking the ligand-binding domain, exhibit a molecular weight of approximately 70-80 kD in Western blots, while full length ligand-dependent ARs have a weight of 110 kD. Following the studies cited above, McPhaul and collaborators analyzed Western blots of genital tissues from spotted (McPhaul et al., 2010) and striped hyenas (striped hyenas have normal internal and external genitalia) and demonstrated ARs at 70 kD and 110 kD (McPhaul, unpublished) using an antibody targeted at the N-terminal of the AR.
McPhaul's comparisons of striped and spotted hyenas call attention to the power of conducting studies in which the unique features of spotted hyenas are compared with the remaining three extant species in the family Hyaenidae. Hammond and his colleagues have initiated such work with sex hormone binding proteins (SHBG's), agents that normally sequester/inactivate steroid hormones in serum. In the studies of Hammond et. al., it was determined that changes in the molecular structure of SHBG result in unusual patterns of steroid hormone binding affinity in all the extant hyaenids (Hammond et al., 2012). Surprisingly, concentrations of SHBG's were lower in spotted hyenas than in the other three species of Hyaenids, resulting in greater abundance of “free” and active steroid hormones in spotted hyenas.
Finally, estrogen receptor activity has also been implicated in prostate cancer (Risbridger et al., 2007; Carruba, 2007), with the nature of the effect varying with the specific ER activated, i.e., ERα facilitating tumor growth and ERα inhibiting tumor growth (Risbridger et al., 2007; Carruba, 2007). All of the unusual phenomena cited in this review might be explained by the existence of ligand-independent AR resulting from deletion of the ligand-binding domain with activation of ERs regulating the abundance of such constitutively active ARs. Alternatively, it is possible that estrogens have assumed some of the roles traditionally assigned to androgens, with regard to an array of epithelial fusion events in the clitoris/penis and scrotum/pseudo-scrotum.
Analysis of development of the ExG of the spotted hyena has demonstrated that the penis and clitoris (known hormone-target organs) can simultaneously exhibit both hormone-dependent as well as hormone-independent developmental mechanisms in so far as the initial formation of the genital tubercle as well as its subsequent growth are largely androgen-independent in both males and females, even though several aspects of internal phallic morphology are clearly androgen-dependent. In addition, study of this fascinating animal has implicated an important role of estrogen or the balance between estrogen-androgen signaling in normal development of the ExG. Given the widespread distribution of ERβ (versus ERα), future research on normal and abnormal development (hypospadias) of ExG should be directed towards events downstream of ERβ signaling, focusing on developmental mechanisms such as epithelial-epithelial fusion events common to development of all mammalian ExG.
An Evolutionary Note
Given that spotted hyenas are the only extant female mammals that in the absence of an external vaginal opening mate and give birth through a penile clitoris, it is not surprising that numerous writers have speculated regarding the evolutionary impetus for this arrangement. As previously noted, Kruuk assigned a critical selective role for the development of an organ in the female spotted hyena that permitted participation in meeting ceremonies without additional speculation regarding physiological substrates (Kruuk, 1972). However, following the role traditionally assigned to androgens in the formation of the masculine phenotype in the ExG (Jost, 1953) and the common assumption that androgens facilitate aggression and dominance (Monaghan and Glickman, 1992), it is not surprising that androgenic involvement has been assumed to play a significant role in the evolution of the ExG of the female spotted hyena. However, the evolutionary scenarios vary markedly among theorists. Gould and Vrba suggest that androgens might originally have been linked with size, dominance and social status of female spotted hyenas, and that “masculinized” ExG were a byproduct (or “exaptation”), albeit that selection could then operate on this “exaptation” (Gould and Vrba, 1982). Hamilton et. al. propose a related hypothesis, but focus on the highly competitive feeding that occurs in spotted hyenas, but not in brown or striped hyenas (Hamilton et al., 1986). They emphasize that since fully “masculinzed” ExG are only observed in spotted hyenas and since among the hyaenids only spotted hyenas engage in such competitive feeding, that female size, aggressiveness and dominance conferred a special advantage among female Crocuta. They then follow Gould in suggesting that such androgenization: “…could lead to initial virilization of the female external genitalia, namely that these ExG rudiments were incorporated into a novel genital display --- or possibly one already occurring in males --- leading to further selection for enlarged and fully erectile phalluses in females” (Hamilton et al., 1986). Others have presented additional interesting and novel evolutionary routes (East et al., 1993; Muller and Wrangham, 2002). However, given the limited role for androgens detailed in this paper and the new focus on estrogenic involvement, it seems likely that a full understanding of the actual mechanisms involved in formation of the extraordinary ExG of the female spotted hyena will both constrain and enrich evolutionary reconstruction of such problems --- particularly when viewed in an appropriately comparative perspective (Autumn et al., 2002).
Supplementary Material
Figure 17.
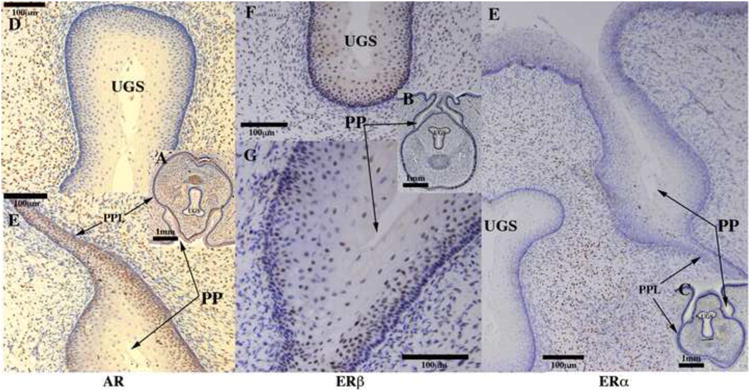
Androgen receptor (AR) (A, D, E), estrogen receptor beta (ERβ) (B, F, G) and estrogen receptor alpha (ERα) immunohistochemistry (C, E) of a 62-day hyena clitoris. AR was detected in the preputial lamina (PPL), preputial skin and mucosa (PP), and mesenchyme surrounding the UGS (D), but was undetectable in the epithelium of the UGS (F). ERβ was detected in the epithelium of the UGS (F) and preputial skin and mucosa (PP). ERα was detected in the mesenchyme surrounding the UGS (E), but was undetectable in the epithelia of the UGS, preputial lamina, and preputial skin and mucosa (PP).
Highlights.
Female spotted hyenas mate and give birth thru a penis-like clitoris.
Formation of male and female external genitalia (ExG) is androgen-independent.
Growth of male and female ExG is androgen-independent.
Sexual dimorphism in ExG is regulated by androgens and estrogens.
Estrogens appear to be involved in epithelial-epithelia fusion.
Acknowledgments
This study was supported by NSF grants IOS-0920793 and HD07684, NIH grants MH39917, DK0581050, NH&MRC grant APP1002733 and Canadian Institutes of Health Research Grant MOP-15261. The authors dedicate this paper to Dr. Penti Siiteri, a dear friend and a brilliant scientist, who had an abiding interest in this study.
Abbreviations
- ExG
external genitalia
- GT
genital tubercle
- DHT
dihydrotestosterone
- T
testosterone
- UGS
urogenital sinus
- IHC
immunohistochemistry
- E1
estrone
- E2
estradiol
- RIA
radioimmunoassays
- GEB
glanular erectile bodies
- 3DR
three-dimensional reconstruction
- HUGE
hyena urogenital glanular extension
- AMH
anti-Müllerian hormone
- AR
androgen receptor
- ER, ERα or ERβ
estrogen receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Autumn K, Ryan MJ, Wake DB. Integrating historical and mechanistic biology enhances the study of adaptation. Quart Rev Biol. 2002;77:383–408. doi: 10.1086/344413. [DOI] [PubMed] [Google Scholar]
- Barnes J. The Complete Works of Aristotle. Vol. 1. Princeton University Press; Princeton: 1985. [Google Scholar]
- Beach FA, Buehler MG, Dunbar IF. Sexual cycles in female dogs treated with androgen during development. Behav Neural Biol. 1983;38:1–31. doi: 10.1016/s0163-1047(83)90339-4. [DOI] [PubMed] [Google Scholar]
- Beach FA, Levinson G. Effects of androgen on the glans penis and mating behavior of male rats. J Exptl Zool. 1950;114:159–168. [Google Scholar]
- Bergerat JP, Ceraline J. Pleiotropic functional properties of androgen receptor mutants in prostate cancer. Human mutation. 2009;30:145–157. doi: 10.1002/humu.20848. [DOI] [PubMed] [Google Scholar]
- Blaschko SD, Mahawong P, Ferretti M, Cunha TJ, Sinclair A, Wang H, Schlomer BJ, Risbridger G, Baskin LS, Cunha GR. Analysis of the effect of estrogen/androgen perturbation on penile development in transgenic and diethylstilbestrol-Treated mice. Anat Rec (Hoboken) 2013;296:1127–1141. doi: 10.1002/ar.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne P, Place NJ, Vidal JD, Moore IT, Cunha GR, Glickman SE, Conley AJ. Endocrine differentiation of ovaries and testes of the spotted hyena (Crocuta crocuta): timing of androgen-independent versus androgen-driven genital development. Reproduction. 2006;132:649–659. doi: 10.1530/rep.1.01120. [DOI] [PubMed] [Google Scholar]
- Carruba G. Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario. J Cell Biochem. 2007;102:899–911. doi: 10.1002/jcb.21529. [DOI] [PubMed] [Google Scholar]
- Catalano S, Avila DM, Marsico S, Wilson JD, Glickman SE, McPhaul MJ. Virilization of the female spotted hyena cannot be explained by alterations in the amino acid sequence of the androgen receptor (AR) Mol Cell Endocrinol. 2002;194:85–94. doi: 10.1016/s0303-7207(02)00179-x. [DOI] [PubMed] [Google Scholar]
- Chapman. Observances on the female generative apparatus of Hyaena crocuta. Proc Acad Natural Sci Philad. 1888:189–191. [Google Scholar]
- Conley AJ, Corbin CJ, Browne P, Mapes SM, Place NJ, Hughes AL, Glickman SE. Placental expression and molecular characterization ofardfcatase cytochrome P450 in the spotted hyena (Crocuta crocuta) Placenta. 2007;28:668–675. doi: 10.1016/j.placenta.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Place NJ, Baskin L, Conley A, Weldele M, Cunha TJ, Wang YZ, Cao M, Glickman SE. The ontogeny of the urogenital system of the spotted hyena (Crocuta crocuta Erxleben) Biol Reprod. 2005;73:554–564. doi: 10.1095/biolreprod.105.041129. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Wang Y, Place NJ, Liu W, Baskin L, Glickman SE. Urogenital system of the spotted hyena (Crocuta crocuta Erxleben): A functional histological study. J Morphol. 2003;256:205–218. doi: 10.1002/jmor.10085. [DOI] [PubMed] [Google Scholar]
- Davis D, Story H. The female external genitalia of the spotted hyena. Fieldiana Zool. 1949;31:277–283. [Google Scholar]
- Dixson AF. Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes, and Human Beings. Oxford University Press; Oxford: 1998. [Google Scholar]
- Drea C, Coscia E, Glickman S. Hyenas. In: Knobil E, Neill J, Licht P, editors. Encyclopedia of Reproduction. Academic Press; San Diego: 1999. pp. 718–724. [Google Scholar]
- Drea CM, Place NJ, Weldele ML, Coscia EM, Licht P, Glickman SE. Exposure to naturally circulating androgens during foetal life incurs direct reproductive costs in female spotted hyenas, but is prerequisite for male mating. Proc R Soc Lond B Biol Sci. 2002;269:1981–1987. doi: 10.1098/rspb.2002.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drea CM, Weil A. External genital morphology of the ring-tailed lemur (Lemur catta): females are naturally “masculinized”. J Morphol. 2008;269:451–463. doi: 10.1002/jmor.10594. [DOI] [PubMed] [Google Scholar]
- Drea CM, Weldele ML, Forger NG, Coscia EM, Frank LG, Licht P, Glickman SE. Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 2. Effects of prenatal anti-androgens. J Reprod Fertil. 1998;113:117–127. doi: 10.1530/jrf.0.1130117. [DOI] [PubMed] [Google Scholar]
- East ML, H H, Wickler W. The erect ‘penis’ is a flag of submission in a female dominated society: greetings in Serengeti spotted hyenas. Behav Ecol Sociobiol. 1993;33:355–370. [Google Scholar]
- Forger NG, Frank LG, Breedlove SM, Glickman SE. Sexual dimorphism of perineal muscles and motoneurons in spotted hyenas. J Comp Neurol. 1996;375:333–343. doi: 10.1002/(SICI)1096-9861(19961111)375:2<333::AID-CNE11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Frank L, Glickman S, Powch I. Sexual dimorphism in the spotted hyaena (Crocuta crocuta) J Zool Lond. 1990;221:308–313. [Google Scholar]
- Frank L, Weldele M, Glickman S. Maculinization costs in hyaenas. Nature. 1995;377:584–585. doi: 10.1038/377584b0. [DOI] [PubMed] [Google Scholar]
- Frank LG, Glickman SE. Giving birth through a penile clitoris: parturition and dystocia in the spotted hyaena (Crocuta crocuta) Journal of Zoology. 1994;234:659–665. [Google Scholar]
- Funk H. Gordon's discovery of the spotted hyeaena's extraordinary genitalia in 1777. J Hist Biol. 2012;45:301–328. [Google Scholar]
- Glickman S, Frank L, Pavgi S, Licht P. Hormonal correlates of ‘masculinization’ in female spotted hyaenas (Crocuta crocuta). 1. Infancy to sexual matuity. J Reprod Fert. 1992;95:451–462. doi: 10.1530/jrf.0.0950451. [DOI] [PubMed] [Google Scholar]
- Glickmai SE. The spotted hyena from Aristotle to The Lion King: Reputation is everything. Social Research. 1995;62:501–537. [Google Scholar]
- Glickman SE, Coscia EM, Frank LG, Licht P, Weldele ML, Drea CM. Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 3. Effects of juvenile gonadectomy. J Reprod Fertil. 1998;113:129–135. doi: 10.1530/jrf.0.1130129. [DOI] [PubMed] [Google Scholar]
- Glickman SE, Cunha GR, Drea CM, Conley AJ, Place NJ. Mammalian sexual differentiation: lessons from the spotted hyena. Trends Endocrinol Metab. 2006;17:349–356. doi: 10.1016/j.tem.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Glickman SE, Short RV, Renfree MB. Sexual differentiation in three unconventional mammals: spotted hyenas, elephants and tammar wallabies. Horm Behav. 2005;48:403–417. doi: 10.1016/j.yhbeh.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Gould SJ, Vrba ES. Exaptation - A missing term in the science of form. Paleobiology. 1982;8:4–15. [Google Scholar]
- Grimpe G. Hyanologische studien. Zool Anz. 1916;48:49–64. [Google Scholar]
- Hamilton WJI, Tilson RL, Frank LG. Sexual monomorphism in spotted hyenas. Ethology. 1986;71:63–73. [Google Scholar]
- Hammond GL, Miguel-Queralt S, Yalcinkaya TM, Underhill C, Place NM, Glickman SE, Drea CM, Wagner AP, Siiteri PK. Unique properties of sex hormone binding globulin in the spotted hyena (Crocuta crocuta) Endocrinol. 2012;153:1435–1443. doi: 10.1210/en.2011-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WCO. Primates: Comparative Anatomy and Taxonomy. Edinburgh University Press; London: 1953. [Google Scholar]
- Holekamp KE, Smale L. Dispersal status influences hormones and behavior in the male spotted hyena. Horm Behav. 1998;33:205–216. doi: 10.1006/hbeh.1998.1450. [DOI] [PubMed] [Google Scholar]
- Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato-McGinley J, Sanchez RS, Spencer JR, Yee B, Vaughan ED. Comparison of the effects of the 5a-reductase inhibitor finasteride and the antiandrogen flutamide on prostate and genital differentiation: dose-response studies. Endocrinology. 1992;131:1149–1156. doi: 10.1210/endo.131.3.1324152. [DOI] [PubMed] [Google Scholar]
- Jost A. Problems of fetal endocrinology: The gonadal and hypophyseal hormones. Rec Prog Horm Res. 1953;8:379–418. [Google Scholar]
- Kruuk H. The Spotted Hyena. University of Chicago Press; Chicago: 1972. [Google Scholar]
- Licht P, Frank LG, Pavgi S, Yalcinkaya TM, Siiteri PK, Glickman SE. Hormonal correlates of ‘masculinization’ in female spotted hyaenas (Crocuta crocuta). 2. Maternal and fetal steroids. J Reprod Fertil. 1992;95:463–474. doi: 10.1530/jrf.0.0950463. [DOI] [PubMed] [Google Scholar]
- Licht P, Hayes T, Tsai P, Cunha G, Kim H, Golbus M, Hayward S, Martin MC, Jaffe RB, Glickman SE. Androgens and masculinization of genitalia in the spotted hyaena (Crocuta crocuta). 1. Urogenital morphology and placental androgen production during fetal life. J Reprod Fertil. 1998;113:105–116. doi: 10.1530/jrf.0.1130105. [DOI] [PubMed] [Google Scholar]
- Lindeque M, Skinner JD. Fetal androgens and sexual mimicry in spotted hyaenas (Crocuta crocuta) J Reprod Fert. 1982;65:405–410. doi: 10.1530/jrf.0.0650405. [DOI] [PubMed] [Google Scholar]
- Matthews L. The oestrus cycle and intersexuality in the female mole (Talpa europaea Linn.) Proc Zool Soc Lond. 1937;1935:347–383. [Google Scholar]
- Matthews L. Reproduction of the spotted hyena (Crocuta crocuta Erxleben) Phil Tran Roy Soc, Lond B. 1939;230:1–78. [Google Scholar]
- McPhaul M, Glickman SE, Marcelli M, Sharifi N, Auchus RJ. Alterations of Androgen Action in Castration-Resistant Prostate Cancer (CRPC) and in Abnormalities of Virilization. Endocr J. 2010;57:S264. [Google Scholar]
- McPhaul MJ. Mechanisms of prostate cancer progression to androgen independence. Best Pract Res Clin Endocrinol Metab. 2008;22:373–388. doi: 10.1016/j.beem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Monaghan EP, Glickman SE. Hormones and aggressive behavior. In: Becker JB, Breedlove SM, Crews D, editors. Behavioral Endocrinology. Cambridge: MIT Press; 1992. pp. 261–286. [Google Scholar]
- Moore KL, Persaud TVN. The Developing Human. Saunders; Philadelphia: 2003. [Google Scholar]
- Muller MN, Wrangham R. Sexual mimicry in hyenas. Quart Rev Biol. 2002;77:3–15. doi: 10.1086/339199. [DOI] [PubMed] [Google Scholar]
- Murakami R. Autoradiographic studies of the localisation of androgen-binding cells in the genital tubercles of fetal rats. J Anat. 1987;151:209–219. [PMC free article] [PubMed] [Google Scholar]
- Neaves WB, Griffin JE, Wilson JD. Sexual dimorphism of the phallus in spotted hyaena (Crocuta crocuta) J Reprod Fertil. 1980;59:509–513. doi: 10.1530/jrf.0.0590509. [DOI] [PubMed] [Google Scholar]
- Neri R, Florance K, Koziol P, Van Cleave S. A biological profile of a nonsteroidal antiandrogen, SCH 13521 (4′-nitro-3′trifluoromethylisobutyranilide) Endocrinology. 1972;91:427–437. doi: 10.1210/endo-91-2-427. [DOI] [PubMed] [Google Scholar]
- Neri RO. Studies on the biology and mechanism of action of nonsteroidal anti-androgens. In: Martini L, Motta L, editors. Androgens and Antiandrogens. 1977. pp. 179–189. [Google Scholar]
- Neumann F, Elger W, Steinbeck H. Antiandrogens and reproductive development. Phil Trans Roy Soc London Ser B. 1970;259:179–184. doi: 10.1098/rstb.1970.0057. [DOI] [PubMed] [Google Scholar]
- Petter-Rousseaux A. Reproductive physiology and behavior of the Lemuroidea. Academic Press; New York: 1964. [Google Scholar]
- Place NJ, Glickman SE. Masculinization of female mammals: lessons from nature. Adv Exp Med Biol. 2004;545:243–253. doi: 10.1007/978-1-4419-8995-6_15. [DOI] [PubMed] [Google Scholar]
- Place NJ, Holekamp KE, Sisk CL, Weldele ML, Coscia EM, Drea CM, Glickman SE. Effects of prenatal treatment with antiandrogens on luteinizing hormone secretion and sex steroid concentrations in adult spotted hyenas, Crocuta crocuta. Biol Reprod. 2002;67:1405–1413. doi: 10.1095/biolreprod.102.004226. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Short RV. Sex determination in marsupials: evidence for a marsupial-eutherian dichotomy. Philos Trans R Soc Lond B Biol Sci. 1988;322:41–53. doi: 10.1098/rstb.1988.0112. [DOI] [PubMed] [Google Scholar]
- Risbridger GP, Ellem SJ, McPherson SJ. Estrogen action on the prostate gland: a critical mix of endocrine and paracrine signaling. J Mol Endocrinol. 2007;39:183–188. doi: 10.1677/JME-07-0053. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Jr, Weiss DA, Ferretti M, Wang H, Menshenia J, Risbridger G, Handelsman D, Cunha G, Baskin L. Specific morphogenetic events in mouse external genitalia sex differentiation are responsive/dependent upon androgens and/or estrogens. Differentiation. 2012:84169–279. doi: 10.1016/j.diff.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein NM, Cunha GR, Wang YZ, Campbell KL, Conley AJ, Catania KC, Glickman SE, Place NJ. Variation in ovarian morphology in four species of New World moles with a peniform clitoris. Reproduction. 2003;126:713–719. doi: 10.1530/rep.0.1260713. [DOI] [PubMed] [Google Scholar]
- Schneider KM. Uber Hyanenzucht. Die Peltierzucht. 1926;2:1–14. [Google Scholar]
- Schoenfeld WA. Primary and secondary sexual characteristics: study of their development in males from birth through maturity, with biometric study of penis and testes. Amer J Diseases of Children. 1943;65:535–549. [Google Scholar]
- Siiteri PK, Thompson EA. Studies of human placental aromatase. J Steroid Biochem. 1975;6:317–322. doi: 10.1016/0022-4731(75)90149-1. [DOI] [PubMed] [Google Scholar]
- Simmons MN, Jones JS. Male genital morphology and function: an evolutionary perspective. J Urol. 2007;177:1625–1631. doi: 10.1016/j.juro.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Steinetz BG, Randolph C, Weldele M, Frank LG, Licht P, Glickman SE. Pattern and source of secretion of relaxin in the reproductive cycle of the spotted hyena (Crocuta crocuta) Biol Reprod. 1997;56:1301–1306. doi: 10.1095/biolreprod56.5.1301. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Fetus into Man. Harvard University Press; Cambridge, MA: 1978. [Google Scholar]
- Watson M. On the female generative organs of Hyaena crocuta. Proc Zool Soc Lond. 1877:369–379. [Google Scholar]
- Watson M. On the male generative organs of Hyaena crocuta. Proc Zool Soc London. 1878;27:416–426. Proc. Zool. Soc. London 27:416-426. [Google Scholar]
- Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, Kim K, Sawyers CL. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, MacLeod DJ, Walker M, Smith LB, Sharpe RM. Critical androgen-sensitive periods of rat penis and clitoris development. Int J Androl. 2010;33:e144–152. doi: 10.1111/j.1365-2605.2009.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;118:1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JD, Griffin JE, Leshin M, George FW. Role of gonadal hormones in development of the sexual phenotypes. Hum Genet. 1981;58:78–84. doi: 10.1007/BF00284153. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Shaw G, Leihy ML, Renfree MB. The marsupial model for male phenotypic development. Trends Endocrinol Metab. 2002;13:78–83. doi: 10.1016/s1043-2760(01)00525-2. [DOI] [PubMed] [Google Scholar]
- Yalcinkaya TM, Cunha GR, Siiteri PK. Functional androgen receptors without androgen binding in the spotted hyena. Society for Gynecologic Investigation 1992 [Google Scholar]
- Yalcinkaya TM, Siiteri PK, Vigne JL, Licht P, Pavgi S, Frank LG, Glickman SE. A mechanism for virilization of female spotted hyenas in utero. Science. 1993;260:1931–1931. doi: 10.1126/science.8391165. [DOI] [PubMed] [Google Scholar]
- Yamada G, Satoh Y, Baskin LS, Cunha GR. Cellular and molecular mechanisms of development of the external genitalia. Differentiation. 2003;71:445–460. doi: 10.1046/j.1432-0436.2003.7108001.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


