Abstract
B cells expressing antibodies of the immunoglobulin E (IgE) isotype are rare, yet are heavily implicated in the pathogenesis of allergies and asthma. This review discusses recent methodological advances that permit sensitive probing of IgE-expressing (IgE+) B cells in vivo and have accordingly clarified the basic behavior and fate of IgE+ B cells during immune responses in mouse models. IgE antibody secreting plasma cells can arise from extrafollicular foci, germinal centers, and memory B cells. However, compared to B cells expressing other isotypes, IgE+ B cells are susceptible to multiple additional regulatory constraints that restrict the size of the IgE+ B cell pool at each stage, coordinately limiting the overall magnitude, affinity, and duration of the IgE antibody response.
Introduction
Immunoglobulin E (IgE) is the least abundant antibody isotype in mammals, yet it can produce remarkably potent inflammatory responses. IgE may contribute to immunity against helminths and venoms [1–5], but it is best known for its critical role in the pathogenesis of allergy and asthma [1,2,6]. Binding of IgE to cognate antigen crosslinks FcεRI on mast cells and basophils, leading to the rapid release of inflammatory mediators [6,7]. Systemic triggering of IgE responses can cause life-threatening anaphylaxis [6,8], but this condition occurs rarely, suggesting that IgE is normally tightly regulated. IgE has a short half-life in serum and is primarily cell bound, but these properties cannot fully explain its low abundance, which is typically several orders of magnitude less than that of IgG [1]. Under optimal in vitro conditions, the production of IgE can approach or even exceed that of IgG, suggesting that additional mechanisms operate in vivo to restrict IgE production [9]. Historically, IgE-expressing (IgE+) B cells have been difficult to study in vivo due to the lack of methods to specifically detect these rare cells. Recently, multiple groups have developed innovative methodologies and tools to detect IgE+ B cells in mouse models, bringing substantial insight into the biology of these cells. In this review, we first describe these technical advancements and then discuss our current understanding of the generation and differentiation of IgE+ B cells in mouse models. Throughout the review, we focus on novel mechanisms that regulate IgE production in vivo. Due to space limitations, we refer the reader to previous reviews [1,2,10] on the regulatory steps involved in the initial class switch recombination (CSR) to IgE, which is a prerequisite for the generation of IgE+ B cells.
New tools and techniques to study IgE+ B cells
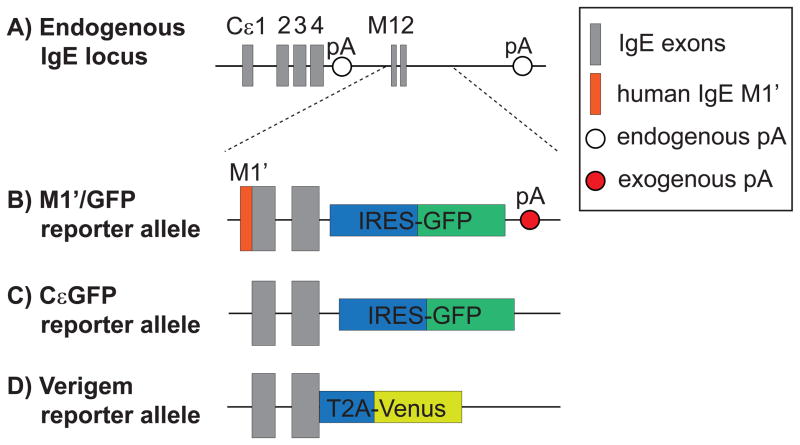
The direct study of IgE+ B cells has been hindered by technical challenges. IgE+ B cells are vastly outnumbered by B cells expressing different isotypes, and numerous other cell types capture secreted IgE on their cell surface, such as mast cells, basophils, and naïve B cells [10]. In order to specifically identify rare IgE+ B cells, three laboratories independently generated mouse strains encoding fluorescent protein reporters for membrane IgE (mIgE) by targeting the endogenous IgE locus (Figure 1) [11**,12**,13**]. In these mice, mIgE and the fluorescent protein are coexpressed in a single transcript, and two different strategies were employed to functionally separate the protein products—an intraribosomal entry site (IRES) or a 2A peptide sequence. Both the IRES and 2A strategies yielded robust reporter expression in B cells expressing the mature mIgE transcript. However, the IRES strategy (Figure 1b, c) also led to reporter expression from two related IgE transcripts that were present in some B cells expressing other isotypes [11**,13**,14]. Specifically, 1) the germline transcript precedes but does not necessarily lead to CSR to IgE, and 2) the post-switch transcript is expressed following CSR to IgE on an inactive immunoglobulin heavy chain locus that has undergone allelic exclusion. IRES-mediated reporter expression occurred at low levels with the germline transcript [11**], but at high levels with the post-switch transcript [13**]. As a consequence, IRES reporter expression alone was inadequate to distinguish mIgE+ B cells from those cells that had undergone CSR to IgE on the inactive allele, as was highlighted in two recent reports, in which as many as half of the reporter positive cells were IgG1+ B cells [13**,14]. The 2A reporter (Figure 1d) is expected to closely correlate with mIgE expression, although this allele unexpectedly exhibited a modest increase in mIgE splicing [12**]. One of the IRES reporter alleles (Figure 1b) also contains a coding sequence for a human M1′ extracellular segment as well as an exogenous polyadenylation signal sequence (pA) [11**,15]. Concerns have been raised that these additional sequences could potentially alter normal mouse IgE responses [16]. Previous studies identified weak, non-canonical pAs in the endogenous IgE locus [17,18] and found that replacement with an exogenous pA led to an increase in the abundance of mIgE transcripts in a transfection system [18]. Basic evaluations of the M1′/GFP reporter mice have not revealed overt differences from wild-type mice [15,19], although mIgE transcript abundance was not compared. Despite these caveats, both the IRES and 2A reporter mice provide powerful tools to study IgE+ B cells by flow cytometry, histology, and dynamic imaging [11**,12**,13**]. Indeed, as we will describe in detail later in this review, novel findings have been made in all three reporter mice, providing key insights into regulation of IgE antibody responses.
Figure 1.
Comparison of fluorescent IgE reporter alleles. (a) Structure of the native, endogenous IgE locus, which generates transcripts that encode secreted IgE versus membrane IgE (mIgE) by alternative splicing and polyadenylation. The secreted IgE transcript has a canonical polyadenylation signal sequence (pA), AAUAAA, in the intron between the CH4 and M1 exons. The mIgE transcript incorporates the M1 and M2 exons and has three potential, endogenous pAs with weak non-canonical sequences (AGUAAA, AAGAAA, or AUUAAA) [17,18]. Another possible pA with a canonical sequence is located further downstream in the intergenic region between IgE and IgA [53], although its relevance is unclear due to its long distance from the IgE M2 exon. (b and c) Schematic of the M1′/GFP and CεGFP reporter alleles, in which insertion of IRES-GFP downstream of the murine M2 exon leads to the generation a bicistronic transcript encoding mIgE and GFP. (d) Schematic of the Verigem reporter allele, in which a T2A site translationally links mIgE expression to the expression of Venus, a yellow fluorescent protein [12**]. In the M1′/GFP allele (b), the coding sequence for a 52 amino acid human M1′segment was inserted directly upstream of the murine M1 exon, and an additional exogenous pA was inserted in the 3′ UTR [11**,15]. In contrast, the CεGFP (c) and Verigem (d) reporter alleles did not introduce an exogenous pA, instead relying on the endogenous pAs in the 3′ UTR [12**,13**].
In addition to reporter mice, alternative methods have been devised to detect IgE+ B cells. Decades ago, an acid-wash procedure was described, in which brief acid treatment strips secreted IgE bound to Fc-receptors [20,21]. This method enhances the specificity of mIgE antibody staining, but may affect other cell surface molecules and appears to lack sufficient sensitivity to detect all IgE+ B cells [10,19,21]. More recently, methods have been described to specifically stain intracellular IgE, which is abundant in IgE-expressing B cells but at low levels in cells that capture secreted IgE. Surface IgE was either removed by trypsin treatment [22*] or blocked by an excess of unconjugated antibody to IgE [12**,23] before the intracellular IgE was detected with fluorophore-conjugated antibodies to IgE. Trypsin treatment has not been utilized for in vivo studies and may cleave other relevant surface markers. With antibody blockade of surface IgE, the intracellular IgE staining method has been used to study wild-type mice [12**,23] and has the potential to be applied to the characterization of IgE responses in other species, including humans. A recent study has also used a monoclonal antibody to IgE that does not recognize IgE bound to Fc receptors [13**], which may also show utility in specific identification of mIgE+ B cells without acid-treatment or fixation, although its specificity and sensitivity need further evaluation. In summary, the new IgE staining methods and fluorescent reporter mice can be used complementarily to definitively identify and study IgE+ B cells in vivo.
The GC phase of IgE+ B cells
Recent studies have focused on the appearance and fate of IgE+ B cells in germinal centers (GCs), sites of antibody affinity maturation which are thought to give rise to long-lived plasma cells (PCs) and memory B cells [24]. With the aid of new fluorescent mIgE reporter mice, two groups identified a population of IgE+ GC B cells by flow cytometry, by immunohistochemistry, and by dynamic imaging with two-photon laser scanning microscopy [11**,12**]. The IgE+ GC B cells were also detected in wild-type mice by flow cytometry with the intracellular IgE staining procedure [12**], and the presence of IgE+ B cells in GCs was recently confirmed in a third fluorescent IgE reporter mouse strain [13**].
All three groups found that the participation of IgE+ B cells in GCs was transient, as numbers of IgE+ GC B cells peaked early in the response and declined steadily thereafter [12**,13**,19]. These kinetics were in sharp contrast with those of IgG1+ GC B cells, which increased in frequency within GCs over time [12**,19]. Two models have been proposed to account for the transient presence of IgE+ B cells in GCs: 1) IgE+ B cells exhibit an increased propensity to terminally differentiate into PCs rather than maintain a GC phenotype [12**] and 2) IgE+ B cells are unable to survive within GCs due to reduced BCR signaling [10,13**]. These models are not mutually exclusive, and key evidence for these models is described below.
In support of the first model, several lines of evidence indicate that IgE+ B cells preferentially differentiate into PCs. A greater proportion of IgE+ cells had a PC phenotype compared with IgG1+ cells in multiple mouse studies [12**,13**,25] and in vitro B cell cultures [12**]. As expected from previous in vitro studies [26], IgG1+ B cells showed increasing PC differentiation with subsequent cell divisions after CSR; however, IgE+ B cells were unusual in that PC differentiation occurred independently of the number of cell divisions [12**]. IgE+ B cells that had not yet undergone PC differentiation in vitro also showed increased expression of the transcription factor Blimp-1 [12**], a master regulator of PC differentiation [27]. Supporting the idea that PC differentiation diverts IgE+ B cells from the GC, blockade of PC differentiation by B-cell deficiency in Blimp-1 led to a selective increase in the frequency of IgE+ B cells within GCs [12**]. Taken together, these findings suggest that IgE+ GC B cells have a greater likelihood of differentiating into PCs compared with their IgG1+ counterparts, thereby depleting the population of IgE+ B cells from GCs over time.
In the second model, IgE+ B cells are unable to survive within GCs due to reduced BCR signaling. This model was based primarily on the observation that the level of surface BCR on IgE+ GC B cells was several-fold lower than that on IgE+ PCs [12**,13**] and on IgG1+ GC B cells [13**]. When cultured ex vivo, compared with IgG1+ B cells, IgE+ GC B cells exhibited increased apoptosis and a reduced capacity for phosphorylation of Syk and BLNK in response to BCR ligation [13**]. While the significance of these assays and BCR signaling in the GC remain unclear [28,29], it seems plausible that low BCR expression could also impair IgE+ GC B cell antigen uptake, processing, and presentation to GC T cells. In related findings, IgE+ GC B cells were reported to express lower surface levels of CD21/35, OX40L and ICOSL than IgG1+ GC B cells [13**], further suggesting that IgE+ GC B cells may show a reduced capacity for antigen processing and costimulation to obtain critical T cell help in the GC. IgE+ B cells were also reported to show reduced localization to the GC light zone, where selection is thought to occur [13**]; however, IgE+ B cells were readily detectable in the GC light zone in another study [12**], and thus these results require further evaluation. Overall, recent studies suggest that IgE+ B cells may have a competitive disadvantage within the GC (Figure 2).
Figure 2.
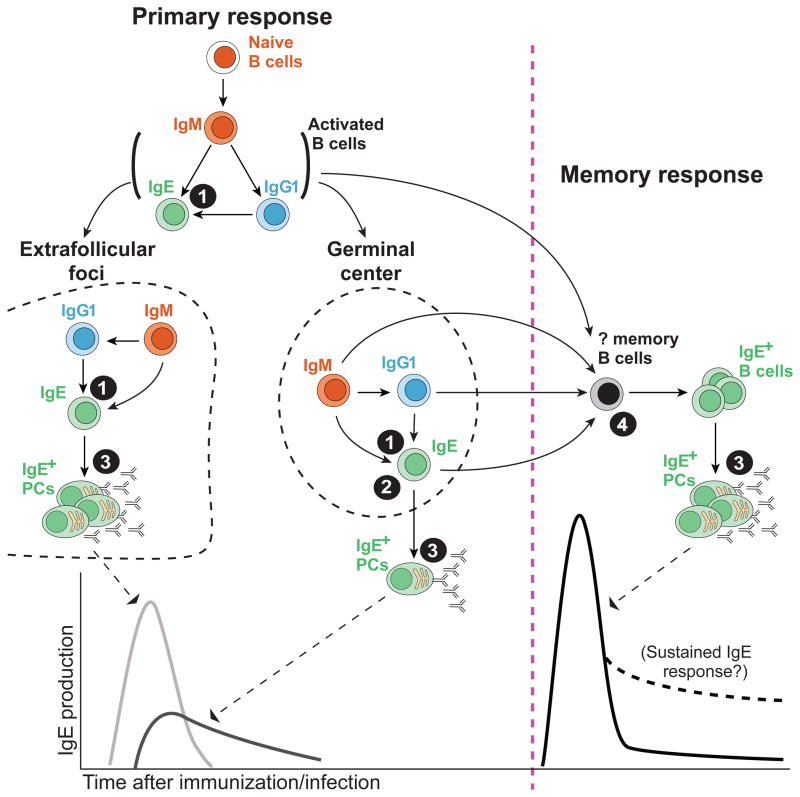
Overview of the cellular differentiation pathways and regulatory mechanisms in IgE antibody production. B cells may undergo CSR to IgE by either direct switching from IgM or sequential switching through an IgG1 intermediate. CSR to IgE may occur in activated B cells before they segregate to form extrafollicular foci and germinal centers, and/or inside these regions after they are formed. IgE+ B cells in extrafollicular foci and GCs give rise to two overlapping waves of IgE+ PCs in primary immune responses. In secondary immune responses, memory B cells may already express IgE or undergo de novo CSR to IgE, generating a new wave of IgE+ PCs. Sustained IgE antibody production may occur in atopic diseases. The filled circles with numbers designate key steps in the regulation of IgE responses: 1) CSR to IgE is infrequent due to intrinsic and extrinsic factors; 2) IgE+ B cells are only transiently present in GCs, limiting IgE affinity maturation; 3) IgE+ B cells are predisposed to differentiate into short-lived PCs; and 4) IgE memory responses are restrained by the low frequency of IgE+ memory B cells and a potential requirement for de novo CSR to IgE.
While the above models postulate mechanisms for the disappearance of existing IgE+ B cells from GCs, it appears this population is not replenished by the generation of new IgE+ B cells. Therefore, although CSR is thought to occur in GCs [24], the data suggest that little to no CSR to IgE occurs in ongoing GC responses. Inhibition of IgE-switching in GCs could be B cell intrinsic, for example, due to the high expression of the transcription factor Bcl-6, which has been reported to inhibit IgE germline transcription [30–32]. Alternatively, inhibition of IgE-switching in GCs could be mediated by extrinsic signals from other cell types, such as from follicular helper T cells secreting the cytokine IL-21 [33], which has also been reported to inhibit IgE germline transcription [32,34]. In summary, we propose that multiple mechanisms act to restrict the frequency of IgE+ B cells in GCs, thereby limiting the generation of high-affinity, long-lived PCs and memory IgE+ B cells.
Differentiation of IgE+ PCs and their fate
Although IgE+ B cells have been observed in GCs, most IgE+ antibody-secreting PCs appear to arise independently from the GC (note that the term PC is used broadly here, as the studies we will discuss have not tried to distinguish PCs from plasmablasts). In primary immune responses, PCs can be generated via two distinct pathways, first from extrafollicular foci and then from GCs [24,35]. The early PCs in extrafollicular foci primarily secrete germline-encoded antibodies of low-to-moderate affinity. In contrast, the ongoing somatic hypermutation and selection process in GCs gives rise to PCs expressing high-affinity antibodies. While these descriptions are not absolute, the origin of PCs can be inferred based on both the kinetics of the response and analysis of antibody variable region mutations. In recent studies, IgE+ PCs appeared at peak numbers early in the immune response [11**,12**,13**] and expressed primarily germline, unmutated antibody sequences [12**], suggesting they were derived via the extrafollicular pathway. At later timepoints, a fraction of IgE+ PCs had numerous mutations and showed evidence of affinity maturation [12**,25,36*], suggesting they were derived from GCs. We therefore propose that two waves of IgE+ PCs are generated in primary antibody responses (Figure 2). The first, major wave of IgE+ PCs are generated in extrafollicular foci and express germline-encoded antibodies. The second wave of IgE+ PCs, arriving later and overlapping with the first wave, are generated from GCs and show some degree of affinity maturation. The contribution of the second wave of GC-derived PCs to the overall IgE antibody response may be quite limited, as the number of IgE+ GC B cells and PCs steadily decreases over time.
As long-lived PCs have been proposed to emerge from GCs at later stages [24], the early differentiation of IgE+ PCs may favor short-term survival. Indeed, IgE+ PCs quickly disappeared from secondary lymphoid organs after the peak of the response [11**,12**,13**], which correlated with a decline in serum IgE titers [11**,12**]. IgE+ PCs were infrequent or undetectable in the bone marrow [11**,12**,13**], where most long-lived plasma cells reside [37]. Prevention of PC apoptosis in Bcl-2 transgenic mice led to a selective increase in the number of IgE+ PCs by an order of magnitude compared with IgG1+ PCs, further suggesting that most IgE+ B cells differentiate into short-lived PCs [12**]. One group argued for a long-lived IgE+ PC population based on adoptive transfer studies [13**], however this approach may alter the normal homing of the cells, and thus its significance is unclear. Regulated egress from secondary lymphoid organs into blood may be an important step in the selection of long-lived PCs [38] and the IgE BCR has also been reported to disfavor migration to the bone marrow [39]. Taken together, these findings suggest that IgE+ B cells are predisposed to a short-lived PC fate, which we propose is an important mechanism to limit the duration and magnitude of the IgE response in vivo (Figure 2).
Direct and sequential CSR origins of IgE+ B cells
CSR to IgE can be achieved either directly from IgM or sequentially via an IgG1 intermediate step (Figure 2) [40–42]. IgG1+ and IgE+ cells appear in extrafollicular foci and GCs with similar kinetics [12**], suggesting that CSR to these isotypes may occur in parallel in these locations, or, alternatively, in common activated B cell precursors (Figure 2). An IgG1 intermediate stage appears to be critical for IgE affinity maturation, as studies of IgG1-deficient mice revealed that the production of high-affinity IgE was compromised [36*], whereas total IgE titers were undiminished [43,44]. A follow-up study found that a minor fraction of IgE+ PCs, but almost none of the IgE+ GC B cells, showed evidence of sequential CSR [13**]. It was concluded from these results that IgE+ PCs must derive from IgG1+ GC B cells, but there are some caveats to such an exclusive interpretation of the data. Since CSR is not exclusive to GCs [24,45–48], it seems plausible that sequential CSR to IgE could have occurred in activated B cell precursors, in extrafollicular foci, and/or during the memory response. The extent of sequential CSR may be underestimated with currently available methods [13**]; nevertheless, the majority of IgE PCs do not show evidence of sequential switching [13**,36*], suggesting that at least a proportion undergo direct CSR. Intriguingly, IgE+ PCs invariably had a lower frequency of high affinity mutations than synchronously isolated IgG1+ PCs and GC B cells [12**,13**,25,36*], suggesting that IgE+ PCs are unlikely to derive exclusively from IgG1+ GC B cells. From current evidence, we propose that both direct and sequential switching pathways give rise to IgE+ GC B cells and PCs, and further that IgE+ GC B cells can differentiate into PCs, although the contributions of these pathways to the IgE antibody response are not necessarily quantitatively or functionally equivalent.
Memory IgE responses
The IgE reporter mice have also provided a new opportunity to determine the cellular source of IgE antibodies in secondary immune responses, which arise faster and reach higher titers than in primary immune responses [49]. A series of classical studies, with experiments involving IL-4 blocking antibodies and IgE-activating antibodies, concluded that secondary IgE antibody responses to conventional immunizations required de novo CSR to IgE, suggesting that a population of previously-switched IgE+ memory B cells was nonexistent or insignificant [49–51]. In contrast to B cells expressing other isotypes, IgE+ B cells failed to develop into memory B cells in a model involving in vitro culture followed by in vivo adoptive transfer [52]. In a mouse model with a monoclonal T- and B-cell repertoire, the adoptive transfer of IgG1+ memory B cells was sufficient to confer memory IgE responses in recipients [25]. Challenging these conclusions, one of the three groups with fluorescent IgE reporter mice detected a small population of IgE reporter-positive cells expressing memory B cell markers [11**]. These putative memory cells generated weak IgE antibody responses upon adoptive transfer. As a caveat to these studies, mIgE expression was not directly demonstrated in this population, raising a potential concern that the IRES reporter expression could reflect CSR to IgE on an inactive allele, as described earlier in this review. Indeed, a recent update indicates that at least half of the reporter-positive memory cells expressed surface IgG1, and the expression of other isotypes, such as IgM, was not excluded [14]. In contrast, another group found via adoptive transfers that IgE+ memory B cells were not necessary for secondary IgE antibody responses, although they did not directly assess or test for a population of IgE+ memory B cells [13**]. Taken together, the existence of a bona fide IgE+ memory B cell population remains controversial and we propose that only small numbers of IgE+ memory B cells may be generated, possibly related to the transient participation of IgE+ B cells in GCs. Memory B cells expressing other isotypes, particularly IgG1, appear to be able to undergo CSR and contribute to IgE antibody production in secondary responses. Therefore, restrictions on the generation of IgE memory B cells and the regulation of de novo CSR to IgE may represent additional mechanisms that limit and regulate IgE responses (Figure 2).
Conclusion
The advent of new techniques and tools has led to significant advances in understanding the biology of IgE+ B cells. Aside from the short serum half-life of IgE, new mechanisms limiting IgE production in vivo have been uncovered (Figure 2). The major constraints imposed on IgE production are 1) the restricted CSR to IgE, 2) the transient presence of IgE+ B cells in GCs, 3) the predisposition of IgE+ B cells toward a short-lived PC fate, and 4) the possible requirement of de novo IgE switching in memory responses. We propose that these mechanisms limit the magnitude, affinity, and duration of normal IgE antibody responses. Variations in these regulatory mechanisms among mouse strains and the human population may influence allergic disease susceptibility. Breaching one or more of these barriers may cause dysregulation of IgE production, leading to dangerous responses such as anaphylaxis. Indeed, it is important to note that the mouse studies discussed above have represented immune responses to protein-based antigens in alum, mimicking vaccines, or acute infections with helminths. However, these responses likely mimic a normal healthy condition, in the absence of allergic disease, in which case IgE responses may only be transient. With the new tools available to identify IgE+ B cells in mice, studies of allergic disease models may provide valuable new insights into IgE-mediated allergic sensitization. In the future, it would be of particular interest to understand the spatiotemporal regulation of the generation of IgE+ B cells, and the actual contribution of different cellular compartments to sustained IgE antibody production.
Highlights (for review).
IgE reporter mice enable specific detection of IgE+ B cells in vivo.
IgE+ B cells appear transiently in germinal centers.
IgE+ plasma cells develop in both extrafollicular foci and germinal centers.
IgE+ B cells are predisposed to a short-lived plasma cell fate.
The existence and importance of IgE+ memory B cells remain controversial.
Acknowledgments
We thank members of the Sandler Asthma Basic Research Center for helpful discussions and L. Kelly for comments on a draft of the review. Research in this laboratory is supported by the UCSF Sandler Asthma Basic Research Center, the Weston Havens Foundation, the UCSF Cardiovascular Research Institute, and grants from the National Institutes of Health R01AI103146 and DP2HL117752. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol. 2003;3:721–732. doi: 10.1038/nri1181. [DOI] [PubMed] [Google Scholar]
- 2.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, Fear D, Smurthwaite L. The biology of IgE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 3.Marichal T, Starkl P, Reber LL, Kalesnikoff J, Oettgen HC, Tsai M, Metz M, Galli SJ. A Beneficial Role for Immunoglobulin E in Host Defense against Honeybee Venom. Immunity. 2013;39:963–975. doi: 10.1016/j.immuni.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39:976–985. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obata-Ninomiya K, Ishiwata K, Tsutsui H, Nei Y, Yoshikawa S, Kawano Y, Minegishi Y, Ohta N, Watanabe N, Kanuka H, et al. The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med. 2013;210:2583–2595. doi: 10.1084/jem.20130761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraft S, Kinet J-P. New developments in Fc(epsilon)RI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 8.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 516–507. [DOI] [PubMed] [Google Scholar]
- 9.Snapper C, Finkelman F, Paul W. Differential regulation of IgG1 and IgE synthesis by interleukin 4. J Exp Med. 1988;167:183–196. doi: 10.1084/jem.167.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong H, Curotto de Lafaille MA, Lafaille JJ. What is unique about the IgE response? Adv Immunol. 2012;116:113–141. doi: 10.1016/B978-0-12-394300-2.00004-1. [DOI] [PubMed] [Google Scholar]
- 11**.Talay O, Yan D, Brightbill HD, Straney EE, Zhou M, Ladi E, Lee WP, Egen JG, Austin CD, Xu M, et al. IgE+ memory B cells and plasma cells generated through a germinal-center pathway. Nat Immunol. 2012;13:396–404. doi: 10.1038/ni.2256. This paper characterized IgE responses in fluorescent IgE reporter mice, which revealed the presence of IgE+ B cells in GCs and identified a putative population of IgE+ memory B cells. [DOI] [PubMed] [Google Scholar]
- 12**.Yang Z, Sullivan BM, Allen CD. Fluorescent In Vivo Detection Reveals that IgE+ B Cells Are Restrained by an Intrinsic Cell Fate Predisposition. Immunity. 2012;36:857–872. doi: 10.1016/j.immuni.2012.02.009. This study described fluorescent IgE reporter mice and an intracellular staining method, based on antibody blockade of surface IgE, to specifically identify IgE+ B cells. These new methodologies revealed the presence of IgE+ B cells in GCs and their predisposition to differentiate into short-lived PCs. [DOI] [PubMed] [Google Scholar]
- 13**.He JS, Meyer-Hermann M, Xiangying D, Zuan LY, Jones LA, Ramakrishna L, de Vries VC, Dolpady J, Aina H, Joseph S, et al. The distinctive germinal center phase of IgE+ B lymphocytes limits their contribution to the classical memory response. J Exp Med. 2013;210:2755–2771. doi: 10.1084/jem.20131539. Using fluorescent IgE reporter mice and T/B monoclonal mice, this study revealed differences in the phenotype, antigen responsiveness and apoptosis rates of isolated IgE+ and IgG1+ GC B cells, and demonstrated that IgE+ B cells were not necessary for the adoptive transfer of IgE memory responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talay O, Yan D, Brightbill HD, Straney EEM, Zhou M, Ladi E, Lee WP, Egen JG, Austin CD, Xu M, et al. Addendum: IgE+ memory B cells and plasma cells generated through a germinal-center pathway. Nat Immunol. 2013;14:1302–1304. doi: 10.1038/ni.2770. [DOI] [PubMed] [Google Scholar]
- 15.Brightbill HD, Jeet S, Lin Z, Yan D, Zhou M, Tan M, Nguyen A, Yeh S, Delarosa D, Leong SR, et al. Antibodies specific for a segment of human membrane IgE deplete IgE-producing B cells in humanized mice. J Clin Invest. 2010;120:2218–2229. doi: 10.1172/JCI40141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lafaille JJ, Xiong H, Curotto de Lafaille MA. On the differentiation of mouse IgE+ cells. Nat Immunol. 2012;13:623. doi: 10.1038/ni.2313. [DOI] [PubMed] [Google Scholar]
- 17.Anand S, Batista FD, Tkach T, Efremov DG, Burrone OR. Multiple transcripts of the murine immunoglobulin [epsilon] membrane locus are generated by alternative splicing and differential usage of two polyadenylation sites. Mol Immunol. 1997;34:175–183. doi: 10.1016/s0161-5890(96)00110-1. [DOI] [PubMed] [Google Scholar]
- 18.Karnowski A, Achatz-Straussberger G, Klockenbusch C, Achatz G, Lamers MC. Inefficient processing of mRNA for the membrane form of IgE is a genetic mechanism to limit recruitment of IgE-secreting cells. Eur J Immunol. 2006;36:1917–1925. doi: 10.1002/eji.200535495. [DOI] [PubMed] [Google Scholar]
- 19.Talay O, Yan D, Brightbill HD, Straney EEM, Zhou M, Ladi E, Lee WP, Egen JG, Austin CD, Xu M, et al. Reply to “On the differentiation of mouse IgE+ cells”. Nat Immunol. 2012;13:623–624. [Google Scholar]
- 20.Ishizaka T, Ishizaka K. Mechanisms of passive sensitization. IV. Dissociation of IgE molecules from basophil receptors at acid pH. Journal of immunology. 1974;112:1078–1084. [PubMed] [Google Scholar]
- 21.Katona IM, Urban JF, Jr, Scher I, Kanellopoulos-Langevin C, Finkelman FD. Induction of an IgE response in mice by Nippostrongylus brasiliensis: characterization of lymphoid cells with intracytoplasmic or surface IgE. J Immunol. 1983;130:350–356. [PubMed] [Google Scholar]
- 22*.Wesemann DR, Magee JM, Boboila C, Calado DP, Gallagher MP, Portuguese AJ, Manis JP, Zhou X, Recher M, Rajewsky K, et al. Immature B cells preferentially switch to IgE with increased direct Smu to Sepsilon recombination. J Exp Med. 2011;208:2733–2746. doi: 10.1084/jem.20111155. This article described an intracellular staining method to identify IgE+ B cells, involving trypsin treatment to remove surface IgE, and showed that immature B cells undergo CSR to IgE at higher rates than mature B cells in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ota T, Aoki-Ota M, Duong BH, Nemazee D. Suppression of IgE B cells and IgE binding to Fc(epsilon)RI by gene therapy with single-chain anti-IgE. J Immunol. 2009;182:8110–8117. doi: 10.4049/jimmunol.0900300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247:52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 25.Erazo A, Kutchukhidze N, Leung M, Christ AP, Urban JF, Jr, Curotto de Lafaille MA, Lafaille JJ. Unique maturation program of the IgE response in vivo. Immunity. 2007;26:191–203. doi: 10.1016/j.immuni.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasbold J, Corcoran LM, Tarlinton DM, Tangye SG, Hodgkin PD. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat Immunol. 2004;5:55–63. doi: 10.1038/ni1016. [DOI] [PubMed] [Google Scholar]
- 27.Calame K. Blimp-1’s maiden flight. J Immunol. 2010;185:3–4. doi: 10.4049/jimmunol.1090044. [DOI] [PubMed] [Google Scholar]
- 28.Khalil AM, Cambier JC, Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science. 2012;336:1178–1181. doi: 10.1126/science.1213368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bannard OM, Cyster JG. Immunology. When less signaling is more. Science. 2012;336:1120–1121. doi: 10.1126/science.1223811. [DOI] [PubMed] [Google Scholar]
- 30.Harris MB, Mostecki J, Rothman PB. Repression of an interleukin-4-responsive promoter requires cooperative BCL-6 function. J Biol Chem. 2005;280:13114–13121. doi: 10.1074/jbc.M412649200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang TT, Makondo KJ, Marshall AJ. p110delta Phosphoinositide 3-Kinase Represses IgE Switch by Potentiating BCL6 Expression. J Immunol. 2012;188:3700–3708. doi: 10.4049/jimmunol.1103302. [DOI] [PubMed] [Google Scholar]
- 32.Kitayama D, Sakamoto A, Arima M, Hatano M, Miyazaki M, Tokuhisa T. A role for Bcl6 in sequential class switch recombination to IgE in B cells stimulated with IL-4 and IL-21. Mol Immunol. 2008;45:1337–1345. doi: 10.1016/j.molimm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Nutt SL, Tarlinton DM. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat Immunol. 2011;12:472–477. doi: 10.1038/ni.2019. [DOI] [PubMed] [Google Scholar]
- 34.Suto A, Nakajima H, Hirose K, Suzuki K, Kagami S, Seto Y, Hoshimoto A, Saito Y, Foster DC, Iwamoto I. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line Cepsilon transcription of IL-4-stimulated B cells. Blood. 2002;100:4565–4573. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- 35.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 36*.Xiong HZ, Dolpady J, Wabl M, Curotto de Lafaille MA, Lafaille JJ. Sequential class switching is required for the generation of high affinity IgE antibodies. J Exp Med. 2012;209:353–364. doi: 10.1084/jem.20111941. Through the analysis of sequential switching and IgG1-deficient mice, this report demonstrated the importance of an IgG1 intermediate stage for the generation of high-affinity IgE memory responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 38.Kabashima K, Haynes NM, Xu Y, Nutt SL, Allende ML, Proia RL, Cyster JG. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J Exp Med. 2006;203:2683–2690. doi: 10.1084/jem.20061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Achatz-Straussberger G, Zaborsky N, Königsberger S, Luger EO, Lamers M, Crameri R, Achatz G. Migration of antibody secreting cells towards CXCL12 depends on the isotype that forms the BCR. Eur J Immunol. 2008;38:3167–3177. doi: 10.1002/eji.200838456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida K, Matsuoka M, Usuda S, Mori A, Ishizaka K, Sakano H. Immunoglobulin switch circular DNA in the mouse infected with Nippostrongylus brasiliensis: evidence for successive class switching from mu to epsilon via gamma 1. Proc Natl Acad Sci USA. 1990;87:7829–7833. doi: 10.1073/pnas.87.20.7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siebenkotten G, Esser C, Wabl M, Radbruch A. The murine IgG1/IgE class switch program. Eur J Immunol. 1992;22:1827–1834. doi: 10.1002/eji.1830220723. [DOI] [PubMed] [Google Scholar]
- 42.Mandler R, Finkelman FD, Levine AD, Snapper CM. IL-4 induction of IgE class switching by lipopolysaccharide-activated murine B cells occurs predominantly through sequential switching. J Immunol. 1993;150:407–418. [PubMed] [Google Scholar]
- 43.Misaghi S, Garris CS, Sun Y, Nguyen A, Zhang J, Sebrell A, Senger K, Yan D, Lorenzo MN, Heldens S, et al. Increased targeting of donor switch region and IgE in Sgamma1-deficient B cells. J Immunol. 2010;185:166–173. doi: 10.4049/jimmunol.1000515. [DOI] [PubMed] [Google Scholar]
- 44.Jung S, Siebenkotten G, Radbruch A. Frequency of immunoglobulin E class switching is autonomously determined and independent of prior switching to other classes. J Exp Med. 1994;179:2023–2026. doi: 10.1084/jem.179.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toellner KM, Gulbranson-Judge A, Taylor DR, Sze DM, MacLennan IC. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J Exp Med. 1996;183:2303–2312. doi: 10.1084/jem.183.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pape KA, Kouskoff V, Nemazee D, Tang HL, Cyster JG, Tze LE, Hippen KL, Behrens TW, Jenkins MK. Visualization of the genesis and fate of isotype-switched B cells during a primary immune response. J Exp Med. 2003;197:1677–1687. doi: 10.1084/jem.20012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen Affinity Controls Rapid T-Dependent Antibody Production by Driving the Expansion Rather than the Differentiation or Extrafollicular Migration of Early Plasmablasts. J Immunol. 2009;183:3139–3149. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- 48.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 50.Katona I, Urban J, Kang S, Paul W, Finkelman F. IL-4 requirements for the generation of secondary in vivo IgE responses. J Immunol. 1991;146:4215–4221. [PubMed] [Google Scholar]
- 51.Le Gros G, Schultze N, Walti S, Einsle K, Finkelman F, Kosco-Vilbois MH, Heusser C. The development of IgE+ memory B cells following primary IgE immune responses. Eur J Immunol. 1996;26:3042–3047. doi: 10.1002/eji.1830261233. [DOI] [PubMed] [Google Scholar]
- 52.Nojima T, Haniuda K, Moutai T, Matsudaira M, Mizokawa S, Shiratori I, Azuma T, Kitamura D. In-vitro derived germinal centre B cells differentially generate memory B or plasma cells in vivo. Nat Commun. 2011;2:465. doi: 10.1038/ncomms1475. [DOI] [PubMed] [Google Scholar]
- 53.Karnowski A, Yu P, Achatz G, Lamers MC. The Road to the Production of IgE Is Long and Winding. Am J Respir Crit Care Med. 2000;162:S71–75. doi: 10.1164/ajrccm.162.supplement_2.ras-3. [DOI] [PubMed] [Google Scholar]