Abstract
Early-stage endometrial carcinoma (EC) patients have a high cure rate; however, those with high-risk factors may have poor prognosis. Thus, there is an urgent need for searching for new prognostic molecules to more accurately predict survival of patients. We detected the Rictor mRNA expression level in 30 fresh EC tissue and 17 normal endometrial tissue samples with real-time quantitative RT-PCR and Rictor protein expression level in 134 (test cohort) and 115 (validation cohort) paraffin tissue samples by immunohistochemistry, analyzed the correlation between variables and overall survival (OS) using Cox proportional hazards regression, compared the prognostic accuracy of Rictor with other clinicopathological risk factors by logistic regression. The results showed that Rictor mRNA expression of EC is higher than that of normal endometrium; Rictor protein expression level was closely correlated with FIGO stage, grade and vascular invasion in both cohorts; a univariate analysis showed that the pathological type, stage, grade, vascular invasion, lymphatic metastasis and Rictor were predictors of OS in both cohorts; furthermore, multivariate Cox proportional hazards regression analysis indicated that vascular invasion and Rictor were independent prognostic factors for EC in both cohorts; an ROX curve comparison showed that the area under the curve (AUC) for Rictor combined with other clinicopathological prognostic factors was higher than any individual factor or other clinicopathological prognostic factors’ combination. Based on the above data, we concluded that Rictor is an independent prognostic factor for EC. It combined with other clinicopathological risk factors was a stronger prognostic model than individual risk factor or their combination.
Keywords: Rictor, prognosis, endometrial carcinoma
Introduction
Endometrial carcinoma (EC) is the fourth most common cancer among women worldwide [1]. Early-stage patients have a high cure rate; however, those who are at old age or low differentiation, lymphatic metastasis, vascular and myometrial invasion of EC [2,3], may have poor prognosis. Thus, there is an urgent need for searching for new prognostic molecules to more accurately predict survival of patients.
mTOR, a highly conserved regulator of cell proliferation and growth in all eukaryotes [4], includes more than two complex, mTORC1 and mTORC2 [5,6]. The mTORC1 complex is sensitive to rapamycin and responds to multiple stimuli, such as energy status, growth factors, amino acids, and inflammation [7]. The mTORC2 complex affecting cell morphology and actin polymerization [6,8] mainly promotes cell proliferation and survival through phosphorylation of Akt and SGK [9,10]. Rictor which is insensitive to rapamycin binds with mLST8, mSin1 and Protor to form a stable structure of the mTORC2 complex [10,11] and also other protein partners that include the unconventional myosin motor Myo1C, Cullin-1, the integrin linked kinase (ILK), PKCz and Hsp70 [11]. Rictor mediates the activities of integrin-linked kinase [12] and controls neutrophil chemotaxis by regulating the activity of Rac/Cdc42 and the actin cytoskeleton [13].
Currently, only a limited number of reports exist regarding the roles of Rictor in benign diseases. Rictor expression has been shown to be up-regulated in tuberous sclerosis complex [14], pituitary adenomas [15] and hypertrophying myocardium [16]. However, Rictor has diverse and complicated biological functions in malignant tumors. For example, Rictor enhances activity of the Rho proteins and cell migration by suppressing RhoGDI2 [17] and induces the expression of c-Myc and cyclin E overexpressed in colorectal cancer by phosphorylation and activation of Akt to promote proliferation. The Rictor expression level is up-regulated in almost all of malignant tumors by now, such as colorectal cancer [18], urothelial carcinoma [19], Glioma [20], oral squamous cell carcinoma [21], prostate cancer [22], breast cancer [23], bladder cancer [24], hepatocellular carcinoma [25] and Oral Cancer [26]. In gynecological cancers, it is reported that miR-152 is a tumor suppressor by targeting Rictor in endometrial cancer [27]. These reports suggested complex roles for Rictor in cancer progression.
Although Rictor has been implicated in cancer progression, its prognostic value in EC remains unclear. By analysis of EC microarray datasets from the GEO database (GSE21882, GSE17025), we found that Rictor was up-regulated in deceased patients or those with low differentiation compared to surviving patients or those with high differentiation, which suggested that Rictor might be involved in cancer progression of EC. However, the prognostic value of Rictor in EC remains obscure.
In the study, we detected the Rictor mRNA expression level in 30 fresh EC tissue samples and 17 normal endometrial tissue samples with real-time quantitative RT-PCR and Rictor protein expression level in 134 paraffin tissue samples (test cohort) and 115 paraffin tissue samples (validation cohort) by immunohistochemistry, analyzed the correlation between variables and overall survival using Cox proportional hazards regression, compared the prognostic accuracy of Rictor with other clinicopathological risk factors by logistic regression and assessed the prognostic efficiency of Rictor in EC patients.
Materials and methods
Samples and clinical database
In this study, 30 fresh EC tissue samples, 17 fresh normal endometrial tissue samples and 249 formalin-fixed paraffin-embedded (FFPE) tissue samples were collected during April 2002 and March 2013 from Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, China, the First People’s Hospital of Huai’an city, Jiangsu, China and the Shanghai Changning District Central Hospital, China. We excluded patients with the history of chemoradiotherapy, other solid tumors, or other anti-cancer therapies before surgery, without complete follow-up and clinicopathologic data, and without completed the ethics committee’s consent and approval of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, or the Shanghai Changning District Central Hospital, or the first People’s hospital of Huai’an city, Jiangsu, China, for the use of samples. Two pathologists reassessed all the samples. We assigned 134 samples for test cohort and 115 samples for validation cohort using computer-generated random numbers (Supplementary Figure 1, Supplementary Table 1). The FFPE tissues samples comprised at least 80% tumor cells. Overall survival (OS) was defined as the time from the date of surgery to the date of the last follow-up examination or death by us.
Tissue microarray construction
Shanghai Zuoli Biotechnology Co., Ltd (Zuoli Biotechnology Co, Shanghai, China) constructed tissue microarrays. Pathologists stained paraffin tissue blocks of EC samples for test and validation cohorts using hematoxylin-eosin to affirm the diagnoses and marked at fixed points including the most typical histological characteristics. We diverted cores of 1.1 mm diameter from per donor block into a recipient block microarray, cut four-micron-thick sections from the recipient block and used with an adhesive tape transfer system to divert them to glass slides so as to ultraviolet cross linkage.
Immunohistochemistry
We de-paraffinized the slides in xylene for 10 min per time for three times, rehydrated with a graded series of ethanol concentrations (in 100%, 95%, 85%, 75% ethanol for 10 min respectively) and performed antigen retrieval in 100°C water with 0.01 M citrate buffer for 30 minutes. The sections were incubated in 37°C with 0.3% hydrogen peroxide/phosphate-buffered saline for 30 minutes and blocked at room temperature with 10% BSA for 1 hour. Rictor antibody (1:100, Abcam Biotechnology, Cambridge, UK) and a labeled polymer-HRP anti-mouse secondary antibody (1:1000, Dako, Carpentaria, CA, USA) were incubated at room temperature for 1 hour. We visualized the slides using DAB substrate liquid (Thermo Scientific, USA), washed them with deionized water before hematoxylin counterstaining and conducted scoring in the light of the staining intensity and percentage of positive-staining cells. 0-5% scored 0; 6-35% scored 1; 36-70% scored 2; more than 70% scored 3. We designated the final score as negative or positive expression group as follows: score 0, negative expression, score 1-3, positive expression. Two senior pathologists determined the scores independently.
Statistical analysis
We conducted statistical analyses with SPSS 17.0 software (Chicago, IL, USA), compared test and validation cohorts using Fisher exact or χ² test for enumeration data, Mann-Whitney U test for ranked data, analyzed the correlation between OS and variables using the Kaplan-Meier method and compared survival curves using the log-rank test for survival analyses. Univariate analyses were based on a Cox proportional hazard regression. Multivariate analyses were used with the Cox proportional hazard regression model with stepwise manner (forward: condition, entry α=0.05, stay α=0.1). ROC curves were used to compare the prognostic accuracy of Rictor with clinicopathological risk factors in test and validation cohorts. Statistical significance was set at 0.05.
Results
The mRNA expression of Rictor is upregulated in EC
To evaluate the mRNA expression of Rictor, we first detected Rictor mRNA expression in 30 fresh EC tissue samples and 17 fresh normal endometrial tissues using real-time quantitative RT-PCR. Rictor mRNA expression of EC is higher than that of normal endometrium (Figure 1A), which suggested that Rictor may be involved in cancer progression in EC.
Figure 1.
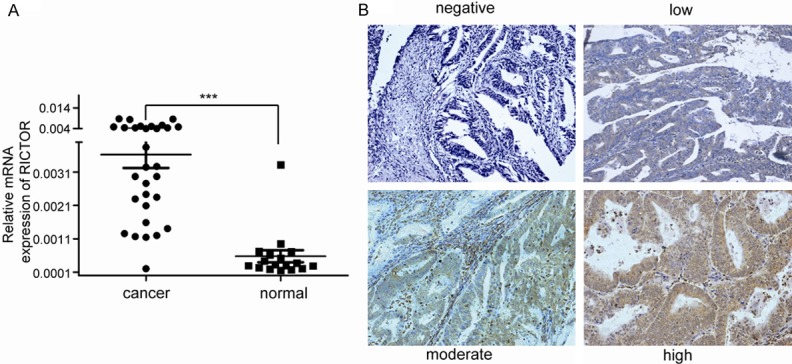
A. Rictor mRNA expression in 30 fresh EC tissue samples and 17 fresh normal endometrial tissues using real-time quantitative RT-PCR. Rictor mRNA expression of EC is higher than that of normal endometrium. The data were analyzed using the ΔΔCt approach and expressed as the Rictor/β-actin ratio [2-ΔCt (Rictor-β-actin)]. B. Representative image of Rictor expression in EC tissues was defined as negative, low, moderate and strong staining. Original magnification: ×200.
The Rictor protein expression level is closely correlated with FIGO stage, grade and vascular invasion
To further investigate the expression and significance of Rictor protein in EC, we first selected 134 paraffin-embedded EC (test cohort) tissue samples and 115 (validation cohort)samples to detect Rictor protein expression with immunochemical methods. The rate of Rictor -positive expression was observed in 59 cases (44%) in the test cohort and in 50 cases (43.5%) in the validation cohort (Supplementary Table 1). As shown in Table 1, we compared the relationship between Rictor expression and the clinicopathological features. Statistical analyses displayed that the expression level of Rictor protein was closely related with the International Federation of Gynecology and Obstetrics (FIGO) stage, grade and vascular invasion but not with pregnancy, pathological type or lymphatic metastasis in the test and validation cohorts. Moreover, the Rictor positive rate was lower in Grade 1 than Grade 2 or Grade 3 in both cohorts. These results suggest that Rictor might be associated with differentiation in EC. However, the prognostic value of Rictor in human EC remains obscure.
Table 1.
Relationship between Rictor expression and clinicopathologic features of endometrial cancer patients in test and validation cohorts
| Variable | Test cohort (n=134) | Validation cohort (n=115) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| RICTOR | P value | RICTOR | P value | ||||
|
|
|
||||||
| negative | positive | negative | positive | ||||
| Pregnancy | |||||||
| No | 4 | 3 | 0.949 | 3 | 3 | 0.741 | |
| Yes | 71 | 56 | 62 | 47 | |||
| Pathological type | |||||||
| Adenocarcinoma | 70 | 51 | 0.181 | 60 | 42 | 0.163 | |
| Squamous carcinoma, Papillay serous carcinoma, clear cell carcinoma | 5 | 8 | 5 | 8 | |||
| Stage | |||||||
| I | 71 | 44 | 0.001** | 61 | 39 | 0.011* | |
| II | 3 | 8 | 3 | 6 | |||
| III, IV | 1 | 7 | 1 | 5 | |||
| Grade | |||||||
| G1 | 62 | 15 | < 0.001** | 55 | 14 | < 0.001** | |
| G2 | 12 | 30 | 9 | 23 | |||
| G3 | 1 | 15 | 1 | 13 | |||
| Vascular invasion | |||||||
| No | 74 | 52 | 0.011* | 64 | 45 | 0.043* | |
| Yes | 1 | 7 | 1 | 5 | |||
| Lymphatic metastasis | |||||||
| No | 73 | 54 | 0.134 | 63 | 47 | 0.446 | |
| Yes | 2 | 5 | 2 | 3 | |||
P < 0.05;
P < 0.001.
Rictor is an independent prognostic factor for OS of EC patients
Because the GEO database showed that Rictor was associated with patients survival status in EC, we next analyzed the correlation between Rictor expression status and overall survival (OS) using the Kaplan-Meier method and compared the survival curves using the log-rank test for EC in the test and validation cohorts. A univariate analysis demonstrated that the pathological type, FIGO stage, grade, vascular invasion, lymphatic metastasis and Rictor were predictors of OS in the test and validation cohorts (Figure 2). In addition, The OS of Rictor-negative group was distinctly better than that of the Rictor-positive one for all 249 samples separated according to pathological type, grade, vascular invasion and lymphatic metastasis (Figure 3). Furthermore, multivariate Cox proportional hazards regression analyses showed that vascular invasion and Rictor were independent prognostic factors for EC in the both cohorts (Table 2). These results suggested that Rictor was a risk prognostic factor for the OS of EC patients.
Figure 2.
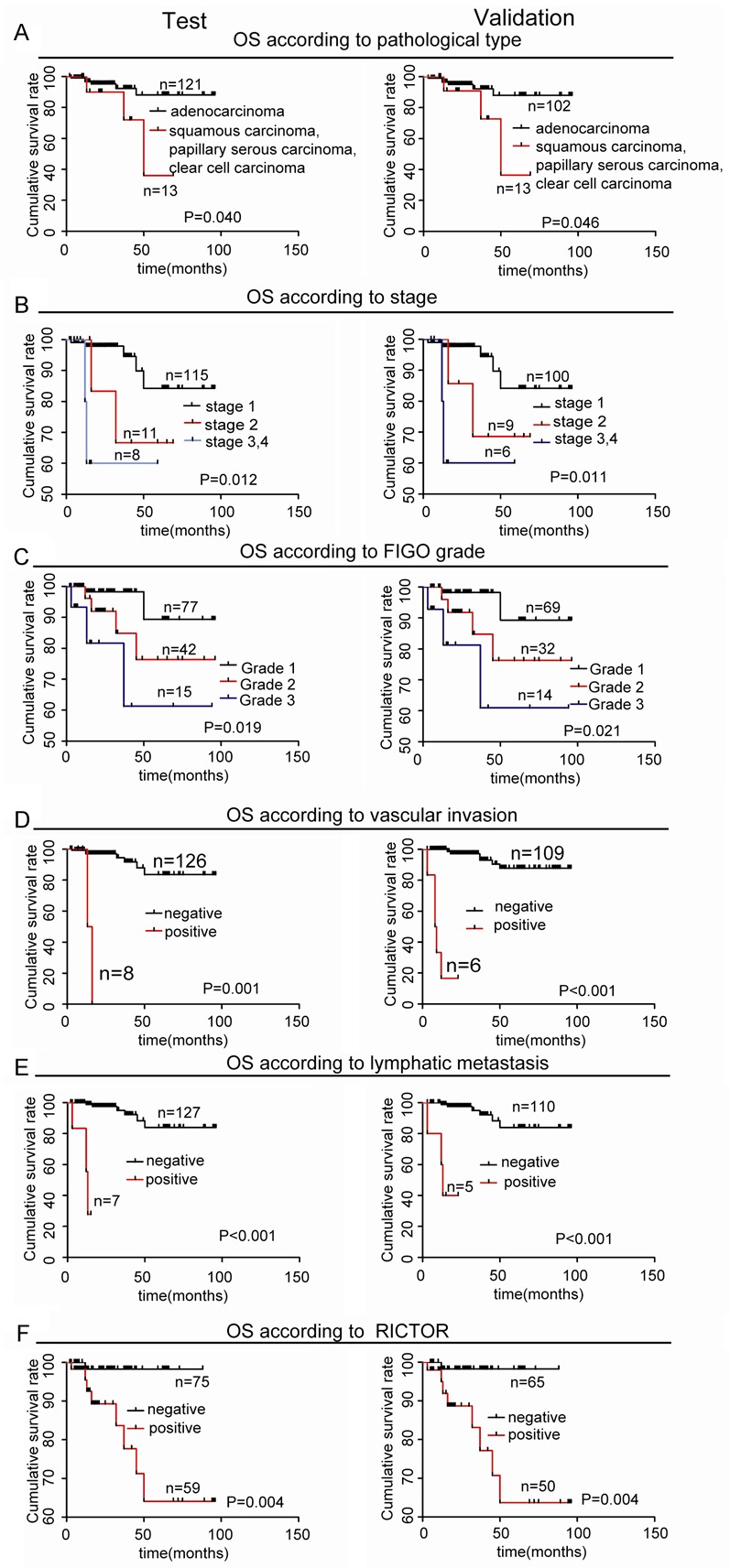
Univariate analyses of factors were associated with OS in test and validation cohorts by Kaplan-Meier method and log-rank test. (*, P < 0.05; **, P < 0.01). A. Comparisons of OS between adenocarcinoma and squamous carcinoma or papillary serous carcinoma or clear cell carcinoma groups in the test and validation cohorts. B. Comparisons of OS between stage I, stage II and stage III/IV groups in the test and validation cohorts. C. Comparisons of OS between G1, G2 and G3 groups in the test and validation cohorts. D. Comparisons of OS with and without vascular invasion groups in the test and validation cohorts. E. Comparisons of OS with and without lymphatic metastasis groups in the test and validation cohorts. F. Comparisons of OS between Rictor negative and positive groups in the test and validation cohorts.
Figure 3.
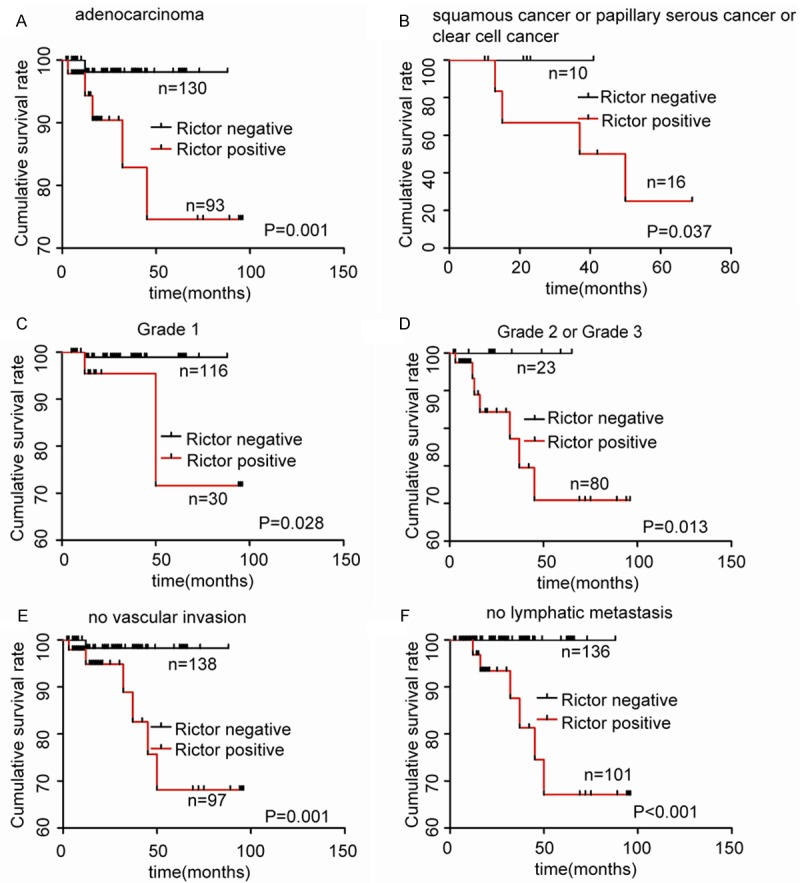
Prognostic significance of Rictor expression was assessed for all 249 samples separated according to pathological type, grade, vascular invasion and lymphatic metastasis by Kaplan-Meier method and log-rank test. A, B. Comparisons of OS between Rictor negative and positive groups in the adenocarcinoma cohort and in the squamous carcinoma or papillary serous carcinoma or clear cell carcinoma cohort. C, D. Comparisons of OS between Rictor negative and positive groups in G1 cohort and in G2 or G3 cohort. E. Comparisons of OS between Rictor negative and positive groups in no vascular invasion cohort. F. Comparisons of OS between Rictor negative and positive groups in no lymphatic metastasis cohort.
Table 2.
Multivariate analyses of factors associated with OS in test and validation cohorts with the Cox proportional hazard regression model with stepwise manner (forward: condition, entry α=0.05, stay α=0.1)
| Variable | Test cohort (n=133) | Validation cohort (n=109) | ||
|---|---|---|---|---|
|
| ||||
| OS hazard ratio (95% CI) | P value | OS hazard ratio (95% CI) | P value | |
| Vascular invasion | 11.961 | 0.009** | 12.034 | 0.008** |
| (Yes vs No) | (1.858-76.980) | (1.895-76.433) | ||
| Rictor | 8.612 | 0.046* | 8.736 | 0.045* |
| (Yes vs No) | (1.034-71.702) | (1.050-72.707) | ||
P < 0.05;
P < 0.01.
The sensitivity and specificity of Rictor for EC prognosis
To further confirm the prognostic efficiency of Rictor, we compared the sensitivity and specificity of Rictor for EC prognosis by logistic regression. We constructed seven models including Rictor, individual clinicopathological risk factor, combination of clinicopathological risk factors and Rictor combined with clinicopathological risk factors in both cohorts. We performed an ROC curve to compare the prognostic accuracy of Rictor with clinicopathological risk factors. As shown in Figure 4, the area under the curve (AUC) was 0.627 in test cohort and 0.619 in validation cohort for pathological type, 0.718 in test cohort and 0.726 in validation cohort for grade, 0.587 in test cohort and 0.592 in validation cohort for vascular invasion, 0.651 in test cohort and 0.657 in validation cohort for lymphatic metastasis, 0.740 in test cohort and 0.746 in validation cohort for Rictor, 0.833 in test cohort and 0.849 in validation cohort for clinicopathological prognostic factors’ combination and 0.855 in test cohort and 0.887 in validation cohort for Rictor combined with clinicopathological prognostic factors. Statistical analyses displayed that AUC for Rictor combined with other clinicopathological prognostic factors was higher than any individual factor or the combination of clinicopathological prognostic factors. The results showed that Rictor combined with other clinicopathological prognostic factors had more sensitivity and specificity and was a stronger prognostic model than the single risk factor or their combination.
Figure 4.
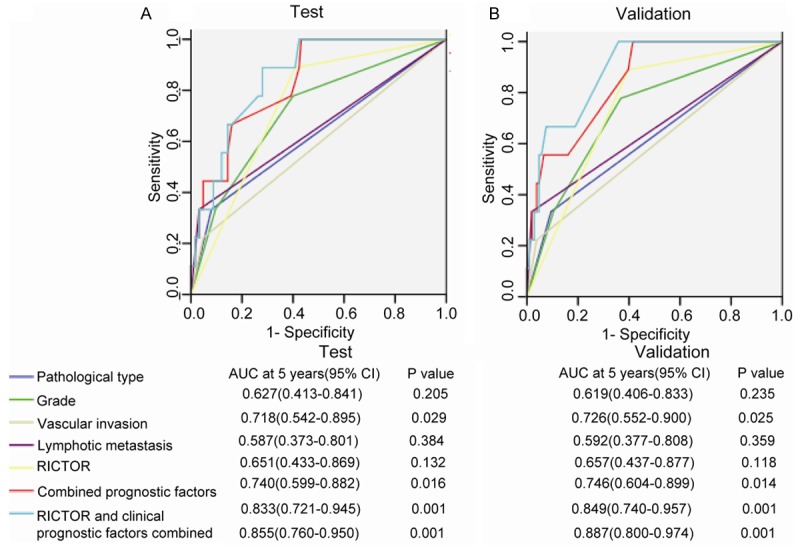
ROC curve compares the prognostic accuracy of Rictor with clinicopathological risk factors in all 249 endometrial carcinoma patients by logistic regression. ROC=receiver operator characteristic. AUC=area under curve. A. Comparisons of the prognostic accuracy by Rictor (positive vs. negative), poor prognostic features (with vs without any poor prognostic feature), pathological type (adenocarcinoma vs squamous carcinoma, papillary serous carcinoma and clear cell carcinoma), stage (I vs II vs III, IV), grade (G1 vs G2 vs G3), vascular invasion (yes vs no), lymphatic metastasis (yes vs no), combined clinicopathological prognostic factors alone, or Rictor and clinicopathological prognostic factors combined. P values show the AUC at 5 years for Rictor vs the AUC at 5 years for other features.
Discussion
Many studies show that multiple genetic molecules characterize the initiation, development, and poor prognosis of EC [28]. Thus, it is urgent to find new prognostic factors involved in EC to improve the survival of patients.
Rictor, a component of the mTORC2 complex, is closely associated with cancer proliferation, migration, invasion, metastasis, EMT and poor prognosis [17,22,23,29]. For example, Integrin-linked kinase (ILK), a focal adhesion adaptor, is a serine/threonine protein kinase that regulates cell proliferation, survival and EMT. The ILK/Rictor complex acts as a potential molecular target for preventing/reversing EMT causing fibrosis, cancer progression and metastasis [29]. Although Rictor mostly carries its role as the key component of mTORC2, the recent studies show that Rictor also exerts the mTORC2-independent functions in cell migration. Rictor alone without other components of mTORC2 interacts with the regulators of cell morphology and migration such as actin based molecular motor myosin 1c and the integrin-linked kinase [30,31]. These data suggested complex roles for Rictor in cancer progression.
Although Rictor is correlated with cancer cell invasion, proliferation, EMT, migration and metastasis, its prognostic value in EC remains unclear. In the study, we found that the expression of Rictor mRNA and protein was higher in EC tissue than in normal endometrium, suggesting that Rictor may be involved in EC progression. Through analysis of the relationship between Rictor and clinicopathologic features of EC patients, we further confirmed Rictor is associated with FIGO stage, grade and vascular invasion; moreover, the Rictor positive rate was higher in the samples of higher stage, low differentiation and vascular invasion. It has been reported that the upregulation of Rictor or EpCAM mRNA expression links with a higher rate of relapse in hepatocellular carcinoma [32]. Taking together, we speculate that Rictor might be associated with cancer proliferation, migration and invasion in EC. It has been reported that the repression of miR-152 is attributed to the upregulation of Rictor in EC [21]. In other words, it is possible that miRNA-mediated Rictor downregulation represses tumor progression. Thus, Rictor evaluated in EC expression potentially offers clinical value in directing personal treatment by targeting miRNAs.
By analysis of the correlation between different variables and overall survival we found that Rictor was an independent prognostic factor for EC. By correlating the expression levels of Rictor with clinicopathological risk factors, we hope to validate the utility of Rictor as a factor to evaluate the prognosis of patients. Some tissue samples did not express high Rictor levels, leading us to suggest that the examination of tissue Rictor levels in combination with the other risk factors would increase the specificity and sensitivity of the prognosis. Thus, we compared the prognostic accuracy of Rictor with other clinicopathological risk factors. Our results showed that Rictor combined with other clinicopathological risk factors was a stronger prognostic model than individual risk factor or their combination.
It has been reported that the myometrium of pregnancy women differently expresses mTOR signaling components including Rictor that could be regulated by progesterone [33]. The status of progesterone receptor (PR) in EC has been confirmed an independent prognostic factor [34]. PR includes two isoforms, PRA and PRB. The absence of one or both were correlated with shorter disease-free or overall survival of EC [35]. PR positive EC patients have more effective with progesterone treatment. Thus, our results provide enlightenment for indentifying whether Rictor combination with PR could exert evaluation of a more accurate prognosis of EC. Clinical research show that Rictor contributes to the resistance of cisplatin in ovarian cancer [36] and increased the sensitivity to vincristine and temozolomide in Glioblastoma [37]. Therefore, our data supplied reference for indentifying whether the enhancement of chemosensitivity by targeting Rictor in EC. Accumulating evidence show Rictor is associated with Glucose metabolism and senescence [38,39], Metformin potentiates the effects of paclitaxel in EC by inhibition of cell proliferation and modulation the activity of the mTOR pathway [40]. The estrogen-dependent EC is often accompanied with diabetes, obesity and age. This could suggest that we should uncover the correlation which could be released into the circulation at the tumor onset stage associated with systematic cancer status.
However, our current study is limited because of its retrospection and all Chinese patient population. In addition, as an initial report of the potential role of Rictor in EC, further work, such as a prospective study and molecular mechanism, should be performed to clarify issues that were not explored in this study. Moreover, we should treat patients in a different way on the basis of Rictor expression. However, there were only qualitative data of Rictor and no quantitative data in our original source.
In conclusion, we clarified that Rictor expression is associated with FIGO stage, grade and vascular invasion; confirmed that Rictor is an independent prognostic factor in EC patients; moreover, we developed and validated that Rictor combined with other clinicopathological risk factors is a more novel prognostic tool than individual risk factor or clinicopathological risk factors’ combination. Thus, Rictor potentially offers clinical value in directing personal treatment for EC patients.
Acknowledgements
This work was supported by the National Science Foundation of China (No. 81372794), Science and Technology Commission of Shanghai Municipality (13JC1404502), Songjiang district of Science and Technology Commission of Shanghai Municipality (No. 10SJGG26), and Shanghai Songjiang District Central Hospital (No. BY10A07).
Disclosure of conflict of interest
The authors declare no conflict of interest.
Supporting Information
References
- 1.Kang S, Lee JM, Lee JK, Kim JW, Cho CH, Kim SM, Park SY, Park CY, Kim KT. How low is low enough? Evaluation of various risk-assessment models for lymph node metastasis in endometrial cancer: a Korean multicenter study. J Gynecol Oncol. 2012;23:251–256. doi: 10.3802/jgo.2012.23.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geels YP, Pijnenborg JM, van den Berg-van Erp SH, Snijders MP, Bulten J, Massuger LF. Absolute depth of myometrial invasion in endometrial cancer is superior to the currently used cut-off value of 50% Gynecol Oncol. 2013;129:285–291. doi: 10.1016/j.ygyno.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Luomaranta A, Leminen A, Loukovaara M. Prediction of lymph node and distant metastasis in patients with endometrial carcinoma: a new model based on demographics, biochemical factors, and tumor histology. Gynecol Oncol. 2013;129:28–32. doi: 10.1016/j.ygyno.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 6.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 7.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, He X, Hung JY, Lai CC, Ding Q, Su JL, Yang JY, Sahin AA, Hortobagyi GN, Tsai FJ, Tsai CH, Hung MC. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 8.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 9.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 10.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 11.Pearce LR, Huang X, Boudeau J, Pawlowski R, Wullschleger S, Deak M, Ibrahim AF, Gourlay R, Magnuson MA, Alessi DR. Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald PC, Oloumi A, Mills J, Dobreva I, Maidan M, Gray V, Wederell ED, Bally MB, Foster LJ, Dedhar S. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res. 2008;68:1618–1624. doi: 10.1158/0008-5472.CAN-07-5869. [DOI] [PubMed] [Google Scholar]
- 13.He Y, Li D, Cook SL, Yoon MS, Kapoor A, Rao CV, Kenis PJA, Chen J, Wang F. Mammalian target of rapamycin and Rictor control neutrophil chemotaxis by regulating Rac/Cdc42 activity and the actin cytoskeleton. Mol Biol Cell. 2013;24:3369–3380. doi: 10.1091/mbc.E13-07-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carson RP, Fu C, Winzenburger P, Ess KC. Deletion of Rictor in neural progenitor cells reveals contributions of mTORC2 signaling to tuberous sclerosis complex. Hum Mol Genet. 2012;22:140–152. doi: 10.1093/hmg/dds414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia W, Sanders AJ, Jia G, Liu X, Lu R, Jiang WG. Expression of the mTOR pathway regulators in human pituitary adenomas indicates the clinical course. Anticancer Res. 2013;33:3123–3131. [PubMed] [Google Scholar]
- 16.Balasubramanian S, Johnston RK, Moschella PC, Mani SK, Tuxworth WJ Jr, Kuppuswamy D. mTOR in growth and protection of hypertrophying myocardium. Cardiovasc Hematol Agents Med Chem. 2009;7:52–63. doi: 10.2174/187152509787047603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal NK, Chen CH, Cho H, Boulbès DR, Spooner E, Sarbassov DD. Rictor regulates cell migration by suppressing RhoGDI2. Oncogene. 2012;32:2521–2526. doi: 10.1038/onc.2012.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Z, Zhou Y, Evers BM, Wang Q. Rictor regulates FBXW7-dependent c-Myc and cyclin E degradation in colorectal cancer cells. Biochem Biophys Res Commun. 2012;418:426–432. doi: 10.1016/j.bbrc.2012.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu MJ, Chang CH, Chiu YT, Wen MC, Shu KH, Li JR, Chiu KY, Chen YT. Rictor-dependent AKT activation and inhibition of urothelial carcinoma by rapamycin. Urol Oncol. 2012;30:69–77. doi: 10.1016/j.urolonc.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Oshima R, Bashir T, Cloninger C, Artinian N, Anderson L, Bernath A, Holmes B, Benavides-Serrato A, Sabha N, Nishimura RN, Guha A, Gera J. Conditional Astroglial Rictor Overexpression Induces Malignant Glioma in Mice. PLoS One. 2012;7:e47741. doi: 10.1371/journal.pone.0047741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uesugi A, Kozaki K, Tsuruta T, Furuta M, Morita K, Imoto I, Omura K, Inazawa J. The tumor suppressive microRNA miR-218 targets the mTOR component Rictor and inhibits AKT phosphorylation in oral cancer. Cancer Res. 2011;71:5765–5778. doi: 10.1158/0008-5472.CAN-11-0368. [DOI] [PubMed] [Google Scholar]
- 22.Kyprianou N, Oneyama C, Kito Y, Asai R, Ikeda JI, Yoshida T, Okuzaki D, Kokuda R, Kakumoto K, Takayama KI, Inoue S, Morii E, Okada M. MiR-424/503-Mediated Rictor Upregulation Promotes Tumor Progression. PLoS One. 2013;8:e80300. doi: 10.1371/journal.pone.0080300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F, Zhang X, Li M, Chen P, Zhang B, Guo H, Cao W, Wei X, Cao X, Hao X, Zhang N. mTOR Complex Component Rictor Interacts with PKC and Regulates Cancer Cell Metastasis. Cancer Res. 2010;70:9360–9370. doi: 10.1158/0008-5472.CAN-10-0207. [DOI] [PubMed] [Google Scholar]
- 24.Kyprianou N, Gupta S, Hau AM, Beach JR, Harwalker J, Mantuano E, Gonias SL, Egelhoff TT, Hansel DE. Mammalian Target of Rapamycin Complex 2 (mTORC2) Is a Critical Determinant of Bladder Cancer Invasion. PLoS One. 2013;8:e81081. doi: 10.1371/journal.pone.0081081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M, Battiston C, Van Laarhoven S, Fiel MI, Di Feo A, Hoshida Y, Yea S, Toffanin S, Ramos A, Martignetti JA, Mazzaferro V, Bruix J, Waxman S, Schwartz M, Meyerson M, Friedman SL, Llovet JM. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–1983. 1983.e1971–1911. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uesugi A, Kozaki Ki, Tsuruta T, Furuta M, Morita KI, Imoto I, Omura K, Inazawa J. The Tumor Suppressive MicroRNA miR-218 Targets the mTOR Component Rictor and Inhibits AKT Phosphorylation in Oral Cancer. Cancer Res. 2011;71:5765–5778. doi: 10.1158/0008-5472.CAN-11-0368. [DOI] [PubMed] [Google Scholar]
- 27.Tsuruta T, Kozaki K, Uesugi A, Furuta M, Hirasawa A, Imoto I, Susumu N, Aoki D, Inazawa J. miR-152 is a tumor suppressor microRNA that is silenced by DNA hypermethylation in endometrial cancer. Cancer Res. 2011;71:6450–6462. doi: 10.1158/0008-5472.CAN-11-0364. [DOI] [PubMed] [Google Scholar]
- 28.Shiozawa T, Konishi I. Early endometrial carcinoma: clinicopathology, hormonal aspects, molecular genetics, diagnosis, and treatment. Int J Clin Oncol. 2006;11:13–21. doi: 10.1007/s10147-005-0546-1. [DOI] [PubMed] [Google Scholar]
- 29.Serrano I, McDonald PC, Lock FE, Dedhar S. Role of the integrin-linked kinase (ILK)/Rictor complex in TGFβ-1-induced epithelial–mesenchymal transition (EMT) Oncogene. 2012;32:50–60. doi: 10.1038/onc.2012.30. [DOI] [PubMed] [Google Scholar]
- 30.McDonald PC, Oloumi A, Mills J, Dobreva I, Maidan M, Gray V, Wederell ED, Bally MB, Foster LJ, Dedhar S. Rictor and Integrin-Linked Kinase Interact and Regulate Akt Phosphorylation and Cancer Cell Survival. Cancer Res. 2008;68:1618–1624. doi: 10.1158/0008-5472.CAN-07-5869. [DOI] [PubMed] [Google Scholar]
- 31.Hagan GN, Lin Y, Magnuson MA, Avruch J, Czech MP. A Rictor-Myo1c Complex Participates in Dynamic Cortical Actin Events in 3T3-L1 Adipocytes. Mol Cell Biol. 2008;28:4215–4226. doi: 10.1128/MCB.00867-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakata A, Tanaka S, Mogushi K, Yasen M, Noguchi N, Irie T, Kudo A, Nakamura N, Tanaka H, Arii S. Gene expression signature of the gross morphology in hepatocellular carcinoma. Ann Surg. 2011;253:94–100. doi: 10.1097/SLA.0b013e3181f9bc00. [DOI] [PubMed] [Google Scholar]
- 33.Foster HA, Davies J, Pink RC, Turkcigdem S, Goumenou A, Carter DR, Saunders NJ, Thomas P, Karteris E. The human myometrium differentially expresses mTOR signalling components before and during pregnancy: Evidence for regulation by progesterone. J Steroid Biochem Mol Biol. 2014;139:166–172. doi: 10.1016/j.jsbmb.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose PG. Endometrial carcinoma. N Engl J Med. 1996;335:640–649. doi: 10.1056/NEJM199608293350907. [DOI] [PubMed] [Google Scholar]
- 35. doi: 10.1507/endocrj.kr-114. <Biological roles of estrogen and progesterone in human endometrial carcinoma--new developments in potential endocrine therapy for endometrial cancer..pdf>. [DOI] [PubMed] [Google Scholar]
- 36.Maki Carl G, Im-aram A, Farrand L, Bae SM, Song G, Song YS, Han JY, Tsang BK. The mTORC2 Component Rictor Contributes to Cisplatin Resistance in Human Ovarian Cancer Cells. PLoS One. 2013;8:e75455. doi: 10.1371/journal.pone.0075455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verreault M, Weppler SA, Stegeman A, Warburton C, Strutt D, Masin D, Bally MB. Combined RNAi-mediated suppression of Rictor and EGFR resulted in complete tumor regression in an orthotopic glioblastoma tumor model. PLoS One. 2013;8:e59597. doi: 10.1371/journal.pone.0059597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 39.Wang CY, Kim HH, Hiroi Y, Sawada N, Salomone S, Benjamin LE, Walsh K, Moskowitz MA, Liao JK. Obesity Increases Vascular Senescence and Susceptibility to Ischemic Injury Through Chronic Activation of Akt and mTOR. Sci Signal. 2009;2:ra11. doi: 10.1126/scisignal.2000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanna RK, Zhou C, Malloy KM, Sun L, Zhong Y, Gehrig PA, Bae-Jump VL. Metformin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and modulation of the mTOR pathway. Gynecol Oncol. 2012;125:458–469. doi: 10.1016/j.ygyno.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


