Abstract
A subset of cancer cells, termed cancer stem cells (CSCs) or tumor-initiating cells (TICs) could initiate tumors and are responsible for tumor recurrence and chemotherapeutic resistance. In this study, we enriched TICs in nasopharyngeal carcinoma (NPC) by the spheres formation and characterized the stem-like signatures such as self-renewal, proliferation, chemoresistance and tumorigenicity. By this method, we investigated that epigallocathechin gallate (EGCG), the major polyphenol in green tea could target TICs and potently inhibit sphere formation, eliminate the stem-like properties and enhance chemosensitivity in NPC through attenuation of STAT3 activation, which could be important in regulating the stemness expression in NPC. Our results demonstrated that STAT3 pathway plays an important role in mediating tumor-initiating capacities in NPC and suggest that inactivation of STAT3 with EGCG may represent a potential preventive and therapeutic approach for NPC.
Keywords: STAT3, EGCG, nasopharyngeal cancer
Introduction
Nasopharyngeal carcinoma (NPC), which arises from the nasopharyngeal epithelial cells, has high prevalence rates in southeast China and Taiwan. Many studies conclude that NPC is a complex disease that is caused by interactions among genetic predisposition, environmental factors, and EBV infection [1]. There is still an obstacle in improving therapeutic efficacy and increasing the survival rate of NPC populations. Although NPC is radiation-sensitive, the treatment effect of advanced stages in patients is not ideal because of recurrence and chemotherapy-resistance. The prognosis remains poor in a significant number of NPC patients with relapse and metastasis diseases [2]. Accumulating studies have shown that tumors contain a small subpopulation of cells, called cancer stem cells (CSCs) or tumor-initiating cells (TICs) [3,4], which possess the ability to reinitiate a tumor for several generations and increase the tumorigenic potential. TICs enable tumor cells to self-renew and proliferate extensively because of the expression of an anti-apoptotic and drug-resistant property that resists the effects of chemotherapeutic drugs, which play important roles in recurrence and treatment difficulty [5,6].
The isolation of TICs from cancer cells has been performed successfully using several techniques. One proposed method for isolating and enriching stem-like cells was growing the sphere-generated cells in a serum-free non-adherent culture. These cells may represent the tumor-initiating subpopulation, with their ability of self-renewal and unlimited proliferation [7,8]. We demonstrated that NPC sphere-generated cells express properties of stemness, chemoresistance and anti-apoptosis compared to parental monolayer cells, and we used these properties to design the experiments.
Natural dietary polyphenols such as quercetin, curcumin, resveratrol, and epigallocatechin-3-gallate (EGCG) have gained considerable attention as substances that may potentially prevent cancer formation and progression [9-12]. Among phenolic compounds, EGCG is the most prevalent polyphenol in green tea which has chemo-preventive properties against various types of cancers because of its potent capacity for inhibiting cancer cell growth through several signaling pathways [13,14].
Signal transducer and activator of transcription 3 (Stat3) is an oncogenic transcript factor that responds to cellular growth signaling and has been implicated in the development and progression of various tumors. The activation of STAT3 results in expression of many target genes required for tumor cell survival, proliferation and metastasis. The activation of STAT3 also contributes to the invasiveness of NPC cells and is correlated with advanced clinical staging in NPC [15]. Recently, STAT3 was found as an important factor in tumor initiation property [16,17]. Although some studies have shown that dietary compounds have the potential to act against the tumor-initiating characteristics of cancers [18,19], the anti-cancer effect of EGCG in target NPC TICs and the possible pathway was not explored largely. In this study, we used sphere-generated cells to investigate the underlying mechanism in blockade of stem-like properties by EGCG and to clarify STAT3-signaling pathway which maybe as a therapeutic target for suppression of tumor-initiating signatures of NPC.
Materials and methods
Cell culture
Parental monolayer cells culture
Two human NPC cell lines, TW01 and TW06 were cultured in 10 cm2 dishes with Dulbecco’s Modified Eagle Medium (DMEM, GIBCO) and 10% FBS, 1% sodium pyruvate, 1% penicillin, streptomycin, amphotericin, and 1% NEAA. The cells were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Non-adherent culture
TW01 and TW06 parental cells were seeded non-adhesively in a 6-well culture dish coated with thin agarose at a density of 2 × 104/mm3 in serum-free DMEM/F12 medium supplemented with 10 ng/mL of basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF). The culture medium was changed every other day until sphere formation took place. To propagate spheres in vitro, sphere cells were collected by filtration through a 70 μm mesh and gentle centrifugation, dissociated into single-cell suspensions, and cultured to allow the regeneration of spheres. Second-generation spheres were used for subsequent experiments.
RNA extraction and real-time RT-PCR
The total RNA was isolated with Trizol reagent. The concentration and purity of the total RNA was determined with a NanoDrop ND-1000 spectrophotometer. First-strand cDNA was reverse-transcribed (RT) according to the manufacturer’s protocols. Relative levels of mRNA were determined by Q-PCR, using a real-time PCR system. Several stemness markers and drug-resistant genes were analyzed. GAPDH was the endogenous reference. The Q-PCR was performed with an ABI PRISM® 7900HT machine. The primer sequences used for real-time RT-PCR are shown as follows:
GAPDH: Forward: 5’-ACGGGAAGCTCACTGGCATGG-3’, Reverse: 5’-GGTCCACCACCCTGTTGCTGTA-3’; Oct-4: Forward: 5’-GTGGAGAGCAACTCCGATG-3’, Reverse: 5’-TGCTCCAGCTTCTCCTTCTC-3’; β-catenin: Forward: 5’-CCAGCCGACACCAAGAAG-3’, Reverse: 5’-CGAATCAATCCAACAGTAGCC-3’; Nanog: Forward: 5’-ATTCAGGACAGCCCTGATTCTTC-3’, Reverse: 5’-TTTTTGCGACACTCTTCTCTGC-3’; ABCG2: Forward: 5’-CATG TACTGGCGAAGAATATTTGGT-3’, Reverse: 5’-CACGTGATTCTTCCACAAGCC-3’; MRP-1: Forward: 5’-GCTTCCTCTTGGTGATATTCG-3’, Reverse: 5’-GCAGTTCAACGCATAGTGG-3’; MDR-1: Forward: 5’-TGGCAAAGAAATAAAGCGACTGA-3’, Reverse: 5’-CAGGATGGGCTCCTGGG-3’; Survivin: Forward: 5’-TCCCTGGCTCCTCTACTGTT-3’, Reverse: 5’-TGTCTCCTCATCCACCTGAA-3’; Bcl-2: Forward: 5’-ATGTGTGTGGAGAGCGTCAACC-3’, Reverse: 5’-TGAGCAGAGTCTTCAGAGACAGCC-3’; c-Myc: Forward: 5’-GGAACGAGCTAAAACGGAGCT-3’, Reverse: 5’-GGCCTTTTCATTGTTTTCCAACT-3’.
Cell viability assay
The effect of chemo-drugs cisplatin and 5-FU on the visibility of NPC parental and sphere cells was examined using a MTT assay. Cells were seeded at 2.5 × 103 per well in 96-well plates and allowed to grow for 24 hours. After 72 hours, 20 μL of MTT solution was added to each well and incubated for 4 hours at 37°C. The MTT formazan crystals were then dissolved in DMSO, and the absorbance was measured with a microplate reader (Bio-Rad 680, USA) at a wavelength of 570 nm. Each treatment was performed in triplicate.
Apoptosis assessed by flow cytometry
TW01, TW06 parental and sphere cells were washed twice with cold PBS and then re-suspended in 1X binding buffer at a concentration of 1 × 106 cells/mL. Next, 100 μL of the solution (1 × 105 cells) was transferred to a 5 mL culture tube, where 5 μL of FITC Annexin V and 5 μL PI were added. The cells were gently vortexed and incubated for 15 minutes at RT (25°C) in the dark. An additional 400 μL of 1X binding buffer was added to each tube. The solution was analyzed using a FC500 flow cytometer to identify subpopulations of the apoptosis cells within 1 hour.
Soft agar clonogenic assay
The bottom of each well (35 mm) of a 6-well culture dish was coated with 2 mL agar mixture (DMEM, 10% [v/v] FCS, 0.6% [w/v] agar). After the bottom layer had solidified, a 2 mL top agar-medium mixture (DMEM, 10% [v/v] FCS, 0.3% [w/v] agar) containing 2 × 104 cells with various concentrations of EGCG was added, and incubated at 37°C for 2 weeks. At the end of the incubation period, the number of colonies with diameters > 0.5 mm was counted using microscopy, after staining with crystal violet.
Western blot analysis
Samples (20 μg) were boiled at 95°C for 5 minutes, separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto a polyvinylidene difluoride membrane, and immunoblotted with specific antibodies. A rabbit anti-GAPDH was used as sample-loading control. Immunoreactive protein bands were detected with the ECL detection system. The primary antibodies against STAT3 and phosphorylated STAT3 (Tyrosine 705), were purchased from Sigma-Aldrich (St Louis, MO) and used at a dilution of 1:1000.
Immunofluorescent staining
Cells were cultured on sterile glass coverslips in 6-well plates and fixed in 4% paraformaldehyde. After washing with PBS, the cells were permeabilized using 0.1% Triton X-100 solution and blocked with 10% BSA. The slides were incubated overnight at 4°C with the primary antibodies, followed by incubation with the fluorescently-labeled secondary antibodies at room temperature for 1 h. Slides were counterstained with DAPI and analyzed under a fluorescent microscope (Leica DM 4000B).
Statistical analysis
Data were expressed as the mean ± SD from at least 3 separate experiments. The Student’s t test or ANOVA was used to compare the differences between groups, and statistical significance was considered as P < .05. Statistical analyses were performed with the SPSS for Windows, version 14.0.
Results
NPC cells forming spheres acquired tumor-initiating capability and showed relative resistance to chemo-agents
To assess the capability of NPC cells to initiate self-renewal, we selected monolayer cells dissociated into single cells growing in serum-free non-adherent culture. After the formation of spheres, sphere cells were dissociated into single cells and the first-generation spheres were found to be capable of generating second-generation spheres, suggesting that NPC sphere-generated cells are capable of self-renewal (Figure 1A). In addition, second-generation spheres were observed with increased sphere-forming efficiency, compared with primary spheres derived from the parental cells (Figure 1B and 1C). These results demonstrated that a considerable percentage of single cells derived from second-generation spheres are self-renewing cells with features of TICs and were therefore used for subsequent experiments. In vitro tumorigenicity, assessed by a soft agar colony formation assay, showed that sphere cells reached more potent clonogenicity compared to parental cells (Figure 1D), and the cell viability analyzed by an MTT assay showed that the sphere cells were relatively resistant to cisplatin and 5-FU (Figure 1E). Similarly, sphere cells were more resistant to cisplatin-induced apoptosis analyzed by flow cytometry with Annexin V and PI staining (Figure 1F). These data indicated that, although chemo drugs induced growth inhibition and apoptosis in the NPC cells in a dose-dependent manner, the toxicity in the spheres was not as effective as in the parental cells. Real-Time RT-PCR analysis revealed that sphere cells grown in the serum-free non-adherent culture showed increased expression of stem cell markers Oct-4, Nanog and β-catenin, compared to parental cells grown in a conventional culture. In addition, the levels transcription factors involved in chemo resistance, including ABCG2, MRP-1, and MDR-1 were higher, compared by the mRNA expression (Figure 1G).
Figure 1.
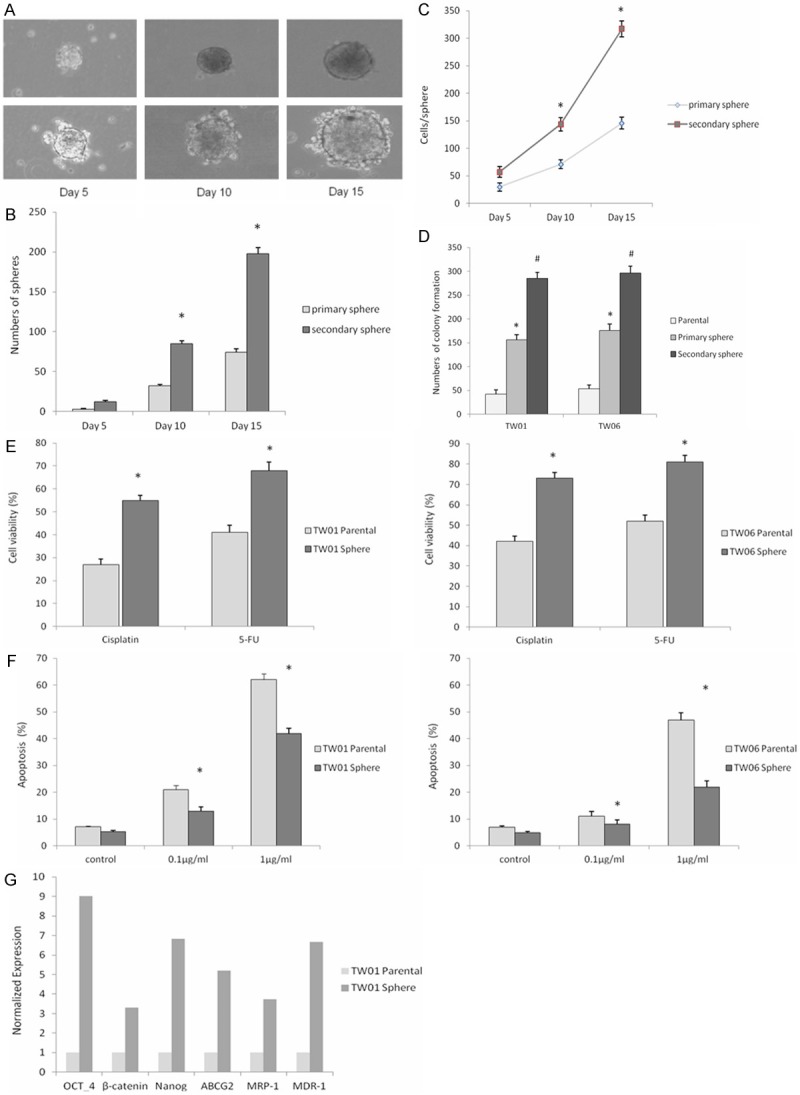
TW01 sphere with self-renewing cells growing in serum-free non-adherent condition. A: Phase-contrast micrographs of primary spheres generated from single-cell cultures of TW01 parental cells (upper panels) and secondary spheres (lower panels) taken on the indicated day of suspension culture. B: Amount of primary spheres generated from parental cells and secondary generated from dissociated primary spheres on the indicated day. *P < 0.05. Data represent mean ± SD. C: Number of cells per sphere generated from the secondary spheres and from primary spheres on the indicated day of culture. *P < 0.05 compared with primary spheres. Data represent mean ± SD. D: Sphere cells revealed significantly higher colony-forming ability than parental cells. Data represent mean ± SD. * or # = significantly different from respective controls, P < 0.05. E: Sphere cells showed relative resistant to chemo agents (cisplatin and 5-fluorouracil, 1 μg/ml respectively) compared to parental cells by MTT assay. Data represent mean ± SD. *means significantly different between two groups, P < 0.05. F: Sphere cells show significantly resistance to cisplatin-induced apoptosis compared to parental cells at different doses. Data are presented as the mean ± SD. *means significantly different between two groups, P < 0.05. G: Real-time RT-PCR revealed the amounts of Oct-4, β-catenin, Nanog and drug resistant genes ABCG2, MRP-1 and MDR-1. The internal control is housekeeping gene-GAPDH and every gene expression of sphere-generated cells was normalized with parental cells.
EGCG attenuates the stem-like properties and enhances the anti-tumor effects of cisplatin in NPC sphere cells
Several studies have demonstrated that STAT3 activation is associated with NPC, the expression of STAT3 or phosphorylated STAT3 (p-STAT3) in TW01 and TW06 sphere cells was further evaluated by Western blot. The results confirmed that the level of activated STAT3 was higher in NPC sphere cells than in parental cells (Figure 2A). It has been demonstrated that STAT3 plays an important role in tumorigenicity and sphere forming capacity in tumor and these results suggest that STAT3 may play a crucial role in maintaining the cancer stem-like characteristics of NPC CSC. Since STAT3 is active in NPC sphere cells, we next examine the effects of EGCG on NPC sphere cells to know if this results from inhibition STAT3. We investigated the effect of EGCG on the ability of TW01 and TW06 to form tumor spheres in anchorage-independent conditions. After the treatments of EGCG, the numbers of sphere were counted. Our results demonstrate that treatment with 20 μM EGCG in TW01 and TW06 cells led to decreases in tumor sphere-formation capacity and further efficiently inhibited with 40 μM EGCG (Figure 2B and 2C). In addition, colony formation assay revealed that the treatment of NPC sphere cells with EGCG also significantly inhibited the numbers of colony formation (Figure 2D). To examine the influence of EGCG on the chemo-resistance of NPC sphere cells, cell viability and apoptosis analysis was performed. We observed a marked decrease in the viability of TW01 and TW06 sphere cells after incubation with cisplatin and 20 μM EGCG, as determined by MTT assay (Figure 2E). The chemoinduced apoptosis effect detected using flow cytometry with Annexin V and PI double staining showed increased apoptotic activity in both TW01 and TW06 sphere cells with 20 μM EGCG added for 48 hours (Figure 2F). Although the sphere cells showed relatively higher anti-apoptosis than the parental cells, their apoptotic change had increased markedly after combination with EGCG. In consistence with these findings, the mRNA levels of several stemness genes were reduced after EGCG treatment examined by qRT-PCR (Figure 2G). These results suggest that EGCG has the potential to suppress stem-like properties, reduce drug resistance and downregulate the aggressiveness of NPC.
Figure 2.
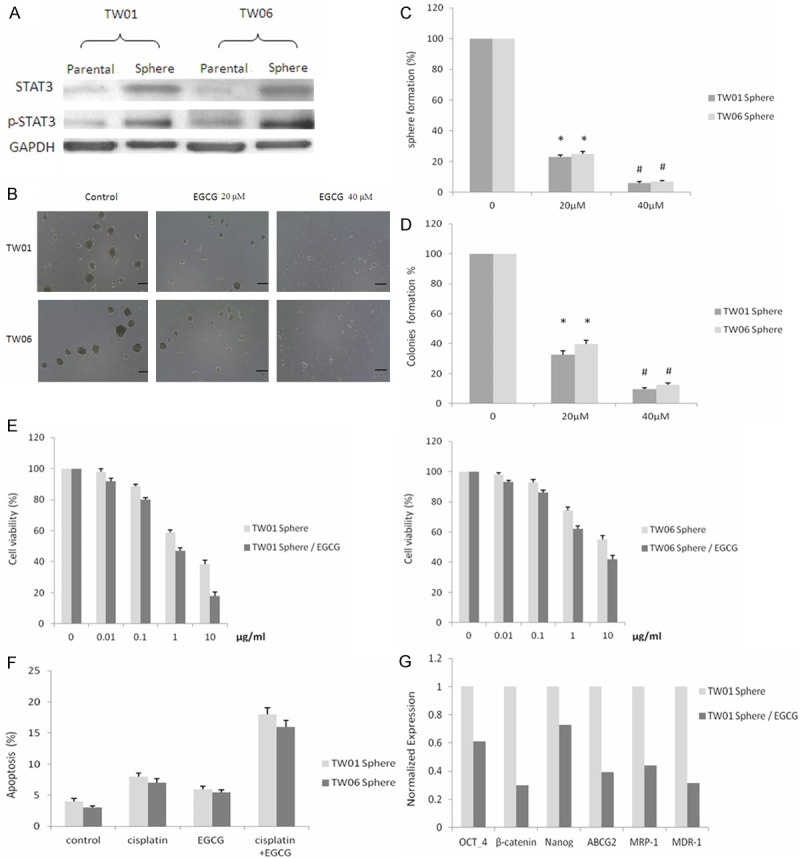
Effects of EGCG on the stem-like properties and chemo-resistance on NPC sphere cells. A: Western blot showing the protein levels of total and phosphorylated STAT3 in NPC parental and sphere cells. B: EGCG-inhibited spheres forming in the serum-free non-adherent culture. (Scale bar: 100 μm). C: Sphere numbers were significantly decreased in EGCG-treated group (sphere diameter > 70 μm). @, * or # means significantly different from controls, P < 0.05. D: Colony numbers were counted after treated with or without EGCG. Data represent mean ± SD. @, * or # means significantly different from controls, P < 0.05. E: EGCG enhanced cisplatin toxicity significantly after incubation with various doses of cisplatin revealed by MTT assay. *P < 0.05. Data shown here are the mean ± SD of three experiments. F: EGCG induced synergistic apoptosis effect with 0.1 μg/ml cisplatin on NPC sphere cells. * or # means significantly different between two groups, P < 0.05. G: The mRNA levels of OCT-4, β-catenin, Nanog, ABCG2, MRP-1 and MDR-1 in TW01 sphere cells in the presence or absence of 20 μM EGCG.
Effects of EGCG on STAT3 activation and downstream genes expressions in NPC sphere cells
Due to the correlation between STAT3 activation and tumor-initiating property of NPC, we next examined the effects of EGCG on STAT3 protein expression. TW01 sphere cells were treated with different concentrations of EGCG and measured the expression of total and p-STAT3 by the Western blot analysis (Figure 3A). We found although the expression level of total STAT3 protein was not change, the p-STAT3 expression was decreased significantly in response to EGCG which were evaluated with Western blotting. To evaluate that this effect of EGCG was specific on the p-STAT3, TW01 sphere cells were grown on a glass slide and were treated with or without EGCG. We found EGCG repressed p-STAT3 activity in a dose dependent manner in TW01 cells, as measured by immunofluorescent staining (Figure 3B), consistent with the EGCG-induced inhibition of STAT3 phosphorylation. Previous studies have shown that activation of STAT3 can regulate the genes expression involved in proliferation and cell survival such as c-Myc and Survivin and is associated with antiapoptotic genes such as BCL2 and BCLXL. We next examine the effects of EGCG on STAT3 downstream genes expression by qRT-PCR analysis of BCL2, Survivin and c-Myc. The data showed these gene expressions were inhibited by EGCG in TW01 sphere cells as illustrated in (Figure 3C). These results suggest that inhibition of sphere growth by EGCG is associated with suppression of STAT3 phosphoryation and thus inactivation of STAT3 may further inhibit the expression of STAT3-regulated genes in NPC cancer cells.
Figure 3.
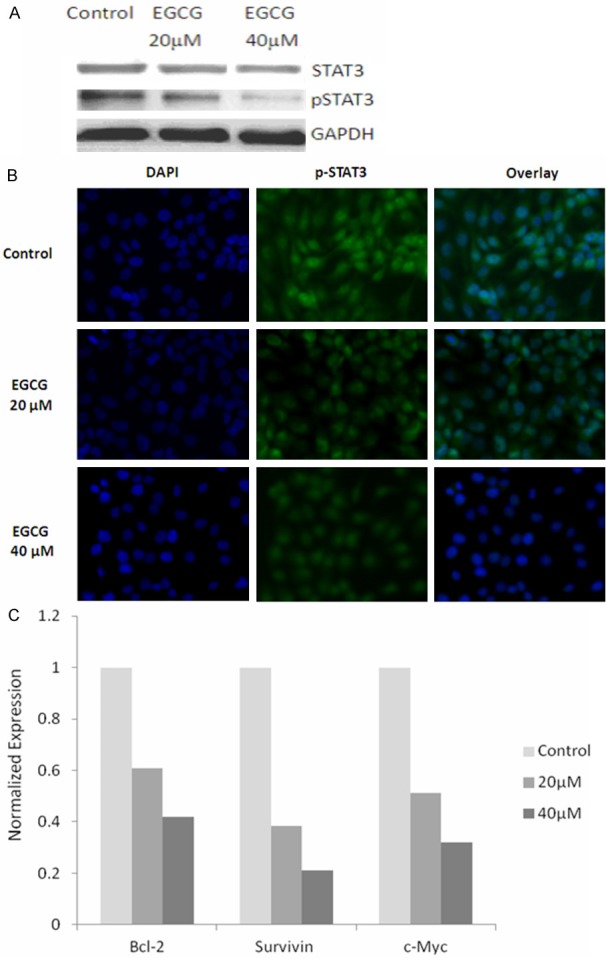
Effects of EGCG on STAT3 expression and downstream genes in NPC spheres. A: Western blot showing levels of total and phosphorylated STAT3 in TW01 sphere cells after EGCG treatment. B: TW01 sphere cells were analyzed for the expression of p-STAT3 by immunofluorescence staining. C: Effects of EGCG on the expression of Bcl-2, Survivin and c-Myc in TW01 sphere cells.
Discussion
Despite the development of advanced therapeutic methods, a significant number of patients diagnosed with NPC receive an unfavorable prognosis, especially those diagnosed at late stage and who had responded poorly to traditional therapy. Major clinical features of NPC include frequent relapse and early metastasis. Even after treatment, an aggressive phenotype and a tendency for recurrence contributes to poor outcomes and mortalities in NPC patients [20]. TICs are considered to play a role in tumor initiation, progression, and metastasis, and are responsible for therapeutic resistance. These cancer cells grow in an anchorage-independent condition, and exhibit resistance to anoikis, resulting in improved ability to survive and proliferate, and higher chemo-resistance and invasiveness, compared to cells grown as a monolayer [21,22].
In this study, we enriched and isolated TICs within NPC cells by spheres formation, and characterized their phenotypical and functional features such as self-renewal, proliferation, chemoresistance and tumorigenicity, compared to parental cells. In addition, we found that STAT3 is activated in these NPC sphere-generated cells and the STAT3 signaling pathway may play important roles in tumor growth, tumor sphere-forming capacity, and drug resistance of these cells. Previous reports have shown STAT3 signaling is an important target in cancer stem-like cells and is responsible for tumor initiation, cell survival and resistance to chemotherapy [23,24]. By this method, we further investigated the functional change that allowed us to observe the inhibitory effects of EGCG on stem-like properties of NPC. Because TICs seem to play an important role in tumor recurrence, resulting in a refractory response to treatment, the effects of EGCG on NPC sphere cells were examined using sphere-forming and colony-formation assays. We found that tumor spheres growing in suspension were affected by EGCG, and the sphere-forming efficiency was markedly suppressed. Similarly, the colony-formation assay revealed that EGCG blocked the clonogenicity of sphere cells. As the spheres contained enriched TICs with the stem-like properties, these results suggest that EGCG may inhibit the self-renewal capacity and tumorigenic potential of NPC TICs.
Concurrent chemotherapy acted as the main method in the treatment of NPC, in addition to radiotherapy, chemotherapeutic agent such as cisplatin is a classic drug that has been clinically widely used. In this study, we found that addition of EGCG with cisplatin markedly inhibited the viability of sphere cells and further enhanced cisplatin-induced apoptosis in these cells. This implies that EGCG may increase the sensitivity of sphere cells to chemotherapeutic drug, and minimizes the toxic side effects of cisplatin. These results indicate that EGCG may play a potential role as a supplementary compound, in combination with current treatments, for the increased efficacy of cancer treatments. Of note, EGCG efficiently suppresses the ability of NPC cells to initiate self-renewal, inhibits the tumorigenic potential of NPC TICs, and downregulates stemness genes expression. We therefore suggest that EGCG is potentially effective in preventing the recurrence and inhibiting the aggressiveness of NPC. Additionally, the data revealed that NPC sphere formation was associated with elevation of STAT3 expression and can be interfered by EGCG, suggesting that EGCG has a potent anti-tumor activity on NPC in part by the inhibition of STAT3 activation. Previous studies have shown that STAT3 was expressed in many types of cancer, and targeting STAT3 represents a promising target for the treatment of human cancers [25].
In this context, we investigated the potential effect of EGCG to downregulate STAT3 expression as a putative mechanism for suppressing the tumor-initiating capability of NPC. A decrease in the expression level of phosphorylated STAT3, an active form of STAT3 in the EGCG-treated TW01 sphere cells was found by Western blotting and immunofluorescence. These results demonstrated that EGCG impairs maintenance and the growth of nasopharyngneal carcinoma sphere cells through attenuation of STAT3 phosphoration.
The activated STAT3 is important for proliferation and invasion in nasopharyngeal carcinoma. Notably, STAT3 is also necessary for proliferation and survival of cancer-initiating cells [26]. In our study, we found STAT3 plays an essential role in NPC cancer-initiating cells and the effect of EGCG on STAT3 inhibition may attenuate their stem-like properties. Furthermore, we also found that EGCG could inhibit the expression of STAT3 downstream genes related to cell growth and survival, including BCL2, c-Myc and Survivin. Previous report has shown that EGCG causes down-regulation NFκB and its regulated signaling pathways that may contribute to the induction of cancer cell apoptotic activity [27]. Besides, EGCG suppress carcinogenesis by reduced expression of c-Myc in association with p53 modulation [28]. Our data give an additional explanation for EGCG inhibitory effect on cancer cell survival, growth and tumorigenicity by target STAT3.
EGCG has been gained much attention recently as a non-toxic natural compound beneficial for human health. Several underlying mechanisms have been proposed by particular studies that EGCG could target stemness characteristics in cancer. In the present study, we provide evidence and emphasize that EGCG has the potential to target NPC TICs through STAT3 inactivation. Overall, we examined the ability of EGCG to target the stem-like tumor-initiating cells of NPC by effectively attenuating their growth and tumorigenicity mediated by inhibition of STAT3 pathway. These conclusions support that further investigation of EGCG as a potential chemopreventive agent which may be used with current treatment strategies to against NPC.
Acknowledgements
This work was supported by research grants from the Taipei Institute of Pathology (Grant No.25), Taipei City Hospital and the Department of Health, Taipei City Government.
Disclosure of conflict of interest
None.
References
- 1.Hildesheim A, Wang CP. Genetic predisposition factors and nasopharyngeal carcinoma risk: a review of epidemiological association studies, 2000-2011: Rosetta Stone for NPC: genetics, viral infection, and other environmental factors. Semin Cancer Biol. 2012;22:107–116. doi: 10.1016/j.semcancer.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004;5:423–428. doi: 10.1016/s1535-6108(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 3.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 4.Lara-Padilla E, Caceres-Cortes JR. On the nature of the tumor-initiating cell. Curr Stem Cell Res Ther. 2012;7:26–35. doi: 10.2174/157488812798483412. [DOI] [PubMed] [Google Scholar]
- 5.Frame FM, Maitland NJ. Cancer stem cells, models of study and implications of therapy resistance mechanisms. Adv Exp Med Biol. 2011;720:105–118. doi: 10.1007/978-1-4614-0254-1_9. [DOI] [PubMed] [Google Scholar]
- 6.Sampieri K, Fodde R. Cancer stem cells and metastasis. Semin Cancer Biol. 2012;22:187–193. doi: 10.1016/j.semcancer.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Chen SF, Chang YC, Nieh S, Liu CL, Yang CY, Lin YS. Nonadhesive culture system as a model of rapid sphere formation with cancer stem cell properties. PLoS One. 2012;7:e31864. doi: 10.1371/journal.pone.0031864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Huang S, Zhao X, Zhang Q, Wu M, Sun F, Han G, Wu D. Enrichment of prostate cancer stem cells from primary prostate cancer cultures of biopsy samples. Int J Clin Exp Pathol. 2014;7:184–193. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou W, Kallifatidis G, Baumann B, Rausch V, Mattern J, Gladkich J, Giese N, Moldenhauer G, Wirth T, Buchler MW, Salnikov AV, Herr I. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int J Oncol. 2010;37:551–561. doi: 10.3892/ijo_00000704. [DOI] [PubMed] [Google Scholar]
- 10.Link A, Balaguer F, Shen Y, Lozano JJ, Leung HC, Boland CR, Goel A. Curcumin modulates DNA methylation in colorectal cancer cells. PLoS One. 2013;8:e57709. doi: 10.1371/journal.pone.0057709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu FW, Tsai LL, Yu CH, Chen PN, Chou MY, Yu CC. Impairment of tumor-initiating stem-like property and reversal of epithelial-mesenchymal transdifferentiation in head and neck cancer by resveratrol treatment. Mol Nutr Food Res. 2012;56:1247–1258. doi: 10.1002/mnfr.201200150. [DOI] [PubMed] [Google Scholar]
- 12.Chen D, Pamu S, Cui Q, Chan TH, Dou QP. Novel epigallocatechin gallate (EGCG) analogs activate AMP-activated protein kinase pathway and target cancer stem cells. Bioorg Med Chem. 2012;20:3031–3037. doi: 10.1016/j.bmc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lui VW, Wong EY, Ho Y, Hong B, Wong SC, Tao Q, Choi GC, Au TC, Ho K, Yau DM, Ma BB, Hui EP, Chan AS, Tsang CM, Tsao SW, Grandis JR, Chan AT. STAT3 activation contributes directly to Epstein-Barr virus-mediated invasiveness of nasopharyngeal cancer cells in vitro. Int J Cancer. 2009;125:1884–1893. doi: 10.1002/ijc.24567. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder A, Herrmann A, Cherryholmes G, Kowolik C, Buettner R, Pal S, Yu H, Mueller-Newen G, Jove R. Loss of androgen receptor expression promotes a stem-like cell phenotype in prostate cancer through STAT3 signaling. Cancer Res. 2014;74:1227–37. doi: 10.1158/0008-5472.CAN-13-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho PL, Lay EJ, Jian W, Parra D, Chan KS. Stat3 activation in urothelial stem cells leads to direct progression to invasive bladder cancer. Cancer Res. 2012;72:3135–3142. doi: 10.1158/0008-5472.CAN-11-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki BT, Hurt EM, Mistree T, Farrar WL. Targeting cancer stem cells with phytochemicals. Mol Interv. 2008;8:174–184. doi: 10.1124/mi.8.4.9. [DOI] [PubMed] [Google Scholar]
- 19.Burnett J, Newman B, Sun D. Targeting Cancer Stem Cells with Natural Products. Curr Drug Targets. 2012;13:1054–64. doi: 10.2174/138945012802009062. [DOI] [PubMed] [Google Scholar]
- 20.Bensouda Y, Kaikani W, Ahbeddou N, Rahhali R, Jabri M, Mrabti H, Boussen H, Errihani H. Treatment for metastatic nasopharyngeal carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:79–85. doi: 10.1016/j.anorl.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Liotta LA, Kohn E. Anoikis: cancer and the homeless cell. Nature. 2004;430:973–974. doi: 10.1038/430973a. [DOI] [PubMed] [Google Scholar]
- 22.Ghods AJ, Irvin D, Liu G, Yuan X, Abdulkadir IR, Tunici P, Konda B, Wachsmann-Hogiu S, Black KL, Yu JS. Spheres isolated from 9L gliosarcoma rat cell line possess chemoresistant and aggressive cancer stem-like cells. Stem Cells. 2007;25:1645–1653. doi: 10.1634/stemcells.2006-0624. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Wang G, Zhao Y, Liu X, Ding Q, Shi J, Ding Y, Wang S. STAT3 mediates resistance of CD44(+)CD24(-/low) breast cancer stem cells to tamoxifen in vitro. J Biomed Res. 2012;26:325–335. doi: 10.7555/JBR.26.20110050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin L, Fuchs J, Li C, Olson V, Bekaii-Saab T, Lin J. STAT3 signaling pathway is necessary for cell survival and tumorsphere forming capacity in ALDH(+)/CD133(+) stem cell-like human colon cancer cells. Biochem Biophys Res Commun. 2011;416:246–251. doi: 10.1016/j.bbrc.2011.10.112. [DOI] [PubMed] [Google Scholar]
- 25.Peyser ND, Grandis JR. Critical analysis of the potential for targeting STAT3 in human malignancy. Onco Targets Ther. 2013;6:999–1010. doi: 10.2147/OTT.S47903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E 3rd, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S, Hastak K, Afaq F, Ahmad N, Mukhtar H. Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappa B and induction of apoptosis. Oncogene. 2004;23:2507–2522. doi: 10.1038/sj.onc.1207353. [DOI] [PubMed] [Google Scholar]
- 28.Manna S, Mukherjee S, Roy A, Das S, Panda CK. Tea polyphenols can restrict benzo[a] pyrene-induced lung carcinogenesis by altered expression of p53-associated genes and H-ras, c-myc and cyclin D1. J Nutr Biochem. 2009;20:337–349. doi: 10.1016/j.jnutbio.2008.04.001. [DOI] [PubMed] [Google Scholar]


