Abstract
Irinotecan is a topoisomerase I inhibitor approved worldwide as a first- and second-line chemotherapy for advanced or recurrent colorectal cancer (CRC). Although irinotecan showed significant survival advantage for patients, a relatively low response rate and severe adverse effects demonstrated the urgent need for biomarkers searching to select the suitable patients who can benefit from irinotecan-based therapy and avoid the adverse effects. In present work, the irinotecan response (IC50 doses) of 20 CRC cell lines were correlated with the basal expression profiles investigated by RNA-seq to figure out genes responsible for irinotecan sensitivity/resistance. Genes negatively or positively correlated to irinotecan sensitivity were given after biocomputation, and 7 (CDC20, CTNNAL1, FZD7, CITED2, ABR, ARHGEF7, and RNMT) of them were validated in two CRC cell lines by quantitative real-time PCR, several of these 7 genes has been proposed to promote cancer cells proliferation and hence may confer CRC cells resistance to irinotecan. Our work might provide potential biomarkers and therapeutic targets for irinotecan sensitivity in CRC cells.
Keywords: Colorectal cancer, irinotecan, RNA-seq, CITED2, CTNNAL1, FZD7
Introduction
Colorectal cancer (CRC) is one of the most common malignant diseases, with 945,000 new cases and approximately 492000 patients’ death annually, and is the fourth cause of cancer-related deaths worldwide [1]. As the probability of developing CRC rises sharply with age, CRC is a great health challenge for countries whose population is ageing, such as the UK and China [2]. Approximately 5-25% of newly diagnosed CRC cases present with advanced disease [3], prognosis for these patients remains poor. Chemotherapy is used as adjuvant therapy for resectable CRC patients or palliative therapy for advanced/metastatic CRC patients, to prevent the recurrence or to improve the survival or quality of life. Irinotecan is a topoisomerase I inhibitor approved worldwide for the treatment of metastatic CRC (mCRC), as a first- and second-line chemotherapy for advanced or recurrent CRC. Irinotecan has demonstrated significant survival advantage for patients with tumors that have progressed on initial 5-fluorouracil (5-FU)-based chemotherapy compared with supportive care alone [4-6]. When used alone, irinotecan exhibits 20-30% of objective response rate (ORR) for advanced CRC patients [7]. Irinotecan-based combinational therapy, such as FOLFIRI (irinotecan together with leucovorin and 5-FU), can reach an ORR of approximately 50% for mCRC patients [8]. However, more than half of the patients response poor to irinotecan-based therapy, in addition, irinotecan has severe toxicities, such as delayed-onset diarrhea, neutropenia, nausea, and vomiting, these demonstrate the urgent need for finding irinotecan biomarkers to improve the therapy.
Previous studies suggested that the mRNA or protein expression levels of several DNA repair-related genes or ubiquitin-like genes or kinase genes were associated with irinotecan sensitivity, including APTX, BRCA1 and ERCC1 [9,10], DNA topoisomerase I (Topo I) [7,11], IFN-stimulated gene 15 (ISG15) [12], thymidine phosphorylase (TP) [13], protein kinase CK2 [14,15]. However, few of these potential biomarkers were validated in prospective clinical trials. Hence it is of great interest to figure out irinotecan biomarkers in CRC cell lines in vitro.
In present work, 21 CRC cell lines with different sensitivities to irinotecan were subjected to RNA-seq. The basal expression of CRC cell lines was correlated with the irinotecan response and genes responsible for irinotecan sensitivity were predicted and validated by quantitative real-time PCR.
Materials and methods
Cell culture
The human CRC cell lines, SW620, LS180, COLO741, COLO205, LOVO, CX-1, GP2D, SW48, RKO, HCT116, HCT15, SW480, SW1116, DLD1, CACO-2, D2, SW837, GP5D, CO115, HT29, and LS174T were purchased from ATCC or China Center for Type Culture Collection. These cell lines were maintained in RPMI 1640 or DMEM medium (Gibco) supplemented with 10% FBS (Hyclone), penicillin (100 IU/ml) and Streptomycin (100 μg/ml) (Life Technologies) in a humidified atmosphere containing 5% CO2 at 37°C. Cells in the exponential growth phase were used for all the experiments.
Determination of IC50 dose by MTS assay
Cells (1×103/each well) were grown in 100 μl of RPMI 1640 or DMEM medium containing serum per well in a 96-well plate. After 24 h, the cells were treated with irinotecan (0, 0.0100, 0.0316, 0.100, 0.316, 1.00, 3.16, 10.0, 31.6, 100 μmol/L, respectively) for 144 h. Every treatment was triplicate in the same experiment. Then 20 μl of MTS (CellTiter 96 AQueous One Solution Reagent; Promega) was added to each well for 1 to 4 h at 37°C. After incubation, the absorbance was read at a wavelength of 490 nm according to the manufacturer’s protocol. The cell viability was calculated relative to the untreated cells, respectively. The IC50 calculation was performed with GraphPad Prism 5.0 software via nonlinear regression.
RNA-seq
Cells (8×104) were grown in 2 ml of RPMI 1640 or DMEM medium containing serum per well in a 6-well plate with duplication. All the samples were homogenized with 1 ml Trizol (Invitrogen, Life Technologies) and total RNAs were extracted according to the manufacturer’s instruction.
Preparation of cDNA followed the procedure described in Trapnell et al. [16]. The cDNA library was size-fractionated on a 2% TAE low melt agarose gel (Lonza catalog # 50080), a narrow slice (~2 mm) of the cDNA lane centered at the 300 bp marker was cut. The slice was extracted using the Qia Ex II kit (Qiagen catalog # 20021), and the extract was filtered over a Microcon YM-100 microconcentrator (Millipore catalog # 42409) to remove DNA fragments shorter than 100 bps. One-sixth of the filtered sample volume was used as template for 15 cycles of amplification using the paired-end primers and amplification reagents supplied with the Illumina ChIP-Seq genomic DNA prep kit. Each library was loaded into its own single Illumina flow cell lane, producing an average of 14.5 million pairs of 51-mer reads per lane (8.4 million purity filtered read pairs), or nearly 1.5 Gb of total sequence for each sample. Transcripts were assembled from the mapped fragments sorted by reference position.
Biocomputation for irinotecan sensitivity-related genes
We applied an elastic net regression algorithm combined with a bootstrapping procedure to derive predictive models that explained the drug sensitivity profiles based on the basal expression profiles investigated by RNA-seq, as described before [17]. Several parameters were calculated: Pearson correlation R between irinotecan IC50 doses and expression profile of some gene; Correlation distance (d = 1-R) between irinotecan response and the expression profile of some gene; Correlation distance (dc) accumulated between irinotecan response and the accumulated expression profiles among a selected set of genes; False discovery rate (FDR) when H (hypothesis) = gene expression significantly correlated with irinotecan response (H = corr); False discovery rate (FDR) when H = gene expression significantly counter-correlated with irinotecan response (H = counter-corr).
Quantitative real-time PCR (qPCR)
Total RNA above isolated was synthesized to cDNA using Prime Script RT reagent kit with gDNA Eraser (Takara, RR074A) for RT-PCR with mixture of oligo-dT and Random Primer (9 mer). The primers used for qPCR validation were list in Supplementary Table 1. Real-time qPCR was performed on CFX-96 (Bio-lab), with endogenous control Actb. Gene expression was calculated relative to expression of Actb endogenous control and adjusted relative to expression in COLO205 cells.
Results
21 CRC cell lines response differentially to irinotecan
21 CRC cell lines were treated with 9 different doses of irinotecan for 144 h, and then the cell viability was examined by MTS assay. The IC50 doses of these cell lines to irinotecan were calculated with the aid of GraphPad Prism 5.0 software via nonlinear regression (Table 1 and Figure 1). The results showed that the sensitivities of these cell lines to irinotecan were distributed in nearly a normal fashion: SW620, LS180, COLO741, COLO205 and LOVO cell lines were sensitive to irinotecan, SW837, GP5D, CO115, HT29, and LS174T cell lines were resistant to irinotecan, while the other 11 CRC cell lines were moderate sensitive to irinotecan. This normal distribution of irinotecan sensitivity is very suitable for biomarkers searching by correlation between irinotecan response and gene expression profile.
Table 1.
IC50 doses of 21 CRC cell lines to irinotecan
| Cell line | Irinotecan IC50 (μM) |
|---|---|
| SW620 | 1.53 |
| LS180 | 3.11 |
| COLO741 | 3.57 |
| COLO205 | 7.23 |
| LOVO | 7.64 |
| CX-1 | 8.69 |
| GP2D | 9.89 |
| SW48 | 10.02 |
| RKO | 10.78 |
| HCT116 | 14.84 |
| HCT15 | 17.81 |
| SW480 | 18.26 |
| SW1116 | 27.22 |
| DLD1 | 28.58 |
| CACO-2 | 30.90 |
| D2 | 36.60 |
| SW837 | 46.42 |
| GP5D | 47.22 |
| CO115 | 53.95 |
| HT29 | 120 |
| LS174T | 150 |
Figure 1.
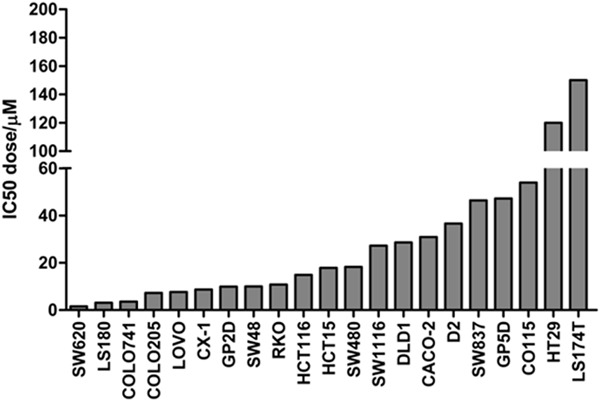
Determination of IC50 doses for 21 CRC cell lines. 21 CRC cell lines were treated with nine doses of irinotecan for 144 h, and then the cell viability was detected by MTS assay. The IC50 doses of these cell lines were calculated with the aid of GraphPad Prism 5.0 software via nonlinear regression.
RNA-seq and biocomputation for irinotecan sensitivity-related genes
And then the basal expression profiles of 20 CRC cell lines (except SW1116 cell line) were investigated by RNA-seq. The gene expression was log2 transformed, the irinotecan sensitivities (IC50 doses) were log10 transformed, and these two sets of data were used for biocomputation of irinotecansensitivity-related genes (Figure 2). The process of data analysis was described in Material and Methods. The top 20 genes negatively and positively correlated to irinotecan sensitivity were list in Table 2 and Table 3, respectively. The expression of CDC20, TP73, CTNNAL1, FZD7, and CITED2 was negatively correlated with irinotecan sensitivity, while the expression of ABR, ARHGEF7, and RNMT was positively correlated with irinotecan sensitivity.
Figure 2.
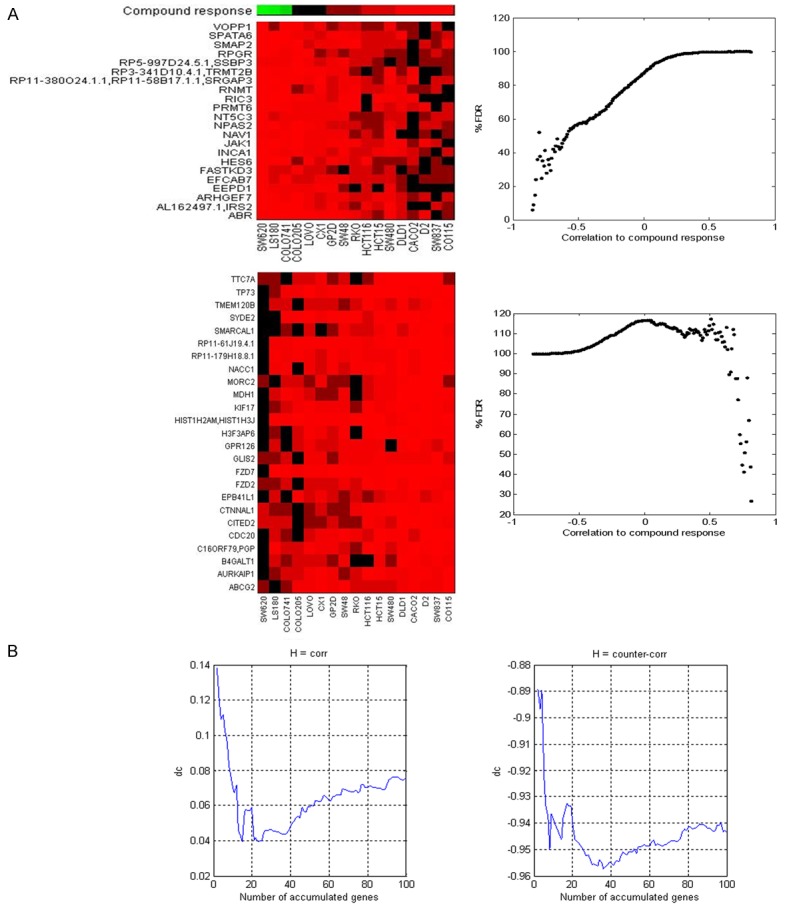
Identification of genes correlated with compound response. A: In upper panel, we showed that genes with expression counter-correlated with compound response (FDR < 20%); in lower panel we showed that genes with expression correlated with compound response (FDR < 30%). B: The correlation/counter-correlation was improved when gene expression were accumulated, where we are able to estimate the size of gene panel in the predictor.
Table 2.
Top 20 genes negatively correlated to irinotecan sensitivity
| Gene symbol | d | dc |
|---|---|---|
| CDC20 | 0.185 | 0.185 |
| RP11-61J19.4.1 | 0.218 | 0.138 |
| TP73 | 0.232 | 0.119 |
| H3F3AP6 | 0.236 | 0.109 |
| KIF17 | 0.239 | 0.112 |
| AURKAIP1 | 0.246 | 0.102 |
| SMARCAL1 | 0.270 | 0.096 |
| CTNNAL1 | 0.292 | 0.083 |
| MDH1 | 0.299 | 0.078 |
| SYDE2 | 0.302 | 0.070 |
| HIST1H2AM, HIST1H3J | 0.322 | 0.067 |
| FZD2 | 0.324 | 0.072 |
| EPB41L1 | 0.326 | 0.046 |
| B4GALT1 | 0.327 | 0.043 |
| GPR126 | 0.328 | 0.040 |
| FZD7 | 0.333 | 0.056 |
| TTC7A | 0.336 | 0.058 |
| RP11-179H18.8.1 | 0.336 | 0.057 |
| MORC2 | 0.340 | 0.057 |
| CITED2 | 0.344 | 0.059 |
Table 3.
Top 20 genes positively correlated to irinotecan sensitivity
| Gene symbol | 1-d | 1-dc |
|---|---|---|
| EFCAB7 | -0.861 | -0.861 |
| INCA1 | -0.795 | -0.889 |
| ABR | -0.792 | -0.897 |
| EEPD1 | -0.791 | -0.890 |
| HES6 | -0.774 | -0.923 |
| RNMT | -0.769 | -0.934 |
| SPATA6 | -0.749 | -0.938 |
| ARHGEF7 | -0.747 | -0.950 |
| AL162497.1, IRS2 | -0.747 | -0.936 |
| NPAS2 | -0.747 | -0.939 |
| RPGR | -0.745 | -0.941 |
| RP5-997D24.5.1, SSBP3 | -0.730 | -0.943 |
| JAK1 | -0.729 | -0.944 |
| SMAP2 | -0.727 | -0.946 |
| NAV1 | -0.725 | -0.938 |
| NT5C3 | -0.724 | -0.935 |
| RP3-341D10.4.1, TRMT2B | -0.722 | -0.933 |
| RP11-380O24.1.1, RP11-58B17.1.1, SRGAP3 | -0.722 | -0.934 |
| VOPP1 | -0.720 | -0.934 |
| RIC3 | -0.719 | -0.940 |
qPCR validation
And then 7 genes (CDC20, CTNNAL1, FZD7, CITED2, ABR, ARHGEF7, and RNMT) were selected to perform qPCR in COLO205 and LS174T, two cell lines sensitive and resistant to irinotecan, respectively. The results showed that all the 7 genes’ expression detected by qPCR assay was in line with the RNA-seq data: CDC20, CTNNAL1, FZD7 and CITED2 genes were highly expressed in LS174T, while ABR, ARHGEF7, and RNMT genes were highly expressed in COLO205 (Figure 3). Although ABR, RNMT and CDC20 genes were not too much differentially expressed between these two cell lines, the expression difference was significant (p < 0.05, data not shown). The most significantly expression-altered genes were ARHGEF7 and CITED2, the expression of these two genes was 0.13-fold and 3.40-fold in LS174T cells compared to that in COLO205 cells.
Figure 3.
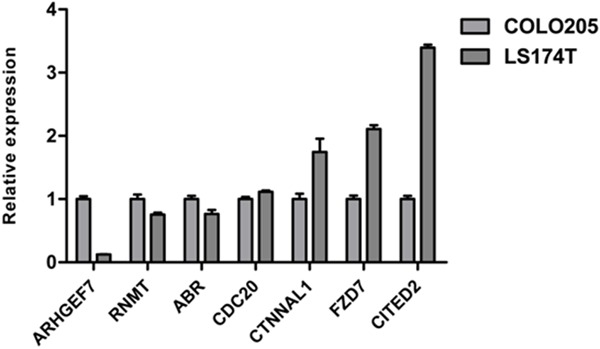
qPCR validation. 7 genes were validated in two CRC cell lines (COLO205 and LS174T) by qPCR. The gene expression was calculated relative to that in COLO205 cells.
Discussion
Irinotecan is a topoisomerase I inhibitorapproved worldwide as a first- and second-line 0.940chemotherapy for advanced or recurrent CRC. Although irinotecan showed significant survival advantage for patients, a relatively low response rate and severe adverse effects demonstrated the urgent need for biomarkers searching to select the suitable patients who can benefit from irinotecan-based therapy and avoid the adverse effects.
In present work, the irinotecan response (IC50 doses) of 20 CRC cell lines were correlated with the basal expression profiles investigated by RNA-seq to figure out genes responsible for irinotecan sensitivity/resistance. Genes negatively or positively correlated to irinotecan sensitivity were given after biocomputation, and 7 of them were validated in two CRC cell lines by qPCR.
Cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 2 (CITED2), a novel MYC-interacting transcriptional modulator, functions as a molecular switch of transforming growth factor-α (TGF-α)-induced proliferation and transforming growth factor-β (TGF-β)-mediated quiescence. Ectopic CITED2 expression enhanced tumor growth in nude mice; furthermore, CITED2 knockdown caused tumor shrinkage and increased overall host mouse survival rates. Expression of CITED2/MYC/E2F3/p21 (CIP1) signaling molecules was associated with poor prognosis of lung cancer patients [18]. Moreover, CITED2 expression has been associated with the resistance to cisplatin in tumor cells [19,20] and in vivo metastasis of breast cancer cells and in vitro invasion of colon cancer cells [21,22]. These reports suggested that CITED2 is implicated in tumor initiation and progression, chemotherapy resistance, metastasis and prognosis of various cancers. In our work, CITED2 was highly expressed in irinotecan-resistant CRC cells, suggesting that CITED2 may serve as a potential biomarker for irinotecan resistance. The underlying mechanism by which high expression of CITED2 confer cancer cells resistance warrants further investigation in more cancer cell lines and tissues. Previous studies have demonstrated that expression of several crucial molecules is regulated by CITED2, such as NF-κB [23], estrogen receptor [24], OCT4 [25], HIF1α [26] and VEGF [27]. In our opinion, TGF-α signaling and NF-κB signaling pathways may be suitable targets for next research to uncover the mechanism of CITED2 function.
Catenin, alpha-like 1 (CTNNAL1), also called as α-Catulin, is proposed to downregulate E-cadherin and hence promote melanoma progression and invasion [28], drive metastasis by activating ILK and driving an αvβ3 integrin signaling axis in non-small cell lung cancer and head and neck squamous cell carcinoma [29,30]. Furthermore, as a Rho signaling component, CTNNAL1 can regulate NF-κB through binding to IKK-beta, and confers resistance to apoptosis [31]. CTNNAL1 promotes tumor growth by preventing cellular senescence, and its knockdown induces senescence in cancer cells [32]. In our results, CTNNAL1 was highly expressed in irinotecan-resistant CRC cell lines, which is in line with previous reports in theory. The mechanism by which CTNNAL1 confer resistance to irinotecan may be lie in EMT signaling and NF-κB signaling pathways, which needs further study.
FZD7, receptor for Wnt signaling proteins, has a critical role in cell proliferation in triple negative breast cancer [33], its downregulation decreases survival, invasion and metastatic capabilities of colon cancer cells [34]. FZD7 has been suggested to be a potential therapeutic target in colorectal cancer [35]. Therefore, it is reasonable that the high expression of FZD7 confer CRC cells resistance to chemotherapy drugs.
Taken together, high expression of CITED2, CTNNAL1 and FZD7 may promote CRC cells proliferation and hence confer CRC cells resistance to irinotecan, which warrants further investigation in more cancer cell lines and tissues. Our work might provide potential biomarkers and therapeutic target for irinotecan sensitivity in CRC cells.
Acknowledgements
This work was granted by China Medical Foundation (No. 201301).
Disclosure of conflict of interest
We have no conflict of interest to declare.
Supporting Information
References
- 1.Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.Hind D, Tappenden P, Tumur I, Eggington S, Sutcliffe P, Ryan A. The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation. Health Technol Assess. 2008;12 doi: 10.3310/hta12150. iii-ix, xi-162. [DOI] [PubMed] [Google Scholar]
- 3.Popowich DA, Halverson AL. Medical oncology: Multimodality therapy in unresectable colorectal cancer. Nat Rev Clin Oncol. 2009;6:305–306. doi: 10.1038/nrclinonc.2009.65. [DOI] [PubMed] [Google Scholar]
- 4.Yim KL, Cunningham D. Chemotherapy: Optimizing irinotecan regimens for colorectal cancer. Nat Rev Clin Oncol. 2009;6:560–561. doi: 10.1038/nrclinonc.2009.140. [DOI] [PubMed] [Google Scholar]
- 5.Vanhoefer U, Harstrick A, Achterrath W, Cao S, Seeber S, Rustum YM. Irinotecan in the treatment of colorectal cancer: clinical overview. J. Clin. Oncol. 2001;19:1501–1518. doi: 10.1200/JCO.2001.19.5.1501. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Maroun J, Vanhoefer U, Van Cutsem E. Optimizing the use of irinotecan in colorectal cancer. Oncologist. 2001;6:17–23. doi: 10.1634/theoncologist.6-suppl_4-17. [DOI] [PubMed] [Google Scholar]
- 7.Ikeguchi M, Arai Y, Maeta Y, Ashida K, Katano K, Wakatsuki T. Topoisomerase I expression in tumors as a biological marker for CPT-11 chemosensitivity in patients with colorectal cancer. Surg Today. 2011;41:1196–1199. doi: 10.1007/s00595-011-4546-7. [DOI] [PubMed] [Google Scholar]
- 8.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 9.Shen J, Wei J, Wang H, Yue G, Yu L, Yang Y, Xie L, Zou Z, Qian X, Ding Y, Guan W, Liu B. A three-gene signature as potential predictive biomarker for irinotecan sensitivity in gastric cancer. J Transl Med. 2013;11:73. doi: 10.1186/1479-5876-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dopeso H, Mateo-Lozano S, Elez E, Landolfi S, Ramos Pascual FJ, Hernandez-Losa J, Mazzolini R, Rodrigues P, Bazzocco S, Carreras MJ, Espin E, Armengol M, Wilson AJ, Mariadason JM, Ramon YCS, Tabernero J, Schwartz S Jr. and Arango D. Aprataxin tumor levels predict response of colorectal cancer patients to irinotecan-based treatment. Clin Cancer Res. 2010;16:2375–2382. doi: 10.1158/1078-0432.CCR-09-3275. [DOI] [PubMed] [Google Scholar]
- 11.Braun MS, Richman SD, Quirke P, Daly C, Adlard JW, Elliott F, Barrett JH, Selby P, Meade AM, Stephens RJ, Parmar MK, Seymour MT. Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. J. Clin. Oncol. 2008;26:2690–2698. doi: 10.1200/JCO.2007.15.5580. [DOI] [PubMed] [Google Scholar]
- 12.Desai SD, Wood LM, Tsai YC, Hsieh TS, Marks JR, Scott GL, Giovanella BC, Liu LF. ISG15 as a novel tumor biomarker for drug sensitivity. Mol Cancer Ther. 2008;7:1430–1439. doi: 10.1158/1535-7163.MCT-07-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meropol NJ, Gold PJ, Diasio RB, Andria M, Dhami M, Godfrey T, Kovatich AJ, Lund KA, Mitchell E, Schwarting R. Thymidine phosphorylase expression is associated with response to capecitabine plus irinotecan in patients with metastatic colorectal cancer. J. Clin. Oncol. 2006;24:4069–4077. doi: 10.1200/JCO.2005.05.2084. [DOI] [PubMed] [Google Scholar]
- 14.Bandyopadhyay K, Gjerset RA. Protein kinase CK2 is a central regulator of topoisomerase I hyperphosphorylation and camptothecin sensitivity in cancer cell lines. Biochemistry. 2011;50:704–714. doi: 10.1021/bi101110e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandyopadhyay K, Li P, Gjerset RA. CK2-mediated hyperphosphorylation of topoisomerase I targets serine 506, enhances topoisomerase I-DNA binding, and increases cellular camptothecin sensitivity. PLoS One. 2012;7:e50427. doi: 10.1371/journal.pone.0050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, Reddy A, Liu M, Murray L, Berger MF, Monahan JE, Morais P, Meltzer J, Korejwa A, Jane-Valbuena J, Mapa FA, Thibault J, Bric-Furlong E, Raman P, Shipway A, Engels IH, Cheng J, Yu GK, Yu J, Aspesi P Jr, de Silva M, Jagtap K, Jones MD, Wang L, Hatton C, Palescandolo E, Gupta S, Mahan S, Sougnez C, Onofrio RC, Liefeld T, MacConaill L, Winckler W, Reich M, Li N, Mesirov JP, Gabriel SB, Getz G, Ardlie K, Chan V, Myer VE, Weber BL, Porter J, Warmuth M, Finan P, Harris JL, Meyerson M, Golub TR, Morrissey MP, Sellers WR, Schlegel R, Garraway LA. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou YT, Hsieh CH, Chiou SH, Hsu CF, Kao YR, Lee CC, Chung CH, Wang YH, Hsu HS, Pang ST, Shieh YS, Wu CW. CITED2 functions as a molecular switch of cytokine-induced proliferation and quiescence. Cell Death Differ. 2012;19:2015–2028. doi: 10.1038/cdd.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu ZZ, Sun NK, Chao CC. Knockdown of CITED2 using short-hairpin RNA sensitizes cancer cells to cisplatin through stabilization of p53 and enhancement of p53-dependent apoptosis. J Cell Physiol. 2011;226:2415–2428. doi: 10.1002/jcp.22589. [DOI] [PubMed] [Google Scholar]
- 20.Wu ZZ, Lu HP, Chao CC. Identification and functional analysis of genes which confer resistance to cisplatin in tumor cells. Biochem Pharmacol. 2010;80:262–276. doi: 10.1016/j.bcp.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 21.van Agthoven T, Sieuwerts AM, Veldscholte J, Meijer-van Gelder ME, Smid M, Brinkman A, den Dekker AT, Leroy IM, van Ijcken WF, Sleijfer S, Foekens JA, Dorssers LC. CITED2 and NCOR2 in anti-oestrogen resistance and progression of breast cancer. Br J Cancer. 2009;101:1824–1832. doi: 10.1038/sj.bjc.6605423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai L, Merchant JL. A role for CITED2, a CBP/p300 interacting protein, in colon cancer cell invasion. FEBS Lett. 2007;581:5904–5910. doi: 10.1016/j.febslet.2007.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lou X, Sun S, Chen W, Zhou Y, Huang Y, Liu X, Shan Y, Wang C. Negative feedback regulation of NF-kappaB action by CITED2 in the nucleus. J Immunol. 2011;186:539–548. doi: 10.4049/jimmunol.1001650. [DOI] [PubMed] [Google Scholar]
- 24.Lau WM, Doucet M, Huang D, Weber KL, Kominsky SL. CITED2 modulates estrogen receptor transcriptional activity in breast cancer cells. Biochem Biophys Res Commun. 2013;437:261–266. doi: 10.1016/j.bbrc.2013.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Ramirez-Bergeron DL, Dunwoodie SL, Yang YC. Cited2 gene controls pluripotency and cardiomyocyte differentiation of murine embryonic stem cells through Oct4 gene. J Biol Chem. 2012;287:29088–29100. doi: 10.1074/jbc.M112.378034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28:941–953. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 27.Agrawal A, Gajghate S, Smith H, Anderson DG, Albert TJ, Shapiro IM, Risbud MV. Cited2 modulates hypoxia-inducible factor-dependent expression of vascular endothelial growth factor in nucleus pulposus cells of the rat intervertebral disc. Arthritis Rheum. 2008;58:3798–3808. doi: 10.1002/art.24073. [DOI] [PubMed] [Google Scholar]
- 28.Kreiseder B, Orel L, Bujnow C, Buschek S, Pflueger M, Schuett W, Hundsberger H, de Martin R, Wiesner C. alpha-Catulin downregulates E-cadherin and promotes melanoma progression and invasion. Int J Cancer. 2013;132:521–530. doi: 10.1002/ijc.27698. [DOI] [PubMed] [Google Scholar]
- 29.Liang CH, Chiu SY, Hsu IL, Wu YY, Tsai YT, Ke JY, Pan SH, Hsu YC, Li KC, Yang PC, Chen YL, Hong TM. alpha-Catulin drives metastasis by activating ILK and driving an alphavbeta3 integrin signaling axis. Cancer Res. 2013;73:428–438. doi: 10.1158/0008-5472.CAN-12-2095. [DOI] [PubMed] [Google Scholar]
- 30.Cao C, Chen Y, Masood R, Sinha UK, Kobielak A. alpha-Catulin marks the invasion front of squamous cell carcinoma and is important for tumor cell metastasis. Mol Cancer Res. 2012;10:892–903. doi: 10.1158/1541-7786.MCR-12-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiesner C, Winsauer G, Resch U, Hoeth M, Schmid JA, van Hengel J, van Roy F, Binder BR, de Martin R. Alpha-catulin, a Rho signalling component, can regulate NF-kappaB through binding to IKK beta, and confers resistance to apoptosis. Oncogene. 2008;27:2159–2169. doi: 10.1038/sj.onc.1210863. [DOI] [PubMed] [Google Scholar]
- 32.Fan LC, Chiang WF, Liang CH, Tsai YT, Wong TY, Chen KC, Hong TM, Chen YL. alpha-Catulin knockdown induces senescence in cancer cells. Oncogene. 2011;30:2610–2621. doi: 10.1038/onc.2010.637. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, Deng X, Chen L, Kim CC, Lau S, Somlo G, Yen Y. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene. 2011;30:4437–4446. doi: 10.1038/onc.2011.145. [DOI] [PubMed] [Google Scholar]
- 34.Ueno K, Hazama S, Mitomori S, Nishioka M, Suehiro Y, Hirata H, Oka M, Imai K, Dahiya R, Hinoda Y. Down-regulation of frizzled-7 expression decreases survival, invasion and metastatic capabilities of colon cancer cells. Br J Cancer. 2009;101:1374–1381. doi: 10.1038/sj.bjc.6605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueno K, Hiura M, Suehiro Y, Hazama S, Hirata H, Oka M, Imai K, Dahiya R, Hinoda Y. Frizzled-7 as a potential therapeutic target in colorectal cancer. Neoplasia. 2008;10:697–705. doi: 10.1593/neo.08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


