Abstract
Intracellular calcium (Ca2+) plays a significant role in many cell signaling pathways, some of which are localized to spatially restricted microdomains. Ca2+ binding proteins (Ca2+ buffers) play an important role in regulating Ca2+ concentration ([Ca2+]). Buffers typically slow [Ca2+] temporal dynamics and increase the effective volume of Ca2+ domains. Because fluctuations in [Ca2+] decrease in proportion to the square-root of a domain’s physical volume, one might conjecture that buffers decrease [Ca2+] fluctuations and, consequently, mitigate the significance of small domain volume concerning Ca2+ signaling. We test this hypothesis through mathematical and computational analysis of idealized buffer-containing domains and their stochastic dynamics during free Ca2+ influx with passive exchange of both Ca2+ and buffer with bulk concentrations. We derive Langevin equations for the fluctuating dynamics of Ca2+ and buffer and use these stochastic differential equations to determine the magnitude of [Ca2+] fluctuations for different buffer parameters (e.g., dissociation constant and concentration). In marked contrast to expectations based on a naive application of the principle of effective volume as employed in deterministic models of Ca2+ signaling, we find that mobile and rapid buffers typically increase the magnitude of domain [Ca2+] fluctuations during periods of Ca2+ influx, whereas stationary (immobile) Ca2+ buffers do not. Also contrary to expectations, we find that in the absence of Ca2+ influx, buffers influence the temporal characteristics, but not the magnitude, of [Ca2+] fluctuations. We derive an analytical formula describing the influence of rapid Ca2+ buffers on [Ca2+] fluctuations and, importantly, identify the stochastic analog of (deterministic) effective domain volume. Our results demonstrate that Ca2+ buffers alter the dynamics of [Ca2+] fluctuations in a nonintuitive manner. The finding that Ca2+ buffers do not suppress intrinsic domain [Ca2+] fluctuations raises the intriguing question of whether or not [Ca2+] fluctuations are a physiologically significant aspect of local Ca2+ signaling.
Introduction
The regulation of intracellular calcium (Ca2+) concentration ([Ca2+]) is a fundamental aspect of many cell signaling pathways (1). In many types of cells, spatially localized Ca2+ signals known as Ca2+ micro- or nanodomains regulate specific cellular processes in different subcellular regions. Significant examples include pre- and postsynaptic signaling in neuronal dendrites (2–4), contraction regulation in cardiac dyadic subspaces (3,5–7), localized control of mitochondria (8–10), and nuclear gene transcription (4,8), and localized mechanical and olfactory sensing in primary cilia (11–13).
Ca2+ buffers play an important role in modulating spatially localizing Ca2+ signals. The term “Ca2+ buffers” is generic and includes endogenous binding proteins, exogenous Ca2+ chelators (e.g., EGTA and BAPTA), indicator dyes (e.g., Fluo-4 and Rhod-2), and other nonspecific Ca2+ binding molecules (e.g., membrane phospholipids). In addition to regulating the levels of free Ca2+ in the cytoplasm, Ca2+ buffers influence the spatiotemporal dynamics of Ca2+ signaling. Because Ca2+ buffer binding rates, affinities, and diffusivities can range over several orders of magnitude (14), it is important to understand the influence of these parameters on Ca2+-dependent signaling. Using deterministic formulations for Ca2+ and buffer dynamics, investigators have derived analytical results pertaining to the influence of rapid mobile and immobile buffers on Ca2+ signaling, demonstrating that buffers can greatly alter the [Ca2+] profile in the proximity of Ca2+ channels (15–19). Immobile buffers have been shown to reduce the effective diffusivity of Ca2+, whereas mobile buffers can facilitate diffusion near an open Ca2+ channel and produce steep [Ca2+] gradients (20). Experimental and simulation studies have shown that the decay of residual Ca2+ after Ca2+ channel closure can be longer for rapid buffers than slow buffers (21,22). The influence of buffers on Ca2+ signaling can be counterintuitive, because it depends subtly on binding rate kinetics and competition between Ca2+-binding sites. For example, analytical results have shown that increased buffer diffusivity may increase or decrease the speed of a propagating Ca2+ wave, depending on the excitability of Ca2+ dynamics and buffer properties (23).
Assuming a rapidly equilibrating bimolecular association reaction between Ca2+ and buffer (B),
| (1) |
the time evolution of [Ca2+], denoted by c below, can be described by the ordinary differential equation (ODE) (24)
| (2) |
where the dot indicates a time derivative, Ω is the compartmental volume, J = Jin – Jout is the net flux of Ca2+ molecules into the compartment, and the buffering factor β is the differential fraction of free-to-total Ca2+ that takes values between 0 and 1,
| (3) |
In this expression, total buffer concentration bT = b + cb is the sum of free and Ca2+-bound buffer concentration, b and cb, respectively; κ = k−/k+ is the dissociation constant for Ca2+; and the ratio w = bTκ/(c + κ)2 is the buffering capacity, i.e., the differential fraction of bound-to-free Ca2+. Concentration balance equations such as Eq. 2 are often written in the form
| (4) |
where the effective volume Ωeff is defined as Ωeff = Ω/β ≥ Ω.
It is well known that the number of molecules in biochemical reaction networks randomly fluctuate, and the resulting concentration fluctuations for each chemical species are larger amplitude when the system size is small (25,26). For a sufficiently large system, i.e., large volume and number of molecules, concentration fluctuations become negligible and deterministic modeling is appropriate (26). However, in physiological settings of local Ca2+ signaling, i.e., Ca2+ microdomains, the system size is often small and concentration fluctuations may be significant. Intracellular [Ca2+] is very low in resting cells, typically 100 nM, and the volume of Ca2+ microdomains or subspaces are ∼10−17–10−15 liters, values that correspond to 0.6–60 Ca2+ ions at rest. A large amount of prior work has focused on the influence of stochastic gating of Ca2+-regulated Ca2+ channels on [Ca2+] (19,27–32), but only a few have considered the role of concentration fluctuations associated with the small number of Ca2+ ions typically present in domains (30,33,34). The relative size of [Ca2+] fluctuations can be characterized by their coefficient of variation (cv), defined as the standard deviation of [Ca2+] divided by the mean [Ca2+]. In a spatially restricted domain and in the absence of Ca2+ buffers, the relative size of [Ca2+] fluctuations is given by (35)
| (5) |
where css is the steady-state [Ca2+] and Ω is the physical volume of the domain (the superscripted 0 indicates no buffers). Because cssΩ is the number of Ca2+ ions in the domain, this expression is consistent with the influence of system size on molecular fluctuations well known in statistical physics (26). For the small volume domains mentioned above, this is in the range of 1.3–0.13. Our prior work suggests that fluctuations in [Ca2+] of this magnitude could significantly influence the gating of Ca2+-regulated Ca2+ channels in Ca2+ microdomains, such as dyadic subspaces or dendritic spines (35).
Although prior work makes it clear that [Ca2+] fluctuations are a nonnegligible aspect of Ca2+ signaling, the influence of Ca2+ buffers on the dynamics of [Ca2+] fluctuations has not previously been studied. Based on the notion of effective volume, one might conjecture that buffers decrease [Ca2+] fluctuations and, consequently, mitigate the significance of small domain volume vis-a-vis downstream Ca2+ signaling (e.g., Ca2+-triggered events). Do Ca2+ buffers influence [Ca2+] fluctuations? If so, how do these fluctuations depend on Ca2+ buffer properties such as dissociation constant and total concentration (Ca2+ free plus bound)? To answer these questions, we develop and analyze a minimal stochastic model that includes Ca2+ and buffer in a domain of small volume.
Our analysis focuses on Ca2+ microdomains that arise from Ca2+ influx into a spatially restricted subcellular compartment that may contain Ca2+ buffers, e.g., a dendritic spine or cardiac dyadic subspace (Fig. 1, A and B). The theory is most directly applicable to Ca2+ signaling complexes with membrane configurations or other obstructions to diffusion (e.g., molecular crowding) resulting in spatially confined Ca2+ signaling, for example, the primary cilium (Fig. 1 C), mitochondria-endoplasmic reticulum (ER) junctions, and presynaptic boutons (2–13). Free Ca2+ and buffer (Ca2+-bound and -free) may exchange between domain and bulk via diffusion represented as a first-order reaction (Fig. 1 D). We specify the volume but not the detailed geometry of the spatially confined region that delimits the domain. Spatial gradients between domain and bulk are minimally represented in the model, but the microdomain itself is treated as a well-stirred compartment. The compartmental model formulation is most applicable and relevant to microdomains for which the time constant for domain escape is longer than the characteristic time for diffusion across the domain (36).
Figure 1.

The analysis of the effect of Ca2+ buffers on domain [Ca2+] fluctuations is applicable to microdomains that arise from Ca2+ influx into a spatially restricted subcellular compartment. (A) In a dendritic spine (Ω denotes volume of spine head), Ca2+ influx occurs via ionotropic receptors (e.g., NMDA) or plasma membrane (PM) Ca2+ channels downstream of metabotropic receptors (e.g., mGluRs) (2). (B) In the cardiac dyad, Ca2+ influx via PM L-type Ca2+ channels trigger Ca2+ influx from Ca2+-activated sarcoplasmic reticulum (SR) Ca2+ channels (ryanodine receptors) (7). Close associations of endoplasmic reticulum (ER) and PM are observed in many cell types. During ER depletion in Jurkat T cells, luminal Ca2+ sensor STIM1 aggregates on the ER membrane and binds the Ca2+ channel Orai1 providing store-operated Ca2+ entry. (C) In olfactory primary cilium, odorant detection induces a rise in second messenger cAMP, activating cyclic nucleotide-gated channels allowing entry of Ca2+ and Na+ ions (11). (D) Model components and fluxes employed in the microdomain model. The stochastic ODE model includes Ca2+ influx, association of Ca2+ and buffer, and passive exchange of Ca2+ and buffer between the domain of volume Ω and the bulk cytosol.
For clarity and readability, we first present the stochastic model representing [Ca2+] fluctuations in a microdomain with no Ca2+ buffer. This preliminary calculation illustrates our approach and provides a reference point in our interpretation of results that include influx and efflux of both Ca2+ and buffer. We perform parameter studies to determine how Ca2+ buffers influence the size of [Ca2+] fluctuations, derive the fluctuating rapid buffer approximation to the dynamics of domain [Ca2+] fluctuations, and illustrate how the influence of buffers on the effective system size and the time course of [Ca2+] fluctuations depend on buffer parameters. We conclude with a discussion of our findings.
Model Formulation
Domain [Ca2+] fluctuations in the absence of buffer
Deterministic ODE formulation
The deterministic ODE that minimally describes the time evolution of [Ca2+] in the domain depicted in Fig. 1 without buffer, constant influx jin, and passive exchange with the bulk is
| (6) |
where kc is the exchange rate (due to diffusion) that has physical dimensions of inverse time. Setting the left-hand side of Eq. 6 to zero, steady-state [Ca2+] is found to be
| (7) |
This deterministic formulation assumes that concentration fluctuations are negligible (i.e., large system size, Ω → ∞). The following section presents the corresponding stochastic ODEs that are valid for small domain volume.
Stochastic ODE (Langevin) formulation
In a domain with physical volume small enough that fluctuations are not negligible, but large enough that molecular concentrations can be modeled continuously, [Ca2+] dynamics may be described by a Langevin equation. This would be a stochastic differential equation (SDE) similar to the deterministic ODE (Eq. 6), but augmented by a fluctuating term, ξc(t), with properties determined by the elementary molecular events that drive concentration fluctuations (26,37,38),
| (8) |
In this stochastic ODE, ξc(t) is Gaussian noise (specifically, a random function of time with zero mean),
| (9) |
and two-time covariance
| (10) |
where δ is the Dirac delta function and γc is given by
| (11) |
Note that the signs in the numerator are correct; γc is proportional to the sum of rates of the elementary processes leading to changes in [Ca2+], which includes increases through influx (jin) as well as decreases and increases due to exchange with the bulk (kcc and kcc∞). The reader who is unfamiliar with chemical Langevin equations may review the general form of the two-time covariance (see Appendix A in the Supporting Material).
Employing a linear noise approximation (26) for fluctuations around the stable steady-state css transforms Eq. 8 into
| (12) |
where δc is the deviation of the fluctuating [Ca2+] from the steady-state value, css, that is,
| (13) |
and we have used f′(css) = −kc and 〈ξcss(t)〉 = 0. The two-time covariance of the random term takes the form
where the constant is found by evaluating Eq. 11 at steady state, that is,
| (14) |
where we have used the balance of Ca2+ influx and efflux at steady state, jin = kc(css – c∞), to express the numerator as 2kccss rather than jin + kc(css + c∞) (compare to Eq. 14).
Analysis of concentration fluctuations
To understand the dynamics of [Ca2+] fluctuations implied by Eq. 12, consider an ensemble of domains with initial state c(0) = css, that is, δc(0) = 0. The time evolution of the ensemble variance of the deviations δc, defined by σc(t) = 〈(t)〉, follows from Eq. 12,
| (15) |
where kc = |f′(css)| is the relaxation rate. By assumption, the ensemble variance is initially zero, that is, δc(0) = 0 implies σc(0) = 0, but as time proceeds, σc(t) is given by the solution of Eq. 15,
| (16) |
where steady-state ensemble variance is found by setting in Eq. 15,
| (17) |
The first equality in Eq. 17 relating and is called the “fluctuation-dissipation theorem” (25,26,39).
Thus, the relative size of the steady-state [Ca2+] fluctuations in the Langevin formulation, given by the coefficient of variation of the fluctuating [Ca2+], is
| (18) |
Using the Langevin equation for a fluctuating Ca2+ microdomain, we have derived the well-known result (35) that the relative magnitude of concentration fluctuations is inversely proportional to the square-root of the system size, i.e., the expected number of Ca2+ ions in the domain at steady-state (cssΩ). This result is a reference point for our analysis of domain [Ca2+] fluctuations in the presence of buffer.
Domain [Ca2+] fluctuations in the presence of buffer
Deterministic ODE formulation
In this section we characterize [Ca2+] fluctuations in a domain model that includes Ca2+ buffers, Ca2+ influx, and exchange of both Ca2+ and buffer with the bulk (see Fig. 1). Assuming mass-action kinetics and bimolecular association of Ca2+ and buffer (Eq. 1), we write the following deterministic system of ODEs,
| (19a) |
| (19b) |
| (19c) |
where kc and kb are the Ca2+ and buffer exchange rates, and the reaction terms are given by R = −k + c × b + k − cb, with c, b, and cb denoting free Ca2+, free buffer, and bound buffer, respectively. We assume that the free and Ca2+-bound buffer have the same exchange rate kb and, furthermore, that the bulk concentrations b∞ and cb∞ are in equilibrium with the bulk free [Ca2+],
Setting the time derivatives of Eq. 19 to 0, we find that the steady-state domain [Ca2+], denoted by css, solves the implicit expression
| (20a) |
where the steady-state Ca2+-free and -bound buffer concentrations are given by
| (20b) |
In the presence of Ca2+ influx, jin > 0, and because ∂cbss/∂css > 0, css > c∞, cbss > c∞, and bss > b∞. Also note that cbss + bss = at this nonequilibrium steady state, because buffer in the bulk is at equilibrium, cb∞ + b∞ = , and the exchange of Ca2+-bound buffer between domain and bulk, balances the exchange of Ca2+-free buffer, kb(cbss − cb∞) = kb(b∞ − bss) > 0.
Stochastic ODE (Langevin) formulation
As in the previous section, the Langevin-type stochastic Ca2+ domain model is found by adding the appropriate random terms to the deterministic ODEs (Eq. 19),
| (21a) |
| (21b) |
| (21c) |
where 〈ξi(t)〉 = 0 for i ∈ {c,b,cb}. Because the ξi(t) are correlated, the two-time covariances are most easily expressed in matrix form,
| (22) |
where ξ is the column vector (ξc, ξb, ξcb)T and Γ is the state-dependent covariance matrix (see Appendix A in the Supporting Material),
| (23a) |
where
| (23b) |
Denoting the fluctuations around steady state by δc = c – css, δb = b – bss, and δcb = cb – cbss, the linear SDE system for the concentration fluctuations in the presence of buffer is
| (24) |
where δ = (δc, δc, δcb)T is a column vector, Hss is the Jacobian of the full system of SDEs evaluated at steady state,
〈ξss(t)〉 = 0, and 〈ξss(t) ξTss(t′) 〉 = Γssδ(t – t′) with steady-state covariance matrix (compare to Eq. 23),
| (25a) |
where
| (25b) |
If one prefers, the Langevin system for the fluctuations (Eq. 24) can be written as
| (26a) |
| (26b) |
| (26c) |
Analysis of concentration fluctuations
Our analysis of concentration fluctuations in the presence of buffer begins by defining a symmetric 3×3 covariance matrix for the fluctuating concentrations, Σ(t) = (σij) for i, j ∈{c,b,cb}, that is,
where each star indicates a redundant entry. The time-dependent dynamics of Σ(t) follows from Eq. 24 (26),
| (27) |
which is the matrix version of Eq. 15. Solving Eq. 27 with the initial condition corresponds to an ensemble of domains with initial state c(0) = css, b(0) = bss, and cb(0) = cbss, that is, δc = δb = δcb = 0. This equation represents a linear system of 6 ODEs simultaneously solved by the six covariances 〈δc2〉, 〈δcδb〉,…, 〈δcb2〉.
The covariances Σ(t) that solve Eq. 27 are the focus of this article’s mathematical and computational analysis of domain [Ca2+] fluctuations in the presence of buffer. The solution of Eq. 27 with Σ(0) = 0 is (26),
| (28) |
The steady-state ensemble variance is the limiting value of this expression as t → ∞,
| (29) |
but the steady-state ensemble variance Σss is often more conveniently found by solving the linear algebraic system for the steady state of Eq. 27, the continuous Lyapunov equation, and the more general form of the fluctuation-dissipation theorem,
| (30) |
Equation 30 can be solved numerically using the command LYAP available in the software MATLAB (The MathWorks, Natick, MA). For symbolic and analytical calculations, we rewrite Eq. 30 as
| (31) |
and then expand in terms of the parameters of the problem and the unknown entries of Σss. In Eq. 31, I is the 3×3 identity matrix, Hss ⊕ Hss is a Kronecker sum given by
where ⊗ is the Kronecker product, the vec operation creates a column vector from a matrix by stacking its column vectors, and we have used
Although Hss ⊗ Hss is 9×9 and vec(Σss) and vec(Γss) are 9×1, Σss and Γss are symmetric and thus Eq. 31 can be contracted to a 6×6 linear system by eliminating rows 4, 7, and 8 of vec(Σss) and vec(Γss) and the corresponding rows and columns of the Hss ⊕ Hss.
The remainder of this article communicates numerical and analytical results obtained using this Langevin formulation for domain [Ca2+] fluctuations in the presence of buffer.
Results
The influence of Ca2+ buffers on domain [Ca2+] fluctuations
Fig. 2 shows parameter studies that characterize the dependence of the relative magnitude of domain [Ca2+] fluctuations on Ca2+ buffer parameters. Results were obtained by numerically solving the continuous Lyapunov equation (Eq. 30) for a range of total buffer concentrations () and rate constants (k+ and k−), fast and slow exchange rates for Ca2+-free and -bound buffer with the bulk (kb), and Ca2+ influx rates leading to steady-state free [Ca2+] of css = 1, 10, and 100 μM. The relative magnitude of domain [Ca2+] fluctuations are characterized by the coefficient of variation
| (32) |
where the steady-state domain concentration css is determined by the Ca2+ influx rate via Eq. 20 and = 〈δc2〉ss is the germane element of Σss found by numerical solution of Eq. 30. Numerically calculated cv values that were generated from Monte Carlo simulations of an ensemble of domains using Gillespie’s stochastic simulation algorithm (40) agree with the linear noise approximation, validating our approach (see Fig. S1 in the Supporting Material).
Figure 2.
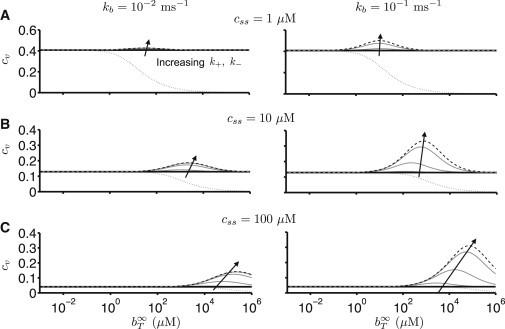
Dependence of the relative magnitude of domain [Ca2+] fluctuations probed by plotting the coefficient of variation (cv) of the deviation of the fluctuating [Ca2+] on buffer parameters. (Shaded lines) cv for different values of buffer binding reaction rate k+. (Solid lines) in the absence of buffer (Eq. 5). (Dotted line) cv predicted from a naive application of the concept of effective volume employed in deterministic models of Ca2+ signaling (Eq. 33). (Dashed lines) , the (correctly derived) influence of buffer on cv in the rapid buffer limit (Eq. 39). Parameters: css = 1 (A), 10 (B), and 100 (C) μM, c∞ = 0.1 μM, κ = 0.2 μM, kc = 0.2 ms−1, k+ = 10−3, and 1 μM−1 ms−1. The domain volume Ω = 10−17L corresponds to 0.01 μm3 ≈ (0.22 μm)3.
Fig. 2 reveals that the relative magnitude of domain [Ca2+] fluctuations (cv) is a biphasic (bell-shaped) function of the total buffer concentration (gray lines). For small or large total buffer concentrations (), the relative fluctuation magnitude (cv) asymptotically approaches the value obtained in the absence of buffer (, solid black line). This asymptotic value represents the minimum fluctuation size; cv is enhanced for intermediate total buffer concentrations. Rapid Ca2+ buffers increase the relative fluctuation magnitude, that is, for fixed κ = k−/k+, the cv increases with increasing k+. High mobility Ca2+ buffer (represented in this compartmental formulation by increasing the exchange rate kb) also enhances domain [Ca2+] fluctuations (compare left and right columns). High Ca2+ influx rates (jin) amplify domain [Ca2+] fluctuations for intermediate, but not extreme, concentrations of buffer. Taken together, these results indicate that domain [Ca2+] fluctuations are significantly enhanced by Ca2+ buffer and nontrivially dependent on Ca2+ buffer properties.
The response of domain [Ca2+] to buffer parameters documented in Fig. 2 are counterintuitive in the sense that our numerical calculations are precisely the opposite of what one would predict using a naive application of the concept of effective volume. As mentioned in the Introduction, the effective volume derived from the rapid buffer limit of the deterministic equations for buffered Ca2+ dynamics (Eq. 19) is Ωeff = Ω/β, where the buffering factor β is given by Eq. 3. If one replaces the physical volume Ω in Eq. 18 with this effective volume, one might conjecture that
| (33) |
where βss = β(css). However, the numerical calculations presented in Fig. 2 show that this is incorrect (dotted line). In fact, this naive application of the concept of buffer-mediated increase in effective volume is quite misleading. Comparison of solid and dotted lines in Fig. 2 show that Eq. 33 incorrectly suggests that the size of [Ca2+] fluctuations is suppressed by buffer (by increasing the system size, cssΩeff > cssΩ). The conjecture of Eq. 33 also incorrectly suggests that domain [Ca2+] fluctuations can be eliminated (cv → 0) as total buffer concentration increases and drives the differential fraction of free to total calcium to zero (βss → 0). On the contrary, the numerical results of this section (and analytical results presented below) are definitive: during periods of Ca2+ influx, Ca2+ buffers enhance domain [Ca2+] fluctuations.
The size of [Ca2+] fluctuations in the rapid buffer limit
Although closed-form analytical solution for the entries of the steady-state covariances Σss (and in particular ) can be obtained beginning with Eq. 31, the algebra is tedious, the result is unwieldy, and little insight is gained from these expressions (not shown). On the other hand, numerical evaluation of symbolic solutions of Eq. 31 confirm the results of the previous section obtained by numerically solving the Lyapunov equation (Eq. 30).
To obtain more insight into the enhancement of domain [Ca2+] fluctuations mediated by Ca2+ buffers, we sought to derive a stochastic version of the rapid buffer approximation (RBA) that correctly accounts for the influence of fast Ca2+ buffering on domain [Ca2+] fluctuations. Two distinct approaches were identified and successfully employed, both yielding the same analytical result. In the first approach, the above-mentioned cumbersome analytical expression for was symbolically evaluated in the limit that k+, k− → ∞ for fixed κ = k−/k+. The second approach involves no symbolic computations, but rather implements a general method for deriving a 0th-order approximation to the size of fluctuations in chemical reaction networks with widely separated timescales (26).
Briefly, beginning with the stochastic ODEs for buffered Ca2+ dynamics (Eq. 26), we identify the fluctuations in total calcium (δcT = δc + δcb) and total buffer (δbT = δb + δcb) as the slow variables. Differentiating these expressions and adding equations, we obtain the fast/slow system
| (34a) |
| (34b) |
| (34c) |
where δc is the fast variable, δcT and δbT are slow variables, and we define
In the rapid buffer limit (k+, k− → ∞ with κ = k−/k+ fixed), a quasistatic approximation for the average value of the [Ca2+] fluctuation that is valid on the slow (outer) timescale is
| (35) |
where we have written νss = css/(css + κ), and 〈⋅〉∗ indicates a time average (as opposed to an ensemble average) and we have used the fact that bss → bTκ/(css + κ) in the RBA limit (compare to Eq. 20b). Replacing δc in the slow equations by this average value gives
| (36a) |
| (36b) |
thereby expressing the slow SDEs in terms of δcT and δbT. Next, the 2×2 covariance matrix for and is calculated, and the 2×2 matrix , whose entries correspond to 〈δ〉, 〈δcTδbT 〉, and 〈δ〉, is found by solving a 2×2 Lyapunov equation (Eq. 30). Finally, the values of 〈δc2〉, 〈δcδcT 〉, and 〈δcδbT〉 consistent with Eq. 34 are found by solving a 3×3 Lyapunov equation for 〈δc2〉, 〈δcδcT〉, and 〈δcδbT〉. From the perspective of analytical work, this two-step process is far easier than solving the Lyapunov equation for the covariance matrix Σss for δc, δb, and δcb, because the fast and slow versions of the Γss and Hss matrices are simpler than those that appear in the full calculation (see Appendix B in the Supporting Material for details).
Using both of the above-mentioned approaches (symbolic and analytical), we find that the variance of the free [Ca2+] fluctuations in the presence of rapid Ca2+ buffer (denoted by ) is given by
| (37) |
where βss and wss are evaluated at the steady-state domain [Ca2+],
| (38) |
and we note that css ≥ c∞ and thus χ ≥ 0. The variance implies that in the rapid buffer limit the coefficient of variation for domain [Ca2+] fluctuations is given by
| (39) |
where is the result in absence of buffer. Noting that χ ≥ 0, we find
| (40) |
and conclude that Ca2+ buffers may enhance but cannot suppress domain [Ca2+] fluctuations.
One way to represent the effect of rapid buffers on domain [Ca2+] fluctuations involves rewriting Eq. 37 as
| (41) |
where Ω′eff denotes an effective volume that appropriately accounts for the influence of rapid Ca2+ buffers on [Ca2+] fluctuations. The fluctuating RBA is readily generalizable to multiple buffers (see Eq. S1 in the Supporting Material). We show that domain [Ca2+] fluctuations in the presence of two rapid buffers with different dissociation constants and exchange rates is given by the multiple buffer fluctuating RBA and not a naive weighted average utilizing total buffer concentration (see Fig. S2 in the Supporting Material).
Interpretation of the fluctuating rapid buffer limit
Some salient observations can be made regarding domain [Ca2+] fluctuations in the rapid buffer limit (Eqs. 37 and 41):
-
1.
The effective volume in the fluctuating RBA is smaller than the physical volume (χ ≥ 0 and thus Ω′eff ≤ Ω). This is in marked contrast to the effective volume for the deterministic RBA that is greater than the physical volume (Ω′eff = Ω/βss > Ω). This is to say, we have analytically confirmed our previous numerical result demonstrating that rapid buffers typically increase the magnitude of domain [Ca2+] fluctuations (Fig. 2).
-
2.
The χ in is proportional to the concentration difference css − c∞ (Eq. 37). That is, the buffer-mediated enhancement of domain [Ca2+] fluctuations is an increasing function of the disequilibrium between the steady-state domain [Ca2+] (css) and the bulk (c∞).
-
3.
The numerically observed biphasic dependence of the relative magnitude of domain [Ca2+] fluctuations (cv in Fig. 2) corresponds to the factor βsswsskb/(kc + wsskb) in χ that is a biphasic function of through βss and wss.
-
4.
In several limiting parameter regimes, the steady-state variance of domain [Ca2+] fluctuations is not affected by Ca2+ buffer (χ = 0 and thus = = css/Ω). These include the absence of Ca2+ influx (jin = 0, css = c∞), immobile buffer that is unable to exchange with the bulk (kb = 0), and extreme values for the total buffer concentration (both → 0 and → ∞). However, even in these limiting parameter regimes, buffers do not decrease [Ca2+] fluctuations to a size less than that observed in the absence of buffer.
-
5.The total buffer concentration that maximizes domain [Ca2+] fluctuations for a given fixed css,
is found as the that zeros ∂(1 + χ)/∂ = ∂χ/∂. This corresponds to(42)
and(43) -
6.
Equation 43 indicates that the maximum fluctuation magnitude is determined in part by the relative exchange rate kb/kc. Because χ∗ is a monotone increasing function of kb/kc, domain [Ca2+] fluctuations can, formally, be made as large as possible by choosing kb ≫ kc and optimal (Eq. 42). However, the molecular weight of Ca2+-bound buffer is greater than Ca2+ and, consequently, one expects Ca2+-bound buffer to diffuse and exchange with the bulk more slowly than free Ca2+. This physical consideration implies that kb ≤ kc and, after imposing this constraint, the physiologically realistic parameter choice that maximizes domain [Ca2+] fluctuations (kb ≈ kc) leads to χ∗ ≈ 1/4(css –c∞)/(c∞ + κ) and thus
-
7.
The buffer dissociation constant κ influences buffer-mediated increase in [Ca2+] fluctuations in a complex manner. For example, the variance of free [Ca2+] fluctuations (, Eqs. 37 and 38) depends on the relative (as opposed to absolute) concentrations of domain (css/κ) and bulk (c∞/κ) Ca2+ and total buffer (/κ) via the dimensionless quantities βss and wss. This is because the buffering capacity can expressed as wss = (/κ)/(1 + css/κ)2 and, similarly, the second factor of χ can be written as (css/κ − c∞/κ)/(1 + c∞/κ). For a given steady-state [Ca2+], css, the total buffer concentration with maximal effect (, Eq. 42) is a u-shaped function of the buffer dissociation constant κ (see Supporting Results and Fig. S3 in the Supporting Material).
Applicability of the fluctuating rapid buffer approximation
In the spatial and deterministic formulation of the buffered diffusion of intracellular Ca2+, the rapid buffer approximation is valid when the equilibration time for buffers is much smaller than the time constant for Ca2+ diffusion. In this minimal model of domain [Ca2+] fluctuations, Ca2+ and buffer diffusion are represented as first-order reactions (rate constants kc and kb) that couple the domain with the bulk. The validity of the fluctuating RBA depends on buffer kinetics being faster than exchange. Consequently, the physical domain volume influences the validity of this approximate result.
The time constant for diffusion-mediated passive exchange between compartments can be characterized by the mean escape time for a Brownian particle with diffusion constant D leaving a domain of volume Ω via a small opening of radius a (36),
| (44) |
As illustrated in Fig. 3 A, a cubical domain (Ω = L3) with an opening whose radius scales with linear dimension (ρ = a/L < 1) would yield rate constant k = 1/τ and thus ki = 4ρDi/L2, where i ∈{c, b} and Dc and Db are the diffusion coefficients for Ca2+ and buffer, respectively. Using parameters for the fast, mobile buffer BAPTA, Fig. 3 B plots the coefficient of variation of domain [Ca2+] fluctuations (cv) and the buffer-mediated relative decrease in system size (/, where = css/Ω) as functions of physical domain volume Ω for different values of the dimensionless escape radius ρ (shaded lines). As expected, both cv and / decrease with system size Ω. Narrowing the dimensionless escape radius (decreasing ρ) leads to an increase in cv and a decrease in / for any physical domain volume Ω. Comparison of the solid and dotted lines in Fig. 3 B shows that the fluctuating RBA agrees with the full calculation when the physical domain volume Ω is sufficiently large and/or the escape opening is sufficiently narrow (small ρ). Both situations lead to exchange rates (kc and kb) that are small compared to rate of buffer equilibration (an increasing function of both k+ and k−).
Figure 3.

The geometric parameters of a Ca2+ domain determine the time constant for escape of Ca2+ and buffer and the range of applicability of the fluctuating rapid buffer approximation (RBA). (A) Diagram of restricted subspace with volume Ω, linear dimension L, and radius of domain escape a. (B) (Shaded lines) Coefficient of variation of domain [Ca2+] fluctuations (cv, top) and buffer-mediated relative decrease in system size (/, bottom) as functions of physical domain volume Ω using four decades of dimensionless escape radii ρ = a/L = 10−4,…,10−1. (Dashed black lines) Fluctuating RBA (Eq. 39) well approximates the full calculation when ρ is small. css = 1 μM. (C) Illustration of three frequent spine shapes adapted from Harris et al. (41). (D, top) Relative increase in cv in the presence of BAPTA, compared with the absence of buffer , for different spine shapes. (D, bottom) Percent difference between cv as calculated using the fluctuating RBA, and the full calculation, cv. css = 10 μM. Parameters: c∞ = 0.1 μM, Dc = 0.2 μm2/ms. BAPTA (72): Db = 0.1 μm2/ms, k+ = 0.6 μM−1 ms−1 , κ = 0.2 μM, and = 100 μM. Spine geometries (41): thin (a = 0.05 μm, Ω = 4 × 10−17L), mushroom (a = 0.1 μm, Ω = 2.9 × 10−16L), and stubby (a = 0.16 μm, Ω = 3 × 10−17L).
[Ca2+] fluctuations in a physiological setting
Neuronal dendritic spines are a good setting to illustrate the influence of Ca2+ buffer on domain [Ca2+] fluctuations and explore the validity of the fluctuating RBA, in part because such spines are highly variable in shape and size. Although it is difficult to define an average spine geometry, most spines have a clearly defined neck (corresponding to the escape opening of radius a in the previous paragraph) and head (corresponding to the microdomain of volume Ω). Fig. 3 C illustrates three frequently observed spine shapes—thin, mushroom, and stubby—whose geometric parameters (a and Ω) have been experimentally quantified (41).
Fig. 3 D shows that over a wide range of concentrations, the fast buffer BAPTA increases the coefficient of variation, compared with the absence of buffer (cv/), by as much as a factor of 2 (top). The largest increase occurs in the case of the mushroom-shaped dendritic spine, for which the ratio of the spine-neck opening cross-sectional area, and the spine-head volume, is smallest (a/Ω).
For small and large BAPTA concentrations, there is a negligible difference between the fluctuating RBA and the corresponding full calculation, even measured in relative terms (Fig. 3 D, bottom). When the BAPTA concentration induces comparatively large [Ca2+] fluctuations, the fluctuating RBA overestimates the cv (depending on spine shape by as much as 50–150% in the worst case). When the steady-state domain [Ca2+] (css) is decreased, the disequilibrium between domain Ca2+ and buffer is attenuated, and the relative deviation between the RBA and full calculation is smaller (not shown). The fluctuating RBA works best for the mushroom-shaped dendritic spine, because a/Ω is smallest.
Influence of buffers on the relaxation time for domain [Ca2+] fluctuations
We have demonstrated that in the presence of Ca2+ influx (jin > 0), Ca2+ buffer may significantly increase the steady-state covariance of [Ca2+] fluctuations, especially when buffers are mobile and rapid (Eq. 37). On the other hand, the fluctuating RBA shows that in the absence of Ca2+ influx (jin = 0, css = c∞), fluctuation amplitude is not influenced by total buffer concentration ( as when = 0), a result that holds generally (e.g., when buffers are slow) in numerical simulations (not shown). This occurs because Γss and Hss scale in such a way that the steady-state covariance matrix Σss is not a function of when jin = 0 (Eq. 30). On the other hand, the time-dependent stochastic dynamics of the fluctuating concentrations (δc, δb, δcb) and the relaxation of the covariance matrix Σ to steady state are a function of buffer properties in both the absence or presence of Ca2+ influx (Eq. 27). For example, the relaxation rates governing the dynamics of Eq. 27 are given by the pairwise sums of the eigenvalues of Hss (by properties of Kronecker sums and Eq. 31 (42)), and these are a function of in both the presence and absence of Ca2+ influx.
Fig. 4 A shows Monte Carlo simulations of domain [Ca2+], c(t), fluctuating around the steady-state css that were generated by numerical integration of Eq. 21 using the Euler-Maruyama method. In the absence of buffer, the fluctuations δc(t) = c(t) – css are typically small and long-lasting (Fig. 4 Aa). Similar dynamics are observed in the presence of slow buffers (Fig. 4 Ab). Conversely, in the presence of rapid buffer, free [Ca2+] fluctuations and subsequent relaxation to steady state occurs on a fast timescale (Fig. 4 Ac).
Figure 4.

Influence of buffer on the time evolution of [Ca2+] fluctuations. (A) Monte Carlo simulation of domain [Ca2+], c(t), for different buffer parameters. (B) Autocorrelation function (ϕc,c (τ), Eq. 45) and (C) power spectrum (sc,c(ω), Eq. 46) in the presence of slow (open circles) and fast (dashed line) buffer and in the absence of buffer (solid line). Autocorrelation function curves are normalized by the steady-state variance such that ϕc,c (0) = 1. Parameters: css = 0.1 μM, c∞ = 0.1 μM, kc = 0.2 ms−1, and Ω = 10−17L. Buffer parameters: μM, κ = 0.2 μM, kb = 10−1 ms−1, k+ = 10−3 (slow), and 1 (fast) μM−1 ms−1.
One characterization of the influence of buffer on the time evolution of [Ca2+] fluctuations is given by the 3×3 steady-state correlation function Φ = (ϕi,j) with i,j ∈ {c,b,cb},
where Φ(0) = Σss. In the presence of buffer the correlation function for the domain [Ca2+] fluctuation δc is given by ϕc,c(τ), where −∞ < τ < ∞ and
| (45) |
whereas in the absence of buffer (26). Fig. 4 B plots ϕc,c(τ) using parameters that correspond to the three previously shown stochastic trajectories (Fig. 4 Aa–c). In the absence of buffer, ϕc,c(τ) decays by 50% when τ ≈ 5 ms, consistent with the time constant for Ca2+ exchange (kc = 0.2 ms−1, solid line). In the presence of slow buffer, ϕc,c(τ) is nearly identical (open circles), but in the presence of fast buffer the 50% decay occurs at a much shorter time (τ ≈ 0.1 ms, dashed line).
The time evolution of the domain [Ca2+] fluctuation δc(t) can also be characterized by its (colored) power spectrum, that is, the 3×3 matrix S(ω) = (si,j),
| (46) |
that reduces to
in the absence of buffer (26). Fig. 4 C shows the power spectrum sc,c(ω) in the absence of buffer and in the presence of slow buffer (solid line, open circles). In both cases, the spectrum is low-pass, but for fast buffer, sc,c(ω) drops off at higher frequency (smaller τ, dashed line), consistent with the higher frequency fluctuations observed in Fig. 4 A. In addition to clarifying the frequency content of domain [Ca2+] fluctuations, this power spectrum analysis illustrates how buffers influence fluctuations even in the absence of Ca2+ influx. Further analysis of the influence of buffers on the timescale of domain [Ca2+] fluctuations is provided in Fig. S4 and Fig. S5.
Discussion
Relation to prior work
The influence of Ca2+ buffers on Ca2+ diffusion, oscillations, propagating Ca2+ waves, and spatially localized Ca2+ elevations has received considerable attention in recent years (for reviews focused on local signaling, see Berridge (2,43), Smith et al. (45), and Augustine et al. (46)). Previous studies have presented theoretical analysis of buffered Ca2+ diffusion and localized Ca2+ elevations (16–18,20,47–49), as well as stochastic aspects of Ca2+-induced Ca2+ release, Ca2+ oscillations, and propagating Ca2+ waves (34,50–53). A subset of these studies utilized a Langevin formulation for the dynamics of intracellular Ca2+ channels (54–56).
Models of Ca2+-regulated channels and stochastic Ca2+ release usually incorporate buffering into the domain description and, more rarely, consider the fluctuations in free [Ca2+] that result from the small volume of subcellular compartments (e.g., the cardiac dyadic subspace) (30,33–35). To our knowledge, this is the first study that formulates Langevin equations for the buffered dynamics of intracellular Ca2+ and, subsequently, characterizes the influence of Ca2+ buffers on [Ca2+] fluctuations that arise from the association and dissociation of Ca2+ and buffer. The analytical results pertaining to the fluctuating rapid buffer approximation (RBA) and the influence of buffers’ properties on fluctuation amplitude is, to our knowledge, entirely novel.
Previous studies have, of course, utilized Langevin equations to simulate concentration fluctuations in other biochemical systems (30,33,57–60) and the stochastic gating of plasma membrane and intercellular ion channels (54,55,61–64), and used the fluctuation-dissipation theorem to characterize concentration fluctuations in biochemical reaction networks, e.g., in models of gene networks and Michaelis-Menten enzyme reactions (65–68). However, to our knowledge, this is the first mathematical analysis of domain [Ca2+] fluctuations in the presence of Ca2+ buffers.
Summary of findings
The fluctuating RBA derived above shows how the Ca2+ and buffer exchange rates (kc and kb), total buffer concentration (), and dissociation constant (κ) influence [Ca2+] fluctuations. Typically, fast buffer binding and high exchange rates increase the size of [Ca2+] fluctuations ( = 〈δc2〉). The fluctuating RBA derived here shows that the relative increase in fluctuation size due to buffers, i.e., the ratio of coefficients of variation (Eq. 40), is an increasing function of the steady-state domain [Ca2+] (css) and thus an increasing function of Ca2+ influx rate (jin). For fixed steady-state domain [Ca2+], css > c∞, Fig. 2 and Eq. 40 show that the relative fluctuation size / is a biphasic function of the total buffer concentration . At low , the fluctuation amplitude approaches that observed in the absence of buffer ( ≈ , wss → 0 in Eq. 37). At high , the ratio / ≈ 1 because increased buffering capacity attenuates the buffer-mediated increase in free [Ca2+] fluctuations (βss → 0 in Eq. 37). The total buffer concentration that maximizes / is given by Eq. 42. For a fixed Ca2+ influx rate jin, css is a decreasing sigmoidal function of that approaches c∞ (Eq. 20). In this case, is a decreasing sigmoidal function that approaches c∞/Ω. However, the buffer-mediated change in fluctuation size as measured by
remains a biphasic function of . For fixed css and for fixed jin, Ca2+ buffers increase the amplitude of domain [Ca2+] fluctuation.
We explore the validity of the RBA for realistic buffer parameters and microdomain geometries and find the fluctuating RBA is a good approximation when buffer kinetics are rapid compared to the Ca2+ and buffer exchange rates. Exchange rates on domain volume depends on the presumed relationship between escape target size and linear dimension of the domain. However, the fluctuating RBA agrees with the full calculation whenever the physical domain volume is sufficiently large and/or the escape passage is sufficiently narrow, because both situations lead to exchange rate constants (kc and kb) that are small compared to the buffer equilibration rate (an increasing function of both k+ and k−). As one would expect based on these considerations, the fluctuating RBA is a better approximation to the full equations for domain [Ca2+] fluctuations for mushroom-shaped (as opposed to thin or stubby) dendritic spine geometries (Fig. 3 D).
Our analysis shows that a buffer-mediated increase in intrinsic [Ca2+] fluctuations requires a nonequilibrium steady state, that is, a gradient between domain and bulk Ca2+ (css > c∞), which implies disequilibrium between Ca2+ and buffer within the domain. However, our primary result that buffers do not suppress intrinsic [Ca2+] fluctuations—due to the buffer’s contribution to these fluctuations—is true in the absence, as well as the presence, of elevated domain [Ca2+]. Although buffers do not influence the steady-state variance of domain [Ca2+] fluctuations in the absence of Ca2+ influx, power spectrum analysis shows that buffers do alter the temporal dynamics of domain [Ca2+] fluctuations. For fixed κ = k−/k+, a faster association rate constant (k+) leads to higher frequency free [Ca2+] fluctuations and shorter autocorrelation times.
Buffers suppress extrinsic (not intrinsic) domain [Ca2+] fluctuations
As discussed in the Introduction, the effective volume that arises in the deterministic equations for the buffered diffusion of intracellular Ca2+ is
where Ωeff ≥ Ω because 0 < β(c) ≤ 1 (24). Because the relative size of concentration fluctuations decreases as system size increases, one might expect that domain [Ca2+] fluctuations would decrease as total buffer concentration increases. In marked contrast to this conjecture, the analysis of domain [Ca2+] fluctuations presented here demonstrates that buffers increase the size of [Ca2+] fluctuations (during periods of Ca2+ influx) or have no influence (when there is no influx). That is, buffers typically lead to domain [Ca2+] fluctuations with variance that is consistent with a domain that lacks buffers but has smaller physical volume.
Some clarity with regard to this counterintuitive result can be obtained by revisiting the fast/slow system of stochastic ODEs for buffered Ca2+ dynamics (Eq. 34) under the restriction that Ca2+-free or Ca2+-bound buffer do not exchange with bulk (kb = 0),
| (47a) |
| (47b) |
Here the fluctuation is free [Ca2+]. The value (δc) is the fast variable and δcT is the only slow variable because the domain total buffer concentration is a conserved quantity given by initial conditions (bT = b(0) + cb(0)) and does not fluctuate (δbT = 0). Setting kb = 0 in Eq. 37, we see that under this restriction χ = 0, and the fluctuating RBA result is identical to the case without buffers,
because immobile buffers (kb = 0) do not change the steady-state variance of domain [Ca2+] fluctuations. Note that when kb = 0, the differential fraction of free to total calcium (βss) appears in the quasistatic approximation,
and the slow equation,
| (48) |
precisely as one would expect. Using the fluctuation-dissipation equation (Eq. 30), we calculate the steady-state covariance of the total calcium concentration,
| (49) |
as well as the covariances that involve the fast variable δc. In the RBA limit with kb = 0, the latter are given by
| (50a) |
and
| (50b) |
| (50c) |
where for the second equality we have used βsswss = 1 – βss and the definition of (Eq. 23).
Equation 49 shows that the covariance of the total [Ca2+] fluctuation (〈δ〉) responds to the elementary processes of Ca2+ influx and exchange with the bulk
The covariance of the free [Ca2+] is also dependent on influx and exchange () through the term 〈δ〉. The value 〈δc2〉 is influenced by the elementary processes of Ca2+ and buffer association and dissociation
whereas 〈δ〉 is not. Increasing buffer concentration () decreases βss and increases 〈δ〉. The value also scales in such a way that 〈δc2〉 is unchanged. Stationary buffers do not influence the steady-state covariance of domain [Ca2+] fluctuations (kb = 0), because the intrinsic nature of the fluctuating force (t) causes and βss to be related in a manner that makes 〈δc2〉 = css/Ω invariant to the buffer parameter and κ.
However, if the influx rate were modulated so that there was an extrinsic component to the fluctuations,
where
where = /Ω, then the fluctuating terms in both Eqs. 47a and 47b would have greater variance and Eqs. 49 and 50b become
and
where we have used the fact that /k+ → 0 in the RBA limit. From the last equality we conclude that the variance of the external drive increases the steady-state variance of domain [Ca2+] fluctuations. The location of the coefficient βss in the term shows that stationary buffers are able to suppress such extrinsic variability of Ca2+ influx/efflux rate, despite the fact that the buffers do not suppress intrinsic fluctuations (compare to Eq. 50b).
Analysis of the fluctuating RBA and intrinsic fluctuations
Analysis of the fluctuating RBA also provides insight into intrinsic (buffer-driven) fluctuations when both Ca2+ and buffer exchange with bulk (kb ≠ 0). The quasistatic approximation for the average value of the [Ca2+] fluctuation in the fluctuating RBA (Eq. 35) shows that the free (δc) and total (δcT) calcium fluctuations are related by the factor βss as expected. Also, Eq. 36a shows that total buffer concentration slows the relaxation of total calcium fluctuations (δcT) through the factor βss. However, the relaxation of total buffer (δbT) fluctuations are not slowed in this manner (Eq. 36b) and, importantly, the entries of the covariance matrix for the random terms , , and , are all proportional to the total buffer concentration (Eq. S18 and Eq. S20 in the Supporting Material).
To understand the interplay between the effect of on the random terms (fluctuation) and the relaxation rate (dissipation) in the case of mobile buffers (kb > 0), we analyze the Langevin domain model when the exchange rates for Ca2+ and buffer are identical (kc = kb = k). Under this restriction, the covariances of the slow variables are given by
| (51) |
where the css + cbss value in the numerator of 〈δ〉 is the steady-state total calcium concentration in the domain. The variance of the fast variable 〈δc2〉 can be written in terms of the covariances of the slow variables,
| (52) |
and it can be shown that 〈δcδbT〉 is negative whereas 〈δcδcT〉 and 〈δc2〉 are positive. Upon substitution of Eq. 51, we have
| (53a) |
| (53b) |
In this case, the fluctuating RBA simplifies to
| (54) |
This analysis of the fluctuating RBA under the restriction kc = kb shows that an increase in total buffer increases the absolute value of the slow covariances (Eq. 51). These covariances (the terms within square brackets in Eq. 53) combine to create a net positive impact on 〈δc2〉 that is attenuated to some extent by a decrease in the βss that scales these terms. The βss/Ω value outside the curly brackets in Eq. 53a might be interpreted as an effective volume that attenuates all of these contributions to 〈δc2〉, but this is misleading because βsswsscss = (1 − βss)css is an increasing function of (compare to Eq. 53b). Furthermore, Eq. 54 shows that the variance of the free [Ca2+] fluctuations in the presence of buffer > 0 is never less than what would occur in the absence of buffer ( = 0).
All of the expressions in this section have counterparts when the fluctuating RBA is derived in full generality (kc ≠ kb, see Appendix B in the Supporting Material). The derivation of the fluctuating RBA when kc = kb is shown in Appendix C in the Supporting Material.
Physiological implications
Ca2+ is an important signaling molecule in many cell types. Intracellular Ca2+ regulates cellular responses through Ca2+-regulated ion channels, Ca2+-dependent kinases, and phosphotases, and key processes in excitable cells, including muscle contraction and neurotransmitter release (1). Many of these signaling pathways occur in small spatially restricted volumes (microdomains) and, consequently, fluctuations in [Ca2+] may be physiologically significant. Our results are broadly applicable to subcellular Ca2+ domains, including dendritic spines, the dyadic cleft, and many other situations where localized Ca2+ elevations occur in spatially restricted subcellular compartments, such as the primary cilium, mitochondria-ER junctions, and endoplasmic reticulum/plasma membrane (ER/PM) junctional regions (Fig. 1). As a concrete example of an application of the theory, we found—using physiological values for dendritic spine shape—that the exogenous Ca2+ buffer BAPTA does not suppress intrinsic domain [Ca2+] fluctuations, and may increase the coefficient of variation (cv) of domain [Ca2+] fluctuations by a factor of 2 or more (Fig. 3 D).
Spatially localized subcellular dynamics can greatly alter whole-cell or even tissue-level physiological processes. For example, in the cardiac dyadic subspace, the regenerative (i.e., positive feedback) nature of Ca2+-induced Ca2+-release creates a subcellular environment where the spontaneous opening of only a few Ca2+ channels can lead to opening of nearly all of the channels in a dyad (essentially an all-or-none response) (69). Ca2+ release that spontaneously initiates within one dyad may activate neighboring dyads resulting in propagating Ca2+ waves. In pathophysiological conditions this may lead to ectopic heart beats that are potentially arrhythmogenic. This is one of many examples where the spatially localized stochastic dynamics of a few Ca2+ channels may influence the global Ca2+ response and, for this reason, the regulation of Ca2+ channels by buffer-modulated [Ca2+] fluctuations may be physiologically significant.
In Weinberg and Smith (35), we developed and analyzed a minimal model of a Ca2+ microdomain that included a stochastically gating Ca2+-regulated Ca2+ channel and accounted for the small number of Ca2+ ions present in the domain at any given time. This prior work demonstrated that [Ca2+] fluctuations due to small domain volumes could alter the open probability of Ca2+ channels, typically reducing the open probability compared with larger volumes. The fluctuating RBA derived here shows that Ca2+ buffers typically enhance domain [Ca2+] fluctuations and, when combined with previous work, this suggests that buffers may influence the stochastic dynamics of Ca2+-regulated Ca2+ channels (the frequency and duration of channel openings) and potentially influence the stochastic properties of local Ca2+ release events (sparks and puffs).
Our analysis of the fluctuating RBA raises the intriguing question of whether or not domain [Ca2+] fluctuations are physiologically significant aspects of local (and perhaps global) Ca2+ signaling. This topic requires further study, in part because our mathematical analysis indicates that Ca2+ chelators and indictor dyes enhance intrinsic domain [Ca2+] fluctuations. The physiological relevance of domain [Ca2+] fluctuations on the dynamics of signaling complexes will presumably depend on the timescale of Ca2+ influx, the geometry of the microdomain, and the kinetics of downstream Ca2+-dependent signaling.
Limitations of this analysis
We have shown that [Ca2+] fluctuations in spatially restricted subcellular compartments cannot be suppressed by increasing the total concentration of Ca2+ buffer (). Of course, this conclusion that Ca2+ buffers typically enhance domain [Ca2+] fluctuations pertains only to buffers whose dynamics are well represented by the bimolecular association reaction that was our starting point (Eq. 1). It is possible that Ca2+ buffers with more complicated and realistic Ca2+-binding kinetics (e.g., cooperative binding) may suppress free [Ca2+] fluctuations. This question could be addressed rigorously using chemical reaction network theory.
Although we consider Ca2+ and buffer transport via exchange between domain and bulk, this work does not use continuum modeling (partial differential equations) to explicitly represent spatial aspects of the buffered diffusion of intracellular Ca2+. Spatial correlations in fluctuations are of interest, but beyond the scope of this study, in part because the mathematical description of diffusion-mediated concentration fluctuations is less accessible than the analysis of stochastic differential equations presented here.
This article has focused on intrinsic Ca2+ and buffer concentration fluctuations around nonequilibrium steady states in the presence of Ca2+ influx (jin > 0) and equilibrium steady states in the absence of Ca2+ influx (jin = 0). Our analytical work is relevant and easily applied whenever a separation of timescales exists between the concentration fluctuations per se (that arise from finite size effects) and temporal variations in Ca2+ influx rate. For example, during the cardiac cycle, triggered release of junctional sarcoplasmic reticulum (SR) Ca2+ elevates dyadic subspace [Ca2+] for durations of ∼300 ms, and the low [Ca2+] of the diastolic interval persists for ∼500 ms. Because domain [Ca2+] fluctuations occur on a faster timescale (1–10 ms), it is straightforward to apply our theory to the cardiac dyad under the assumption that domain concentrations (expected values) are in quasistatic equilibrium with the time-varying influx rate, i.e., css(t) = css(jin(t)) through Eq. 20 and similarly for bss(t) and cbss(t). Even when no such timescale separation exists, domain [Ca2+] fluctuations in the context of time-varying Ca2+ influx can be calculated by numerical integration of Eq. 27, with Hss(t) and Γss(t) evaluated at the time-varying expected values of the domain Ca2+ and buffer concentrations (26). Such quasistatic and nonstationary calculations agree with numerical simulations of domain [Ca2+] fluctuations during triggered release (see Fig. S6). Perhaps most importantly, such calculations confirm that buffers increase intrinsic domain [Ca2+] fluctuations during systole, and buffers do not suppress [Ca2+] fluctuations during diastole, just as one would expect from the nonequilibrium steady-state analysis that has been our focus.
In other physiological settings, domain [Ca2+] is elevated for longer or shorter durations. In pancreatic β-cells, for example, insulin secretion is regulated by minute-long duration [Ca2+] elevations in nuclear (8) and subplasma membrane (70) Ca2+ microdomains. In pituitary cells, minute-long spontaneous [Ca2+] oscillations have been observed in mitochondrial microdomains (71). In such cases, it is straightforward to apply our nonequilibrium steady-state analysis (e.g., the fluctuating RBA). This article’s conclusions are also directly applicable to the case of pulsatile Ca2+ influx with characteristic time shorter than the domain equilibration time (for buffers and escape). When the timescale for pulsatile influx is comparable to the domain time constant, quantitative studies will require simulation of the full system of stochastic ODEs. In numerical simulation of a stochastically gated influx with mean open time of 1 ms and mean closed time of 9 ms, we find that buffers do not decrease intrinsic domain [Ca2+] fluctuations (see Fig. S7), consistent with our analysis of constant and slowly changing Ca2+ influx.
It would be interesting to understand the impact of intrinsic fluctuations (due to the finite number of Ca2+ ions in a domain) on Ca2+-mediated effectors, including Ca2+-activated or inactivated channels, initiation of Ca2+ sparks, and so on. In complementary projects we have made some progress in that direction (35). It is important to understand that even when [Ca2+] fluctuations are much faster than the timescale of a downstream Ca2+-mediated process (e.g., opening of a Ca2+-activated channel), these fluctuations may (34) or may not (35) average-out over the slower timescale. Further studies could address such issues by including downstream signaling events in model formulations, but this article’s primary conclusion—that Ca2+ buffers do not suppress intrinsic [Ca2+] fluctuations—is an important first step in our developing understanding of the physiological significance of domain [Ca2+] fluctuations.
Acknowledgments
The authors thank the referees for helpful and constructive comments.
The work was supported by National Science Foundation grant No. DMS 1121606, the Biomathematics Initiative at The College of William & Mary, and a 2013 Plumeri Award for Faculty Excellence to G.D.S.
Supporting Material
References
- 1.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signaling: dynamics, homeostasis and remodeling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M.J. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 3.Berridge M.J. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. doi: 10.1016/j.ceca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Carrasco M.A., Hidalgo C. Calcium microdomains and gene expression in neurons and skeletal muscle cells. Cell Calcium. 2006;40:575–583. doi: 10.1016/j.ceca.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Demuro A., Parker I. Imaging single-channel calcium microdomains. Cell Calcium. 2006;40:413–422. doi: 10.1016/j.ceca.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh X., Srinivasan B., Levchenko A. A 3D Monte Carlo analysis of the role of dyadic space geometry in spark generation. Biophys. J. 2006;90:1999–2014. doi: 10.1529/biophysj.105.065466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winslow R.L., Greenstein J.L. Cardiac myocytes and local signaling in nano-domains. Prog. Biophys. Mol. Biol. 2011;107:48–59. doi: 10.1016/j.pbiomolbio.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso M.T., Villalobos C., García-Sancho J. Calcium microdomains in mitochondria and nucleus. Cell Calcium. 2006;40:513–525. doi: 10.1016/j.ceca.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Dedkova E.N., Blatter L.A. Calcium signaling in cardiac mitochondria. J. Mol. Cell. Cardiol. 2013;58:125–133. doi: 10.1016/j.yjmcc.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohlhaas M., Maack C. Calcium release microdomains and mitochondria. Cardiovasc. Res. 2013;98:259–268. doi: 10.1093/cvr/cvt032. [DOI] [PubMed] [Google Scholar]
- 11.Lindemann B. Predicted profiles of ion concentrations in olfactory cilia in the steady state. Biophys. J. 2001;80:1712–1721. doi: 10.1016/S0006-3495(01)76142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pazour G.J., Witman G.B. The vertebrate primary cilium is a sensory organelle. Curr. Opin. Cell Biol. 2003;15:105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 13.Delling M., DeCaen P.G., Clapham D.E. Primary cilia are specialized calcium signaling organelles. Nature. 2013;504:311–314. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith G.D. Computational Neuroscience. CRC Press; Boca Raton, FL: 2001. Modeling local and global calcium signals using reaction diffusion equations. [Google Scholar]
- 15.Smith G.D., Wagner J., Keizer J. Validity of the rapid buffering approximation near a point source of calcium ions. Biophys. J. 1996;70:2527–2539. doi: 10.1016/S0006-3495(96)79824-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith G.D. Analytical steady-state solution to the rapid buffering approximation near an open Ca2+ channel. Biophys. J. 1996;71:3064–3072. doi: 10.1016/S0006-3495(96)79500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith G.D., Dai L., Sherman A. Asymptotic analysis of buffered calcium diffusion near a point source. SIAM J. Appl. Math. 2001;61:1816–1838. [Google Scholar]
- 18.Naraghi M., Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J. Neurosci. 1997;17:6961–6973. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shuai J., Pearson J.E., Parker I. Modeling Ca2+ feedback on a single inositol 1,4,5-trisphosphate receptor and its modulation by Ca2+ buffers. Biophys. J. 2008;95:3738–3752. doi: 10.1529/biophysj.108.137182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stern M.D. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992;13:183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- 21.Dargan S.L., Parker I. Buffer kinetics shape the spatiotemporal patterns of IP3-evoked Ca2+ signals. J. Physiol. 2003;553:775–788. doi: 10.1113/jphysiol.2003.054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeller S., Rüdiger S., Falcke M. Modeling of the modulation by buffers of Ca2+ release through clusters of IP3 receptors. Biophys. J. 2009;97:992–1002. doi: 10.1016/j.bpj.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai J.-C., Sneyd J. Traveling waves in the buffered FitzHugh-Nagumo model. SIAM J. Appl. Math. 2011;71:1606–1636. [Google Scholar]
- 24.Wagner J., Keizer J. Effects of rapid buffers on Ca2+ diffusion and Ca2+ oscillations. Biophys. J. 1994;67:447–456. doi: 10.1016/S0006-3495(94)80500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Kampen N.G. North-Holland Publishing Company; Amsterdam, The Netherlands: 1981. Stochastic Processes in Physics and Chemistry. [Google Scholar]
- 26.Keizer J. Springer; Berlin, Germany: 1987. Statistical Thermodynamics of Nonequilibrium Processes. [Google Scholar]
- 27.Rüdiger S., Shuai J.W., Falcke M. Hybrid stochastic and deterministic simulations of calcium blips. Biophys. J. 2007;93:1847–1857. doi: 10.1529/biophysj.106.099879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huertas M.A., Smith G.D. The dynamics of luminal depletion and the stochastic gating of Ca2+-activated Ca2+ channels and release sites. J. Theor. Biol. 2007;246:332–354. doi: 10.1016/j.jtbi.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Groff J.R., Smith G.D. Calcium-dependent inactivation and the dynamics of calcium puffs and sparks. J. Theor. Biol. 2008;253:483–499. doi: 10.1016/j.jtbi.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 30.von Wegner F., Fink R.H.A. Stochastic simulation of calcium microdomains in the vicinity of an L-type calcium channel. Eur. Biophys. J. 2010;39:1079–1088. doi: 10.1007/s00249-009-0504-3. [DOI] [PubMed] [Google Scholar]
- 31.Williams G.S.B., Chikando A.C., Jafri M.S. Dynamics of calcium sparks and calcium leak in the heart. Biophys. J. 2011;101:1287–1296. doi: 10.1016/j.bpj.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rüdiger S., Jung P., Shuai J.-W. Termination of Ca²+ release for clustered IP3R channels. PLOS Comput. Biol. 2012;8:e1002485. doi: 10.1371/journal.pcbi.1002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winslow R.L., Tanskanen A., Greenstein J.L. Multiscale modeling of calcium signaling in the cardiac dyad. Ann. N. Y. Acad. Sci. 2006;1080:362–375. doi: 10.1196/annals.1380.027. [DOI] [PubMed] [Google Scholar]
- 34.Hake J., Lines G.T. Stochastic binding of Ca2+ ions in the dyadic cleft; continuous versus random walk description of diffusion. Biophys. J. 2008;94:4184–4201. doi: 10.1529/biophysj.106.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg S.H., Smith G.D. Discrete-state stochastic models of calcium-regulated calcium influx and subspace dynamics are not well-approximated by ODEs that neglect concentration fluctuations. Comput. Math. Methods Med. 2012;2012:897371. doi: 10.1155/2012/897371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuss Z., Singer A., Holcman D. The narrow escape problem for diffusion in cellular microdomains. Proc. Natl. Acad. Sci. USA. 2007;104:16098–16103. doi: 10.1073/pnas.0706599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillespie D.T. The chemical Langevin equation. J. Chem. Phys. 2000;113:297–306. [Google Scholar]
- 38.Higham D.J. Modeling and simulating chemical reactions. SIAM Rev. 2008;50:347–368. [Google Scholar]
- 39.Gardiner C.W. Springer; New York: 1997. Handbook of Stochastic Methods for Physics, Chemistry, and the Natural Sciences. [Google Scholar]
- 40.Gillespie D.T. Stochastic simulation of chemical kinetics. Annu. Rev. Phys. Chem. 2007;58:35–55. doi: 10.1146/annurev.physchem.58.032806.104637. [DOI] [PubMed] [Google Scholar]
- 41.Harris K.M., Jensen F.E., Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J. Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laub A.J. Matrix Analysis for Scientists and Engineers. Society for Industrial and Applied Mathematics (SIAM); Philadelphia, PA: 2004. Kronecker products. [Google Scholar]
- 43.Berridge M.J. Elementary and global aspects of calcium signaling. J. Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reference deleted in proof.
- 45.Smith G.D., Pearson J., Keizer J. Modeling intracellular Ca2+ waves and sparks. In: Fall C., Marland E., Wagner J., Tyson J., editors. Computational Cell Biology. Springer; Berlin, Germany: 2002. pp. 200–232. [Google Scholar]
- 46.Augustine G.J., Santamaria F., Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- 47.Neher E. Concentration profiles of intracellular Ca2+ in the presence of diffusible chelator. Exp. Brain Res. 1986;14:80–96. [Google Scholar]
- 48.Reference deleted in proof.
- 49.Falcke M. Buffers and oscillations in intracellular Ca2+ dynamics. Biophys. J. 2003;84:28–41. doi: 10.1016/S0006-3495(03)74830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shuai J.W., Jung P. Understanding Calcium Dynamics. Springer; Berlin, Germany: 2003. Langevin modeling of intracellular calcium dynamics; pp. 231–252. [Google Scholar]
- 51.Nguyen V., Mathias R., Smith G.D. A stochastic automata network descriptor for Markov chain models of instantaneously coupled intracellular Ca2+ channels. Bull. Math. Biol. 2005;67:393–432. doi: 10.1016/j.bulm.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Jafri M.S., Keizer J. On the roles of Ca2+ diffusion, Ca2+ buffers, and the endoplasmic reticulum in IP3-induced Ca2+ waves. Biophys. J. 1995;69:2139–2153. doi: 10.1016/S0006-3495(95)80088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thul R., Falcke M. Waiting time distributions for clusters of complex molecules. EuroPhys. Lett. 2007 38003. [Google Scholar]
- 54.Shuai J.W., Jung P. Stochastic properties of Ca2+ release of inositol 1,4,5-trisphosphate receptor clusters. Biophys. J. 2002;83:87–97. doi: 10.1016/S0006-3495(02)75151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shuai J.W., Jung P. Optimal ion channel clustering for intracellular calcium signaling. Proc. Natl. Acad. Sci. USA. 2003;100:506–510. doi: 10.1073/pnas.0236032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hinch R. A mathematical analysis of the generation and termination of calcium sparks. Biophys. J. 2004;86:1293–1307. doi: 10.1016/S0006-3495(04)74203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qian H. Nonlinear stochastic dynamics of mesoscopic homogeneous biochemical reaction systems—an analytical theory. Nonlinearity. 2011;24:R19–R49. [Google Scholar]
- 58.Rovetti R., Das K.K., Shiferaw Y. Macroscopic consequences of calcium signaling in microdomains: a first-passage-time approach. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2007;76:051920. doi: 10.1103/PhysRevE.76.051920. [DOI] [PubMed] [Google Scholar]
- 59.Wieder N., Fink R.H.A., Wegner F. Exact and approximate stochastic simulation of intracellular calcium dynamics. J. Biomed. Biotechnol. 2011;2011:572492. doi: 10.1155/2011/572492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wegner F., Wieder N., Fink R.H.A. Springer; Dordrecht, The Netherlands: 2012. Simulation Strategies for Calcium Microdomains and Calcium-Regulated Calcium Channels; pp. 553–567. [DOI] [PubMed] [Google Scholar]
- 61.Fox R.F., Lu Y. Emergent collective behavior in large numbers of globally coupled independently stochastic ion channels. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics. 1994;49:3421–3431. doi: 10.1103/physreve.49.3421. [DOI] [PubMed] [Google Scholar]
- 62.White J.A., Rubinstein J.T., Kay A.R. Channel noise in neurons. Trends Neurosci. 2000;23:131–137. doi: 10.1016/s0166-2236(99)01521-0. [DOI] [PubMed] [Google Scholar]
- 63.Kepler T.B., Elston T.C. Stochasticity in transcriptional regulation: origins, consequences, and mathematical representations. Biophys. J. 2001;81:3116–3136. doi: 10.1016/S0006-3495(01)75949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chow C.C., White J.A. Spontaneous action potentials due to channel fluctuations. Biophys. J. 1996;71:3013–3021. doi: 10.1016/S0006-3495(96)79494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tao Y., Jia Y., Dewey T.G. Stochastic fluctuations in gene expression far from equilibrium: ω expansion and linear noise approximation. J. Chem. Phys. 2005;122:124108. doi: 10.1063/1.1870874. [DOI] [PubMed] [Google Scholar]
- 66.Grima R. Investigating the robustness of the classical enzyme kinetic equations in small intracellular compartments. BMC Syst. Biol. 2009;3:101. doi: 10.1186/1752-0509-3-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomas, P., H. Matuschek, and R. Grima. 2012. Computation of biochemical pathway fluctuations beyond the linear noise approximation using iNA. In 2012 IEEE International Conference on Bioinformatics and Biomedicine (BIBM). IEEE, Piscataway, NJ. 1–5.
- 68.Pahle J., Challenger J.D., McKane A.J. Biochemical fluctuations, optimization and the linear noise approximation. BMC Syst. Biol. 2012;6:86. doi: 10.1186/1752-0509-6-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bers D.M. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 70.Rutter G.A., Tsuboi T., Ravier M.A. Ca2+ microdomains and the control of insulin secretion. Cell Calcium. 2006;40:539–551. doi: 10.1016/j.ceca.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 71.Villalobos C., Núñez L., García-Sancho J. Mitochondrial [Ca2+] oscillations driven by local high [Ca2+] domains generated by spontaneous electric activity. J. Biol. Chem. 2001;276:40293–40297. doi: 10.1074/jbc.C100465200. [DOI] [PubMed] [Google Scholar]
- 72.Pethig R., Kuhn M., Jaffe L.F. On the dissociation constants of BAPTA-type calcium buffers. Cell Calcium. 1989;10:491–498. doi: 10.1016/0143-4160(89)90026-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


