Abstract
Transplantation of human neural progenitor cells (NPCs) into the brain or spinal cord to replace lost cells, modulate the injury environment or create a permissive milieu to protect and regenerate host neurons is a promising therapeutic strategy for neurological diseases. Deriving NPCs from human fetal tissue is feasible, though problematic issues include limited sources and ethical concerns. Here we describe a new and abundant source of NPCs derived from human induced pluripotent stem cells (iPSCs). A novel chopping technique was used to transform adherent iPSCs into free-floating spheres that were easy to maintain and were expandable (EZ spheres) (Ebert et al., 2013). These EZ spheres could be differentiated towards NPC spheres with a spinal cord phenotype using a combination of all-trans retinoic acid (ATRA) and epidermal growth factor (EGF) and fibroblast growth factor-2 (FGF-2) mitogens. Suspension cultures of NPCs derived from human iPSCs or fetal tissue have similar characteristics, though they were not similar when grown as adherent cells. In addition, iPSC-derived NPCs (iNPCs) survived grafting into the spinal cord of athymic nude rats with no signs of overgrowth and with a very similar profile to human fetal-derived NPCs (fNPCs). These results suggest that human iNPCs behave like fNPCs and could thus be a valuable alternative for cellular regenerative therapies of neurological diseases.
INTRODUCTION
Fetal neural progenitor cells (fNPCs) can be isolated from different regions of the developing human brain, expanded in culture and then differentiated into neurons and glia (Conti and Cattaneo, 2010; Kriegstein and Alvarez-Buylla, 2009). We have previously shown that fNPCs transplanted into the brain, spinal cord or retina in animal models of disease can survive and migrate, and provide beneficial effects in some cases (Andres et al., 2011; Nichols et al., 2013; Wang et al., 2008). Moreover, we have genetically engineered these cells to produce therapeutic molecules for neuroprotection following transplantation in animal models of Parkinson’s disease and amyotrophic lateral sclerosis (ALS) (Ebert et al., 2008; Suzuki et al., 2007). Other groups have also generated and shown the potential of human fNPCs, which in some cases have been taken forward into Federal Drug Administration-approved clinical trials for a number of neurological disorders with no reported serious adverse effects (Azienda Ospedaliera Santa Maria et al., 2012; Glass et al., 2012; Neuralstem Inc. and Emory University, 2011; ReNeuron Limited, 2010; Robberecht and Philips, 2013; StemCells, 2006; StemCells, 2011; StemCells, 2012; Tamaki et al., 2002; Taupin, 2006). However, due to the limited supply, concerns of chromosomal aberrations (aneuploidies) during expansion (Sareen et al., 2009), and ethical concerns associated with the use of aborted human fetal tissues there is a pressing need for alternative sources.
Human pluripotent stem cells (hPSCs) including, embryonic stem cells (ESCs) derived from the blastocyst of a developing embryo and induced PSCs (iPSCs) derived from reprogrammed adult somatic cells have great potential for generating cells for use in regenerative and cell replacement strategies (Okano et al., 2013; Robinton and Daley, 2012; Yamanaka, 2012). They are essentially immortal allowing limitless cellular expansion and banking, and extremely plastic allowing differentiation into any cell type. Human iPSCs also offer an unprecedented opportunity for autologous transplantation, possibly circumventing the complexities surrounding immunological rejection with allogeneic human cell transplantation (Araki et al., 2013; Kaneko and Yamanaka, 2013; Liu et al., 2013; Okita et al., 2011c; Zhao et al., 2011). Human iPSCs can efficiently develop into neural cells (Chetty et al., 2013; Ebert et al., 2013; Kobayashi et al., 2012; Lee et al., 2012; Zhou et al., 2010), however, before iPSC-derived neural cells can be used in clinical transplantation trials they must 1) be shown to be safe, 2) maintain a normal cytogenetic status, 3) be devoid of residual pluripotent cells to avoid possible malignant tumor formation, 4) be reproducibly expanded in large numbers, and finally 5) survive and integrate into relevant central nervous system regions.
Neuronal replacement is one strategy to use for future clinical transplantation trials. However, in fact, astroglial cells are the most abundant cell type in the human brain and spinal cord and are now understood to be as important as neurons for brain function (Oberheim et al., 2006). They have also been implicated in a number of neurodegenerative diseases, with perhaps the best example being ALS, where glial dysfunction has been shown to lead to non-cell autonomous death of the motor neurons (Di Giorgio et al., 2007; Haidet-Phillips et al., 2011; Nagai et al., 2007; Yamanaka et al., 2008). Replacement of astrocytes (Lepore et al., 2011; Lepore et al., 2008; Nichols et al., 2013), either naive or secreting growth factors (Suzuki et al., 2007), has been shown to be beneficial in ALS models. We have previously shown that fNPCs can give rise to astroglial progenitors that then differentiate to immature and mature astrocytes within the rodent brain and spinal cord over long time periods (Gowing et al., 2013; Klein et al., 2005; Suzuki et al., 2007; Svendsen et al., 1997). Human PSCs can also be directed into more mature astrocytes (Juopperi et al., 2012; Krencik et al., 2011; Yuan et al., 2013). While such PSC-derived mature astrocytes may survive transplantation, immature NPCs generated from iPSCs may provide cells that are easier to culture and expand in vitro and better suited to migrate, integrate and restore function in vivo.
Here, we report a new protocol for the production of expandable human iPSC-derived neural progenitor cells (iNPCs). These human iNPCs could be easily propagated over long-term as suspension cultures and had similar profile to human fNPCs. Following direct parenchymal injection, iNPCs successfully engrafted into athymic nude rats with no signs of tumor formation or overgrowth, and again appeared similar to fNPC transplants. Our results describe a new source of human neural progenitor cells that do not have the supply, expansion and ethical concerns of fNPCs, and hence could be ideal for stem cell-based therapeutic approaches.
METHODS
Generation of non-integrating human iPSCs using episomal plasmids
Apparently healthy human fibroblast cell lines (GM05400, 03814 and 02183) were obtained from the Coriell Institute for Medical Research, under their consent and privacy guidelines. All protocols were performed in accordance with the institutional review board’s guidelines at the Cedars-Sinai Medical Center under the auspice IRB-SCRO Protocols Pro00028429 (Transplantation iPS derived human neural progenitors), Pro00021505, and Pro00032834. Upon iPSC generation at Cedars Sinai, they were renamed 00iCTR-n2, 15iCTR-n5, and 83iCTR-n1 to reflect catalog number, control line and clone number (Luong et al., 2011; Sareen et al., 2012). Fibroblasts were reprogrammed into virus-free iPSC lines using the Amaxa Human Dermal Fibroblast Nucleofector Kit to express episomal plasmids with 6 factors: OCT4, SOX2, KLF4, L-MYC, LIN28, and p53 shRNA (Addgene) (Okita et al., 2011a). This method has a significant advantage over viral transduction, because exogenously introduced genes do not integrate and are instead expressed episomally in a transient fashion. Briefly, fibroblasts (0.8 × 106 cells per nucleofection) were harvested, centrifuged at 200g for 5 minutes, re-suspended carefully in Nucleofector® Solution (VPD-1001, Lonza) and the U-023 program was applied. All cultures were maintained under norm-oxygen conditions (5%O2) during reprogramming, which further enhance the efficiency of iPSC generation. The media was kept on for 48 hours and gradually changed to chemically-defined mTeSR®1 medium containing small molecules to enhance reprogramming efficiency. The small molecules used were1) sodium butyrate (0.5 mM), 2) glycogen synthase kinase 3β inhibitor of the Wnt/β-catenin signaling pathway (CHIR99021, 3 µM), 3) MEK pathway inhibitor (PD 0325901, 0.5 µM), 4) Selective inhibitor of TGF-β type I receptor ALK5 kinase, type I activin/nodal receptor ALK4 and type I nodal receptor ALK7 (A 83-01, 0.5 µM). Colonies with ES/iPSC-like morphology appeared 25–31 days later. Subsequently, colonies with the best morphology were transferred onto a feeder-independent 6 BD Matrigel™ Matrix and maintained in mTeSR®1 medium. The iPSC clones were further expanded and cryopreserved according to previously published protocols (Yu et al., 2007).
Human iPSC characterization
Human iPSCs were rigorously characterized at the Cedars-Sinai iPSC core using several assays. G-Band karyotyping (see below) ensured normal a karyotype, and genomic DNA PCR confirmed the absence of episomal plasmid genes, as previously described (Muller et al., 2011; Okita et al., 2011b; Sareen et al., 2012). Pluripotency was assessed by immunostaining with surface and nuclear pluripotency markers for subsequent flow cytometry quantification (> 80% SSEA4 and Oct3/4 double positivity), by quantitative RT-PCR of endogenous pluripotency genes, and by gene-chip and bioinformatics-based PluriTest assays. Spontaneous embryoid body differentiation confirmed the capacity to form all germ layers.
Karyotype
Spheres were incubated in Colcemid (100 ng/mL; Life Technologies) for 30 minutes at 37°C and then dissociated using TrypLE for 10 minutes. They were then washed in phosphate buffered saline (PBS) and incubated at 37°C in 5mL hypotonic solution (1g KCl, 1g Na Citrate in 400mL water) for 30 minutes. The cells were centrifuged for 2.5 minutes at 1500 RPM and resuspended in fixative (methanol: acetic acid, 3:1) at room temperature for 5 minutes. This was repeated twice, and finally cells were resuspended in 500 µl of fixative solution and submitted to the Cedars-Sinai Clinical Cytogenetics Core for G-Band karyotyping.
EZ sphere generation for astrocyte formation
A 6-well plate of iPSCs was cultured to ~ 80% confluence and differentiating colonies were removed prior to generating neural stem cell aggregates (termed EZ spheres). To generate EZ spheres, media was aspirated, iPSC colonies were washed with PBS, treated with dispase (1 mg/mL) at 37°C for 15 minutes, gently washed with PBS, and gently collected in Stemline Neural Expansion media (Sigma) with a 5 ml pipette into a 15mL conical tube. After the primarily intact colonies settled to the bottom by gravity, media was aspirated and the colonies were resuspended in Stemline media supplemented with epidermal growth factor (EGF; 100 ng/ml; Millipore), fibroblast growth factor-2 (FGF-2; 100 ng/ml; Millipore), and heparin (5 µg/ml; Sigma) (termed StemHi E/F/H) and placed into one T75 flask coated with poly-HEMA to prevent attachment at 20% O2 in 37°C incubator. Fresh media was replaced after the first 2 days of EZ sphere generation and subsequently every 3–4 days. The spheres were maintained in suspension, passaged by chopping with an automated tissue chopper into 200 µm chunks every 7–10 days as described previously (Ebert et al., 2013; Svendsen et al., 1998), and were efficiently cryopreserved and thawed for banking. EZ spheres after passage 10 are a stable population of neural stem cell aggregates with an excellent propensity for directed differentiation towards an astrocyte lineage (see below).
Neural progenitor cell generation from iPSC-derived EZ spheres
EZ spheres generated from iPSCs can be differentiated to a culture of neural progenitor cells in suspension (iNPCsSU), with astroglial predisposition. After EGF/FGF2/heparin withdrawal, EZ spheres were caudalized using retinoic acid RA (0.5µM) in Neural Induction Media (NIM) (DMEM/F12, 1% NEAA, 1% N2, heparin 2µg/ml; Sigma). This media was replaced every 2 days for the next 11 days, after which a stable population of iNPCsSU was reintroduced into StemHi E/F/H for expansion by weekly chopping (similar to EZ spheres). The iNPCsSU maintain their proliferative potential and astroglial generation propensity for 26–30 passages and can be efficiently cryopreserved. In addition, an adherent format of iNPCs grown as adherent cultures and termed iNPCsAD, were generated by accutase dissociation of EZ spheres for plating on growth-factor reduced Matrigel (Corning) at a density of 10,000 cells/cm2 in StemHi E/F/H and passaged weekly using TrypLE (Life Technologies). For differentiation to astrocytes, iNPCsSU were dissociated to single cells with accutase (BD Biosciences) or iNPCsAD harvested with TrypLE were plated on poly-l-ornithine/Matrigel coated glass coverslips at 25000 cells/cm2 in NIM for 7–21 days.
Fetal-derived human neural progenitors
Human fetal neural progenitor cells (fNPCs) isolated from 8 week old fetal cortex were maintained and expanded as free floating spheres as previously described (Sareen et al., 2009; Svendsen et al., 1997; Svendsen et al., 1998). Fetal NPC spheres were maintained in Stemline media supplemented with EGF (100 ng/ml), leukemia inhibitory factor (LIF; 100 ng/ml), Anti-Anti penicillin-streptomycin (1%, Life Technologies) and passaged by mechanical chopping (Svendsen et al., 1998). Following expansion, cells were prepared for transplantation at p28.
Cryopreservation
Spheres were collected and settled by gravity in a 15mL conical tube. The media was aspirated and the spheres were resuspended in serum-free, 8.7% DMSO-supplemented cell freezing media (Sigma). Cells were frozen at −80°C in an isopropyl alcohol chamber for 24 hours, followed by long-term storage in liquid nitrogen.
Antibody Characterization
Antibodies utilized in this study for immunocytochemical and histological analyses are summarized in Table 1 and have been tested for specificity. The anti-A2B5 monoclonal antibody (MAB312; Clone A2B5-105; Millipore) has been characterized by others to recognize A2B5 positive cells in fluorescence activated cell sorting (Windrem et al., 2004) and immunocytochemistry (Jiang et al., 2013). The anti-Aldh1L1 monoclonal antibody (73–140; clone N103/39; Antibodies, Inc.) recognizes a specific band at a molecular weight of 100 kDa on adult rat brain immunoblots (manufacturer’s technical information). The anti-Aquaporin 4 (AQP4) polyclonal antibody (HPA014784, Sigma) has been validated by the manufacturer with two or more antibodies showing similar staining patterns consistent with gene characterization data and a band of predictive size ~37 KDa observed on immunoblot obtained from various cell lysates. The GLAST (EAAT-1) polyclonal antibody has been characterized by the manufacturer with predictive bands obtained at 60 and 120 kDa on immunoblots. The anti-Nestin (AB5922; Millipore) polyclonal antibody corresponds to a detected 200–220-kDa band in human and monkey brain cell lysates (Messam et al., 2000). The anti-GFAP (Z0334; Dako) polyclonal antibody has been solid-phase absorbed with human and cow serum proteins and shows one distinct precipitate (GFAP) with cow brain extract via Coomassie brilliant blue (manufacturer’s technical information) and labels a major band at the expected 50kDa on Western blots of mouse retina (Smith et al., 1997). The STEM123 (Stem Cells, Inc.) monoclonal antibody reacts specifically with human GFAP but not rodent cells by immunohistochemistry (Gowing et al., 2013). Moreover, laboratory testing has shown that co-localization of signal is obtained by immunocytochemistry when using STEM123 and anti-GFAP antibodies (Z0334; Dako) on human cells. The anti-Ki67 (ab27619; clone SP6, Abcam) monoclonal antibody is a FITC conjugated equivalent to ab16667. Ab16667 recognizes a single band at 359 kDa on immunoblots from cell lysates (Manufacturer’s technical information) and an increase in Ki67 labeling has been previously shown to correlate with increased BrdU labeling, another marker of cell proliferation (Bonzo et al., 2012). The anti-βIII-tubulin (Tuj1, Clone SDL.3D10, T8660, Sigma) monoclonal antibody was shown to specifically recognize the protein on immunoblots (Lee et al., 1990). The anti-S100B (S2532; Clone SH-B1, Sigma) monoclonal antibody has been extensively characterized by immunohistochemistry and western blotting (20 kDa band in mouse homogenates) (Liao et al., 2008). The STEM101 (Stem Cells, Inc.) monoclonal antibody reacts specifically with the Ku80 Protein located in nuclei of human cells only. It has been shown to specifically label the nuclei of human cells, but not rodent or monkey cells by immunohistochemistry (Mattis et al., 2014; Salazar et al., 2010). The STEM121 (Stem Cells, Inc.) has been shown to specifically recognize the cytoplasm of human but not rodent cells or primate cells by immunohistochemistry (Cummings et al., 2005; Guzman et al., 2007; Kelly et al., 2004; Kriks et al., 2011; Mattis et al., 2014; Piltti et al., 2013; Salazar et al., 2010; Uchida et al., 2012). All pluripotency antibodies (OCT3/4; SOX2, NANOG, SSEA4, TRA-1-60, TRA-1-81; Table 1) have been extensively tested and validated in studies for immunocytochemistry (Sareen et al., 2013). The OCT3/4-PE conjugated antibody (561556; BD Biosciences) and SSEA4-AF647 conjugated antibody (560796; BD Biosciences) have been extensively tested for flow cytometry applications (Sareen et al., 2013).
Table 1.
Antibody Characterization for Immunohisto/cytochemistry
| Antigen | Immunogen / Band size on immunoblots | Dilution | Host/ Isotype |
Company |
|---|---|---|---|---|
| A2B5 | Embryonic Chicken Retinal Cells | 1:500 | Mouse IgM | Millipore |
| Aldh1L1 | Full length protein(aa 1–902). Recognizes a band of 100 kDa. | 1:25 | Mouse IgG1 | Antibodies, Inc. |
| Aquaporin 4 (AQP4) | CPDVEFKRRFKEAFSKAAQQTKGSYMEVEDNRSQVETDDLILKPGVVHVIDVDRGEEKKGKDQSGEVLSSV. Recognizes a band of 37 kDa. | 1:2500 | Rabbit | Sigma |
| GFAP | GFAP isolated from cow spinal cord. Recognizes a band of 50 kDa. | 1:1000 | Rabbit | Dako |
| GLAST (EAAT-1) | E. coli-derived recombinant human EAAT1/GLAST-1. His146-Ser237. Specific bands were detected at approximately 60 and 120 kDa for human neuroblastoma cell lines. | 1:20 | Sheep IgG | R&D Systems |
| Ki67 | Synthetic peptide from C terminus of human Ki67. Recognizes a single band at 359 kDa. | 1:500 | Rabbit IgG | Abcam |
| NANOG | Synthetic peptide corresponding to residues near the amino terminus of human Nanog protein. Recognizes bands of a 42 kDa of 35kDa on iPS cell lysates. | 1:400 | Rabbit IgG monoclonal | Cell Signaling Technology |
| Nestin | Fusion protein. Recognizes 200–220 kDa band. | 1:10,000 | Rabbit IgG | Millipore |
| OCT3/4 | Synthetic peptide (residues 300 to the C-terminus of human Oct4) conjugated to KLH. | 1:250 | Rabbit IgG polyclonal | Stemgent |
| OCT3/4-PE | Human Oct3/4 Isoform A Recombinant protein | 1:100 | Mouse IgG1, κ | BD Biosciences |
| S100B | Bovine brain S-100b. Recognizes an epitope located on the β chain (i.e. in S-100a and S-100b) but not on theα chain of S-100 (i.e. in S-100a and S-100ao). Predicted to recognize a 20 kDa band. | 1:250 | Mouse IgG1 | Sigma |
| SOX2 | Recombinant protein fragment containing a sequence corresponding to a region within amino acid 45 and 261 of human Sox2 | 1:100 | Rabbit IgG polyclonal | Stemgent |
| SSEA4 | Human embryonic carcinoma cell line 2102 Ep. | 1:100 | Mouse IgG3 | Stemgent |
| SSEA4-AF647 | Human Teratocarcinoma Cell Line. | 1:100 | Mouse IgG3 | BD Biosciences |
| STEM101 (Ku80) | Human brain cell suspension. | 1:200 | Mouse IgG1 | Stem Cell Inc. |
| STEM121 | Recognizes human cytoplasm (see references in materials and methods). | 1:2000 | Mouse IgG1 | Stem Cell Inc. |
| STEM123 (hGFAP) | Human peptide for sequence specific to human GFAP. | 1:2000 | Mouse IgG1 | Stem Cell Inc. |
| TRA-1-60 | Human embryonal carcinoma cell line 2102Ep | 1:100 | Mouse IgM | Stemgent |
| TRA-1-81 | Human embryonal carcinoma cell line 2102Ep | 1:100 | Mouse IgM, κ | Stemgent |
| Tuj1 (βIII-tubulin) | Synthetic peptide corresponding to the C-terminal sequence of human β-tubulin isotype III coupled to BSA | 1:1000 | Mouse IgG2b | Sigma |
Immunocytochemistry
The spheres were dissociated using accutase, and plated on coverslips at a density of 70,000 cells/well in Stemline media with appropriate mitogens for differentiation. Following differentiation in culture, plated cells were fixed with paraformaldehyde (PFA), rinsed in PBS, blocked in 5–10% goat or donkey serum with 0.2% Triton X-100 and incubated with primary antibodies; OCT3/4, NANOG, SOX2, SSEA4, TRA-1-60, TRA-1-81, GFAP, Ald1hL1, S100B, A2B5, Nestin, and Tuj1 at dilutions indicated in Table 1. After 1 hour at ambient temperature or overnight incubation at 4°C, cultures were rinsed and incubated in species-specific AF488 or AF594-conjugated secondary antibodies followed by Hoechst 33258 (0.5 µg/ml; Sigma) to counterstain nuclei. Cells were imaged using Nikon/Leica microscopes and quantified using MetaMorph Offline software (Molecular Devices).
Quantitative RT-PCR
Total RNA was isolated using the RNeasy Mini Kit (Qiagen), and RNA (1 µg) was first DNase treated and then reverse transcribed to cDNA with oligo(dT) using the Promega Reverse Transcriptase System. Reactions were performed in three technical replicates using SYBR Green master mix (Applied Biosystems) using specific primer sequences (Table 2). Each PCR cycle consisted of 95°C for 10 min, 95°C 30 s −> 58°C for 60 s, for 50 cycles, and 72°C 5 min. The melting curve was measured and recorded from 65°C to 95°C in increments of 0.05°C to 0.5°C. Genes of interest were normalized to RPL13 ribosomal Protein L13A, and calculated by 2−ΔΔCT method (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008)
Table 2.
Primer sets qRT-PCR.
| Genes | Accession | Sequences |
|---|---|---|
| POU5F1 / OCT3/4 (CDS) |
NM_002701.5 NM_203289.5 |
CCC CAG GGC CCC ATT TTG GTA CC |
| ACC TCA GTT TGA ATG CAT GGG AGA GC | ||
| POU5F1/OCT3/4 (pla) | CAT TCA AAC TGA GGT AAG GG | |
| TAG CGT AAA AGG AGC AAC ATA G | ||
| SOX2 (CDS) | NM_003106.3 | TTC ACA TGT CCC AGC ACT ACC AGA |
| TCA CAT GTG TGA GAG GGG CAG TGT GC | ||
| SOX2 (pla) | TTC ACA TGT CCC AGC ACT ACC AGA | |
| TTT GTT TGA CAG GAG CGA CAA T | ||
| KLF4 (CDS) | NM_004235.4 | ACC CAT CCT TCC TGC CCG ATC AGA |
| TTG GTA ATG GAG CGG CGG GAC TTG | ||
| KLF4 (pla) | CCA CCT CGC CTT ACA CAT GAA GA | |
| TAG CGT AAA AGG AGC AAC ATA G | ||
| LIN28 (CDS) | NM_024674.4 | AGC CAT ATG GTA GCC TCA TGT CCG C |
| TCA ATT CTG TGC CTC CGG GAG CAG GGT AGG | ||
| LIN28 (pla) | AGC CAT ATG GTA GCC TCA TGT CCG C | |
| TAG CGT AAA AGG AGC AAC ATA G | ||
| L-MYC (CDS) |
NM_001033082.2 NM_001033081.2 |
GCG AAC CCA AGA CCC AGG CCT GCT CC |
| CAG GGG GTC TGC TCG CAC CGT GAT G | ||
| L-MYC (pla) | GGC TGA GAA GAG GAT GGC TAC | |
| TTT GTT TGA CAG GAG CGA CAA T | ||
| GAPDH |
NM_002046.4 NM_001256799.1 |
ACC ACA GTC CAT GCC ATC AC |
| TCC ACC ACC CTG TTG CTG TA | ||
| EBNA1 (pla) | ATC AGG GCC AAG ACA TAG AGA TG | |
| GCC AAT GCA ACT TGG ACG TT | ||
| AQP4 |
NM_001650.4 NM_004028.3 |
GCG AGG ACA GCT CCT ATG AT |
| ACT GGT GCC AGC ATG AAT C | ||
| GFAP | NM_002055.4 | CAC CAC GAT GTT CCT CTT GA |
| GTG CAG ACC TTC TCC AAC CT | ||
| GLAST/SLC1A3 |
NM_001166695.1 NM_004172.4 |
ATC CTT GGA TTT ACC CTC CGA |
| CGC CAT TCC TGT GAC AAG AC | ||
| S100B | NM_006272.2 | TCC ACA ACC TCC TGC TCT TT |
| GGA GAC AAG CAC AAG CTG AA | ||
| SLC26A7 |
NM_052832.3 NM_134266.1 |
AGA AGG CGA CTG CCC ATT TT |
| ACT GCC AAC ATT ATC CCA GAC A | ||
| SLC38A1 |
NM_030674.3 NM_001077484.1 |
TGA CAG TGC CCG AGG ATG ATA |
| TGG CTG TTT GTG AGA CTT CTT C | ||
| KROX20 (EGR2) |
NM_000399.3 NM_001136177.1 |
TGG CCG GAG ATG GCA TGA |
| TAG GTG CAG AGA CGG GAG CA | ||
| ISL1 | NM_002202.2 | TCA CGA AGT CGT TCT TGC TG |
| CAT GCT TTG TTA GGG ATG GG | ||
| HOXB3 | NM_002146.4 | CCA GTG CCA CTA GCA ACA G |
| CGT TTG CCT CGA CTC TTT CAT C | ||
| HOXB4 | NM_024015.4 | ACG AGT CAG GGG TCG GAA TA |
| CAT GGA GGG AAC TTG GGG TC | ||
| SPRACL1 |
NM_001128310.1 NM_004684.4 |
GCA CCT GAC AAC ACT GCA ATC |
| TTT CAG CCT TAT GGT GGG AAT C | ||
| NTRK2 |
NM_006180.3 NM_001018064.1 |
TGT TCA GCA CAT CAA GCG ACA |
| GCT CAG GAC AGA GGT TAT AGC AT | ||
| FOXG1 | NM_005249.4 | TGC GCA AAT GCC GCA TAA AT |
| ACA CGG GCA TAT GAC CAC AG | ||
| SIX3 | NM_005413.3 | GTC CAT GGT ATT CCG CTC CC |
| ATG GAG CGG TGG TGA GAA TC | ||
| NKX2.1 |
NM_001079668.2 NM_003317.3 |
GCC ATC TCC CGC TTC ATG |
| CAG CGG GGC CAT GTT CTT G | ||
| RPL13A |
NM_012423.3 NM_001270491.1 |
CCT GGA GGA GAA GAG GAA AGA GA |
| TGA GGA CCT CTG TGT ATT TGT CAA |
CDS; for detection of coding sequence gene expression.
Pla; for detection of plasmid vector-derived expression.
Cell preparation for transplantation
Cells were incubated in TrypLE (Life Technologies), rinsed with DMEM, incubated in DNase/DMEM (50%/50%), rinsed and dissociated with fire polished glass Pasteur pipette into a single cell suspension. Cells were passed through a 30 µm filter (Miltenyi Biotec, Auburn, CA, USA), centrifuged (1000 RPM, 5 min) and resuspended in hibernate media (2.2g/L KCl, 0.9 g/L Glucose, 0.05g/L MgCl2․6H2O, 1.51 g/L NaH2PO4․H2O, 0.89 g/L Na2HPO4․2H2O, 0.2% Lactic Acid, pH7.2 with KOH Pellets, 25.5 g/L Sorbitol) at the appropriate cell concentration and kept on ice for transplantation.
Cell transplantation
All animal procedures were carried out in accordance with the Cedars-Sinai Medical Center Institutional Animal Care and Use Committee under protocol #3133 (Neural Stem Cells for Amyotrophic Lateral Sclerosis) and National Institutes of Health standards of animal care. Athymic nude rats (Hsd:RH-Foxn1rnu; Harlan Laboratories) were transplanted with 2 µl of fNPCs, iNPCsAD or iNPCsSU in 3 distinct sites, 1 mm apart at a concentration of 20,000 cells/µl, 60,000 cells/µl or 100,000 cells/µl. Briefly, rats were anesthetized with isofluorane and transferred to a stereotaxic frame (David Kopf instruments, Tujunga, CA, USA). The 12th rib of the rat was identified and an incision was performed in the skin and muscle to expose the lumbar vertebrae. A hemi-laminectomy was performed on the side of the surgery to expose the spinal cord and dura was cut. Cells were loaded into a 45° beveled glass micropipette connected to a 10 µl Hamilton syringe and a microinjection pump. Cells were then injected directly into the parenchyma (0.8 mm medio-lateral, 1.8 mm dorso-ventral) at a rate of 1 µl/minute.
Tissue collection and histology
Rats were anesthetized and transcardially perfused with 0.9% NaCl and fixed with 4% PFA (EMS 1224SK-SP). Tissues were collected and post-fixed overnight in 4% PFA and transferred into 30% sucrose for 48 hours prior to sectioning (35 µm) on a sliding microtome. Prior to sectioning, the side contralateral to surgery was identified by notching the dorsal horn. Every 1/12th sample through the lumbar spinal cord was immuno-stained with the following antibodies: STEM101, STEM121, STEM123, Nestin, Ki67, and Aquaproin-4 (AQP4) (Table 1) according to standard techniques. Sections were stained with the Alexa-488 or Alexa-594 coupled secondary antibodies (Life Technologies) and nuclei were counterstained with 4', 6-diamidino-2-phenylindole (DAPI, Life Technologies).
Stereology for immunohistological quantifications
Stereological quantifications of STEM 101 and Ki67 were performed using the optical fractionator method from StereoInvestigator software (MBF Biosciences) associated with an Axio Imager M2 microscope (Zeiss) at 60× magnification. Stereological analysis was performed at 1/12th sample interval for STEM101 and Ki67 cell counts and the entire ipsi-lateral side of the spinal cord sections within the grafted area that was traced. The counting parameters were distance between counting frames (500 µm); counting frame size (75 µm × 75 µm); dissector height (24 µm) and the guard zone (2 µm). The range of coefficient of error (CE, Gundersen m=1) and number of markers counted are reported in figures associated with stereological counts. Overall, the lowest number of markers counted and highest CE were observed in the data obtained from iNPCAD cultures and associated with the overall small size and localized nature of the surviving graft. To assess absolute cell migration, the total number of sections (out of 1/12th series) containing STEM101+ cells were counted and multiplied by a factor of 12 (series) and by the section thickness (35 µm).
Statistical analysis
Prism software (GraphPad software, La Jolla, CA) was used for statistical analyses. Immunocyto/histochemical analyses and cell survival quantification were expressed as mean values ± SEM and analyzed by two-tailed t-test or two-way ANOVA with Bonferroni post hoc test. Differences were considered significant when p<0.05.
RESULTS
Neural stem cell spheres are generated from human iPSCs
Skin fibroblasts were reprogrammed from multiple healthy individuals to iPSCs using a non-integrating system based on the oriP/EBNA1 (Epstein-Barr nuclear antigen-1). This episomal plasmid vector system was used to avoid potential deleterious effects of proviral sequences randomly inserted into the genome (Okita et al., 2011a; Sareen et al., 2012; Yu et al., 2009). Multiple clonal iPSC lines were generated showing typical PSC-like morphology with a high nuclear-cytoplasmic ratio (Sareen et al., 2012), alkaline phosphatase activity and surface and nuclear expression of pluripotency markers, SSEA4, TRA-1-81, TRA-1-60, OCT3/4, SOX2, NANOG (Fig. 1A). An iPSC line from one individual (00iCTR-n1) was picked for further in-depth studies and characterized using a battery of pluripotency assays. The iPSC population maintained greater than 80% OCT3/4 and SSEA4 double positive cells (Fig. 1B) and a normal karyotype over many passages (Fig. 1C). PluriTest analysis, a validated open-access bioinformatics assay for assessing pluripotency using transcriptome profiling, showed that the 00iCTR line had a high pluripotency score and low novelty score, similar to other PSC lines and in contrast to differentiated fibroblasts and NPCs (Fig. 1D). Importantly, qRT-PCR and Southern blot analyses confirmed that the 00iCTR line lacked expression of exogenous transgenes, demonstrating that that the oriP/EBNA1 plasmid-based method generated “footprint-free” iPSC lines (Fig. 1E and F).
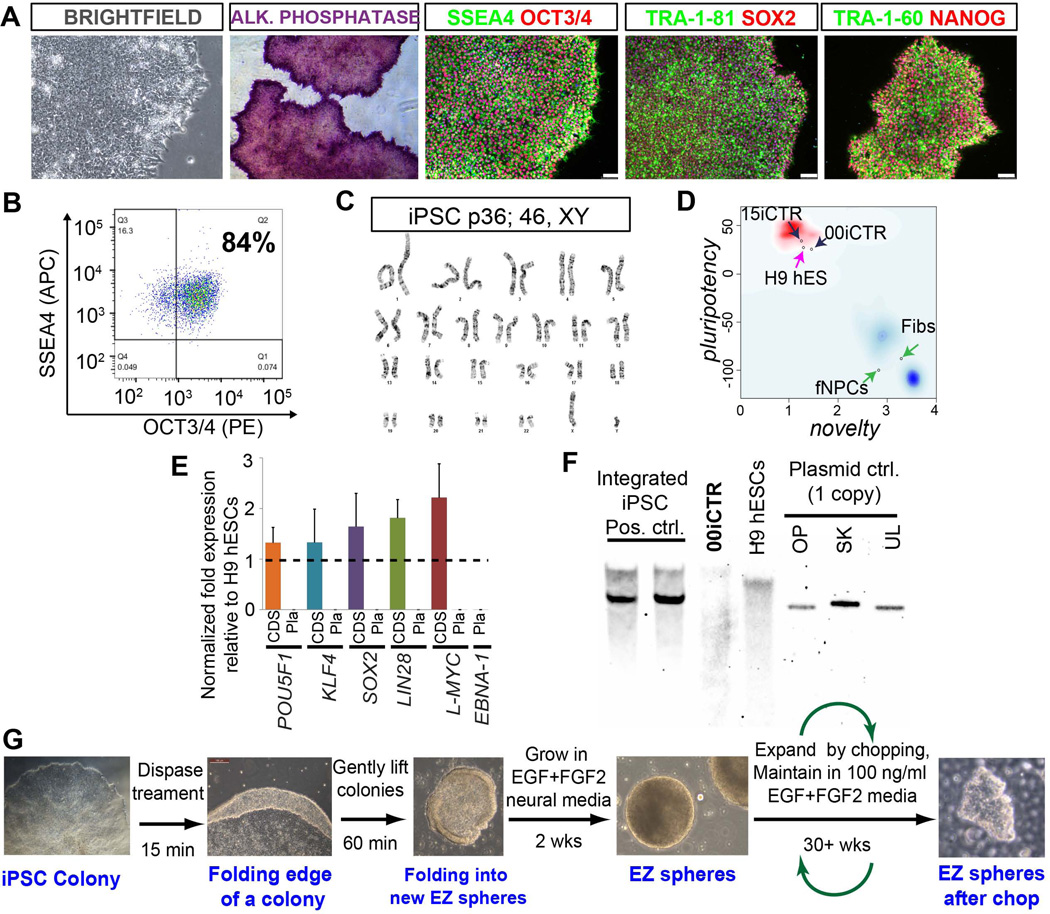
Figure 1. Human iPSC and EZ sphere generation.
(A) Bright-field images of the reprogrammed iPSC colonies from healthy subject fibroblasts maintained on Matrigel/mTeSR1 show high nuclear-to-cytoplasmic ratio, typical of standard pluripotent stem cells (hESCs and hiPSCs). Successfully reprogrammed iPSC lines show positive staining for alkaline phosphatase. Immunostaining shows expression of embryonic and pluripotency stem cell surface antigens, SSEA4, TRA-1-60, TRA-1-81; and nuclear pluripotency markers OCT3/4, SOX2 and NANOG. (B) Flow cytometry analysis scatter plot shows that 00iCTR maintained SSEA4 and OCT3/4 double positive pluripotent cell population > 80% over long-term passaging. (C) The 00iCTR line maintained a normal G-band karyotype. (D) Transciptomics and bioinformatics based PluriTest characterization (Muller et al., 2011) confirmed pluripotency of 00iCTR. Chart combines pluripotency score on y-axis and novelty score on x-axis. The red and blue background hint to the empirical distribution of the pluripotent (red) and non-pluripotent (blue) samples in test data set (Muller et al., 2011). (E) Quantitative RT–PCR analyses of POU5F1 (OCT4), KLF4, SOX2, LIN28, L-MYC, and EBNA-1 expression in 00iCTR iPSCs relative to H9 hESCs. “CDS” indicates that primers designed for the coding sequence measured expression of the total endogenous gene expression only, whereas “Pla” indicates that primers designed for the plasmid transgene expression, including EBNA-1 from plasmid backbone, were not detected. Data are represented as mean ± SEM. (F) Southern blot analysis of genomic DNA from 00iCTR with a plasmid back-bone specific probe common to the reprogramming plasmids used shows lack of exogenous transgene-integration. Negative control: H9 hESC line. Positive controls: Other integrating iPSC lines generated using the episomal plasmid technique showing plasmid-based gene integration. Technical control: A single copy of the plasmid DNA can be detected using this technique. (G) Schematic depicting EZ sphere generation from 00iCTR iPSCs. Representative data and images for iPSC and EZ sphere generation here are depicted from a healthy individual iPSC line (00iCTR) with similar results for other iPSC lines.
We have recently developed an easy ("EZ") and robust method to generate multipotent neural stem cells (NSCs) from human iPSCs without utilizing embryoid body formation, which we termed EZ spheres (Ebert et al., 2013). We used the same approach here where iPSC colonies were lifted from feeder-free Matrigel and cultured in suspension medium containing high concentrations of EGF (100 ng/ml) and FGF2 (100 ng/ml) supplemented with heparin. Spherical aggregates of pre-rosette NSCs formed within 2 weeks and could be expanded as EZ spheres for over 40 weeks in culture (Fig. 1G).
Human iPSC-derived EZ spheres can be differentiated into neural progenitor cells (iNPCs)
We previously published that EZ spheres are a very primitive type of neural stem cell at a stage prior to early neural tube (rosette) formation (Ebert et al., 2013) and that such cells do not engraft well into the spinal cord (Fig. 6 and other unpublished data). In order to generate a cell type more similar to neural progenitor cells isolated from fetal tissues we directed EZ spheres towards a caudalized spinal cord fate by withdrawing EGF and FGF2 and applying retinoic acid for 11 days, a morphogen known to drive hindbrain and spinal cord development (Li et al., 2005; Novitch et al., 2003; Pierani et al., 1999; Sockanathan and Jessell, 1998; Wichterle et al., 2002). Mitogen withdrawal and retinoic acid addition was used with two different approaches shown in Fig. 2A. Either iNPCs were cultured on Matrigel substrate as an adherent monolayer (iNPCsAD) or cultured as aggregates in suspension (iNPCsSU). Following caudalization in retinoic acid, iNPC monolayers and spheres were re-introduced into high EGF and FGF-2 mitogens for subsequent expansion.
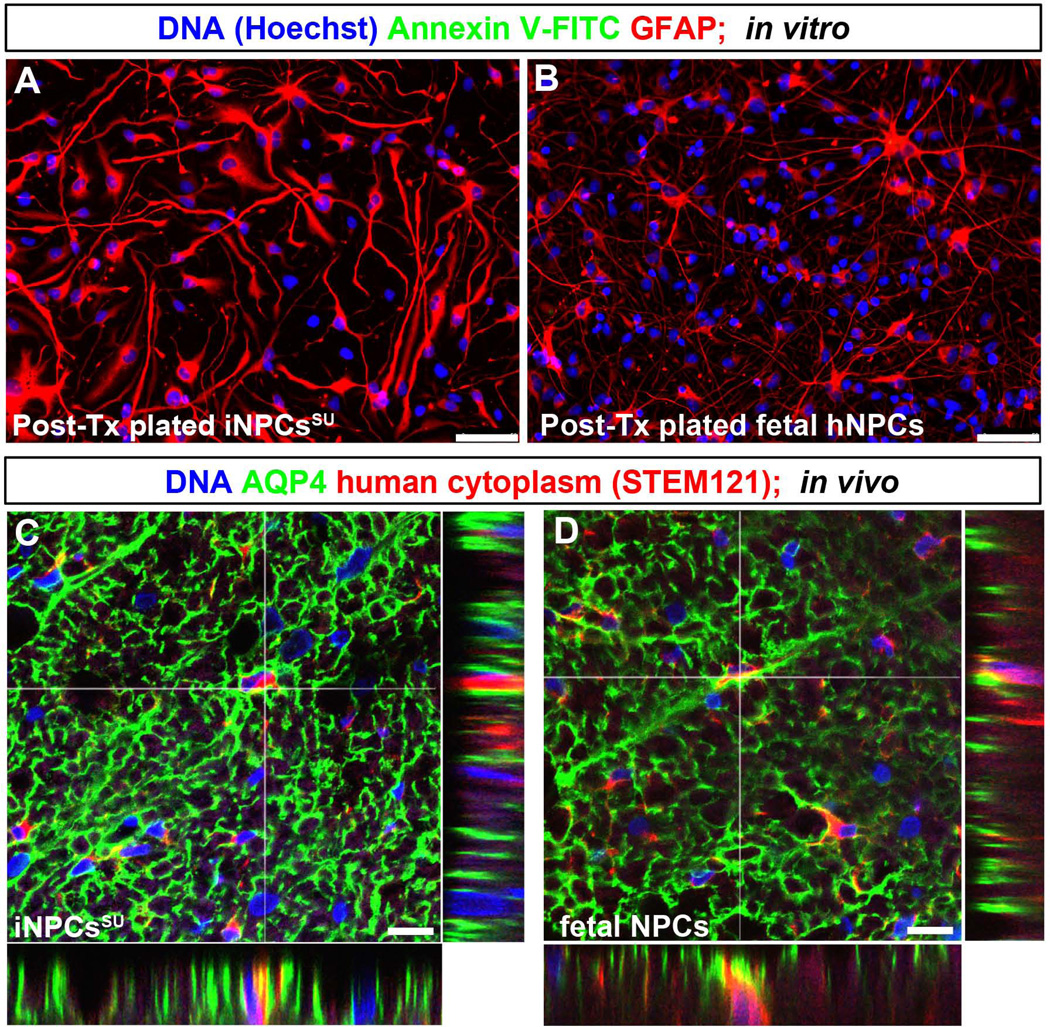
Figure 6. Survival and integration of transplanted human iNPCsSU in the rodent spinal cord.
(A–B) In vitro immunostaining of (A) iNPCsSU and (B) fNPCs from identical batches of cells transplanted in rat spinal cords did not show FITC-conjugated Annexin V staining, nor pyknotic or condensed apoptotic nuclei (Hoechst), suggesting low levels of cell death. Rather the differentiated cells displayed robust astrocyte differentiation by GFAP staining. Scale bars: A, B = 50 µm. (C–D) Representative confocal microscopy images of spinal cord histological sections from athymic nude rats (n=3–4 per group) transplanted with (C) iNPCsSU and (D) fNPCs show adjacent co-staining of human cytoplasm (red, STEM123) and AQP4 (green). White cross-hairs show transplanted human cells that stained for both AQP4 and human cytoplasm-specific STEM121 markers in the same focal plane. Adjacent panels show orthogonal views in yz and xz planes at the location of the white cross-hairs, confirming co-staining in three dimensions. Scale bars = 25 µm.
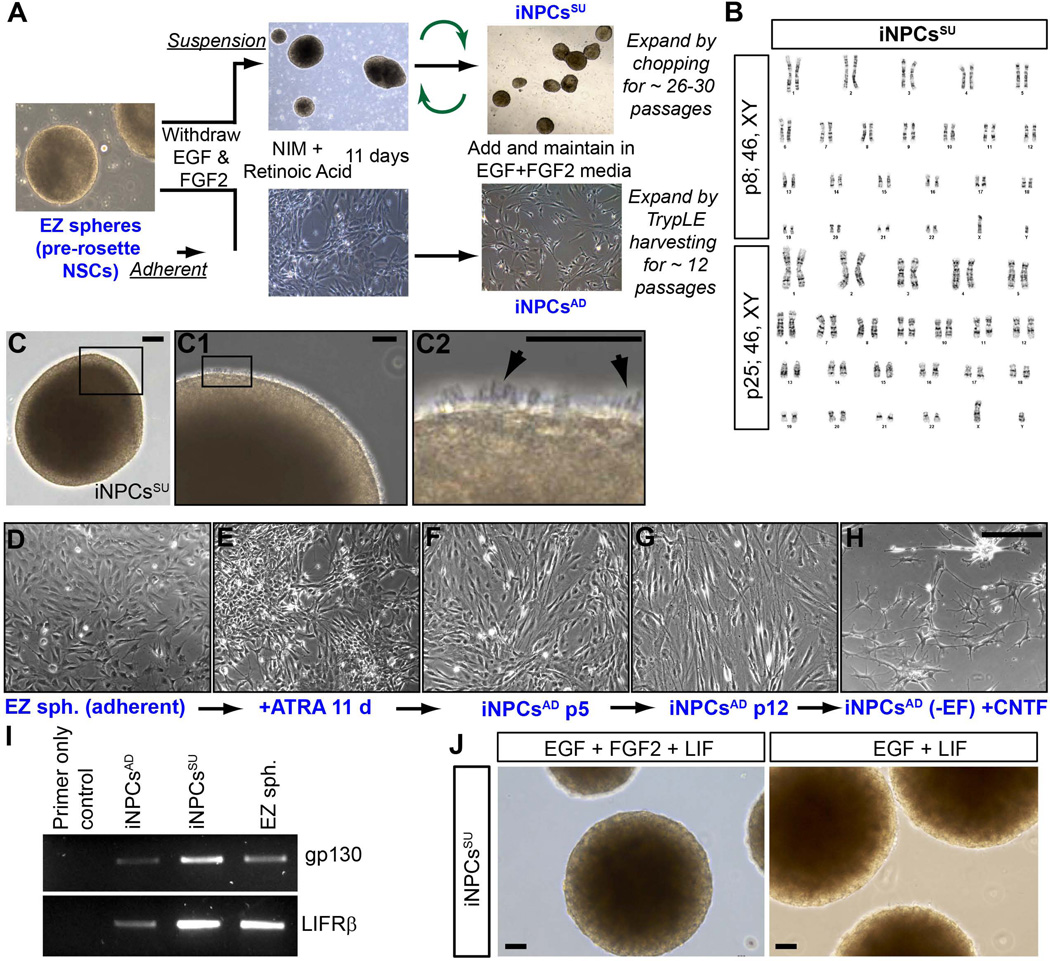
Figure 2. Generation of iPSC-derived neural progenitor cells (iNPCs).
(A) Schematic illustrating iNPC generation from iPSCs via EZ spheres formation using an adherent (iNPCsAD) and a suspension (iNPCsSU) culture protocols. (B) The 00iCTR-derived iNPCsSU line subjected to G-Band karyotyping at multiple passages (p) 8 and 25 stably maintained a normal male 46, XY karyotype. (C) Light microscopy image of a representative iNPCSU sphere in suspension. Black box in (C) shows a region on the sphere magnified in the right adjacent image (C1). Black box in (C1) shows a representative region on the sphere with pseudopodia extensions protruding from an iNPCSU sphere and magnified in the right adjacent image (C2). Black arrows in (C2) point to the pseudopodia extensions of the iNPCsSU. (D–H) Light microscopy images depicting temporal progression of iNPCsAD formation from (D) EZ spheres dissociated and plated on Matrigel at p16, (E) after all-trans retinoic acid (ATRA) addition for 11 days, (F) removal of ATRA, re-introduction of EGF+FGF2, iNPCsAD formation, and culture at p5, and (G) at p12 after which they senesce. (H) Morphology of mature astrocytes generated from iNPCsAD after differentiation following mitogen withdrawal and CNTF addition showing more complex astroglial processes and ramifications. (I and J) Effect of LIF on iNPCsSU, (I) RT-PCR for iNPCsAD, iNPCsSU, and EZ spheres show strong gene expression of LIF receptors, gp130 and LIFRβ in iNPCSU cultures. (J) Light microscopy image of representative iNPCSU spheres after LIF addition to EGF+FGF2 cultures or EGF cultures. Both cultures senesced rapidly after LIF addition and lost the pseudopodia extensions of iNPCSU spheres maintained in EGF+FGF2 media shown in Figure 2C-C2. Scale bars: C = 50 µm, C1–C2 = 10 µm, D–H, and J = 50 µm. Results are representative of at least one of three independent biological samples with similar results.
The iNPCsAD cultures could only be expanded enzymatically for 12–15 weeks, while the iNPCsSU expanded using the chopping technique (similar to fNPCs) maintained proliferative capacity without senescing for 26–30 weeks and a stable normal karyotype at different passages (Figure 2B). The iNPCsSU showed many pseudopodia or cilium-like structures (Figure 2C-C2), as has also been observed with fNPCs (Lobo et al., 2003; Svendsen et al., 1998). The iNPCsAD were healthy following plate-down from EZ spheres (Fig. 2D) and during retinoic acid patterning Fig. 2E). Upon reintroduction of mitogens for expansion, their morphological features on Matrigel substrate appeared similar to adherent neural stem and progenitor cells in other studies (Koch et al., 2009; Tamaki et al., 2002; Taupin, 2006) (Fig. 2F). After over 10 weeks in culture they adopted a more mature cellular morphology with larger cell size and visible cytoskeletal structures (Fig. 2G). Mitogen withdrawal and ciliary neurotropic factor (CNTF) addition could differentiate the iNPCsAD into cell types with a typical mature astrocyte morphology and processes (Fig. 2H), comparable to protoplasmic astrocytes reported to possess dense ramifications and ‘spongiform’ morphology (Bushong et al., 2004).
Ultimately, both cultures displayed senescence following continual passaging suggesting that these cells are more similar to fNPCs (Ostenfeld et al., 2000; Wright et al., 2006) rather than immortal PSCs (Takahashi et al., 2007; Thomson et al., 1998). We have previously shown that early senescence of fNPCs can be avoided by adding leukemia inhibitory factor (LIF) to the cultures (Wright et al., 2003; Wright et al., 2006). In order to establish if iNPCs could also respond to LIF, we first confirmed that both suspension and adherent iNPCs expressed LIF receptors (Fig. 2I). Though the LIF receptor was expressed, it appeared that LIF addition did not change the senescence pattern observed for cultures of either the iNPCsAD or iNPCsSU, ultimately losing the pseudopodia-like structures (Fig. 2J). This interesting difference in response to LIF suggests that while some aspects of the iNPC and fNPC profiles are similar, they are certainly not identical.
Molecular and cellular characterization of iNPCs reveals a predominant astroglial progenitor composition
As the plated iNPCsAD appeared morphologically like astrocytes, we wanted to further establish how similar these cultures were to neural progenitors or astroglia. To do this, we assessed gene expression of multiple astrocyte markers, Aquaporin 4 (AQP4), glial-fibrillary associated protein (GFAP), glial high affinity glutamate transporter (GLAST/SLC1A3 /EAAT-1), S100B, and functional astroglia genes SLC26A7 (Anion exchange transporter/Solute Carrier Family 26, Member 7) and SLC38A1 (Sodium-coupled neutral amino acid transporter 1/Solute Carrier Family 38, Member 1) (Krencik et al., 2011). Analysis by qRT-PCR showed iNPCsAD had increased levels of GFAP mRNA compared to iPSC and EZ spheres, however, the other markers were expressed in similar levels to EZ spheres. Interestingly, expression of AQP4, the primary water conductance transport protein in astrocytes, was not detected in iNPCsAD (Fig. 3A). In contrast, both iNPCsSU and fNPCs showed a clear up-regulation of these astroglia genes when compared to iPSCs and EZ spheres (Fig. 3A).
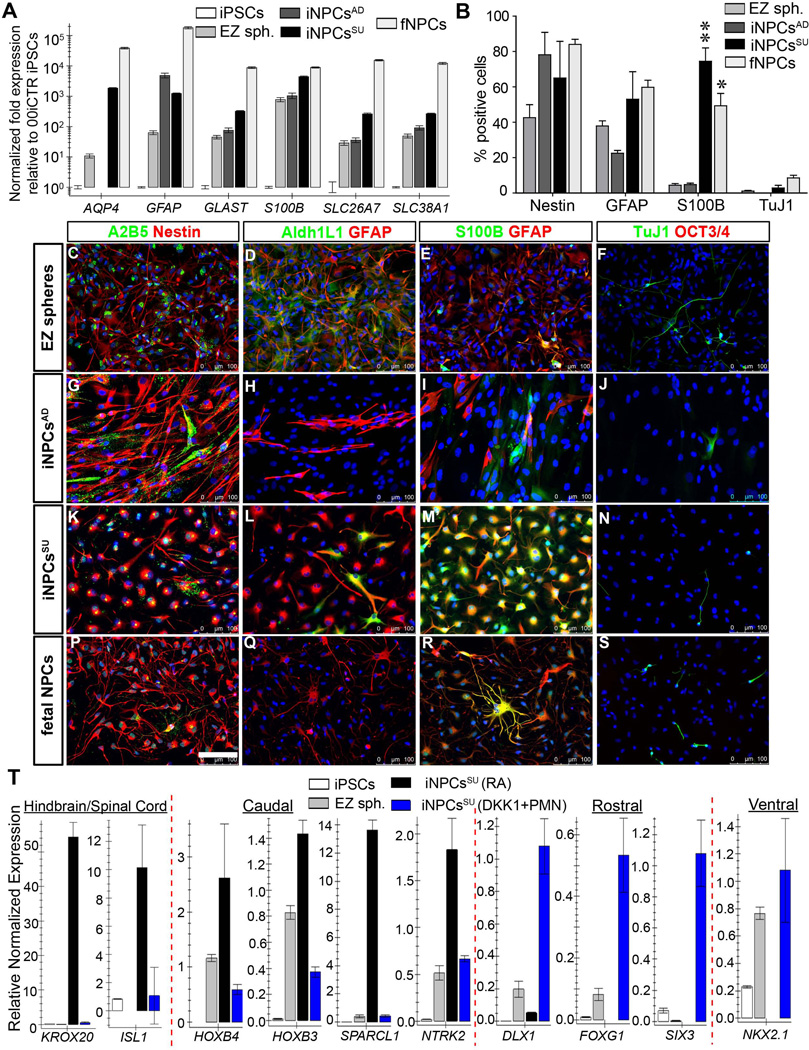
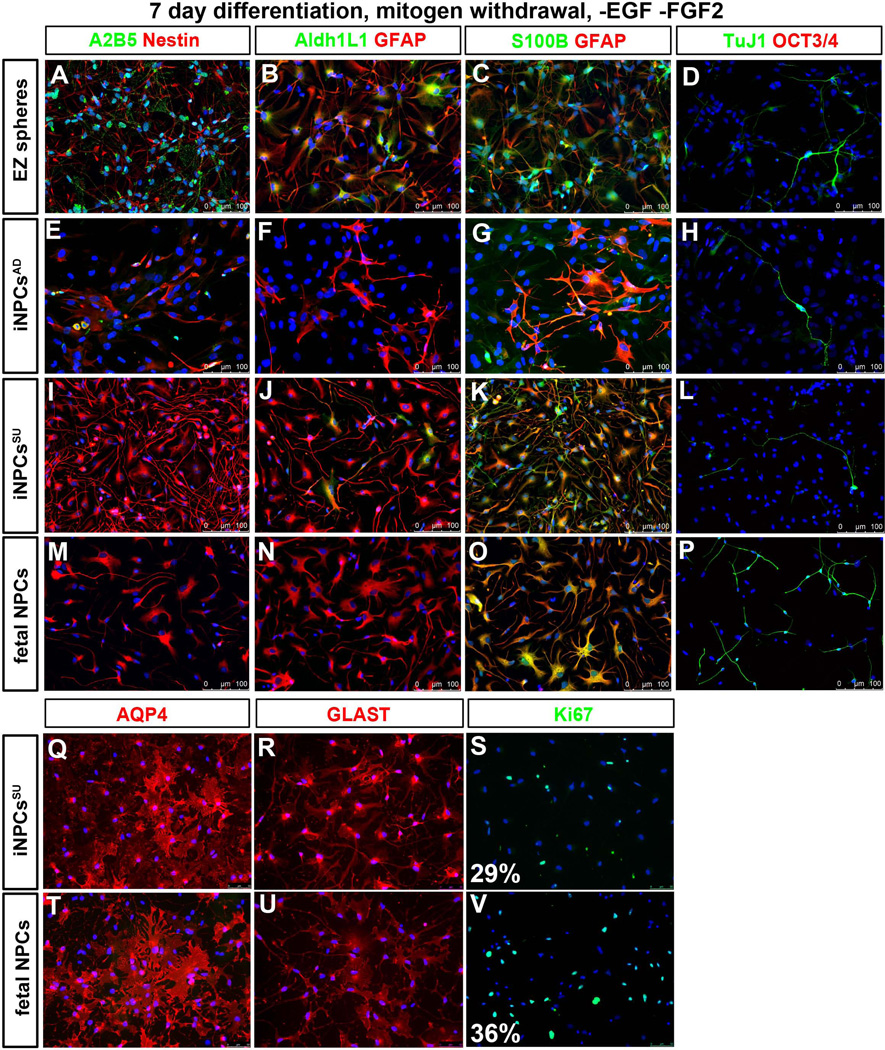
Figure 3. Phenotype characterization of iNPCs.
(A) Quantitative RT-PCR characterization of known astroglia developmental and functional genes, AQP4, GFAPGLAST (SLC1A3/EAAT-1), S100B, SLC26A7 and SLC38A1 showed strong expression of these genes in iNPCsSU as in fNPCs. AQP4, S100B, GLAST, and SLC26A7 were expressed at significantly greater levels (101 – 104 fold) in iNPCsSU vs. iNPCsAD. Error bars are mean ± SEM of three technical replicates. (B) Immunocytochemical staining cell counts of nestin, GFAP, S100B and Tuj1 (βIII-tubulin) positive cells following short-term (24 hours) plate-down of EZ spheres, iNPCsSU, iNPCsAD and fNPCs. There were significantly more S100B+ cells in iNPCsSU and fNPCs vs. iNPCsAD or EZ spheres. ** = p<0.01; * = p<0.05; two-way ANOVA (Bonferroni post-test). Error bars are mean ± SEM of three biological replicates. Representative immunostaining images of (C–F) EZ spheres, (G–J) iNPCsAD, (K–N) iNPCsSU, and (P–S) fNPCs, using protein markers for astroglial progenitors, (C, G, K, P) A2B5 and nestin, (D, H, L, Q) ADH1L1 and GFAP; differentiated astroglia, (E, I, M, R) S100B and GFAP; (F, J, N, S) pan-neurons, Tuj1 and pluripotency, OCT3/4. Scale bars = 100 µm. (T) Determination of regional identity (rostro-caudal, hindbrain/spinal cord, ventral) of iNPCsSU. CNS patterning morphogens promoting caudal/posterior (RA), ventral (PMN), and rostral/anterior (Dkk1) fates generated iNPCsSU with distinct expression of region-specific genes as shown in qRT-PCR expression histogram plots. The iNPCsSU generated with RA patterning, iNPCsSU (RA) (in black bars), expressed hindbrain/spinal cord (KROX20, ISL1) and caudal (HOXB4, HOXB3, SPARCL1, NTRK2) genes. The iNPCsSU (DKK1+PMN) made with DKK1 and PMN (in blue bars), expressed genes of rostral (DLX1, FOXG1, SIX3) and ventral (NKX2.1) identities, and lacked spinal cord or caudal genes. Experiments on all cell types were performed at similar passage numbers (between p8–25) using independent biological samples in at least three replicates. Results are mean ± SEM from representative experiments.
In order to assess the cell types and their morphology present within the NPC cultures, we plated cells onto Matrigel coated coverslips for 24hrs and used immunocytochemistry to detect the neural progenitor and astrocyte markers nestin, GFAP, S100B, Aldh1L1, the pluripotency marker OCT3/4, and the neuronal marker TuJ1. As shown previously by Ebert et al. (2013), cells plated directly from EZ spheres mainly expressed nestin and GFAP, with minimal S100B, TuJ1 or OCT3/4 staining (Fig. 3B–F). Qualitatively there was low expression of A2B5 (Fig. 3C, G, K and P) and Aldh1L1 (Fig. 3D, H, L Q) in iNPCs and fNPCs when compared to the EZ spheres. The iNPCsAD showed an increase in nestin, however, no increase was observed for GFAP and S100B protein levels compared to EZ spheres (Fig. 3E and I), which is in contrast to the increased GFAP mRNA expression (Fig. 3A). Both iNPCsSU and fNPCs showed an increase in nestin (Fig. 3K and P), GFAP and S100B (Fig. 3M and R) expression compared to EZ spheres (Fig. 3B, C and E). In addition to this similar protein expression profile for iNPCsSU and fNPCs, the morphology of astroglia from iNPCsSU and fNPCs resembled each other closer, while the astroglia from iNPCsAD displayed a more spindly morphology. None of the cultures expressed OCT3/4 positive cells (Fig. 3F, J, N and S) and very few stained for TuJ1 positive neurons (Fig. 3B, F, J, N and S). Together these data show that both iNPCsAD and iNPCsSU have a predominately astroglial progenitor composition, though the molecular and morphological astroglial characteristics of iNPCsSU are more akin to fNPCs.
Since our goal was to obtain transplantable iNPCs with a CNS caudalized/posterior regional identity, we determined whether our protocol for generating iNPCsSU led to specification of rostro-caudal, hindbrain/spinal cord, and/or ventral fates by examining distinct expression of region-specific genes, including caudal homeodomain transcription factors, HOXB3 and HOXB4. Differential treatment of iPSC-derived EZ spheres with patterning morphogens promoting different CNS-specific regional fates including, caudal/posterior (retinoic acid), ventral (purmorphamine (PMN); Shh agonist) and rostral/anterior (Dikkopf1 (Dkk1); WNT signaling antagonist) generates different iNPCsSU with distinct expression of region-specific genes confirmed by qRT-PCR gene expression analysis (Fig. 3T). The iNPCsSU produced using retinoic acid, iNPCsSU (RA), significantly up-regulate genes specific to hindbrain/spinal cord (KROX20, ISL1) and caudal (HOXB4, HOXB3, SPARCL1, NTRK2) identities. In contrast, iNPCsSU produced by patterning EZ spheres with DKK1 and PMN, iNPCsSU (DKK1+PMN), up-regulate gene expression specific to rostral (DLX1, FOXG1, SIX3) and ventral (NKX2.1) identities, and lack signatures of spinal cord and caudal gene expression (Fig. 3T).
Differentiation of iNPCs leads to maturation of astroglia
To determine phenotypes upon differentiation, we assessed astroglial progenitor and mature astrocyte marker profile of iPSC-derived neural stem and progenitor cells (EZ spheres and iNPCs) to fNPCs by plating them on laminin/matrigel coated coverslips in mitogen-free media (withdrawal of EGF and FGF2) for 7 days. We observed that astroglial progenitor markers (A2B5, Aldh1L1) were down-regulated in differentiated iNPCsAD (Fig. 4E, F), iNPCsSU (Fig. 4I, J) and fNPCs (Fig. 4M, N) when compared to EZ spheres (Fig. 4A, B). The developmental astrocyte markers, nestin and GFAP, and mature marker, S100B, were strongly expressed in both iNPCsSU and fNPCs (Fig. 4K, O) when compared to EZ spheres and iNPCsAD Fig. 4C and G). The pluripotency marker was undetectable and the TuJ1 pan-neuronal marker was at similar levels in all cell types (Fig. 4D, H, L, and P). Significantly, differentiation led to robust expression of mature and functional astrocyte genes, AQP4 and GLAST (SLC1A3/EAAT-1), which was similar between iNPCsSU and fNPCs (Fig. 4Q, R, T and U). Both iNPCsSU and fNPCs contained proliferating populations of cells, 29±2% and 36±4%, respectively, as determined by Ki67 marker staining and counts (Fig. 4S and V).
Figure 4. Differentiation of iNPCs into astrocytes.
Differentiation phenotypes of EZ spheres, iNPCs and fNPCs were compared by immunostaining for astroglial and cell proliferation markers at similar passage numbers (between p8–25) following dissociation, plate-down and differentiation by mitogen withdrawal (EGF and FGF2) for 7 days. Astroglial progenitor (A2B5, Aldh1L1) markers were down- regulated in differentiated (E–F) iNPCsAD, (I–J) iNPCsSU, and (M–N) fNPCs when compared to (A–B) EZ spheres, while astrocyte developmental (nestin, GFAP) and mature markers (S100B) were robustly and equivalently expressed in (K) iNPCsSU and (O) fNPCs when compared to (G) iNPCsAD and (C) EZ spheres. (D, H, L, and P) OCT3/4 (pluripotency) protein was undetectable and TuJ1 (pan-neurons) protein was at similar levels in all cell types examined. (Q, R, T, and U) Functional astrocyte genes, AQP4 and GLAST were actively expressed at similar levels between iNPCsSU and fNPCs. (S, V) Human iNPCsSU (29%) and fNPCs (36%) maintained cell proliferation rates as determined by Ki67 staining and counting. Representative images are depicted from at least three independent biological replicates with similar results.
Transcriptome profiling shows that suspension culture iNPCsSU and human fNPCs are similar
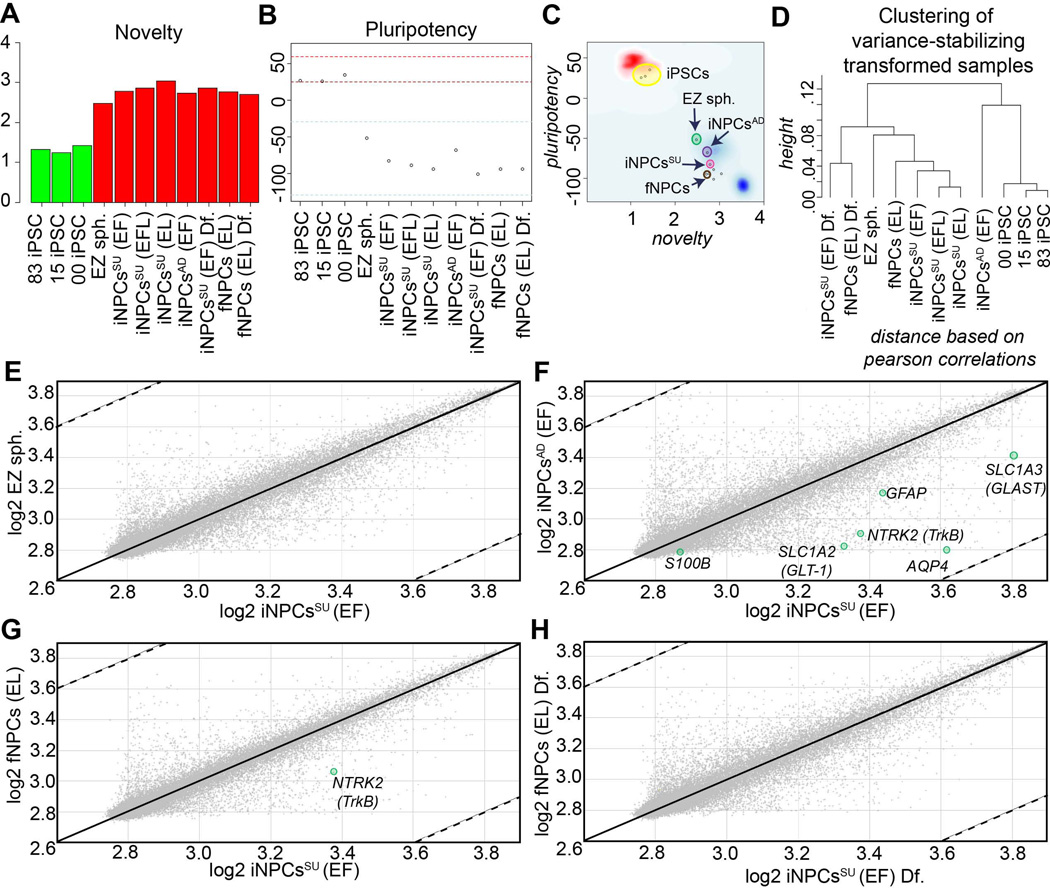
We further investigated the relationship between all the fNPCs and iNPCs (adherent and suspension) by whole transcriptome analysis and bioinformatics testing based on a pluripotency characterization assay, known as PluriTest at www.pluritest.org (Muller et al., 2011). In this test human microarrays are performed and mRNA expression values of all probes including pluripotency-associated genes are scored against samples in the stem cell model matrix, consisting of 264 pluripotent cell lines (223 hESC and 41 human iPSC) and 204 samples derived from somatic cells and tissues. A “novelty” score is generated based on overall gene expression patterns of well-characterized pluripotent samples against the sample of interest. A low novelty score indicates that the test sample can be well reconstructed based on existing data from other well-characterized iPSC and ESC lines. A high novelty score indicates that there are patterns in the tested sample that are not concordant with gene expression patterns of PSC, and instead of more variant cell types, thus making them “novel”. As expected, iPSC lines, including 001iCTR, expressed few novel genes and mostly pluripotent genes leading to a score of < 1.6 in this test (Fig. 5A, Green bars). In contrast, EZ spheres, iNPCsSU and AD, and fNPCs had higher novelty scores (Fig. 5A, Red bars). Another way to plot the gene expression data is to rank pluripotency based on a positive score index. Samples with positive values are more similar to the pluripotent samples in the model matrix than to all other classes of samples in the matrix. The area between the red lines indicates the range that contains ~95 percent of the pluripotent samples tested, while the blue lines indicate those scores that were observed in ~95 percent of the non-pluripotent samples (Fig. 5B). Again iPSCs, including 001iCTR, were between the red dashed lines, whereas all NPCs scored below the blue dashed lines. Interestingly, EZ spheres were found to maintain a pluripotency score (−51.8) higher and closer to PSCs than any non-PSC sample tested thus falling in-between PSCs and more differentiated somatic cells, further supporting their pre-rosette NSC status (Ebert et al, 2013). Both the fNPCs (−93.9) and iNPCsSU (−82.7) had similar scores much lower on this scale as did their differentiated counterparts, 00iNPCsSU Df. and fNPCs Df. (Fig. 5B). The combination of the two scores provides a more comprehensive perspective of this data by combining the pluripotency score on the y-axis and the novelty score on the x-axis. The red and blue background show the empirical distribution of the pluripotent (red) and non-pluripotent (blue) samples in the stem cell model matrix. Using this analysis, it confirmed that the iNPCsSU and fNPCs were similar to each other, while the iNPCsAD were further away (Fig. 5C). The EZ spheres were not pluripotent, but remained closer to the iPSCs (Fig. 5C). This paradigm was also validated by hierarchical clustering analysis that grouped the fNPCs and iNPCsSU together (Fig. 5D).
Figure 5. Transcriptome profiling of iNPCs.
(A) Novelty score graph: A score based in well-characterized pluripotent samples in the stem-cell model matrix. Samples are color coded, green (pluripotent) and red (not pluripotent or closer to somatic). All iPSC-derived neural derivatives, including EZ spheres, iNPCsSU, iNPCsAD, and fNPCs (red bars) were more dissimilar to the pluripotent samples in the model matrix than other pluripotent samples (green bars). EZ spheres were the least novel from all neural derivatives tested. (B) The Pluripotency score giving an indication if a sample contains a pluripotent signature above the red-dotted line shows that all iPSC-derived neural derivatives were not pluripotent. The EZ spheres possessed the most and iNPCsAD second-most pluripotent signature closer to PSCs than iNPCsSU and other NPC derivatives. (C) Chart combines pluripotency score on y-axis and novelty score on x-axis. The red and blue background hint to the empirical distribution of the pluripotent (red) and non-pluripotent (blue) samples The iNPCsSU were closer to fNPCs and other somatic cells (blue cloud) in the PluriTest test dataset. (D) Hierarchical clustering of the samples based on PluriTest Gene Expression profile shows that fNPCs and iNPCsSU clustered close to each other. Whole-transcriptome gene expression scatter plots of (E) EZ spheres vs. iNPCsSU, (F) iNPCsAD vs. iNPCsSU, (G) fNPCs vs. iNPCsAD, and (H) differentiated fNPCs vs. differentiated iNPCsSU showed that even though EZ spheres, iNPCsAD and iNPCsSU were derived from the same individual, the iNPCsSU were more similar to fNPCs derived from a different individual along with their respective differentiate cell types. The gene expression profiles depicted were performed with independent biological samples in duplicate.
Next, we utilized whole-genome transciptomics profiling and scatter plot analysis to compare iNPCsSU with EZ spheres (Fig. 5E), iNPCsAD (Fig. 5F), and fNPCs (Fig. 5G). When the differentiated iNPCsSU were also compared with differentiated fNPCs, their profiles coincided significantly (Fig. 5H). The iNPCsSU had acquired different transcription profiles making them more distant from EZ spheres and iNPCsAD (Fig. 5E and F) and rather much closer in gene expression patterns to fNPCs (Fig. 5G), further supporting the idea that they were being patterned towards a human fNPC phenotype. In this array data the astroglia-related genes, AQP4, S100B, SLC1A2, NTRK2, and SLC1A3, were found to be highly expressed in iNPCsSU compared to iNPCsAD (Fig. 5F), confirming previous observations (Fig. 3A).
Suspension culture iNPCsSU exhibit greater survival in rat spinal cords
Prior to transplantation, the dissociation of NPC sphere cultures may compromise cell viability. To address this, we assessed cell death and integration parameters for identical batches of NPCs transplanted into athymic rat spinal cords. For in vitro evaluation, the NPCs used for transplantation studies were plated and differentiated for 7 days. Immunostaining with FITC conjugated Annexin V, an early cell death marker, did not show any cytotoxicity (Fig. 6A and B), and quantification demonstrated >98% cell viability (data not shown). Further, pyknotic or condensed apoptotic nuclei were undetectable by nuclear Hoechst dye labeling (Fig. 6A and B), a previously validated cell death assay (Sareen et al., 2006). Instead, robust GFAP+astrocyte differentiation and cell survival were observed in both NPC cultures (Fig. 6A and B). In addition, spinal cord sections from animals transplanted with the same batch of NPCs were analyzed using a well-known functional astrocyte marker (AQP4) and human-specific cytoplasmic antibody (STEM121). Careful examination by confocal microscopy confirmed that the transplanted human iNPCsSU and fNPCs can express the functional AQP4 astroglial marker in vivo and can anatomically integrate in the rodent spinal cord also (Fig. 6C and D).
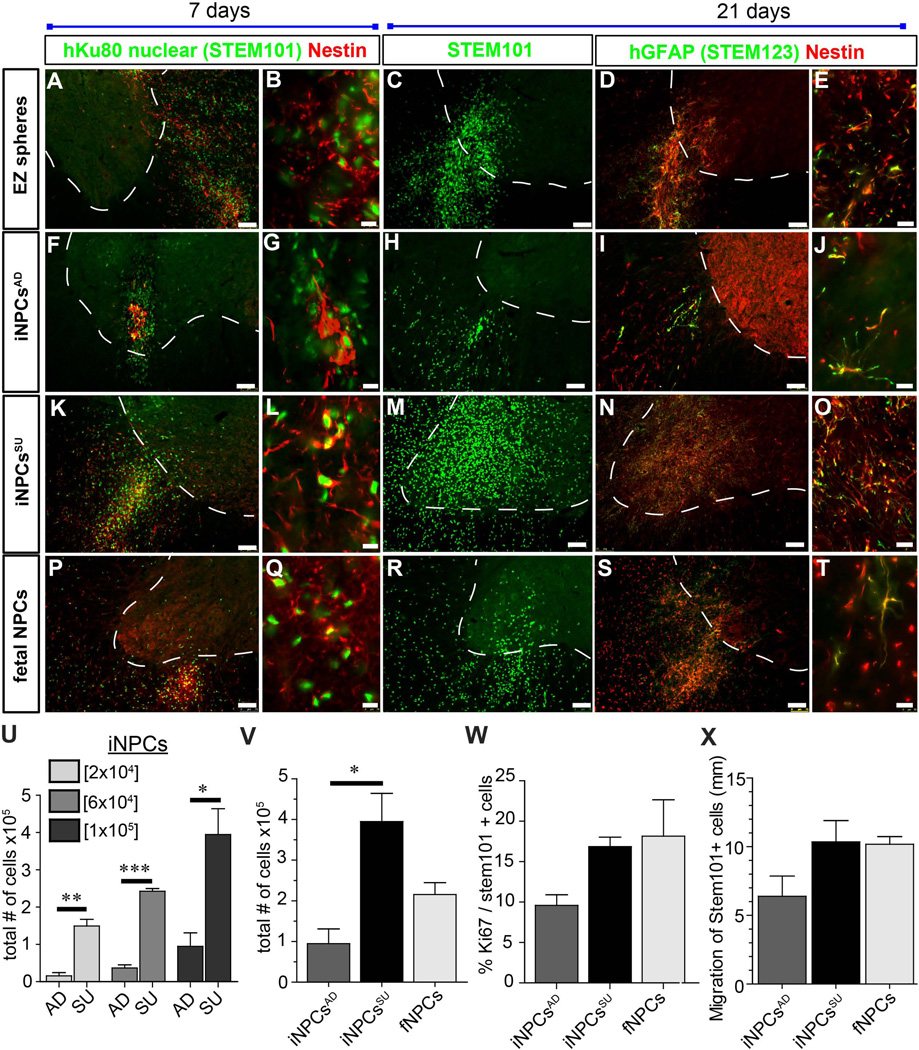
To next establish whether the iNPCs behaved in a similar way to fNPCs in vivo, we transplanted EZ spheres, iNPCsAD, iNPCsSU and fNPCs into the spinal cord of athymic nude rats at three different cell doses (20,000, 60,000 and 100,000) (Fig. 7). At three weeks after transplantation there were clear differences in cell survival and distribution. All graft types labeled with STEM101 for human-specific nuclear staining and STEM123 for human-specific GFAP staining and expressed nestin. EZ spheres (Fig. 7A–D) and iNPCsAD (Fig. 7F–J) showed much poorer graft survival compared to iNPCsSU (Figs. 7K–O). The iNPCsSU also showed robust survival comparable to fNPC grafts (Figs. 7P–T). These observations were confirmed by stereological counts showing that cell survival of iNPCsSU was significantly greater compared to iNPCsAD and similar to (or slightly greater than) that seen with fNPC transplants (Fig. 7U). Indeed, the significantly greater cell survival of iNPCsSU compared to iNPCsAD was maintained at each transplant dose (Fig. 7V). Interestingly, the numbers of proliferating cells detected at the time of sacrifice using the proliferative marker Ki67 was not different between the three groups (Fig. 7W). This suggests that the increased number of cells was a result of increased survival rather than proliferation, though, we cannot rule out early proliferation of the cells prior to sacrifice. There was no significant difference in cell migration as the iNPCsSU appeared similar to fNPCs (Fig. 7X).
Figure 7. Engraftment of neural stem and progenitor cells in the rodent spinal cord.
(A–E) EZ spheres, (F–J) iNPCsAD, (K–O) iNPCsSU, and (P–T) fNPCs were transplanted into the spinal cord of athymic nude rats. Transplanted cells from each cell type survived to varying degrees and were observed at 7 and 21 days post-transplantation by STEM101, nestin and GFAP immunofluorescence staining in histological sections. Representative images with similar results are shown from n=3–4 animals that were transplanted in each group. (U) Graph showing cell concentration dosage (2×104, 6×104 and 1×105) and cell survival of transplanted iNPCsSU vs. iNPCsAD as determined by stereological counting of STEM101+ cells and 1/12th section sampling in 7 day post-transplantation spinal cords. Much greater cell survival was noted at all cell dosages in iNPCsSU. *** = p<0.001, ** = p<0.01; * = p<0.05; unpaired t-test (two-tailed). (V) Greater cell survival (STEM101+) was also observed in iNPCsSU vs. iNPCsAD at 21 day post-transplantation. However, no significant difference in survival was observed between fNPCs vs. iNPCsSU or iNPCsAD by stereological counting and 1/12th section sampling.* = p<0.05; one-way ANOVA (Bonferroni post-test). (U, V) STEM101 counts: Coefficient of error (CE) range was 0.03–0.08 and markers counted were 193–2956. One sample in the 2×104 iNPCsAD group had CE of 0.28 and 13 markers counted. (W) Proliferation of grafted cells (Ki67) was analyzed on tissue from animals having received cells at a concentration of 60,000 cells/µl. No significant differences were observed in cell proliferation by Ki67. CE ranges were 0.1–0.22 (Ki67) and 0.08–0.17 (STEM101). Markers counted 22–83 (Ki67) and 193–585 (STEM101). As expected, in all stereological counts in these data sets, the lowest CE and number of markers counted were observed in the iNPCsAD samples associated with the very small grafts. (X) No significant differences were observed in cell migration between iNPCsAD, iNPCsSU, and fNPCs; one-way ANOVA. *** = p<0.001, ** = p<0.01; * = p<0.05; unpaired t-test (two-tailed). Scale bars: A, C, D, F, H, I, K, M, N, P, R, S = 75 µm and B, E, G, J, L, O, Q, T = 10 µm. (U–X) n=3–4 rats per group were transplanted and analyzed.
DISCUSSION
Neural stem and progenitor cell transplantation holds great potential for providing cell replacement and support to the brain or spinal cord afflicted by neurodegenerative disorders or injury. Fetal-derived neural progenitor cells (fNPCs) grown either as aggregates or monolayer cultures have been around for many years, can be successfully propagated and have advanced into human clinical trials (Neuralstem Inc. and Emory University, 2011; StemCells, 2006; StemCells, 2011; StemCells, 2012; Taupin, 2006). However, they are limited by ethical and cell expansion considerations. By using EZ spheres (Ebert et al., 2013) as the starting source of material we found that both adherent and suspension NPC cultures could be generated from immortal iPSCs that could be proliferated for extensive periods. Therefore, this study provides a much-needed simple protocol for the generation of a stable, easily maintained, highly expandable and bankable population of human iPSC-derived NPCs (iNPCs) and describes their properties in culture and upon transplantation.
To generate iNPCs with ease and in abundance, we avoided embryoid body formation and instead used EZ spheres, a stable source of pre-rosette multipotent stem cell spheres generated from human iPSCs (Ebert et al., 2013). The EZ spheres were directed through caudal patterning using all-trans retinoic acid to produce iNPCs, which were then expandable after addition of EGF and FGF-2 mitogens. The iNPCs were maintained either as adherent cultures (iNPCsAD) that were passaged enzymatically for up to 15 weeks past EZ sphere stage or as suspension cultures (iNPCsSU) that were passaged for up to 30 weeks using a chopping technique we have described previously for fNPCs (Svendsen et al., 1998) and EZ spheres (Ebert et al., 2013). An interesting difference found in culture was related to chromosomal abnormalities and aneuploidies which occur stochastically in ES and iPS cell cultures (trisomy 12, 17, and X) and in various fNPC (trisomy 7 and 19) lines (Sareen et al., 2009), but were not detected in the iNPC line even after extensive culturing. Following reprogramming, none of the episomal plasmid genes were still expressed, which importantly demonstrated that the genome is free from potentially deleterious exogenous transgenes. This fact, along with the stable karyotype overtime, makes these iPSC-derived NPCs reliable for researchers and potentially safe for future clinical trials.
A number of different culture protocols exist for the generation of PSC-derived neural progenitor cells, including a complex four-stage culture system involving RA-mediated induction (Yuan et al., 2013), stromal cell co-culture, adherent culture in EGF and FGF-2 (Koch et al., 2009; Sun et al., 2008), conditioned and serum-free medium induction (Daadi et al., 2008), and neural rosette isolation. However, these methods can be lengthy, laborious, and costly, provide low yields, and none of these studies have reliably shown good cell engraftment capacity in the spinal cord. Retinoic acid has been demonstrated to promote human ESC differentiation into neural stem cells, but efficiency was low and the survival capacity was limited (Baharvand et al., 2007; Bain et al., 1995; Fraichard et al., 1995; Guan et al., 2001; Rohwedel et al., 1999; Strubing et al., 1995; Zhou et al., 2008). In contrast, only a few hESC or hiPSC protocols have been described that produce neural progenitor cells capable of generating astrocytes, and the most thorough method required over 100 days of differentiation (Krencik et al., 2011; Krencik and Zhang, 2011).
Astrocytes comprise the majority of the brain, play a prominent role in protection through glutamate update and are known to be defective in some neurological disorders, like ALS. Providing a population of cells that can easily generate astrocytes would facilitate both researchers interested in further understanding the role of astrocytes and clinicians wanting to assess their benefits in trials. Here we investigated human induced pluripotent stem cells (iPSCs) as a promising alternative to provide NPCs that would be abundantly available and then go on to produce astrocytes following transplantation. Upon differentiation in culture, both iNPCsAD and iNPCsSU showed a propensity towards an astroglial lineage, with iNPCsSU being more propitious and resembling a differentiation pattern similar to what was previously reported for fNPCs. In addition, iNPCsSU could be regionalized to caudal spinal cord fate with retinoic acid and also shared similar transcriptomes and gene expression profiles with fNPCs. The propensity towards an astroglial lineage makes these iNPCs a promising choice for transplantation trials needing astrocytes.
The transplantation of neural progenitors that can mature into astrocytes in the spinal cord is being pursued as a novel treatment for ALS where the astrocytes may protect dying motor neurons (Lepore et al., 2011; Neuralstem Inc. and Emory University, 2011; Suzuki et al., 2007; Suzuki and Svendsen, 2008). Here we show that iNPCs survived in the spinal cord of athymic nude rats, with expression of mainly nestin after 1 week and GFAP after 3 weeks. These results show that grafted iNPCs survive well in the spinal cord and have the potential to differentiate into astrocytes. However, 3 weeks is likely insufficient for complete differentiation, as we have previously shown with fNPCs it may take up to 3 months to completely differentiate into GFAP-positive astrocytes in large numbers (Ostenfeld et al., 2000). Interestingly, we found that the iNPCsSU and fNPCs grown as free-floating spheres survive and integrate better following transplantation than the iNPCsAD, grown as a monolayer, suggesting that maintaining a three-dimensional cellular aggregate niche may provide better culture conditions for long-term survival and cell engraftment in the host. Importantly, both human iNPCsSU and fNPCs also express GFAP and another putative astroglial marker Aquaporin 4 (a water channel protein) in vivo post-transplantation, suggesting that they mature to astroglia and anatomically integrate in the rodent spinal cord. In contrast, three-dimensional EZ sphere suspension cultures did not survive well in the spinal cord suggesting that differentiation towards a neural progenitor phenotype is required to optimize transplant survival. Overall these data suggest iNPCs may be an ideal candidate for transplantation, in particular for diseases such as ALS where cell therapy is being actively pursued (Gowing and Svendsen, 2011).
Clearly we were concerned with possible tumor formation from remaining pluripotent cells within the iNPC cultures as this has been an going concern for the field (Blum and Benvenisty, 2009; Bonnamain et al., 2012; Ghosh et al., 2011; Kawai et al., 2010; Zhang et al., 2012; Zhang et al., 2011). However, in no case following iNPC transplantation did we find any histopathological evidence of increased cell proliferation and tumor forming capacities. One possible reason for this is because prior to transplantation the pluripotent cells were first converted to EZ spheres and cultured for greater than 10 weeks and then subsequently driven to iNPC cultures for a further 10 weeks. However, three weeks is certainly not long enough to fully assess potential tumor development, and hence long-term testing of iNPC safety profile is still required.
In summary, we demonstrated that human iPSCs can be used to efficiently derive NPCs that are easy to culture and bank, have an astroglial differentiation propensity and survive transplantation. These characteristics, along with their transcriptome profiles, pluripotency and novelty scores, are all very similar to the human fetal-derived NPCs that our lab has extensively characterized (Ostenfeld et al., 2000; Svendsen et al., 1998; Wright et al., 2003; Wright et al., 2006). In addition to avoiding the ethical issues of using human fetal tissues, iPSC-derived NPCs permit the future possibility of autologous transplantation thus avoiding complex immune suppression problems that currently plague neural transplantation therapies. As such, human iPSC-derived NPCs are a novel and promising resource for cell-based therapies aiming to replace cells or to modulate the environment for host neuron protection or regeneration in injury and disease.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Soshana Svendsen for critical review and editing of the manuscript.
FUNDING: This work was supported by Cedars-Sinai Institutional startup funds (CNS), California Institute for Regenerative Medicine grants, RT-02040 (CNS, DS) and DR2A-05320 (CNS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
COMPETING INTERESTS: The authors have declared that no competing interests exist.
AUTHOR CONTRIBUTIONS: Conceived and designed the experiments: DS, GG, CNS. Performed the experiments: DS, GG, AS, KS, RP, PA, JL, LO, LG. Analyzed the data: DS, GG, CNS. Contributed reagents/materials/analysis tools: DS, GG, CNS. Wrote the paper: DS, GG, CNS.
REFERENCES
- Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, Schaar BT, Svendsen CN, Bliss TM, Steinberg GK. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, Sugiura M, Ideno H, Shimada A, Nifuji A, Abe M. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- Azienda Ospedaliera Santa Maria TI, Azienda Ospedaliero Universitaria Maggiore della Carita, Università di Padova I. ClinicalTrials gov [Internet] Bethesda (MD): National Library of Medicine; 2012. [cited 2012 July 11]. Human Neural Stem Cell Transplantation in Amyotrophic Lateral Sclerosis (ALS) (hNSCALS) (US) 2000- Available from: http://www.clinicaltrials.gov/ct2/show/NCT01640067 NLM Identifier: NCT01640067. [Google Scholar]
- Baharvand H, Mehrjardi NZ, Hatami M, Kiani S, Rao M, Haghighi MM. Neural differentiation from human embryonic stem cells in a defined adherent culture condition. Int J Dev Biol. 2007;51:371–378. doi: 10.1387/ijdb.72280hb. [DOI] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Blum B, Benvenisty N. The tumorigenicity of diploid and aneuploid human pluripotent stem cells. Cell Cycle. 2009;8:3822–3830. doi: 10.4161/cc.8.23.10067. [DOI] [PubMed] [Google Scholar]
- Bonnamain V, Neveu I, Naveilhan P. Neural stem/progenitor cells as a promising candidate for regenerative therapy of the central nervous system. Front Cell Neurosci. 2012;6:17-. doi: 10.3389/fncel.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzo JA, Ferry CH, Matsubara T, Kim JH, Gonzalez FJ. Suppression of hepatocyte proliferation by hepatocyte nuclear factor 4alpha in adult mice. J Biol Chem. 2012;287:7345–7356. doi: 10.1074/jbc.M111.334599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Ellisman MH. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci. 2004;22:73–86. doi: 10.1016/j.ijdevneu.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Chetty S, Pagliuca FW, Honore C, Kweudjeu A, Rezania A, Melton DA. A simple tool to improve pluripotent stem cell differentiation. Nat Methods. 2013;10:553–556. doi: 10.1038/nmeth.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nat Rev Neurosci. 2010;11:176–187. doi: 10.1038/nrn2761. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daadi MM, Maag AL, Steinberg GK. Adherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke model. PLoS One. 2008;3:e1644-. doi: 10.1371/journal.pone.0001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Beres AJ, Barber AE, Svendsen CN. Human neural progenitor cells over-expressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson's disease. Exp Neurol. 2008;209:213–223. doi: 10.1016/j.expneurol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Shelley BC, Hurley AM, Onorati M, Castiglioni V, Patitucci TN, Svendsen SP, Mattis VB, McGivern JV, Schwab AJ, Sareen D, Kim HW, Cattaneo E, Svendsen CN. EZ spheres: a stable and expandable culture system for the generation of pre-rosette multipotent stem cells from human ESCs and iPSCs. Stem Cell Res. 2013;10:417–427. doi: 10.1016/j.scr.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraichard A, Chassande O, Bilbaut G, Dehay C, Savatier P, Samarut J. In vitro differentiation of embryonic stem cells into glial cells and functional neurons. J Cell Sci. 1995;108(Pt 10):3181–3188. doi: 10.1242/jcs.108.10.3181. [DOI] [PubMed] [Google Scholar]
- Ghosh Z, Huang M, Hu S, Wilson KD, Dey D, Wu JC. Dissecting the oncogenic and tumorigenic potential of differentiated human induced pluripotent stem cells and human embryonic stem cells. Cancer Res. 2011;71:5030–5039. doi: 10.1158/0008-5472.CAN-10-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman EL. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells. 2012;30:1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- Gowing G, Shelley B, Staggenborg K, Hurley A, Avalos P, Victoroff J, Latter J, Garcia L, Svendsen CN. Glial cell line-derived neurotrophic factor-secreting human neural progenitors show long-term survival, maturation into astrocytes, and no tumor formation following transplantation into the spinal cord of immunocompromised rats. Neuroreport. 2013 doi: 10.1097/WNR.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing G, Svendsen CN. Stem cell transplantation for motor neuron disease: current approaches and future perspectives. Neurotherapeutics. 2011;8:591–606. doi: 10.1007/s13311-011-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K, Chang H, Rolletschek A, Wobus AM. Embryonic stem cell-derived neurogenesis. Retinoic acid induction and lineage selection of neuronal cells. Cell Tissue Res. 2001;305:171–176. doi: 10.1007/s004410100416. [DOI] [PubMed] [Google Scholar]
- Guzman R, Uchida N, Bliss TM, He D, Christopherson KK, Stellwagen D, Capela A, Greve J, Malenka RC, Moseley ME, Palmer TD, Steinberg GK. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci U S A. 2007;104:10211–10216. doi: 10.1073/pnas.0608519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, Rao M, Eagle A, Kammesheidt A, Christensen A, Mendell JR, Burghes AH, Kaspar BK. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29:824–828. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Chen C, Wang R, Chechneva OV, Chung SH, Rao MS, Pleasure DE, Liu Y, Zhang Q, Deng W. hESC-derived Olig2+ progenitors generate a subtype of astroglia with protective effects against ischaemic brain injury. Nat Commun. 2013;4:2196-. doi: 10.1038/ncomms3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juopperi TA, Kim WR, Chiang CH, Yu H, Margolis RL, Ross CA, Ming GL, Song H. Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington's disease patient cells. Mol Brain. 2012;5:17-. doi: 10.1186/1756-6606-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Yamanaka S. To be immunogenic, or not to be: that's the iPSC question. Cell Stem Cell. 2013;12:385–386. doi: 10.1016/j.stem.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Kawai H, Yamashita T, Ohta Y, Deguchi K, Nagotani S, Zhang X, Ikeda Y, Matsuura T, Abe K. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J Cereb Blood Flow Metab. 2010;30:1487–1493. doi: 10.1038/jcbfm.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SM, Behrstock S, McHugh J, Hoffmann K, Wallace K, Suzuki M, Aebischer P, Svendsen CN. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Okada Y, Itakura G, Iwai H, Nishimura S, Yasuda A, Nori S, Hikishima K, Konomi T, Fujiyoshi K, Tsuji O, Toyama Y, Yamanaka S, Nakamura M, Okano H. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012;7:e52787-. doi: 10.1371/journal.pone.0052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P, Opitz T, Steinbeck JA, Ladewig J, Brustle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci U S A. 2009;106:3225–3230. doi: 10.1073/pnas.0808387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Zhang SC. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat Protoc. 2011;6:1710–1717. doi: 10.1038/nprot.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Ramirez CN, Kim H, Zeltner N, Liu B, Radu C, Bhinder B, Kim YJ, Choi IY, Mukherjee-Clavin B, Djaballah H, Studer L. Large-scale screening using familial dysautonomia induced pluripotent stem cells identifies compounds that rescue IKBKAP expression. Nat Biotechnol. 2012;30:1244–1248. doi: 10.1038/nbt.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- Lepore AC, O'Donnell J, Kim AS, Williams T, Tuteja A, Rao MS, Kelley LL, Campanelli JT, Maragakis NJ. Human glial-restricted progenitor transplantation into cervical spinal cord of the SOD1 mouse model of ALS. PLoS One. 2011;6:e25968. doi: 10.1371/journal.pone.0025968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Liao CW, Fan CK, Kao TC, Ji DD, Su KE, Lin YH, Cho WL. Brain injury-associated biomarkers of TGF-beta1, S100B, GFAP, NF-L, tTG, AbetaPP, and tau were concomitantly enhanced and the UPS was impaired during acute brain injury caused by Toxocara canis in mice. BMC Infect Dis. 2008;8:84-. doi: 10.1186/1471-2334-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Chen S, Li X, Qin L, Huang K, Wang L, Huang W, Li S, Jia B, Zhong M, Pan G, Cai J, Pei D. Low immunogenicity of neural progenitor cells differentiated from induced pluripotent stem cells derived from less immunogenic somatic cells. PLoS One. 2013;8:e69617-. doi: 10.1371/journal.pone.0069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lobo MV, Alonso FJ, Redondo C, Lopez-Toledano MA, Caso E, Herranz AS, Paino CL, Reimers D, Bazan E. Cellular characterization of epidermal growth factor-expanded free-floating neurospheres. J Histochem Cytochem. 2003;51:89–103. doi: 10.1177/002215540305100111. [DOI] [PubMed] [Google Scholar]
- Luong MX, Auerbach J, Crook JM, Daheron L, Hei D, Lomax G, Loring JF, Ludwig T, Schlaeger TM, Smith KP, Stacey G, Xu RH, Zeng F. A call for standardized naming and reporting of human ESC and iPSC lines. Cell Stem Cell. 2011;8:357–359. doi: 10.1016/j.stem.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Mattis VB, Wakeman DR, Tom C, Dodiya HB, Yeung SY, Tran AH, Bernau K, Ornelas L, Sahabian A, Reidling J, Sareen D, Thompson LM, Kordower JH, Svendsen CN. Neonatal immune-tolerance in mice does not prevent xenograft rejection. Exp Neurol. 2014 doi: 10.1016/j.expneurol.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messam CA, Hou J, Major EO. Coexpression of nestin in neural and glial cells in the developing human CNS defined by a human-specific anti-nestin antibody. Exp Neurol. 2000;161:585–596. doi: 10.1006/exnr.1999.7319. [DOI] [PubMed] [Google Scholar]
- Muller FJ, Schuldt BM, Williams R, Mason D, Altun G, Papapetrou EP, Danner S, Goldmann JE, Herbst A, Schmidt NO, Aldenhoff JB, Laurent LC, Loring JF. A bioinformatic assay for pluripotency in human cells. Nat Methods. 2011;8:315–317. doi: 10.1038/nmeth.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuralstem Inc., Emory University. ClinicalTrials gov [Internet] Bethesda (MD): National Library of Medicine (US); 2011. [cited 2013 May 8]. Human Spinal Cord Derived Neural Stem Cell Transplantation for the Treatment of Amyotrophic Lateral Sclerosis (ALS) 2000- Available from: http://www.clinicaltrials.gov/ct2/show/NCT01348451 NLM Identifier: NCT01348451. [Google Scholar]
- Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, Mulcrone PL, McHugh J, Svendsen CN, Mitchell GS. Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 2013;187:535–542. doi: 10.1164/rccm.201206-1072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, Ikeda E, Yamanaka S, Miura K. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112:523–533. doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011a;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011b;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Okita K, Nagata N, Yamanaka S. Immunogenicity of induced pluripotent stem cells. Circ Res. 2011c;109:720–721. doi: 10.1161/RES.0b013e318232e187. [DOI] [PubMed] [Google Scholar]
- Ostenfeld T, Caldwell MA, Prowse KR, Linskens MH, Jauniaux E, Svendsen CN. Human neural precursor cells express low levels of telomerase in vitro and show diminishing cell proliferation with extensive axonal outgrowth following transplantation. Exp Neurol. 2000;164:215–226. doi: 10.1006/exnr.2000.7427. [DOI] [PubMed] [Google Scholar]
- Pierani A, Brenner-Morton S, Chiang C, Jessell TM. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell. 1999;97:903–915. doi: 10.1016/s0092-8674(00)80802-8. [DOI] [PubMed] [Google Scholar]
- Piltti KM, Salazar DL, Uchida N, Cummings BJ, Anderson AJ. Safety of epicenter versus intact parenchyma as a transplantation site for human neural stem cells for spinal cord injury therapy. Stem Cells Transl Med. 2013;2:204–216. doi: 10.5966/sctm.2012-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ReNeuron Limited. ClinicalTrials gov [Internet] Bethesda (MD): National Library of Medicine (US); 2010. [cited 2013 March 27]. Pilot Investigation of Stem Cells in Stroke (PISCES) 2000- Available from: http://www.clinicaltrials.gov/ct2/show/NCT01151124 NLM Identifier: NCT01151124. [Google Scholar]
- Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013;14:248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwedel J, Guan K, Wobus AM. Induction of cellular differentiation by retinoic acid in vitro. Cells Tissues Organs. 1999;165:190–202. doi: 10.1159/000016699. [DOI] [PubMed] [Google Scholar]
- Salazar DL, Uchida N, Hamers FP, Cummings BJ, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS One. 2010;5:e12272-. doi: 10.1371/journal.pone.0012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen D, Ebert AD, Heins BM, McGivern JV, Ornelas L, Svendsen CN. Inhibition of apoptosis blocks human motor neuron cell death in a stem cell model of spinal muscular atrophy. PLoS One. 2012;7:e39113-. doi: 10.1371/journal.pone.0039113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen D, McMillan E, Ebert AD, Shelley BC, Johnson JA, Meisner LF, Svendsen CN. Chromosome 7 and 19 trisomy in cultured human neural progenitor cells. PLoS One. 2009;4:e7630-. doi: 10.1371/journal.pone.0007630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen D, O'Rourke JG, Meera P, Muhammad AK, Grant S, Simpkinson M, Bell S, Carmona S, Ornelas L, Sahabian A, Gendron T, Petrucelli L, Baughn M, Ravits J, Harms MB, Rigo F, Bennett CF, Otis TS, Svendsen CN, Baloh RH. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med. 2013;5:208ra149-. doi: 10.1126/scitranslmed.3007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen D, van Ginkel PR, Takach JC, Mohiuddin A, Darjatmoko SR, Albert DM, Polans AS. Mitochondria as the primary target of resveratrol-induced apoptosis in human retinoblastoma cells. Invest Ophthalmol Vis Sci. 2006;47:3708–3716. doi: 10.1167/iovs.06-0119. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Smith SB, Brodjian S, Desai S, Sarthy V. Glial fibrillary acidic protein (GFAP) is synthesized in the early stages of the photoreceptor cell degeneration of the mivit/mivit (vitiligo) mouse. Exp Eye Res. 1997;64:645–650. doi: 10.1006/exer.1996.0249. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Jessell TM. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- StemCells I. linicalTrials gov [Internet] Bethesda (MD): National Library of Medicine (US); 2006. [cited 2010 November 5]. Study of HuCNS-SC Cells in Patients With Infantile or Late Infantile Neuronal Ceroid Lipofuscinosis (NCL) 2000- Available from: http://www.clinicaltrials.gov/ct2/show/NCT00337636 NLM Identifier: NCT00337636. [Google Scholar]
- StemCells I. ClinicalTrials gov [Internet] Bethesda (MD): National Library of Medicine (US); 2011. [cited 2012 November 7]. Long-Term Follow-Up Study of Human Stem Cells Transplanted in Subjects With Connatal Pelizaeus-Merzbacher Disease (PMD) 2000- Available from: http://www.clinicaltrials.gov/ct2/show/NCT01391637 NLM Identifier: NCT01391637. [Google Scholar]
- StemCells I. ClinicalTrials gov [Internet] Bethesda (MD): National Library of Medicine (US); 2012. [cited 2012 November]. Long-Term Follow-Up of Transplanted Human Central Nervous System Stem Cells (HuCNS-SC) in Spinal Cord Trauma Subjects. 2000-Available from: http://www.clinicaltrials.gov/ct2/show/NCT01725880 NLM Identifier: NCT01725880. [Google Scholar]
- Strubing C, Ahnert-Hilger G, Shan J, Wiedenmann B, Hescheler J, Wobus AM. Differentiation of pluripotent embryonic stem cells into the neuronal lineage in vitro gives rise to mature inhibitory and excitatory neurons. Mech Dev. 1995;53:275–287. doi: 10.1016/0925-4773(95)00446-8. [DOI] [PubMed] [Google Scholar]
- Sun Y, Pollard S, Conti L, Toselli M, Biella G, Parkin G, Willatt L, Falk A, Cattaneo E, Smith A. Long-term tripotent differentiation capacity of human neural stem (NS) cells in adherent culture. Mol Cell Neurosci. 2008;38:245–258. doi: 10.1016/j.mcn.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One. 2007;2:e689-. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Svendsen CN. Combining growth factor and stem cell therapy for amyotrophic lateral sclerosis. Trends Neurosci. 2008;31:192–198. doi: 10.1016/j.tins.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, Caldwell MA, Shen J, ter Borg MG, Rosser AE, Tyers P, Karmiol S, Dunnett SB. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp Neurol. 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, Caldwell MA. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tamaki S, Eckert K, He D, Sutton R, Doshe M, Jain G, Tushinski R, Reitsma M, Harris B, Tsukamoto A, Gage F, Weissman I, Uchida N. Engraftment of sorted/expanded human central nervous system stem cells from fetal brain. J Neurosci Res. 2002;69:976–986. doi: 10.1002/jnr.10412. [DOI] [PubMed] [Google Scholar]
- Taupin P. HuCNS-SC (StemCells) Curr Opin Mol Ther. 2006;8:156–163. [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Uchida N, Chen K, Dohse M, Hansen KD, Dean J, Buser JR, Riddle A, Beardsley DJ, Wan Y, Gong X, Nguyen T, Cummings BJ, Anderson AJ, Tamaki SJ, Tsukamoto A, Weissman IL, Matsumoto SG, Sherman LS, Kroenke CD, Back SA. Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci Transl Med. 2012;4:155ra136. doi: 10.1126/scitranslmed.3004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Girman S, Lu B, Bischoff N, Holmes T, Shearer R, Wright LS, Svendsen CN, Gamm DM, Lund RD. Long-term vision rescue by human neural progenitors in a rat model of photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2008;49:3201–3206. doi: 10.1167/iovs.08-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, Roy NS, Goldman SA. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- Wright LS, Li J, Caldwell MA, Wallace K, Johnson JA, Svendsen CN. Gene expression in human neural stem cells: effects of leukemia inhibitory factor. J Neurochem. 2003;86:179–195. doi: 10.1046/j.1471-4159.2003.01826.x. [DOI] [PubMed] [Google Scholar]
- Wright LS, Prowse KR, Wallace K, Linskens MH, Svendsen CN. Human progenitor cells isolated from the developing cortex undergo decreased neurogenesis and eventual senescence following expansion in vitro. Exp Cell Res. 2006;312:2107–2120. doi: 10.1016/j.yexcr.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10:678–684. doi: 10.1016/j.stem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yuan T, Liao W, Feng NH, Lou YL, Niu X, Zhang AJ, Wang Y, Deng ZF. Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurological function in a rat model of middle cerebral artery occlusion. Stem Cell Res Ther. 2013;4:73. doi: 10.1186/scrt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Shang B, Yang P, Cao Z, Pan Y, Zhou Q. Induced pluripotent stem cell consensus genes: implication for the risk of tumorigenesis and cancers in induced pluripotent stem cell therapy. Stem Cells Dev. 2012;21:955–964. doi: 10.1089/scd.2011.0649. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Chen M, Yang B, Zhang F, Cao K. Intramyocardial transplantation of undifferentiated rat induced pluripotent stem cells causes tumorigenesis in the heart. PLoS One. 2011;6:e19012. doi: 10.1371/journal.pone.0019012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- Zhou J, Su P, Li D, Tsang S, Duan E, Wang F. High-efficiency induction of neural conversion in human ESCs and human induced pluripotent stem cells with a single chemical inhibitor of transforming growth factor beta superfamily receptors. Stem Cells. 2010;28:1741–1750. doi: 10.1002/stem.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JM, Chu JX, Chen XJ. An improved protocol that induces human embryonic stem cells to differentiate into neural cells in vitro. Cell Biol Int. 2008;32:80–85. doi: 10.1016/j.cellbi.2007.08.015. [DOI] [PubMed] [Google Scholar]