Abstract
Bacterial lipoproteins are peripherally anchored membrane proteins that play a variety of roles in bacterial physiology and virulence in monoderm (single membrane-enveloped, e.g., grampositive) and diderm (double membrane-enveloped, e.g., gram-negative) bacteria. After export of prolipoproteins through the cytoplasmic membrane, which occurs predominantly but not exclusively via the general secretory or Sec pathway, the proteins are lipid-modified at the cytoplasmic membrane in a multistep process that involves sequential modification of a cysteine residue and cleavage of the signal peptide by the signal II peptidase Lsp. In both monoderms and diderms, signal peptide processing is preceded by acylation with a diacylglycerol through preprolipoprotein diacylglycerol transferase (Lgt). In diderms but also some monoderms, lipoproteins are further modified with a third acyl chain through lipoprotein N-acyl transferase (Lnt). Fully modified lipoproteins that are destined to be anchored in the inner leaflet of the outer membrane (OM) are selected, transported and inserted by the Lol (lipoprotein outer membrane localization) pathway machinery, which consists of the inner-membrane (IM) ABC transporterlike LolCDE complex, the periplasmic LolA chaperone and the OM LolB lipoprotein receptor. Retention of lipoproteins in the cytoplasmic membrane results from Lol avoidance signals that were originally described as the “+2 rule”. Surface localization of lipoproteins in diderms is rare in most bacteria, with the exception of several spirochetal species. Type 2 (T2SS) and type 5 (T5SS) secretion systems are involved in secretion of specific surface lipoproteins of γ-proteobacteria. In the model spirochete Borrelia burgdorferi, surface lipoprotein secretion does not follow established sorting rules, but remains dependent on N-terminal peptide sequences. Secretion through the outer membrane requires maintenance of lipoproteins in a translocation-competent unfolded conformation, likely through interaction with a periplasmic holding chaperone, which delivers the proteins to an outer membrane lipoprotein flippase.
1. Introduction
Since the description of the first prokaryotic lipoprotein in the cell envelope of Escherichia coli by Braun and colleagues over four decades ago [1, 2], this class of peripherally anchored membrane proteins has been increasingly recognized to play important roles in basic bacterial physiology such envelope stability, cell division, sporulation, conjugation, nutrient acquisition, signal transduction, transport and protein folding, but also in bacterial pathogenic mechanisms such as adhesion, colonization, invasion and persistence through immune evasion. Proper localization of these lipoproteins is of utmost importance for their function and hinges on an efficient lipoprotein modification and transport pathway and accurate lipoprotein sorting machinery. This review will focus on cis and trans factors that help compartmentalize the bacterial lipoproteome according to individual lipoprotein function within the bacterial envelope. Mechanistically, localization is relatively simple in monoderm (or single membrane-enveloped) bacteria such as the firmicutes, where only export through the cytoplasmic membrane and acylation is required for proper and stable localization on the bacterial surface. In diderm (or double membrane-enveloped) bacteria such as the γ-proteobacteria, acylated and therefore partially hydrophobic proteins destined for the outer membrane face a formidable hurdle in the aqueous periplasmic space, which is overcome with the help of a lipoprotein-specific chaperoned pathway. Surface localization of lipoproteins utilizes specific outer membrane porins and is rare in most eubacterial species, with the exception of some spirochetes, where it appears to be the norm.
2. Lipoprotein domain structure-function
All lipoproteins are translated in the cytoplasm as preprolipoprotein precursors with several structural and functional domains that can be recognized at the primary, secondary and tertiary structural level (Fig. 1). The most N-terminal domain is on average 20 amino acids in length and forms the signal (or leader) peptide [3]. In contrast to the signal peptides of secreted soluble proteins, the C-termini of lipoprotein signal peptides contain a four-amino-acid motif called the “lipobox” [4], which forms the molecular basis for several in silico algorithms that are used to predict lipoprotein genes in bacterial genomes [5, 6]. Maybe not surprisingly, the originally canonical lipobox sequence has degenerated as more and more lipoproteins were identified, not in a small part powered by the exponential increase in sequenced bacterial genomes and the associated proteomic analyses. So are lipobox sequences a bit more degenerate in spirochetes than in gram-positive and –negative bacteria [6] (Fig. 1). Today, the only conserved residue within the motif remains a cysteine that will become the target of acylation and the new Nterminal amino acid of the mature lipoprotein, i.e., the residue at position +1. In silico predictions as well as structural information on a number of lipoproteins indicate that the residues following the +1 cysteine lack any predicted or observed secondary structure. This indicates that this second domain is intrinsically disordered and forms a “tether” that links the lipid anchor to the third domain, which folds into a tertiary and sometimes quaternary structure and executes the protein-specific functions (Fig. 1). Tether lengths can vary quite dramatically from lipoprotein to lipoprotein [7]. As extreme examples, the crystal structure of Braun’s lipoprotein Lpp does not reveal any significant N-terminal disorder [8], while the Borrelia burgdorferi surface lipoprotein BBA66 has stretch of about 170 disordered N-terminal amino acids [9]. As discussed in more detail below, the tether peptides contain lipoprotein sorting information, but they are also thought - by means of their extension - to properly position lipoproteins for optimal function within the at times complex bacterial envelope architecture [10].
Figure 1. Lipoprotein domain structure.
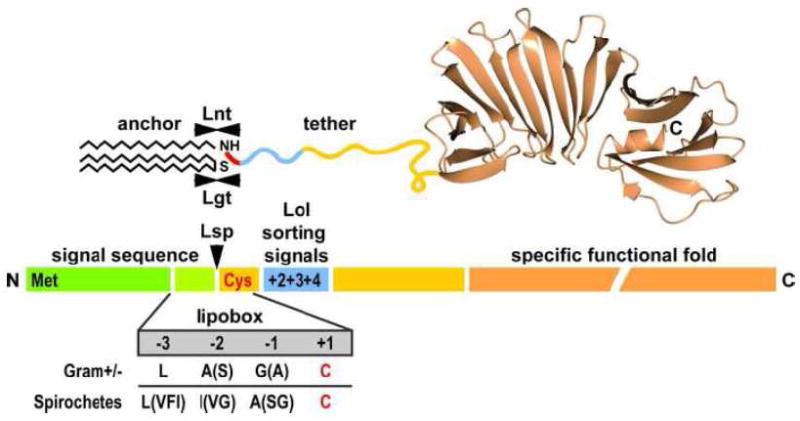
Lipoproteins are translated in the bacterial cytoplasm as preprolipoprotein precursors. An Nterminal signal peptide (in green) targets the protein for export of the protein through the cytoplasmic membrane. Diacylation at a conserved Cys residue (in red) is mediated by Lgt via the Cys sulfhydryl group. Lsp recognizes the lipobox residues and cleaves the signal peptide. This makes the N-terminal amine group available for Lnt-mediated modification with a third acyl chain, which completes the membrane anchor. The N-terminus of the mature lipoprotein contains sorting signals (in blue) recognized by the Lol pathway in diderm bacteria. Generally, the Nterminus is also intrinsically disordered, providing a flexible “tether” (in yellow) for proper positioning and function in the bacterial envelope. The C-terminal portion of the polypeptide (in orange) assumes a fold specific for the protein’s function. The structure of B. burgdorferi OspA (PBD Accession # 1osp) is used for illustrative purposes. See text for details.
3. Lipoprotein modification
Preprolipoproteins generally cross the cytoplasmic membrane membrane as unfolded proteins via the general secretory (Sec) pathway with the help of YidC [11], but can also cross in an already folded conformation via the twin-arginine translocation (TAT) pathway [12-16] or with the help of a SecA variant [17] (see Fig. 2 for an overall model of lipoprotein secretion; also see other chapters in this issue). Upon this translocation event, preprolipoproteins are targeted for a two- or three-step posttranslational modification by three essential enzymes that are associated with the cytoplasmic membrane. Multiple paralogs can be found in some bacteria [18], but their functions remain to be determined.
Figure 2. Modular model of lipoprotein secretion pathways in monoderm and diderm bacteria.
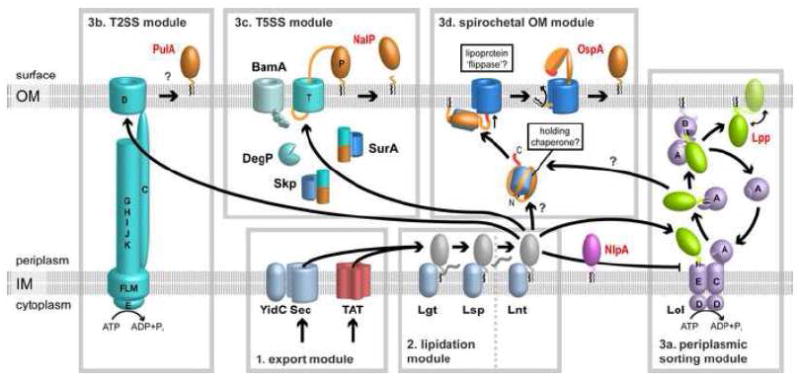
Lipoprotein secretion is mediated by a sequence of pathway modules. (1) The first module exports lipoprotein precursors through the cytoplasmic or inner membrane via the Sec or TAT pathways. (2) The second module processes the N-terminus of the proteins to yield a mature lipidated protein; in monoderm bacteria, the last modification step is dispensable, as indicated by a vertical dashed line. In diderm bacteria, IM lipoproteins like E. coli Nlp are retained by failure to interact with downstream pathways. (3) In diderm bacteria, OM lipoproteins can subsequently interact with three modules: (a) the Lol periplasmic sorting module uses the energy from ATP hydrolysis to release lipoproteins like E. coli Lpp from the IM, provides a carrier chaperone for transport through the periplasm and an OM membrane receptor for insertion into the inner leaflet of the OM. Lpp also assumes an integral membrane protein conformation leading to surface exposure of its C terminus. (b) The T2SS module uses assembly of a pseudopilus on a periplasmic platform to drive secretion of a specific surface lipoprotein such as K. oxytoca PulA through an OM pore. (c) The T5SS module involves the BAM complex in the OM and the integral membrane protein chaperones Skp, SurA and DegP as well to facilitate OM insertion and pore formation of NalP’s translocator domain (T) and subsequent translocation of its N-terminal passenger domain (P) through the OM. N. gonorrhoeae NalP is released from the cell by autolytic cleavage. (d) A proposed module mediates complete surface localization of spirochetal lipoproteins such as B. burgdorferi OspA by interaction with a holding chaperone and an outer membrane lipoprotein flippase complex. Any specific involvement of the Lol pathway remains to be resolved. See text for details.
3.1. Lipoprotein diacylglyceryl transferase Lgt
Preprolipoprotein diacylglyceryl transferase (Lgt) serves as the committing enzyme and was shown in vitro to catalyze the attachment of a negatively charged diacylglycerol moiety, particularly phosphatidylglycerol, to the thiol group of the conserved +1 position cysteine via a thioester bond [19]. The same experiments also showed that recognition of the signal peptide alone is sufficient for the reaction to occur. Structure-function information on Lgt is limited. Based on in silico predictions and C-terminal tagging with localization-sensitive reporters PhoA (periplasmic activity only) and GFP (cytoplasmic fluorescence only), Escherichia coli Lgt is a multipass integral protein with five transmembrane (TM) helices and a C terminus that is exposed to the cytoplasm [20]. Mutation of a conserved histidine residue (His103) within the predicted TM helix 3 inactivated the enzyme. Mutations of two additional residues, Tyr235 in the predicted TM helix 4 and His196 in a predicted large cytoplasmic loop, also affected activity [21, 22]. Pending the complete confirmation of Lgt topology, this suggests that the first step of lipoprotein modification may occur within the cytoplasmic membrane or at its interface with the cytoplasm. Somewhat puzzling has been the finding that recombinant Lgt retained full specific activity in an aqueous environment [23].
3.2. Signal peptidase II Lsp
In the next posttranslational modification step, the (partially) acylated prolipoproteins are cleaved by lipoprotein signal peptidase (Lsp), also known as signal peptidase II (SPase II) to distinguish it from signal peptidase I that processes non-lipidated exported proteins. Cleavage of the prolipoprotein occurs N terminally of the +1 position cysteine residue, i.e., within the lipobox [24]. Early topology experiments using fusions of Lsp fragments to PhoA and LacZ (cytoplasmic activity only) reporters produced a model with 4 TM helices that has remained unchallenged in later experiments and predictions [20, 25]. In the Bacillus subtilis Lsp, five conserved residues within conserved domains (Asn99, Asp102, Asn126, Ala128 and Asp129) were shown to be functionally important, with residues Asp102 and Asp129 most likely involved in catalysis [26]. The positioning of the latter two residues at both ends of an extracytoplasmic loop indicates that signal peptide II cleavage occurs at the interface of the cytoplasmic membrane with the cell wall or periplasm. Of experimental interest is the function of globomycin as a noncompetitive inhibitor of Lsp function [27], which can be exploited to help establish posttranslational modification of a suspected lipoprotein.
3.3. N-acyl transferase Lnt
A third posttranslational modification step occurs in diderms and some monoderms (high-GC gram-positive and mycobacteria; [28, 29] and is catalyzed by lipoprotein N-acyl transferase (Lnt). Lnt attaches an acyl group to the now available amino group of the +1 cysteine via an amide linkage. The studied E. coli enzyme is rather promiscuous in its incorporation of phospholipids [30-32], and the amide-linked acyl chain of a lipoprotein may thus reflect availability of a membrane phospholipid in a particular bacterium and niche [33]. A topology study using E. coli Lnt fusions to LacZ and PhoA indicated seven TM helices and a large extracytoplasmic loop that assumes a CN hydrolase family fold and contains all conserved residues crucial for enzyme activity: the catalytic site residues (a typical triad of Glu267-Lys335-Cys387), the hydrophobic pocket (Tyr388, Glu389) and two residues involved in binding/release of the apolipoprotein and/or the phospholipid (Trp237, Glu343) [28, 34].
3.4. Additional biological roles of lipoprotein signal peptides
In this context, the fate of the signal peptide that is cleaved from the maturing lipoprotein by Lsp deserves some attention. Lipoprotein signal peptides are commonly thought to be discarded and its amino acids recycled for the synthesis of new proteins. Yet, some lipoprotein signal peptides have a quite intriguing “afterlife”. As one instructive example in the monoderm pathogen Enterococcus faecalis, the signal peptide of lipoprotein CcfA is further processed by Eep. Eep is a predicted metalloprotease that cleaves its substrates by regulated intramembrane proteolysis. In concert with a still unknown carboxy exopeptidase, Eep trims the CcfA signal peptide to an 8-amino-acid peptide, cCF10, which serves as a pheromone regulating horizontal gene transfer by conjugation [35-37]. Additional pheromone peptides are produced from other enterococcal signal peptides, and similar processing has been shown in a related streptococcal species [38]. Interestingly, cCF10 is titrated from the system by the cytoplasmic membrane-bound protease PrgY [37], whose homologs can be found in many bacteria as well as higher organisms [39, 40].
4. Rules and pathways of lipoprotein sorting within the periplasm
4.1. Lipoprotein sorting and the evolution of the “+2” rule
How diderm bacteria localize lipoproteins such as the major E. coli envelope component Lpp to their outer membrane long remained a mystery. Insight into the cis sorting determinants was first provided by fusions of N-terminal peptides of E. coli Lpp and cytoplasmic membrane lipoprotein NlpA to a β-lactamase reporter. Reporter fusions to the wild type (w.t.) N-terminal lipopeptides behaved like the w.t. lipoproteins, showing that the lipoproteins’ N-termini were sufficient for proper localization, i.e., contained the in cis sorting information [41, 42]. In a more detailed and targeted analysis, swapping the residues immediately following the N-terminal cysteine between NlpA (Asp+2) and Lpp (Ser+2) resulted in mislocalization of the respective mutants to the opposite periplasmic leaflet [42]. These seminal experiments gave rise to the formulation of the “+2 rule” for lipoprotein localization, where Asp+2 predicts localization to the cytoplasmic membrane, but any other residue targets lipoproteins to the outer membrane. Subsequent studies confirmed the +2 rule as a guiding principle, but also showed that it is far from universal. In the E. coli system, His and Lys residues at position +3 were found to lower the stringency of cytoplasmic membrane retention by an Asp+2 signal [43]. A systematic study using the maltose binding protein MalE as a localization reporter showed that Phe, Trp, Tyr, Gly and Pro also could serve as +2 inner membrane retention signals when next to an Asn at position +3 [44]; yet, none of the E. coli lipoproteins naturally employ these 5 residues at position +2. The E. coli-derived sorting rules held true for several Enterobacteriaceae such as Salmonella enterica serovar Typhimurium, Shigella flexneri, Yersinia pseudotuberculosis, Erwinia carotovora, and Klebsiella oxitoca [45], but didn’t fully extend to other γ-proteobacteria. Studies in Pseudomonas aeruginosa showed that an artificially introduced Asp+2 could function as IM retention signal, but that residues at position 3 and 4 were innately responsible for retention of lipoprotein MexA (Gly+2Lys+3Ser+4) in the cytoplasmic membrane [46].
4.2. The Lol pathway
4.2.1. The periplasmic chaperone LolA and outer membrane lipoprotein receptor LolB
The related challenge was to conceptualize the crossing of a lipidated, i.e., partially hydrophobic protein through the hydrophilic gel-like periplasmic space. One model bypassed this aqueous barrier altogether by directly transferring lipoproteins from the cytoplasmic membrane to the outer membrane via the localized fusion of their periplasmic leaflets (a.k.a. “zones of adhesion” or “Bayer’s junctions”; [47, 48]. Yet, the interpretation and functional relevance of these electronmicroscopic observations remained controversial [49]. The puzzle was finally solved with the identification and characterization of the lipoprotein outer membrane localization (Lol) pathway by Tokuda and colleagues. The first identified component of this pathway was the lipoprotein-specific periplasmic carrier protein LolA, which released Lpp and several other known OM lipoproteins (Pal, Bam, Slp and RlpA), but not IM lipoproteins (AcrA and NlpA), from E. coli spheroplasts, forming complexes with a 1:1 stoichiometry [50]. A conditional lolA knockout strain accumulated OM lipoproteins in the inner membrane under depleting conditions and was non-viable under non-permissive conditions [51], confirming LolA’s in vivo role as an essential factor in periplasmic lipoprotein transport and cell growth.
Like for LolA, purification and in vitro reconstitution of the pathway was key in identifying the OM lipoprotein LolB as the downstream OM lipoprotein receptor. LolA-lipoprotein complexes incubated with LolB-containing proteoliposomes showed specific transfer of lipoproteins to from the soluble to the membrane fraction [52]. In accordance with these in vitro results, deletion of lolB caused in vivo accumulation of lipoproteins in a complex with LolA as well as in the cytoplasmic membrane of E. coli [53].
The functional similarity of LolA and LolB in their interaction with lipoproteins first became apparent when a non-acylated soluble LolB derivative was shown to form lipoprotein complexes that were identical to those with LolA [52]. X-ray crystallography revealed a high degree of structural homology despite a low degree of primary sequence identity. Both LolA and LolB fold into an incomplete (β-barrel consisting of 11 antiparallel β-strands with a lid of 3 α-helices [54], which together form a hydrophobic cavity that provides a potential binding site for lipoprotein acyl chains. The periplasmic and thus energy-independent one-way transfer of lipoproteins from LolA to LolB is facilitated by four differences between the two proteins. (i) Compared to aromatic residues in LolA, the hydrophobic cavity of LolB is lined by more flexible Leu and Ile residues [54]. (ii) LolA has an extra C-terminal loop domain with a short α-helix and extra β-strand, which blocks the carrier protein’s interaction with phospholipids and therefore prevents retrograde transfer of lipoproteins to the cytoplasmic membrane lipid bilayer [55]. (iii) Access to the hydrophobic cavity in LolA is more restricted by hydrogen bonding of an Arg residue in the second β-sheet with several α-helical lid residues. This intramolecular clasp allows for the LolA lid to open and close reversibly [56]. If this restriction is eased by mutation of the Arg residue in a LolAR43L mutant, LolA is no longer able to efficiently transfer lipoproteins to LolB [57, 58]. Conversely, locking the LolA lid in a closed conformation via a disulfide bond in a LolAI93C lid residue cysteine substitution mutant is toxic, causes envelope stress and activates the Cpx two-component system [59, 60]. (iv) LolB has a protruding internal loop that was shown by site-directed mutagenesis to be involved in anchoring lipoproteins in the OM [61].
No co-crystals of LolA or LolB with lipoproteins have been obtained so far, so there currently are two competing hypotheses for the interaction of the lipoprotein acyl chains with LolA/B, both indirectly supported by experimental data [62]. On one hand, the hydrophobic cavity of one of the two obtained LolB crystal contained a polyethylene glycol methyl ether molecule, which roughly corresponds in its dimensions to a single acyl chain [54]. This would indicate that only one of the three chains is bound internally, while the other two chains would have to interact with the proteins via surface hydrophobic patches, as proposed for the P. aeruginosa LolA [63]. On the other hand, a structural LolA/B homolog, the Mycobacterium tuberculosis LprG lipoprotein, was shown to bind three acyl chains in a hydrophobic cavity of about the same volume as that of LolA in its open conformation [64]. This would indicate that all three chains could be accommodated. A unifying postulate for both inferences would be that the LolA/B fold is somewhat flexible which would allow the proteins to wrap around presenting hydrophobic molecules within a certain dimensional range. Interestingly, LolB homologs are only found in β- and γ-proteobacteria [62], which begs the question whether LolA plays a broader role in LolB-deficient systems. Support for such expanded functionality comes from a study demonstrating that the non-acylated soluble LolB variant was able to compensate for loss of wild type LolB, albeit at reduced efficiency [65].
4.2.2. The inner membrane ABC transporter-like LolCDE complex
The availability of a conditional LolA knockout allowed for the trapping of OM lipoproteins in the inner membrane and identification of the lipoprotein release complex using purification and reconstitution of the pathway in a spheroplast/proteoliposome release assay. The ABC transporter-like complex was shown to consist of LolC, LolD and LolE subunits in a 1:2:1 stoichiometry [66-68]. LolC and LolE are structurally related integral membrane proteins with four TM-spanning helices and one large periplasmic loop [69], while LolD serves as the nucleotide-binding subunit with an ABC transporter signature as well as Walker A and B motifs. In vitro and in vivo experiments demonstrated that interaction of lipoproteins with the LolCDE complex depended on full triacylation of lipoproteins by Lnt. OM lipoprotein Pal in its apolipoprotein form with an N-terminal, diacylated cysteine was not released from proteoliposomes even in the presence of LolCDE and LolA [70], while a temperature-sensitive S. enterica Lnt mutant [71] and a conditional E. coli Lnt knockout mutant [34] accumulated apolipoproteins in the IM at nonpermissive temperatures or non-inducing conditions, respectively. Lnt was only dispensable in the absence of a functional version of the major lipoprotein Lpp and overexpression of LolCDE [72].
Especially recent studies using site-specific photocrosslinking have provided more and quite detailed insight into the sequence of events at the inner membrane, but also the modalities of lipoprotein transfer in the periplasm and at the outer membrane. In a first step, LolE captures an OM lipoprotein from the inner membrane [73]. The precise mechanism remains to be determined, but it involves LolE’s periplasmic loop, which may fold into a hydrophobic domain similar to the hydrophobic cavities found in LolA/B. This committing step in periplasmic lipoprotein transport leads to allosteric structural changes that trickle down to LolD and increase its affinity for ATP [66]. At the same time, LolC procures an empty LolA carrier molecule [74]. In a second step, ATP binding to LolD weakens the hydrophobic interaction of LolE with the OM lipoprotein [73]. In the third and final step at the IM, ATP hydrolysis leads to the transfer of the lipoprotein to LolA and the release of the lipoprotein:LolA complex into the periplasm, where it is captured at the OM by LolB [74]. The spatial arrangements of the crosslinked residues support a “mouth-to-mouth” transfer of lipoproteins from LolE to LolA and from LolA to LolB, where the openings of the hydrophobic cavities are in close proximity during the transfer of the acyl chains [73, 74]. Of note, the N-termini of lipoproteins with an Asp+2 residue appear to simply avoid interaction with the LolCDE sortase complex due to a competing interaction of the negatively charged Asp with phosphatidylethanolamine in the IM [75, 76].
5. Secretion through the outer membrane: Rare in most, but common in few
Lipoproteins that are secreted through the outer membrane and localize to the bacterial surface are rare in most diderm bacterial genera. Some examples of lipid-modified surface-associated proteins in diderm pathogens are Klebsiella oxytoca pullulanase PulA [77], Neisseria gonorrhoeae iron-scavenging proteins LbpB and TbpB [78-80] or peptidyl-prolyl cis/trans isomerase Ng-MIP [81], Porphyromonas gingivalis hemin-binding protein IhtB [82], Vibrio cholerae lipid-scavenging phospholipase VolA [83], Neisseria meningitidis surface protease NalP [84, 85], Bordetella pertussis maturation subtilisin SphB1 [86], Campylobacter jejuni adhesin JlpA, which interestingly has a “catcher’s mitt”-like structure similar to LolB [87, 88], or several Capnocytophaga canimorsus surface lipoproteins associated with glycan foraging systems [89]. In the model organism E. coli, only four of the about 90 lipoproteins [90, 91] have been demonstrated to be at least partially surface exposed [92-95]. In contrast, about two thirds of the over 120 lipoproteins expressed by the spirochetal pathogen Borrelia burgdorferi localize to the surface (S. Chen, A.S. Dowdell, M.D. Murphy, C.B. Azodi and W.R. Zückert, unpublished), among them OspA and OspC, which are essential for bacterial persistence in the disease-transmitting Ixodes tick or subsequent infection of the mammalian reservoir host (reviewed in [10] and [96]). Few of the surface lipoprotein secretion mechanisms have been studied in detail. Instructive examples are K. oxytoca PulA, N. meningitidis NalP, B. burgdorferi OspA and OspC, and maybe surprisingly, E. coli Lpp.
5.1. Klebsiella oxytoca PulA
The 116 kDa amylolytic enzyme PulA is secreted by the Pul secreton, the terminal branch of a prototypical T2SS consisting of a multi-protein complex that is conserved among bacteria, spans the entire diderm envelope [97] and is discussed in detail elsewhere in this edition. Current experimental data support a model where PulA already folds at the IM to form and display a T2SS signal “patch” from at least three discontinuous secretion determinants, although formation of an identified stabilizing disulfide bond appears dispensable for proper secretion [98-101]; in the absence of a functional T2SS machinery, PulA’s Asp+2 residue functions as IM retention/Lol avoidance signal [102]. ATP hydrolysis by the PulE ATPase is then thought to lead to assembly of PulG pseudopilin fibers on a periplasmic IM-anchored assembly platform, which is pushing the folded PulA protein from the periplasm through an OM pore formed by a dodecamer of PulD secretin subunits [103-105]. Intriguingly, proper localization and stability of PulD is dependent on interaction with its cognate chaperone, the pilotin PulS, which in turn is a lipoprotein and itself interacts with its chaperone LolA on the way from the IM to the OM [106-110]. This suggests that single PulD subunits traverse the periplasm in a complex with PulS and LolA before assembling into a pore in the OM in an apparently BAM-independent manner [111]. Although it is clear that at least the functional enzymatic domain of PulA localizes to the bacterial surface and can be released into the milieu in lipidated form as part of membrane vesicles or micelles [112], the molecular events allowing PulA to disengage from the IM and to traverse the OM remain to be defined. In constrast to other secretins, the N-terminal domain of PulD appears not involved in recognition of its specific substrate [113]. Whether PulA is ultimately anchored in the periplasmic or apical OM leaflet, i.e., assumes an “in-out” or an “out-out” lipoprotein topology [114], its full release from the PulD channel into the membrane would likely require a lateral opening for which there is currently no experimental evidence [115].
5.2. Neisseria meningitidis NalP
N. meningitidis NalP, like B. pertussis SphB1, is a lipoprotein with the C-terminal translocator and an N-terminal passenger domain structure of an “autotransporter” (AT), which accordingly is secreted by the T5SS pathway [84] (see other articles in this edition). NalP is responsible for the release of several surface-exposed immunogenic proteins from the bacterial surface, including the N. meningitidis LbpB homolog [116], while it is itself released from the bacterial envelope after further N-terminal processing [84]. Recent studies expressing a non-lipidated mutant showed that lipidation is not required for NalP biogenesis and secretion. However, the non-lipidated protein was much less efficient in cleaving its target proteins and also underwent more rapid autocatalytic processing [85]. Thus, the lipid moiety delays the release of NalP from the bacterial surface, which in turn optimizes the cleavage of its target proteins. The C-terminal translocator domain forms a 12-stranded β-barrel with a narrow hydrophilic pore containing an N-terminal α-helix [117]. The orientation of this α-helix - with its N-terminus facing the extracellular space - suggests that the NalP N-terminal passenger domain is entirely surface-localized, i.e., that the lipid anchor is inserted in the surface leaflet of the neisserial OM, but specific anchor topology information is not available.
5.3. Escherichia coli Lpp
E. coli Lpp was long known to exist in two conformations: the “bound” form and the “free” form. The “bound” form was shown to be tethered to the OM via its N-terminal lipid moiety and covalently attached to the peptidoglycan cell wall by the ε-amino group of its C-terminal Lys58 residue, thereby stabilizing the bacterial envelope [118, 119]. The “free” form had been harder to pin down, until Cowles and colleagues demonstrated that it assumed an integral OM protein topology, with the protein’s C terminus exposed to the cell surface [95]. Interestingly, BamC (a.k.a. NlpB), one of the lipoproteins associated with the OM β-barrel assembly machinery (BAM) complex [120, 121], was subsequently shown to assume a similar “N-in/C-out” topology [122]. How Lpp switches between the two vastly different conformations remains unknown. As demonstrated for T5SS “autotransporter” proteins [123], peptide insertion into the OM is unlikely to be spontaneous and may depend on a yet cryptic activity of the BAM complex [124].
5.4. Borrelia burgdorferi OspA and OspC
Our own work has focused on lipoprotein secretion in the spirochetal model organism Borrelia burgdorferi. Although Borrelia are occasionally described as a gram-negative bacteria due to their Gram stain properties and diderm envelope, a closer examination reveals significant differences in composition and architecture, such as the lack of lipopolysaccharide in the OM or the presence of periplasmic flagella (reviewed in [125]). A unique envelope feature of Borrelia is the prevalence of major immunodominant and serotype-defining lipoproteins on the bacterial surface. It is therefore not surprising that surface lipoproteins mediate the majority of the known interactions of Borrelia spirochetes with their tick vectors and mammalian hosts and are therefore considered major virulence factors and attractive vaccine targets. Using the monomeric OspA and dimeric OspC as two model surface lipoproteins and the monomeric red fluorescent protein derivative mRFPΔ4 as a faithful localization reporter, we first demonstrated that sorting determinants of Borrelia surface lipoproteins localized within the N-terminal disordered tether peptides, but that variations of the “+2/+3/+4” lipoprotein sorting rule identified for periplasmic lipoproteins in other eubacteria did not apply [7, 126-128]. Surface lipoprotein tether mutants were predominantly mislocalized to the periplasmic leaflet of the OM [7, 126-128]. Further studies indicated that this mislocalization was due to premature folding, and that secretion of lipoproteins through the OM required the maintenance of the substrates in an at least partially unfolded, translocation-competent state [7, 129]. Accordingly, OspC appeared to traverse the periplasm as monomer to assume its final quaternary fold only after reaching the cell exterior [128]. Intriguingly, translocation through the OM could be initiated by mutational or conditional unfolding of a lipoprotein’s C-terminus, indicating a transient anchoring of surface lipoproteins in the periplasmic OM leaflet and N to C terminus directionality of translocation through the OM. Yet, this process appeared to be independent of primary C-terminal amino acid sequence [7, 114, 129]. This led us to hypothesize a periplasmic mechanism in which the various surface-targeted prolipoprotein peptides interact with a periplasmic “holding” chaperone, as they emerge tether-first from the IM Sec complex on the periplasmic side of the IM. Such a mechanism may be analogous to the “high affinity, low specificity” interaction of the proteobacterial chaperone SecB with the diverse peptides released from the ribosome [130-133]. In the model, the holding chaperone then delivers surface lipoproteins to an OM lipoprotein “flippase” complex, which facilitates translocation through the OM and leads to the ultimate anchoring of surface lipoproteins in the surface leaflet of the OM [7, 114, 129]. In agreement with this model, depletion of a BamA homolog reduced the amount of B. burgdorferi OM proteins, including lipoproteins [134].
Any involvement of the Borrelia Lol pathway homologs in surface lipoprotein secretion remains to be determined. Preliminary data so far have been instructive but inconclusive. Like other bacteria outside the β- and γ-proteobacteria branches, Borrelia lack a LolB homolog [135]. Conditional overexpression of a B. burgdorferi LolDG41D ATPase Walker A motif mutant using a hybrid tetracycline-responsive promoter [136] led to a significant B. burgdorferi growth defect, most likely due to a dominant negative effect on the function of the LolCDE complex in releasing OM lipoproteins from the IM. Yet, there was no detectable reduction in surface lipoprotein localization. At the same time, the B. burgdorferi homolog of the periplasmic LolA lipoprotein chaperone was unable to complement an E. coli mutant, but appeared to interact selectively with lipoproteins (K.O. Bridges, S. Chen, J.L. Kueker, J. Liu & W.R. Zückert, unpublished). Current studies focus on trapping lipoprotein secretion intermediates, in part through the generation of conditional knockouts in lolA and other pathway candidate genes.
6. Conclusions
Over the last decade, our understanding of lipoprotein secretion in bacteria has significantly deepened, as the molecular mechanisms of lipoprotein modification and sorting were identified and novel tools allowed us to discover details of protein-protein interactions during the associated molecular events. However, our horizons also have been broadened by numerous studies that used a variety of experimental systems and approaches. New paradigms in proteins secretion emerged and existing models had to be modified or retired. As such, the current overall picture of lipoprotein secretion is a puzzle of a mosaic where several pieces remain to be placed or are missing altogether. The assumption that canonical secretion pathways, sorting rules or structurefunction relationships can be dogmatically deduced from a small and rather limited number of established model systems is tempting in the interest of simplification. At the same time, such an approach is fraught with pitfalls and simply doesn’t do justice to the broad diversity of the bacterial world. As we continue following the paths of lipoprotein secretion and venture deeper and farther into the bacterial envelope, we would be wise to heed the past and to continue expecting the unexpected.
Highlights.
Bacterial lipoproteins are anchored in membranes via N-terminal lipids.
Lipoprotein signal sequences contain a lipobox motif with a conserved cysteine.
Lipid modification occurs at the cytoplasmic membrane after export by Sec or TAT.
Sorting information is generally encoded in the lipoproteins’ disordered N termini.
Targeting pathways include Lol, T2SS, T5SS and a proposed OM lipoprotein flippase.
Acknowledgments
The author wishes to thank Hajime Tokuda, Tony Pugsley and Joe Lutkenhaus for advice and inspiration, the past and current members of his laboratory for their effort in deciphering the mysteries of lipoprotein secretion in spirochetes, and Tassos Economou and Ross Dalbey for their patience. Current work in the author’s laboratory is supported by NIH Grant P30 GM103326.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braun V, Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969;10:426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- 2.Hantke K, Braun V. Covalent binding of lipid to protein. Diglyceride and amidelinked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973;34:284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- 3.von Heijne G. The structure of signal peptides from bacterial lipoproteins. Protein Eng. 1989;2:531–534. doi: 10.1093/protein/2.7.531. [DOI] [PubMed] [Google Scholar]
- 4.Sankaran K, Wu HC. Bacterial lipoproteins. In: Schlesinger MJ, editor. Lipid Modifications of Proteins. CRC Press; Boca Raton: 1993. pp. 163–181. [Google Scholar]
- 5.Babu MM, Priya ML, Selvan AT, Madera M, Gough J, Aravind L, Sankaran K. A database of bacterial lipoproteins (DOLOP) with functional assignments to predicted lipoproteins. J Bacteriol. 2006;188:2761–2773. doi: 10.1128/JB.188.8.2761-2773.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setubal JC, Reis M, Matsunaga J, Haake DA. Lipoprotein computational prediction in spirochaetal genomes. Microbiology. 2006;152:113–121. doi: 10.1099/mic.0.28317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulze RJ, Chen S, Kumru OS, Zückert WR. Translocation of Borrelia burgdorferi surface lipoprotein OspA through the outer membrane requires an unfolded conformation and can initiate at the C-terminus. Mol Microbiol. 2010;76:1266–1278. doi: 10.1111/j.1365-2958.2010.07172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu W, Liu J, Ji H, Lu M. Core structure of the outer membrane lipoprotein from Escherichia coli at 1.9 A resolution. J Mol Biol. 2000;299:1101–1112. doi: 10.1006/jmbi.2000.3776. [DOI] [PubMed] [Google Scholar]
- 9.Brangulis K, Petrovskis I, Kazaks A, Tars K, Ranka R. Crystal structure of the infectious phenotype-associated outer surface protein BBA66 from the Lyme disease agent Borrelia burgdorferi. Ticks Tick Borne Dis. 2014;5:63–68. doi: 10.1016/j.ttbdis.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Zückert WR. A call to order at the spirochaetal host-pathogen interface. Mol Microbiol. 2013;89:207–211. doi: 10.1111/mmi.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Froderberg L, Houben EN, Baars L, Luirink J, de Gier JW. Targeting and translocation of two lipoproteins in Escherichia coli via the SRP/Sec/YidC pathway. J Biol Chem. 2004;279:31026–31032. doi: 10.1074/jbc.M403229200. [DOI] [PubMed] [Google Scholar]
- 12.De Buck E, Lebeau I, Maes L, Geukens N, Meyen E, Van Mellaert L, Anne J, Lammertyn E. A putative twin-arginine translocation pathway in Legionella pneumophila. Biochem Biophys Res Commun. 2004;317:654–661. doi: 10.1016/j.bbrc.2004.03.091. [DOI] [PubMed] [Google Scholar]
- 13.Dilks K, Gimenez MI, Pohlschröder M. Genetic and biochemical analysis of the twin-arginine translocation pathway in halophilic archaea. J Bacteriol. 2005;187:8104–8113. doi: 10.1128/JB.187.23.8104-8113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimenez MI, Dilks K, Pohlschröder M. Haloferax volcanii twin-arginine translocation substates include secreted soluble, C-terminally anchored and lipoproteins. Mol Microbiol. 2007;66:1597–1606. doi: 10.1111/j.1365-2958.2007.06034.x. [DOI] [PubMed] [Google Scholar]
- 15.Widdick DA, Dilks K, Chandra G, Bottrill A, Naldrett M, Pohlschröder M, Palmer T. The twin-arginine translocation pathway is a major route of protein export in Streptomyces coelicolor. Proc Natl Acad Sci U S A. 2006;103:17927–17932. doi: 10.1073/pnas.0607025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arambula D, Wong W, Medhekar BA, Guo H, Gingery M, Czornyj E, Liu M, Dey S, Ghosh P, Miller JF. Surface display of a massively variable lipoprotein by a Legionella diversity-generating retroelement. Proc Natl Acad Sci U S A. 2013;110:8212–8217. doi: 10.1073/pnas.1301366110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feltcher ME, Gibbons HS, Ligon LS, Braunstein M. Protein export by the mycobacterial SecA2 system is determined by the preprotein mature domain. J Bacteriol. 2013;195:672–681. doi: 10.1128/JB.02032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchings MI, Palmer T, Harrington DJ, Sutcliffe IC. Lipoprotein biogenesis in Gram-positive bacteria: knowing when to hold ‘em, knowing when to fold ‘em. Trends Microbiol. 2008 doi: 10.1016/j.tim.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Sankaran K, Wu HC. Lipid modification of bacterial prolipoprotein. Transfer of diacylglyceryl moiety from phosphatidylglycerol. J Biol Chem. 1994;269:19701–19706. [PubMed] [Google Scholar]
- 20.Daley DO, Rapp M, Granseth E, Melen K, Drew D, von Heijne G. Global topology analysis of the Escherichia coli inner membrane proteome. Science. 2005;308:1321–1323. doi: 10.1126/science.1109730. [DOI] [PubMed] [Google Scholar]
- 21.Qi HY, Sankaran K, Gan K, Wu HC. Structure-function relationship of bacterial prolipoprotein diacylglyceryl transferase: functionally significant conserved regions. J Bacteriol. 1995;177:6820–6824. doi: 10.1128/jb.177.23.6820-6824.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sankaran K, Gan K, Rash B, Qi HY, Wu HC, Rick PD. Roles of histidine-103 and tyrosine-235 in the function of the prolipoprotein diacylglyceryl transferase of Escherichia coli. J Bacteriol. 1997;179:2944–2948. doi: 10.1128/jb.179.9.2944-2948.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvan AT, Sankaran K. Localization and characterization of prolipoprotein diacylglyceryl transferase (Lgt) critical in bacterial lipoprotein biosynthesis. Biochimie. 2008;90:1647–1655. doi: 10.1016/j.biochi.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Tokunaga M, Tokunaga H, Wu HC. Post-translational modification and processing of Escherichia coli prolipoprotein in vitro. Proc Natl Acad Sci U S A. 1982;79:2255–2259. doi: 10.1073/pnas.79.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munoa FJ, Miller KW, Beers R, Graham M, Wu HC. Membrane topology of Escherichia coli prolipoprotein signal peptidase (signal peptidase II) J Biol Chem. 1991;266:17667–17672. [PubMed] [Google Scholar]
- 26.Tjalsma H, Zanen G, Venema G, Bron S, van Dijl JM. The potential active site of the lipoprotein-specific (type II) signal peptidase of Bacillus subtilis. J Biol Chem. 1999;274:28191–28197. doi: 10.1074/jbc.274.40.28191. [DOI] [PubMed] [Google Scholar]
- 27.Dev IK, Harvey RJ, Ray PH. Inhibition of prolipoprotein signal peptidase by globomycin. J Biol Chem. 1985;260:5891–5894. [PubMed] [Google Scholar]
- 28.Vidal-Ingigliardi D, Lewenza S, Buddelmeijer N. Identification of essential residues in apolipoprotein N-acyl transferase, a member of the CN hydrolase family. J Bacteriol. 2007;189:4456–4464. doi: 10.1128/JB.00099-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tschumi A, Nai C, Auchli Y, Hunziker P, Gehrig P, Keller P, Grau T, Sander P. Identification of apolipoprotein N-acyltransferase (Lnt) in mycobacteria. J Biol Chem. 2009;284:27146–27156. doi: 10.1074/jbc.M109.022715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackowski S, Rock CO. Transfer of fatty acids from the 1-position of phosphatidylethanolamine to the major outer membrane lipoprotein of Escherichia coli. J Biol Chem. 1986;261:11328–11333. [PubMed] [Google Scholar]
- 31.Gupta SD, Dowhan W, Wu HC. Phosphatidylethanolamine is not essential for the N-acylation of apolipoprotein in Escherichia coli. J Biol Chem. 1991;266:9983–9986. [PubMed] [Google Scholar]
- 32.Hillmann F, Argentini M, Buddelmeijer N. Kinetics and phospholipid specificity of apolipoprotein N-acyltransferase. J Biol Chem. 2011;286:27936–27946. doi: 10.1074/jbc.M111.243519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakayama H, Kurokawa K, Lee BL. Lipoproteins in bacteria: structures and biosynthetic pathways. FEBS J. 2012;279:4247–4268. doi: 10.1111/febs.12041. [DOI] [PubMed] [Google Scholar]
- 34.Robichon C, Vidal-Ingigliardi D, Pugsley AP. Depletion of apolipoprotein N-acyltransferase causes mislocalization of outer membrane lipoproteins in Escherichia coli. J Biol Chem. 2005;280:974–983. doi: 10.1074/jbc.M411059200. [DOI] [PubMed] [Google Scholar]
- 35.An FY, Sulavik MC, Clewell DB. Identification and characterization of a determinant (eep) on the Enterococcus faecalis chromosome that is involved in production of the peptide sex pheromone cAD1. J Bacteriol. 1999;181:5915–5921. doi: 10.1128/jb.181.19.5915-5921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antiporta MH, Dunny GM. ccfA, the genetic determinant for the cCF10 peptide pheromone in Enterococcus faecalis OG1RF. J Bacteriol. 2002;184:1155–1162. doi: 10.1128/jb.184.4.1155-1162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandler JR, Dunny GM. Characterization of the sequence specificity determinants required for processing and control of sex pheromone by the intramembrane protease Eep and the plasmid-encoded protein PrgY. J Bacteriol. 2008;190:1172–1183. doi: 10.1128/JB.01327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denham EL, Ward PN, Leigh JA. Lipoprotein signal peptides are processed by Lsp and Eep of Streptococcus uberis. J Bacteriol. 2008;190:4641–4647. doi: 10.1128/JB.00287-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bazan JF, Macdonald BT, He X. The TIKI/TraB/PrgY family: a common protease fold for cell signaling from bacteria to metazoa? Dev Cell. 2013;25:225–227. doi: 10.1016/j.devcel.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandler JR, Flynn AR, Bryan EM, Dunny GM. Specific control of endogenous cCF10 pheromone by a conserved domain of the pCF10-encoded regulatory protein PrgY in Enterococcus faecalis. J Bacteriol. 2005;187:4830–4843. doi: 10.1128/JB.187.14.4830-4843.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghrayeb J, Inouye M. Nine amino acid residues at the NH2-terminal of lipoprotein are sufficient for its modification, processing, and localization in the outer membrane of Escherichia coli. J Biol Chem. 1984;259:463–467. [PubMed] [Google Scholar]
- 42.Yamaguchi K, Yu F, Inouye M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell. 1988;53:423–432. doi: 10.1016/0092-8674(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 43.Gennity JM, Inouye M. The protein sequence responsible for lipoprotein membrane localization in Escherichia coli exhibits remarkable specificity. J Biol Chem. 1991;266:16458–16464. [PubMed] [Google Scholar]
- 44.Seydel A, Gounon P, Pugsley AP. Testing the ‘+2 rule’ for lipoprotein sorting in the Escherichia coli cell envelope with a new genetic selection. Mol Microbiol. 1999;34:810–821. doi: 10.1046/j.1365-2958.1999.01647.x. [DOI] [PubMed] [Google Scholar]
- 45.Lewenza S, Vidal-Ingigliardi D, Pugsley AP. Direct visualization of red fluorescent lipoproteins indicates conservation of the membrane sorting rules in the family Enterobacteriaceae. J Bacteriol. 2006;188:3516–3524. doi: 10.1128/JB.188.10.3516-3524.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewenza S, Mhlanga MM, Pugsley AP. Novel inner membrane retention signals in Pseudomonas aeruginosa lipoproteins. J Bacteriol. 2008;190:6119–6125. doi: 10.1128/JB.00603-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bayer ME. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968;53:395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- 48.Bayer ME. Zones of membrane adhesion in the cryofixed envelope of Escherichia coli. J Struct Biol. 1991;107:268–280. doi: 10.1016/1047-8477(91)90052-x. [DOI] [PubMed] [Google Scholar]
- 49.Kellenberger E. The ‘Bayer bridges’ confronted with results from improved electron microscopy methods. Mol Microbiol. 1990;4:697–705. doi: 10.1111/j.1365-2958.1990.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 50.Matsuyama S, Tajima T, Tokuda H. A novel periplasmic carrier protein involved in the sorting and transport of Escherichia coli lipoproteins destined for the outer membrane. EMBO J. 1995;14:3365–3372. doi: 10.1002/j.1460-2075.1995.tb07342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tajima T, Yokota N, Matsuyama S, Tokuda H. Genetic analyses of the in vivo function of LolA, a periplasmic chaperone involved in the outer membrane localization of Escherichia coli lipoproteins. FEBS Lett. 1998;439:51–54. doi: 10.1016/s0014-5793(98)01334-9. [DOI] [PubMed] [Google Scholar]
- 52.Matsuyama S, Yokota N, Tokuda H. A novel outer membrane lipoprotein, LolB (HemM), involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of Escherichia coli. EMBO J. 1997;16:6947–6955. doi: 10.1093/emboj/16.23.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanaka K, Matsuyama SI, Tokuda H. Deletion of lolB, encoding an outer membrane lipoprotein, is lethal for Escherichia coli and causes accumulation of lipoprotein localization intermediates in the periplasm. J Bacteriol. 2001;183:6538–6542. doi: 10.1128/JB.183.22.6538-6542.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeda K, Miyatake H, Yokota N, Matsuyama S, Tokuda H, Miki K. Crystal structures of bacterial lipoprotein localization factors, LolA and LolB. EMBO J. 2003;22:3199–3209. doi: 10.1093/emboj/cdg324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okuda S, Watanabe S, Tokuda H. A short helix in the C-terminal region of LolA is important for the specific membrane localization of lipoproteins. FEBS Lett. 2008;582:2247–2251. doi: 10.1016/j.febslet.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 56.Oguchi Y, Takeda K, Watanabe S, Yokota N, Miki K, Tokuda H. Opening and closing of the hydrophobic cavity of LolA coupled to lipoprotein binding and release. J Biol Chem. 2008;283:25414–25420. doi: 10.1074/jbc.M804736200. [DOI] [PubMed] [Google Scholar]
- 57.Miyamoto A, Matsuyama S, Tokuda H. Mutant of LolA, a lipoprotein-specific molecular chaperone of Escherichia coli, defective in the transfer of lipoproteins to LolB. Biochem Biophys Res Commun. 2001;287:1125–1128. doi: 10.1006/bbrc.2001.5705. [DOI] [PubMed] [Google Scholar]
- 58.Taniguchi N, Matsuyama S, Tokuda H. Mechanisms underlying energyindependent transfer of lipoproteins from LolA to LolB, which have similar unclosed {beta}-barrel structures. J Biol Chem. 2005;280:34481–34488. doi: 10.1074/jbc.M507388200. [DOI] [PubMed] [Google Scholar]
- 59.Tao K, Watanabe S, Narita S, Tokuda H. A periplasmic LolA derivative with a lethal disulfide bond activates the Cpx stress response system. J Bacteriol. 2010;192:5657–5662. doi: 10.1128/JB.00821-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe S, Oguchi Y, Takeda K, Miki K, Tokuda H. Introduction of a lethal redox switch that controls the opening and closing of the hydrophobic cavity in LolA. J Biol Chem. 2008;283:25421–25427. doi: 10.1074/jbc.M804737200. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi Y, Tsurumizu R, Tsukahara J, Takeda K, Narita SI, Mori M, Miki K, Tokuda H. Roles of the protruding loop of factor B essential for the localization of lipoproteins (LolB) in the anchoring of bacterial triacylated proteins to the outer membrane. J Biol Chem. 2014 doi: 10.1074/jbc.M113.539270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okuda S, Tokuda H. Lipoprotein sorting in bacteria. Annu Rev Microbiol. 2011;65:239–259. doi: 10.1146/annurev-micro-090110-102859. [DOI] [PubMed] [Google Scholar]
- 63.Remans K, Pauwels K, van Ulsen P, Buts L, Cornelis P, Tommassen J, Savvides SN, Decanniere K, Van Gelder P. Hydrophobic surface patches on LolA of Pseudomonas aeruginosa are essential for lipoprotein binding. J Mol Biol. 2010;401:921–930. doi: 10.1016/j.jmb.2010.06.067. [DOI] [PubMed] [Google Scholar]
- 64.Drage MG, Tsai HC, Pecora ND, Cheng TY, Arida AR, Shukla S, Rojas RE, Seshadri C, Moody DB, Boom WH, Sacchettini JC, Harding CV. Mycobacterium tuberculosis lipoprotein LprG (Rv1411c) binds triacylated glycolipid agonists of Toll-like receptor 2. Nat Struct Mol Biol. 2010;17:1088–1095. doi: 10.1038/nsmb.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsukahara J, Mukaiyama K, Okuda S, Narita S, Tokuda H. Dissection of LolB function--lipoprotein binding, membrane targeting and incorporation of lipoproteins into lipid bilayers. FEBS J. 2009;276:4496–4504. doi: 10.1111/j.1742-4658.2009.07156.x. [DOI] [PubMed] [Google Scholar]
- 66.Ito Y, Matsuzawa H, Matsuyama S, Narita S, Tokuda H. Genetic analysis of the mode of interplay between an ATPase subunit and membrane subunits of the lipoprotein-releasing ATP-binding cassette transporter LolCDE. J Bacteriol. 2006;188:2856–2864. doi: 10.1128/JB.188.8.2856-2864.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yakushi T, Yokota N, Matsuyama S, Tokuda H. LolA-dependent release of a lipid-modified protein from the inner membrane of Escherichia coli requires nucleoside triphosphate. J Biol Chem. 1998;273:32576–32581. doi: 10.1074/jbc.273.49.32576. [DOI] [PubMed] [Google Scholar]
- 68.Yakushi T, Masuda K, Narita S, Matsuyama S, Tokuda H. A new ABC transporter mediating the detachment of lipid-modified proteins from membranes. Nat Cell Biol. 2000;2:212–218. doi: 10.1038/35008635. [DOI] [PubMed] [Google Scholar]
- 69.Yasuda M, Iguchi-Yokoyama A, Matsuyama S, Tokuda H, Narita S. Membrane topology and functional importance of the periplasmic region of ABC transporter LolCDE. Biosci Biotechnol Biochem. 2009;73:2310–2316. doi: 10.1271/bbb.90451. [DOI] [PubMed] [Google Scholar]
- 70.Fukuda A, Matsuyama S, Hara T, Nakayama J, Nagasawa H, Tokuda H. Aminoacylation of the N-terminal cysteine is essential for Lol-dependent release of lipoproteins from membranes but does not depend on lipoprotein sorting signals. J Biol Chem. 2002;277:43512–43518. doi: 10.1074/jbc.M206816200. [DOI] [PubMed] [Google Scholar]
- 71.Gupta SD, Gan K, Schmid MB, Wu HC. Characterization of a temperaturesensitive mutant of Salmonella typhimurium defective in apolipoprotein N-acyltransferase. J Biol Chem. 1993;268:16551–16556. [PubMed] [Google Scholar]
- 72.Narita S, Tokuda H. Overexpression of LolCDE allows deletion of the Escherichia coli gene encoding apolipoprotein N-acyltransferase. J Bacteriol. 2011;193:4832–4840. doi: 10.1128/JB.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mizutani M, Mukaiyama K, Xiao J, Mori M, Satou R, Narita S, Okuda S, Tokuda H. Functional differentiation of structurally similar membrane subunits of the ABC transporter LolCDE complex. FEBS Lett. 2013;587:23–29. doi: 10.1016/j.febslet.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 74.Okuda S, Tokuda H. Model of mouth-to-mouth transfer of bacterial lipoproteins through inner membrane LolC, periplasmic LolA, and outer membrane LolB. Proc Natl Acad Sci USA. 2009;106:5877–5882. doi: 10.1073/pnas.0900896106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Masuda K, Matsuyama S, Tokuda H. Elucidation of the function of lipoprotein-sorting signals that determine membrane localization. Proc Natl Acad Sci U S A. 2002;99:7390–7395. doi: 10.1073/pnas.112085599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hara T, Matsuyama S, Tokuda H. Mechanism underlying the inner membrane retention of Escherichia coli lipoproteins caused by Lol avoidance signals. J Biol Chem. 2003;278:40408–40414. doi: 10.1074/jbc.M307836200. [DOI] [PubMed] [Google Scholar]
- 77.d’Enfert C, Ryter A, Pugsley AP. Cloning and expression in Escherichia coli of the Klebsiella pneumoniae genes for production, surface localization and secretion of the lipoprotein pullulanase. EMBO J. 1987;6:3531–3538. doi: 10.1002/j.1460-2075.1987.tb02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anderson JE, Sparling PF, Cornelissen CN. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol. 1994;176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ostberg KL, DeRocco AJ, Mistry SD, Dickinson MK, Cornelissen CN. Conserved regions of gonococcal TbpB are critical for surface exposure and transferrin iron utilization. Infect Immun. 2013;81:3442–3450. doi: 10.1128/IAI.00280-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pettersson A, Prinz T, Umar A, van der Biezen J, Tommassen J. Molecular characterization of LbpB, the second lactoferrin-binding protein of Neisseria meningitidis. MolMicrobiol. 1998;27:599–610. doi: 10.1046/j.1365-2958.1998.00707.x. [DOI] [PubMed] [Google Scholar]
- 81.Leuzzi R, Serino L, Scarselli M, Savino S, Fontana MR, Monaci E, Taddei A, Fischer G, Rappuoli R, Pizza M. Ng-MIP, a surface-exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol Microbiol. 2005;58:669–681. doi: 10.1111/j.1365-2958.2005.04859.x. [DOI] [PubMed] [Google Scholar]
- 82.Dashper SG, Hendtlass A, Slakeski N, Jackson C, Cross KJ, Brownfield L, Hamilton R, Barr I, Reynolds EC. Characterization of a novel outer membrane hemin-binding protein of Porphyromonas gingivalis. J Bacteriol. 2000;182:6456–6462. doi: 10.1128/jb.182.22.6456-6462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pride AC, Herrera CM, Guan Z, Giles DK, Trent MS. The outer surface lipoprotein VolA mediates utilization of exogenous lipids by Vibrio cholerae. MBio. 2013;4:e00305–e00313. doi: 10.1128/mBio.00305-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Ulsen P, van Alphen L, ten Hove J, Fransen F, van der Ley P, Tommassen J. A Neisserial autotransporter NalP modulating the processing of other autotransporters. Mol Microbiol. 2003;50:1017–1030. doi: 10.1046/j.1365-2958.2003.03773.x. [DOI] [PubMed] [Google Scholar]
- 85.Roussel-Jazede V, Grijpstra J, van Dam V, Tommassen J, van Ulsen P. Lipidation of the autotransporter NalP of Neisseria meningitidis is required for its function in the release of cell-surface-exposed proteins. Microbiology. 2013;159:286–295. doi: 10.1099/mic.0.063982-0. [DOI] [PubMed] [Google Scholar]
- 86.Coutte L, Willery E, Antoine R, Drobecq H, Locht C, Jacob-Dubuisson F. Surface anchoring of bacterial subtilisin important for maturation function. Mol Microbiol. 2003;49:529–539. doi: 10.1046/j.1365-2958.2003.03573.x. [DOI] [PubMed] [Google Scholar]
- 87.Jin S, Joe A, Lynett J, Hani EK, Sherman P, Chan VL. JlpA, a novel surfaceexposed lipoprotein specific to Campylobacter jejuni, mediates adherence to host epithelial cells. Mol Microbiol. 2001;39:1225–1236. doi: 10.1111/j.1365-2958.2001.02294.x. [DOI] [PubMed] [Google Scholar]
- 88.Kawai F, Paek S, Choi KJ, Prouty M, Kanipes MI, Guerry P, Yeo HJ. Crystal structure of JlpA, a surface-exposed lipoprotein adhesin of Campylobacter jejuni. J Struct Biol. 2012;177:583–588. doi: 10.1016/j.jsb.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manfredi P, Renzi F, Mally M, Sauteur L, Schmaler M, Moes S, Jeno P, Cornelis GR. The genome and surface proteome of Capnocytophaga canimorsus reveal a key role of glycan foraging systems in host glycoproteins deglycosylation. Mol Microbiol. 2011;81:1050–1060. doi: 10.1111/j.1365-2958.2011.07750.x. [DOI] [PubMed] [Google Scholar]
- 90.Brokx SJ, Ellison M, Locke T, Bottorff D, Frost L, Weiner JH. Genome-wide analysis of lipoprotein expression in Escherichia coli MG1655. J Bacteriol. 2004;186:3254–3258. doi: 10.1128/JB.186.10.3254-3258.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tokuda H, Matsuyama S, Tanaka-Masuda K. Structure, function and transport of lipoproteins in Escherichia coli. In: Ehrmann M, editor. The Periplasm. ASM Press; Herndon, VA: 2007. pp. 67–79. [Google Scholar]
- 92.Manning PA, Beutin L, Achtman M. Outer membrane of Escherichia coli: properties of the F sex factor traT protein which is involved in surface exclusion. J Bacteriol. 1980;142:285–294. doi: 10.1128/jb.142.1.285-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Drummelsmith J, Whitfield C. Translocation of group 1 capsular polysaccharide to the surface of Escherichia coli requires a multimeric complex in the outer membrane. EMBO J. 2000;19:57–66. doi: 10.1093/emboj/19.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robinson LS, Ashman EM, Hultgren SJ, Chapman MR. Secretion of curli fibre subunits is mediated by the outer membrane-localized CsgG protein. Mol Microbiol. 2006;59:870–881. doi: 10.1111/j.1365-2958.2005.04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cowles CE, Li Y, Semmelhack MF, Cristea IM, Silhavy TJ. The free and bound forms of Lpp occupy distinct subcellular locations in Escherichia coli. Mol Microbiol. 2011;79:1168–1181. doi: 10.1111/j.1365-2958.2011.07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pugsley AP. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pugsley AP, Poquet I, Kornacker MG. Two distinct steps in pullulanase secretion by Escherichia coli K12. Mol Microbiol. 1991;5:865–873. doi: 10.1111/j.1365-2958.1991.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 99.Sauvonnet N, Pugsley AP. Identification of two regions of Klebsiella oxytoca pullulanase that together are capable of promoting beta-lactamase secretion by the general secretory pathway. Mol Microbiol. 1996;22:1–7. doi: 10.1111/j.1365-2958.1996.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 100.Sauvonnet N, Pugsley AP. The requirement for DsbA in pullulanase secretion is independent of disulphide bond formation in the enzyme. Mol Microbiol. 1998;27:661–667. doi: 10.1046/j.1365-2958.1998.00722.x. [DOI] [PubMed] [Google Scholar]
- 101.Francetic O, Pugsley AP. Towards the identification of type II secretion signals in a nonacylated variant of pullulanase from Klebsiella oxytoca. J Bacteriol. 2005;187:7045–7055. doi: 10.1128/JB.187.20.7045-7055.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pugsley AP, Kornacker MG, Ryter A. Analysis of the subcellular location of pullulanase produced by Escherichia coli carrying the pulA gene from Klebsiella pneumoniae strain UNF5023. Mol Microbiol. 1990;4:59–72. doi: 10.1111/j.1365-2958.1990.tb02015.x. [DOI] [PubMed] [Google Scholar]
- 103.Campos M, Nilges M, Cisneros DA, Francetic O. Detailed structural and assembly model of the type II secretion pilus from sparse data. Proc Natl Acad Sci U S A. 2010;107:13081–13086. doi: 10.1073/pnas.1001703107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sauvonnet N, Vignon G, Pugsley AP, Gounon P. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J. 2000;19:2221–2228. doi: 10.1093/emboj/19.10.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Py B, Loiseau L, Barras F. An inner membrane platform in the type II secretion machinery of Gram-negative bacteria. EMBO Rep. 2001;2:244–248. doi: 10.1093/embo-reports/kve042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Collin S, Guilvout I, Nickerson NN, Pugsley AP. Sorting of an integral outer membrane protein via the lipoprotein-specific Lol pathway and a dedicated lipoprotein pilotin. Mol Microbiol. 2011;80:655–665. doi: 10.1111/j.1365-2958.2011.07596.x. [DOI] [PubMed] [Google Scholar]
- 107.Collin S, Krehenbrink M, Guilvout I, Pugsley AP. The targeting, docking and anti-proteolysis functions of the secretin chaperone PulS. Res Microbiol. 2013;164:390–396. doi: 10.1016/j.resmic.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 108.D’Enfert C, Pugsley AP. Klebsiella pneumoniae pulS gene encodes an outer membrane lipoprotein required for pullulanase secretion. J Bacteriol. 1989;171:3673–3679. doi: 10.1128/jb.171.7.3673-3679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hardie KR, Lory S, Pugsley AP. Insertion of an outer membrane protein in Escherichia coli requires a chaperone-like protein. EMBO J. 1996;15:978–988. [PMC free article] [PubMed] [Google Scholar]
- 110.Nickerson NN, Tosi T, Dessen A, Baron B, Raynal B, England P, Pugsley AP. Outer membrane targeting of secretin PulD protein relies on disordered domain recognition by a dedicated chaperone. J Biol Chem. 2011;286:38833–38843. doi: 10.1074/jbc.M111.279851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Collin S, Guilvout I, Chami M, Pugsley AP. YaeT-independent multimerization and outer membrane association of secretin PulD. Mol Microbiol. 2007;64:1350–1357. doi: 10.1111/j.1365-2958.2007.05743.x. [DOI] [PubMed] [Google Scholar]
- 112.Pugsley AP, Chapon C, Schwartz M. Extracellular pullulanase of Klebsiella pneumoniae is a lipoprotein. J Bacteriol. 1986;166:1083–1088. doi: 10.1128/jb.166.3.1083-1088.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guilvout I, Hardie KR, Sauvonnet N, Pugsley AP. Genetic dissection of the outer membrane secretin PulD: are there distinct domains for multimerization and secretion specificity? J Bacteriol. 1999;181:7212–7220. doi: 10.1128/jb.181.23.7212-7220.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen S, Kumru OS, Zückert WR. Determination of borrelia surface lipoprotein anchor topology by surface proteolysis. J Bacteriol. 2011;193:6379–6383. doi: 10.1128/JB.05849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huysmans GH, Guilvout I, Pugsley AP. Sequential steps in the assembly of the multimeric outer membrane secretin PulD. J Biol Chem. 2013;288:30700–30707. doi: 10.1074/jbc.M113.489112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roussel-Jazede V, Jongerius I, Bos MP, Tommassen J, van Ulsen P. NalPmediated proteolytic release of lactoferrin-binding protein B from the meningococcal cell surface. Infect Immun. 2010;78:3083–3089. doi: 10.1128/IAI.01193-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oomen CJ, van Ulsen P, van Gelder P, Feijen M, Tommassen J, Gros P. Structure of the translocator domain of a bacterial autotransporter. EMBO J. 2004;23:1257–1266. doi: 10.1038/sj.emboj.7600148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Magnet S, Bellais S, Dubost L, Fourgeaud M, Mainardi JL, Petit-Frere S, Marie A, Mengin-Lecreulx D, Arthur M, Gutmann L. Identification of the L,D-transpeptidases responsible for attachment of the Braun lipoprotein to Escherichia coli peptidoglycan. J Bacteriol. 2007;189:3927–3931. doi: 10.1128/JB.00084-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang WY, Wu HC. Alterations of the carboxyl-terminal amino acid residues of Escherichia coli lipoprotein affect the formation of murein-bound lipoprotein. J Biol Chem. 1992;267:19560–19564. [PubMed] [Google Scholar]
- 120.Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299:262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- 121.Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ, Kahne D. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell. 2005;121:235–245. doi: 10.1016/j.cell.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 122.Webb CT, Selkrig J, Perry AJ, Noinaj N, Buchanan SK, Lithgow T. Dynamic association of BAM complex modules includes surface exposure of the lipoprotein BamC. J MolBiol. 2012;422:545–555. doi: 10.1016/j.jmb.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ieva R, Bernstein HD. Interaction of an autotransporter passenger domain with BamA during its translocation across the bacterial outer membrane. Proc Natl Acad Sci U S A. 2009;106:19120–19125. doi: 10.1073/pnas.0907912106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bernstein HD. The double life of a bacterial lipoprotein. Mol Microbiol. 2011;79:1128–1131. doi: 10.1111/j.1365-2958.2011.07538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bergström S, Zückert WR. Structure, Function and Biogenesis of the Borrelia Cell Envelope. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Caister Academic Press; Norwich, UK: 2010. pp. 139–166. [Google Scholar]
- 126.Schulze RJ, Zückert WR. Borrelia burgdorferi lipoproteins are secreted to the outer surface by default. Mol Microbiol. 2006;59:1473–1484. doi: 10.1111/j.1365-2958.2006.05039.x. [DOI] [PubMed] [Google Scholar]
- 127.Kumru OS, Schulze RJ, Slusser JG, Zückert WR. Development and validation of a FACS-based lipoprotein localization screen in the Lyme disease spirochete Borrelia burgdorferi. BMC Microbiol. 2010;10:277. doi: 10.1186/1471-2180-10-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kumru OS, Schulze RJ, Rodnin MV, Ladokhin AS, Zückert WR. Surface Localization Determinants of Borrelia OspC/Vsp Family Lipoproteins. J Bacteriol. 2011;193:2814–2825. doi: 10.1128/JB.00015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen S, Zückert WR. Probing the Borrelia burgdorferi surface lipoprotein secretion pathway using a conditionally folding protein domain. J Bacteriol. 2011;193:6724–6732. doi: 10.1128/JB.06042-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lilly AA, Crane JM, Randall LL. Export chaperone SecB uses one surface of interaction for diverse unfolded polypeptide ligands. Protein Sci. 2009;18:1860–1868. doi: 10.1002/pro.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu G, Topping TB, Randall LL. Physiological role during export for the retardation of folding by the leader peptide of maltose-binding protein. Proc Natl Acad Sci U S A. 1989;86:9213–9217. doi: 10.1073/pnas.86.23.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Park S, Liu G, Topping TB, Cover WH, Randall LL. Modulation of folding pathways of exported proteins by the leader sequence. Science. 1988;239:1033–1035. doi: 10.1126/science.3278378. [DOI] [PubMed] [Google Scholar]
- 133.Randall LL, Hardy SJ. SecB, one small chaperone in the complex milieu of the cell. Cell Mol Life Sci. 2002;59:1617–1623. doi: 10.1007/PL00012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lenhart TR, Akins DR. Borrelia burgdorferi locus BB0795 encodes a BamA orthologue required for growth and efficient localization of outer membrane proteins. Mol Microbiol. 2010;75:692–709. doi: 10.1111/j.1365-2958.2009.07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zückert WR, Lloyd JE, Stewart PE, Rosa PA, Barbour AG. Cross-species surface display of functional spirochetal lipoproteins by recombinant Borrelia burgdorferi. Infect Immun. 2004;72:1463–1469. doi: 10.1128/IAI.72.3.1463-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Whetstine CR, Slusser JG, Zückert WR. Development of a single-plasmid-based regulatable gene expression system for Borrelia burgdorferi. Appl Environ Microbiol. 2009;75:6553–6558. doi: 10.1128/AEM.02825-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


