Abstract
Usage of nicotine-only formulations, such as transdermal patches, nicotine gum, or electronic nicotine delivery systems is increasing, as they are perceived as healthier alternatives to traditional cigarettes. Unfortunately, there is little data available on the effect of isolated nicotine on myocardial and aortic remodeling, especially in the setting of cardiovascular disease risk factors, such as hypertension. We hypothesized that nicotine would exacerbate cardiovascular remodeling induced by angiotensin-II (Ang II) treatment. Subcutaneous osmotic minipumps were implanted to administer Ang II, Nic, nicotine plus ANG II or saline to C57Bl/6 mice for 4 weeks. Heart weights were increased by all treatments, with control < nicotine < Ang II < nicotine + Ang II. Activity levels of matrix metalloproteinase (MMP-2) mirrored these changes and demonstrated clear additivity between nicotine and Ang II. Histopathological analysis of aortas revealed that mice receiving combined nicotine and Ang II treatment induced significant hypertrophy compared to all other groups. This study reveals possible cardiotoxic interactions between nicotine and a common model of systemic hypertension. Safety testing of novel nicotine delivery devices should consider that hypertension is a common impetus to begin smoking cessation therapy, and potential interactions should be more thoroughly studied.
Keywords: E-cigarettes, electronic nicotine delivery systems, cardiovascular toxicity, vascular remodeling, angiotensin
INTRODUCTION
The use of tobacco is an important cardiovascular risk factor that has been the focus of intense public health campaigns for over a decade. Despite efforts aimed at cessation, currently 19.3% of all Americans (45.3 million people) report smoking at least 9 cigarettes daily, and 52% of those describe attempting to quit at least once in the last year (1). Patients with hypertension, a risk factor for the development of heart failure, are invariably urged to quit smoking to slow disease progression.
To aid smoking cessation, nicotine replacement therapy (NRT) products such as transdermal delivery patches and nicotine gum have been developed and become commonplace. More recently, electronic nicotine delivery systems, or “e-cigarettes,” have been increasing in popularity. Unlike the previously noted NRT products, electronic cigarettes have not been evaluated for safety by the FDA, and little information is available to patients or their physicians as to the health impact of nicotine alone. While administration of pure nicotine avoids numerous toxicants found in traditional cigarette smoke including carbon monoxide, benzene, and acrolein, it remains an agent with well-known detrimental pharmacological and toxicological characteristics. There is conflicting data on how exposure to pure nicotine impacts cardiovascular disease, specifically.
Both smoking and hypertension are established drivers of cardiovascular disease, specifically aortic aneurysm formation and atherosclerosis (2). Cigarette exposure is shown to cause a moderate, transient worsening of hypertension (3). However, the relationship between nicotine alone and hypertension is complex. Some studies demonstrate increased heart rate and BP (2,4–7), whereas others find no effect (8–10). In addition to nicotine, complete cigarette smoke contains other vasoactive compounds, some of which are present in significant levels and have potent vasodilatory affects, including carbon monoxide (CO; 11). Thus, the cardiovascular effects of nicotine alone, without other toxicants that may antagonize or enhance nicotine effects, require further investigation to ensure safety for pertinent at-risk subpopulations.
In an effort to address these gaps in knowledge, a model of subchronic hypertension was used to evaluate the effects of nicotine exposure on myocardial and aortic remodeling. Studies of long-term tobacco smoke have shown cardiac enlargement in healthy rats, noting ultrastructural cardiomyocyte remodeling by electron microscopy (12). More recent work by Meurrens and colleagues with cigarette smoke in a rat model of spontaneous hypertension noted pronounced cardiac hypertrophy with associated increases in ventricular mRNA for atrial natriuretic factor and ornithine decarboxylase; the specific components of cigarette smoke that contributed to the cardiac enlargement were not identified (13). Therefore, we tested the hypothesis that nicotine exposure augments cardiac remodeling as evidenced by increased matrix metalloproteinase-2 (MMP-2) activity resulting in cardiac growth and increased aortic wall thickness, in an angiotensin-II (Ang II) model of hypertension.
METHODS
Animals
Mice (male, C57BL/6; 8 weeks old) were obtained from a commercial vendor (Taconic) and placed in 7 day quarantine prior to experimentation. Mice were housed in an AAALAC-approved facility with a 12h light:dark cycle and food and water available ad libitum throughout the study protocol. On day zero, mice were implanted with pumps containing saline, nicotine, Ang II, or nicotine+Ang II (N=6 per group) and followed for 4 weeks. All procedures were conducted with full approval of the University of New Mexico Institutional Care and Use Committee.
Experimental Protocol
Osmotic pumps (Alzet model 2ML4) were filled with sterile-filtered saline, nicotine and/or Ang II under aseptic conditions. Nicotine was delivered at a target rate of 5 mg/kg/day, and Ang II was delivered at 21.6 µg/d, calculated based on initial weight and assumed weight gain of 0.5 g per week (normal chow diet), for a total of 4 weeks. Pumps were primed in saline at 37°C for 4 hours prior to implantation. Subcutaneous pump implantation was conducted with mice briefly anesthetized with isofluorane. Mice were shaved at the incision site and scrubbed with betadine. A small dermal incision was made between the scapulae and the pump was gently inserted. The incision was closed with wound clips, which were removed 5 days later. After 4 weeks, the mice were anesthetized with isoflurane and intubated for ventilator support during a thoracotomy. A saline-filled catheter was inserted into the left ventricle to obtain cardiac pressure readings (ADInstruments, Inc, Colorado Springs, CO). Mice were then euthanized and hearts and aortas carefully dissected, weighed, and divided for freezing in liquid nitrogen.
Gelatinase Zymography
1–2mm2 samples of cardiac tissue were homogenized in 0.1% Triton X-100/Phosphate buffer, pH 7.4. A Bradford assay was performed on homogenate, to allow equal amounts of protein to be loaded onto a Tris/SDS 10% Polyacrylamide gel, with 0.1% Gelatin (Sigma #G-2500) substrate. Equal amounts of protein were diluted 1:1 with non-reducing loading buffer, loaded (without boiling) and run at 80V for 1.5 hours. Gel was transferred to LSCB buffer (0.05M Tris, pH 7.6; 0.02M NaCl; 5mM CaCl2; 0.02% Brij-35) and allowed to incubate for 48 hours at 37°C. The gel was then placed in Coomassie-G dye solution (0.8% Coomassie-G, 50% Methanol, 10% Acetic Acid in water) for one hour, followed by a 2.5-hour incubation in 10% acetic acid to destain. Gel was imaged and quantification of bands was performed using Image J.
Gene Analysis
Following euthanasia, mouse hearts were removed and stored in a −80° freezer. 1ml of Trizol reagent was used to homogenize a 0.5mm3 sample of apical myocardial muscle using Dynabeads in a Magnalyser for 120 seconds. RNA was extracted using the common Trizol protocol, resuspended in 50ul RNase free water and quantified on a Nanodrop 2000. cDNA was synthesized (Applied Biosystems) with standard cycling parameters using 100µg RNA per reaction. Primer/Probe sets were purchased from Applied Biosystems, (MMP-2, MMP-9, Ang2 Receptor, and ACE) and were used to analyze gene expression after dynamic range analysis was performed.
Histology
Abdominal aortas were dissected out and mounted on-end in OCT and snap frozen on dry ice. Thereafter, 4 µm cryosections were cut and placed on Fisher Superfrost slides. Sections were heat fixed overnight on a slide warmer at 37°C. Sections were stained with a standard Hematoxalin and Eosin stain, and imaged on the Olympus BX51 with an Olympus DP72 camera. Aortic wall thicknesses for each animal were measured using Image J (NIH, Bethesda) and determined by the mean area of three sections of each aorta. Diameters were determined by normalizing perimeter tracings to a true circle, as in vivo morphologies are closer to this assumption.
Statistics
All data were tested for normality and Guassian distributions were confirmed in all cases. For all data, a two-factor (nicotine vs. Ang II) ANOVA with Bonferroni post hoc testing (GraphPad Prism v5.02) was used to ascertain differences among groups. Probability values less than 0.05 were considered significant.
RESULTS
General
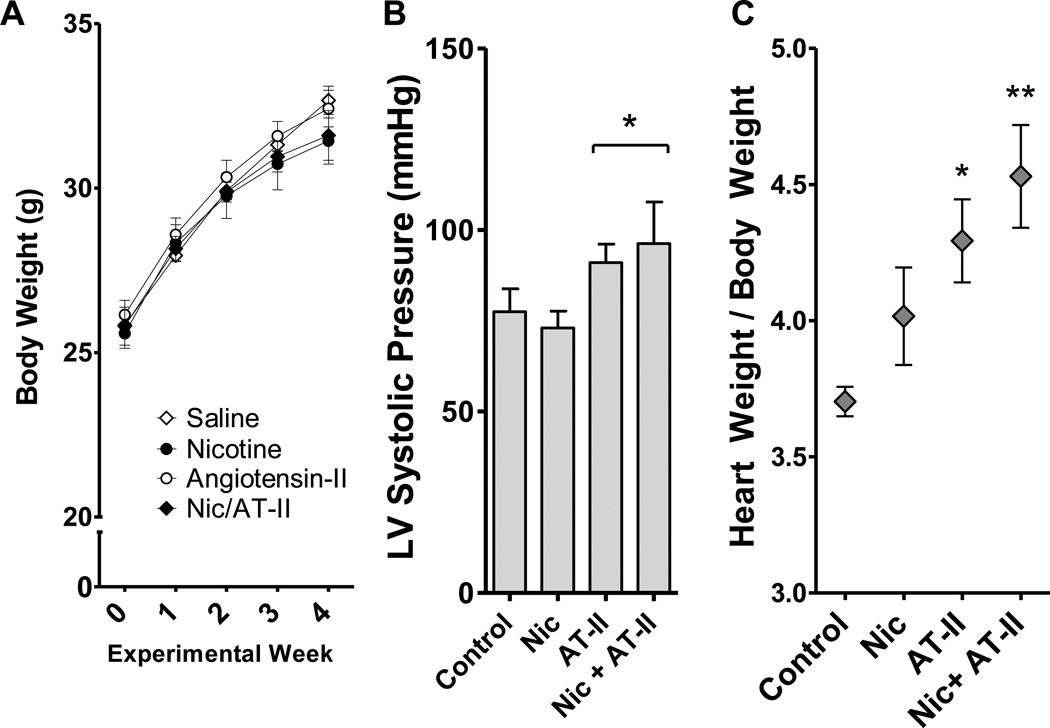
Mice in all four groups (saline control, nicotine alone, Ang II alone, nicotine+Ang II) gained appropriate weight during the study. A slight trend for reduced growth was noted in the mice receiving nicotine (Figure 1A), although it did not reach statistical significance by a 2-factor ANOVA. One mouse from the nicotine+Ang II group died early in the study (Day 9) and gross necropsy revealed substantial hemorrhage limited to the pericardial sac. Left ventricular pressures were determined at sacrifice under anesthesia, confirming that the Ang II treatment elevated systemic blood pressure (Figure 1B). While the data confirmed the efficacy of the Ang II model, the determination of arterial pressures under anesthesia was suboptimal. This caveat, combined with potentially inadequate statistical power, did not reveal potential interactions with Ang II and nicotine, which may be a crucial factor in the interpretation of this study.
Figure 1. Body and Heart Weight Changes Induced by Nic and ANG II.
A. Body weight (mean ± SEM) in four groups of mice throughout the experimental period: saline control (n=6), Nic-treated (n=6), ANG II-treated (n=6), and Nic + ANG II-treated (n=5). B. Left ventricular pressures obtained under anesthesia prior to euthanasia revealed a probable pressor effect of ANG II (p<0.05 for ANG II by 2-way ANOVA). Values overall were low due to the suboptimal methodology for collection, thus conclusions on a possible interaction with Nic are not feasible. C. The ratio of heart weight to body weight was calculated. Animals treated with ANG II alone and ANG II with Nic had a statistically significant increase in heart weight compared to controls when adjusted for body weight (*,** p<0.05, p<0.01, by 2-way ANOVA). No statistical interaction was noted for the combination treatment.
Cardiac Changes
Cardiac weights were elevated in both groups treated with Ang II (Figure 1C). The addition of nicotine appeared to further increase the cardiac mass relative to body weight, but this effect was not significantly elevated above Ang II, alone, and may have been largely related to the potential anorectic effects of nicotine, rather than an impact on the heart. Without normalizing to body weight, it is more evident that Ang II is the only factor that increases cardiac mass (not shown).
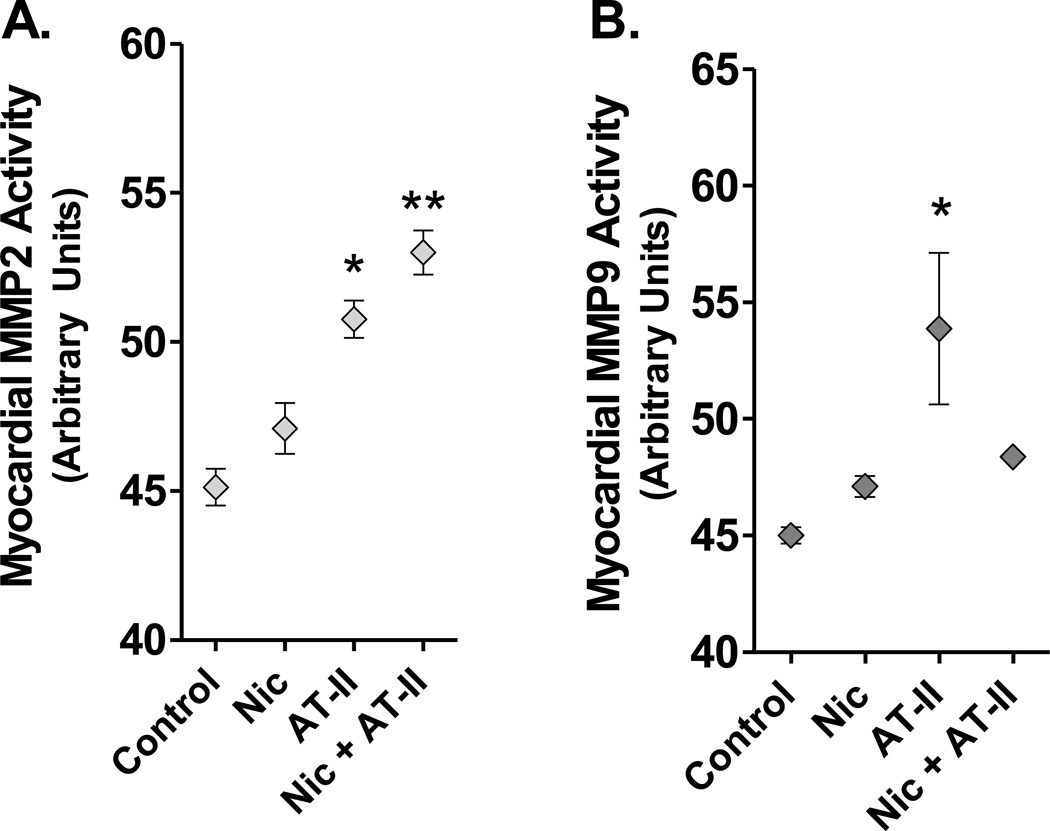
Activities of MMP-2 and -9 were determined in cardiac homogenates by gelatin zymography. Cardiac MMP-2 activity was substantially increased in Ang II-treated mice, with a further independent, additive effect induced by nicotine (Figure 2). Cardiac MMP-9 activity also was elevated by Ang II, but the addition of nicotine to this treatment appeared to downregulate MMP-9 activity, resulting in a differential balance of gelatinase activity. Gene expression analysis did not demonstrate increases in MMP-2, MMP-9, ACE, or Ang II receptor in response to exogenous Ang II, nicotine, or the combination thereof.
Figure 2. Cardiac Metalloproteinase Effects Induced by Nic and ANG II.
A. Activity of MMP-2 in myocardial homogenates. Exposure to Nic alone did not increase MMP-2 activity above baseline. The presence of Angiotensin II increased MMP-2 activity (*, p<0.05), which was further augmented by the presence of Nic(**, p<0.05). B. Activity of MMP-9 in myocardial homogenates. ANG II treatment significantly upregulated MMP-9 activity, but this was reduced when combined with Nic (*,** p<0.05, p<0.01, compared to control by 2-way ANOVA)..
Aortic Changes
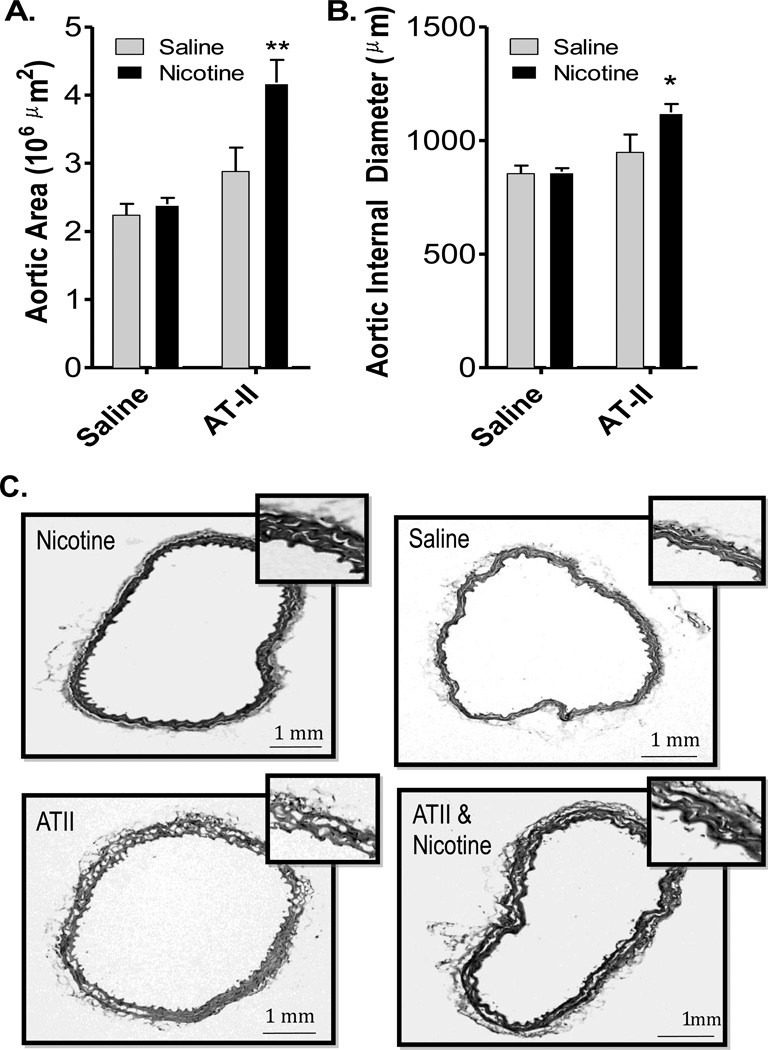
One mouse treated with Ang II, alone, was noted as having evidence of an aortic aneurism. Beyond that, no gross pathological changes were observed in any group. Aortic wall thickness was analyzed from cross sections outlining the inside vessel diameter and subtracting that from the outlined outside diameter. Neither nicotine nor Ang II alone induced thickening of the aortic wall. However, the combination treatment of nicotine +Ang II led to substantial thickening, nearly doubling the cross-sectional area of the vessels (Figure 3).
Figure 3. Interactive Effects of Nic and ANG II on Vascular Remodeling.
A. Cross-sectional aortic wall thickness (mean±SE) in micrometers2 in four treatment groups. B. Normalized internal diameter for aortic cross-sections, suggesting an eccentric remodeling in the Nic + ANG II group. Combined treatment of Nic + ANG II was the only permutation that significantly drove aortic hypertrophy. C. Representative images at 10× and 40× are shown for each group.
DISCUSSION
The data from the present study reveal an interactive relationship between nicotine and hypertension on cardiovascular remodeling. While the net arterial vascular pressure was not significantly increase above Ang II, alone, we observed a clear interaction between Nic and Ang II in terms of thickening of the aortic wall and indications of a complex alteration in cardiac growth. While the study design is descriptive, these interactions highlight the need to consider potentially additive impacts of nicotine in the setting of hypertension, as a number of cardiovascular pathologies may be adversely affected.
Numerous studies specifically implicate the renin-angiotensin system (RAS) in the development of hypertension and hypertension-mediated cardiovascular remodeling (14–16). The final product of the RAS, Ang II, regulates blood pressure through its effects on salt and volume balance, increased myocyte contractility, and stimulation of the vascular tone via the sympathetic nervous system (17). Other functions of Ang II include increases in cellular levels of reactive oxygen species (ROS) through its effects on mitochondria, inducing fibroblast proliferation, and intensified extracellular matrix deposition, all of which are features of heart failure. Clinically, it has been shown that Ang II expression is increased in humans following a myocardial infarction and in those with congestive heart failure (14,15). Furthermore, pharmacologic inhibition of Ang II inhibits detrimental cardiac remodeling in congestive heart failure, preserves renal function in diabetics and prevents the development of hypertension.
Previously published work suggests that exposure to nicotine alone can impact the vasculature by driving changes in endothelial and vascular smooth muscle cell physiology. Wang et al. (18) demonstrated that, in a mouse model of atherosclerosis, nicotine exposure increases the incidence of abdominal aortic aneurysms nearly two-fold in a MMP-2-dependent manner. This work is supported by previous data indicating that nicotine exposure drives proliferation of vascular smooth muscle and neointima formation following vascular injury (19). Other groups have published data linking nicotine exposure to endothelial cell gene expression of other markers of vascular dysfunction, such as eNOS, angiotensin converting enzyme (ACE) and plasminogen activator inhibitor-1 (20). The data presented in this paper adds to this body of work and has potentially important implications as to which patients should receive screening for abdominal aortic aneurism, as it is currently only recommended for all current or former cigarette smokers.
Little is known regarding the relationship of nicotine and the RAS to cardiac and vascular remodeling and hypertrophy. Aortic wall thickness has been linked to hypertension and older age (21). Cigarette smoking was first reported to be associated with cardiac hypertrophy more than 20 years ago (22,23), but determining which components of cigarette smoke are responsible has proved challenging. Li et al. (22) reported that cultured vascular smooth muscle cells and fibroblasts display a potent mitogenic response to nicotine and angiotensin, either when administered together or separately. Interestingly, Loennechen et al. (24) evaluated the effects of nicotine and CO on ventricular hypertrophy and concluded that cardiac remodeling is driven by CO mediated activation of endothelin-1. Nicotine alone, when uncoupled from vasodilatory factors such as CO and NO found in traditional cigarette smoke, has the potential to induce greater vascular constriction and worsen cardiac outcomes. Gene expression analysis of ACE and the Ang II receptor indicate this effect does not appear to be mediated through upregulation of these products as no detectable change between groups was observed.
Increased MMP-2 activity in the response to Ang II is not a new finding but how this relationship is impacted by nicotine exposure was not previously known. Castardeli and colleagues (25) reported MMP-2 or -9 activity is not increased in normotensive rats chronically exposed (4 months) to cigarette smoke. Our data support this finding, as nicotine alone did not have a statistically significant impact on MMP-2 or -9 activity. However, increased activity was demonstrated when nicotine is combined with Ang II. This unique finding points to an interactive relationship between Ang II and nicotine in cardiac remodeling. Other studies have reported induction of MMP-2 gene expression in response to mechanical stress in cultured vascular smooth muscle cells (26). Elevated LV pressures in animals receiving Ang II is likely partially driving the MMP-2 activity; however, LV pressures were not different in the group that received nicotine and Ang II compared to those that received Ang II alone. Because MMP-2 activation is augmented in the combined nicotine/Ang II group, this further supports the assertion there is an interactive relationship between Ang II and nicotine that is not simply due to hemodynamic effects.
It is important to note that the dosage used in the present study is likely high compared to the typical high initial dose of nicotine in a patch form (20–25 mg/day in a 70 kg subject). However, the mouse metabolism of nicotine is notoriously high (27) and the mice also insensitive to many of the neurobehavioral effects of nicotine, and thus we predict that the level used for the present study is a reasonable starting point for assessing potential adverse cardiovascular effects. Additionally, while nicotine delivery via patches is well-controlled, nicotine inhalation via electronic cigarettes may have greater potential for higher doses. Future research at various levels and in different species will clearly be necessary to fully understand the potential nicotine impact on human cardiovascular remodeling.
The present study focused primarily on gross morphological changes of the aorta and activation of specific remodeling pathways in the myocardium. These findings indicate that the effects of nicotine alone, independent of other vasoactive substances found in cigarette smoke, may promote vascular remodeling pathways, especially in the setting of systemic hypertension. This has important implications regarding the safety of nicotine-only delivery systems. Although these are publicly perceived as a safe alternative to smoking, substantial risks likely persist, especially to those with hypertension or other cardiovascular diseases. The present findings, along with that published by Wang et al., raise public health questions regarding the need for similar monitoring guidelines for those using alternative nicotine delivery systems as those that smoke regular cigarettes (18). Smokers that utilize the patch or switch to electronic cigarettes should be encouraged to use these modalities as a means to cessation, not a permanent replacement. Safety labeling should also be considered with special attention to those patients with uncontrolled hypertension and/or a history of coronary artery disease, congestive heart failure, or aneurysm formation.
Acknowledgements
This project was funded by a grant from the National Institutes of Health (ES014639).
Abbreviations
- Ang II
Angiotensin-2
- AAA
Abdominal Aortic Aneurysm
- ACE
Angiotensin-Converting Enzyme
- CO
Carbon Monoxide
- MMP
Matrix Metalloproteinase
- NRT
Nicotine Replacement Therapy
- RAS
Renin-Angiotensin System
- ROS
Reactive Oxygen Species
Footnotes
The authors declare they have no known conflicts of interest with the present work.
References
- 1.CDC Vital Signs: Current Cigarette Smoking Among Adults Aged ≥ 18 Years – United States, 2005–2010. [Accessed 12/10/2012]; www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/quitting/index.htm.
- 2.Krupski WC, Olive GC, Weber CA, Rapp JH. Comparative effects of hypertension and nicotine on injury induced myointimal thickening. Surg. 1987;102:409–415. [PubMed] [Google Scholar]
- 3.Benowitz NL, Hansson A, Jacob P., 3rd Cardiovascular effects of nasal and transdermal nicotine and cigarette smoking. Hypertens. 2002;39:1107–1112. doi: 10.1161/01.hyp.0000018825.76673.ea. [DOI] [PubMed] [Google Scholar]
- 4.Fishebein L, O’Brien P, Hutson A, Theriaque D, Stacpoole PW, Flotte T. Pharmacokinetics and pharmacodynamic effects fo nicotine nasal spray devices on cardiovascular and pulmonary function. J. Investig. Med. 2000;48:435–440. [PubMed] [Google Scholar]
- 5.Martins LC, Ferreira-Melo SE, Sabha M, Coelho OR, Yugar-Toledo JC, Quinaglia T, Moreira MM, Coca A, Moreno H., Jr Acute effects of pharmacotherapies in blood pressure in normotensive moderate smokers. Blood Pressure. 2009;18(4):255–260. doi: 10.3109/08037050903289606. [DOI] [PubMed] [Google Scholar]
- 6.Mundal HH, Hjemdahl P, Ghesdal K. Acute effects of low dose nicotine gum on platelet function in non-smoking hypertensive and normotensive men. Eur. J. Clin. Pharmacol. 1995;41:411–416. doi: 10.1007/BF00196854. [DOI] [PubMed] [Google Scholar]
- 7.Yugar-Toledo JC, Ferreira-Melo E, Sabha M, Nogueira EA, Coelho O, Colombo FMC, Irigoyen MC, Moreno H., Jr Blood pressure circadian rhythm and endothelial function in heavy smokers: Acute effects of transdermal nicotine. J. Clin. Hypertension. 2005;7:721–729. doi: 10.1111/j.1524-6175.2005.04597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury Z, Comans P, Lerer K, Gavish A, Tzivoni D. Effects of transdermal nicotine patches on ambulatory ecg monitoring findings: A double-blind study in healthy smokers. Cardiovasc. Drug Ther. 1996;10:179–184. doi: 10.1007/BF00823596. [DOI] [PubMed] [Google Scholar]
- 9.Mahmarian JJ, Moye LA, Nasser GA, Nagueh SF, Bloom MF, Benowitz NL, Verani M, Byrd W, Pratt C. Nicotine patch therapy in smoking cessation reduces the extent of exercise-induced myocardial ischemia. J. Am. Coll. Cardiol. 1997;30:125–130. doi: 10.1016/s0735-1097(97)00128-9. [DOI] [PubMed] [Google Scholar]
- 10.Tanus-Santos JE, Toledo JC, Cittadiono M, Sabha M, Rocha JC, Moreno H., Jr Cardiovascular effects of transdermal nicotine in mildly hypertensive smokers. Am. J. Hypertension. 2001;14:601–614. doi: 10.1016/s0895-7061(01)01301-2. [DOI] [PubMed] [Google Scholar]
- 11.Thorup C, Jose CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am. J. Physiol. 1999;277:F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- 12.Zornoff LA, Matsubara LS, Matsubara BB, Okoshi MP, Okoshi K, Dal Pai-Silva M, Carvalho RF, Cicogna AC, Padovani CR, Novelli EL, Novo R, Campana AO, Paiva SA. Beta-Carotene Supplementation Attenuates Cardiac Remodeling Induced by One-Month Tobacco-Smoke Exposure in Rats. Toxicol. Sci. 2006;90:259–266. doi: 10.1093/toxsci/kfj080. [DOI] [PubMed] [Google Scholar]
- 13.Meurrens K, Ruf S, Ross G, Schleef R, von Holt K, Schlüter KD. Smoking accelerates the progression of hypertension-induced myocardial hypertrophy to heart failure in spontaneously hypertensive rats. Cardiovasc. Res. 2007;76:311–322. doi: 10.1016/j.cardiores.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Danser J, van Kesteren C, Bax W, Tavenier M, Derkx F, Saxena P, Schalekamp M. Prorenin, renin, angiotensinogen, and angiotensin-converting enzyme in normal and failing human hearts: Evidence for renin binding. Circ. 1997;96:220–226. doi: 10.1161/01.cir.96.1.220. [DOI] [PubMed] [Google Scholar]
- 15.Iwata M, Cowling R, Yeo S, Greenberg B. Targeting the ACE2-Ang-(1–7) pathway in cardiac fibroblasts to treat cardiac remodeling and heart failure. J. Mol. Cell. Cardiol. 2011;51:542–547. doi: 10.1016/j.yjmcc.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malayeri AA, Natori S, Bahrami H, Bertoni AG, Kronmal R, Lima JA, Bluemke DA. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the Multi-Ethnic Study of Atherosclerosis (MESA)) Am. J. Cardiol. 2008;102:491–496. doi: 10.1016/j.amjcard.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schluter KD, Wenzel S. Angiotensin II: A hormone involved in and contributing to pro-hypertrophic cardiac networks and target of anti-hypertrophic cross-talks. Pharmacol. Therapeut. 2008;119:311–325. doi: 10.1016/j.pharmthera.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Zhang C, Zhang M, Liang B, Zhu H, Lee J, Voillet B, Xia L, Zhang Y, Zou MH. Activation of AMP-activated protein kinase α2 by Nic instigates formation of abdominal aortic aneurysms in mice in vivo. Nat. Med. 2012;18:902–910. doi: 10.1038/nm.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Day I, Ye S. Nicotine-induced changes in gene expression by human coronary artery endothelial cells. Atheroscler. 2001;154:277–283. doi: 10.1016/s0021-9150(00)00475-5. [DOI] [PubMed] [Google Scholar]
- 20.Osergren JB. Angiotensin receptor blockade with candesartan in heart failure: Findings from the Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity (CHARM) Programme. J. Hypertension, suppl. 2006;24:S3–S7. doi: 10.1097/01.hjh.0000220400.08128.fa. [DOI] [PubMed] [Google Scholar]
- 21.Fernie JM, Douglas AN, Lamb D, Ruckley VA. Right ventricular hypertrophy in a group of coalworkers. Thorax. 1983;38:436–442. doi: 10.1136/thx.38.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li JM, Cui TX, Shiuch T, Liu HW, Min LJ, Okumura M, Jinno T, Wu L, Iwai M, Horiuchi M. Arterio. Thromb. Vasc. Biol. 2004;24:80–84. doi: 10.1161/01.ATV.0000104007.17365.1c. [DOI] [PubMed] [Google Scholar]
- 23.Shasha SM, Damal H, Kristal B, Galetzky G, Roguin N, Shkolnik T. Red cell filterability in cigarette smokers and its relations to cardiac hypertrophy. Atherosclerosis. 1993;98:91–98. doi: 10.1016/0021-9150(93)90226-k. [DOI] [PubMed] [Google Scholar]
- 24.Loennechen J, Nilsen O, Arbo I, Aadahl P, Nilsen T, Waldum H, Sandvik A, Ellingsen O. Chronic exposure to carbon monoxide and nicotine: endothelin ETA receptor antagonism attenuates carbon monoxide-induced myocardial hypertrophy in rat. Toxicol. Appl. Pharmacol. 2002;178:8–14. doi: 10.1006/taap.2001.9300. [DOI] [PubMed] [Google Scholar]
- 25.Castardeli E, Duarte D, Minicucci M, Azevedo P, Matsubara B, Matusbara L, Campana A, Paiva SA, Zornoff L. Tobacco smoke-induced left ventricular remodelling is not associated with metalloproteinase-2 or -9 activation. Euro. J. Heart Fail. 2007;9:1081–1085. doi: 10.1016/j.ejheart.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 26.O’Callagan C, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells. Hypertension. 2000;36:319–324. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- 27.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]