Abstract Abstract
Pentraxin-3 (PTX3) is a protein mediator of innate immunity that is elevated in the setting of left heart disease and pulmonary arterial hypertension. The relationship between PTX3 and right ventricular (RV) structure and function is not known. We included men and women with magnetic resonance imaging assessment of RV structure and function and measurement of PTX3 from the Multi-Ethnic Study of Atherosclerosis, a study of individuals free of clinical cardiovascular disease. Multivariable linear regression estimated associations between PTX3 protein levels and RV measures after adjusting for demographic characteristics, anthropometrics, smoking status, diabetes mellitus, hypertension, and corresponding left ventricular (LV) parameters. Instrumental variable analysis exploiting Mendelian randomization was attempted using two-stage least squares regression. The study sample included 1,779 participants with available PTX3 levels, RV measures, and all covariables. Mean PTX3 level was 2.1 ng/mL. Higher PTX3 was independently associated with greater RV mass and larger RV end-diastolic volume with and without adjustment for the corresponding LV parameters or C-reactive protein (all P < .05). There was no association between PTX3 and RV ejection fraction or stroke volume. Single-nucleotide polymorphisms were not associated with PTX3 protein levels or RV measures after accounting for race. Instrumental variable analysis could not be reliably performed. Higher PTX3 protein levels were associated with greater RV mass and larger RV end-diastolic volume. These associations were independent of common cardiovascular risk factors and LV morphologic changes. Inflammation is associated with differences in the pulmonary circulation-RV axis in adults without clinical cardiovascular disease.
Keywords: pulmonary hypertension, heart failure, inflammation, right ventricle, Mendelian randomization
Introduction
Pentraxin-3 (PTX3) is a protein mediator of innate immunity produced by vascular smooth muscle cells, endothelial cells, and fibroblasts. PTX3 may dampen the immune response through interruption of the complement cascade, apoptotic clearance, and mitigation of atherosclerosis.1 PTX3 is found in atherosclerotic plaques, and increased circulating levels are seen in diastolic dysfunction, metabolic syndrome, and myocarditis.2-5 High PTX3 levels also suggest poor prognosis in chronic heart failure and other diseases.6-8
PTX3 may also be important in pulmonary vascular disease and right ventricular (RV) dysfunction. High PTX3 levels are reported in patients with pulmonary hypertension,9 and local control of inflammation is hypothesized to influence RV adaptation.10 Furthermore, specific PTX3 genotypes are associated with increased risk for primary graft dysfunction after lung transplant, for which pulmonary hypertension is also a known risk factor.11,12 The relationship between PTX3 and RV structure and function has not been studied.
We examined the relationship of PTX3 with magnetic resonance imaging (MRI) measures of RV structure and function in a multiethnic cohort of adults free of clinical cardiovascular disease. We hypothesized that higher PTX3 protein levels would be independently associated with greater RV mass, larger end-diastolic volume, and reduced RV ejection fraction.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter prospective cohort study designed to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease in whites, African Americans, Hispanics, and Chinese Americans.13 In 2000–2002, MESA recruited 6,814 men and women aged 45–84 years old from 6 US communities: Forsyth County, NC; northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St. Paul, MN; Chicago, IL; and Los Angeles, CA. Exclusion criteria included clinical cardiovascular disease (physician-diagnosed heart attack, stroke, transient ischemic attack, heart failure, angina, current atrial fibrillation, and any cardiovascular procedure), weight greater than 136 kg (300 lb), pregnancy, or impediment to long-term participation. The institutional review boards of all participating institutions approved the protocols of MESA and all studies described herein. The MESA-Right Ventricle Study, which includes this investigation, is an ancillary study with funding to interpret approximately 4,200 cardiac MRIs for RV morphology in MESA participants at the baseline examination.
Cardiac MRI measures
The cardiac MRI protocol has been previously described.14 All imaging was performed on 1.5-tesla magnets. Imaging consisted of fast-gradient echo cine images with temporal resolution of 50 ms or less. Cardiac MRI examination findings were analyzed at the reading center at Johns Hopkins University in Baltimore, Maryland. Image analysis was performed by two independent analysts on Windows workstations using QMASS software (Medis, Leiden, the Netherlands). Images were magnified to 250%, contrast and brightness were set to 55, and window width and level were set with auto function in QMASS.
Methods for interpretation of left ventricle (LV) and RV parameters have been reported elsewhere.15,16 Endocardial and epicardial borders of the RV were manually traced on MRI short-axis cine images at end-systole and end-diastole. Full visualization of the correct placement of RV contours relied on evaluation of cine images to determine the demarcation between the right atrium and the RV. Contours were modified at basal slices of the heart by careful identification of the tricuspid valve to exclude the right atrium and to avoid overestimation of the volumes. The outflow tract was included in RV volume. Papillary muscles and trabeculae were included in RV volumes and excluded from RV mass, as is commonly done for LV mass.17,18
RV end-systolic volume and RV end-diastolic volume (RVEDV) were calculated using Simpson’s rule by summation of areas on each slice multiplied by the sum of slice thickness and image gap. RV mass was determined at the end-diastole phase as the difference between end-diastolic epicardial and endocardial volumes of the RV free wall multiplied by the specific gravity of the heart (1.05 g/mL). RV stroke volume (SV) was calculated by subtracting RV end-systolic volume from the RVEDV. RVEF was calculated by dividing RVSV by RVEDV.
Our protocol included random blinded rereads by the same reader. The intrareader intraclass correlation coefficient for RV mass was 0.94 (229 scans); for RVEDV, it was 0.99 (230 scans); and for RVEF, it was 0.89 (230 scans). In addition, blinded rereads by a second reader were performed. The interreader intraclass correlation coefficients on 240 scans for RV mass, RVEDV, and RVEF were 0.89, 0.96, and 0.80, respectively.
Biomarkers and covariables
Fasting blood samples were obtained, processed, and stored using standardized procedures.19 PTX3 was measured as part of another ancillary study involving 2,880 MESA participants. Participants underwent novel biomarker testing and were selected to achieve balanced representation from all four race/ethnic groups to allow sufficient power to conduct subgroup analyses in each racial/ethnic group. PTX3 was measured at the Laboratory for Clinical Biochemistry Research at University of Vermont (Burlington) using a sandwich enzyme-linked immunosorbent assay (PTX3 [human] detection set; Alexis Biochemicals, San Diego, CA). The analytic coefficient of variation was 10.2%.
Genotyping was attempted in a random sample of participants in MESA who gave consent for genetic analysis and from whom DNA was extracted from peripheral leukocytes using a commercially available isolation platform (Puregene; Minneapolis, MN).20 Genotyping was performed by Illumina Genotyping Services (San Diego, CA) using the GoldenGate Assay. After removal of failed single-nucleotide polymorphisms (SNPs) and samples, the genotyping call rate was 99.93%. The human PTX3 gene, located on chromosome 3q25.32, consists of 3 exons separated by 2 introns and covering 7 kilobases.21 Several SNPs have been described in the PTX3 gene, including SNPs with potential functional significance. The GAG haplotype for sites rs2305619, rs3816527, and rs1840680, in particular, has been associated with increased PTX3 protein levels,22 a reduced risk of tuberculosis,23 a reduced risk of Pseudomonas species colonization in cystic fibrosis,21 and improved female fertility.22 There were 6 available SNPs in the PTX3 gene locus considered for analysis.
Other covariables, including race/ethnicity, height, weight, presence of hypertension or diabetes mellitus, C-reactive protein (CRP), smoking status, spirometry, and emphysema, were measured as described elsewhere (see Appendix).24
Statistical analysis
We used linear regression to characterize the relationship between PTX3 and RV parameters. PTX3 was log transformed to achieve normality. All models were adjusted for height and weight, so it was not necessary to index RV parameters to account for differences in body size. Generalized additive models were used to assess possible nonlinearity. Covariables were chosen on the basis of known associations with ventricular size, heart disease, and comorbidities. In limited models, we adjusted for age, sex, race/ethnicity, height, and weight. In fully adjusted models, we included educational level, income, smoking status, hypertension, diabetes mellitus, cholesterol, and impaired glucose tolerance. We adjusted for LV parameters to evaluate for independence from LV abnormalities (e.g., increased LV mass causing pulmonary venous hypertension leading to increased RV mass) and to better account for differences in body size. Because RVSV and LVSV are interdependent, we adjusted for LV mass in this case. We adjusted for both structural and functional lung disease using percentage emphysema on chest computed tomography, forced expiratory volume at 1 second, and forced vital capacity, when available (n = 1,360). Because of theoretical antagonism between CRP and PTX3, we performed additional analyses adjusted for CRP level (n = 1,772).
In participants with available SNPs in the PTX3 gene locus (n = 1,582), departures from Hardy-Weinberg equilibrium were assessed using the Fisher exact test. Multiple linear regression was used to characterize the relationship between candidate genotypes/haplotypes and PTX3 protein levels or RV measures in models with and without adjustment for race and principal components.25 Because the true nature of inheritance is unknown, both additive and dominant models were assessed. Instrumental variable analysis exploiting Mendelian randomization was attempted using two-stage least squares regression.26
Statistical significance was defined as P < .05 for all nongenetic analyses. For genetic analyses, we used a Bonferroni correction for 12 comparisons so that P < .004 was considered significant. Analyses were performed using STATA 12.0 (StataCorp, College Station, TX). Results of genetic analyses were confirmed using the Golden Helix software package (Bozeman, MT).
Results
There were 6,814 men and women enrolled in MESA (Fig. 1), of whom 5,098 underwent cardiac MRI and 5,004 (98%) had interpretable examinations for the LV. The RV was successfully interpreted in 4,204 participants (95% of 4,424 attempted). PTX3 was measured in 1,856 of these participants under another ancillary study. Seventy-seven participants were excluded for missing covariables, leaving 1,779 in the study sample. Table 1 shows characteristics of the study sample compared with those excluded. The mean age of the study sample was 60.7 years, and 46.1% of the participants were men. As compared with MESA participants not included in the analysis, the study sample had a larger proportion of Chinese race and fewer whites, which reflects oversampling of this racial group in the PTX3 ancillary study design. Formal statistical testing is not appropriate for such comparisons, which are descriptive rather than inferential. Mean RV mass in the study sample was 20.9 ± 4.5 g, mean RVEDV was 121.3 ± 30.5 mL, mean RVSV was 84.6 ± 20.2 mL, and mean RVEF was 70.3% ± 6.6%. Mean PTX3 level was 2.1 ng/mL, with an intraquartile range of 1.4–2.5 ng/mL.
Figure 1.
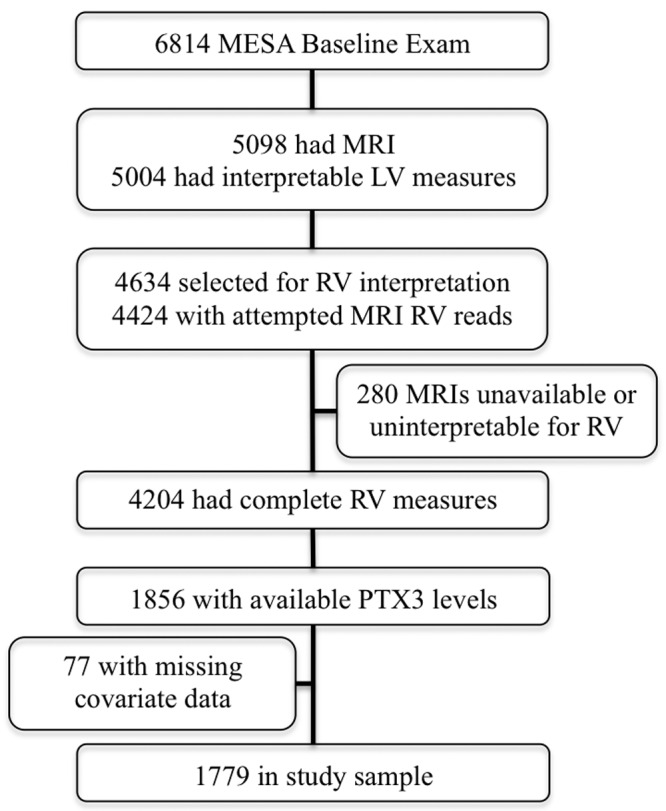
Study sample. LV: left ventricle; MESA: Multi-Ethnic Study of Atherosclerosis; MRI: magnetic resonance imaging; PTX3: pentraxin-3; RV: right ventricle.
Table 1.
Characteristics of the study sample compared with participants not included in the analysis
| Variable | Study sample (n = 1,779) | Excluded (n = 5,035) |
|---|---|---|
| Age, mean ± SD, years | 60.7 ± 10.0 | 62.7 ± 10.3 |
| Male sex | 46.1 | 47.5 |
| Race/ethnicity | ||
| White | 26.6 | 42.7 |
| Chinese American | 26.5 | 6.6 |
| African American | 23.5 | 29.3 |
| Hispanic | 23.4 | 21.4 |
| Educational attainment | ||
| No high school degree | 19.2 | 17.6 |
| High school degree | 17.6 | 18.4 |
| Some college | 14.4 | 17.0 |
| Bachelor’s degree | 18.1 | 17.0 |
| Higher than bachelor’s degree | 17.9 | 18.1 |
| Height, mean ± SD, cm | 165.1 ± 9.8 | 166.8 ± 10.1 |
| Weight, mean ± SD, kg | 74.5 ± 16.3 | 80.1 ± 17.4 |
| Body mass index, mean ± SD | 27.2 ± 5.0 | 28.7 ± 5.6 |
| Cigarette smoking status | ||
| Never | 57.2 | 47.9 |
| Former | 30.0 | 38.9 |
| Current | 12.8 | 13.2 |
| Hypertension | 40.7 | 46.5 |
| Systolic blood pressure, mean ± SD, mmHg | 124.6 ± 21.0 | 127.3 ± 21.6 |
| Diastolic blood pressure, mean ± SD, mmHg | 71.7 ± 10.0 | 72.0 ± 10.3 |
| Diabetes mellitus | 12.9 | 13.8 |
| PTX3, mean ± SD, ng/mL | 2.1 ± 1.2 | 2.2 ± 1.6a |
Data are % of patients, unless otherwise indicated. Body mass index was defined as weight in kilograms divided by the square of height in meters. PTX3: pentraxin-3; SD: standard deviation.
A total of 1,007 participants with measured PTX3 were not included in the study sample because of unavailable magnetic resonance imaging or missing covariables.
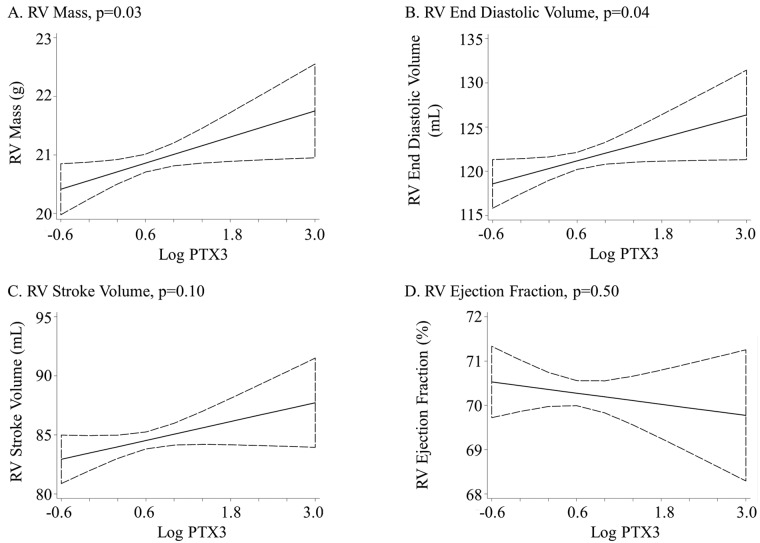
Higher PTX3 was associated with greater RV mass (0.3-g increase per log increase in PTX3, P = .04). This relationship was not altered by adjustment for cardiovascular risk factors (P = .03) or LV mass (P = .02; Table 2; Fig. 2). Further adjustment for lung structure and function did not alter the relationship between PTX3 and RV mass in the 1,360 participants with these measures (0.4-g increase per log increase in PTX3, P = .03). Adjustment for CRP did not change the relationship between PTX3 and RV mass (0.4-g increase per log increase in PTX3, P = .02; Table 3).
Table 2.
Change in right ventricular (RV) measures per log increase in pentraxin-3 (PTX3) protein level
| Per log increase in PTX3 | |||
|---|---|---|---|
| Variable | Coefficient | 95% CI | P |
| RV mass, g | |||
| Limited model | 0.3 | 0.0–0.7 | .04 |
| Adjusted model | 0.4 | 0.0–0.7 | .03 |
| Adjusted plus LV mass | 0.4 | 0.1–0.7 | .02 |
| RVEDV, mL | |||
| Limited model | 1.8 | −0.3 to 3.9 | .09 |
| Adjusted model | 2.2 | 0.1–4.3 | .04 |
| Adjusted plus LVEDV | 2.3 | 0.8–3.8 | .003 |
| RVSV, mL | |||
| Limited model | 1.0 | −0.5 to 2.6 | .19 |
| Adjusted model | 1.3 | −0.2 to 2.9 | .10 |
| Adjusted plus LV mass | 1.3 | −0.2 to 2.8 | .08 |
| RVEF, % | |||
| Limited model | −0.2 | −0.9 to 0.4 | .44 |
| Adjusted model | −0.2 | −0.8 to 0.4 | .50 |
| Adjusted plus LVEF | −0.2 | −0.7 to 0.4 | .55 |
The limited model includes age, sex, race/ethnicity, height, and weight. The adjusted model includes the limited model plus education, income, smoking status, presence of hypertension or diabetes mellitus, cholesterol level, and impaired glucose tolerance. CI: confidence interval; EDV: end-diastolic volume; EF: ejection fraction; LV: left ventricle; SV: stroke volume.
Figure 2.
Association between the log of pentraxin-3 (PTX3) protein level and right ventricle (RV) parameters. Linear regression adjusted for age, sex, race/ethnicity, education, income, height, weight, smoking status, presence of hypertension or diabetes mellitus, cholesterol, and impaired glucose tolerance.
Table 3.
Change in right ventricle (RV) measures per log increase in pentraxin-3 (PTX3) protein level accounting for differences in lung structure, function, and C-reactive protein level
| Per log increase in PTX3 | |||
|---|---|---|---|
| Variable | Coefficient | 95% CI | P |
| Adjusted for lung structure and function (n = 1,360) | |||
| RV mass, g | 0.4 | 0.0–0.8 | .03 |
| RVEDV, mL | 2.0 | −0.3 to 4.4 | .09 |
| RVSV, mL | 1.1 | −0.7 to 2.8 | .23 |
| RVEF, % | −0.3 | −1.0 to 0.4 | .39 |
| Adjusted for C-reactive protein (n = 1,772) | |||
| RV mass, g | 0.4 | 0.1–0.7 | .02 |
| RVEDV, mL | 2.4 | 0.3–4.5 | .03 |
| RVSV, mL | 1.4 | −0.1 to 2.9 | .07 |
| RVEF, % | −0.2 | −0.9 to 0.4 | .45 |
Lung structure and function adjustment included the adjusted model plus forced expiratory volume at 1 second, forced vital capacity, and percentage emphysema on chest computed tomography. C-reactive protein adjustment included the adjusted model plus C-reactive protein level. CI: confidence interval; EDV: end-diastolic volume; EF: ejection fraction; LV: left ventricle; RV: right ventricle; SV: stroke volume.
Higher PTX3 protein level had a borderline association with larger RVEDV after limited adjustment (1.8-mL increase per log increase in PTX3, P = .09). Full adjustment accounting for confounding by cardiovascular risk factors strengthened the relationship (P = .04), as did additional adjustment for LVEDV (P = .003; Table 2; Fig. 2). Additional adjustment for lung structure and function attenuated the relationship (2.0-mL increase per log increase in PTX3, P = .09), and additional adjustment for CRP did not change the relationship between PTX3 and RVEDV (2.4-mL increase per log increase in PTX3, P = .03; Table 3).
PTX3 protein level was not associated with RVSV or RVEF (Table 2; Fig. 2). Adjustment for cardiovascular risk factors, LV mass or ejection fraction, CRP level, or lung structure and function did not alter these results (Table 2, 3).
In exploratory analyses, there was no effect modification by age, sex, race/ethnicity, or LV parameters. No significant nonlinearity was detected using generalized additive models (all P > .2).
Of 1,779 participants, 1,582 had genotypes available for analysis (86.4%). Of available PTX3 SNPs, rs3845978, rs9289983, and rs2614 were not in Hardy-Weinberg equilibrium (Table S1; Tables S1–S5 available online). When stratified by race/ethnicity, only rs2614 was not in Hardy-Weinberg equilibrium (Table S2). None of the 5 candidate genes with a minor allele frequency greater than 5% (rs3845978, rs2305619, rs2120243, rs1456099, and rs9289983) were associated with PTX3 protein levels, RV mass, or RVEDV after adjustment for principal components (Table 4). Additional adjustment for race/ethnicity did not change these results. There was no significant interaction with race/ethnicity. Exploratory relationships stratified by race/ethnicity approached but did not achieve significance for some evaluated SNPs (Tables S3, S4). The first-stage F statistic in all instrumental variable analyses was less than 10, which suggests a poor instrument. The weak association between selected SNPs and PTX3 protein level precluded meaningful analysis using this technique (Table S5).27
Table 4.
Single-nucleotide polymorphism (SNP) association with pentraxin-3 (PTX3), right ventricle (RV) mass, and RV end-diastolic volume (EDV)
| Variable | Genetic model (P values) | |||||
|---|---|---|---|---|---|---|
| Association with PTX3 protein level | Association with RV mass | Association with RVEDV | ||||
| Additive | Dominant | Additive | Dominant | Additive | Dominant | |
| rs3845978 | ||||||
| Unadjusted | .03 | .07 | .007 | .02 | .03 | .08 |
| Adjusted | .24 | .46 | .23 | .38 | .48 | .76 |
| rs2305619 | ||||||
| Unadjusted | .57 | .37 | .008 | .03 | .005 | .01 |
| Adjusted | .85 | .83 | .76 | .80 | .53 | .46 |
| rs2120243 | ||||||
| Unadjusted | .75 | .70 | .11 | .10 | .07 | .04 |
| Adjusted | .68 | .83 | .95 | .82 | .75 | .44 |
| rs1456099 | ||||||
| Unadjusted | .49 | .73 | .32 | .27 | .14 | .29 |
| Adjusted | .55 | .75 | .96 | .97 | .56 | .94 |
| rs9289983 | ||||||
| Unadjusted | .36 | .59 | .19 | .10 | .07 | .10 |
| Adjusted | .37 | .57 | .97 | .87 | .59 | .84 |
Adjusted model includes principal components.
Discussion
We have shown that higher PTX3 protein levels are associated with greater RV mass and larger RVEDV in a multicity cohort of adults without clinical cardiovascular disease. The relationship with RV mass was not strongly affected by adjustment for differences in pulmonary structure and function or by differences in respective LV mass, which suggests an RV-specific relationship. MESA participants had a 0.3-g (1.4%) increase in RV mass per log increase in PTX3. This is similar to the difference seen in LV mass (2.4%) in MESA participants with and without diabetes and may support the clinical and biological relevance of this association.28 In addition, RV hypertrophy in MESA is associated with a threefold higher risk for cardiovascular death or heart failure.29
Our findings suggest the importance of PTX3 in the pulmonary circulation–RV axis. An increasingly nuanced understanding of inflammation in the pulmonary circulation and RV is necessary as immunomodulators such as FK506 are considered as treatments for PAH.30 Inflammation is broadly implicated in pulmonary vascular disease, and recent work confirms that PTX3 is elevated in pulmonary arterial hypertension.9,31 Our findings may reflect subclinical pulmonary vascular disease with decreased ability to recruit the pulmonary vasculature, leading to increased afterload, RV hypertrophy, and dilation. The LV undergoes similar hypertrophy even in response to mild systemic hypertension; hypertrophy in the thin-walled RV may be more pronounced.32 Unlike systemic hypertension, however, small changes in the pulmonary vasculature typically do not result in increased resting pulmonary arterial pressure due to the large-flow volume reserve of the pulmonary vasculature.33
A direct relationship between PTX3 and RV structure independent of pulmonary vascular disease may also contribute to our findings. Myocardial inflammation can promote LV hypertrophy both in the presence and absence of pathologic afterload.34-36 The pathophysiologic role of inflammation in determining RV structure may explain the variable susceptibility to right heart failure that exists among patients with identical afterload.37
Although CRP and PTX3 plasma levels are both elevated in pulmonary hypertension, their relationship with the RV differs in adults without clinical cardiovascular disease. High levels of CRP are associated with lower RV mass and a smaller RVEDV, which is opposite to the greater mass and larger RVEDV that we currently describe with high levels of PTX3.9,38,39 Animal models have found that CRP potentiates and PTX3 dampens myocardial inflammation and that, in patients with heart failure, rosuvastatin decreases CRP levels while increasing PTX3 protein levels.7,40-42 The different relationships of CRP and PTX3 to RV structure cautiously support this paradigm of distinct and opposing biologic roles for CRP and PTX3 in cardiac pathophysiology and expand this paradigm to the RV.
Finally, we evaluated several SNPs in the PTX3 gene locus and found no association with PTX3 protein level or RV structure after accounting for race/racial stratification. Previous analyses of similar PTX3 genotypes have shown increased risk for Pseudomonas in patients with cystic fibrosis and increased PTX3 protein levels and primary graft dysfunction in patients who undergo lung transplant.11,21 Both cystic fibrosis and lung transplant are pathologically inflamed states. The strong relationship of PTX3 genotype to aspects of these diseases, not seen in our largely healthy cohort, may suggest that the studied PTX3 genotypes have a greater effect on PTX3 levels only in the setting of robust inflammation. The lack of association with previously reported genotypes may therefore reflect the lack of pathologic inflammation in the relatively healthy MESA cohort and the relevance of these genotypes only in the setting of such inflammation.
This study has limitations. As is possible in all observational studies, residual or unmeasured confounding could account for the results. Our study was cross-sectional, and causality cannot be assessed. Unfortunately, measurement of invasive pulmonary hemodynamics, which may have informed the mechanism underlying our results, was not feasible in this large study of community-dwelling participants free of cardiovascular disease. Finally, this cohort is reasonably healthy, leading to relatively small effect estimates. However, such estimates are similar in scale to other clinically significant associations in MESA.28
Summary. Higher PTX3 levels are associated with greater RV mass and larger RVEDV. These relationships are independent of left-sided cardiovascular disease. This study contributes to the understanding of inflammation in the pulmonary circulation-RV axis at a time when immunomodulators are being considered for use in pulmonary hypertension.
Acknowledgments
This manuscript was reviewed by the Multi-Ethnic Study of Atherosclerosis (MESA) investigators for scientific content and consistency of data interpretation with previous MESA publications, and significant comments were incorporated before submission for publication. We thank the other investigators, staff, and participants of the MESA and MESA-Lung Studies for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
This work was presented in part as an abstract at the American Thoracic Society Meeting (Philadelphia; May 2013).
Appendix. Supplemental Material
Supplemental Methods: Covariables
Race/ethnicity was self-reported during the baseline Multi-Ethnic Study of Atherosclerosis (MESA)–Right Ventricle Study examination using categories from the 2000 US Census. Height was measured to the nearest 0.1 cm with the participant in stocking feet, and weight was measured to the nearest pound with the participant in light clothing using a balance scale. Education was self-reported in 8 categories that ranged from no schooling to graduate or professional school. Gross family income was self-reported in 13 categories that ranged from a minimum of less than $5,000 to a maximum of greater than $100,000. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current use of anti-hypertension medication. C-reactive protein level was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring, Deerfield, IL). Presence of diabetes mellitus was based on self-report of a physician diagnosis, use of medication for hyperglycemia, or fasting glucose value ≥126 mg/dL, measured by rate reflectance spectrophotometry (Johnson & Johnson Clinical Diagnostics, Rochester, NY). Fasting glucose level of 100–125 mg/dL was considered impaired fasting glucose. Smoking status was classified as current, past, or never. Spirometry and computed tomographic lung density measures of percentage emphysema were available for the subset of coparticipants in a separate ancillary study (MESA-Lung).43,44
Table S1.
Hardy-Weinberg equilibrium (HWE) of the single-nucleotide polymorphisms (SNPs) in the pentraxin-3 gene locus
| SNP | Observed genotype counts | Observed allele counts | Minor allele frequency, % | Expected counts under HWE | Exact P |
|---|---|---|---|---|---|
| rs3845978 (n = 1,585) | AA = 34; AG = 306; GG = 1,245 | A = 374; G = 2,796 | 12 | AA = 22; AG = 330; GG = 1,233 | .01 |
| rs2305619 (n = 1,583) | AA = 335; AG = 764; GG = 484 | A = 1,434; G = 1,732 | 45 | AA = 325; AG = 784; GG = 474 | .31 |
| rs2120243 (n = 1,580) | AA = 237; AC = 740; CC = 603 | A = 1,214; C = 1,946 | 38 | AA = 233; AC = 748; CC = 599 | .71 |
| rs1456099 (n = 1,582) | AA = 330; AT = 744; TT = 508 | A = 1,404; T = 1,760 | 44 | AA = 312; AT = 781; TT = 490 | .06 |
| rs9289983 (n = 1,582) | AA = 311; AG = 719; GG = 554 | A = 1,341; G = 1,827 | 42 | AA = 284; AG = 773; GG = 525 | .01 |
| rs2614 (n = 1,582) | AA = 5; AG = 55; GG = 1,522 | A = 65; G = 3,099 | 2 | AA = 1; AG = 64; GG = 1,518 | .00 |
Table S2.
Hardy-Weinberg equilibrium of the single-nucleotide polymorphisms (SNPs) in the pentraxin-3 gene locus stratified by race
| SNP | White (n = 412) | Chinese American (n = 441) | African American (n = 341) | Hispanic (n = 391) |
|---|---|---|---|---|
| rs3845978 | .99 | .31 | .48 | .41 |
| rs2305619 | .49 | .60 | .38 | .61 |
| rs2120243 | .77 | .74 | .49 | .52 |
| rs1456099 | .43 | .45 | .27 | .76 |
| rs9289983 | .43 | .57 | .86 | .61 |
| rs2614 | .00 | .00 | .06 | .00 |
Exact P values are reported.
Table S3.
Unadjusted single-nucleotide polymorphism (SNP) association with pentraxin-3 (PTX3), right ventricle (RV) mass, and RV end-diastolic volume (EDV) stratified by race using an additive genetic model of inheritance
| P value | |||||
|---|---|---|---|---|---|
| SNP | Interaction P | White (n = 412) | Chinese American (n = 441) | African American (n = 341) | Hispanic (n = 391) |
| PTX3 | |||||
| rs3845978 | .16 | .16 | .008 | .91 | .76 |
| rs2305619 | .31 | .50 | .79 | .20 | .52 |
| rs2120243 | .70 | .98 | .22 | .91 | .59 |
| rs1456099 | .31 | .63 | .03 | .70 | .56 |
| rs9289983 | .37 | .63 | .03 | .98 | .60 |
| RV mass | |||||
| rs3845978 | .69 | .46 | .17 | .49 | .42 |
| rs2305619 | .76 | .47 | .87 | .10 | .93 |
| rs2120243 | .68 | .44 | .54 | .26 | .67 |
| rs1456099 | .23 | .33 | .21 | .03 | .49 |
| rs9289983 | .20 | .33 | .20 | .01 | .61 |
| RVEDV | |||||
| rs3845978 | .78 | .83 | .17 | .87 | .92 |
| rs2305619 | .98 | .77 | .33 | .39 | .94 |
| rs2120243 | .93 | .65 | .57 | .80 | .48 |
| rs1456099 | .53 | .66 | .74 | .07 | .61 |
| rs9289983 | .47 | .66 | .71 | .02 | .83 |
Table S4.
Adjusted single-nucleotide polymorphism (SNP) association with pentraxin-3 (PTX3), right ventricle (RV) mass, and RV end-diastolic volume (EDV) stratified by race using an additive genetic model of inheritance
| P value | |||||
|---|---|---|---|---|---|
| SNP | Interaction P | White (n = 412) | Chinese American (n = 441) | African American (n = 341) | Hispanic (n = 391) |
| PTX3 | |||||
| rs3845978 | .19 | .18 | .01 | .68 | .96 |
| rs2305619 | .35 | .46 | .84 | .20 | .69 |
| rs2120243 | .67 | .92 | .24 | .83 | .66 |
| rs1456099 | .38 | .62 | .04 | .59 | .89 |
| rs9289983 | .45 | .62 | .04 | .80 | .96 |
| RV mass | |||||
| rs3845978 | .57 | .77 | .14 | .40 | .27 |
| rs2305619 | .80 | .35 | .75 | .15 | .96 |
| rs2120243 | .76 | .34 | .49 | .38 | .60 |
| rs1456099 | .37 | .33 | .14 | .10 | .32 |
| rs9289983 | .36 | .33 | .13 | .05 | .41 |
| RVEDV | |||||
| rs3845978 | .77 | .74 | .13 | .97 | .82 |
| rs2305619 | .99 | .59 | .42 | .54 | .86 |
| rs2120243 | .91 | .51 | .65 | .99 | .43 |
| rs1456099 | .73 | .67 | .55 | .24 | .33 |
| rs9289983 | .76 | .67 | .51 | .11 | .47 |
Adjusted model includes principal components.
Table S5.
Mendelian randomization using single-nucleotide polymorphisms (SNPs) in the pentraxin-3 gene locus as the instrumental variable in 2-stage least squares regression of the relationship with right ventricle (RV) mass and end-diastolic volume (EDV)
| Relationship with RV mass | Relationship with RVEDV | |||||
|---|---|---|---|---|---|---|
| SNP | P | First-stage F statistic | Bias likely | P | First-stage F statistic | Bias likely |
| Unadjusted | ||||||
| rs3845978 | .06 | 3.2 | Yes | .09 | 3.2 | Yes |
| rs2305619 | .42 | 0.5 | Yes | .40 | 0.5 | Yes |
| rs2120243 | .71 | 0.1 | Yes | .70 | 0.1 | Yes |
| rs1456099 | .64 | 0.3 | Yes | .47 | 0.3 | Yes |
| rs9289983 | .57 | 0.5 | Yes | .39 | 0.5 | Yes |
| Adjusted | ||||||
| rs3845978 | .28 | 1.4 | Yes | .30 | 1.4 | Yes |
| rs2305619 | .93 | 0.2 | Yes | .84 | 0.2 | Yes |
| rs2120243 | .77 | 0.1 | Yes | .79 | 0.1 | Yes |
| rs1456099 | .92 | 0.2 | Yes | .56 | 0.2 | Yes |
| rs9289983 | .87 | 0.5 | Yes | .58 | 0.5 | Yes |
Adjusted model includes principal components.
References Cited Only in the Appendix
- 43.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society–recommended spirometry reference values in a multiethnic sample of adults: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Chest 2010;137:138–145. [DOI] [PMC free article] [PubMed]
- 44.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, Reinhardt J, et al. Reproducibility and validity of lung density measures from cardiac CT scans: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol 2009;16:689–699. [DOI] [PMC free article] [PubMed]
Source of Support: The National Institutes of Health supported this work (RC1-HL100543, R01-HL086719, K24-103844, R01-HL077612, N01-HC95159 through HC95165, and N01-HC95169). This publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR000421.
Conflict of Interest: None declared.
Supplements
Appendix: Supplemental MaterialPulmCirc-004-250.s001.pdf (410.7KB, pdf)
References
- 1.Inforzato A, Jaillon S, Moalli F, Barbati E, Bonavita E, Bottazzi B, Mantovani A, Garlanda C. The long pentraxin PTX3 at the crossroads between innate immunity and tissue remodelling. Tissue Antigens 2011;77(4):271–282. [DOI] [PubMed]
- 2.Savchenko A, Imamura M, Ohashi R, Jiang S, Kawasaki T, Hasegawa G, Emura I, et al. Expression of pentraxin 3 (PTX3) in human atherosclerotic lesions. J Pathol 2008;215(1):48–55. [DOI] [PubMed]
- 3.Liu Q, Tu T, Bai Z, Zhou S. Pentraxin 3 as a new biomarker of diastolic dysfunction. Int J Cardiol 2011;152(1):106–107. [DOI] [PubMed]
- 4.Zanetti M, Bosutti A, Ferreira C, Vinci P, Biolo G, Fonda M, Valente M, Cattin L, Guarnieri G, Barazzoni R. Circulating pentraxin 3 levels are higher in metabolic syndrome with subclinical atherosclerosis: evidence for association with atherogenic lipid profile. Clin Exp Med 2009;9(3):243–248. [DOI] [PubMed]
- 5.Nebuloni M, Pasqualini F, Zerbi P, Lauri E, Mantovani A, Vago L, Garlanda C. PTX3 expression in the heart tissues of patients with myocardial infarction and infectious myocarditis. Cardiovasc Pathol 2011;20(1):e27–e35. [DOI] [PubMed]
- 6.Dubin R, Shlipak M, Li Y, Ix J, de Boer IH, Jenny N, Peralta CA. Racial differences in the association of pentraxin-3 with kidney dysfunction: the Multi-Ethnic Study of Atherosclerosis. Nephrol Dial Transplant 2011;26(6):1903–1908. [DOI] [PMC free article] [PubMed]
- 7.Latini R, Gullestad L, Masson S, Nymo SH, Ueland T, Cuccovillo I, Vårdal M, et al. Pentraxin-3 in chronic heart failure: the CORONA and GISSI-HF trials. Eur J Heart Failure 2012;14(9):992–999. [DOI] [PubMed]
- 8.Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM. Associations of pentraxin 3 with cardiovascular disease and all-cause death: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 2009;29(4):594–599. [DOI] [PMC free article] [PubMed]
- 9.Tamura Y, Ono T, Kuwana M, Inoue K, Takei M, Yamamoto T, Kawakami T, et al. Human pentraxin 3 (PTX3) as a novel biomarker for the diagnosis of pulmonary arterial hypertension. PLoS ONE 2012;7(9):e45834. [DOI] [PMC free article] [PubMed]
- 10.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 2009;135(3):794–804. [DOI] [PubMed]
- 11.Diamond JM, Meyer NJ, Feng R, Rushefski M, Lederer DJ, Kawut SM, Lee JC, et al. Variation in PTX3 is associated with primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2012;186(6):546–552. [DOI] [PMC free article] [PubMed]
- 12.Fang A, Studer S, Kawut SM, Ahya VN, Lee J, Wille K, Lama V, et al. Elevated pulmonary artery pressure is a risk factor for primary graft dysfunction following lung transplantation for idiopathic pulmonary fibrosis. Chest 2011;139(4):782–787. [DOI] [PMC free article] [PubMed]
- 13.Bild DE David A. Bluemke, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156(9):871–881. [DOI] [PubMed]
- 14.Natori S. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. Am J Roentgenol 2006;186(6 suppl 2):S357–S365. [DOI] [PubMed]
- 15.Chahal H, Johnson C, Tandri H, Jain A, Hundley WG, Barr RG, Kawut SM, Lima JAC, Bluemke DA. Relation of cardiovascular risk factors to right ventricular structure and function as determined by magnetic resonance imaging (results from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 2010;106(1):110–116. [DOI] [PMC free article] [PubMed]
- 16.Bluemke DA, Kronmal RA, Lima JAC, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events. J Am Coll Cardiol 2008;52(25):2148–2155. [DOI] [PMC free article] [PubMed]
- 17.Vogel-Claussen J, Finn JP, Gomes AS, Hundley GW, Jerosch-Herold M, Pearson G, Sinha S, Lima JAC, Bluemke DA. Left ventricular papillary muscle mass: relationship to left ventricular mass and volumes by magnetic resonance imaging. J Comput Assist Tomogr 2006;30(3):426–432. [DOI] [PubMed]
- 18.Winter MM, Bernink FJ, Groenink M, Bouma BJ, van Dijk APJ, Helbing WA, Tijssen JGP, Mulder BJM. Evaluating the systemic right ventricle by CMR: the importance of consistent and reproducible delineation of the cavity. J Cardiovasc Magn Reson 2008;10:40. [DOI] [PMC free article] [PubMed]
- 19.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 1995;41(2):264–270. [PubMed]
- 20.Bielinski SJ, Pankow JS, Li N, Hsu FC, Adar SD, Jenny NS, Bowden DW, Wasserman BA, Arnett D. ICAM1 and VCAM1 polymorphisms, coronary artery calcium, and circulating levels of soluble ICAM-1: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2008;201(2):339–344. [DOI] [PMC free article] [PubMed]
- 21.Chiarini M, Sabelli C, Melotti P, Garlanda C, Savoldi G, Mazza C, Padoan R, et al. PTX3 genetic variations affect the risk of Pseudomonas aeruginosa airway colonization in cystic fibrosis patients. Genes Immun 2010;11(8):665–670. [DOI] [PMC free article] [PubMed]
- 22.May L, Kuningas M, van Bodegom D, Meij HJ, Frolich M, Slagboom PE, Mantovani A, Westendorp RGJ. Genetic variation in pentraxin (PTX) 3 gene associates with PTX3 production and fertility in women. Biol Reprod 2010;82(2):299–304. [DOI] [PubMed]
- 23.Olesen R, Wejse C, Velez DR, Bisseye C, Sodemann M, Aaby P, Rabna P, et al. DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immun 2007;8(6):456–467. [DOI] [PubMed]
- 24.Leary PJ, Barr RG, Bluemke DA, Bristow MR, Hough CL, Kronmal RA, Lima JA, McClelland RL, Tracy RP, Kawut SM. Von Willebrand factor and the right ventricle (the MESA-Right Ventricle Study). Am J Cardiol 2012;110(12):1846–1851. [DOI] [PMC free article] [PubMed]
- 25.Collins-Schramm HE, Chima B, Morii T, Wah K, Figueroa Y, Criswell LA, Hanson RL, et al. Mexican American ancestry-informative markers: examination of population structure and marker characteristics in European Americans, Mexican Americans, Amerindians and Asians. Hum Genet 2004;114(3):263–271. [DOI] [PubMed]
- 26.Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008;27(8):1133–1163. [DOI] [PubMed]
- 27.Burgess S, Thompson SG, CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 2011;40(3):755–764. [DOI] [PubMed]
- 28.Heckbert SR, Post W, Pearson GDN, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol 2006;48(11):2285–2292. [DOI] [PMC free article] [PubMed]
- 29.Kawut SM, Barr RG, Lima JAC, Praestgaard A, Johnson WC, Chahal H, Ogunyankin KO, et al. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the Multi-Ethnic Study of Atherosclerosis (MESA)–Right Ventricle Study. Circulation 2012;126(14):1681–1688. [DOI] [PMC free article] [PubMed]
- 30.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 2013;123:3600–3613. [DOI] [PMC free article] [PubMed]
- 31.Chami El H, Hassoun PM. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Prog Cardiovasc Dis 2012;55(2):218–228. [DOI] [PMC free article] [PubMed]
- 32.Milan A, Caserta MA, Dematteis A, Naso D, Pertusio A, Magnino C, Puglisi E, et al. Blood pressure levels, left ventricular mass and function are correlated with left atrial volume in mild to moderate hypertensive patients. J Hum Hypertens 2009;23(11):743–750. [DOI] [PubMed]
- 33.Burrowes KS, Clark AR, Tawhai MH. Blood flow redistribution and ventilation-perfusion mismatch during embolic pulmonary arterial occlusion. Pulm Circ 2011;1(3):365–376. [DOI] [PMC free article] [PubMed]
- 34.Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, Kassiri Z, et al. Tumor necrosis factor-α mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation 2007;115(11):1398–1407. [DOI] [PubMed]
- 35.Kubota T, Bounoutas GS, Miyagishima M, Kadokami T, Sanders VJ, Bruton C, Robbins PD, McTiernan CF, Feldman AM. Soluble tumor necrosis factor receptor abrogates myocardial inflammation but not hypertrophy in cytokine-induced cardiomyopathy. Circulation 2000;101(21):2518–2525. [DOI] [PubMed]
- 36.Thaik CM, Calderone A, Takahashi N, Colucci WS. Interleukin-1β modulates the growth and phenotype of neonatal rat cardiac myocytes. J Clin Invest 1995;96(2):1093–1099. [DOI] [PMC free article] [PubMed]
- 37.Voelkel NF, Gomez-Arroyo JG, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Resp J 2012;40(6):1555–1565. [DOI] [PMC free article] [PubMed]
- 38.Quarck R, Nawrot T, Meyns B, Delcroix M. C-reactive protein: a new predictor of adverse outcome in pulmonary arterial hypertension. J Am Coll Cardiol 2009;53(14):1211–1218. [DOI] [PubMed]
- 39.Harhay MO, Tracy RP, Bagiella E, Barr RG, Pinder D, Hundley WG, Bluemke DA, Kronmal RA, Lima JAC, Kawut SM. Relationship of CRP, IL-6, and fibrinogen with right ventricular structure and function: the MESA-Right Ventricle Study. Int J Cardiol 2013;168(4):3818–3824. [DOI] [PMC free article] [PubMed]
- 40.Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JGF, Cornel JH, Dunselman P, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007;357(22):2248–2261. [DOI] [PubMed]
- 41.Bisoendial RJ, Boekholdt SM, Vergeer M, Stroes ESG, Kastelein JJP. C-reactive protein is a mediator of cardiovascular disease. Eur Heart J 2010;31(17):2087–2091. [DOI] [PubMed]
- 42.Salio M, Chimenti S, De Angelis N, Molla F, Maina V, Nebuloni M, Pasqualini F, Latini R, Garlanda C, Mantovani A. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation 2008;117(8):1055–1064. [DOI] [PubMed]