Abstract
Purpose
It has been demonstrated that large numbers of tumor-specific T cells for adoptive cell transfer (ACT) can be manufactured by retroviral genetic engineering of autologous peripheral blood lymphocytes and expanding them over several weeks. In mouse models, this therapy is optimized when administered with dendritic cell (DC) vaccination. We developed a short one-week manufacture protocol to determine the feasibility, safety and antitumor efficacy of this double cell therapy.
Experimnetal Design
A clinical trial (NCT00910650) adoptively transferring MART-1 T cell receptor (TCR) transgenic lymphocytes together with MART-1 peptide pulsed DC vaccination in HLA-A2.1 patients with metastatic melanoma. Autologous TCR transgenic cells were manufactured in 6 to 7 days using retroviral vector gene transfer, and re-infused with (n = 10) or without (n = 3) prior cryopreservation.
Results
14 patients with metastatic melanoma were enrolled and nine out of 13 treated patients (69%) showed evidence of tumor regression. Peripheral blood reconstitution with MART-1-specific T cells peaked within two weeks of ACT indicating rapid in vivo expansion. Administration of freshly manufactured TCR transgenic T cells resulted in a higher persistence of MART-1-specific T cells in the blood as compared to cryopreserved. Evidence that DC vaccination could cause further in vivo expansion was only observed with ACT using non-cryopreserved T cells.
Conclusion
Double cell therapy with ACT of TCR engineered T cells with a very short ex vivo manipulation and DC vaccines is feasible and results in antitumor activity, but improvements are needed to maintain tumor responses.
Keywords: MART-1 TCR, short ex vivo transgenic T cell manufacture, adoptive cell therapy, DC vaccine, clinical trial
Introduction
The genetic transfer of alpha and beta chains of the T cell receptor (TCR) endows recipient T cells with the specificity of donor T cells (1), allowing the generation of large numbers of T cells with uniform antigen. Initial clinical experiences testing ACT of TCR engineered T cells in humans have provided clear evidence of antitumor efficacy in patients with metastatic melanoma and sarcoma (2-4). Pioneering TCR engineering clinical trials by Steven A. Rosenberg and colleagues at the Surgery Branch, National Cancer Institute (Bethesda, MD) (2, 3, 5) included a 2-4-week ex vivo T cell culture. Preclinical models suggest that extended ex vivo expansion of lymphocytes before ACT results in more terminally differentiated cells with limited proliferation ability in vivo and lower antitumor activity (6, 7). Provision of antigen in the form of a vaccine is required in some animal models to support the antitumor activity of adoptively transferred T cells (8-10). This may be because exposure to antigen while undergoing homeostatic proliferation can stimulate further T cell expansion (11, 12).
To test this combined cell therapy approach in the clinic, the UCLA/Caltech F5 clinical trial was designed with a short, one-week, cell manipulation that included initial lymphocyte activation followed by retroviral transduction and limited further ex vivo cell expansion. We also provided autologous MART-126-35 peptide-loaded dendritic cell (DC), a vaccine that in our prior experience had resulted per se in two durable complete responders out of 25 patients with metastatic melanoma. These responses are durable over 10 years later (13, 14).
Patients and Methods
Study design and conduct
A Simon optimal two-stage phase II clinical trial design (15) was used to allow for the simultaneous testing of three co-primary endpoints, safety, feasibility and objective tumor response. Patients were enrolled in the clinical trial after signing a written informed consent approved by the UCLA IRB (#08-02-020 and #10-001212) under an investigational new drug (IND) filed with the US Food and Drug Administration (IND# 13859). The study was conducted in accordance with local regulations, the guidelines for Good Clinical Practice (GCP), and the principles of the current version of the Declaration of Helsinki. The study had the clinical trial registration number NCT00910650.
Trial eligibility and screening procedures
Eligible patients were HLA-A*0201 by molecular subtyping, had progressive locally advanced (stage IIIc) or metastatic melanoma (stage IV) with either no available standard therapeutic options with a curative intent, or who had progressed on standard options like chemotherapy, high dose IL-2, interferon and experimental therapies as listed in table 1, the melanoma was MART-1-positive by immunohistochemistry (IHC), age greater than or equal to 18, ECOG performance status 0 or 1, life expectancy greater than 3 months, adequate organ function as routinely required to receive high dose IL-2 (16), and seronegative for HIV, Hepatitis B and C. Patients with clinically active brain metastases were excluded. Baseline radiological documentation of absence of active brain metastases was required for all patients, but previously treated brain metastases were acceptable. All patients underwent formal ophthalmologic and otological exams at baseline and periodically after TCR engineered ACT.
Table 1.
Patient demographics and outcomes.
| Pt Study# | Sex (M/F) | Age | Prior Treatments for sIII-IV | Active Metastasis Sites | Stage | Protocol amendment | # F5 TCR transgenic cells | Cryo or Fresh | # Doses IL-2 | # Doses DCs | Evidence of transient tumor response | Response at EOS (day 90) | PFS (mo) | OS (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F5-1 | M | 60 | - | Lung, Stomach, Liver, Pancreas, Peritoneum, Soft tissues, bone |
M1c | 1-5 | 1 × 109 | Cryo | 12/14 | 3/3 | Yes by PET/CT | Progression | 3 | 5 |
| F5-2 | F | 46 | HD IFN-a2b MKC prime- boost vaccine, HD IL-2 |
Skin, LN, Bone | M1c | 1 × 109 | 6/14 | 3/3 | Yes by PET/CT | Stable disease |
6 | 10 | ||
| F5-3 | M | 61 | - | Lung, Liver | M1c | 1 × 109 | 13/14 | 3/3 | Yes by PET/CT | Stable disease |
5 | 46+ | ||
| F5-4 | M | 50 | HD IL-2 | Lung, LN, SC | M1c | 0.6 × 109 | 14/14 | 3/3 | No | Progression | 2 | 22 | ||
| F5-5 | M | 53 | HD IFN | Lung | M1c | NA | NA | NA | NA | NA | NA | NA | ||
| F5-6 | M | 59 | - | Lung, LN | M1b | 1 × 109 | 13/14 | 3/3 | Yes by PE | Stable disease | 3 | 4 | ||
| F5-7 | M | 48 | HD IL-2 | SC, Bone | M1c | 1 × 109 | 9/14 | 3/3 | Yes by CT | Stable disease | 4 | 11 | ||
| F5-8 | M | 44 | - | LN, Liver, lung | M1c | 1 × 109 | 11/14 | 3/3 | Yes by PET/CT | Stable disease | 4 | 11 | ||
| F5-9 | F | 46 | - | Skin, LN | M1a | 1 × 109 | 11/14 | 3/3 | No | Progression | 3 | 16 | ||
| F5-10 | F | 47 | - | Liver, Adrenal, SC, LN, Orbit |
M1c | 6-7 | 4.8 × 109 | 14/14 | 2/3 | Yes by PET/CT | Progression | 2 | 8 | |
| F5-11 | F | 56 | - | Lung, LN | M1b | 1.8 × 109 | 8/14 | 3/3 | Yes by PET/CT | Stable disease | 4 | 4 | ||
| F5-12 | M | 40 | HD IFN-a2b, Temodar, ipilimumab, CR-011, Taxol, MLN- 4924, AMG- 337 |
Lung, LN | M1b | 8-9 | 3.9 × 109 | Fresh | 6/9 | 3/3 | Yes by PET/CT | Stable disease | 5 | 6 |
| F5-13 | M | 60 | HD IFN, HD IL-2, ipilimumab |
Lung, Abdomen, SC |
MI1b | 4.41 × 109 | 4/9 | 3/3 | Yes by PET/CT | Progression | 3 | 6 | ||
| F5-14 | F | 50 | Ipilimumab | Lung, Liver, Adrenal gland, LN, bone |
M1c | 3.93 × 109 | 3/9 | 1/3 | Yes by CXR | Progression | 0 | 1 |
Legend: #: number; M: male; F: female; LN: lymph nodes; SC: subcutaneous; N: normal; Neg: negative; HD: high dose; IFN: interferon alpha 2b. IL-2: interleukin-2; NA: not available; Cryo: cryopreserved TCR transgenic cells; PET: Positron Emission Tomography; CXR: chest X-ray; PE: physical exam.
Study outline
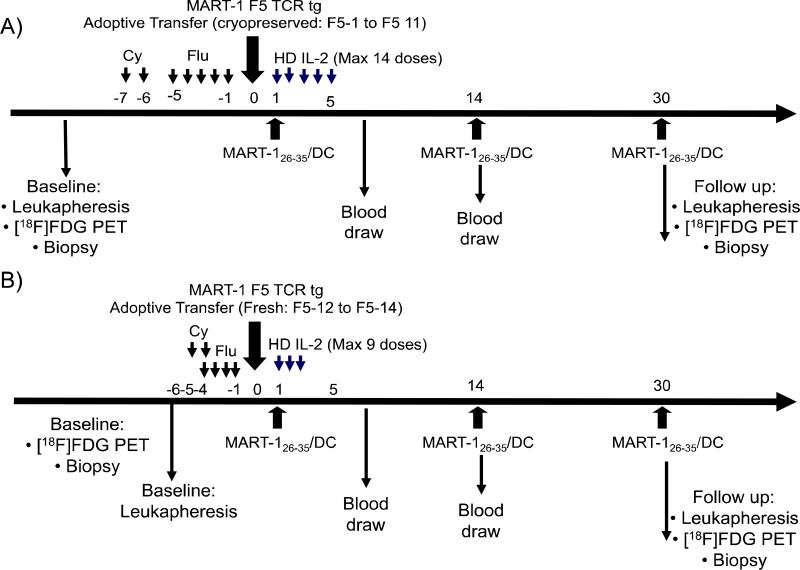
The study outline is included in Figure 1. Patients underwent baseline leukapheresis for the manufacture of the two cell therapies, the F5 TCR gene modified T cells and the MART-1 peptide pulsed DC (see Supplemental Online Methods). Patients received conditioning chemotherapy as inpatients consisting of cyclophosphamide 60 mg/kg/day × 2 days i.v. and fludarabine 25 mg/m2/day i.v. daily for 4 or 5 days as previously described (2, 3, 5, 17). On day 0, patients received the ACT of TCR engineered lymphocytes as an i.v. infusion with the cells thawed at bedside under the initial study protocol. Patients were transferred for monitoring to the intensive care unit (ICU) to start high dose IL-2 on the next morning, and then they received the first intradermal administration of MART-126-35 peptide pulsed DC. IL-2 (Proleukin, Prometheus, San Diego, CA) was given at 600,000 IU/kg i.v. every eight hours for up to 14 (amendments 1-7) or 9 (amendments 8-10) doses, as tolerated, following the standard high dose IL-2 UCLA protocol (16). Standard supportive care was provided including filgrastim (Neupogen, Amgen, Thousand Oaks, CA), antibiotics for neutropenic fever, and red blood cell and platelet transfusions. When patients had recovered peripheral blood cell counts and were transfusion-free they were discharged from the hospital. Patients received two more doses of DC vaccines at two weeks intervals. Patients were eligible to receive 3 more booster DC vaccines after study day 90 if the level of MART-1 TCR transgenic cells was below 5% of total lymphocytes by MHC tetramer or dextramer assay. A research [18F]FDG PET scan and biopsy was planned at around day 30, and formal restaging PET CT scans were performed on day 90.
Figure 1. F5 study outline.
A) Schedule of events for patients F5-1 to F5-11 who received cryopreserved TCR transgenic cells under amendments 1-7. B) Schedule of events for patients F5-12 to F5-14 who received freshly manufactured TCR transgenic cells after amendment 8.
Safety assessments
Safety was determined in stage one, and if 3 out of 8 patients have MART-1 F5 TCR-induced dose limiting toxicities (DLT), then further accrual would not be warranted. Adverse events were analyzed following NCI CTCAE v3.0. The known toxicities and side effects of the chemotherapy preparative regimen, or from the administration of IL-2 or G-CSF, as listed in the protocol or package insert, were not considered for the assessment of DLTs. Analysis of patient blood samples for potential replication competent retrovirus (RCR) and cytokine storm is described in the Supplemental Methods.
Assessment of feasibility
Feasibility was also determined in the first stage, and if 3 out of 8 patients could not receive the intended cellular therapies, or if they resulted in suboptimal TCR transgenic cell in vivo persistence, further accrual would not be warranted to the protocol as originally designed. Feasibility was assessed after the first 8 patients were followed up for a minimum of 3 months after the last subject had received the infusion of the MART-1 F5 TCR transgenic cells.
Assessment of antitumor activity
Quantification of changes in PET imaging for the intratumoral accumulation of [18F]FDG was performed by counting the total number of FDG avid lesions as well as the maximum standardized uptake value (SUVmax) averaged over up to 5 hottest lesions at baseline, at day 30 and day 90. Objective clinical response rate was assessed on study day 90 and recorded following a modified Response Evaluation Criteria in Solid Tumors (RECIST) (18).
MHC tetramer immunological monitoring
MHC tetramer analysis in cryopreserved PBMC collected at different time points were performed as previously described (19, 20). Our previous definitions for a positive or negative immunological response using standardized MHC tetramer assays were used, which are based on the assay performance specifications by defining changes that were beyond the assay variability with a 95% confidence level (19).
Statistical design and analysis
The Simon optimal two-stage design (15) was used to determine the sample size, using the co-primary endpoint of response rate as the criterion for the clinical trial statistical design. This clinical trial was set up to rule out the null hypothesis that p0 ≤ 0.10 (i.e. to rule out that this combined therapy has a response beyond 10%, since several current treatment approaches achieve response rates of 10% in patients with advanced melanoma) versus the alternative that the effect-size = p1 – p0 > 0.25 (α ≤ 0.05 β ≤ 0.20). The alternative hypothesis of a response rate of 35% was chosen since it was felt to represent a clinically meaningful difference and results in a study sample size that is feasible to be conducted within a pilot single-institution study. Using this statistical design, if 2 or more of 8 patients in stage one had an objective response at day 90, the study would proceed to stage two and accrue a total of 22 patients. If 5 or more patients in the overall study had a complete response (CR) or a partial response (PR), defined as the objective response rate (ORR) at day 90, the study would be declared positive. Due to the small sample size the statistical analyses are mostly descriptive. Descriptive statistics such as mean, standard deviation, median, minimum, maximum and frequency of variables of interest are calculated. Responses of individual patients are plotted over time to present the data.
Results
Patient characteristics
Between April 2009 and September 2011, 14 HLA-A*0201 positive patients with MART-1 positive metastatic melanoma were accrued. Patients had a median age of 50 years. Nine patients had M1c metastatic melanoma with visceral and/or bone metastases, four had lung metastases (M1b) and one had skin, nodal and subcutaneous only metastases (M1a). Half of the patients had received prior therapy for metastatic disease, including high dose IL-2 in four patients and ipilimumab in three patients (Table 1).
Study conduct and protocol changes
This study underwent nine protocol amendments during its conduct, with some significantly changing the delivered cell therapies; therefore, we analyzed patients in subgroups based on the protocol amendments. The first nine patients (F5-1 to F5-9) were treated under the original protocol (amendments 1-5) administering up to 1 × 109 previously cryopreserved TCR transgenic lymphocytes following the full cyclophosphamide-fludarabine conditioning regimen and up to 14 infusions of high dose IL-2. One patient (F5-5) had brain metastases at the baseline MRI screening exam and did not receive the TCR transgenic infusion. Therefore, this patient is not accounted in the safety, feasibility or efficacy evaluations. After the planned study endpoint assessment based on the first eight patients receiving the full protocol, it was deemed that the study was safe and feasible, but the antitumor activity assessed on day +90 was suboptimal. Therefore, patients F5-10 and F5-11 were treated under amendments 6-7 that allowed an increase in the cell number of previously cryopreserved TCR transgenic lymphocytes up to 1 × 1010. Due to an event of delayed pancytopenia in patient F5-10 who had continuing evidence of durable antitumor activity at that time, and fludarabine being the most likely cause of marrow toxicity, the study was further amended (amendments 8-9, patients F5-12 to F5-14) to decrease the conditioning regimen (one fewer day of fludarabine). Since this patient also had received 14 doses of IL2, a potential adverse contributor to the marrow aplasia, which was more than what the other patients tolerated except for F5-4, the number of potential IL-2 doses was also limited to a maximum of 9. Studies in murine model of ACT showed that cryopreservation has a profound detrimental effect on the in vivo long0term survival of the adoptively transferred T cells and their ability to have a secondary response to antigen exposure. Therefore, we amended to administer non-cryopreserved, freshly produced TCR transgenic lymphocytes (Figure 1b).
Peripheral blood reconstitution with cryopreserved TCR transgenic cells
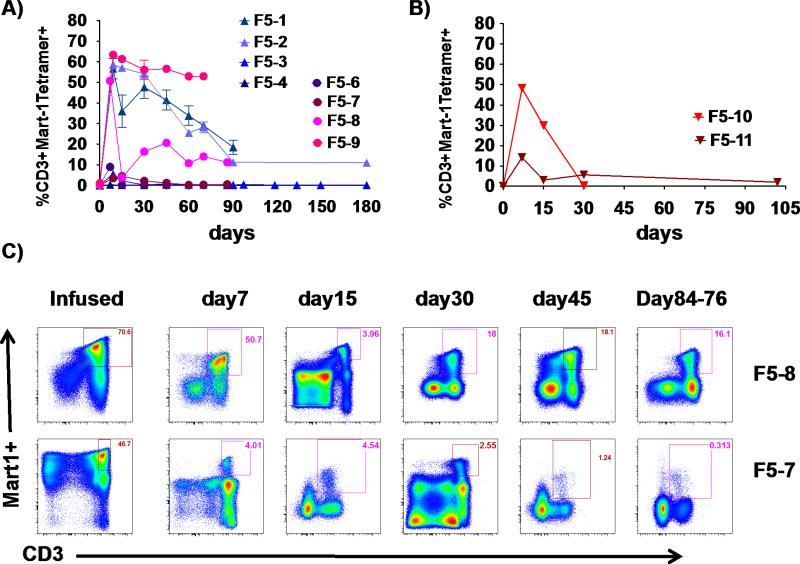
TCR transgenic cell preparations were manufactured for all patients that met the lot release criteria of viability >70%, negative for mycoplasma, Gram and fungal stain, endotoxin level of ≤ 5 EU/kg body weight, and >10% MART-1 tetramer positive CD3 lymphocytes (Supplemental Figure 1). On average, transduction efficiency was 64% (range 42-84%, Supplemental Figures 2 and 3). The first 10 patients received cryopreserved TCR engineered cells, with the first 8 receiving up to 1 × 109 cells and the next two patients receiving up to 1 × 1010 cells. However, there was not much difference in the absolute number of delivered MART-1 TCR transgenic cells (Supplemental Figure 3). Despite administering a similar number of cells to these patients, peripheral cell reconstitution varied widely (Figure 2). In this group of patients, the mean and median peak blood levels of TCR transgenic cells was 31%, with a maximum of 63% and a minimum of 0.67% of total CD3+ T lymphocytes. The peak of TCR transgenic cell frequency was early, within the first two weeks after ACT, and the percentage and absolute number of TCR transgenic cells in peripheral blood decreased over time in all patients. There was no clear evidence of enhancing number or function of peripheral blood TCR transgenic cells with the delivery of MART-1 peptide pulsed DC vaccines on study days 14 and 28.
Figure 2. Post-infusion peripheral blood levels of MART-1 TCR transgenic cells at various time points in patients receiving cryopreserved transgenic cells.
A) F5-1 to F5-9 receiving up to 109 cryopreserved transgenic cells. B) F5-10 and F5-11 receiving up to 1010 cryopreserved transgenic cells. C) Representative dot plots of MART-1 MHC tetramer analysis of infused cells and post-infusion peripheral blood PBMC in F5-7 and F5-8.
Antitumor activity with cryopreserved TCR transgenic cells
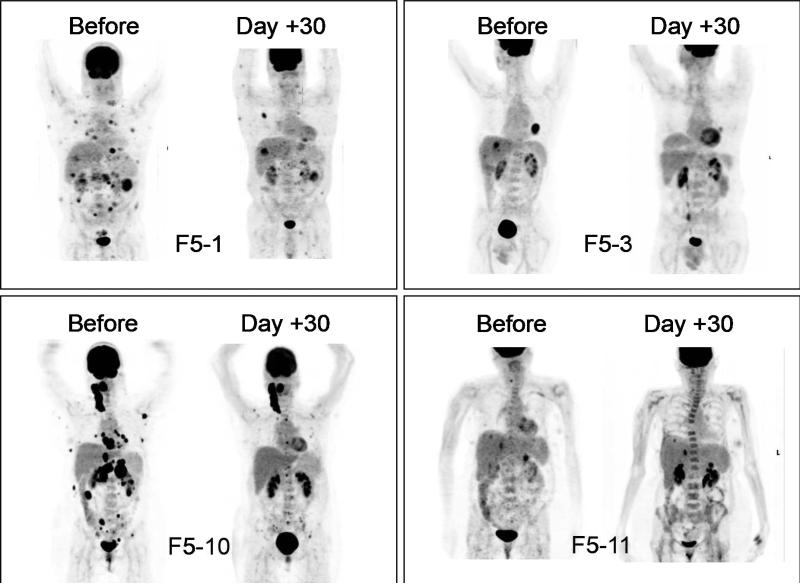
There was evidence of initial antitumor activity in 8 out of 10 patients with metastatic melanoma regression detected at day 30 PET scans (examples in Figures 3 and 4, and additional explanation in Supplemental Methods) or physical examination. However, the initial antitumor activity was incomplete and transient. At the formal restaging PET CT scans on day 90, none of the patients had evidence of a sustained tumor response by RECIST criteria. Therefore, after the first 8 patients had been enrolled it was deemed that improvements in the protocol were needed. This led to the testing of a higher TCR transgenic cell dose, and then the testing of the infusion of freshly manufactured cells, without cryopreservation.
Figure 3. Pre- and post-treatment day 30 PET scans indicating initial antitumor activity.
Representative scans of F5-1 and F5-3 receiving up to 109 cryopreserved transgenic cells, F5-10 and F5-11 receiving up to 1010 cryopreserved transgenic cells.
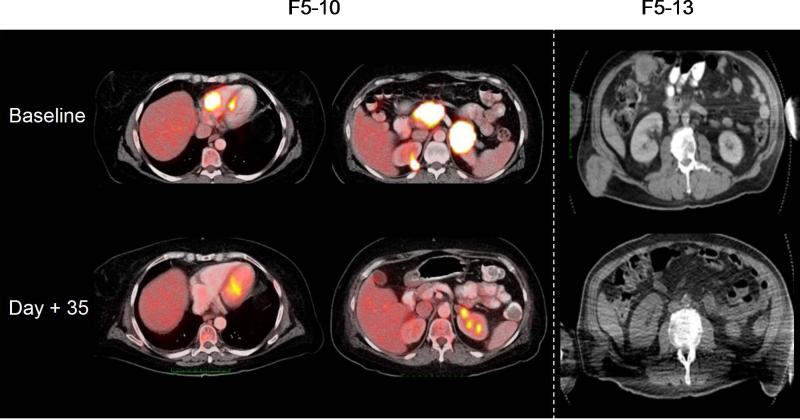
Figure 4. Pre- and post-treatment day 35 PET/CT (F5-10) and CT (F5-13) showing evidence of initial antitumor activity.
Representative scans of F5-10 receiving up to 1010 cryopreserved transgenic cells and F5-13 receiving up to 1010 freshly harvested transgenic cells.
Safety evaluation of cryopreserved TCR transgenic cells
There were no unanticipated serious toxicities in the first 8 patients in the protocol beyond the ones that would be expected with the conditioning chemotherapy and high dose IL-2 therapy. Of note, there were no findings of otologic, vestibular or ophthalmologic toxicities after repeated specialist visits. Therefore, the protocol was deemed to meet the co-primary endpoint of safety when administered as 1 × 109 cryopreserved cells. Since the antitumor activity did not meet the pre-specified criteria to proceed to the second stage of study, the next two patients were treated with up to 1 × 1010 cryopreserved cells. However, the outcomes did not improve since one patient had a transient tumor response and the other had stable disease (Supplemental Figures 4-6).
Safety and antitumor activity with non-cryopreserved TCR transgenic cells
A new protocol amendment was introduced to administer 1 × 1010 TCR engineered cells to be infused fresh after harvesting from the ex vivo culture, without a cryopreservation step. The baseline leukapheresis was performed on the day of hospital admission, and cells were manufactured concurrent to patients receiving conditioning chemotherapy. The conditioning chemotherapy was shortened by one day and the number of potential IL-2 doses was limited at nine. Three patients were enrolled and had evidence of transient tumor responses by serial X-rays and PET scans, but also had a more pronounced whole body erythematous skin rash compared to the majority of prior patients in this protocol. Furthermore, two of the patients (F5-12 and F5-14) had serious adverse events (SAEs) of acute respiratory distress requiring intubation associated with patchy pulmonary infiltrates within one week of cell infusion (Supplemental Figure 7), resulting in the discontinuation of this cohort due to increased toxicities. Plasma from peripheral blood was analyzed for the production of multiple cytokines to study the potential development of a cytokine storm (Supplemental Figure 8). Both patients received corticosteroid therapy and recovered their baseline respiratory function within two weeks.
Despite this increased toxicities with the infusion of fresh TCR transgenic cells and the administration of corticosteroids in these two patients and lack of administration of further MART-1/DC vaccinations, this group of three patients all had evidence of antitumor activity. The serial chest X-rays shown in Supplemental Figure 7 document a time-course decrease in size of lung metastases in patients F5-12 and F5-14, and the CT scan images from patient F5-13 in Figure 4, demonstrate the regression of large subcutaneous/muscle metastases. However, none of these responses were durable with all three patients having disease progression within 6 months from study start.
Peripheral blood reconstitution and DC boosting effect with fresh TCR transgenic cells
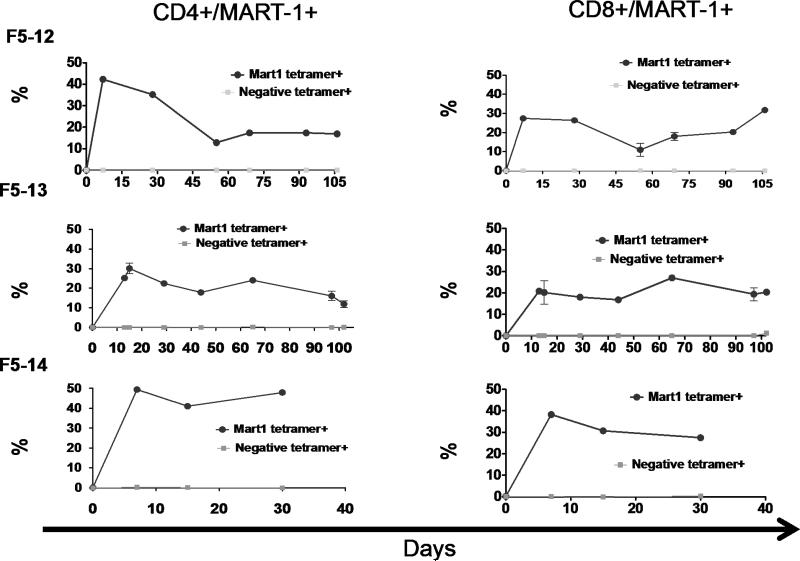
The infusion of fresh TCR transgenic lymphocytes resulted in a more prolonged persistence of circulating TCR transgenic cells in blood in the three patients from whom we had samples for analysis compared to the previous experience with cryopreserved cells (Figure 5). It is remarkable that both CD4+ and CD8+ TCR transgenic cells were approximately 20% of the peripheral T lymphocytes at three months after ACT in F5-12 and F5-13. Furthermore, patient F5-13, who did not have a SAE and received the three scheduled MART-1/DC administrations, had evidence of recall whole body rash and re-expansion of the TCR transgenic cells in peripheral blood demonstrating in vivo activation impact of MART-1/DC vaccination (Supplemental Figure 9).
Figure 5. Post-infusion peripheral blood levels of MART-1 TCR transgenic cells at various time points in patients receiving freshly harvested transgenic cells.
MART-1 tetramer positive CD4+ and CD8+ levels in F5-12, F5-13 and F5-14 receiving up to 1010 freshly harvested transgenic cells.
Discussion
The MART-1 TCR ACT protocol described herein resulted in a high rate of transient tumor responses. These results are not too different from the studies at the NCI Surgery Branch despite multiple differences in the cell manufacture protocol (see Supplemental Online Discussion), primarily our short one-week ex-vivo culture using basic equipment available in any facility, resulting in younger cells and the use of DC vaccination. The generation of transgenic T cells, TILs and endogenous antigen specific T cells that have been used in the trials so far involved complex processes and at least 4 to 8 weeks of culture, thereby feasible only in a few specialized centers and many of these patients progressing rapidly don't have this long a time to wait. Tumor progression after an initial response may be due to loss of antitumor activity of the TCR transgenic cells given to patients or changes in tumor cells resulting in acquired resistance to this mode of immunotherapy. The decrease in frequency of TCR transgenic T cells after the initial brisk expansion is a logical evolution of an effective immune response, as is commonly noted with T cell responses to viral infections (21). In other work (22), we analyzed TCR transgenic T cells administered and recovered from three of the patients in this series using new generation microfluidics-based miniaturized assays able to simultaneously study multiple functional responses of T cells selected based on defined antigen specificities (23, 24). These studies showed that the initial polyfunctionality resulting in high antitumor activity of the administered TCR transgenic T cells is gradually lost over time in vivo, which is temporarily associated with the clinical course of initial tumor response followed by progression. Therefore, there is a need to better maintain the TCR transgenic cell polyfunctionality upon ACT. This could be achieved pharmacologically using monoclonal antibodies blocking negative immune checkpoints (CTLA4, PD-1) (25), with small molecules fostering T memory cell function (26, 27), or by endogenously generating fully active TCR transgenic cells from stem cell precursors (28-31).
Patients who experienced respiratory distress with the administration of non-cryopreserved TCR transgenic cells demonstrated an increase in circulating cytokines and chemokines, nevertheless lower than the levels noted in two clinical reported cases of life-threatening cytokine storm (32, 33). In fact, these cytokine levels are comparable to those observed in acute pneumonia (34). Despite these toxicities, this group of patients had favorable antitumor activity with evidence of longer persistence of circulating TCR transgenic cells and in vivo expansion following DC vaccination.
In conclusion, a short ex vivo manufacture protocol was able to generate large numbers of tumor-specific TCR transgenic T cells. Administration of these in combination with DC vaccination is feasible and has high initial antitumor activity. These two cell therapies can be concurrently manufactured while patients undergo conditioning with lymphodepleting chemotherapy over a period of one week. The administration of freshly manufactured cells without cryopreservation results in higher in vivo persistence, but also in a higher incidence of side effects when targeting the self-melanosomal antigen MART-1. However, improvements in the conditions to maintain TCR transgenic cell functionality, tumor responses and to lower toxic side effects are needed.
Supplementary Material
Statement of translational relevance.
This manuscript describes an investigator-initiated clinical trial using two cell therapies, one genetically modified and one pulsed with peptides, for the treatment of melanoma. We report on a high response rate in patients with melanoma, attesting to the high antitumor activity of adoptively transferred T cell receptor engineered lymphocytes administered with dendritic cell vaccination and high dose IL-2. However, it also points out to the need to improve the durability of the tumor responses, which is the basis of further research in this protocol. With the evidence that adoptive cell transfer (ACT) therapy is a viable option for patients with advanced cancers there is an increasing need in establishing ACT programs at multiple institutions. We think that this work demonstrating the feasibility of a short one-week manufacture protocol and detailed clinical observations will help other groups in establishing similar programs.
Acknowledgements
We thank Steven A. Rosenberg, Richard Morgan, Laura Johnson and Mark Dudley (all from the NCI Surgery Branch, Bethesda, MD) for their guidance in establishing the TCR engineered ACT protocol at UCLA and allowing access to their clinical grade retroviral vector master cell bank. We acknowledge the contributions of Erika von Euw, Joanne Cox and Narsis Attar in some of the cell therapy preparations.
Financial support: This work was funded by the National Cancer Institute grants P01 CA132681 (DB, JSE, ONW, AR, DBK, JAZ), U54 CA119347 (JRH, AR), P50 CA086306 (AR) and RO1 CA129816 (JSE), the California Institute for Regenerative Medicine New Faculty Award RN2-00902-1 (AR), the Eli & Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA (ONW, AR), the Samuel Waxman Foundation (JSE, DB, ONW), the Keck Foundation (JSE, DB, ONW), The Seaver Institute (AR), the PhaseOne Foundation (AR), the Louise Belley and Richard Schnarr Fund (AR), the Wesley Coyle Memorial Fund (AR), the Garcia-Corsini Family Fund (AR), the Bila Alon Hacker Memorial Fund (AR), the Fred L. Hartley Family Foundation (AR), the Ruby Family Foundation (AR), the Joy and Jerry Monkarsh Fund (JSE), the Caltech/UCLA Joint Center for Translational Medicine (DB, OWN, AR) and the Melanoma Research Alliance (AR, DB, JRH). ONW is an Investigator of the Howard Hughes Medical Institute. RCK was supported in part by the V Foundation-Gil Nickel Family Endowed Fellowship in Melanoma Research. The UCLA Jonsson Comprehensive Cancer Center (JCCC) Flow Cytometry Core Facility is supported by National Institutes of Health awards CA-16042 and AI-28697.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Dembic Z, Haas W, Zamoyska R, Parnes J, Steinmetz M, von Boehmer H. Transfection of the CD8 gene enhances T-cell recognition. Nature. 1987;326:510–511. doi: 10.1038/326510a0. [DOI] [PubMed] [Google Scholar]
- 2.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson LA, Heemskerk B, Powell DJ-, Jr, Cohen CJ, Morgan RA, Dudley ME, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lou Y, Wang G, Lizee G, Kim GJ, Finkelstein SE, Feng C, et al. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783–6790. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koya RC, Mok S, Comin-Anduix B, Chodon T, Radu CG, Nishimura MI, et al. Kinetic phases of distribution and tumor targeting by T cell receptor engineered lymphocytes inducing robust antitumor responses. Proc Natl Acad Sci U S A. 2010;107:14286–14291. doi: 10.1073/pnas.1008300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrello I, Sotomayor EM, Rattis FM, Cooke SK, Gu L, Levitsky HI. Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing tumor vaccines. Blood. 2000;95:3011–3019. [PubMed] [Google Scholar]
- 12.Cui Y, Kelleher E, Straley E, Fuchs E, Gorski K, Levitsky H, et al. Immunotherapy of established tumors using bone marrow transplantation with antigen gene--modified hematopoietic stem cells. Nat Med. 2003;9:952–958. doi: 10.1038/nm882. [DOI] [PubMed] [Google Scholar]
- 13.Butterfield LH, Ribas A, Dissette VB, Amarnani SN, Vu HT, Oseguera D, et al. Determinant Spreading Associated with Clinical Response in Dendritic Cell-based Immunotherapy for Malignant Melanoma. Clin Cancer Res. 2003;9:998–1008. [PubMed] [Google Scholar]
- 14.Ribas A, Glaspy JA, Lee Y, Dissette VB, Seja E, Vu HT, et al. Role of dendritic cell phenotype, determinant spreading, and negative costimulatory blockade in dendritic cell-based melanoma immunotherapy. J Immunother. 2004;27:354–367. doi: 10.1097/00002371-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 16.Figlin R, Gitlitz B, Franklin J, Dorey F, Moldawer N, Rausch J, et al. Interleukin-2-based immunotherapy for the treatment of metastatic renal cell carcinoma: an analysis of 203 consecutively treated patients [see comments]. Cancer J Sci Am. 1997;3(Suppl 1):S92–97. [PubMed] [Google Scholar]
- 17.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Comin-Anduix B, Gualberto A, Glaspy JA, Seja E, Ontiveros M, Reardon DL, et al. Definition of an immunologic response using the major histocompatibility complex tetramer and enzyme-linked immunospot assays. Clin Cancer Res. 2006;12:107–116. doi: 10.1158/1078-0432.CCR-05-0136. [DOI] [PubMed] [Google Scholar]
- 20.Comin-Anduix B, Lee Y, Jalil J, Algazi A, de la Rocha P, Camacho LH, et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. J Transl Med. 2008;6:22. doi: 10.1186/1479-5876-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 22.Ma C, Cheung AF, Chodon T, Koya RC, Wu Z, Ng C, et al. Multifunctional T-cell Analyses to Study Response and Progression in Adoptive Cell Transfer Immunotherapy. Cancer discovery. 2013;3(4):1–12. doi: 10.1158/2159-8290.CD-12-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwong GA, Radu CG, Hwang K, Shu CJ, Ma C, Koya RC, et al. Modular nucleic acid assembled p/MHC microarrays for multiplexed sorting of antigen-specific T cells. J Am Chem Soc. 2009;131:9695–9703. doi: 10.1021/ja9006707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma C, Fan R, Ahmad H, Shi Q, Comin-Anduix B, Chodon T, et al. A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells. Nature medicine. 2011;17:738–743. doi: 10.1038/nm.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. The Journal of experimental medicine. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Rao RR, Araki K, Pollizzi K, Odunsi K, Powell JD, et al. A central role for mTOR kinase in homeostatic proliferation induced CD8+ T cell memory and tumor immunity. Immunity. 2011;34:541–553. doi: 10.1016/j.immuni.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nature medicine. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Qin XF, Baltimore D, Van Parijs L. Generation of functional antigen-specific T cells in defined genetic backgrounds by retrovirus-mediated expression of TCR cDNAs in hematopoietic precursor cells. Proc Natl Acad Sci U S A. 2002;99:6204–6209. doi: 10.1073/pnas.092154599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Baltimore D. Long-term in vivo provision of antigen-specific T cell immunity by programming hematopoietic stem cells. Proc Natl Acad Sci U S A. 2005;102:4518–4523. doi: 10.1073/pnas.0500600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vatakis DN, Koya RC, Nixon CC, Wei L, Kim SG, Avancena P, et al. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2011;108:E1408–1416. doi: 10.1073/pnas.1115050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. The New England journal of medicine. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 34.Endeman H, Meijvis SC, Rijkers GT, van Velzen-Blad H, van Moorsel CH, Grutters JC, et al. Systemic cytokine response in patients with community-acquired pneumonia. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2011;37:1431–1438. doi: 10.1183/09031936.00074410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.