Abstract
Septins are guanosine-5′-triphosphate-binding proteins involved in wide-ranging cellular processes including cytokinesis, vesicle trafficking, membrane remodeling and scaffolds, and with diverse binding partners. Precise roles for these structural proteins in most processes often remain elusive. Identification of small molecules that inhibit septins could aid in elucidating the functions of septins and has become increasingly important, including the description of roles for septins in pathogenic phenomena such as tumorigenesis. The plant growth regulator forchlorfenuron (FCF), a synthetic cytokinin known to inhibit septin dynamics, likely represents an informative probe for septin function. This report deals with septins of the human blood fluke Schistosoma mansoni and their interactions with FCF. Recombinant forms of three schistosome septins, SmSEPT5, SmSEPT7.2 and SmSEPT10, interacted with FCF, leading to rapid polymerization of filaments. Culturing developmental stages (miracidia, cercariae, adult males) of schistosomes in FCF at 50 – 500 μM rapidly led to paralysis, which was reversible upon removal of the cytokinin. The reversible paralysis was concentration-, time- and developmental stage-dependent. Effects of FCF on the cultured schistosomes were monitored by video and/or by an xCELLigence-based assay of motility, which quantified the effect of FCF on fluke motility. The findings implicated a mechanism targeting a molecular system controlling movement in these developmental stages: a direct effect on muscle contraction due to septin stabilization might be responsible for the reversible paralysis, since enrichment of septins has been described within the muscles of schistosomes. This study revealed the reversible effect of FCF on both schistosome motility and its striking impact in hastening polymerization of septins. These novel findings suggested routes to elucidate roles for septins in this pathogen, and exploitation of derivatives of FCF for anti-schistosomal drugs.
Keywords: Schistosome, Forchlorfenuron, Septin, Cytokinin, Miracidium, Cercariae, xCELLigence, Phenylurea
1. Introduction
The septins are cytoskeletal proteins and filaments involved in diverse cellular processes (Cao et al., 2009; Mostowy and Cossart, 2012). The assembly of septins into higher-order structures such as filaments, rings and gauzes (Rodal et al., 2004) is central to their functions, such as in establishing membrane diffusion barriers and scaffolds as well as in vesicle trafficking (Beites et al., 1999; Longtine and Bi, 2003; Dobbelaere and Barral, 2004; Kinoshita, 2006; Spiliotis et al., 2008) and cytokinesis, for which they were first described in yeast (Hartwell, 1971). The filaments formed by these guanosine-5′-triphosphate (GTP)-binding proteins are ordered assemblies of hetero-oligomeric complexes (Sirajuddin et al., 2007). The number of septin genes within the genomes of eukaryotes varies, ranging from one in some algae to 13 in humans (Nishihama et al., 2011; Russell and Hall, 2011), and splice variants occur (Russell and Hall, 2011). Accordingly, there can arise numerous combinations of septins in functional filaments in different species and tissues.
This has allowed for the recent description of four Schistosoma mansoni septins, which have received the names SmSEPT5, SmSEPT7.1, SmSEPT7.2 and SmSEPT10, based on their sequence similarity with human homologues (Zeraik et al., 2013). However, the assembly and specific roles of these proteins in schistosomes has yet to be investigated.
Forchlorfenuron, (FCF; N-(2-chloro-4-pyridyl)-N′-phenylurea (C12H10ClN3O)), a small molecule synthetic plant cytokinin, interferes with the assembly of septin filaments in both yeast and mammalian cells (Iwase et al., 2004; Hu et al., 2008; DeMay et al., 2010). FCF stabilizes septin structures in a specific and reversible way, while not affecting other cytoskeletal components such as actin filaments and microtubules. How FCF alters septin organization in metazoan cells is not understood (DeMay et al., 2010) but, nevertheless, it represents a tractable, promising tool for the investigation of septin function. In yeast, FCF induced the appearance of septin filaments outside of the bud neck, which vanished upon removal of FCF (Iwase et al., 2004). In the filamentous fungus Ashbya gossypii, similar ectopic septin structures were observed. These septin fibers induced by FCF were shown to be stable structures. Therefore FCF was considered a septin stabilizing drug, since septin fibers, highly dynamic structures, were no longer dynamic upon FCF treatment and thus do not recover from photo-bleaching (DeMay et al., 2010). These fibers, although stable, were readily dissolved upon FCF removal, indicating that FCF is necessary to induce ectopic septin polymerization and necessary for its maintenance (DeMay et al., 2010). The effect promoted by FCF in mammalian cells has shown to phenocopy the small interfering (si)RNA treatment (Hu et al., 2008), providing a simpler and reliable alternative to study septin function. It also has the compelling advantages that its effects are reversible once FCF is washed out of the culture, and it has a low level of cytotoxicity for cultured human tumor cells (Hu et al., 2008). Decreased cell migration was observed upon FCF treatment (Hu et al., 2008), and the motility of amoeboid T lymphocytes was seriously affected by septin depletion (Tooley et al., 2008). It has been postulated that septin filaments function as a molecular corset, providing rigidity at the cell cortex to support efficient motion of T cells and that septin might tune actomyosin forces during motility (Tooley et al., 2008).
Here, we report on the interaction of FCF with schistosome septins and its consequences for filament assembly. This phenylurea derivative enhanced the formation of higher-order structures by S. mansoni septins in vitro. Moreover, putative stabilization of septin polymers within the schistosome correlated with the motility of its developmental stages. The impact of FCF on the motor activity of schistosomes might therefore contribute to the elucidation of the function of septins in this pathogen and may be worthy of further investigation for potential anti-schistosomal activity.
2. Materials and methods
2.1. Ethics statement
Mice infected with S. mansoni were obtained from the Biomedical Research Institute (BRI), Rockville, MD, USA and housed at the Animal Research Facility of the George Washington University Medical School, USA which is accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC no. 000347) and has an Animal Welfare Assurance on file with the National Institutes of Health (NIH), USA, Office of Laboratory Animal Welfare, OLAW assurance number A3205-01. All procedures employed were consistent with the Guide for the Care and Use of Laboratory Animals. Maintenance of the mice and recovery of schistosomes were approved by the Institutional Animal Care and Use Committee of the George Washington University.
2.2. Developmental stages of schistosomes
Biomphalaria glabrata snails and Swiss-Webster mice infected with the NMRI (Puerto Rican) strain of S. mansoni were supplied by Drs. Matt Tucker and Fred Lewis, BRI under NIH-NIAID contract HHSN272201000005I. Four developmental stages were investigated - adult worms, eggs, miracidia and cercariae. In brief, adult worms were recovered from mice by portal perfusion; schistosome eggs were isolated from livers of the mice (Dalton et al., 2007) and miracidia obtained by hatching the eggs. Miracidia were used immediately. Cercariae were obtained by shedding infected snails under bright light for 2 h at 23 °C. Adult worms were cultured under 5% CO2 at 37 °C in DMEM, supplemented with 10% FBS and 1×penicillin/streptomycin as described (Dalton et al., 1997; Mann et al., 2009; Zeraik et al., 2013).
2.3. Expression and purification of septin complexes
Aiming to express a protein complex comprising three S. mansoni septins, the gene encoding SmSept10 was ligated in a plasmid pACYCDuet-1 vector (Novagen, EMD Millipore Billerica MA, USA) and those encoding SmSept5 and SmSept7.2 into pETDuet-1 (Novagen). SmSept7.2 was expressed as a histidine (His)-Tag fusion at the N-terminus whereas fusion peptides were not introduced into the two other septin constructs. This strategy was used to facilitate the purification by affinity chromatography of the septin complex by eliminating forms of SmSept5 and SmSept10 not bound to SmSept7.2. The septins were co-expressed in Escherichia coli BL21 (strain DE3) with induction by isopropyl β-D-1-thiogalactopyranoside (IPTG) at 0.4 mM for 16 h at 18 °C in Luria Broth (LB). Cells were harvested by centrifugation at 4,000 g for 40 min at 4 °C and resuspended in 50 mM Tris-HCl pH 8.0, 500 mM NaCl, 12% glycerol, 10 mM β-mercaptoethanol (binding buffer). After sonication, the lysates were centrifuged at 20,000 g for 30 min at 4 °C, after which supernatants were subjected to affinity chromatography on 1 ml of TALON cobalt-resin (Clontech, Mountain View, CA, USA). After several washes with binding buffer, complexes were eluted from the resin with 500 mM imidazole in the same buffer. A sequential purification was undertaken using a column of Superdex-200 (HR 10/30 GE Healthcare Life Sciences, Pittsburgh, PA, USA) fitted to an AKTA purifier liquid handling system (GE Healthcare Life Sciences). Eluates were analyzed by SDS-PAGE. High molecular mass fractions containing the heterocomplex were pooled and examined by MS, dynamic light scattering (DLS) and electron microscopy (see Sections 2.4 – 2.6).
2.4. MS of the purified complexes
Samples for MS were obtained from the polyacrylamide gel containing the recombinant septin complex purified from size exclusion chromatography. The gel was stained with Bio-safe Coomassie Blue G250 (Bio-Rad, Hercules, CA, USA) and destained with water. Gel strips corresponding to detected bands were further destained with 50% acetonitrile solution, dehydrated in 100% acetonitrile and rehydrated in 100 mM ammonium bicarbonate. The gel strips were diced and incubated with DTT (10 mM), followed by alkylation with iodoacetamide (55 mM). After washing steps with ammonium bicarbonate and 50% acetonitrile, the gel pieces were dehydrated in 100% acetonitrile, dried in a Savant SpeedVac concentrator and rehydrated in a solution of trypsin (Promega, Madison, WI, USA) for 16 h at 37 °C to fragment the proteins into peptides. Solutions containing peptides were purified using a C18 ZipTips (EMD Millipore, Billerica, MA, USA) according to the manufacturer's instructions followed by direct infusion electrospray tandem MS (micrOTOF-QII, Bruker, Karlsruhe, Germany) to obtain fragmentation spectra for the liberated peptides. The spectra were interrogated using MASCOT (Matrix Science, Boston, MA, USA), utilizing a database of the proteins predicted encoded by the genome of S. mansoni to identify the peptides.
2.5. Transmission electron microscopy (TEM) of septin complexes
The recombinant S. mansoni septin complex at 2.2 mg/ml in binding buffer was dialyzed against 15 mM Tris-HCl pH 8.0 containing 40 mM NaCl, 2 mM MgCl2, 0.5 mM GTP, 2 mM DTT in the presence of FCF (25 and 75 μM) or DMSO (control) for 30 min at 4 °C. To visualize polymerization of septins, aliquots (10 μl) were applied to glow-discharged carbon-coated grids and stained with 2% uranyl acetate. The grids were examined in a Tecnai-12 TEM (FEI, Hillsboro, OR, USA) under regular-dose conditions at an accelerating voltage of 80 keV.
2.6. DLS
The S. mansoni septin complex(es) at 0.2 mg/ml in binding buffer containing 5 mM GTP was dialyzed against 15 mM Tris-HCl pH 8.0, 40 mM NaCl, and 15 mM DTT in the presence of 75 μM of FCF or DMSO for 30 min at 4 °C. Samples were dispensed into 12 μl quartz cuvettes, inserted into a Zetasizer μV detector (Malvern Instruments, Worcestershire, UK) controlled with Zetasizer version 6.32 software (Malvern Instruments), and DLS from an 830 nm laser was measured at an angle of 90° and at 22°C. DLS was used to determine molecular size.
2.7. Effects of FCF on cultured schistosomes
FCF (Sigma-Aldrich, St. Louis, MO, USA) solution (250 mM) was prepared in DMSO (or ethanol). Aliquots of this stock were dispensed into culture medium, in increasing concentrations from 0 to 500 μM, for cercariae and adult stages of S. mansoni, and into water, from 0 to 200 μM, for miracidia. Control groups containing DMSO were included. To assess the reversibility of the FCF effect, the worms were transferred to fresh culture media without FCF. Phenotypic changes were documented with the assistance of a Zeiss Axio Observer A.1 inverted microscope coupled to a Zeiss AxioCam ICc3 camera. Bright field movies were recorded using the time series mode of the Carl Zeiss LSM 710 system equipped with a Zeiss EC Plan-Neofluar 5x/0.16NA objective. The images were captured consecutively, at 10 frames per second. In some experiments, samples of the schistosomes were removed from the culture and incubated in the fluorophores fluorescein diacetate (FDA), at 2.0 μg/ml, which stains live schistosomes, and propidium iodide (PI), at 0.5 μg/ml, which stains dead schistosomes, using previously reported methods (Peak et al., 2010; Rinaldi et al., 2012). In order to assess the effect of FCF on the F-actin structure, cercariae exposed to 500 μM FCF for 3 h were fixed in 4% paraformaldehyde for 1 h at 4 °C. Subsequently, the worms were stained with Alexa Fluor 568 phalloidin (Invitrogen, Life Technologies, Grand Island, NY, USA) at 165 nM in PBS containing 1 % BSA for 30 min at 25 °C. The slides were mounted with Fluoromount-G (EMS) after which the samples were air-dried and confocal micrographs collected with the Carl Zeiss LSM 710 system equipped with a Zeiss Plan-Apochromat 63x/1.40 oil DIC objective.
2.8. Real time assessment of motility of schistosomes and of egg hatching
The effect of FCF on fluke motility and egg hatching/miracidium motility was evaluated and quantified employing an xCELLigence DP system (ACEA Biosciences, San Diego, CA, USA) originally designed to monitor cellular events in real time by measuring electrical impedance across inter-digitated microelectrodes integrated on the bottom of tissue culture E-plates, see http://www.aceabio.com/main.aspx (Ke et al., 2011). This real time cell assay (RTCA) platform is useful for evaluating motility and egg hatching of other helminth parasites (Smout et al., 2010). The motility test with adult male schistosomes was performed as described (Smout et al., 2010) with the addition of a motility baseline determined over 16 h with 100 μl of media alone before individual flukes were introduced into to each well in three 16 well E-plates. This motility baseline step is a modification to the procedure of Smout et al. (2010) and is desirable due to elevated inter-well baseline variability of the xCELLigence DP platform in comparison with the xCELLigence SP instrument (Smout et al., 2010). In brief, the motility of flukes was registered for ∼24 h before adding 500 μM FCF, or DMSO as vehicle control, in 200 μl media per well. Approximately 6 h later FCF was removed, after which the schistosomes were washed with 1x PBS before fresh medium free of FCF was added to the wells. Recovery of motility after removal of FCF was assessed for ∼16 h. Thereafter, the worms were removed from the E-plates and the final baseline for the plate ascertained for ∼16 h. Controls with individual male schistosomes cultured in media with DMSO (or ethanol) but not FCF and wells with no flukes (‘background’ wells) were included in the assays. To evaluate the motility of miracidia following hatching/release from eggs, 5,000 eggs in 200 μl of 0.1x PBS, 50 μM or 200 μM FCF per well in E-plates were induced to hatch by transfer from physiological medium into 0.1x PBS under bright light at 23 °C for ∼16 h. Controls included wells of eggs exposed to DMSO (or ethanol) in 0.1x PBS and non-viable eggs - eggs that had been killed immediately before the assay by incubation at 80 °C, 15 min. A motility baseline with 100 μl of 0.1X PBS was performed, dispensing schistosome eggs into E-plates. The motility for both adult flukes and miracidia hatching from eggs was monitored every 15 s and the motility index was calculated as described (Smout et al., 2010). The motility index for each E-plate well, i.e. individual flukes or 5,000 eggs, was calculated as the S.D. over 800 data points of the cell index (CI) difference from the rolling average over 20 data points (Smout et al., 2010). Statistical analyses were performed with Graphpad prism 5.0 (Smout et al., 2010). Triplicate biological replicates were undertaken. In parallel, eggs incubated and induced to hatch under these same conditions, as described for the xCELLigence assay, were maintained in 24-well plastic tissue culture plates (Corning Life Sciences, Corning, NY, USA) for microscopical observation (Zeiss Axio Observer A.1 inverted microscope fitted with a Zeiss AxioCam ICc3 camera) of hatching.
3. Results
3.1. Schistosome septins form heterocomplexes
The recently described septins from S. mansoni display traits commonly verified in septins, including a conserved GTPase domain containing the G1, G3 and G4 motifs, the septin unique element (SUE), prior to the C-terminal region and the prediction of a coiled-coil structure at the C-terminal domain (Zeraik et al., 2013). Accordingly, it was anticipated that they organize into filamentous heterocomplexes, as in yeast, Drosophila and human cells (Barral and Kinoshita, 2008). In order to investigate whether S. mansoni septins form such hetero-filaments, the SmSEPT5, SmSEPT10 and SmSEPT7.2 were co-expressed in E. coli; we envisioned the formation of a heterocomplex concomitantly with their expression. After lysis, procedures were performed in a high concentration of NaCl, which dismantles septin filaments into oligomers (Sirajuddin et al., 2007). Only SmSEPT7.2 had a fused His-tag and could be expected to bind directly to the cobalt affinity matrix column during chromatographic purification; the other two recombinant septins could only be co-purified as a consequence of molecular interaction with this septin. The eluate from the affinity column was submitted to gel filtration chromatography; and the resulting profile indicated several populations, suggesting heterogeneity in the composition of the purified complex of the three recombinant septins. Elution fractions corresponding to higher apparent molecular masses of ∼300-400 kDa contained the hetero-complex comprised of the three proteins (Fig. 1). Analysis by MS of that fraction confirmed that the septin hetero-complex comprised all three recombinant S. mansoni septins; peptides corresponding to each of them were detected (Supplementary Table S1). Analysis of the high molecular mass fraction by TEM revealed short rod-like structures (Fig. 2) of similar appearance to those of human septin hetero-oligomers in solution with a high salt concentration (Sirajuddin et al., 2007).
Fig. 1.
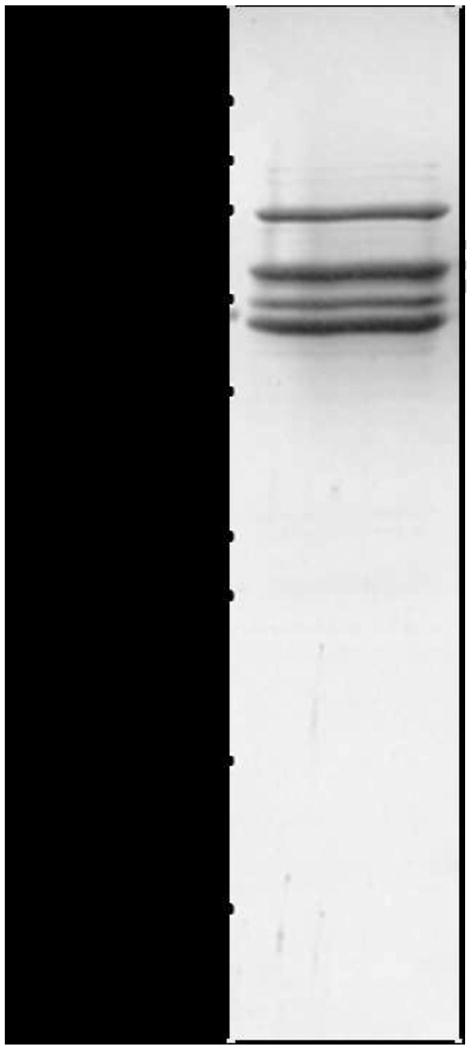
Schistosoma mansoni septins form hetero-oligomeric complexes. SDS-PAGE analysis of the purified hetero-complex. Note that three bands display sizes similar to those expected for SmSEPT5 (51.3 kDa), SmSEPT10 (48.1 kDa) and SmSEPT7.2 (59.2 kDa). The band at ∼75 kDa is indicative of the presence of bacterial GroEL protein. Size standards in kilodaltons (kDa) are shown.
Fig. 2.
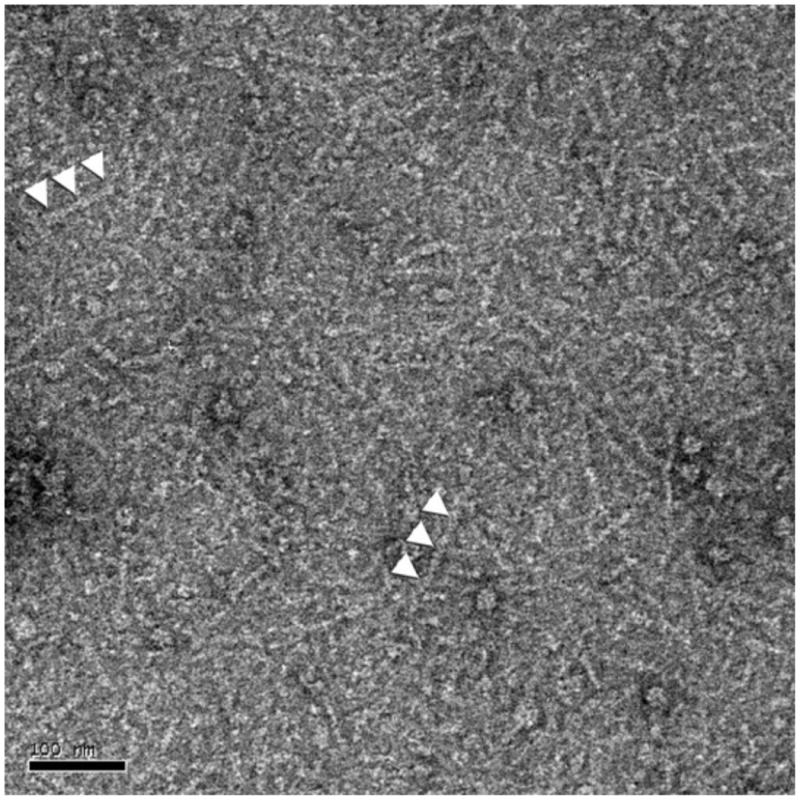
Transmission electron micrographs of negatively stained Schistosoma mansoni septins showing heterogeneous complexes that had assembled (indicated by white arrowheads). Scale bar, 100 nm.
3.2. Polymerization of schistosome septin is hastened by FCF
The cytokinin FCF is a small organic compound that affects septin dynamics in mammalian cells, inducing the assembly of larger structures, among other attributes (Hu et al., 2008). Aiming to verify how schistosome septin assembly is affected by this synthetic phenylurea, septin filament formation was compared in the presence or absence of FCF at 25 μM (Hu et al., 2008). The septin complexes were dialyzed into low salt buffer to initiate polymerization into higher-order structures and the process monitored over time using TEM (Fig. 3). Polymerization of septins has a lag phase and even in the presence of FCF, long filaments were not observed within the first minute of dialysis (Fig. 3A, F, respectively). By 20 min, small quantities of long single filaments were evident in the control (Fig. 3B), as were numerous long filaments in the presence of FCF (Fig. 3G). Similar enhancement in polymerization of septin complexes was observed in FCF at 75 μM (not shown). After dialysis for 3 h, mature bundles were formed regardless of the presence of FCF or not (Fig. 3C - E and H - I). This suggested that whereas FCF hastened the polymerization of schistosome septins, the cytokinin did not influence how these septins interacted with each other within higher-ordered structures. TEM micrographs indicated that septins of S. mansoni form two types of bundles - curved and straight. Septin filaments can switch from one shape to the other within the same bundle, which suggests that the lateral interactions between the filaments within the bundle are polymorphic. To illustrate this phenomenon, we imaged characteristic shapes of bundles of filaments formed in the absence of FCF (Fig. 3C-E). Fig. 3C revealed a mostly straight bundle that terminates in a ring, Fig. 3D shows a long, straight bundle, and Fig. 3E presents an ensemble bundle of both rings and straight filaments. Similar structures were observed in bundles that assembled in FCF (Fig. 3H, I).
Fig. 3.
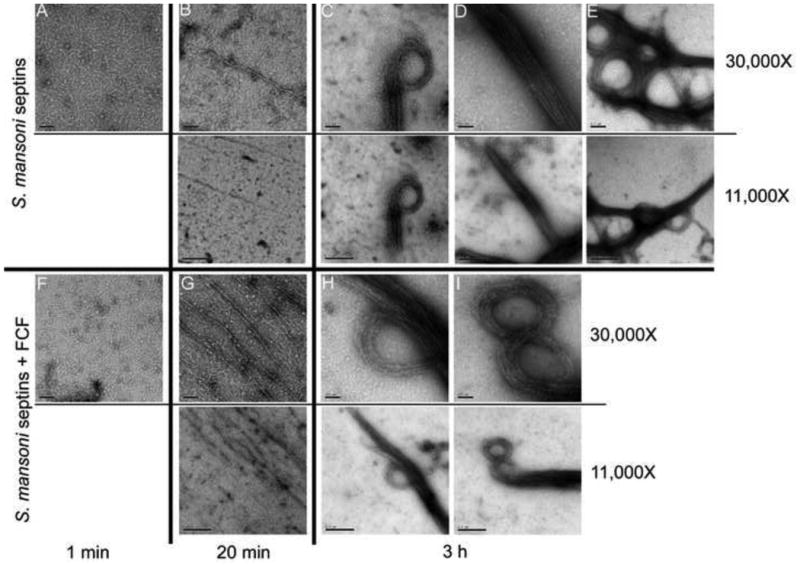
Schistosoma mansoni septin complexes assemble into filaments in vitro. Transmission electron micrographs of negatively stained septin complexes after dialysis against low salt solution with DMSO (A - E) or Forchlorfenuron (FCF) (F - I) for: 1 min (A, F), 20 min (B, G) or 3 h (C - E, H, I). The diversity of higher-order structures formed by S. mansoni septins is especially evident after 3 h of dialysis. Nominal magnification for each panel is shown on the right.
DLS was performed to assess the size of filament particles formed early during septin polymerization. Particle size distributions were obtained for the S. mansoni septin complexes before the dialysis and after dialysis for 30 min in the presence or absence of FCF. After dialysis, larger particles were present in the solution, compared with the sample maintained at high ionic strength, both in the presence or absence of FCF (Fig. 4). However, the solution with FCF showed larger particles in solution compared with the control sample. This indicated that schistosome septins undergo expedited polymerization induced by the decrease of ionic strength and that this process was hastened even further by FCF.
Fig. 4.
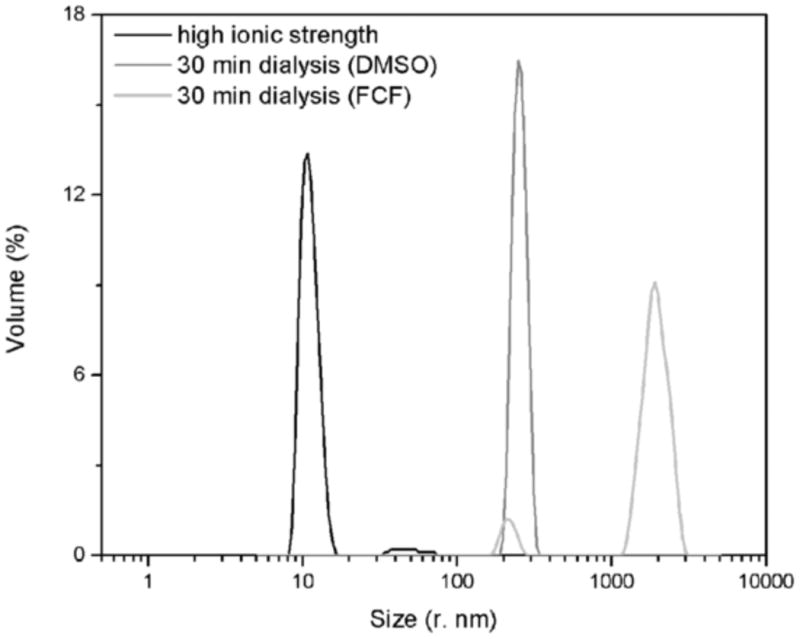
Forchlorfenuron (FCF) hastens polymerization of complexes of schistosome septins. A volume weighted differential size distribution is shown for the purified Schistosoma mansoni septin complexes maintained in high ionic strength (black) and after 30 min dialysis against low salt buffer containing DMSO (dark gray) or 75 μM of FCF) (light gray).
3.3. FCF reversibly paralyzes schistosomes
In view of the influence of FCF on the dynamics of polymerization of schistosome septins, additional investigation was undertaken to examine potential phenotypic effects of this small molecule on developmental stages of schistosomes in vitro. The addition of FCF to culture media had a striking effect on adult stages of these schistosomes – it paralyzed both adult female and male flukes. The worms ceased to move, as observed by light microscopy, in a dose- and time-dependent manner. In 500 μM FCF, paralysis was apparent within 10 min, and by 3 h in 300 μM FCF, all movement by adult schistosomes ceased (Supplementary Movie S1). Yet, strikingly, the paralysis reversed quickly (almost immediately) when the flukes were transferred to culture medium free of FCF (Supplementary Movie S1).
FCF at 500 μM promoted paralysis of cercariae within a few minutes, clearly evident by loss of motor activity of the tail (Supplementary Movie S2). After 3 h in 500 μM FCF, motility could be restored after removal of FCF (Supplementary Movie S2). At 200 μM FCF, reduction of movement was also pronounced, but only after ∼ 3 h (Supplementary Movie S2). Miracidia exhibited exquisite sensitivity; by 30 min in 50 μM FCF, the otherwise highly motile larvae stopped moving and/or displayed only lethargic swimming (Supplementary Movie S3). FCF at 200 μM for 30 min caused miracidia to clump together, with minimal movement discernible in the clumps (Supplementary Movie S3). After 1 h in 200 μM FCF, miracidia had died, as determined by the inability to both exclude PI and uptake FDA, fluorophores that distinguish dead and live schistosomes, respectively (Peak et al., 2010) (Supplementary Fig. S1).
A notable phenomenon evident in schistosomes that were exposed to FCF was that the paralysis was readily reversed when FCF was removed from the culture. In cercariae and adult males, after 24 h in contact with FCF, at 200 and 300 μM, respectively, motility was restored upon removal of FCF. Lower concentrations of 50 to 100 μM did not cause visible effects in cercariae and adults when evaluated for up to 24 h. On the other hand, FCF at concentrations ≥1 mM killed cercariae within minutes. FCF treatment did not appreciably impact the actin filament structure, as phalloidin staining was performed on cercariae after 3 h in the presence of 500 μM FCF, and the F-actin structure appeared unaffected (Supplementary Fig. S2).
The effects of incubation time and FCF concentration for these several, morphologically discrete, developmental stages are summarized in the Table 1. Control groups of these stages (adults, miracidia, cercariae) cultured with vehicle (DMSO) alone exhibited the expected movements for their developmental stages; none of the developmental stages examined displayed paralysis in the absence of FCF (Supplementary Movie S1).
Table 1. Effects of forchlorfenuron (FCF) on developmental stages of Schistosoma mansoni.
| Stage | FCF | 10 min | 30 min | 1 h | 3 h | 24 h | 2-3 h removeda | 24 h removeda |
|---|---|---|---|---|---|---|---|---|
| Adults | <100 μM | ne | ne | ne | ne | ne | ||
| 300 μM | ne | ne | ne | paralysis | paralysis | recovery | ||
| 500 μM | paralysis | paralysis | paralysis | paralysis | death | recovery | ||
| Cercariae | <100 μM | ne | ne | ne | ne | ne | ||
| 200 μM | ne | ne | ab | paralysis | paralysis | recovery | ||
| 500 μM | paralysis | paralysis | paralysis | paralysis | death | recovery | ||
| Miracidia | 50 μM | ab | ab | |||||
| 200 μM | ab | paralysis, clumping | death |
P, paralysis; ne, no effect apparent; ab, abnormal behavior (including slowed movement, lethargy).
Phenotype observed after incubation with FCF for the indicated period followed by removal of FCF and replacement with FCF-free medium.
3.4. Paralysis of schistosomes and inhibition of egg hatching quantified by xCELLigene RTCA
Motility of adult flukes was monitored by RTCA, before and during the incubation with 500 μM FCF for ∼ 6 h and after removal of the phenylurea from the media. ACEA's xCELLigence system uses E-plates, multi-well microtitre plates bearing gold electrodes across which changes in conductivity are monitored and output reported as a CI (Ke et al., 2011). While originally designed for monitoring cultured cells attached to the E-plate surface, we revealed in 2010 that this system is ideal for monitoring movements of parasitic helminths as they move or writhe in vitro (Smout et al., 2010). A motile worm will constantly change the degree of contact with the E-plate and hence the CI output varies with each twitch. The amplitude of this variation is used to monitor the degree of schistosome motility in response to external stimuli such as a drug or xenobiotic, or a pharmacological agent.
The flukes incubated in FCF exhibited near complete paralysis (∼3 - 7% motility) in comparison with flukes cultured in its absence (92-116% motility) (Fig. 5). After removal of FCF, the flukes recovered near complete motility (87% motility) within 10 h. It was noted that after the treatments were removed and fresh media applied, the vehicle control also showed reduced motility (67%) followed by complete recovery. The reason for this is unclear, but changing the medium may be disruptive in this assay such that movement of the schistosomes is perturbed temporarily until the culture conditions stabilize.
Fig. 5.
Motility (%) of adult male schistosomes before, during and after incubation in 500 μM Forchlorfenuron (FCF). The percentage of motility is calculated after subtraction of the background signal detected in the wells before worms were added and relative to worm motility prior to treatment. Treatments were added at time 0 h and removed at 7 h (indicated by the red arrow). Data points are the average of ≥5 replicates ± S.E.M. For clarity, data points are shown at hourly intervals (symbol points have been nudged 0.2 h left or right to reduce overlaps).
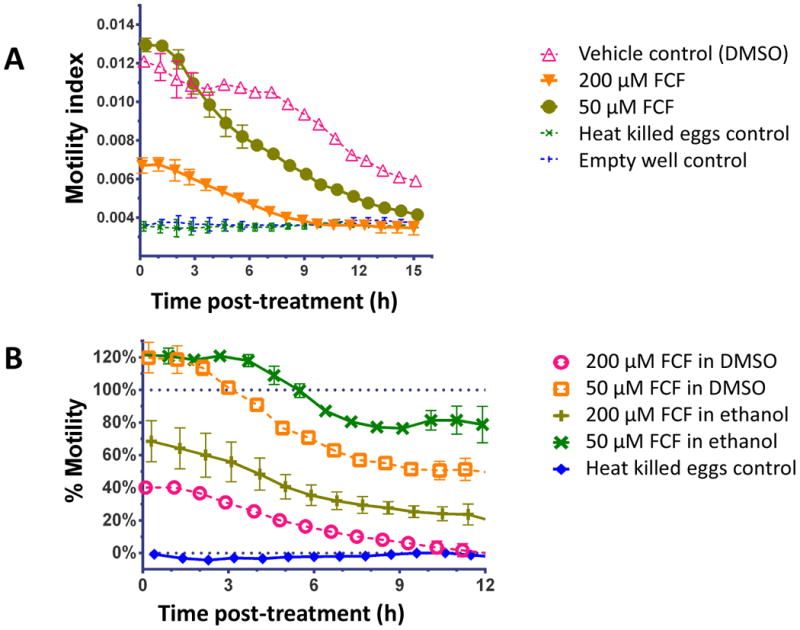
The egg hatch method developed by Smout et al. (2010) directly measures the numbers of hatched larval nematodes that migrate through mesh and fall onto the xCELLigence electrodes to be detected. This technique was employed with caution for schistosomes because the hatched egg releases a ciliated, motile miracidium that does not maintain contact with the electrodes on the base the well of the E-plate. Despite this limitation, we anticipated that the system could monitor egg hatching indirectly. Akin to the assay of motility for adult schistosomes (above), the motility of 5,000 eggs in 0.1 X PBS was measured and the output represented the combination of the number and amount of movement of the hatching eggs and miracidia. Initially we confirmed there were no significant differences in the FCF solvents on egg hatching by comparing ethanol and DMSO without dissolved FCF (Supplementary Fig. S3A). The reduced motility index with 50 μM or 200μM FCF was apparent (Fig. 6A shows FCF in DMSO and Supplementary Fig. S3B shows FCF in ethanol). Converting the motility index to % motility allowed comparisons between the solvents (DMSO versus ethanol) (Fig. 6B). This graph has been limited to 12 h duration; after this point the vehicle control motility index (100% motility) drops below twice the empty well motility index (0% motility) and artificially amplifies data variability % motility as a proportion of the vehicle control. Whereas all FCF treatments significantly reduced hatching/movement over time (P < 0.001), the FCF in DMSO was ∼25% more effective than FCF in ethanol (Fig. 5B). FCF at 200 μM in DMSO induced the most pronounced reduction (down to 37%) within 1 h after induction of egg hatching, and caused complete cessation of hatching and miracidial movement by 11 h. It was also noted that 50 μM FCF in ethanol or DMSO initially induced ∼120% motility (hyper-motility response?) that waned over the next ∼ 4 h. In parallel, these effects of FCF on hatching of eggs and motility of miracidia released from these eggs, as registered and quantified by RTCA, were directly observed and confirmed by microscopical examination (not shown).
Fig. 6.
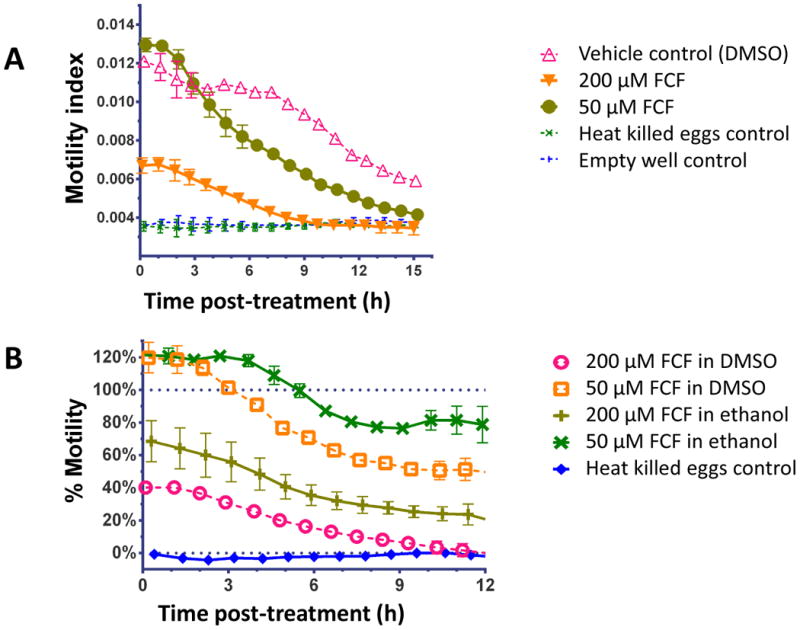
Motility of schistosome miracidia during the egg hatching quantified by the xCELLigence Real Time Cell Analyzer DP platform. (A) Motility Index of miracidia hatching from the eggs exposed to light in 0.1X PBS with Forchlorfenuron (FCF) at 50 μM or 200 μM FCF prepared in DMSO as solvent. (B) Motility (%) of miracidia hatching from eggs with FCF prepared in ethanol or DMSO. These curves range from 0% empty well background to 100% vehicle control.
4. Discussion
The septins are classified into four subgroups and it has been postulated that the septin heterocomplexes contain septin representatives from different subgroups in specific positions (Kinoshita, 2003). Schistosoma mansoni only displays proteins from three of these four subgroups, which are those with members present in the complex whose structures have been previously determined by X-ray diffraction (Sirajuddin et al., 2007). Successful observation of oligomers composed of recombinant septins from schistosomes from these three groups suggests that their assembly into higher-order structures is similar to that reported for orthologous septins (Kinoshita et al., 2002; Versele and Thorner, 2005; John et al., 2007).
Filaments of schistosome septins examined here organized themselves into curved and straight bundles, similar to those observed in other species (see Kinoshita, 2006), suggesting a conserved form of organization. Kinoshita et al. (2002) reported that upon actin disruption, septins tend to reorganize from long linear bundles to uniform rings, and considered that the rings could represent a default assembly or a septin storage form. Ring forms are abolished and septin recovers the linear structures after removal of cytochalasin, a fungal metabolite that depolymerizes actin filaments. It is possible that discrete forms of organization are also relevant in septins of S. mansoni. To address these kinds of questions, functional analyses in studies reported previously have employed antibody probes and RNA interference (Correnti et al., 2005; Morales et al., 2008) but the existence of multiple septin genes, splice isoforms, potential redundancy, plasticity and the wide diversity of septin functions can obfuscate the interpretation of results. It would be informative to determine the presence of these complexes in the parasite itself, perhaps with antisera to pull down such complexes from extracts of schistosomes. This would be challenging, however, because cytoskeletal proteins generally are difficult to solubilize and harsher treatments tend to destroy associations of septins.
The synthetic phenylurea FCF can stabilize septins, although its mechanism of action is unknown (Kim et al., 2010; Hagiwara et al., 2011; Wasik et al., 2012). The stabilized septin filaments are not functional and previous studies have shown that FCF treatments phenocopy the effects of septin depletion by RNAi, such as mitotic and cell migration defects (Hu et al., 2008). Nonetheless, here we used FCF to probe schistosome septins in vitro as well as cultured schistosomes in order to determine if the approach could identify whether the compound also interacts with septin/septin filaments of schistosomes and/or results in an informative phenotype. These assays revealed that FCF enhances the formation of septin fibers early in polymerization of S. mansoni septins, further confirming the specific effect of this cytokinin on a wide range of septins and species. The responses to exposure to FCF by the developmental stages of S. mansoni were striking. Motility of the flukes was severely compromised but, similarly strikingly, its effect was reversible - fully or partially - when FCF was removed from the culture medium. This swift reversal of the phenotype suggested a specific mechanism of action rather than a generalized toxicity. In like fashion, the effects of FCF on fungi and yeast are rapid and reversible (Iwase et al., 2004; De May et al., 2010). Since the action of FCF was relatively slow (10 min - 3 h) whereas the reversal of its effects was swift, the kinetics of binding of FCF to septins might be significantly slower than that of dissociation.
Compared with adult worms and cercaria, the miracidia showed the most marked sensitivity to FCF; FCF was lethal for miracidia after exposure for 1 h to 200 μM of the phenylurea. This might suggest that septins have a critical role in this developmental stage. This is consistent with the earlier findings that septins exhibit distinctive patterns of localization among developmental stages of the schistosome, indicating they perform development-related functions (Zeraik et al., 2013). Also, the motility of miracidia exiting hatching eggs appeared to be influenced by the solvent used as vehicle for FCF, with formulations with DMSO enhancing efficiency of FCF over those with ethanol. A possible explanation for this phenomenon may be related to the ability of DMSO to perturb lipid bilayers (Anchordoguy et al., 1992), and hence its presence might favor uptake of FCF by the schistosome cells.
Immunolocalization of septins in schistosomes revealed the prevalence of septin filaments in the muscles of this blood fluke – septin co-localized with F-actin fibers (Zeraik et al., 2013). Accordingly, we now hypothesize that the paralysis phenotype from exposure to FCF may result from interference of muscle contraction in which interaction between actin and septin filaments is expected to be essential for normal function of muscular fibers. Given that the phenotype was promptly reversed upon removal of FCF, there is likely to be a specific, defined mechanism involved rather than generalized disorganization of the filaments. This mechanism may be related to the perturbed polymerization of septin filaments observed in the presence of FCF.
In view of the striking reversible paralysis phenotype described here, and increased understanding of septins in the pathobiology of human diseases ranging from neurodegeneracy to oncogenesis (Kartmann and Roth, 2001), deeper investigation is warranted to elucidate the exact mechanism of action of this phenylurea derivative on schistosome septins. Considering the paralysis phenotype, it is possible to propose the therapeutic use of FCF for schistosomiasis. Indeed, an analogous paralysis is displayed when worms are cultured in praziquantel (Chavasse et al., 1979; You et al., 2013). A drawback is that high doses of FCF were required to achieve a visible phenotype in schistosomes, which would limit utility of the compound as an oral medication. Nonetheless, developing derivatives of FCF with higher affinity to schistosome septins may be warranted, thus facilitating a tractable pharmacological regimen. FCF has horticultural value; for example, the European Food Safety Authority approves its use for foliar outdoor treatment of commercial crops of grapes, kiwi fruits and other plants, and its toxicity has been assessed, http://www.efsa.europa.eu/en/efsajournal/doc/2862.pdf. In rats, FCF displays an acute oral toxic dose of 4,918 mg/kg, close to the threshold of 5,000 mg/kg at which a substance is considered non-toxic. Chronic oral exposure to FCF in animal models caused minimal side effects, limited to loss of body weight and kidney inflammation in long-term treatments (2 years with ∼100 mg/kg/day dosage) (United States Environmental Protection Agency, 2004). These findings indicate that FCF does not harm mammals.
Lastly, the development of an automated and quantitative schistosome egg hatch assay based on the xCELLigence system presented here is also noteworthy. This novel application expands the catalogue of species of helminth parasites and developmental stages amenable (e.g. Smout et al., 2010; Silbereisen et al., 2011; Tritten et al., 2013; You et al., 2013) to functional analysis in real time without the need for laborious, and more subjective, assessment by microscopy.
Supplementary Material
Supplementary Fig. S1. Viability of schistosome miracidia after exposure to 200 μM forchlorfenuron (FCF) for 1 h. (A) Propidium iodide (PI; 2.0 μg/ml) and fluorescein diacetate (FDA; 0.5 μg/ml) stained miracidia; dead cells stained in red by PI and live cells in green by FDA. (B) Bright field micrograph of miracidia. Scale bar, 100 μm.
Supplementary Fig. S2. Forchlorfenuron did not alter F-actin filaments in schistosomes. Cross-section confocal imaging of a cercaria of Schistosoma mansoni stained with phalloidin conjugated to Alexa Fluor 568. Scale bar, 20 μm.
Supplementary Fig. S3. Additional findings dealing with schistosome egg hatching/ miracidial motility. (A) Comparisons of egg hatch motility index with differing solvents (DMSO and ethanol alone) for forchlorfenuron (FCF). (B) Motility index for miracidia hatching from the eggs of Schistosoma mansoni exposed to light in 0.1 X PBS in FCF (dissolved in ethanol).
Highlights.
Schistosome septins assemble into complexes and higher-order structures, including filaments and rings
Polymerization of schistosome septins hastened in forchlorfenuron (FCF), a phenylurea cytokinin
FCF led to a striking paralysis phenotype in cercariae and adult schistosomes
The paralysis was reversible, but FCF dose- and time-dependent
Acknowledgments
The authors gratefully acknowledge Drs. Anastas Popratiloff and Danielle E. Skinner for their technical assistance. This work was supported by NIH, USA, Shared Instrumentation Grant S10RR025565 and by CNPq, Brazil and FAPESP, Brazil to INCT/Instituto Nacional de Biologia Estrutural e Química Medicinal em Doenças Infecciosas, Brazil. AEZ received a CNPq and CAPES, Brazil fellowship (BEX: 9193/11-1). RDM, RCG and APUA are recipients of productivity fellowships from CNPq.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anchordoguy TJ, Carpenter JF, Crowe JH, Crowe LM. Temperature-dependent perturbation of phospholipid bilayers by dimethylsulfoxide. Biochim Biophys Acta. 1992;1104:117–122. doi: 10.1016/0005-2736(92)90139-d. [DOI] [PubMed] [Google Scholar]
- Barral Y, Kinoshita M. Structural insights shed light onto septin assemblies and function. Curr Opin Cell Biol. 2008;20:12–18. doi: 10.1016/j.ceb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Beites CL, Xie H, Bowser R, Trimble WS. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci. 1999;2:434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- Cao L, Yu W, Wu Y, Yu L. The evolution, complex structures and function of septin proteins. Cell Mol Life Sci. 2009;66:3309–3323. doi: 10.1007/s00018-009-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavasse CJ, Brown MC, Bell DR. Schistosoma mansoni: activity responses in vitro to praziquantel. Z Parasitenkd. 1979;58:169–174. doi: 10.1007/BF01951341. [DOI] [PubMed] [Google Scholar]
- Correnti JM, Brindley PJ, Pearce EJ. Long-term suppression of cathepsin B levels by RNA interference retards schistosome growth. Mol Biochem Parasit. 2005;143:209–215. doi: 10.1016/j.molbiopara.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Dalton JP, Day SR, Drew AC, Brindley PJ. A method for the isolation of schistosome eggs and miracidia free of contaminating host tissues. Parasitology. 1997;115:29–32. doi: 10.1017/s0031182097001091. [DOI] [PubMed] [Google Scholar]
- DeMay BS, Meseroll RA, Occhipinti P, Gladfelter AS, Goode B. Cellular requirements for the small molecule forchlorfenuron to stabilize the septin cytoskeleton. Cytoskeleton. 2010;67:383–399. doi: 10.1002/cm.20452. [DOI] [PubMed] [Google Scholar]
- Dobbelaere J, Barral Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science. 2004;305:393–396. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- Estey MP, Di Ciano-Oliveira C, Froese CD, Bejide MT, Trimble WS. Distinct roles of septins in cytokinesis: SEPT9 mediates midbody abscission. J Cell Biol. 2010;191:741–749. doi: 10.1083/jcb.201006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority. Reasoned opinion on the review of the existing maximum levels (MRLs) for forchlorfenuron according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2012;10(8):2862. Parma, Italy http://www.efsa.europa.eu/en/efsajournal/doc/2862.pdf. [Google Scholar]
- Hagiwara A, Tanaka Y, Hikawa R, Morone N, Kusumi A, Kimura H, Kinoshita M. Submembranous septins as relatively stable components of actin-based membrane skeleton. Cytoskeleton. 2011;68:512–525. doi: 10.1002/cm.20528. [DOI] [PubMed] [Google Scholar]
- Hartwell L. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- Hu Q, Nelson WJ, Spiliotis ET. Forchlorfenuron alters mammalian septin assembly, organization, and dynamics. J Biol Chem. 2008;283:29563–29571. doi: 10.1074/jbc.M804962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase M, Okada S, Oguchi T, Toh-e A. Forchlorfenuron, a phenylurea cytokinin, disturbs septin organization in Saccharomyces cerevisiae. Genes Genet Syst. 2004;79:199–206. doi: 10.1266/ggs.79.199. [DOI] [PubMed] [Google Scholar]
- John CM, Hite RK, Weirich CS, Fitzgerald DJ, Jawhari H, Faty M, Schläpfer D, Kroschewski R, Winkler F, Walz T, Barral Y, Steinmetz M. The Caenorhabditis elegans septin complex is nonpolar. EMBO J. 2007;26:3296–3307. doi: 10.1038/sj.emboj.7601775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartmann B, Roth D. Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J Cell Sci. 2001;114:839–844. doi: 10.1242/jcs.114.5.839. [DOI] [PubMed] [Google Scholar]
- Ke N, Wang X, Xu X, Abassi YA. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol Biol. 2011;740:33–43. doi: 10.1007/978-1-61779-108-6_6. [DOI] [PubMed] [Google Scholar]
- Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, Wallingford JB. Planar Cell Polarity Acts Through Septins to Control Collective Cell Movement and Ciliogenesis. Science. 2010;329:1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self and actin template assembly of mammalian septins. Dev Cell. 2002;3:791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- Kinoshita M. Assembly of mammalian septins. J Biochem. 2003;134:491–496. doi: 10.1093/jb/mvg182. [DOI] [PubMed] [Google Scholar]
- Kinoshita M. Diversity of septin scaffolds. Curr Opin Cell Biol. 2006;18:54–60. doi: 10.1016/j.ceb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Bi E. Regulation of septin organization and function in yeast. Trends Cell Biol. 2003;13:403–409. doi: 10.1016/s0962-8924(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Mann VH, Morales ME, Rinaldi G, Brindley PJ. Culture for genetic manipulation of developmental stages of Schistosoma mansoni. Parasitology. 2009;137:451–62. doi: 10.1017/S0031182009991211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales ME, Rinaldi G, Gobert GN, Kines KJ, Tort JF, Brindley PJ. RNA interference of Schistosoma mansoni cathepsin D, the apical enzyme of the hemoglobin proteolysis cascade. Mol Biochem Parasit. 2008;157:160–168. doi: 10.1016/j.molbiopara.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S, Cossart P. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:183–194. doi: 10.1038/nrm3284. [DOI] [PubMed] [Google Scholar]
- Nishihama R, Onishi M, Pringle JR. New insights into the phylogenetic distribution and evolutionary origins of the septins. Biol Chem. 2011;392:681–687. doi: 10.1515/BC.2011.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peak E, Chalmers IW, Hoffmann KF. Development and Validation of a Quantitative, High-Throughput, Fluorescent-Based Bioassay to Detect Schistosoma Viability. PLoS Negl Trop Dis. 2010;4:e759. doi: 10.1371/journal.pntd.0000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi G, Suttiprapa S, Tort JF, Folley AE, Skinner DE, Brindley PJ. An antibiotic selection marker for schistosome transgenesis. Int J Parasitol. 2012;42:123–130. doi: 10.1016/j.ijpara.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal AA, Kozubowski L, Goode BL, Drubin DG, Hartwig JH. Actin and Septin Ultrastructures at the Budding Yeast Cell Cortex. Mol Biol Cell. 2004;16:372–384. doi: 10.1091/mbc.E04-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SEH, Hall PA. Septin genomics: a road less travelled. Biol Chem. 2011;392:763–767. doi: 10.1515/BC.2011.079. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Nichols BJ. Functional interdependence between septin and actin cytoskeleton. BMC Cell Biol. 2004;5:43. doi: 10.1186/1471-2121-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereisen A, Tritten L, Keiser J. Exploration of novel in vitro assays to study drugs against Trichuris spp. J Microb Methods. 2011;87:169–175. doi: 10.1016/j.mimet.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Hauer F, Kühlmann D, Macara IG, Weyand M, Stark H, Wittinghofer A. Structural insight into filament formation by mammalian septins. Nature. 2007;449:311–315. doi: 10.1038/nature06052. [DOI] [PubMed] [Google Scholar]
- Smout MJ, Kotze AC, McCarthy JS, Loukas A. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLOS Negl Trop Dis. 2010;4:e885. doi: 10.1371/journal.pntd.0000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis ET, Hunt SJ, Hu Q, Kinoshita M, Nelson WJ. Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J Cell Biol. 2008;180:295–303. doi: 10.1083/jcb.200710039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley AJ, Gilden J, Jacobelli J, Beemiller P, Trimble WS, Kinoshita M, Krummel MF. Amoeboid T lymphocytes require the septin cytoskeleton for cortical integrity and persistent motility. Nat Cell Biol. 2008;11:17–26. doi: 10.1038/ncb1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritten L, Braissant O, Keiser J. Comparison of novel and existing tools for studying drug sensitivity against the hookworm Ancylostoma ceylanicum in vitro. Parasitology. 2012;139:348–357. doi: 10.1017/S0031182011001934. [DOI] [PubMed] [Google Scholar]
- Office of Prevention Pesticides Toxic Substances, United States Environmental Protection Agency. Pesticide Fact Sheet Name of Chemical: Forchlorfenuron. USA. 2004 Retrieved from http://www.epa.gov/pesticides/chemsearch/regactions/registration/fsPC-12881901-Sep-04.pdf.
- Versele M, Thorner J. Some assembly required: yeast septins provide the instruction manual. Trends Cell Biol. 2005;15:414–424. doi: 10.1016/j.tcb.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasik AA, Polianskyte-Prause Z, Dong MQ, Shaw AS, Yates JR, Farquhar MG, Lehtonen S. Septin 7 forms a complex with CD2AP and nephrin and regulates glucose transporter trafficking. Mol Biol Cell. 2012;23:3370–3379. doi: 10.1091/mbc.E11-12-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, McManus DP, Hu W, Smout MJ, Brindley PJ, Gobert GN. Transcriptional responses of in vivo praziquantel exposure in schistosomes identifies a functional role for calcium signalling pathway member CamKII. PLoS Pathog. 2013;9(3):e1003254. doi: 10.1371/journal.ppat.1003254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeraik AE, Rinaldi G, Mann VH, Popratiloff A, Araujo APU, DeMarco R, Brindley PJ. Septins of Platyhelminths: Identification, Phylogeny, Expression and Localization among Developmental Stages of Schistosoma mansoni. PLoS Negl Trop Dis. 2013;7:e2602. doi: 10.1371/journal.pntd.0002602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. S1. Viability of schistosome miracidia after exposure to 200 μM forchlorfenuron (FCF) for 1 h. (A) Propidium iodide (PI; 2.0 μg/ml) and fluorescein diacetate (FDA; 0.5 μg/ml) stained miracidia; dead cells stained in red by PI and live cells in green by FDA. (B) Bright field micrograph of miracidia. Scale bar, 100 μm.
Supplementary Fig. S2. Forchlorfenuron did not alter F-actin filaments in schistosomes. Cross-section confocal imaging of a cercaria of Schistosoma mansoni stained with phalloidin conjugated to Alexa Fluor 568. Scale bar, 20 μm.
Supplementary Fig. S3. Additional findings dealing with schistosome egg hatching/ miracidial motility. (A) Comparisons of egg hatch motility index with differing solvents (DMSO and ethanol alone) for forchlorfenuron (FCF). (B) Motility index for miracidia hatching from the eggs of Schistosoma mansoni exposed to light in 0.1 X PBS in FCF (dissolved in ethanol).


