Abstract
Gaining insight into the impact of anthropogenic change on ecosystems requires investigation into interdependencies between multiple drivers of ecological change and multiple biotic responses. Global environmental change drivers can act simultaneously to impact the abundance and diversity of biota, but few studies have also measured the impact across trophic levels. We firstly investigated whether climate (using temperature differences across a latitudinal gradient as a surrogate) interacts with habitat fragmentation (measured according to fragment area and distance to habitat edges) to impact a New Zealand tri-trophic food chain (plant, herbivore and natural enemy). Secondly, we examined how these interactions might differentially impact both the density and biotic processes of species at each of the three trophic levels. We found evidence to suggest that these drivers act non-additively across trophic levels. The nature of these interactions however varied: location synergistically interacted with fragmentation measures to exacerbate the detrimental effects on consumer density; and antagonistically interacted to ameliorate the impact on plant density and on the interactions between trophic levels (herbivory and parasitoid attack rate). Our findings indicate that the ecological consequences of multiple global change drivers are strongly interactive and vary according to the trophic level studied and whether density or ecological processes are investigated.
Keywords: antagonistic, climate, density, land-use change, synergistic, trophic level
1. Introduction
Concern has been mounting for over a decade about the ability of environmental systems to absorb multiple anthropogenic pressures [1,2]. Despite this, global environmental change research is a young branch of ecological knowledge [3], and only a few studies have empirically investigated [4,5], conceptually modelled [6] or reviewed [2,7,8] the effects of simultaneous pressures explicitly.
Drivers of global change include nitrogen deposition, CO2 enrichment, biotic invasions, climate change and land-use change [9]. Alone, these drivers each impose considerable influence on biodiversity around the world. For example, Zavaleta et al. [10] found that three (elevated CO2, nitrogen deposition and precipitation) of four (warming) global change variables exerted a rapid impact on the diversity of Californian grasslands. Carbon dioxide (ambient plus 300 ppm) and nitrogen deposition (increased by 7 g m−2 yr−1) diminished diversity after 3 years, precipitation (increase of 50%, including a growing-season extension of 20 days) increased it and warming (soil-surface warming 0.8–1°C) had no effect. Understanding how multiple drivers interact—do they simply add-up in a linear fashion or does one non-additively interact with the other?—is increasingly imperative given the challenges facing contemporary conservation science [11].
Two types of non-additive outcomes are possible from interactions among global change drivers: either they combine in a synergistic fashion (total effect is amplified), or they combine in an antagonistic way (total effect is reduced) [12]. The former, synergistic interactions, was exemplified by Mora et al. [4], who found that declines in experimental populations of rotifers occurred 50 times faster when fragmentation, environmental warming and overexploitation acted simultaneously than when these same threats occurred in isolation. The latter, antagonistic interactions, was exemplified by Zvereva & Kozlov [13], who found that the adverse impact of carbon dioxide elevation on herbivore performance was offset by the favourable impact of increased temperature. Systems subjected to multiple, usually sequential stressors such as imposed by global change, may therefore enter alternative abnormal stable states, and hence ‘ecological surprises’ can be expected when attempting to predict effects [14].
Climate change and land-use modification are the two dominant drivers of global environmental change [11,15], altering population dynamics [16], restricting species distributions [17] and influencing food web dynamics [9]. While these drivers have globally recognized impacts on the persistence of biodiversity, relatively few studies have empirically investigated their simultaneous impacts [2,8] and have instead focused on one or the other driver [18,19]. Initial studies into their interactive impact do however suggest that climate change and habitat destruction have the potential to produce a ‘deadly anthropogenic cocktail’ [20]. Here, we investigate the impact of two indicators of these global change drivers, temperature differences and habitat fragmentation in New Zealand, the last major land mass to be colonized by humans [21]. In the approximately 730 years of human occupation [22], the clearance of native habitat for food provisions and transport networks [23] has reduced indigenous forest cover from 82% [24] to 24% of the total land area leaving the remnants heavily fragmented [23]. These environments are simultaneously undergoing the additional pressure of rapid climate change. Temperatures in New Zealand have risen by about 0.9°C from 1908 to 2006 [25], slightly higher than the average global rise of 0.4–0.8°C over the past 100 years [26]. This trend is projected to continue with an additional national average rise in temperature of between 0.2 to 2°C expected by 2040, and 0.7 to 5.1°C temperature rises expected by 2090 [25].
There are a number of approaches to study the impact of climate on biota including: climate models on individual species or habitats; long-term studies at a particular site; or experiments that compare slightly warmer or cooler sites [27]. The latter approach is particularly appropriate for a relatively short-term study in an environment with pronounced latitudinal and altitudinal temperature differences (which also vary within the range of potential climatic changes, as mentioned above). A number of researchers have used a latitudinal surrogate approach such as this [27–29], however as Andrew & Hughes [30] point out, a number of studies [31,32] confound differences in habitat and evolutionary lineages with climate impacts. To reduce the impact of these variables, here we use latitudinal environmental gradients while selecting sites with similar habitat types and elevation, and study the same focal species along the gradient.
A large number of studies have measured the impacts of global change on ecological systems through changes in population abundance [33], diversity [34], organism physiology [35] and distributional shifts of suites of species [36]. More recently, the role of biotic interactions—whether they be pollination, competition, herbivory, predation and parasitism—between networks of interacting species has been recognized as being fundamental in shaping and maintaining biodiversity [37] and in determining responses to multiple drivers [1,2]. The impact of global change drivers on biotic interactions may be different than the impact on abundance or diversity measures alone due to the compounding impacts of multiple phenological, physiological and behavioural responses of those interacting species [9,38]. In addition to recording measures such as abundance or density, incorporating process-based measures into ecological change research is likely to enhance the accuracy of predictions on the response of species to global change [39].
Here, we monitor a New Zealand tri-trophic system in order to examine how organisms at different levels of the food chain (density measures) respond and how the frequency of biotic interactions (process measures) alters under multiple drivers of environmental change. Specifically, the study seeks to investigate whether two drivers of global environmental change—habitat fragmentation and climate (latitudinal temperature surrogate)—exert an additive or interactive effect on the various components of this tri-trophic system, and whether simultaneous global change impacts are experienced uniformly across trophic levels and species interactions.
2. Material and methods
(a). Study system
The native Kawakawa tree (Macropiper excelsum, Piperales: Piperaceae) is a common understory plant throughout the mixed native scrub environments of New Zealand [40]. The species is confined to coastal areas of the New Zealand mainland and a number of its offshore islands and reaches its southern limit at a latitude of 43°46′ S (Banks Peninsula) on the Pacific coast of New Zealand. A range of biochemical defences stored within M. excelsum leaves deters widespread generalist feeding activity. However, despite the tree's range of anti-herbivory bio-compounds [41] it has a non-specialist primary herbivore, Cleora scriptaria (Lepidoptera: Geometridae). This moth is effective at sequestering these compounds and feeds extensively on the plant. Cleora scriptaria is responsible for the conspicuous shot hole appearance of herbivory scars, which is highly distinguishable in M. excelsum. The two most commonly associated parasitoid species, which feed on this geometrid moth, are Aleiodes declanae (Brachonidae: Rogadinae) and Meteorus pulchricornis (Brachonidae: Euphorinae). The former is a native non-specialist koinobiont endoparasitoid, which attacks the moth larvae at the first and second instars and mummifies at the fourth and fifth instars. The latter is a globally widespread generalist endoparasitoid accidentally introduced into New Zealand in 1996 [42]. This invasive koinobiont species also attack larvae at the second instar and emerge at the fourth.
(b). Sampling design
We collected data on the tri-trophic system across a fragmentation and climatic gradient, using latitude as a surrogate for a gradient of temperature. We selected five field locations spanning seven degrees of latitude from Banks Peninsula on the South Island (43°46′ S) up to Auckland on the North Island (36°51′S) (figure 1). According to NIWA weather station data, this corresponds to a 4.1°C average temperature gradient; this value is within the range of temperature increases projected for each region by 2090 (ranging from 0.7 to 5.1°C [5]; figure 2). Three additional locations were selected at intervals between the latitudinal extremes, located in the Waikato district (37°54′ S), Wellington district (41°12′ S), and the Nelson district (41°15′ S). Two of these locations (Wellington and Nelson) lie at similar latitudes, but are sited on different land masses (North and South Island, respectively). To verify that this temperature gradient was evident at field sites, microclimate air temperature data were collected using iButton dataloggers positioned every 10 m across an edge gradient (0–40 m) at each field site during the period research was taking place. Similar to the 10-year NIWA climate data, we found a temperature gradient across latitude (figure 2a). Elevation did not differ significantly between locations (F4,35 = 1.603, p = 0.196) across the sites on the two main islands (mean m.a.s.l.; Auckland: 75, Hamilton: 150, Wellington: 93, Nelson: 74 and Christchurch: 80). Indigenous forest cover (in 2002) in the five regions varied between 6 and 31% (Auckland: 13%, Waikato: 22%, Wellington: 20%. Nelson: 31% and Canterbury: 6%; [23])
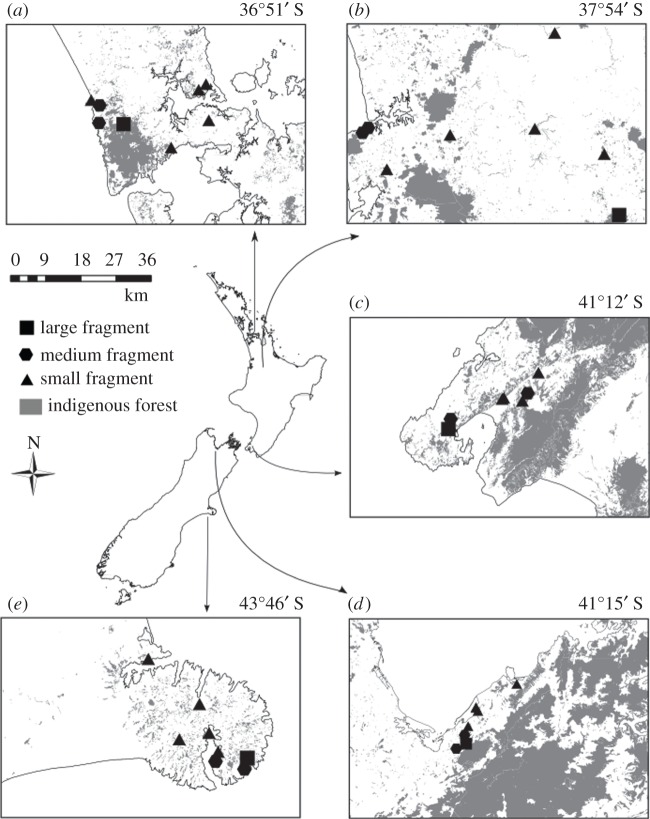
Figure 1.
Field site localities. Five sites span the distribution of M. excelsum (national map modified from Gardner [43]) and extend across a latitudinal gradient of approximately 7° over the North and South Island of New Zealand: Auckland (a), Waikato (b), Wellington (c), Nelson (d) and Banks Peninsula (e). Grey shading in panels (a–e) shows the distribution of indigenous forest in the five study localities. At each locality, eight forest fragments (average latitude given above insets) were sampled, with the fragments categorized according to area: large more than 100 ha (squares), medium 10–100 ha (hexagons) and small less than 10 ha (triangles).
Figure 2.
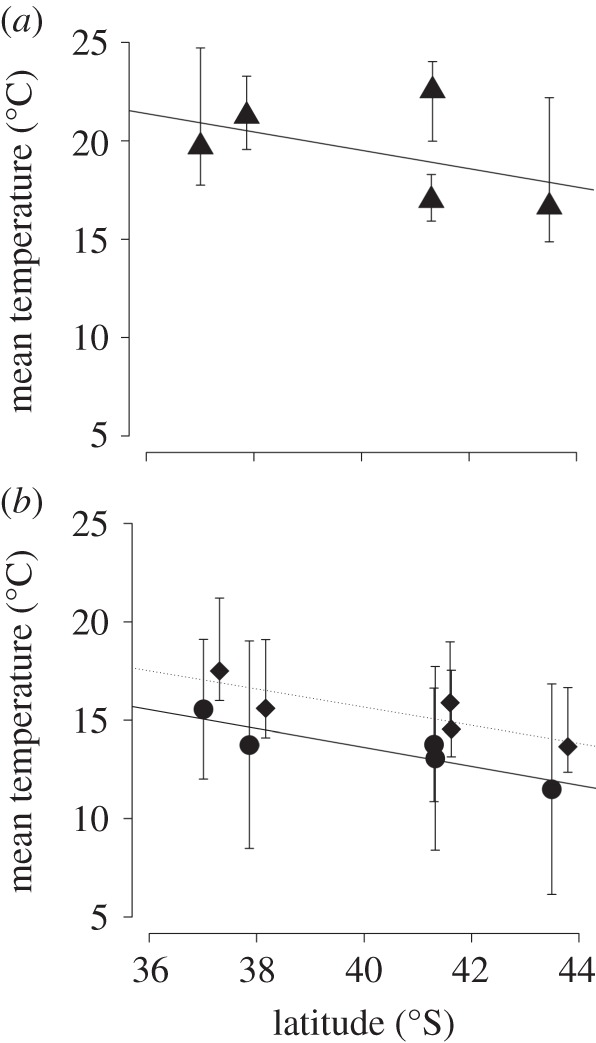
Temperature variation across latitude in New Zealand. Microhabitat data (a) collected at field sites with data loggers positioned across 0–40 m of the edge of the forest fragment over a one-week period during biological data collection in 2009/2010. Triangles represent average values, and whiskers represent the average minimum and maximum temperatures across sites. Longer term climate readings (b). Circles represent mean air temperature averaged over a 10-year period 2002–2011 from NIWA automatic weather stations (or airport stations where AWS are not present) which are situated closest to the field locations, and whiskers represent the average minimum and average maximum daily air temperatures over this period [44]. Diamonds represent future temperatures derived from 12 model projections of A1B scenario 2090 mean temperature [25]. Values represent the average projected mean annual temperature for each latitude in 2090, and whiskers represent the minimum and maximum model predictions.
At each of the five locations (approx. latitude), eight forest fragments were selected on the basis of M. excelsum presence, landowner permission, accessibility and size. To reflect the size distribution of fragments in real landscapes (the relative abundance of small patches of forest in comparison to large), we surveyed more small than large fragments and surveyed an equal number of small, medium and large fragments at each location. This comprised one large fragment (more than 100 ha), two medium fragments (10–100 ha) and five small (less than 10 ha) fragments. A single transect in each forest fragment was established, extending from the edge (denoted by the dripline marking the extent of overhanging branches) to the interior of the forest fragment along a north to south trajectory. Sampling plots (i.e. individual plants) were situated every 10 m along transects. The length of a transect was dependent upon the size of fragment: transects were 300 m in large fragments (N = 31 plots × 1 fragment), 150 m in medium fragments (N = 16 plots × 2 fragments) and 60 m long in small fragments (N = 7 plots × 5 fragments). This sampling design ensured that the total number of sample plots in each of the three fragment size categories were approximately equal in each region (31 ≤ N ≤ 35).
(c). Field data collection
Data collection took place in the austral summer of 2009/10. One M. excelsum tree was sampled per plot, with the tree selected being the one that was nearest to the centre of the plot. All trees selected were located within 2 m of the transect. For each tree, we recorded the density of surrounding M. excelsum individuals (number of individuals in a 2 m radius centred on the target tree) and plant growth (number of young leaves visible at time of sampling, with young leaves identified by their light green coloration and small size). Density of the caterpillar C. scriptaria was determined by beating each tree for 10 s (approximately one beat per second) and counting the number of larvae collected on a 1 m2 beating tray extended beneath the tree. Herbivory levels were estimated by quantifying the percentage of the leaves exhibiting evidence of feeding. From each tree, we live-collected a maximum of 10 larvae, which were reared in the laboratory to determine parasitism rates. If less than five larvae were sourced from the target tree, we collected additional larvae from the tree's nearest neighbours until we had collected five caterpillars in total. In some cases, we were unable to collect five caterpillars from trees within the specified distance from the forest edge, so we reduced sample size at these sample plots (mean = 2.9 caterpillars per plot; range 0–10). In the laboratory, caterpillars were fed a diet of M. excelsum and raised in transparent plastic containers through to either adult development or parasitoid emergence in controlled conditions of 20°C and 65% relative humidity (following Hodge et al. [45] and Schnitzler et al. [46]). The parasitoids that emerged from C. scriptaria were then identified and parasitism rates calculated by comparing the number of caterpillars that were found to be attacked against the number that were not attacked.
(d). Analysis
To analyse (i) whether habitat fragmentation and climate (latitudinal temperature gradient) exert an additive or interactive effect on forest ecosystems and (ii) whether simultaneous global change impacts are experienced uniformly across trophic levels and interactions, we use generalized linear mixed effect models (GLMM) performed using the lmer function in the lme4-library [47] of the R v. 2.11.1 statistical environment [48]. Owing to the hierarchical nature of this experimental design, the random effect of fragment (eight fragments at each of five locations, N = 40) was included in the model to account for the close proximity of sites within fragments. As we were interested in investigating the impact of latitude (a surrogate for temperature differences) and fragmentation, we included location (categorical), fragment area (loge-transformed, continuous) and distance from edge (continuous) as fixed effects. We treated location as a categorical variable and relied on post hoc interpretation to identify trends across the latitudinal temperature gradient. Model assessments followed Zuur et al. [49], beginning with a full model and simplified using Akaike's information criterion (AICc) adjusted for small sample size [50]. The effects of multiple drivers were considered non-additive if models including interaction terms had the lowest AICc values, or conversely, were considered additive if the additive models had lower AICc values.
The nature of interactions was determined by comparing effect sizes between additive and non-additive interactions. Although detection of higher order interactions typically precludes the requirement to analyse lower order interactions [51], here, similar to Christensen et al. [52], we used these models comprising individual effects and lower order interactions to help interpret higher order interaction models. Interaction effect sizes (F-values) were subtracted from the sum of main effect sizes producing values representing the difference between additive and interactive effects. If the value was positive (i.e. additive effect was greater than the interactive), then the relationship was considered to be antagonistic (the impact of the drivers was reduced when acting interactively); if however, the value was negative (i.e. additive effect was less than the interactive) then the relationship was considered to be synergistic (the impact of the drivers was increased when acting interactively).
To measure the effect of location and fragmentation on the tri-trophic feeding system, we analysed data within and among multiple trophic levels, with data collected on a measure of both density variables and process variables at each level. At trophic level one, we recorded plant density (count of abundance per unit area) and plant growth (growth process: quantified as the number of new leaves present at time of observation); at trophic level two, the caterpillar density (count of abundance per tree) and herbivory (feeding process: quantified as the percentage of leaves exhibiting signs of attack) were recorded; and at the third trophic level, the parasitoid density (count of the abundance: quantified as the total number of parasitoids that emerged from caterpillars collected at each plot, with the number of caterpillars collected at that plot included as an offset in the model) and the parasitoid attack rate (predation process: quantified as the proportion of caterpillars with parasitoids). All density response variables were count data, and as such a Poisson link function was applied to the GLMMs. We also used a Poisson error when analysing plant growth. By contrast, herbivory rates were percentage data and as such arcsine square root transformations were applied and data analysed using Gaussian errors, and the parasitism rate represented the number of attacked caterpillars versus number not attacked and, as such, was analysed using a binomial link function.
3. Results
For all dependent variables (except herbivory), the optimal model with lowest AICc included an interaction term between at least two of the independent variables (edge, area and location), suggesting that location and fragmentation exert non-additive influences on this tri-trophic system (electronic supplementary material, appendix S1). For all but one of those dependent variables, the optimal model included interaction terms between all three main effects, and for the other (caterpillar density), the optimal model included interactions between edge and location (electronic supplementary material, appendix S1). Habitat fragmentation and latitudinal differences both exerted considerable influence over the density of species and the frequency of interactions between those species (table 1 and figure 3). Location tended to exert larger effects on the density of organisms than fragmentation, whereas the opposite was generally true of the ecological processes (table 1). Interactions between location and fragmentation were stronger than interactions between the two attributes of fragmentation (edge and area) for all response variables except caterpillar herbivory (table 1).
Table 1.
Individual and interactive impacts of habitat fragmentation (edge and area effects) and temperature (using location across a latitudinal gradient as a surrogate) on interacting New Zealand forest species: the host plant M. excelsum, the herbivore C. scriptaria and the parasitoid species A. declanae and M. puchricornis. Abundance variables (dark grey shading) give a measure of density (per 2 × 2 m for plants, per plant for caterpillars and per plant for parasitoids emerging from caterpillars). Process variables (light grey shading) give a measure of ecosystem functioning (number of new leaves present at time of sampling for plants, percentage of leaves exhibiting signs of herbivory and number of caterpillars attacked by parasitoids). Linear mixed effect model F-values are reported, with significance denoted by asterisks.
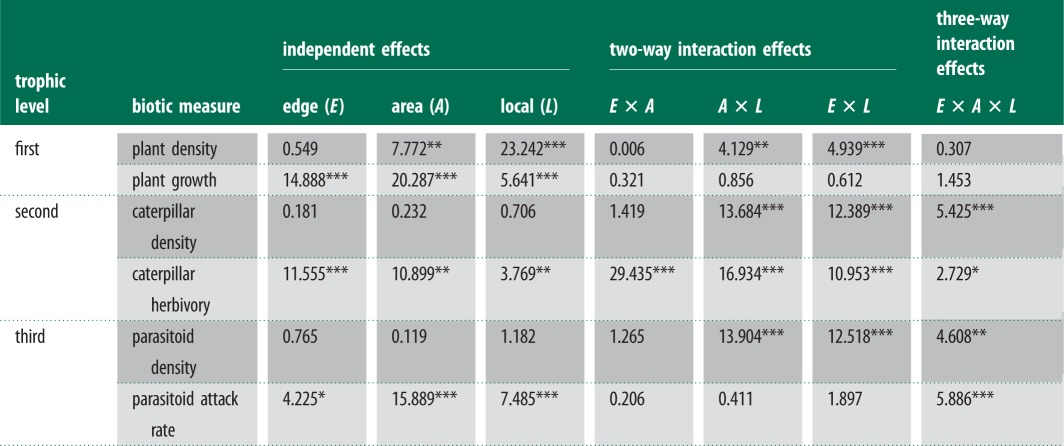 |
***p < 0.001, **p < 0.01, *p < 0.05.
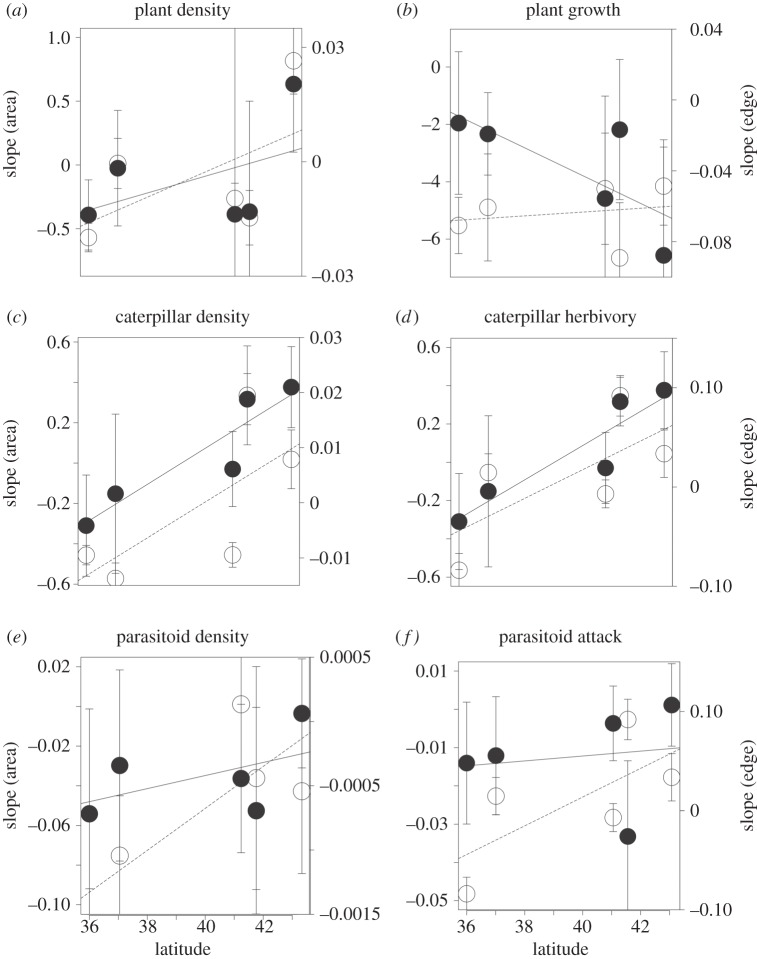
Figure 3.
Interactive effects of habitat fragmentation (fragment area and edge effect) and temperature (along a surrogate gradient of latitude) on three trophic levels (plant: (a,b); herbivore: (c,d); parasitoid: (e,f)) across two biotic measures (density: (a,c,e); and process: (b,d,f)). Values represent the slope (±95% CI) of the relationship between the biotic measure and either fragment area (solid line and filled circle) or distance from edge (dashed line, hollow circle), as calculated at each location ranging from the warmest (Auckland; 36° S) to the coolest (Banks Peninsula; 43° S). Negative slope values represent biotic measures that are highest in fragmented environments (in small fragments or near habitat edges), and positive values represent biotic measures that are highest in non-fragmented environments (in large fragments or far from habitat edges).
Significant effects of individual driver attributes (edge effects, small patch area and locations with higher temperatures) were found on all process variables (table 1; light grey shading). When non-additive effects of multiple drivers were included, we detected a significant two-way interaction between fragmentation and location for both area × location and edge × location in all density variables (table 1; dark grey shading). The two measures of fragmentation significantly interacted in only one dependent variable, synergistically exacerbating the negative effect of fragmentation on herbivory (table 1 and figure 4). Significant three-way interactions were detected between location, area and edge for both density and process variables in all but the lowest trophic level (table 1).
Figure 4.
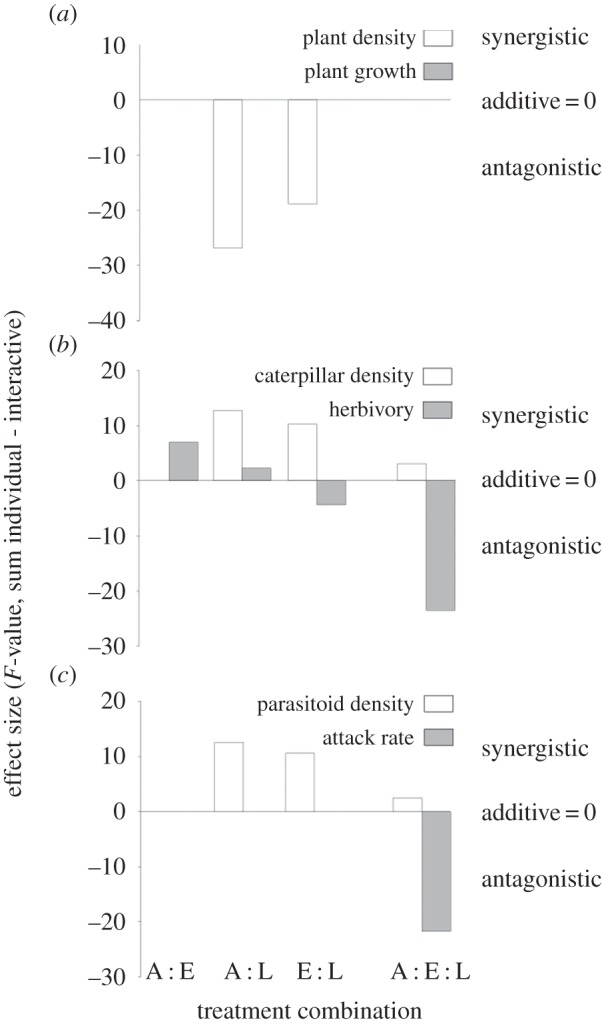
The nature and magnitude of interaction effects among fragment area (A), distance to habitat edges (E) and temperature differences as represented by a latitudinal gradient of study locations (L) on three trophic levels: (a) the host plant M. excelsum, (b) the herbivore C. scriptaria and (c) the parasitoids A. declanae and Meteorus puchricornis. Effects were classified by calculating the difference between the sum of individual effect sizes and the interactive effect sizes, with positive values representing synergistic effects and negative values representing antagonistic effects. Only treatment combinations that exhibited significant interactions (p < 0.05) in linear mixed effect models are displayed.
For all three trophic levels, densities and biotic processes (excluding plant growth) increased in fragmented environments (near the edge and in small fragments) at the warm location (Auckland; 36°), but this effect either disappeared or was reversed in cool locations (figure 3 indicated by negative slope values at low latitude and positive/less negative slope values at higher latitudes). There were, however, important differences in species responses to the individual and interactive effects of the global change drivers.
The first trophic level appeared to be significantly impacted by independent measures of both fragmentation and location, but only plant density was impacted by these two drivers simultaneously (table 1). The reduced density in smaller fragment sizes in cooler locations was offset by higher densities in smaller fragments in warmer locations (figure 3), thereby producing antagonistic responses in plant density (figure 4a).
At the second trophic level, only herbivory was significantly impacted by individual drivers. There were, however, significant interaction effects that influenced both caterpillar density and herbivory rates (table 1 and figure 3). The nature of this interaction differed: caterpillar density had synergistic two- and three-way interactions among fragmentation and location variables, whereas herbivory principally (except for area × location) exhibited antagonistic interactions (figure 4b). The one exception was that fragment area appeared to synergistically combine with location to depress herbivory rates relative to large fragments in cool locations.
At the third trophic level, effect sizes suggest parasitoid attack rates were significantly influenced by individual drivers, but parasitoid density was not (table 1). By contrast, parasitoid density was impacted by two-way interactions between fragmentation and location variables but parasitoid attack rates were not. Both variables had significant three-way interactions (table 1), with the interaction effect on parasitoid density being synergistic but the effect being antagonistic on parasitoid attack rates (figure 4c).
4. Discussion
Contemporary ecological research recognizes the importance of studying the simultaneous impact of multiple drivers of global environmental change [6–9], and our results suggest that focusing on a single driver will provide only restricted insight into the impacts of global changes on species and their interactions. Here, we found evidence that temperature (using latitude as a surrogate) and the degree of habitat fragmentation (represented simultaneously by proximity to fragment edges and the area of forest fragments) both exerted influences on the density and trophic interactions of all levels of a tri-trophic food chain in New Zealand. The two drivers of environmental change interacted non-additively across all trophic levels. The nature of these interactions however, depended upon the biotic measure and the trophic level.
(a). Simultaneous global environmental change drivers
In the majority of cases, the optimal model analysing habitat fragmentation and location included a three-way interaction term; and in many cases, significant two-way interactions were found. This suggests that non-additive interactions are the norm rather than the exception in this study system. In the wider literature, non-additive impacts of multiple drivers are also considered to dominate studies that have investigated the simultaneous impacts of multiple drivers [2,7]. Although these non-additive interactions are often assumed to be synergistic in nature [11,53], several meta-analyses have found similar numbers of studies displaying antagonistic interactions between drivers [2,7]. In one of these reviews, community-level studies largely exhibited antagonistic interactions, whereas studies at population level largely exhibited synergistic responses [2]. Our results parallel these general findings, with fragmentation and location mostly exerting antagonistic interactions on the ecological processes we examined, yet exerting synergistic interactions on the density of consumers (figure 4b,c).
(b). Trophic-level responses
Altered dynamics of biotic interactions are an ‘insidious and functionally important hidden effect’ of anthropogenic environmental modification [54]. Non-additive effects have been analysed with respect to differential effects on trophic levels and reviewed by Mullan Crain et al. [2]. They found that autotrophs exhibited antagonistic responses, whereas heterotrophs exhibited synergistic responses. Here, we found this was also the case: multiple drivers did exert antagonistic impacts on plant density and growth, and synergistic responses were found for insect densities (figure 4b,c). This may perhaps be related to the trophic-level hypothesis [55], whereby there is a loss of biological insurance (i.e. the capacity for diverse communities to be more resilient to perturbations (sensu [56]) as taxonomic, physiological and genetic diversity is reduced towards apex populations [52]). Multiple drivers have been found to exert more negative impacts on these top trophic-level organisms [2].
For the higher trophic levels, the synergistic nature of global change impacts on the herbivore and parasitoid may be explained by a combination of factors. Firstly, as fragmentation occurs, the proportion of habitat near edges increases geometrically [57] and environmental conditions in the remaining habitat are altered (such as air temperature and moisture, vapour pressure deficit, soil moisture and light intensity) [58]. This is commonly hypothesized to impact species that require habitat interior microclimate conditions [58]. Cleora scriptaria is known to typically inhabit forested areas [59] where conditions are cool and moist. A change in the quantity of core habitat will result in microclimatic alterations, and it has therefore been suggested [59] that herbivory rates may be negatively impacted by degradation of this habitat. If C. scriptaria do not fare well in the more extreme temperatures that are typical of matrix environments, then it is likely that they will suffer from habitat fragmentation associated with microclimates that are warmer and less humid, and from warmer and more extreme environmental conditions. Secondly, any ecological effect that reduces the density of the herbivore is likely to have a cascading effect on the density of the parasitoids. This is likely to be the case for one of the species analysed, M. pulchricornis, as this species responds positively to increased larval densities of C. scriptaria as a result of frequency-dependent prey-searching behaviour [60]. As such, the synergistic interaction between habitat fragmentation and location on the herbivore C. scriptaria (figure 4) is likely to pre-define a similar synergistic interaction for the parasitoid M. pulchricornis, assuming this species does not switch between hosts. Our data suggest this trophic interaction between the herbivore and parasitoid is more heavily controlled by bottom-up, rather than top-down, processes because the pattern of global change interactions on the two trophic levels is consistent.
Our data have shown that non-additive interactions more effectively explain the simultaneous impacts of habitat fragmentation and climate (latitudinal surrogate) on biota than the additive effects of single drivers, in line with the majority of global change studies that have investigated multiple drivers [7]. Furthermore, our data lend support to more traditional concerns that the detrimental effects of global change drivers are exacerbated in higher trophic levels. What remains uncertain, however, is whether these synergistic effects at high trophic levels may be ameliorated by the antagonistic nature of global change drivers acting on species interactions. Bringing this into a global context, whether investigating individuals, species, functional groups or communities there will always be relative ‘winners’ and ‘losers’ with global change. With human activities exerting increasing and more numerous pressures on ecosystems, the overall magnitude of change is likely to be considerable. If this study provides insights into widespread trends, the impacts on biota are likely to be predominantly detrimental. As Paine et al. [14] describe, and as we have empirically demonstrated, ecological surprises are likely to be commonplace. This research highlights the requirement to consider multiple drivers of global environmental change and multiple biotic measures when predicting the effects of, and solutions to, anthropogenic impacts on biodiversity.
Supplementary Material
Acknowledgements
We thank the University of Canterbury for providing research facilities, to Raphael Didham, Jenny Ladley and Dave Conder for providing advice and logistical assistance. For access to field sites, we thank the Department of Conservation, the Christchurch, Nelson, Wellington, Hamilton and Auckland Councils, as well as private land owners Hugh Wilson, Sonia and Mark Armstrong and the Pukemokemoke, Maungatautari and Brook Waimarama Trusts. For identification of parasitoid samples, we appreciate the work of Rudolph Schnitzler. Thanks go to Ben Rodriguez, Amy Webster, Isey Lane, Judith and Chris Lakeman Fraser and Colin Bradley for assisting in (and off) the field. Graham Banton and Michael Lewis were instrumental in supporting the experimental set-up and data collection in New Zealand.
Funding statement
We would like to acknowledge the Grantham Institute for Climate Change and the Gilchrist Educational Trust for funding this research.
Data accessibility
Data available from the Dryad Digital Repository: doi:10.5061/dryad.gs3r8.
References
- 1.Breitburg DL, Sanders JG, Gilmour CC, Hatfield CA, Osman RW, Riedel GF, Seitzinger SP, Sellner KG. 1999. Variability in responses to nutrients and trace elements, and transmission of stressor effects through an estuarine food web. Limnol. Oceanogr. 44, 837–863. ( 10.4319/lo.1999.44.3_part_2.0837) [DOI] [Google Scholar]
- 2.Mullan Crain C, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315. ( 10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 3.Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM. 2007. Interactive effects of habitat loss and species invasion on native species decline. Trends Ecol. Evol. 22, 489–496. ( 10.1016/j.tree.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 4.Mora C, Metzger R, Rollo A, Myers RA. 2007. Experimental simulations about the effects of overexploitation and habitat fragmentation on populations facing environmental warming. Proc. R. Soc. B 274, 1023–1028. ( 10.1098/rspb.2006.0338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forister ML, McCall AC, Sanders NJ, Fordyce JA, Thorne JH, O'Brien J, Waetjen DP, Shapiro AM. 2010. Compounded effects of climate change and habitat alteration shift patterns of butterfly diversity. Proc. Natl Acad. Sci. USA 107, 2088–2092. ( 10.1073/pnas.0909686107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinebrooke RD, Cottingham KL, Norberg J, Scheffer M, Dodson SI, Maberly C, Sommer U. 2004. Impacts of multiple stressors on biodiversity and ecosystem functioning: the role of species co-tolerance. Oikos 104, 451–457. ( 10.1111/j.0030-1299.2004.13255.x) [DOI] [Google Scholar]
- 7.Darling ES, Côté IM. 2008. Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286. ( 10.1111/j.1461-0248.2008.01243.x) [DOI] [PubMed] [Google Scholar]
- 8.Mantyka-Pringle CS, Martin TG, Rhodes JR. 2012. Interactions between climate and habitat loss effects on biodiversity: a systematic review and meta-analysis. Glob. Change Biol. 18, 1239–1252. ( 10.1111/j.1365-2486.2011.02593.x) [DOI] [Google Scholar]
- 9.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363. ( 10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 10.Zavaleta ES, Shaw RM, Chiariello NR, Mooney HA, Field CB. 2003. Additive effects of simulated climate changes, elevated CO2, and nitrogen deposition on grassland diversity. Proc. Natl Acad. Sci. USA 100, 7650–7654. ( 10.1073/pnas.0932734100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sala OE, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774. ( 10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 12.Folt CL, Chen CY, Moore MV, Burnaford J, Henry R, Hall J, Baumgartner K. 1999. Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877. ( 10.4319/lo.1999.44.3_part_2.0864) [DOI] [Google Scholar]
- 13.Zvereva EL, Kozlov MV. 2006. Consequences of simultaneous elevation of carbon dioxide and temperature for plant–herbivore interactions: a meta-analysis. Glob. Change Biol. 12, 27–41. ( 10.1111/j.1365-2486.2005.01086.x) [DOI] [Google Scholar]
- 14.Paine RT, Tegner MJ, Johnson EA. 1998. Compounded perturbations yield ecological surprises. Ecosystems 1, 535–545. ( 10.1007/s100219900049) [DOI] [Google Scholar]
- 15.Pereira HM, et al. 2010. Scenarios for global biodiversity in the 21st century. Science 330, 1496–1501. ( 10.1126/science.1196624) [DOI] [PubMed] [Google Scholar]
- 16.Franco AM, Hill JK, Kitschke C, Collingham YC, Roy DB, Fox R, Huntley B, Thomas CD. 2006. Impacts of climate warming and habitat loss on extinctions at species’ low-latitude range boundaries. Glob. Change Biol. 12, 1545–1553. ( 10.1111/j.1365-2486.2006.01180.x) [DOI] [Google Scholar]
- 17.Warren MS, et al. 2001. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 414, 65–69. ( 10.1038/35102054) [DOI] [PubMed] [Google Scholar]
- 18.Valladares G, Salvo A, Cagnolo L. 2006. Habitat fragmentation effects on trophic processes of insect–plant food webs. Conserv. Biol. 20, 212–217. ( 10.1111/j.1523-1739.2006.00337.x) [DOI] [PubMed] [Google Scholar]
- 19.Andrew NR, Hughes L. 2007. Potential host colonization by insect herbivores in a warmer climate: a transplant experiment. Glob. Change Biol. 13, 1539–1549. ( 10.1111/j.1365-2486.2007.01393.x) [DOI] [Google Scholar]
- 20.Travis JMJ. 2003. Climate change and habitat destruction: a deadly anthropogenic cocktail. Proc. R. Soc. Lond. B 270, 467–473. ( 10.1098/rspb.2002.2246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duncan RP, Young JR. 2000. Determinants of plant extinction and rarity 145 years after European settlement of Auckland, New Zealand. Ecology 81, 3048–3061. ( 10.1890/0012-9658(2000)081[3048:DOPEAR]2.0.CO;2) [DOI] [Google Scholar]
- 22.Wilmshurst JM, Anderson AJ, Higham TFG, Worthy TH. 2008. Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proc. Natl Acad. Sci. USA 105, 7676–7680. ( 10.1073/pnas.0801507105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewers RM, Kliskey AD, Walker S, Rutledge D, Harding JS, Didham RK. 2006. Past and future trajectories of forest loss in New Zealand. Biol. Conserv. 133, 312–325. ( 10.1016/j.biocon.2006.06.018) [DOI] [Google Scholar]
- 24.Leathwick J, McGlone M, Walker S. 2004. New Zealand's potential vegetation pattern. Lincoln, New Zealand: Whenua Press. [Google Scholar]
- 25.Ministry for the Environment. 2008. Climate change effects and impacts assessment: a guidance manual for local government in New Zealand, 2nd edn Wellington, New Zealand: Ministry for the Environment. [Google Scholar]
- 26.IPCC. 2002. Climate change and biodiversity: IPCC technical paper V. Working Group II, H Gitay, A Suárez, RT Watson, DJ Dokken (eds). Geneva, Switzerland: IPCC. [Google Scholar]
- 27.Howard-Williams C, Peterson D, Lyons WB, Cattaneo-Vietti R, Gordon S. 2006. Measuring ecosystem response in a rapidly changing environment: the Latitudinal Gradient Project. Antarct. Sci. 18, 465 ( 10.1017/S0954102006000514) [DOI] [Google Scholar]
- 28.Fleishman E, Fay JP, Murphy DD, Biology C. 2000. Upsides and downsides: contrasting topographic gradients in species richness and associated scenarios for climate change. J. Biogeogr. 27, 1209–1219. ( 10.1046/j.1365-2699.2000.00455.x) [DOI] [Google Scholar]
- 29.Weatherhead PJ, Sperry JH, Carfagno GLF, Blouin-Demers G. 2012. Latitudinal variation in thermal ecology of North American ratsnakes and its implications for the effect of climate warming on snakes. J. Therm. Biol. 37, 273–281. ( 10.1016/j.jtherbio.2011.03.008) [DOI] [Google Scholar]
- 30.Andrew NR, Hughes L. 2004. Species diversity and structure of phytophagous beetle assemblages along a latitudinal gradient: predicting the potential impacts of climate change. Ecol. Entomol. 29, 527–542. ( 10.1111/j.0307-6946.2004.00639.x) [DOI] [Google Scholar]
- 31.Moran VC, Southwood TR. 1982. The guild composition of arthropod communities in trees. J. Anim. Ecol. 51, 289–306. ( 10.2307/4325) [DOI] [Google Scholar]
- 32.Majer AJD, Kitching RL, Heterick BE, Hurley K, Brennan KEC. 2001. North–South patterns within arboreal ant assemblages from rain forests in Eastern Australia. Biotropica 33, 643–661. [Google Scholar]
- 33.Bowen ME, McAlpine CA, House APNN, Smith GC. 2009. Agricultural landscape modification increases the abundance of an important food resource: mistletoes, birds and brigalow. Biol. Conserv. 142, 122–133. ( 10.1016/j.biocon.2008.10.005) [DOI] [Google Scholar]
- 34.Fahrig L. 2003. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 34, 487–515. ( 10.1146/annurev.ecolsys.34.011802.132419) [DOI] [Google Scholar]
- 35.Chown SL, Hoffmann AA, Kristensen TN, Angilletta MJ, Stenseth NC, Pertoldi C. 2010. Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 43, 3–15. ( 10.3354/cr00879) [DOI] [Google Scholar]
- 36.Pearson RG, Dawson TP. 2003. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 12, 361–371. ( 10.1046/j.1466-822X.2003.00042.x) [DOI] [Google Scholar]
- 37.Bascompte J, Jordano P, Olesen JM. 2006. Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312, 431–433. ( 10.1126/science.1123412) [DOI] [PubMed] [Google Scholar]
- 38.Suttle KB, Thomsen MA, Power ME. 2007. Species interactions reverse grassland responses to changing climate. Science 315, 640–642. ( 10.1126/science.1136401) [DOI] [PubMed] [Google Scholar]
- 39.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. 2010. A framework for community interactions under climate change. Trends Ecol. Evol. 25, 325–331. ( 10.1016/j.tree.2010.03.002) [DOI] [PubMed] [Google Scholar]
- 40.Smith AC. 1975. The genus Macropiper (Piperaceae). Bot. J. Linn. Soc. 71, 1–38. ( 10.1111/j.1095-8339.1975.tb00936.x) [DOI] [Google Scholar]
- 41.Hodge S, Barron M, Wratten SD. 2007. Cleora scriptaria larvae exhibit reduced growth on a diet of kawakawa leaves subjected to artificial wounding. J. Econ. Entomol. 16, 11–16. [Google Scholar]
- 42.Berry JA, Walker GP. 2004. Meteorus pulchricornis (Wesmael) (Hymenoptera: Braconidae: Euphorinae): an exotic polyphagous parasitoid in New Zealand. NZ J. Zool. 31, 33–44. ( 10.1080/03014223.2004.9518357) [DOI] [Google Scholar]
- 43.Gardner RO. 1997. The genus Macropiper (Piperaceae) in the south-west Pacific. NZ J. Bot. 35, 293–307. ( 10.1080/0028825X.1997.10410155) [DOI] [Google Scholar]
- 44.NIWA. 2012. The National Climate Database See http://cliflo.niwa.co.nz/.
- 45.Hodge S, Barron M, Wratten SD, Macropiper K, Keesing VF. 2000. Induced defences in kawakawa (Macropiper excelsum): do caterpillars avoid previous leaf damage? NZ J. Ecol. 24, 87–89. [Google Scholar]
- 46.Schnitzler F-R, Sarty M, Lester PJ. 2004. Larval parasitoids reared from Cleora scriptaria (Geometridae: Ennominae). Weta 28, 13–18. [Google Scholar]
- 47.Bates D, Maechler M, Bolker B. 2011. lme4: linear mixed-effects models using S4 classes See http://cran.r-project.org/web/packages/lme4/index.html.
- 48.R Development Core Team. 2010. R: a language and environment for statistical computing See http://www.r-project.org/. [Google Scholar]
- 49.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. Statistics 1, 209–243. ( 10.1007/978-0-387-87458-6) [DOI] [Google Scholar]
- 50.Ritz C, Spiess AN. 2008. qpcR: an R package for sigmoidal model selection in quantitative real-time polymerase chain reaction analysis. Bioinformatics 24, 1549–1551. ( 10.1093/bioinformatics/btn227) [DOI] [PubMed] [Google Scholar]
- 51.Zar JH. 1999. Biostatistical analysis, 4th edn Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- 52.Christensen MR, Graham MD, Vinebrooke RD, Findlay DL, Paterson MJ, Turner MA. 2006. Multiple anthropogenic stressors cause ecological surprises in boreal lakes. Glob. Change Biol. 12, 2316–2322. ( 10.1111/j.1365-2486.2006.01257.x) [DOI] [Google Scholar]
- 53.Myers N. 1995. Environmental unknowns. Science 269, 358–360. ( 10.1126/science.269.5222.358) [DOI] [PubMed] [Google Scholar]
- 54.Tylianakis JM, Tscharntke T, Lewis OT. 2007. Habitat modification alters the structure of tropical host–parasitoid food webs. Nature 445, 202–205. ( 10.1038/nature05429) [DOI] [PubMed] [Google Scholar]
- 55.Holt RD, Lawton JH, Polis GA, Martinez ND. 1999. Trophic rank and the species–area relationship. Ecology 80, 1495–1504. [Google Scholar]
- 56.Yachi S, Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468. ( 10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Temple SA, Cary J. 1988. Modelling dynamics of habitat-interior bird populations in fragmented landscapes. Conserv. Biol. 2, 340–347. ( 10.1111/j.1523-1739.1988.tb00198.x) [DOI] [Google Scholar]
- 58.Murcia C. 1995. Edge effects in fragmented forests: implications for conservation. Trends Ecol. Evol. 10, 58–62. ( 10.1016/S0169-5347(00)88977-6) [DOI] [PubMed] [Google Scholar]
- 59.Schnitzler FR. 2008. Hymenopteran parasitoid diversity and tri-trophic interactions: the effects of habitat fragmentation in Wellington, New Zealand. PhD thesis, Victoria University of Wellington, New Zealand. [Google Scholar]
- 60.Schnitzler F-RR, Hartley S, Lester PJ. 2011. Trophic-level responses differ at plant, plot, and fragment levels in urban native forest fragments: a hierarchical analysis. Ecol. Entomol. 36, 241–250. ( 10.1111/j.1365-2311.2011.01266.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: doi:10.5061/dryad.gs3r8.