Abstract
Background
Chronic pressure overload (such as arterial hypertension) may cause left ventricular (LV) remodeling, alterations in cardiac function, and the development of diastolic heart failure. Changes in the composition of the myocardial extracellular matrix (ECM) may contribute to the development of pressure-overload (PO) induced LV remodeling. We hypothesized that a specific pattern of plasma biomarker expression that reflected changes in these pathophysiologic mechanisms would have diagnostic application to identify: 1-patients who have developed LV hypertrophy and 2-patients with LV hypertrophy who have developed diastolic heart failure.
Methods and Results
Plasma concentration of 17 biomarkers (MMP-1, 2, 3, 7, 8, 9, TIMP-1, 2, 3, 4, NT-proBNP, cardiotrophin, osteopontin, sRAGE, CITP, PINP, PIIINP), an echocardiogram, and 6-minute hall walk were performed on 241 referent control subjects, 144 patients with LV hypertrophy (LVH) but no evidence of heart failure, and 61 patients with LV hypertrophy and diastolic heart failure (DHF). A plasma multi-biomarker panel consisting of increased MMP-7, MMP-9, TIMP-1, PIIINP, and NT-proBNP predicted the presence of LVH with an AUC of 0.80. A plasma multi-biomarker panel consisting of increased MMP-2, TIMP-4, PIIINP and decreased MMP-8 predicted the presence of DHF with an AUC of 0.79. These multi-biomarkers panels performed better than any single biomarker including NT-proBNP, and better than using clinical co-variates alone (AUC = 0.73 for LVH, 0.68 for DHF).
Conclusions
Plasma biomarkers reflecting changes in ECM fibrillar collagen homeostasis, combined into a multi-biomarker panel, have discriminative value in identifying the presence of structural remodeling (LVH) and clinical disease (DHF).
Keywords: hypertrophy, heart failure, extracellular matrix, biomarkers
Despite significant advances in diagnosis and management, chronic pressure-overload (such as arterial hypertension) remains one of the most important risk factors for the development of cardiovascular disease and a leading cause of cardiovascular morbidity and mortality.1,2 Chronic pressure-overload (PO) results in significant changes in left ventricular (LV) structure and function, collectively termed myocardial remodeling.3-6 This remodeling includes significant changes in myocytes, fibroblasts and the extracellular matrix (ECM), all of which contribute to one of the structural manifestations of PO; the development of LV chamber hypertrophy (LVH).7 Once established, PO-induced LVH has significant long term consequences because it serves as a major independent risk factor for the development of chronic heart failure (CHF).
While LVH is a pivotal step in the development of PO-induced heart disease, the detection of LVH is made difficult by several factors. First, in and of itself, the clinical disease processes causing PO do not cause symptoms, consequently, LV remodeling may remain an unrecognized and insidious process for a prolonged period of time. Second, LVH is not readily detectable using standard clinical means such as a history, physical exam or ECG, but rather requires costly, specialized testing approaches, and subspecialty expertise to perform and interpret.8-13 Therefore, the first goal of this study was to develop a plasma biomarker profile which could be utilized to identify patients with LVH.
There is a substantial rate of progression from PO-induced LVH to abnormal diastolic function and CHF. Myocardial remodeling, particularly changes in the structure and composition of the ECM result in abnormal LV filling, a stiff noncompliant left ventricle, and increased diastolic pressures. A common form of this PO-induced heart failure occurs with a preserved ejection fraction, and is referred to as diastolic heart failure (DHF).14-22 However, identification of patients with LVH who have developed this form of DHF is difficult, requires extensive non-invasive and/or invasive testing, and complex algorithmic diagnostic criteria. Therefore, the second goal of this study was to develop a plasma biomarker profile which could be utilized to identify the patients with DHF.
The plasma biomarkers examined in the current study were those that reflect changes in the underlying mechanisms effecting structural and functional remodeling in PO-induced disease states. One such pathophysiologic mechanism is a change in ECM composition, particularly fibrillar collagen.14,21,23-30 Changes in collagen homeostasis are reflected in measurements or determinants of the rates of collagen synthesis, processing, post-translational modification, and degradation.23-30 We hypothesized that a specific pattern of plasma biomarker expression would have diagnostic application in: 1-patients who have developed LVH and 2-patients with LVH who have developed DHF. To test these hypotheses, we used high sensitivity assays to examine a large array of plasma proteins and peptides that reflect ECM collagen homeostasis in patients with LVH and DHF.
Methods
Subjects
Four hundred and forty six subjects were enrolled in the present study. There were 241 referent control subjects with no evidence of cardiovascular disease and no evidence of LV structural or functional changes (referent control group). There were 205 subjects with LV hypertrophy; of these, 144 subjects had LV hypertrophy but without evidence of heart failure (LVH group), and 61 subjects with LV hypertrophy and diastolic heart failure (n=61, DHF group).
Subject recruitment methods
Study subjects were recruited from locally sponsored health fairs, response to multimedia stories, physician referral, and echocardiographic studies.
Study Protocol
Each subject underwent the following evaluation: a complete medical history, comprehensive physical exam, 12-lead electrocardiogram, echocardiogram, 6 minute hall walk, and the plasma biomarkers enumerated below.
Compliance
The research protocol used in this study was reviewed and approved by the institutional review board at the Medical University of South Carolina. Written informed consent was obtained from all participants.
Definitions used to define study groups
LVH was defined echocardiographically as an increase in LV wall thickness of > 1.2 cm and/or an increase in LV mass index > 95 in women and ≥ 115 gm/m2 in men.31
DHF was defined using the criteria of the Lahey Clinic and the Heart Failure and Echocardiography Associations of the European Society of Cardiology.32-34 Each requires: 1) signs or symptoms of heart failure that provide clinical evidence of heart failure (may include Framingham or Boston Criteria, exercise testing, quality of life questionnaire), 2) preserved ejection fraction (EF ≥ 50 %), 3) normal LV end-diastolic volume index (LVEDVI < 90 mL/m2, 4) evidence of diastolic LV dysfunction obtained invasively (using left or right heart catheterization) or non-invasively (using Doppler, tissue Doppler or left atrial measurements) and 5) exclusion of non-myocardial diseases.
Subjects in the LVH or DHF group were excluded if they had a clinical condition that would potentially change plasma biomarker profiles independent of the presence of LVH or DHF: 1) chronic obstructive pulmonary disease requiring oral steroids and/or oxygen therapy, 2) poorly controlled diabetes, HbA1c >8.5 within the past 6 months, 3) cardiac surgery, EP ablation, or PCI within the past year, 4) major surgical procedures (defined as requiring a hospital stay of greater than 3 days) in the past 6 months, 5) ST segment elevation myocardial infarction, or non-ST segment elevation myocardial infarction (by history, ECG or review of patient record), or a wall motion abnormality by echocardiography, 6) end-stage renal disease, creatinine > 2.0 mg/dL, 7) active or ongoing malignancy, 8) severe rheumatological disease (i.e., scleroderma, lupus, sarcoidosis), 9) an ejection fraction of <50 %, or an LV end-diastolic volume > 90 mL/M2, 10) valve disease more extensive than mild, 11) severe liver disease, 12) amyloidosis, hypertrophic cardiomyopathy, restrictive or constrictive cardiomyopathy, HIV, 13) significant medication changes within the previous 4 weeks, 14) significant anemia with hemoglobin < 10.5 grams, 15) active or ongoing severe infection or 16) age was < 50 years.
Referent control subject were excluded if: 1) any exclusion listed for the LVH and DHF groups were present, 2) there was abnormal LV function, volume, or mass as assess by echocardiography, or 3) age was < 50 years.
Echocardiographic Methods
All echocardiograms were performed by one experienced sonographer (CDM) using a Sonos 5500 system (Agilent Technologies, Andover, Mass) with an S4 2-4MHz ultrasound transducer. Measurements were made using American Society of Echocardiography criteria.31,34 Ejection fraction was calculated using the standard formula. Left ventricular mass was calculated using the formula of Reichek and Devereux.35 Volume and mass were indexed for body surface area. Doppler measurements of mitral inflow E wave velocity, A wave velocity, the E/A ratio. Tissue Doppler measurements of mitral E’ and A’ wave velocity were made. Pulmonary capillary wedge pressure (PCWP) was calculated using the formula: 2 + 1/3 E/E’.23,36 An average of 3 beats was used for every measurement. Images were coded and read in a blinded fashion by at least two of three authors (SMM, MRZ, CDM).
Plasma Biomarker Measurements
Biomarkers were chosen that reflected selected measurements of or determinants of changes in ECM homeostasis such as the rates of collagen: synthesis (collagen I n-terminal propeptides [PINP], collagen III n-terminal propetide [PIIINP]), processing (osteopontin), post translational modification (soluble receptor for advanced glycation end products [sRAGE]), and degradation (matrix metalloproteinases [MMPs], their tissue inhibitors [TIMPs], telopeptides [collagen I teleopeptide, CITP]). In addition, n-terminal propeptide of brain naturetic peptide (NT-proBNP) and, cardiotrophin were measured.14,15,24,25,28,29,37-39 Four classes of MMPs: gelatinases (MMP-2 and MMP-9), collagenase (MMP-1 and 8), stromelysin (MMP-3), and matrilysin (MMP-7); and all 4 tissue inhibitors of MMPs (TIMP-1, -2, -3, -4) were examined.23,26,40
Blood was collected after the subject had remained supine for 20 minutes. Samples were immediately centrifuged and the plasma layer removed. The separated plasma was divided into 3 equal aliquots and frozen at −70°C. Samples were not thawed and refrozen. For measurement, the plasma samples were thawed on ice. All measurements were performed in duplicate. For the MMP and TIMP assays, a multiplex suspension array approach was utilized. For PINP and PIIINP, a radioimmunoassay was utilized. For the remaining plasma proteins, enzyme-linked immunoassays were employed. For the MMP and TIMP measurements, the plasma was first diluted 1:100, incubated with either the MMP platform (MMP Base Kit LMP000) or the TIMP platform (TIMP MSA kit, LKT003), and then the samples were subjected to 2-laser flow cytometric detection system (Bio-Plex 200, BioRad Laboratories). Using pre-calibrated standards and regression modeling, the fluorescence emission specific to each MMP and TIMP type was converted to an absolute concentration. For the PINIP and PIIINP assays, the plasma was diluted 1:2, incubated with I125 labeled targeted antisera, specifically bound conjugates separated by differential centrifugation, and scintillation counting performed (Wallac 1480 Wizard, Waltham, MA). The final plasma concentrations were calculated from a competitive semi-log calibration curve. To measure plasma cardiotrophin, osteopontin, and sRAGE levels, a sandwich enzyme linked immunoassay was performed. In general terms, plasma was added to a 96-well plate containing the targeted antisera, following rigorous washing steps and conjugation with a secondary detection antisera, and the resultant colorimetric reaction was read by scanning spectrophotometry (VersaMax, Molecular Devices, Sunnyvale, CA). For the CITP measurements, a competitive immunoassay was performed and the resultant reaction analyzed by scanning spectrophotometry. The specific concentration of the plasma proteins in each sample were then determined by comparison to a standard curve generated from known concentrations of each plasma protein. The NT-proBNP assay was performed using VITROS Products NT-proBNP Reagent Pack and Calibrators on the VITROS ECi/ECiQ Immunodiagnostic System (Ortho-Clinical Diagnostics). The assay uses an immunometric technique where the NT-proBNP present in 40 uL of EDTA-plasma specimen reacts simultaneously with a biotinylated antibody (sheep anti-NT-proBNP) and a horseradish peroxidase (HRP)-labeled conjugate (sheep anti-NT-proBNP). The sensitivity for all of the assays was in pg/mL with intra-assay coefficient of variations less than 15%.
Statistical Analysis
Statistical analyses were performed with SAS (version 9.2; SAS Institute, Inc, Cary, NC). The distribution of measurements derived from demographic variables, echocardiograms, and plasma biomarkers were tested for normality based on tests of skewness and kurtosis.
Demographics and group differences
Comparisons were made between 3 groups: referent control, LVH, and DHF using an ANOVA F-test. All pair-wise comparisons were adjusted for multiple comparisons using the Tukey and Kramer methods. Values are presented as means ± standard error of the mean as well as medians and interquartile ranges (please see Table A in supplemental materials). Because of right skewness, for analytical purposes, biomarker values were log base 10 transformed. A p value < 0.05 was considered significant. In Tables 1 and 2, referent control subjects (n=241) were compared to patients with LVH but no DHF (n=166) and patients with LVH and DHF (n=61).
Table 1.
Demographics and LV Function in Referent Control Subjects, Subjects with Left Ventricular Hypertrophy, and Subjects with Diastolic Heart Failure
| Referent Control |
LVH | DHF | F (χ2) | p-Value | |
|---|---|---|---|---|---|
|
Demographics Age, Years |
58 ± 1 | 60 ± 1 | 66 ± 1*† | 11.1 | <0.001 |
| Female, number (%) | 169 (70) | 79 (55) | 36 (59) | 9.7 | 0.008 |
| Black, number (%) | 49 (20) | 55 (38) | 21 (34) | 15.7 | <0.001 |
| Diabetes Mellitus, number (%) | 22 (9) | 23 (16)* | 19 (31)* † | 19.7 | <0.001 |
|
Functional Characteristics Systolic Blood Pressure, mmHg |
128 ± 1 | 134 ± 1* | 139 ± 2*† | 23.4 | <0.001 |
| Diastolic Blood Pressure, mmHg | 76 ± 1 | 79 ± 1* | 77 ± 1 | 8.9 | <0.001 |
| Body Mass Index, kg/m2 | 30 ± 2 | 31 ± 1* | 33 ± 1* | 35.8 | <0.001 |
| LV EDV Indexed, mL/m2 | 52 ± 1 | 53 ± 1 | 54 ± 2 | 0.7 | 0.496 |
| LV EDD, ml | 4.6 ± 0.1 | 4.8 ± 0.1* | 4.8 ± 0.1 | 7.6 | <0.001 |
| LV Ejection Fraction, % | 68 ± 1 | 69 ± 1 | 69 ± 1 | 0.4 | 0.694 |
| LV Mass Indexed, g/m2 | 80 ± 1 | 117 ± 2* | 123 ± 3* | 220.3 | <0.001 |
| LV wall thickness, cm | 0.9 ± 0.1 | 1.3 ± 0.1* | 1.3 ± 0.1* | 385 | <0.001 |
| Mitral inflow E velocity, cm2 | 68 ± 1 | 64 ± 1 | 73 ± 2*† | 7.0 | <0.001 |
| E/A ratio | 0.9 ± 0.1 | 0.8 ± 0.1* | 0.9 ± 0.1 | 5.4 | <0.001 |
| Tissue Doppler E’ velocity, cm2 | 12 ± 2 | 9 ± 1 | 7 ± 1 | 1.3 | 0.250 |
| PCWP, mmHg | 11 ± 1 | 12 ± 1* | 17 ± 1*† | 84.8 | <0.001 |
| Six Minute Walk, m | 387 ± 6 | 370 ± 8 | 240 ± 17*† | 51.9 | <0.001 |
| Sample size, n | 241 | 144 | 61 |
Data = Mean ± SEM.
All continuous data compared using ANOVA F test
All categorical data compared using χ2 test statistic.
= p<0.05 compared with referent control
= p<0.05 compared with LVH
Abbreviations: LVH = patients with left ventricular hypertrophy but no heart failure, DHF = patients with left ventricular hypertrophy and diastolic heart failure, E and A = Pulsed Wave Doppler mitral valve E and A wave inflow velocities. E’ = Tissue Doppler (lateral mitral annulus) E’ velocity. PCWP = pulmonary capillary wedge pressure calculated from the formula 2+1.3(E/E’), LV = left ventricular, EDV = end diastolic volume, EDD = end diastolic dimension.
Table 2.
Myocardial Matrix Biomarker Profiles in Referent Control Subjects, Subjects with Left Ventricular Hypertrophy, and Subjects with Diastolic Heart Failure
| Patient Grouping | F (χ2) | p-Value | |||
|---|---|---|---|---|---|
| Referent Control | LVH | DHF | |||
| MMP-1, ng/mL | 0.78 ± 0.06 | 0.89 ± 0.07 | 0.94 ± 0.17 | 1.1 | 0.325 |
| MMP-2, ng/mL | 339.7 ± 9.3 | 326.4 ± 11.8 | 421.4 ± 21.6*† | 9.6 | <0.001 |
| MMP-3, ng/mL | 10.08 ± 0.39 | 9.33 ± 0.42 | 11.47 ± 0.74† | 3.0 | 0.049 |
| MMP-7, ng/mL | 1.65 ± 0.08 | 1.84 ± 0.10 | 2.14 ± 0.18* | 4.2 | 0.016 |
| MMP-8, ng/mL | 2.63 ± 0.22 | 3.29 ± 0.32 | 1.79 ± 0.18† | 4.4 | 0.014 |
| MMP-9, ng/mL | 95.0 ± 3.8 | 126.7 ± 7.2* | 123.8 ± 8.6* | 10.7 | <0.001 |
| TIMP-1, ng/mL | 72.2 ± 1.4 | 82.5 ± 2.1* | 90.3 ± 3.4* | 18.5 | <0.001 |
| TIMP-2, ng/mL | 78.6 ± 0.9 | 83.6 ± 1.1* | 85.3 ± 1.6* | 8.9 | <0.001 |
| TIMP-3, ng/mL | 7.5 ± 0.5 | 9.2 ± 0.8 | 6.6 ± 0.9 | 2.8 | 0.063 |
| TIMP-4, ng/mL | 1.47 ± 0.04 | 1.46 ± 0.05 | 1.85 ± 0.09*† | 8.9 | <0.001 |
| PINP, ng/mL | 37.1 ± 1.3 | 34.5 ± 1.9 | 39.4 ± 4.0 | 1.1 | 0.327 |
| PIIINP, ng/mL | 7.2 ± 0.1 | 7.6 ± 0.2 | 9.1 ± 0.4*† | 18.4 | <0.001 |
| CITP, ng/mL | 3.01 ± 0.12 | 3.56 ± 0.17 | 3.93 ± 0.42* | 5.0 | 0.007 |
| CTP-1, ng/mL10−3 | 51.1 ± 6.9 | 43.3 ± 6.8 | 22.8 ± 5.7 | 2.4 | 0.096 |
| sRAGE, ng/mL | 3.46 ± 0.17 | 3.15 ± 0.25 | 2.97 ± 0.27 | 1.2 | 0.315 |
| Osteopontin, ng/mL | 76.2 ± 1.9 | 86.5 ± 4.7* | 92.6 ± 5.5* | 5.1 | 0.007 |
| NT-proBNP, pg/m | 87.4 ± 6.4 | 109.4 ± 12.4 | 214.2 ± 33.6*† | 17.7 | <0.001 |
| Sample Size, n | 241 | 144 | 61 | ||
Abbreviations: The table presents means ± standard errors. ANOVA F-tests and p-values are reported for log10 biomarker values and post hoc pairwise statistical comparisons were performed.
LVH = patients with left ventricular hypertrophy but no heart failure, DHF = patients with left ventricular hypertrophy and diastolic heart failure, MMP = matrix metalloproteinase, TIMP = tissue inhibitor of MMP, NT-proBNP = n-terminal propeptides of brain naturetic peptide, sRAGE = soluble receptor for advanced glycation endproduct, PINP = n-terminal collagen I propeptide, PIIINP = n-terminal collagen III propeptide, CITP = collagen I telopeptide, CTP = Cardiotropin-1.
= p<0.05 compared to Referent Control
= p<0.05 compared to LVH.
Predictive Modeling
In Tables 3 and 4 univariable and multivariable analyses were performed: 1) comparing all patients with LVH (n= 205) to referent controls (n=241); and 2) comparing patients with LVH but no DHF (n=144) were compared to patients with LVH and DHF (n=61). Multiple logistic regression was used to analyze binary indicators of LVH and DHF. To determine relevant predictors of these outcomes, plasma biomarkers were first assessed for univariable associations with clinical outcomes. An AUC analysis was performed on each individual biomarker with a p value < 0.15 for association with LVH or DHF. Those biomarkers with a Wald p<0.15 were considered for inclusion in a multiple logistic regression model. Using forward stepwise variable selection, biomarkers were added to the model. In forward stepwise variable selection, the variable most strongly associated with outcome (with respect to statistical significance) was added to the model and this process was continued until no more variables meet the entry criterion of alpha = 0.10. The alpha was set at 0.10 to ensure that even marginally predictive biomarkers were captured. The univariable and multivariable logistic regression models were adjusted for the following clinical covariates: systolic blood pressure, diastolic blood pressure, age quartile, sex, and body mass index. Quadratic and cubic age terms were assessed but ultimately, age quartile was determined to be the most relevant predictor. Betas and p-values for each biomarker are presented for the adjusted models (supplemental table). The effects of anti-hypertensive medications were analyzed by drug class: angiotensin converting enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB), β-blockers, calcium-channel blockers, and diuretics. None of these medications were significantly associated with any of the biomarkers used in the final multivariable analyses for LVH or DHF patients.
Table 3.
Univariable Analysis of Myocardial Matrix Biomarker Profiles
| AUC Biomarker Alone |
p | Adjusted AUC Biomarker and Clinical Variable |
Adjusted p-value | |
|---|---|---|---|---|
| LVH | ||||
| TIMP-1 | 0.65 | <0.001 | 0.77 [0.72,0.81] | <0.001 |
| MMP-9 | 0.62 | <0.001 | 0.76 [0.71,0.80] | 0.001 |
| TIMP-2 | 0.61 | <0.001 | 0.75 [0.71,0.80] | 0.001 |
| PIIINP | 0.60 | <0.001 | 0.75 [0.70,0.79] | 0.002 |
| MMP-7 | 0.58 | 0.020 | 0.74 [0.70,0.79] | 0.080 |
| NT-proBNP | 0.57 | 0.008 | 0.75 [0.71,0.80] | 0.003 |
| sRAGE | 0.57 | 0.150 | 0.73 [0.69,0.78] | 0.290 |
| Osteopontin | 0.57 | 0.004 | 0.73 [0.69,0.78] | 0.080 |
| CITP | 0.56 | 0.004 | 0.75 [0.70,0.79] | 0.003 |
| TIMP-4 | 0.54 | 0.100 | 0.73 [0.69,0.78] | 0.300 |
| Cardiotrophin | 0.53 | 0.120 | 0.73 [0.69,0.78] | 0.420 |
| DHF | ||||
| MMP-2 | 0.68 | 0.003 | 0.74 [0.66,0.82] | 0.001 |
| TIMP-4 | 0.66 | 0.006 | 0.71 [0.63,0.79] | 0.008 |
| NT-proBNP | 0.65 | 0.002 | 0.71 [0.63,0.79] | 0.050 |
| PIIINP | 0.64 | 0.006 | 0.71 [0.63,0.80] | 0.010 |
| Cardiotrophin | 0.60 | 0.090 | 0.71 [0.63,0.79] | 0.100 |
| TIMP-1 | 0.58 | 0.050 | 0.69 [0.61,0.77] | 0.230 |
| TIMP-3 | 0.58 | 0.060 | 0.70 [0.62,0.78] | 0.040 |
| MMP-8 | 0.57 | 0.008 | 0.73 [0.66,0.80] | 0.005 |
Abbreviations: LVH = patients with left ventricular hypertrophy but no heart failure, DHF = patients with left ventricular hypertrophy and diastolic heart failure, MMP = matrix metalloproteinase, TIMP = tissue inhibitor of MMP, NT-proBNP = n-terminal propeptides of brain naturetic peptide, sRAGE = soluble receptor for advanced glycation end product, PINP = n-terminal collagen I propeptide, PIIINP = n-terminal collagen III propeptide, CITP = collagen I telopeptide. AUC = area under the curve, Adjusted AUC values (with 95% confidence intervals) and p-value indicates the model was adjusted for clinical covariates: systolic blood pressure, diastolic blood pressure, age quartile, sex, and body mass index. For LVH, analyses were performed comparing all patients with LVH (n= 205) to referent controls (n=241). For DHF, analyses were performed comparing patients with LVH but no DHF (n=144) were compared to patients with LVH and DHF (n=61). For LVH, the AUC using the clinical covariates alone was 0.73 [0.69, 0.78]. For DHF, the AUC using the clinical covariates alone was 0.68 [0.60, 0.76].
Table 4.
Multivariable, Multi-Biomarker Analysis for LVH and DHF Detection adjusted for covariates
| LVH | ||||||
|---|---|---|---|---|---|---|
| Algorithm | Clinical variables plus 1 biomarker |
Clinical variables plus 2 biomarkers |
Clinical variables plus 3 biomarkers |
Clinical variables plus 4 biomarkers |
Clinical variables plus 5 biomarkers |
Clinical variables plus NT-pro BNP |
| AUC [95%CI] |
0.77[0.72,0.81] | 0.78[0.74,0.83] | 0.78[0.74,0.83] | 0.80[0.75,0.84] | 0.80[0.76,0.84] | 0.75[0.71,0.80] |
| CV AUC | 0.75[0.69,0.80] | 0.76[0.71,0.81] | 0.76[0.72,0.80] | 0.77[0.72,0.82] | 0.77[0.72,0.83] | 0.74[0.67,0.79] |
| TIMP-1 | X | X | X | X | X | |
| MMP-9 | X | X | X | X | ||
| PIIINP | X | X | X | |||
| NT- proBNP |
X | X | ||||
| MMP-7 | X | |||||
| DHF | |||||
|---|---|---|---|---|---|
| Algorithm | Clinical variables plus 1 biomarker |
Clinical variables plus 2 biomarkers |
Clinical variables plus 3 biomarkers |
Clinical variables plus 4 biomarkers |
Clinical variables plus NT-pro BNP |
| AUC | 0.74[0.66, 0.82] | 0.75[0.67,0.82] | 0.79[0.72,0.85] | 0.79[0.73,0.86] | 0.71[0.63,0.79] |
| CV AUC | 0.68[0.58,0.77] | 0.67[0.56,0.77] | 0.72[0.6,0.80] | 0.72[0.63,0.82] | 0.64[0.54,0.74] |
| MMP-2 | X | X | X | X | |
| TIMP-4 | X | X | X | ||
| MMP-8 | X | X | |||
| PIIINP | X | ||||
Abbreviations: LVH = patients with left ventricular hypertrophy, DHF = patients with left ventricular hypertrophy and diastolic heart failure, MMP = matrix metalloproteinase, TIMP = tissue inhibitor of MMP, NT-proBNP = n-terminal propeptides of brain naturetic peptide, PIIINP = n-terminal collagen III propeptide, AUC = area under the curve (with 95% confidence intervals), CV = cross validated. All models were adjusted for: systolic blood pressure, systolic blood pressure, diastolic blood pressure, age quartile, sex, and BMI. For LVH, analyses were performed comparing all patients with LVH (n= 205) to referent controls (n=241). For DHF, analyses were performed comparing patients with LVH but no DHF (n=144) were compared to patients with LVH and DHF (n=61).
Cross validation
The discriminative value of the models for LVH and DHF was assessed by calculating the area under the curve (AUC) (Figures 1 and 2). The model AUC was estimated using five-fold cross validation across 30 simulated splits of the data. For this procedure, the data were divided into approximately five equal parts and the model was fitted to 80% of the data. AUC was then calculated based on validating the model on the remaining 20% of the data. This was repeated for all 5 splits of the data, leaving one split out each time for the purpose of validation. The procedure was conducted 30 times for simulated data splits, and an average cross-validated AUC was reported. This data simulation methodology for validating a predictive model avoids biasing the study toward a higher AUC that one would obtain if the model was validated on the same data originally used to derive the model. In addition, observation of the simulated AUC curves allows researchers to determine whether any parts of the curve are unstable. Finally, the CV AUC for LVH and DHF were compared to that resulting from a logistic regression model with clinical covariates alone, and with clinical covariates plus NT-proBNP. Results are reported as AUC or CV AUC [95%CI].
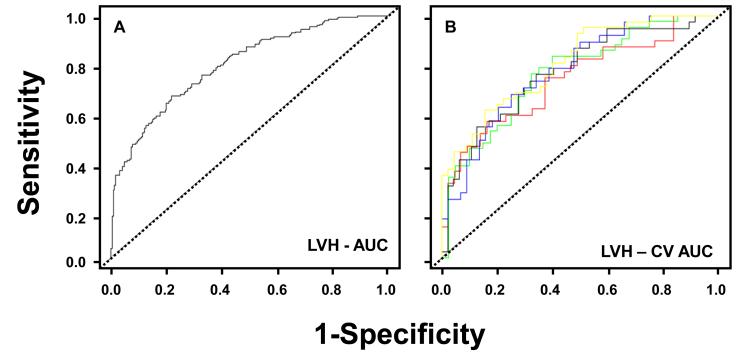
Figure 1.
A: Receiver operator curve analysis for plasma biomarker detection of left ventricular hypertrophy (LVH). Observed area under the curve (AUC) for LVH using clinical covariates plus the 5 biomarker panel = 0.80 [0.76, 0.84].
B: Cross-validated analysis for plasma biomarker detection of LVH. The ROC curves generated from a 30 random simulated simulations of a 5-fold split of the data are presented. The cross validated ROC curves are similar in shape to those obtained in Figure 1A.
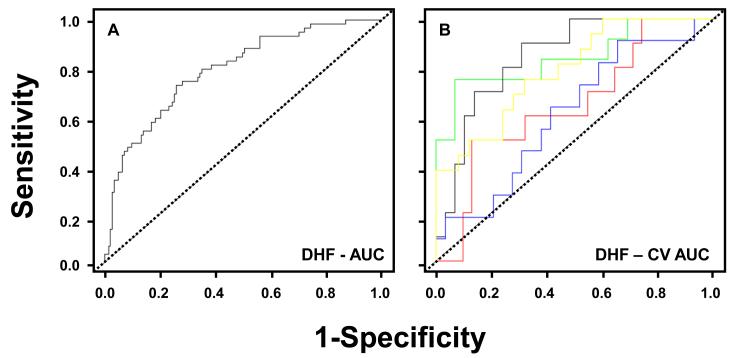
Figure 2.
A: Receiver operator curve analysis for plasma biomarker detection of diastolic heart failure (DHF). Observed area under the curve (AUC) for DHF using clinical covariates plus the 4 biomarker panel = 0.79 [0.73,0.86].
B: The ROC curves generated from 30 random simulated simulations of a 5-fold split of the data are presented. The cross-validated ROC curves are similar in shape to those obtained in Figure 2A.
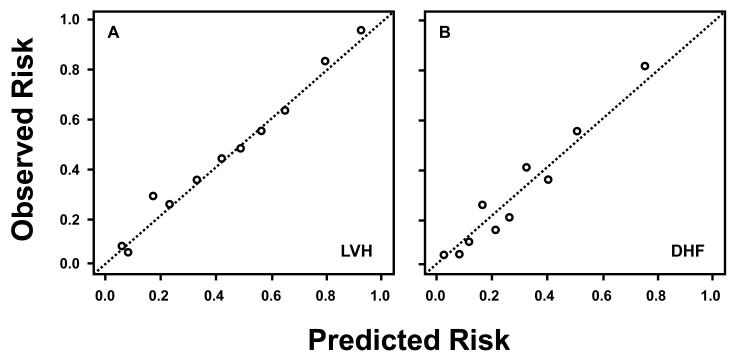
A test of calibration and goodness of fit as suggested by the Hlatky et al in the AHA statement on biomarkers was performed 41. Predicted risk based on the clinical covariates plus 5 biomarker panel and clinical covariates plus 4 biomarker panel, for LVH and DHF, respectively, were grouped into deciles. The predicted versus the observed proportion (risk) of events were plotted at each decile (Figure 3), and finally, Hosmer Lemeshow Chi square goodness of fit tests were conducted.
Figure 3.
The calibration of risk predictors for LVH and DHF. Predicted risk based on the clinical covariates plus 5 biomarker panel and clinical covariates plus 4 biomarker panel, for LVH and DHF, respectively, were grouped into deciles. Observed versus predicted risk of LVH (or DHF) was plotted for each decile. Both calibration curves show a linearly increasing relationship between predictive and observed risk.
Results
Patient Demographics and Echocardiography
Demographics and LV echocardiographic data are presented in Table 1. In the LVH group, body surface area and blood pressure were increased compared with referent control values. In the DHF group, body surface area and blood pressure were increased compared with the referent controls and the DHF subjects were older and had a diminished six minute hall walk compared with both the control and LVH groups. While blood pressure values were elevated in both the LVH and DHF groups, these values remained within the therapeutic range suggested by JNC IV guidelines1. There were no significant differences between referent control, LVH, and DHF subjects with respect to LV end diastolic volume or ejection fraction. Consistent with the criteria for LVH, LV end-diastolic wall thickness and mass were increased in both the LVH and DHF groups compared with referent controls. In the DHF group, E/E’ and PCWP were increased compared with the LVH and referent controls.
Plasma Biomarkers
The absolute values for all of the plasma biomarkers measured in the 3 groups of subjects are shown in Table 2. Compared to referent control values, plasma MMP-9, TIMP-1, TIMP-2, and osteopontin were increased in the LVH group, the other biomarkers (including MMP-1) were not significantly different. In the DHF group, MMP-2, MMP-3, MMP-7, MMP-9, TIMP-1, TIMP-2, TIMP-4, PIINP, CITP, osteopontin and NT-proBNP were increased compared with referent control values, whereas MMP-8 values were reduced, the other biomarkers (including MMP-1) were not significantly different. These plasma analytes were next considered as univariable predictors of LVH and/or DHF before and after adjustment for clinical variables and the results from this analysis are presented in Table 3 and supplemental Tables B and C.
Multivariable Predictors
One of the major objectives of this study was to develop a multivariable model of plasma biomarkers that would discriminate the presence of LVH as well as for DHF. Accordingly, a number of forward modeling and cross-validation iterations were performed both unadjusted (please see Table B in the supplement materials) and adjusted for clinical variables (Table 4). For LVH, the best predictive fit was obtained when a total of 5 biomarkers were utilized in the model. For DHF, the best fit from forward variable selection was achieved when 4 biomarkers were utilized in the model. In light of the fact that plasma profiles of NT-proBNP have been considered a benchmark as a biomarker for CHF,10,15,42 these models were compared to the AUC for NT-proBNP alone.
LVH
The AUCs resulting from 1, 2, 3, 4, and 5 biomarkers panels are presented in Table A in the supplemental materials. The best fit model was achieved using 5 biomarkers consisting of NT-proBNP, MMP-7, MMP-9, TIMP-1 and PIIINP. This regression model adjusted for systolic blood pressure, diastolic blood pressure, age quartile, sex, and body mass index is presented in Table 4. The receiver operator curve for this 5 biomarker model is shown in Figure 1A. The observed area under the curve (AUC) for LVH using clinical covariates plus the 5 biomarker panel was 0.80 [with 95% confidence intervals of 0.76, 0.84], which is improved over the observed AUC using the clinical covariates alone, 0.73 [0.69, 0.78]. The observed AUC value for LVH using clinical covariates plus NT-proBNP was 0.75 [0.71, 0.80]. The cross validated AUC for the multiple logistic regression model using clinical covariates plus the 5 biomarker panel was 0.77 [0.72, 0.83], implying good discriminative ability of the biomarker panel (Figure 1B). The sensitivity and specificity at the “shoulder” of the ROC curves where the sum of sensitivity and specificity was the highest was examined. For LVH, to detect 80% of true positives based on these biomarkers (sensitivity), there will be a 45% false positive (1-specificity) rate. The calibration of the risk predictors were made by comparing the predicted versus observed frequency of events. The resultant analysis showed that the average observed risk versus predicted risk calculated within deciles of predicted risk followed a linear pattern for LVH (Figure 3). These results along with an insignificant Hosmer-Lemeshow goodness of fit statistic (χ2 = 4.68, p = 0.79) confirm a high degree of goodness of fit of the model.
DHF
The AUCs resulting from 1, 2, 3, and 4 biomarkers are presented in the Table A in the supplemental materials. The best fit model was achieved using 4 biomarkers consisting of MMP-2, MMP-8, TIMP-4 and PIIINP. This regression model adjusted for systolic blood pressure, diastolic blood pressure, age quartile, sex, and body mass index is presented in Table 4. The receiver operator curve for this 4 biomarker model is shown in Figure 2A. The observed area under the curve (AUC) for DHF using clinical covariates plus the 4 biomarker panel was 0.79 [0.73, 0.86], which is improved over the observed AUC using the clinical covariates alone, 0.68 [0.60, 0.76]. The observed AUC value for DHF using clinical covariates plus NT-proBNP was 0.71 [0.63, 0.79]. The cross validated AUC for the multiple logistic regression model using clinical covariates plus the 4 biomarker panel was 0.72 [0.63, 0.82], implying good discriminative ability of the biomarker panel (Figure 1B). The highest sensitivity and specificity at the “shoulder” of the ROC curves was examined for DHF: to detect 80% of true positives based on these biomarkers, there will be a 40% false positive rate. The resultant analysis showed that the average observed versus predicted risk calculated within deciles of predicted risk followed a linear pattern for DHF (Figure 3). These results along with an insignificant Hosmer-Lemeshow goodness of fit statistic (χ2 = 3.63, p = 0.89) confirm a high degree of goodness of fit of the model.
Therefore, the use of a multi-biomarker panel improved detection of LVH and DHF compared with clinical covariates alone, NT-proBNP alone, or any single biomarker.
Discussion
The development of LVH is an important risk factor for the progression to DHF. Both LVH and DHF have been associated with an increase in cardiovascular morbidity and mortality 5,6,16-22,43. However, a simple and specific analytical approach to detect the presence of LVH, and more importantly the progression to DHF in an ambulatory/outpatient context, remains elusive. We hypothesized that specific multi-biomarker panels measured in the plasma could be used to identify patients with LVH and DHF. Generically, biomarkers consist of peptides, proteins and enzymes which reflect the presence and status of an underlying pathophysiologic condition 24-26,38-40,42. Biomarkers are well accepted analytical adjunctive tools in the context of hematological, infectious, neoplastic, and metabolic disease. However, the use of biomarkers to identify the presence of LVH and the progression to DHF have only recently been considered, and most studies examined only a single biomarker in their analysis 14,15,25,38,39,42,44,45. The goal of this project was to test the discriminative value of a large portfolio of candidate biomarkers in patients with LVH and/or DHF which were relevant to their underlying pathophysiology. These candidate biomarkers were focused on myocardial extracellular matrix (ECM) remodeling pathways, since ECM remodeling is a common event in the development of LVH and progression to DHF 14,20-26. In addition, these candidate biomarkers were compared to values obtained from a reference, univariable biomarker that has been used in previous CHF studies; the N-terminal peptide of pro-brain natriuretic factor (NT-proBNP) 15,42.
The unique, important findings in this study were 2-fold. First, a specific panel of biomarkers, reflective of the matrix remodeling process, could be utilized in predictive modeling algorithms for both LVH and DHF. Specifically, a multi-biomarker panel which included MMP-7, MMP-9, TIMP-1, PIIINP, and NT-proBNP provided a prediction algorithm for LVH (AUC=0.80), and a multi-biomarker panel which included MMP-2, MMP-8, TIMP-4, and PIIINP provided a prediction algorithm for DHF both with good sensitivity and acceptable specificity (AUC=0.79). Second, the multi-biomarkers panels for LVH and DHF provided better predictive models than when each biomarker was considered as a single entity, better than using clinical co-variates alone, and better than measuring NT-proBNP alone. While these biomarker panels require more rigorous study in a larger, more diverse patient population, these findings demonstrate the proof-of-concept that a multi-biomarker panel measured from a peripheral blood sample holds promise as a diagnostic tool to identify the presence of LVH and DHF.
Biomarkers of ECM homeostasis
Recently published guidelines for the development of biomarkers have emphasized the importance of the link between underlying pathophysiology and the predictive capabilities of the biomarkers chosen. Accordingly, biomarkers of ECM homeostasis were selected for this study because past studies demonstrated that the adaptive response to PO includes structural and biochemical “remodeling” of both cellular and extracellular compartments 14,20-26.
Both collagen type I and III must undergo a series of sequential post-synthetic processing steps in order to become a mature structural collagen fibril. One of these steps is cleavage of the N-terminal propeptide of procollagen I and III (PINP, PIIINP, respectively). When collagne is degraded one proteolytic fragment is the collagen type I telopeptide (CITP). Both the propeptides and telopeptides are of small molecular weight and thereby move from the interstitial space into the vascular compartment, which in turn allows for quantitation in a systemic blood sample 14,24,25. In the present study, univariable analysis demonstrated that PIIINP was increased in patients with LVH and DHF; CITP was increased in DHF patients. Thus, consistent with past reports, PO was associated with increased indices of collagen synthesis and degradation indicative of accelerated ECM turnover 14,24-26. Importantly however, the present study built upon these observations using multivariable modeling to demonstrate that PIIINP but not CITP was an important variable in the predictive model for LVH as well as DHF. This result emphasizes the concept that the use of a single measurement or a univariable approach in a dynamic, diverse and complex structure such as the myocardial ECM may be insufficient to assess the utility of biomarkers for the detection and prediction of LVH and/or DHF.
One of the determinants effecting ECM degradation is the induction and activation of a large family of zinc-dependent interstitial proteases, the MMPs. In general terms, the classification of MMPs is based upon substrate affinity and specificity.Tthe present study measured plasma levels of MMPs from each of the soluble classes- the collagenases (MMP-1,-8), gelatinases (MMP-2,-9), and stomelysins/matrilysins (MMP-3,-7).26 A systematic approach to measuring a full profile of MMPs and performing multivariable predictive analysis had not been previously performed. Recent community based studies demonstrated an approximately 2-fold increased risk of hypertension progression in subjects with elevated MMP-9 levels.46-49 Moreover, these elevated MMP-9 plasma levels were associated with higher PIIINP levels. In the present study, both PIIINP and MMP-9 were important components of the multi-biomarker prediction modeling for LVH. Taken together, previous and present studies suggest that PIIINP and MMP-9 are important biomarkers for identifying patients at high risk for LVH and adverse ECM remodeling.
The present study demonstrated that a different plasma MMP profile emerged for DHF patients compared with LVH patients. Specifically, plasma MMP-2 and MMP-7 were increased, whereas MMP-8 levels were decreased. This likely reflects a local shift in cell type activation as well as ECM proteolytic events occurring in DHF patients. Specifically, increased plasma MMP-2 levels would imply a more generalized proteolytic state which in turn would heighten ECM instability and turnover 26,46. On the other hand, increased MMP-7 levels have been associated with the wound healing response, are expressed in resident cells such as macrophages and may be indicative of local alterations in the cell types and expression patterns occurring within the ECM 26. In addition, the decrease in MMP-8, in the DHF patients suggests a phenotypic change in cell types and MMP expression patterns. MMP-8, a neutrophil collagenase, may be reflective of changes in neutrophil synthesis and activation.
One of the critical control points for MMP proteolytic activity is the expression and binding of endogenous tissue inhibitors of MMPs (TIMPs). TIMPs bind to the activated MMP or proMMP thus regulating MMP activity in vivo.26,50-52 In addition, TIMPs may be potent fibroblast growth factors and stimulate profibrotic signaling cascades.26,50-52 All of 4 known TIMPs are expressed within the human myocardium, with TIMP-4 having a predominantly cardiovascular expression.26,51 The present study is the first to comprehensively measure all 4 TIMPs within the plasma of a large cohort of referent control, LVH, and DHF subjects. Consistent with past reports, plasma TIMP-1 levels were increased in LVH patients.23,53 In patients with DHF, TIMP-4 levels were increased compared with both referent control and LVH subjects and would imply a further induction of a myocardial profibrotic state.
Diagnostic Utility of Plasma Biomarkers and Current Diagnostic Strategies
Epidemiologic studies have identified clinical and demographic factors that increase the risk of developing LVH; these include increased age, female gender, obesity, and uncontrolled blood pressure 8-20. However, knowledge of these risk factors alone cannot provide the means to predict the presence of LVH, hold no real specificity and sensitivity for predicting LVH, and are not useful in a clinically relevant context. As such, analytical measurements that would be easy to use and be cost effective for the detection of LVH, in an ambulatory setting would have significant clinical import. Unfortunately, outside of advanced imaging methodologies, the diagnosis of LVH can not currently be made with sufficient accuracy. For example, electrocardiographic (ECG) methods, even when rigorously applied, can provide 70% specificity but less than 30% sensitivity 12,13. Therefore, to date, the determination of LVH requires non-invasive imaging using an echocardiogram, magnetic resonance imaging or multi slice computed tomography. The present study demonstrated that a 5 analyte biomarker panel could be developed to predict the presence of LVH. The sensitivity and specificity of this plasma multi-biomarker profile exceeded that of any single biomarker (including NT-proBNP) and exceeds any currently available screening algorithm (e.g.: clinical exam, ECG) for LVH.
A number of criteria have been proposed for the diagnosis of DHF including: symptoms and signs of heart failure, a preserved ejection fraction, a normal LV end diastolic volume, clear evidence of abnormal diastolic function, and no evidence of non-myocardial disease.32-34 These were the criteria used in the current study to make the diagnosis of DHF. There are however, a number of limitations to these diagnostic criteria. For example, the symptoms and signs of heart failure may be non-specific and may be caused by factors other than heart failure. Detecting abnormalities in diastolic function, measuring ejection fraction and LV volume require non-invasive imaging such as an echocardiogram or magnetic resonance imaging which are costly and require subspecialty expertise to perform and interpret. In addition, many indices of diastolic function become abnormal with what may be considered normal aging. Thus, there are no available adjunctive analytical tests that can be applied in the ambulatory care setting to identify the presence of DHF. The ability to utilize a plasma profile to screen, diagnose and follow patients with DHF would provide a significant clinical advancement.
The most advanced use of a single biomarker in the context of CHF is that of the natriuretic peptides, which include NT-proBNP.10,15,42 However, the sensitivity and specificity of natriuretic peptide levels such as NT-proBNP are significantly affected by co-morbidities and other demographic factors.10,15,42 These confounding factors include age, obesity, gender, renal, pulmonary and liver dysfunction.42,54 Nevertheless, NT-proBNP has become an accepted biomarker to provide additional supportive evidence for the presence of systolic heart failure.42 However, whether and to what degree NT-proBNP may be useful in patients with DHF, particularly in ambulatory patients with compensated stable DHF, remains unclear.20,21 Accordingly, the present study evaluated plasma NT-proBNP within the multivariable model and the univariable model for comparative purposes. In the multivariable models, NT-proBNP was one of the 5 final variables that provided the highest AUC value for LVH. Interestingly, in patients with DHF, NT-proBNP was not useful in the multivariable predictive model, where the final multivariable biomarker model (MMP-2, MMP-8, TIMP-4, PIIINP) provided a sensitivity and specificity which was approximately 20% higher than NT-proBNP alone.
Limitations, Future Directions and Conclusion
Recent reviews and an AHA scientific statement emphasized the importance for developing biomarkers to enhance diagnostic methods and provide surrogate measures of treatment efficacy.41,55-57 Some of these considerations have been successfully addressed in the present study, whereas others have not. For example, it has been suggested that biomarkers should reflect the underlying pathophysiologic mechanisms responsible for outcomes examined.55-57 The biomarkers measured in the present study fulfill this requirement. In addition, it is clear that no single biomarker is optimal for screening, diagnosis and potentially disease management and therefore the multivariable modeling utilized in the present study addressed this issue 23,38,40,47-49,55-57. Finally, the current study clearly fulfilled the recommended first “phase of evaluation” outlined by the AHA. However, as such the outcomes from the current study represent an initial proof of concept and must be evaluated in a more rigorous fashion before they can become a clinically viable analytical test. Prospective validation, clinical utility, clinical outcomes and cost-effectiveness are necessary next steps in the evaluation of the biomarkers proposed herein. The methods used to recruit the study patients did not result in a completely random sample but selected a group with disproportionate representation of women and blacks and patients with obesity, hypertension and LVH. Therefore, it was not possible to control for demographic distribution or test for incremental value over and above the risk presented by the demographic characteristics of the study population. The additional phases of evaluation will now be possible to pursue because the current study has provided a robust proof of concept.
The current study was designed and conducted to provide a proof-of-concept analysis that would then lead to further large, pivotal, definitive studies. Accordingly, a conservative approach of cross validation in each step (including for variable selection) was used so that any variables that were marginally associated with outcome would not be missed and could be more definitively examined in future studies. It is possible that different splits of the data would result in retention of different covariates if a uniformly cross validated approach had been taken; however, it is unlikely that this outcome would have occurred because there were no additional biomarkers for LVH or DHF that came close to the 0.10 alpha level when added to the final adjusted models. Therefore, the current study provides the proof-of-concept necessary to conduct a large, pivotal, definitive study.
Furthermore, in the current study, patients with significant co-morbidities were excluded. These circumstances avoided the effects of transient changes in biomarkers associated with acute decompensation and avoided the possibility that other disease process may confound these results. Nevertheless, the present study provides a clinical proof of concept that a multi-biomarker panel has discriminative value in identifying the presence of structural and clinical manifestations of PO-induced heart disease.
Supplementary Material
Table A: Myocardial Matrix Biomarker Profiles in Referent Control Subjects, Subjects with Left Ventricular Hypertrophy, and Subjects with Diastolic Heart Failure
Table B: Effect size (beta), Wald T statistics, and p-value for adjusted models obtained by setting forward variable selection entry criteria at 0.10.
Table C: Multivariate, Multi-Biomarker Analysis for LVH and DHF Detection
Supplemental Figure A Panel A: Receiver operator curve analysis for plasma NT-proBNP in left ventricular hypertrophy (LVH). Observed area under the curve (AUC) for LVH using clinical covariates plus NT-proBNP = 0.75 [0.71, 0.80].
Panel B: Cross-validated analysis for NT-proBNP in LVH. The ROC curves generated from a 30 random simulated simulations of a 5-fold split of the data are presented. The cross validated ROC curves are similar in shape to those obtained in Panel A.
Supplement Figure B Panel A: Receiver operator curve analysis for NT-proBNP in diastolic heart failure (DHF). Observed area under the curve (AUC) for DHF using clinical covariates plus NT-proBNP = 0.71 [0.63, 0.79].
Panel B: The ROC curves generated from 30 random simulated simulations of a 5-fold split of the data are presented. The cross-validated ROC curves are similar in shape to those obtained in Panel A.
Acknowledgments
Sources of Funding This study was supported by NIH grants HL87134, HL81692, HL85531 (F.G. Spinale), the Research Service of the Department of Veterans Affairs (M.R. Zile, F.G. Spinale), a research grant provided by Ortho Clinical Diagnostics (M.R. Zile, F.G. Spinale), Health Sciences South Carolina, and the Doris Duke Foundation (M.R. Zile)
Footnotes
Disclosures Dr. Zile and Dr. Spinale report receiving research funds from Ortho Clinical Diagnostics. No other potential conflicts of interest relevant to this article are reported.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 2002;287:1003–10. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KKL. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure. The Framingham Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 5.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, Porcellati C. Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy in essential hypertension. J Am Coll Cardiol. 1998;31:383–90. doi: 10.1016/s0735-1097(97)00493-2. [DOI] [PubMed] [Google Scholar]
- 6.de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J. 2008;29:741–7. doi: 10.1093/eurheartj/ehm605. [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:1144–6. doi: 10.1161/01.cir.81.3.815. [DOI] [PubMed] [Google Scholar]
- 8.Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the framingham offspring study. Circulation. 2009;119:3085–92. doi: 10.1161/CIRCULATIONAHA.108.824243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okin PM, Oikarinen L, Viitasalo M, Toivonen L, Kjeldsen SE, Nieminen MS, Edelman JM, Dahlöf B, Devereux RB, LIFE Study Investigators Prognostic value of changes in the electrocardiographic strain pattern during antihypertensive treatment: the Losartan Intervention for End-Point Reduction in Hypertension Study (LIFE) Circulation. 2009;119:1883–91. doi: 10.1161/CIRCULATIONAHA.108.812313. [DOI] [PubMed] [Google Scholar]
- 10.de Lemos JA, McGuire DK, Khera A, Das SR, Murphy SA, Omland T, Drazner MH. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: results from the Dallas Heart Study. Am Heart J. 2009;157:746–53. doi: 10.1016/j.ahj.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Solomon SD, Appelbaum E, Manning WJ, Verma A, Berglund T, Lukashevich V, Papst C Cherif, Smith BA, Dahlöf B, Aliskiren in Left Ventricular Hypertrophy (ALLAY) Trial Investigators Effect of the direct Renin inhibitor aliskiren, the Angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119:530–7. doi: 10.1161/CIRCULATIONAHA.108.826214. [DOI] [PubMed] [Google Scholar]
- 12.Wachtell K, Okin PM, Olsen MH, Dahlöf B, Devereux RB, Ibsen H, Kjeldsen SE, Lindholm LH, Nieminen MS, Thygesen K. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE Study. Circulation. 2007;116:700–5. doi: 10.1161/CIRCULATIONAHA.106.666594. [DOI] [PubMed] [Google Scholar]
- 13.Westerhout CM, Lauer MS, James S, Fu Y, Wallentin L, Armstrong PW, GUSTO IV ACS Investigators Electrocardiographic left ventricular hypertrophy in GUSTO IV ACS: an important risk marker of mortality in women. Eur Heart J. 2007;28:2064–9. doi: 10.1093/eurheartj/ehm223. [DOI] [PubMed] [Google Scholar]
- 14.Mak GJ, Ledwidge MT, Watson CJ, Phelan DM, Dawkins IR, Murphy NF, Patle AK, Baugh JA, McDonald KM. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J Am Coll Cardiol. 2009;54:1674–82. doi: 10.1016/j.jacc.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Grewal J, McKelvie RS, Persson H, Tait P, Carlsson J, Swedberg K, Ostergren J, Lonn E. Usefulness of N-terminal pro-brain natriuretic Peptide and brain natriuretic peptide to predict cardiovascular outcomes in patients with heart failure and preserved left ventricular ejection fraction. Am J Cardiol. 2008;102:733–7. doi: 10.1016/j.amjcard.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 16.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–7. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, I-PRESERVE Investigators Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 18.Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourcière Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JM, Thomas JD, Zile MR, Aurigemma GP, Valsartan In Diastolic Dysfunction (VALIDD) Investigators Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–87. doi: 10.1016/S0140-6736(07)60980-5. [DOI] [PubMed] [Google Scholar]
- 19.Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–41. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 20.Zile MR, Lewinter MM. Left ventricular end-diastolic volume is normal in patients with heart failure and a normal ejection fraction: a renewed consensus in diastolic heart failure. J Am Coll Cardiol. 2007;49:982–5. doi: 10.1016/j.jacc.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 22.Gaasch WH, Zile MR. Left ventricular diastolic dysfunction and diastolic heart failure. Annu Rev Med. 2004;55:373–94. doi: 10.1146/annurev.med.55.091902.104417. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–96. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 24.Díez J. Towards a new paradigm about hypertensive heart disease. Med Clin North Am. 2009;93:637–45. doi: 10.1016/j.mcna.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 25.González A, López B, Ravassa S, Beaumont J, Arias T, Hermida N, Zudaire A, Díez J. Biochemical markers of myocardial remodelling in hypertensive heart disease. Cardiovasc Res. 2009;81:509–18. doi: 10.1093/cvr/cvn235. [DOI] [PubMed] [Google Scholar]
- 26.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 27.Freed DH, Borowiec AM, Angelovska T, Dixon IM. Induction of protein synthesis in cardiac fibroblasts by cardiotrophin-1: integration of multiple signaling pathways. Cardiovasc Res. 2003;60:365–75. doi: 10.1016/s0008-6363(03)00534-0. [DOI] [PubMed] [Google Scholar]
- 28.Kuwahara K, Saito Y, Harada M, Ishikawa M, Ogawa E, Miyamoto Y, Hamanaka I, Kamitani S, Kajiyama N, Takahashi N, Nakagawa O, Masuda I, Nakao K. Involvement of cardiotrophin-1 in cardiac myocyte-nonmyocyte interactions during hypertrophy of rat cardiac myocytes in vitro. Circulation. 1999;100:1116–24. doi: 10.1161/01.cir.100.10.1116. [DOI] [PubMed] [Google Scholar]
- 29.Singh M, Foster CR, Dalal S, Singh K. Osteopontin: Role in extracellular matrix deposition and myocardial remodeling post-MI. J Mol Cell Cardiol. 2010;48:538–43. doi: 10.1016/j.yjmcc.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto H. Osteopontin and cardiovascular system. Mol Cell Biochem. 2007;300:1–7. doi: 10.1007/s11010-006-9368-3. [DOI] [PubMed] [Google Scholar]
- 31.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Yturralde RF, Gaasch WH. Diagnostic criteria for diastolic heart failure. Prog Cardiovasc Dis. 2005;47:314–9. doi: 10.1016/j.pcad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 34.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–93. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 35.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 36.Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quiñones MA, Zoghbi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue doppler imaging. Circulation. 1998;98:1644–50. doi: 10.1161/01.cir.98.16.1644. [DOI] [PubMed] [Google Scholar]
- 37.Lindsey JB, Cipollone F, Abdullah SM, McGuire DK. Receptor for advanced glycation end-products (RAGE) and soluble RAGE (sRAGE): cardiovascular implications. Diab Vasc Dis Res. 2009;6:7–14. doi: 10.3132/dvdr.2009.002. [DOI] [PubMed] [Google Scholar]
- 38.López B, González A, Querejeta R, Barba J, Díez J. Association of plasma cardiotrophin-1 with stage C heart failure in hypertensive patients: potential diagnostic implications. J Hypertens. 2009;27:418–24. doi: 10.1097/HJH.0b013e32831ac981. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg M, Zugck C, Nelles M, Juenger C, Frank D, Remppis A, Giannitsis E, Katus HA, Frey N. Osteopontin, a new prognostic biomarker in patients with chronic heart failure. Circ Heart Fail. 2008;1:43–9. doi: 10.1161/CIRCHEARTFAILURE.107.746172. [DOI] [PubMed] [Google Scholar]
- 40.Bonnema DD, Webb CS, Pennington WR, Stroud RE, Leonardi AE, Clark LL, McClure CD, Finklea L, Spinale FG, Zile MR. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) J Card Fail. 2007;13:530–40. doi: 10.1016/j.cardfail.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE, Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O’Donnell CJ, Smith SC, Jr, Wilson PW, American Heart Association Expert Panel on Subclinical Atherosclerotic Diseases and Emerging Risk Factors and the Stroke Council Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boerrigter G, Costello-Boerrigter LC, Burnett JC., Jr. Natriuretic peptides in the diagnosis and management of chronic heart failure. In: Braunwald E, editor. Biomarkers in heart failure. Elsevier; Philadelphia, PA: 2009. pp. 501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2152. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barasch E, Gottdiener JS, Aurigemma G, Kitzman DW, Han J, Kop WJ, Tracy RP. Association between elevated fibrosis markers and heart failure in the elderly: the cardiovascular health study. Circ Heart Fail. 2009;2:303–10. doi: 10.1161/CIRCHEARTFAILURE.108.828343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunlay SM, Gerber Y, Weston SA, Killian JM, Redfield MM, Roger VL. Prognostic value of biomarkers in heart failure: application of novel methods in the community. Circ Heart Fail. 2009;2:393–400. doi: 10.1161/CIRCHEARTFAILURE.109.849299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martos R, Baugh J, Ledwidge M, O’Loughlin C, Murphy NF, Conlon C, Patle A, Donnelly SC, McDonald K. Diagnosis of heart failure with preserved ejection fraction: improved accuracy with the use of markers of collagen turnover. Eur J Heart Fail. 2009;11:191–7. doi: 10.1093/eurjhf/hfn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhingra R, Pencina MJ, Schrader P, Wang TJ, Levy D, Pencina K, Siwik DA, Colucci WS, Benjamin EJ, Vasan RS. Relations of matrix remodeling biomarkers to blood pressure progression and incidence of hypertension in the community. Circulation. 2009;119:1101–7. doi: 10.1161/CIRCULATIONAHA.108.821769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sundström J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Sutherland P, Wilson PW, Vasan RS. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 2004;109:2850–6. doi: 10.1161/01.CIR.0000129318.79570.84. [DOI] [PubMed] [Google Scholar]
- 49.Vorovich EE, Chuai S, Li M, Averna J, Marwin V, Wolfe D, Reilly MP, Cappola TP. Comparison of matrix metalloproteinase 9 and brain natriuretic peptide as clinical biomarkers in chronic heart failure. Am Heart J. 2008;155:992–7. doi: 10.1016/j.ahj.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: ’Embracing the MMP-independent-side of the family’. J Mol Cell Cardiol. 2010;48:445–53. doi: 10.1016/j.yjmcc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Melendez-Zajgla J, Del Pozo L, Ceballos G, Maldonado V. Tissue inhibitor of metalloproteinases-4. The road less traveled. Mol Cancer. 2008;7:85–96. doi: 10.1186/1476-4598-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1:1–9. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tayebjee MH, Nadar SK, MacFadyen RJ, Lip GY. Tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9 levels in patients with hypertension Relationship to tissue Doppler indices of diastolic relaxation. Am J Hypertens. 2004;17:770–4. doi: 10.1016/j.amjhyper.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 54.Wu AH, Smith A, Wieczorek S, Mather JF, Duncan B, White CM, McGill C, Katten D, Heller G. Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol. 2003;92:628–31. doi: 10.1016/s0002-9149(03)00741-0. [DOI] [PubMed] [Google Scholar]
- 55.Richards AM. What we may expect from biomarkers in heart failure. In: Braunwald E, editor. Biomarkers in heart failure. Elsevier; Philadelphia, PA: 2009. pp. 501–514. [Google Scholar]
- 56.Fortmann SP, Ford E, Criqui MH, Folsom AR, Harris TB, Hong Y, Pearson TA, Siscovick D, Vinicor F, Wilson PF, CDC. AHA CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the population science discussion group. Circulation. 2004;110:e554–9. doi: 10.1161/01.CIR.0000148982.95775.BF. [DOI] [PubMed] [Google Scholar]
- 57.Smith SC, Jr, Anderson JL, Cannon RO, 3rd, Fadl YY, Koenig W, Libby P, Lipshultz SE, Mensah GA, Ridker PM, Rosenson R, CDC. AHA CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the clinical practice discussion group. Circulation. 2004;110:e550–3. doi: 10.1161/01.CIR.0000148981.71644.C7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A: Myocardial Matrix Biomarker Profiles in Referent Control Subjects, Subjects with Left Ventricular Hypertrophy, and Subjects with Diastolic Heart Failure
Table B: Effect size (beta), Wald T statistics, and p-value for adjusted models obtained by setting forward variable selection entry criteria at 0.10.
Table C: Multivariate, Multi-Biomarker Analysis for LVH and DHF Detection
Supplemental Figure A Panel A: Receiver operator curve analysis for plasma NT-proBNP in left ventricular hypertrophy (LVH). Observed area under the curve (AUC) for LVH using clinical covariates plus NT-proBNP = 0.75 [0.71, 0.80].
Panel B: Cross-validated analysis for NT-proBNP in LVH. The ROC curves generated from a 30 random simulated simulations of a 5-fold split of the data are presented. The cross validated ROC curves are similar in shape to those obtained in Panel A.
Supplement Figure B Panel A: Receiver operator curve analysis for NT-proBNP in diastolic heart failure (DHF). Observed area under the curve (AUC) for DHF using clinical covariates plus NT-proBNP = 0.71 [0.63, 0.79].
Panel B: The ROC curves generated from 30 random simulated simulations of a 5-fold split of the data are presented. The cross-validated ROC curves are similar in shape to those obtained in Panel A.