Abstract
Moringa (Moringa oleifera Lam.) is an edible plant used as food and medicine throughout the tropics. A moringa concentrate (MC) made by extracting fresh leaves with water utilized naturally occurring myrosinase to convert four moringa glucosinolates (1–4) into moringa isothiocyanates (5–8). Optimum conditions maximizing MC yield, compound 5 (4-[(α-L-rhamnosyloxy)benzyl]isothiocyanate), and compound 8 (4-[(4’-O-acetyl-α-L-rhamnosyloxy)benzyl]isothiocyanate) content were established (1:5 fresh leaf weight to water ratio at room temperature). The optimized MC contained 1.66% isothiocyanates and 3.82% total polyphenols. Compound 8 exhibited 80% stability at 37 °C for 30 days. MC, 5, and 8 significantly decreased gene expression and production of inflammatory markers in RAW macrophages. Specifically, 5 and 8 attenuated expression of iNOS and IL-1β and production of nitric oxide and TNFβ at 1 and 5 µM. Our results suggest a potential for stable and concentrated moringa isothiocyanates (5–8), delivered in MC as a food-grade product, to alleviate low-grade inflammation associated with chronic diseases.
Keywords: Moringa oleifera, Moringaceae, chronic inflammation, isothiocyanates, 4-[(α-L-rhamnosyloxy)benzyl]isothiocyanate, 4-[(4'-O-acetyl-α-L-rhamnosyloxy)benzyl]isothiocyanate
1. Introduction
Moringa (Moringa oleifera Lam.) is a fast growing tropical tree known as the “drumstick” or “horseradish tree.” It is one of 13 species in the monogenic family, Moringaceae, within the order Brassicales, to which broccoli and the other cruciferous vegetables belong. Moringa leaves are historically used as nutritious foods and traditional medicine in Asia and Africa. Elevated nutrient content in their leaves can partly be attributed to the relatively low moisture content (ca. 76%) of fresh leaves compared with ca. 90% moisture content of most vegetables. Moringa leaves contain approximately 27% protein by dry weight, and all essential amino acids. In addition, they contain high levels of vitamins and beneficial phytoactives (Pandey et al., 2012). The latter include polyphenols and, most interestingly, four unique sugar-modified aromatic glucosinolates (1–4) (Bennett et al., 2003). In both Brassicaceae and Moringaceae, isothiocyanates (ITCs) are formed from their glycosylated precursors, glucosinolates (GLSs), via a reaction carried out by myrosinase (thioglucoside glucohydrolase), an enzyme activated during plant tissue wounding or digestion. Myrosinase cleaves the thio-linked glucose in the GLS, leaving the aglycone which rearranges quickly to form the active ITC.
Despite well documented health benefits of ITCs from crucifers, such as sulforaphane (SF) from broccoli and phenethyl isothiocyanate from winter cress on inflammation and cancer, their clinical and dietary use is somewhat restricted because of their inherent chemical instability. For example SF, formed from broccoli glucoraphanin, its GLS precursor, is rapidly converted to several degradation products, mainly dimethyl disulfide and S-methyl methylthiosulfinate, making it difficult to formulate and deliver by means other than eating fresh vegetables (Franklin et al., 2013). Consuming ITCs from crucifers in their non-active, but more stable, precursor form as GLSs remains an option. However, GLSs undergo an uncertain and variable degree of enzymatic conversion to ITCs by host gut microbiota (Traka & Mithen, 2009).
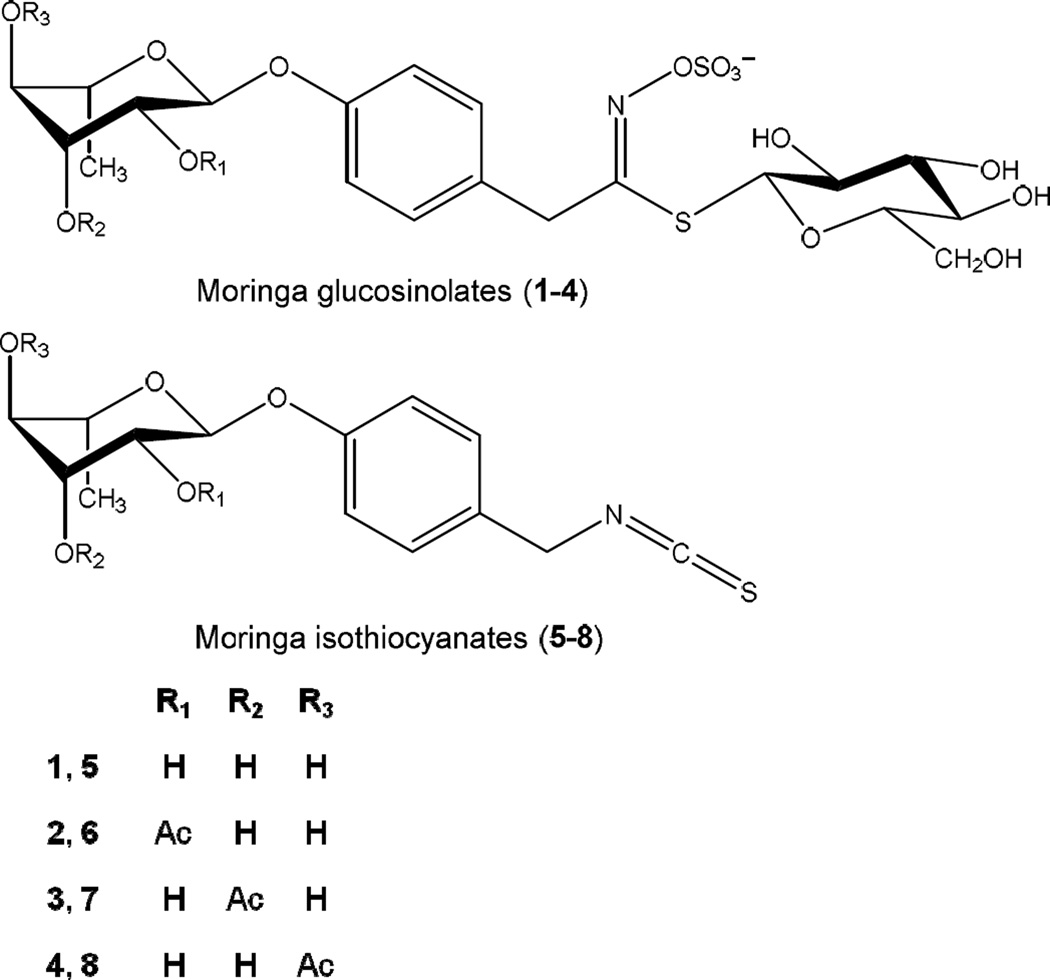
In contrast to crucifers, moringa GLSs (1–4) (Fig. 1) contain an additional sugar moiety in the aglycone/ITC portion of the molecule. They can be converted in situ to four bioactive and relatively stable moringa ITCs (5–8) (Fig. 1). Of these, compound 5 (4-[(α-L-rhamnosyloxy)benzyl]isothiocyanate) and compound 8 (4-[(4’-O-acetyl-α-L-rhamnosyloxy)benzyl]isothiocyanate) are the most abundant isothiocyanates formed from GLS 1 and 4, usually making up over 95% of the total ITCs present. In contrast, compound 6 (4-[(2’-O-acetyl-α-L-rhamnosyloxy)benzyl]isothiocyanate) and 7 (4-[(3’-O-acetyl-α-L-rhamnosyloxy)benzyl]isothiocyanate) are only formed in small quantities from trace amounts of their respective GLSs precursors, compounds 2 and 3 (Amaglo et al., 2010; Bennett et al., 2003). Our optimized moringa concentrate extract (MC) contained 1.15% of 5, 0.51% of 8 and approximately 0.06% of 6 and 7 combined. The moringa ITCs are solid and relatively stable compounds at room temperature, in contrast to volatile ITCs from crucifers that are mostly viscous liquids. The retained rhamnose sugar moiety found in moringa ITCs is extremely unique in nature and likely responsible for their high stability and solid appearance (Brunelli et al., 2010). Previous research with moringa extracts has predominantly utilized commercially available dried leaf powder for experimentation. This powder usually contains much lower levels of ITCs (5–8) due to the destruction of myrosinase in the drying process. Preparing a MC extract with high ITCs (5–8) content takes advantage of endogenous myrosinase in fresh moringa leaves to convert GLSs (1–4) to ITCs (5–8); making MC a useful vehicle for delivering these compounds in the human diet.
Fig. 1.
Chemical structures of moringa glucosinolates (GLSs) 1–4 and isothiocyanates (ITCs) 5–8. Molecular masses: 1 = 570, monoacetylated 2–4 = 612, 5 = 353, monoacetylated 6–8 = 311.
Moringa has been used medicinally throughout the centuries to treat a multitude of acute and chronic conditions. In vitro and in vivo studies with the plant have suggested its effectiveness in treating conditions including inflammation, hyperglycemia, and hyperlipidemia (Bennett et al., 2003; Mbikay, 2012; Fahey, 2005). Moringa’s therapeutic effects were linked to the antiinflammatory, antibacterial and antioxidant properties of its phytochemicals, such as flavonols and phenolic acids (Mbikay, 2012). However, there has been minimal effort focused on the therapeutic activity of GLSs and ITCs present in moringa, even though ITCs from crucifers are some of the most well researched phytoactive therapeutics in human health.
We are particularly interested in the activity of MC and ITCs related to type 2 diabetes mellitus (T2DM) and other chronic conditions associated with metabolic syndrome (MetS). These conditions pose serious and growing health concerns worldwide (Alberti et al., 2009). Effective approaches to combat MetS/T2DM are to maintain a healthy diet and exercise regime. Unfortunately, rates of obesity and T2DM, especially among children, continue to grow. Genetic, social, and economic factors have certainly influenced the epidemic (Wang & Beydoun, 2007). Identifying foods which can help prevent and mitigate manifestations of these conditions is of great interest. Recently, the search for food components that regulate the inflammatory response has attracted particular attention due to evidence linking chronic low-grade inflammation to insulin resistance and obesity. Several key biomarkers of inflammation have been identified as hallmark signs of the pro-inflammatory response found in obesity-induced diabetes. These include cytokines: tumor necrosis factor alpha (TNFα), interleukin-1 beta (IL-1P), interleukin-6 (IL-6), as well as inducible nitric oxide synthase (iNOS), and nitric oxide (NO), an important cellular signaling molecule in insulin signaling catalyzed by iNOS (Bhargava & Lee, 2012; Ferrante, 2007; Xu et al., 2003). iNOS expression and NO overproduction have been implicated in the pathogenesis of disease states, particularly associated with chronic inflammation (Hobbs, Higgs, & Moncada, 1999). TNFα has been shown to directly interfere with insulin signaling (Hotamisligil, Murray, Choy, & Spiegelman, 1994). Interestingly, deregulated overexpression of TNFα (Mocellin & Nitti, 2008) and other inflammatory regulators (IL-1β, IL-6, C-reactive protein (CRP), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), peroxisome proliferator-activated receptor (PPAR), and cyclooxygenase-2 (COX-2)) have been connected to both metabolic syndrome and cancer development (Bao et al., 2011; Mirza et al., 2012). For example SF inhibits NF-κB transcription in many cancer models (Srivastava & Singh, 2004; Xu C, 2005). Activation of NF-κB, an upstream regulator of many inflammatory cytokines, has also been well linked to insulin resistance and diabetes (Mariappan et al., 2010).
Studies of moringa ITCs (5–8) show their pharmacological similarity to well-studied crucifer ITCs. For example, compound 5 was a stronger inhibitor of NF-κB expression and myeloma growth in nude mice than SF (Brunelli et al., 2010). Compounds 5–8 also reduced NO formation at low micromolar concentrations in macrophages (Cheenpracha et al., 2010). Isothiocyanate 6 specifically attenuated NO formation more effectively than SF and benzyl isothiocyanate (Park et al., 2011). However, these publications only examined the activity of purified moringa ITCs. This study describes a simple reagent-free method for bioconversion of moringa GLSs (1–4) into ITCs (5–8) and preparation of a stable isothiocyanate-enriched, food-grade extract from moringa leaves (MC). Additionally evidence of in vitro anti-inflammatory activity of MC, 5, and 8 is presented.
2. Results and Discussion
2.1. Optimization of extract
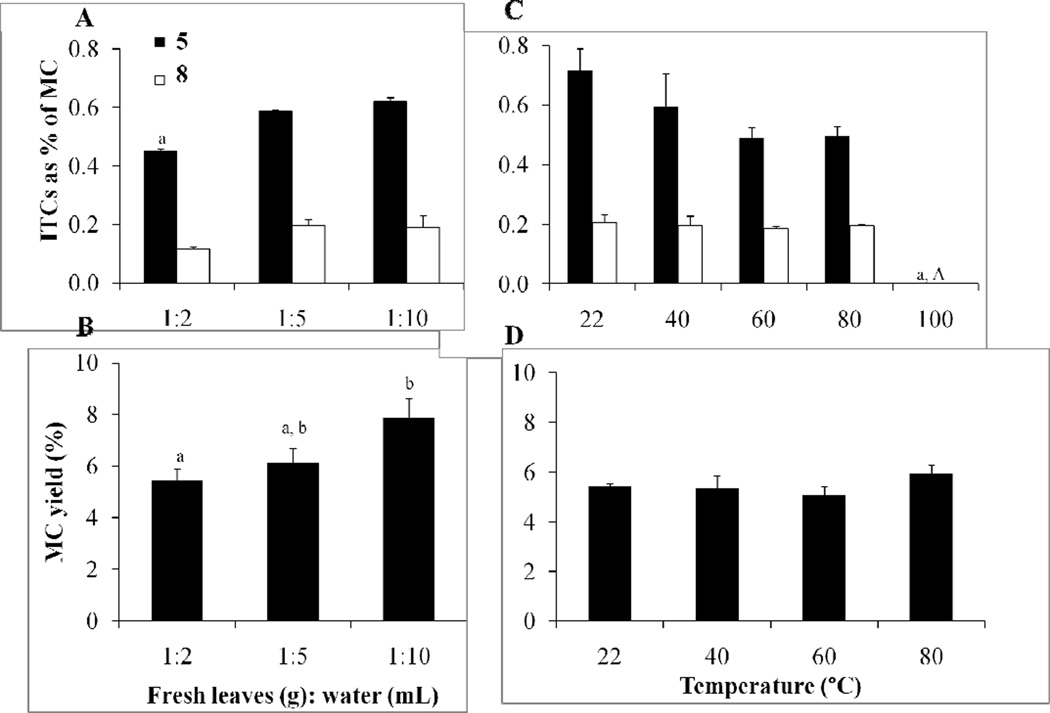
Experiments were performed to optimize in situ biotransformation of moringa GLSs (1–4) into ITCs (5–8) by myrosinase and to maximize the yield of MC from fresh leaves. The solvent ratio (weight of fresh leaves to volume of water) and temperature (22 to 100 °C) were tested to determine the optimal conditions for MC yield and isothiocyanates (determined by quantifying 5 and 8). The solvent ratio affected both the concentrations of ITCs 5–8 and the percent yield (Fig. 2A & 2B). The 1:2 solvent ratio resulted in a lower average of 5 content (0.45% of MC) compared with the 1:5 and 1:10 dilution, (0.59% and 0.62% of MC, respectively). The amount of 8 was higher in the 1:5 and 1:10 dilutions, but not statiscally significant (0.12% of MC compared with 0.20% and 0.19%, respectivly). Larger dilutions resulted in a proportional percent yield increase of MC: (1:2) 5.47%, (1:5) 6.13%, (1:10) 7.87%. The 1:5 dilution factor was selected as optimum to maximize the amount of compounds 5–8 captured in MC, while minimizing the amount of water used for extraction.
Fig. 2.
Effect of dilution factor and temperature on concentration of 5 and 8 and percent yield of MC. A. Effect of dilution ratio of fresh leaves (g):H2O (mL) on ITC concentration (mg of ITC/100 mg of MC). B. Effect of dilution ratio on MC percent yield (mg of MC/100 mg of fresh leaves). All extractions in A and B were performed at room temperature (22 °C). C. Effect of temperature on ITC concentration (mg of ITC/100 mg of MC). D. Effect of temperature on MC percent yield (mg of MC/100 mg of fresh leaves). All extractions in C and D were performed in a dilution ratio of 1:5. Graphs represent the mean ± SEM (n = 3). Comparisons were made by a 1-way ANOVA followed by Tukey’s posthoc test. Significant differences (p < 0.05) between sample sets are signified by letters; different letters indicate significant difference between sample sets, while the same letter or absence of a letter indicates no difference.
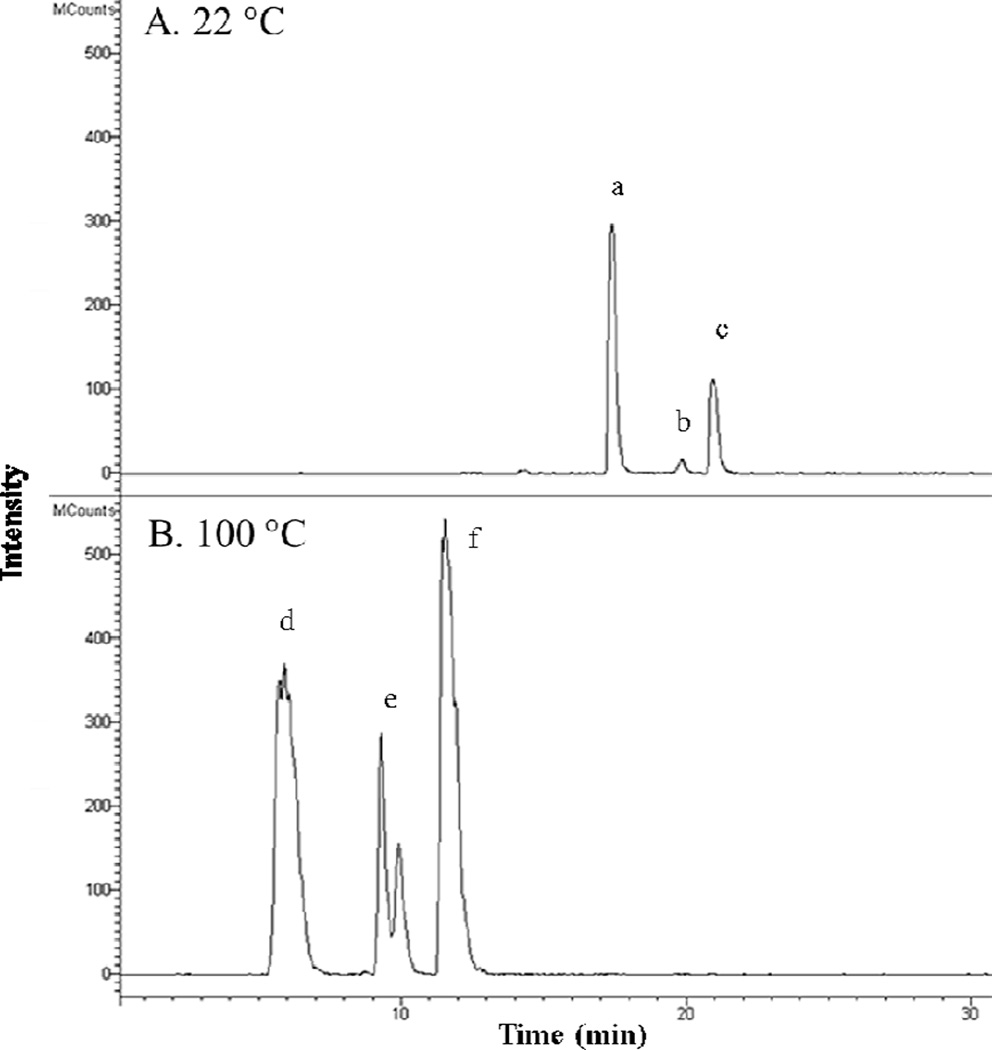
The 1:5 solvent ratio was used to evalute the effect of water temperature on ITC concentration and percent yield. There was a significant difference in the amount of ITCs (5–8) extracted at water temperatures between 22 to 80 °C (5: 0.49 to 0.72% of MC, 8: 0.19 to 0.21% of MC) with undetectable amounts at 100 °C (Fig. 2C). The LCMS mass chromatogram of both GLSs (1–4) and ITCs (5–8) in a 22 °C and 100 °C extraction shows the heat sensitivity of myrosinase activity at high temperatures (Fig. 3). At 22 °C, myrosinase converted GLSs (1–4) to ITCs (5–8) by thioglucose cleavage. At 100 °C, myrosinase was inactivated and GLSs were not converted to their respective ITCs. Although the isoform of the myrosinase enzyme in moringa may be different than the form in broccoli, the thermal stability of moringa myrosinase is similar to broccoli myrosinase, with a reported thermal stability at 50–60 °C (Eylen, Oey, Hendrickx, & Loey, 2008) and complete destruction of the enzyme above 80 °C (Gallaher, Gallaher, & Peterson, 2012). Extraction with the 1:5 solvent ratio at room temperature (22 °C) was adopted to maintain full enzymatic conversion of GLSs into ITCs.
Fig. 3.
Mass chromatogram of GLSs and ITCs after extraction at 22 °C (A) and 100 °C (B). m/z ions for 1 (570) [M-H]−, 2, 3, 4 (612) [M-H]−, 5 (370) [M (311) + C2H4O2 (60)-H]− and 6, 7, 8 (420) [M (311) + C2H4O2 (60)-H]− were selected for in both figures. A. MC prepared at 22 °C (0.1 mg MC injected) showing the presence of: a (5), b (overlapping peaks of 6 & 7) and c (8) and the absence of any ion trace for GLSs. B. MC prepared at 100 °C (0.6 mg MC injected) showing the presence of d (1), e (overlapping peaks of 2 & 3), and f (4), and the absence of any ion trace for ITCs.
Once MC preparation had been optimized over temperature and solvent ratio, larger scale production of MC required the use of a blender instead of a coffee grinder. This unintended parameter of scaling up the extraction resulted in a significant increase in ITC content. Under the same conditions, 1:5 solvent ratio at 22 °C, the coffee grinder produced a lower amount of 5 and 8, approximately 1.00% total in MC while the blender increased the concentration of total ITCs to 1.66% (1.15% 5 and 0.51% 8) of MC. This was likely due to finer crushing of the leaves and the presence of water at the time of blending rather than grinding prior to combining with water in the case of the coffee grinder. Preparation of MC using the blender was performed with three separate batches of moringa leaves and subjected to ITC quantification by LCMS to ensure reproducibility of the extraction method. The content of ITCs in MC (1.66%) is approximately 3 times higher than the SF content delivered in broccoli sprouts (calculated using a reported 61% conversion rate of glucoraphanin to SF and converted to dry weight factoring 89% moisture content (Force, O’Hare, Wong, & Irving, 2007; Pereira et al., 2002; Song & Thornalley, 2007)). MC, prepared in large batches, was subsequently evaluated for chemical stability of ITCs, total polyphenol content, oxygen radical absorbance capacity and anti-inflammatory activity in vitro.
2.2. Stability of compounds
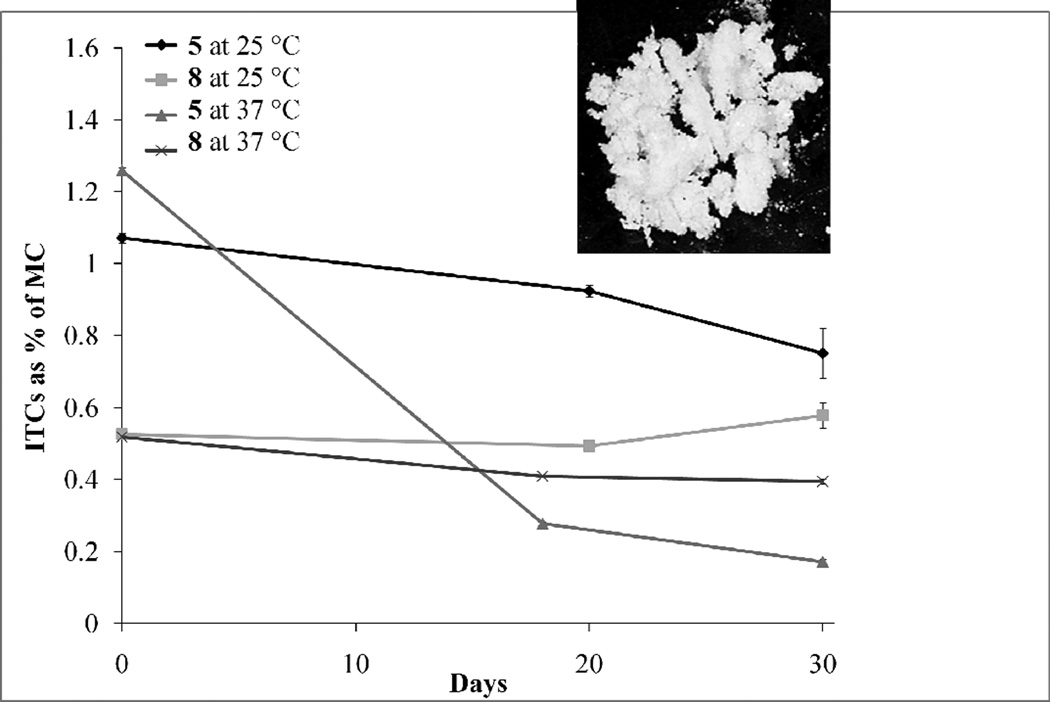
When MC was stored at room temperature, 5 and 8 exhibited exceptional stability of 70% and 100% respectively, over 30 days. The accelerated stability studies of MC at 37 °C for 30 days showed approximately 80% and 20% degradation of 5 and 8 respectively (Fig. 4). The higher stability of 8 is possibly due to the monoacetylation at the 4' position of the rhamnose sugar. Greater acetylation is well known to increase the stability of glycosylated molecules such as anthocyanins (Giusti & Wrolstad, 2003). Both 5 and 8, demonstrated superior stability compared to reported values of SF, the main broccoli isothiocyanate, which has been reported to degrade by 75% after 6 days at 37 °C (Franklin et al., 2013). SF is a volatile, viscous liquid, whereas moringa ITCs, purified by HPLC, appear as solids at room temperature (Fig. 4 insert shows >98% pure 8 as a solid material). This is likely due to the higher molecular weight and unique rhamnose substitution compared with other isothiocyanates.
Fig. 4.
Effect of storage of MC at 25 °C and 37 °C on ITC stability and photograph of purified 8 at 10X magnification (insert). Each bar represents the mean ± SEM (n = 3).
2.3. Total polyphenol content
Total polyphenol (TP) content of MC, determined by the Folin–Ciocalteu method (Singleton & Rossi, 1965) was 3.82 mg of gallic acid equivalents /100 mg of MC (± 0.22); which is similar to reported TP of dried moringa leaves (3.6 to 4.5% DW) (Sreelatha & Padma, 2009). This suggests that the aqueous MC extraction we developed captured the majority of polyphenols present in fresh leaves. Predominant polyphenols identified in moringa include rutin, chlorogenic acid, and quercetin-malonyl-glucoside (Amaglo et al., 2010; Bennett et al., 2003). The molecular weights of these compounds were detected in our LCMS analysis of MC, but quantification of specific polyphenols was not performed.
2.4. Oxygen radical absorbance capacity
The oxygen radical absorbance capacity (ORAC) of MC was 3.6 mmol Trolox equivalents (TE)/g of MC (±0.69 SEM), suggesting it has direct antioxidant potential. The ORAC of MC is greater than reported values on spices with high ORAC, such as dried cinnamon powder (2.6 mmol TE/g) (Wu et al., 2004). Fresh and dried moringa leaf extracts were previously reported to contain high levels of antioxidant compounds including phenolics, flavonols, carotenoids and ascorbic acid (Siddhuraju & Becker, 2003; Dillard & German, 2000). Antioxidants in various moringa leaf extracts (total polyphenols, total flavonoids) have been shown in vitro to possess free radical scavenging activity and ferric reducing power (Vongsak et al., 2012). Reported ORAC data do not preclude the possibility of indirect antioxidant activity of polyphenols and moringa ITCs in MC. Flavonoids and isothiocyanates have been well documented for their indirect antioxidant activity by activating the Keap1/Nrf2/ARE pathway and inducing production of protective phase 2 enzymes (Dinkova-Kostova & Talalay, 2008; Tsuji et al., 2013). The direct, and possible indirect, antioxidant capacities of secondary metabolites present in MC may contribute to the observed reduction of pro-inflammatory markers in our in vitro studies (see below).
2.5. Anti-inflammatory activity
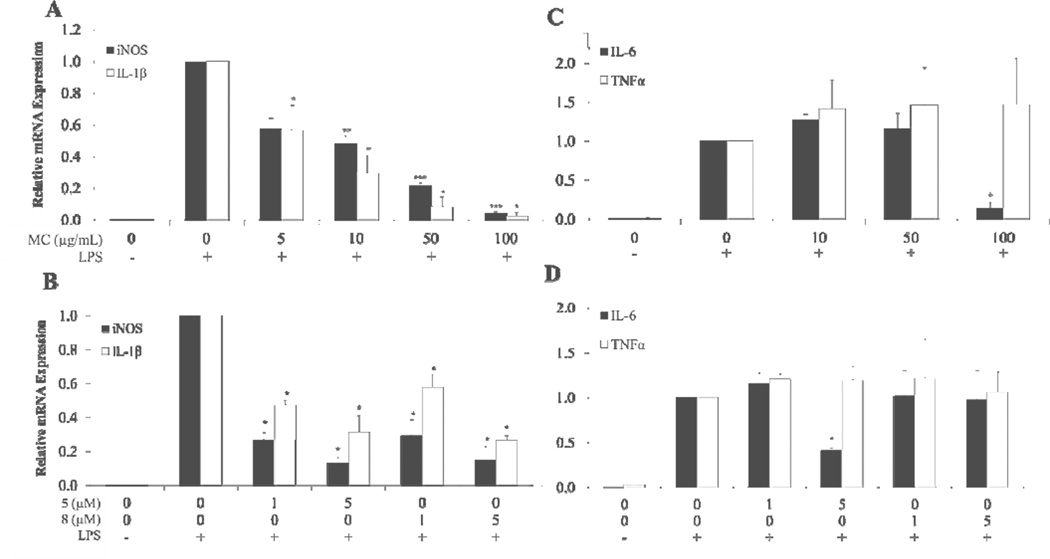
MC demonstrated a dose dependent inhibitory effect on iNOS and IL-1β gene expression (Fig. 5A). Tested concentrations of MC ranged from 5 to 100 µg/mL (0.08% to 1.66% total ITC content). The molar concentration of total ITCs in MC at the various doses ranged from approximately 0.28 µM to 5.1 µM. Almost complete suppression of iNOS and IL-1β gene expression was observed at 100 µg/mL of MC (3.7 µM 5 and 1.4 µM 8).
Fig. 5.
Anti-inflammatory effects of MC, 5 and 8 on LPS-induced iNOS, IL-1β IL-6 and TNFα gene expression in RAW 264.7 macrophage cells. Cells were pretreated for 2 h with MC, 5, or 8 and then induced with LPS for 6 h. Values show relative gene expression compared to vehicle with LPS control determined by comparative ΔΔCt analysis. A: Effect of MC on iNOS and IL-1β B: Effect of 5 and 8 on iNOS and IL-1β C: Effect of MC on IL-6 and TNF±. D: Effect of 5 and 8 on IL-6 and TNFα. Each bar represents the mean ± SEM (n = 4), except for TNFα in D where n = 2. *** = p < 0.001, ** = p < 0.01, * = p < 0.05. Comparisons to controls were made by Dunnett’s test for iNOS measurements or Wilcoxon’s test in all other experiments.
Compounds 5 and 8, tested at 1 and 5 µM, also showed significant reduction of mRNA expression of iNOS and IL-1β (Fig. 5B). These concentrations were much lower than effective concentrations reported for polyphenols, such as quercetin (100 µM), required to inhibit iNOS gene expression in a similar experimental design (Wadsworth & Koop, 1999). Additionally, MC at 100 µg/mL (Fig. 5C) and 5 at 5 µM (Fig. 5D) were able to decrease IL-6 gene expression significantly. However, no reduction of TNFa gene expression was seen at any of the concentrations of MC and ITCs and tested.
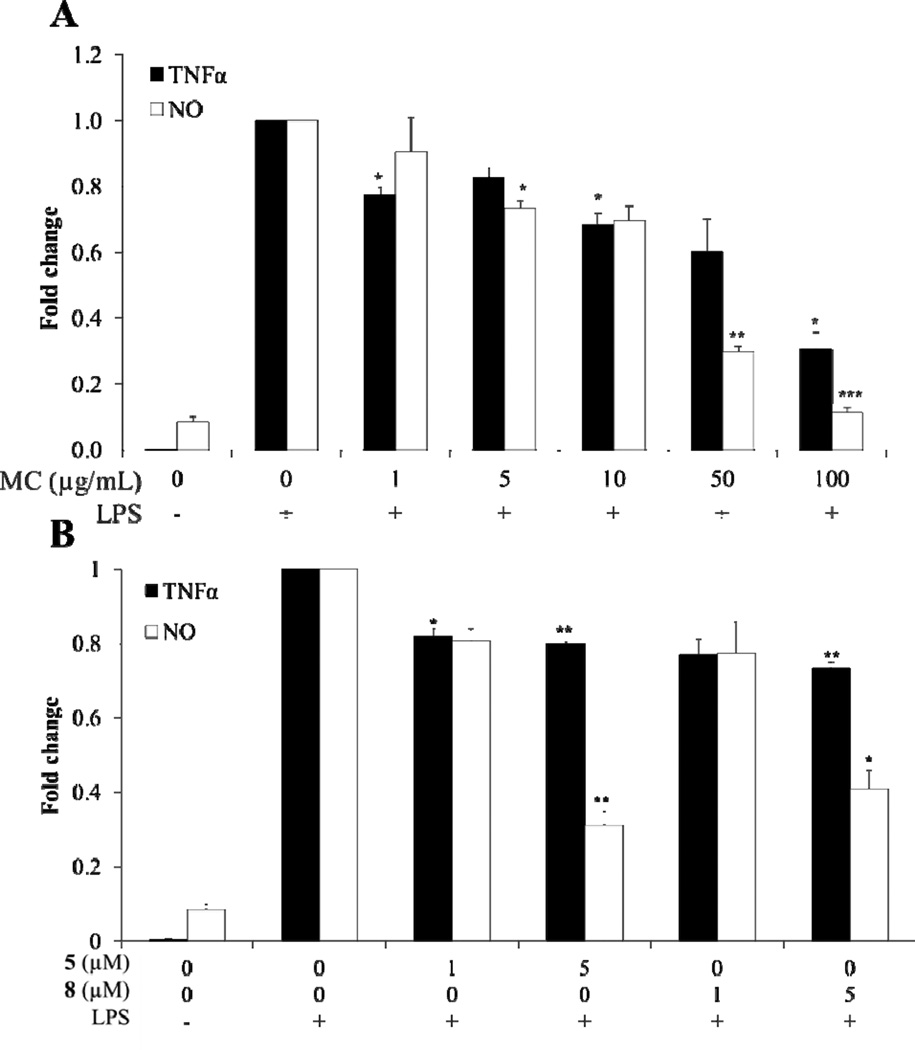
NO and TNF± cytokine production were reduced by MC, 5 and 8 (Fig. 6A & B). MC at 100 µg/mL, containing 1.15% 5 and 0.51% 8, inhibited TNF± production by 70% compared to the control. Compounds 5 and 8 at 5 µM reduced TNF± production by 20% and 27%, respectively. The enhanced anti-inflammatory activity of MC compared with ITCs alone needs further investigation. Improved reduction of TNFα by MC may be explained by the presence of polyphenols in combination with compounds 5–8, including less abundant, but perhaps, highly active 6 and 7. The stability and bioaccessibility of bioactive compounds may also be improved when delivered in a complex mixture. These results demonstrate the plausible advantage of delivering compounds 5–8 in a food-grade product, such as MC. TNFα mRNA expression was not significantly inhibited by MC, 5, or 8, suggesting that moringa phytochemicals may inhibit TNF± production at the translational level or at the level of TNFα turnover.
Fig. 6.
Anti-inflammatory effect of MC, 5, or 8 on LPS-induced NO and TNFα protein production in RAW 264.7 macrophage cells. Cells were pretreated for 2 h with MC or ITCs and then induced with LPS for 6 h. Values show relative fold change in production of NO or TNFα protein measured by the Greiss method and ELISA, respectively. A: Effect of MC on NO and TNF± protein production. B: Effect of ITCs on NO and TNF± protein production. Each bar represents the mean ± SEM (n = 3). *** = p < 0.001, ** = p < 0.01, * = p < 0.05. Comparisons to vehicle with LPS controls were made by a Dunnett’s test.
MC, 5, and 8 inhibited the production of NO significantly (Fig. 6 A & B). This is consistent with previously reported NO inhibition by 5 and 8 (IC50 of 14.43 and 2.71 µM, respectively) (Cheenpracha et al., 2010). MC, at 100 µg/mL, was able to inhibit NO formation by 90%. ITCs 5 and 8 are at least partially responsible for this effect, because they inhibited NO formation at 5 µM by 69% and 39%, respectively. Reduced iNOS expression and NO production by inducers of the phase 2 response, such as the isothiocyanate SF, have been correlated to suppression of inflammation (Liu, Dinkova-Kostova, & Talalay, 2008). The improved reduction by MC treatment over individual ITCs was also evident in NO production, but to a lesser degree than observed in the TNFa production experiments. Again, this may be explained by the trace amounts of 6 and 7, approximately 0.06% of MC. These two compounds have been reported to inhibit NO formation at low micromolar concentrations (IC50 of 1.67 µM and 2.66 µM, respectively (Cheenpracha et al., 2010)). MC, 5, and 8 showed no signs of cytotoxicity at the concentrations tested in anti-inflammatory assays, as demonstrated in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-based cell viability assays (data not shown).
3. Conclusion
A rapid and efficient method was developed for bioconversion of moringa GLSs into ITCs and optimization of conditions for producing an aqueous moringa leaf concentrate (MC) containing 1.15% of 5 and 0.51% of 8. The ORAC value for MC was relatively high, even when compared to spices such as oregano, thyme, and cinnamon, often considered gold standards as direct antioxidants. MC, 5, and 8 demonstrated anti-inflammatory activities at the gene level using an in vitro murine neoplastic mononuclear cell model of lipopolysaccharide (LPS)-induced inflammation. 5 and 8 were isolated from MC and shown to be relatively stable, solid compounds, likely due to the presence of rhamnose residues. Moreover, 8 demonstrated high stability when compared to volatile isothiocyanates from Brassicaceae. This stability presumably arises from the monoacetylation at the 4' position of the rhamnose sugar. The mediation of selected anti-inflammatory biomarkers by MC, and its stable ITC constituents, provides preliminary support for the use of MC as a food ingredient in the prevention and treatment of conditions associated with chronic inflammation. Moringa ITCs (5–8) appear to have similar pharmacological potential as well-known ITCs from crucifers. However, moringa ITCs (5–8) offer two key advantages over cruciferous ITCs such as SF. Firstly, moringa ITCs (5–8) are concentrated in MC to much higher concentrations than other ITCs in foods such as SF in broccoli sprouts. Secondly, solid ITCs possess superior chemical stability and shelf life than liquid and volatile ITCs from crucifers.
4. Experimental
4.1. Plant material and sample preparation
Fresh leaves and seeds from M. oleifera (Indian PKM-1 variety) were shipped overnight from Moringa Farms, Sherman Oaks, CA. The leaves were extarcted on the day of arrival to produce MC. Moringa seeds were cultivated at Rutgers University greenhouse until the plants flowered. A voucher specimen (CW1) was prepared and deposited at the Chrysler Herbarium of Rutgers University (CHRB).
Fresh M. oleifera leaves were blended (Vitamix 5200 Blender, Cleveland, OH) thoroughly with room temperature Millipore H2O in a ratio of leaves (1 g) to H2O (5 mL) for batch reproducibility of MC used in stability tests and all biological assays. Micro preparation of MC for temperature/dilution optimization was performed by grinding fresh leaves in a coffee grinder (Krups, Millville, NJ) and then placing them in H2O. Leaf extracts (either prepared with the blender or coffee grinder) were placed on a shaker (Lab Line Orbit Shaker, Kochi, India) for 30 min at room temperature. In temperature experiments, the extracts were placed in water baths at designated temperatures for 30 min. Following incubation, the extracts were filtered through Miracloth (Calibiochem, Billerica, MA) and centrifuged for 10 min at 3200 g and 4 °C. The supernatants, which appeared as transparent brown “teas,” were individually decanted and lyophilized to produce MCs. In some cases, particularly with larger batches, centrifugation was repeated in order to clear all solid materials from the supernatant.
4.2. Extraction and isolation
Compounds 5 and 8 were extracted from fresh moringa leaves using a modified approach to previously published methods (Cheenpracha et al., 2010). Briefly, 5 and 8 were initially extracted from ground leaves (200 g) in 100% MeOH (400 mL) for 4 hours at room temperature. The MeOH extract was evaporated to dryness under vacuum and partitioned in H2O and hexanes (1:1 v/v) three times with 200 mL of each solvent. Equal volumes of EtOAc were then added to the H2O fraction and partitioned. The EtOAc fraction was dried down and resuspended in CH3CN-H2O (1:1), sonicated briefly, and filtered through a 0.45 µm filter prior to purification by preparative high-performance liquid chromatography (HPLC).
Replicate HPLC injections of an aliquot of the EtOAc fraction (100 µL, 200 mg/mL) were eluted with CH3CN-H2O-TFA (50:50:0.05) to yield 5 (Rt = 8.2 min) and 8 (Rt = 17.5 min). The eluent of HPLC solvent at these Rt was collected and dried initially by rotary-evaporation, followed by lyophilization. The compounds appeared as white solids. Compound 8 was scraped and placed on a petri dish for imaging at 10X magnification (Fig 4 insert). The chemical purity of isolated ITCs was confirmed by liquid chromatography mass spectrometry (LCMS) and 1H NMR. Reversed phase HPLC was carried out on a Waters System consisting of a four channel Waters 616 pump with semi-preparative pump heads operated by a Waters 600 Controller; Waters 490E Programmable Multiwavelength Detector set to monitor at 222 nm; and Waters 717 Plus Autosampler. Compounds were separated on a Phenomenex semi-preparative Synergi Hydro column (4 µM, 250 × 20 mm, 80 Å) with a mobile phase flow rate of 10 mL/min.
4.3. Compound quantification
UV peak area of LCMS injections of 5 and 8 (> 98% purity) at 3 concentrations (3x) were averaged and used to generate standard curves to quantify ITC content in MC preparations. 1 µL injections (3x) of 5 at 20, 100, and 200 ng/µL dissolved in CH3CN-H2O (1:1) generated a standard curve (y = 123x−0.098, R2 = 1) and 8 at 10, 50, and 100 ng/µL generated a standard curve (y = 104.32x−0.098, R2 > 0.99). The content of compounds 6 and 7 were estimated by using the standard curve of 8, but was not included when total ITC content was calculated.
LCMS analysis was performed using the Dionex® UltiMate 3000 RSLC ultra-high pressure liquid chromatography system, consisting of a workstation with Dionex® Chromeleon v. 6.8 software package, solvent rack/degasser SRD-3400, pulseless chromatography pump HPG-3400RS, autosampler WPS-3000RS, column compartment TCC-3000RS, and photodiode array detector DAD-3000RS. After the photodiode array detector, the eluent flow was guided to a Varian 1200L (Varian Inc., Palo Alto, CA) triple quadrupole mass detector with electrospray ionization interface, operating in the negative ionization mode. The voltage was adjusted to −4.5 kV, heated capillary temperature is 280oC, and sheath gas (zero grade compressed air) for the negative ionization mode. The mass detector was used in scanning mode from 65 to 1500 atomic mass units. Data from the Varian 1200L mass detector was collected, compiled and analyzed using Varian’s MS Workstation, v. 6.9, SP2. Compounds were separated on a Phenomenex™ C8 reversed phase column, size 150 × 2 mm, particle size 3 µm, pore size 100 Å. The mobile phase consisted of 2 components: Solvent A (0.5% ACS grade AcOH in double distilled de-ionized H2O, pH 3–.5), and Solvent B (100% CH3CN). The initial conditions of the gradient were 95% A and 5% B. The gradient progressed linearly to 5% A and 95% B over 30 min and then remained isocratic for the next 8 min. During the following 4 min, the ratio was brought to initial conditions linearly. An 8 min equilibration interval was included between subsequent injections. 1H NMR spectra were recorded in MeOH-d4 on a 500 Varian VNMRS 500 MHz.
4.4. Optimization and reproducibility of extraction
MC was prepared in ratios of 1:2, 1:5, and 1:10 (g of fresh leaves: mL H2O) for optimization of ITC content and percent yield. Triplicate samples of fresh moringa leaves (8 g) were ground in a coffee grinder, diluted accordingly in H2O at room temperature and mixed for 30 min. For temperature experiments, fresh moringa leaves (8 g) were ground in a coffee grinder and added to H2O (40 mL) at 22, 40, 60, 80, and 100 °C as triplicate samples. The mixtures were maintained at these temperatures for 30 min in hot water baths. Following 30 min of incubation, each MC was prepared as described above. Analysis for percent yield (weight of MC as a percent of starting fresh weight of leaves) and ITC content was determined.
Once the optimum temperature (22 °C) and dilution factor (1:5) were established using micro preparations, larger batches of MC were made using the Vitamix blender (200 g: 1000 mL). Triplicate samples of MC prepared in this manner from 3 separate batches of moringa leaves were compared for reproducibility tests.
4.5. Compound stability
Triplicate samples (100 mg) of optimized MC were placed at room temperature (ca.25 °C ) and in a 37 °C dark incubator and subjected to LCMS analysis for quantification of ITCs at 0, 20 and 30 days for room temperature experiments and 0, 18 and 30 days for 37 °C experiments.
4.6. Characterization of extract
The optimized MC preparation, prepared with 22 °C H2O at a ratio 1:5 (w/v) of fresh leaves to H2O was additionally characterized for TP content and ORAC. TP content was quantified by the Folin–Ciocalteu method (Singleton & Rossi, 1965) and sample absorbance was read at 726 nm against a gallic acid standard curve. ORAC was determined as µM TE using fluorescein as the fluorescent probe and 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH) as a peroxyl radical generator in a procedure adapted from previously published methods (Prior et al., 2003).
4.7. Cell culture
All reagents were supplied from Sigma-Aldrich Co. (St. Louis, MO) unless otherwise noted. RAW 264.7 macrophages (ATCC TIB-71) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Caisson, North Logan, UT) supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% fetal bovine serum. Cells were incubated at 37 °C with 5% CO2 humidified atmosphere and subcultured by cell scraping. For experiments, RAW cells were plated at a density of 4 × 105 cells/mL in 24-well plates. Cells were incubated overnight (18 h), washed with warm PBS, and replaced with fresh DMEM media. Cells were pretreated with designated doses of MC, 5, 8 or vehicle (EtOH-H2O, 1:1, v/v). LPS (1 µg/mL) was added after 2 h incubation with treatments to elicit inflammatory responses. Cells were treated in duplicate or triplicate. After an additional 6 h incubation period, media were collected and then cells were washed with PBS prior to collection in TRIzol® Reagent (Life Technologies, Carlsbad, CA). Samples were stored at −80 °C prior to processing.
4.8. Gene expression analyses
Total RNA was extracted from RAW macrophage cells according to manufacturer’s specifications. Briefly, CHCl3 (200 µL) was added to TRIzol harvested samples (600 µL). Samples were vigorously mixed for 30 s, incubated at room temperature for 5 min, centrifuged at 12,400 g and i-PrOH was added to the aqueous phase to obtain a ratio of 0.7 supernatant to i-PrOH. Samples were mixed by inverting, vortexed briefly and incubated for 10 min at −20 °C. Samples were centrifuged at 12,400 g for 15 min at 4 °C. Next, supernatant was removed and the sample was washed twice with EtOH-H2O (75:25, v/v) and centrifuged at 6000 g for 10 min. Samples were allowed to dry and resuspended in diethylpyrocarbonate (DEPC) treated-H2O. RNA integrity was evaluated by running ca.1 µg of RNA on a 1% agarose gel.
RNA was then treated with Deoxyribonuclease I Amplification grade (Life Technologies), following the manufacturer’s guidelines. RNA quality was checked on the NanoDrop 1000 system (NanoDrop Technologies, Wilmington, DE). A ratio of OD 260/280 ≥ 2.0 and OD 260/230 ≥ 1.8 was considered to be good quality RNA. First strand cDNA synthesis was performed using ABI High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) with RNAse I inhibitor, according to the manufacturer’s instructions using RNA (1 µg). The thermal cycle program was set as follows: 10 min, 25 °C; 60 min, 37 °C; 60 min, 37 °C; 5 s, 85 °C, and final hold at 4 °C.
Synthesized cDNAs were diluted 25 fold and the diluted sample (5 µL) was used for qPCR with Power SYBR Green PCR master mix (12.5 µL, Applied Biosystems), primers (0.5 µL, 6 µM) and Biotechnology Performance Certified (BPC) grade H2O (Sigma) to a final reaction volume (25 µL). Exon-spanning primer sequences were previously designed (Dey et al., 2006) on Primer Express® (Life Technologies) and are as follows: β-actin forward 5'- AAC CGT GAA AAG ATG ACC CAG AT - 3', reverse: 5'- CAC AGC CTG GAT GGC TAC GT-3', IL-1β forward 5'-CAA CCA ACA AGT GAT ATT CTC CAT - 3', reverse 5'- GAT CCA CAC TCT CCA GCT GCA - 3', iNOS forward 5'- CCC TCC TGA TCT TGT GTT GGA - 3', reverse 5'- TCA ACC CGA GCT CCT GGA A-3', COX-2 forward 5' – TGG TGC CTG GTC TGA TGA TG -3', reverse 5'- GTG GTA ACC GCT CAG GTG TTG-3', TNFα forward 5' – TGG GAG TAG ACA AGG TAC AAC CC – 3', reverse 5'- CAT CTT CTC AAA ATT CGA GTG AGA A - 3', IL-6 forward 5' - TCG GAG GCT TAA TTA CAC ATG TTC – 3', reverse 5' TGC CAT TGC ACA ACT CTT TTC T – 3'. All primers were validated by analyzing amplification efficiencies and melt curve profiles.
Quantitative PCR amplifications were performed on an ABI 7300 Real-Time PCR System (Applied Biosystems) with the following thermal cycler profile: 2 min, 50 °C; 10 min, 95 °C; 15 s, 95 °C; 1 min, 60 °C for the dissociation stage; 15 s, 95 °C; 1 min, 60 °C; 15 s, 95 °C. Inflammatory marker mRNA expressions were analyzed by the comparative ΔΔCt method and normalized with respect to the average Ct value of β-actin. Vehicle with LPS treatment served as the calibrator for ΔΔCt analysis and was assigned a value of 1.0. Lower values indicate inhibition of gene expression relative to vehicle treated with LPS control. All experimental samples were run in triplicate and each reaction plate included no template controls.
4.9. TNFα secretion analysis
RAW 264.7 macrophages were cultured, treated with MC or ITCs, and subjected to LPS-induced inflammation as stated above. After treatments, media (1 mL) was collected and immediately centrifuged at 13,500 g and 4 °C for 10 min. The supernatant was preserved at −80 °C until further processing with the BD OptEIA™ Mouse TNF ELISA kit (BD Bioscience, San Jose, CA) following the manufacturer’s protocol. All samples were assayed in duplicate. TNFα levels were quantified using a reference standard curve provided with the kit. Absorbance was read at 450 nm and corrected at 570 nm.
4.10. Nitric oxide production analysis
Collected media, as described above, was assayed in duplicate following the Griess Reagent System provided by Promega (Promega Corporation; Madison, WI). The nitrite standard (0.1 M NaNO2) reference curve was built performing a serial dilution (0 to 100 µM). Absorbance was read at 540 nm.
4.11. Cell viability
Effect of treatments on cell viability was measured using MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide] (TCI, Portland, OR) (Mosmann, 1983). MTT (5 mg/mL) was dissolved in PBS (Cayman Chemical, Ann Arbor, MI), filtered through a 0.22 µm membrane and added to treated cells during the last 3–4 h of treatment. Media were carefully aspirated; cells were dissolved in DMSO and the absorbance was read at 570 nm.
4.12. Statistical analysis
Data were expressed as mean ± SEM. Statistical comparisons for optimization experiments were made by use of 1-way analysis of variance (ANOVA) followed Tukey’s post hoc test in the MC optimization and stability experiments. Statistical comparisons for anti-inflammatory experiments were made by use of ANOVA followed by a Dunnett’s or Wilcoxon test as indicated and p < 0.05 were considered significant. *** = p < 0.001, ** = p < 0.01, * = p < 0.05. For statistical analysis GraphPad Prism version 6.02 for Windows (GraphPad Software, Inc.) was employed.
Highlights.
A moringa concentrate (MC) was made by extracting fresh leaves with water.
Optimized MC contained 1.66% stable moringa isothiocyanates (5–8).
Compound 8 exhibited 80% stability when stored at 37°C for 30 days.
Compounds 5, 8, and MC significantly reduced inflammatory markers in macrophages.
MC is a potential food-grade product to deliver stable and concentrated isothiocyanates.
Acknowledgements
This project was supported by P50AT002776-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS), which funds the Botanical Research Center and Botanical Research Center Pilot Program Sub award 5P50AT002776-08 S12-50318. CW and DC were also supported by NIH training grant T32: 5T32AT004094-04. PRS was supported by the SENESCYT 2011 Fellowship. We would like to thank Rodney Perdew from Moringa Farms, CA for supplying high-quality moringa leaves. The photo of moringa leaves in the graphical abstracts was provided courtesy of ilovemoringa.com. IR and MAL have equity in Nutrasorb LLC, which licensed moringa-related intellectual property from Rutgers University.
Abbreviations
- ARE
Antioxidant response elements
- GLSs
Glucosinolates
- IL-1β
Interleukin-1 beta
- IL-6
Interleukin-6
- iNOS
Inducible nitric oxide synthase
- ITCs
Isothiocyanates
- Keap1
Kelch-like ECH-associated protein 1
- MC
Moringa concentrate
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- Nfr2
Nuclear factor (erythroid-derived 2)-like 2
- NO
Nitric oxide
- SF
Sulforaphane
- TE
Trolox equivalents
- TNFα
Tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WPT, Loria CM, Smith SC. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Amaglo NK, Bennett RN, Lo Curto RB, Rosa EAS, Lo Turco V, Giuffrida A, Curto AL, Crea F, Timpo GM. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem. 2010;122:1047–1054. [Google Scholar]
- Bao B, Wang Z, Li Y, Kong D, Ali S, Banerjee S, Ahmad A, Sarkar FH. The complexities of obesity and diabetes with the development and progression of pancreatic cancer. Biochim. Biophys. Acta - Reviews on Cancer. 2011;1815:135–146. doi: 10.1016/j.bbcan.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RN, Mellon FA, Foidl N, Pratt JH, Dupont MS, Perkins L, Kroon PA. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003;51:3546–3553. doi: 10.1021/jf0211480. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Lee C. Role and function of macrophages in the metabolic syndrome. Biochem. J. 2012;442:253–262. doi: 10.1042/BJ20111708. [DOI] [PubMed] [Google Scholar]
- Brunelli D, Tavecchio M, Falcioni C, Frapolli R, Erba E, Iori R, Rollin P, Barillari J, Manzotti C, Morazzoni P, D’Incalci M. The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo . Biochem. Pharmacol. 2010;79:1141–1148. doi: 10.1016/j.bcp.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Cheenpracha S, Park E-J, Yoshida WY, Barit C, Wall M, Pezzuto JM, Chang LC. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorgan. Med. Chem. 2010;18:6598–6602. doi: 10.1016/j.bmc.2010.03.057. [DOI] [PubMed] [Google Scholar]
- Dey M, Ribnicky D, Kurmukovl AG, Raskin I. In vitro and in vivo antiinflammatory activity of a seed preparation containing phenethylisothiocyanate. J. Pharm. Exp. Ther. 2006;317:326–333. doi: 10.1124/jpet.105.096511. [DOI] [PubMed] [Google Scholar]
- Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health. J. Sci. Food Agric. 2000;80:1744–1756. [Google Scholar]
- Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008;52:S128–S138. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- Eylen DV, Oey I, Hendrickx M, Loey AV. Effects of pressure/temperature treatments on stability and activity of endogenous broccoli (Brassica oleracea L. cv. Italica) myrosinase and on cell permeability. J. Food Eng. 2008;89:178–186. [Google Scholar]
- Fahey JW. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Trees for Life J. 2005;1:5. [Google Scholar]
- Ferrante AW. Obesity-induced inflammation: A metabolic dialogue in the language of inflammation. J. Intern. Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- Force LE, O’Hare TJ, Wong LS, Irving DE. Impact of cold storage on glucosinolate levels in seed-sprouts of broccoli, rocket, white radish and kohl-rabi. Postharvest Bio. Tech. 2007;44:175–178. [Google Scholar]
- Franklin SJ, Dickinson SE, Karlage KL, Bowden GT, Myrdal PB. Stability of sulforaphane for topical formulation. Drug Dev. Ind. Pharm. 2013;0:1–9. doi: 10.3109/03639045.2013.768634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher CM, Gallaher DD, Peterson S. Development and validation of a spectrophotometric method for quantification of total glucosinolates in cruciferous vegetables. J. Agric. Food Chem. 2012;60:1358–1362. doi: 10.1021/jf2041142. [DOI] [PubMed] [Google Scholar]
- Giusti MM, Wrolstad RE. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003;14:217–225. [Google Scholar]
- Hobbs AJ, Higgs A, Moncada S. Inhibition of nitric oxide synthase as a potential therapeutic target. Annu. Rev. Pharmacol. Toxicol. 1999;39:191–220. doi: 10.1146/annurev.pharmtox.39.1.191. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Dinkova-Kostova AT, Talalay P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc. Natl. Acad. Sci. 2008;105:15926–15931. doi: 10.1073/pnas.0808346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J. NF-κB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc. Res. 2010;85:473–483. doi: 10.1093/cvr/cvp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbikay M. Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: A review. Front. Pharmacol. 2012;3:1–12. doi: 10.3389/fphar.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza S, Hossain M, Mathews C, Martinez P, Pino P, Gay JL, Rentfro A, McCormick JB, Fisher-Hoch SP. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: A cross-sectional study. Cytokine. 2012;57:136–142. doi: 10.1016/j.cyto.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocellin S, Nitti D. TNF and cancer: the two sides of the coin. Front. Biosci. 2008;13:2774–2783. doi: 10.2741/2884. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Pandey A, Pandey RV, Tripathi P, Gupta PP, Haider J, Bhatt S, Singh AV. Moringa oleifera Lam. (Sahijan) - A plant with a plethora of diverse therapeutic benefits: an updated retrospection. Medicinal & Aromatic Plants. 2012;1:1–8. [Google Scholar]
- Park EJ, Cheenpracha S, Chang LC, Kondratyuk TP, Pezzuto JM. Inhibition of lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression by 4-[(2’-O-acetyl-a-L-rhamnosyloxy)benzyl]isothiocyanate from Moringa oleifera . Nutr. Cancer. 2011;63:971–982. doi: 10.1080/01635581.2011.589960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FMV, Rosa E, Fahey JW, Stephenson KK, Carvalho R, Aires A. Influence of temperature and ontogeny on the levels of glucosinolates in broccoli (Brassica oleracea Var. italica) sprouts and their effect on the induction of mammalian phase 2 enzymes. J. Agric. Food Chem. 2002;50:6239–6244. doi: 10.1021/jf020309x. [DOI] [PubMed] [Google Scholar]
- Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, Hampsch-Woodill M, Huang D, Ou B, Jacob R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J. Agric. Food Chem. 2003;51:3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Singleton V, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965;16:144–158. [Google Scholar]
- Song L, Thornalley PJ. Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food Chem. Toxicol. 2007;45:216–224. doi: 10.1016/j.fct.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Sreelatha S, Padma PR. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009;64:303–311. doi: 10.1007/s11130-009-0141-0. [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Singh SV. Cell cycle arrest, apoptosis induction and inhibition of nuclear factor kappa B activation in anti-proliferative activity of benzyl isothiocyanate against human pancreatic cancer cells. Carcinogenesis. 2004;25:1701–1709. doi: 10.1093/carcin/bgh179. [DOI] [PubMed] [Google Scholar]
- Traka M, Mithen R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009;8:269–282. [Google Scholar]
- Tsuji PA, Katherine K, Stephenson KK, Wade KL, Liu H, Fahey JW. Structure-activity analysis of flavonoids: direct and indirect antioxidant, and antiinflammatory potencies and toxicities. Nutr. Cancer. 2013;65:1014–1025. doi: 10.1080/01635581.2013.809127. [DOI] [PubMed] [Google Scholar]
- Vongsak B, Sithisarn P, Mangmool S, Thongpraditchote S, Wongkrajang Y, Gritsanapan W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crop. Prod. 2012;44:566–571. [Google Scholar]
- Wadsworth TL, Koop DR. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem. Pharmacol. 1999;57:941–949. doi: 10.1016/s0006-2952(99)00002-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Beydoun MA. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol. Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004;52:4026–4037. doi: 10.1021/jf049696w. [DOI] [PubMed] [Google Scholar]
- Xu SCG, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NFkappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]