Abstract
All terrestrial life is influenced by multi-directional flows of information about its environment, enabling malleable phenotypic change through signals, chemical processes, or various forms of energy that facilitate acclimatization. Billions of biological co-inhabitants of the earth, including all plants and animals, collectively make up a genetic/epigenetic ecosystem by which adaptation/survival (inputs and outputs) are highly interdependent on one another. As an ecosystem, the solar system, rotation of the planets, changes in sunlight, and gravitational pull influence cyclic epigenetic transitions and chromatin remodeling that constitute biological circadian rhythms controlling senescence. In humans, adverse environmental conditions such as poverty, stress, alcohol, malnutrition, exposure to pollutants generated from industrialization, man-made chemicals, and use of synthetic drugs can lead to maladaptive epigenetic-related illnesses with disease-specific genes being atypically activated or silenced. Nutrition and dietary practices are one of the largest facets in epigenetic-related metabolism, where specific “epi-nutrients” can stabilize the genome, given established roles in DNA methylation, histone modification, and chromatin remodeling. Moreover, food-based “epi-bioactive” constituents may reverse maladaptive epigenetic patterns, not only prior to conception and during fetal/early postnatal development but also through adulthood. In summary, in contrast to a static genomic DNA structure, epigenetic changes are potentially reversible, raising the hope for therapeutic and/or dietary interventions that can reverse deleterious epigenetic programing as a means to prevent or treat major illnesses.
Ecological epigenetics
All terrestrial living systems are self-organizing entities subject to diverse environmental influences that mediate transformation through biological signals, chemical processes, or various forms of energy. Diverse plants and animals collectively make up an epigenetically symbiotic ecosystem wherein adaptation/survival (inputs and outputs) are highly interdependent on one another (Latzel et al. 2013; Vandegehuchte and Janssen 2013). Epigenetics is the basis for continual transformation of almost every biological process within the ecosystem required for adaptation to a dynamic environment. The molecular events associated with transformation involve heritable, sustainable, or reversible chemical modifications of the histones that surround DNA, leading to altered tertiary, higher-ordered nucleosomal positions stabilizing either euchromatin (for transcribed genes) or compact heterochromatin (silenced genes). This allows for short-term flexible phenotypic changes to be controlled by the environment without changing the sequence of base pairs within slowly evolving genomic DNA. Analogous epigenetic mechanisms reportedly occur in bacteria, fungi, worms, flies, yeast, plants, algae, fish, mammals, and other biological entities. A combination of slow evolutionary genetic transformation of DNA, concomitant to rapid epigenetic flexibility has enabled terrestrial survival throughout millions of years, despite incredible transitions of atmosphere, where life formed in the absence of oxygen. (Jeltsch 2013)
An epigenetic relay within living systems can be triggered by just about any environmental signal, including UV light (Dong et al. 2012), atmospheric pressure, food supply, plant nutrients, water supply (Gayacharan and Joel 2013), oxygen (Brand and Ratan 2013), chemicals, temperature (Cao et al. 2013), and climatic change. Moreover, in today’s society, there is an ever-growing accumulation of non-natural environmental products derived from man-made ingenuity that contribute largely to the atmosphere around us (Combs-Orme 2013). This is evidenced by the ascent in pollution, changes in climate or in emissions of gas, the accumulation of non-biodegradable materials (Rossi et al. 2013; Vandegehuchte and Janssen 2013) and alterations in the protective ozone layer (Andersen et al. 2013). With man’s domination of earth, we are now entering an unprecedented change within the environmental ecosystem, not only by introduction of synthetic biomass, but also man-made genetic manipulations of meat and plant products, which are modified for superior traits such as to eliminate natural variation in the vitamin content of crops (Miret and Munne-Bosch 2013) or to promote stress-tolerant, disease-resistant transgenic plants. (Bawa and Anilakumar 2013; Kujur et al. 2013)
On a macromolecular scale, the solar system, planetary rotation, and gravitational pull constitute major epigenetic influences on all biological life, manifest as circadian rhythms within every species, tissue organ, and cell (Mitteldorf 2013). Circadian epigenetic phenotype transitions involve timed patterns of DNA methylation or histone modifications of genes associated with reproduction, sleep, and seasonal memory (Satake and Iwasa 2012; Stevenson and Prendergast 2013) and are responsible for the chronology of life (life cycle), a facet still not well understood but likely involving silencing/expression of clock genes such as Bmal1 (Aguilar-Arnal et al. 2013; Eckel-Mahan and Sassone-Corsi 2013). That said, the field of epigenetics requires not only the study of genetics and biochemistry, but also medicine, astronomy, nutrition, and history all of which investigate the transformation and survival of life; past, present, and future.
Evolution: origin of the species
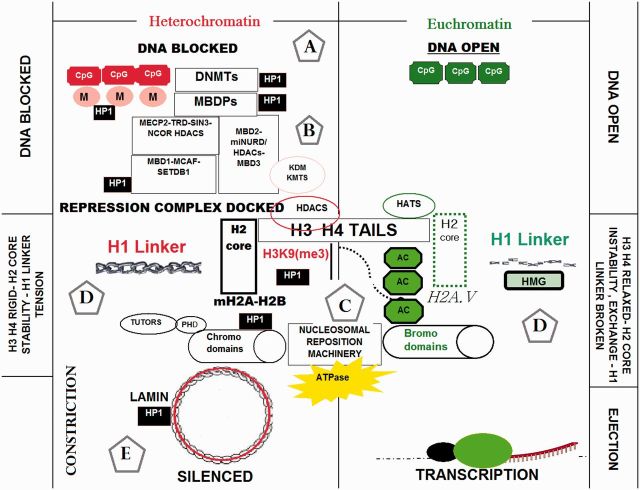
Although scientific advances in epigenetics are recent, natural observation describing the same have been recorded by man throughout history. For example, after Darwin published the literary work entitled “On the Origin of Species” (1859) in which he described natural selection (Crews 2011), he continued to question himself on the unknown elements responsible for superior trait inheritance with changes in the environment. In a hypothetical manifesto on “Pathogenesis,” he proposed that small entities called “gemmules” could communicate information from the environment to an organism, being capable of modification or rearrangement, enabling transfer of superior traits in progeny during embryonic development (Geison 1969). Darwin was also conceptually correct, albeit not current with modern technology or instrumental medical capabilities, when he described the term “gemmules.” These are similar to what is now called DNA, which was not elucidated until the 1950s. Prior to modern technology, Darwin was struggling to conceptualize what could account for rapid heritable phenotypic change in response to the environment. This field of study has matured from obscure definition, with discovery of DNA methylation and histone acetylation in the 1960s to the current molecular sequencing capabilities that have elucidated genomic imprinting, X-chromosome inactivation, exchange of histone octomer cores, histone-tail modifications (e.g., acetylation, methylation, phosphorylation, and biotinylation), chromatin remodeling complexes, high-mobility group proteins, prions, lamins, and non-coding RNAs—all of which contribute to epigenetic controls over phenotype (Mazzio and Soliman 2012). The separation between genomic sequence and epigenetic control originates with methylation of DNA at CpGs, compounded by a complex series of co-coordinated epigenetic proteins, enzymes, repression platforms, nucelosomal remodeling complexes, and dynamic forces that control the flexibility of histones surrounding DNA. The pliability of histones surrounding DNA either results in ejection/expansion where genes are to be transcribed or nucleosomal constriction/mechanical barricade surrounding genes are to be silenced (Fig. 1).
Fig. 1.
Expression of genomic DNA is controlled by heritable epigenetic patterns that result in either transcriptional activated “euchromatin” (right panel) or silenced “heterochromatin” (left panel). [(A) The epigenome diverges from the genome at the level of DNA where CpG sites are methylated by methyltransferases DNMT1, DNMT 3a, and DNMT 3b (which block promoter gene regions to be silenced). (B) Methylated CpGs then attach to methyl-binding proteins (containing methyl-binding domains) “MBDPs” which then recruit diverse transcription repression complexes containing histone modification constriction elements and enzymes such as HDACs and HKMTs. (C) Diverse histone modification enzymes can directly alter histone H3 and H4 tails (tetramers) of the histone unit that corresponds to complementary influences by nucleosomal ATPase reposition machinery containing chromodomains or bromodomains. (D) For gene expression, nucleosomal reposition elements destabilize H2 cores and mobilize nucleosomes through core variant exchange, which initiate nucleosomal ejection, histone H1 linker loss of integrity by several high mobility group proteins (HMG) and a loss of DNA–histone affinity near transcription start sites. In contrast, gene silencing requires H2 core stable variant exchange and recruitment of constrictive nucleosomal remoding complexes, thereby creating mechanical blockage near transcription start sites. (E) Silencing elements are brought together by heterochromatin proteins HP1 α,β which adjoins a number of silencing components in heterochromatin ranging from methylated CpGs, DNMTs, repression complexes such as MBD1-SETDB1-CAF1, silencing marks such as H3K9me3, and nucleosomal reposition machinery. These collective effects reinforce compression of nucleosomes close to the methylated DNA to ensure silencing, when silent heterochromatin is located throughout the lamina circumscribing the nuclear envelope.
In this century, there exists a new paradigm called the DOHaD (developmental origins of adult diseases) hypothesis (de Boo and Harding 2006). The DOHaD hypothesis has similarities to Darwin’s observations, but reflects changes in current scientific understanding and considers the future of scientific applications for human disease. While our historical relatives were focused on ensuring provision of basic foods through hunting and farming, our current generation, having an ample supply of food and modern medicine, can focus on quality of life and on the influence of environment on the risk of disease. The DOHaD hypothesis suggests that negative cues such as stress or malnutrition during fetal development, can alter the outcome of birth and augment the risk of fetal mortality and of morbidities in offspring, such as diabetes, cardiovascular disease, and cancers (Duque-Guimaraes and Ozanne 2013) that affect approximately two-thirds of the world’s population. (James 2006)
The DOHaD hypothesis introduces the concept that the fetus experiences phenotypic epigenetic adjustments based on maternal environmental signals serving to prepare itself for survival in the outside world (de Boo and Harding 2006; Bezek et al. 2008; Brenseke et al. 2013). Likewise, paternal epigenetic transference of environmental information occurs during sperm development from a maturing germ cell to the fertilized zygote carrying forward changes in DNA methylation, histone modification, chromosome organization, and non-coding RNAs (Castillo et al. 2014; DelCurto et al. 2013; Soubry et al. 2014). Subsequently, peri-natal and early post-natal periods constitute one of the most critical windows of opportunity for epigenetic transformation in both the fetus and development of the fetal reproductive system, which affect future progeny (Fig. 2). In human development, epigenetic modification of neurological and cognitive functions are critically important, given an intellectually based environment (Li et al. 2013a) in which learning, behavioral conditioning, language, and capacity for communication are needed for human survival (Schneider et al. 2014). Even the process of language itself is considered an external epigenetic trait of learned behaviors as a part of soft programing within a hard-wired DNA genome (Boeckx and Leivada 2013).
Fig. 2.
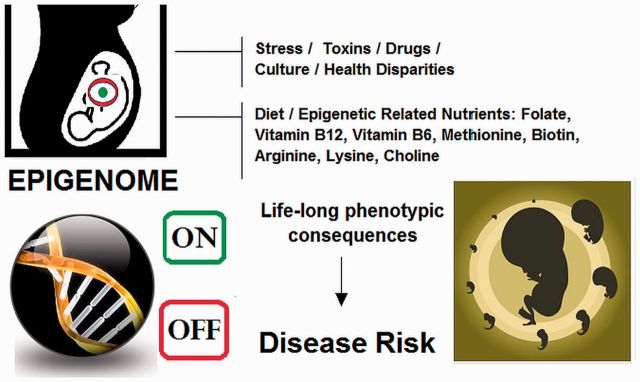
The epigenome is flexible, most impressionable during prenatal and early developmental periods, impacted by environment, replicated through mitosis and can yield long-lasting phenotypic effects that remain stable through adulthood. Adverse impact on epigenetic development can yield maladaptive phenotypic consequences, potentially contributing greater risk of developing adult-onset disease in offspring and subsequent progeny.
From childhood to adulthood, both developmental and learned epigenetic patterns remain etched and stable but are reversible and easily modified by dramatic events and/or epi-modifying chemicals such as 5-aza-cytidine trichostatin A, suberoylanilide (Zolinza), sodium butyrate (Li and Chen 2012; Seo et al. 2013) and epigenetic nutrients which are a diverse class of natural polyphenolics or food-based nutrients that control DNA methylation and histone modifications (Zhang and Chen 2011; Ouedraogo et al. 2011; Park et al. 2012; Henning et al. 2013; Guerrero-Bosagna and Skinner 2014). While epi-nutrients can provide a means of stabilizing the genome, adverse environmental influences brought about by poverty, malnutrition, life-related stress, lack of education, and disparities in health (Thayer and Kuzawa 2011; Brockie et al. 2013; Combs-Orme 2013; Owen et al. 2013), physical inactivity, smoking, and alcohol consumption or use of drugs, can shift the adult epigenome toward morbidity (Maccani and Knopik 2012)
Epigenetics can extend to several generations. For example, the longevity of transgenerational epigenomic inheritance patterns (if not repeated) will dissipate after the first or second generation (e.g., diethylstilbesterol, DES) or up to the fourth generation (e.g., methoxychlor and vinclozolin) (Titus-Ernstoff et al. 2008; Skinner and Guerrero-Bosagna 2009).
Nutritional environment: signal of major consequences
One of the most rudimentary influences on the epigenome is dietary composition, simply because food is essential for survival of all biological entities. It is estimated that >60% of deaths are related to how, and what, we eat, rather than to environmental triggers or, in some cases, even to genetic predispositions (Palou 2006). Nutritional status and biological outcome have long been reported in literature describing the negative effects of malnutrition/vitamin deficiencies on health status and generational effects in offspring (Jordan et al. 1977; Lumey 1992; Locksmith and Duff 1998). Nutritional deficits during pregnancy are particularly critical, often precipitating low weight or length at birth, preterm births, and greater risk of transgenerational pathologies manifest as anemia, resistance to leptin or insulin, hypertension, hepatic steatosis, cardiovascular disorders, cancer, and neurological disorders (Wu et al. 2012; Lukaszewski et al. 2013). Low weight at birth is in and of itself reciprocally correlated to increased susceptibility to diabetes, obesity, and cardiovascular disease in later life (Barnes and Ozanne 2011; Koletzko et al. 2012; Paternain et al. 2012; Li et al. 2013b), and is indicative of dysfunctional epigenetic regulatory activity (DNA methylation/histone modifications) of fetal developmental genes. (Haggarty et al. 2013)
Epi-nutrients
Specific nutrients required for metabolic methylation pathways can directly affect the epigenome to a greater degree than others due to their functional roles in driving DNA methylation/histone modifications (Kussmann and Van Bladeren 2011; DelCurto et al. 2013). These include dietary amino acids such as lysine (required for histone tail residue modifications); methionine [a precursor of S-adenosylmethionine (SAM) methyl donor]; vitamins B6, B12, and B2; folic acid; biotin choline/betaine; and specific minerals (Mg, Zn) (Muskiet 2005; Oommen et al. 2005; Rush et al. 2014) that collectively drive 1-carbon metabolism and nucleotide biosynthesis. These epi-nutrients maintain chromatin epigenetic architecture throughout life, and when lacking, particularly during fetal development (Muskiet 2005), can precipitate dysfunctional DNA silencing patterns associated with greater risk of serious neural-tube defects, insulin resistance (Rush et al. 2014), autism-spectrum disorders (ASD), maladaptive social/language skills, or other diseases that in common have distinct aberrant epigenetic chromatin marks, histone variants, and DNA methylation on disease-relevant gene promoters (Lasalle 2013). Epi-nutrients also include other food-based substances, such as tryptophan and niacin, that are required for nicotinamide adenine dinucleotide-mediated poly (ADP-ribosylation) of histones or DNA repair (Oommen et al. 2005), or carbs/proteins to provide acetyl donors that can effect global methylation and expression of DNMT1, DNMT3a, and DNMT3b during fetal development (Altmann et al. 2012).
One-carbon metabolism
The importance of maternal epi-nutrient status on the outcome of pregnancy was originally demonstrated in the Agouti mouse model, where offspring of maternally epi-nutrient deficient dams had distinctive aberrant phenotypes and color coats correlated to loci-specific DNA methylation (Kim et al. 2009). In humans, epi-nutrient deficits can result in grave birth defects such as autism, neural-tube defects; impaired memory, learning, and cognitive and behavioral function; and psychiatric illnesses, including schizophrenia, bipolar syndrome, and depression, (Sugden 2006; Kubota et al. 2012; Miyake et al. 2012; Lasalle 2013; Stolk et al. 2013), in addition to substantiating elevated risk for adult-onset diseases (Kim 2005).
One of the most important nutrient regulators of 1-carbon metabolism is folic acid, which must be supplied through the diet as a requirement for methylation, the synthesis of purine nucleotides/thymidylate for DNA, and chromosomal stability (Oommen et al. 2005; Stover 2011; Gueant et al. 2013). An in utero folate deficiency correlates to elevated risk of neural-tube defects such as spina bifida in addition to poor birth-outcome, still births, preterm births, hyperglycemia, anemia, learning disabilities, insulin intolerance, and potential transgenerational transmission of maladaptive phenotypes carried to adulthood (Muskiet 2005; Gueant et al. 2013; Krishnaveni et al. 2014). Mutations of methylenetetrahydro-folate reductase can mimic many of the aforementioned effects in offspring, where oral supplementation of folate can effectively reduce infant mortality, and render a higher incidence of twin births associated with in vitro fertilization (Haggarty et al. 2006).
In adults, a major consequence of folate deficiency involves impaired methylation concomitant to a build-up of homocysteine “hyperhomocysteinemia.” This deficiency corresponds to a greater risk of developing cardiovascular disease (Fiorito et al. 2014), neurodegenerative disease, and diabetes (Ji and Kaplowitz 2004) and consequently administration of foliates to adults could be beneficial for overall health and serve to stabilize the epigenome. This treatment would counter the genome-wide DNA hypomethylation that occurs with gene-specific promoter hypermethylation and that, in turn, leads to age-related epigenetic illnesses. (Park et al. 2012)
Additional dietary nutrients involved with 1-carbon metabolism include choline and betaine, also compulsory to drive methylation of homocysteine to methionine (requiring folate and vitamin B12). Choline must be supplied through the diet by nutrient-rich foods such as lecithin, broccoli, and wheat germ. Adequate maternal intake of choline during pregnancy can reduce risk of impaired learning and memory in offspring (Zeisel 2007). Choline is unique in having a dual function, not only in methylation but as a precursor to acetylcholine (required for learning and memory), with capability to protect against hippocampal neurophysiological defects (Lucassen et al. 2013), to promote postnatal spatial memory, enhance dendritic density in the dentate gyrus (required for memory) and avert the decline of memory observed in aged rats (Meck et al. 2007). Choline-rich supplements could yield promising epigenetic prevention of Alzheimer’s disease in aging adults (Fuso and Scarpa 2011) and autistic disorders in children (Hamlin et al. 2013). A deficiency of choline in adults can precipitate epigenetic dysfunction and hyperhomocysteinemia associated with organ dysfunction, particularly in fatty liver (Zeisel 2008; Corbin and Zeisel 2012).
Dietary methionine is also a component of 1-carbon metabolism; readily converted to SAM by methionine adenosyltransferase driving epigenetic modification (Krzystanek et al. 2011). SAM has a number of roles in human biochemistry, not only as a major methyl donor, but also as a precursor for cysteine—a rate limiting amino acid for endogenous production of glutathione (James 2013). This bi-functional requirement for SAM suggests that oxidative stress may rob the epigenetic cycle of needed elements, where reactive oxygen species are evident not only in the process of aging, but also in the pathology of preeclampsia and preterm birth leading to poor birth outcome (D'Souza et al. 2013). Likewise, obesity during pregnancy can lead to transcriptional activation of oxidative stress-related genes which correlate to abnormal epigenetic marks such as H4 (Ac)/(me2) in male offspring (Strakovsky et al. 2014).
It is important to understand that epi-nutrient deficiencies can indirectly occur through external environmental cues operating on the developing fetus, such as infections, stress, metals or drugs like marijuana that deplete biological nutrients (Brown 2011). In many cases, secondary epi-nutrient deficiencies can arise from lifestyle habits such as excessive alcohol intake and cigarette smoking, which correlate to alternations in DNA methylation during fetal development (Reinius et al. 2013; Steegers-Theunissen et al. 2013) possibly arising from secondary deficiencies of B-vitamins and/or hyperhomocysteinemia (Heese et al. 2012; Varela-Rey et al. 2013). Non-nutrient factors can also directly impact epigenetic function (independent of epi-nutrient deficiencies) such as exposure to heavy metals (Cheng et al. 2012; Tsang et al. 2012), combustive toxins (Papoutsis et al. 2013), synthetic chemicals such as perfluoroalkyl acids (Watkins et al. 2014), pesticides, hydrocarbons, dioxins, and endocrine disrupting chemicals that can impair epigenetic machinery directly (Vandegehuchte and Janssen 2013).
The complexities of nutrition on the developing fetus and epigenome can also extend beyond our understanding of epi-nutrients, where nutrients that have a lesser understood role in DNA methylation, histone modifications, ncRNA, etc. can also predict the health of a developing fetus. A good example of this is a maternal vitamin A deficiency being associated with improper silencing of important genes such as GATA4, rendering greater risk of cardiovascular disease in offspring (Feng et al. 2013).
Nutrients that can modify or reverse epigenetic change
In adults, the reversible nature of the epigenome can be subject to modifications by nutrient-rich bioactive foods termed epi-bioactives (DelCurto et al. 2013). Adult-onset diseases often involve profound global genome-wide changes in DNA methylation and chromosomal instability (Choi et al. 2013); these could be reversed by epi-nutrients or drugs that serve as epigenetic modifiers on DNA methyltransferases (DNMTs) or histone deacetylases (HDACs). A growing area of research is in the identification of epi-bioactives that directly modify the epigenome, some of which include phytoestrogens, fatty acids, garlic, resveratrol, curcumin, sulforaphane, proanthocyanidins, genistein and green-tea compounds; these can influence DNA methylation, alter histone structure (Ouedraogo et al. 2011; Park et al. 2012; Zhang and Chen 2011; Henning et al. 2013; Guerrero-Bosagna and Skinner 2014) and augment repair of damaged DNA (Shankar et al. 2013), thereby attenuating risk of adult human diseases. (Malireddy et al. 2012; Shankar et al. 2013). Other epi-bioactives capable of modifying chromatin include coffee polyphenols, ellagitannin, indol-3-carbinol, diindolylmethane, ursodeoxycholic, mahanine, nordihydroguaiaretic acid, lycopene, dihydrocoumarin, cambinol, anacardic acid, garcinol, parthenolide, and chaetocin. (Huang et al. 2011)
Dietary practices/and the macromolecular composition of the diet can also influence chromosomal stability. Recent studies suggest that adhering to caloric restriction or to the Mediterranean diet can positively influence epigenetic stability in adults and correlate to reduced incidence of pathological phenotypes (Chrysohoou and Stefanadis 2013; Tollefsbol 2014), with attenuation of age-related histone modifications, DNA methylation or modulating microRNAs that regulate disease-specific genes (Shah et al. 2012; Martin et al. 2013). Other potential avenues for development of epi-bioactives include identification of those that could combat obesity in adults and fat deposition, such as apple polyphenols (Boque et al. 2013), or new drugs that could target epigenetic regulatory mechanisms in adipose tissue of individuals who are resistant to weight-loss intervention (Moleres et al. 2013) or hypocaloric diets (Milagro et al. 2011).
Future work likely will continue to reveal more information on epigenetic machinery, potentially new epi-nutrients and their functional roles, and epi-bioactives with capability to reverse or change DNA methylation, and higher-order chromatin around critical genes associated with pathologic processes from embryonic development to adulthood. The possibilities for future research on the nutrition of developing fetuses are limitless. In current literature, most studies involve, in one way or another, a correlation of an environmental impact with changes in histone modification marks or with the status of DNA methylation in promoter regions of candidate genes, and a resulting phenotype established in subsets of diseased versus healthy populations.
Funding
Grant from NIH NIMHD RCMI program (G12 MD007582) and NIH P20 MD006738.
References
- Aguilar-Arnal L, Hakim O, Patel VR, Baldi P, Hager GL, Sassone-Corsi P. Cycles in spatial and temporal chromosomal organization driven by the circadian clock. Nat Struct Mol Biol. 2013;20:1206–13. doi: 10.1038/nsmb.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann S, Murani E, Schwerin M, Metges CC, Wimmers K, Ponsuksili S. Maternal dietary protein restriction and excess affects offspring gene expression and methylation of non-SMC subunits of condensin I in liver and skeletal muscle. Epigenetics. 2012;7:239–52. doi: 10.4161/epi.7.3.19183. [DOI] [PubMed] [Google Scholar]
- Andersen SO, Halberstadt ML, Borgford-Parnell N. Stratospheric ozone, global warming, and the principle of unintended consequences–an ongoing science and policy success story. J Air Waste Manag Assoc. 2013;63:607–47. doi: 10.1080/10962247.2013.791349. [DOI] [PubMed] [Google Scholar]
- Barnes SK, Ozanne SE. Pathways linking the early environment to long-term health and lifespan. Progr Biophysics Molecular Biol. 2011;106:323–36. doi: 10.1016/j.pbiomolbio.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Bawa AS, Anilakumar KR. Genetically modified foods: safety, risks and public concerns-a review. J Food Sci Technol. 2013;50:1035–46. doi: 10.1007/s13197-012-0899-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezek S, Ujhazy E, Mach M, Navarova J, Dubovicky M. Developmental origin of chronic diseases: toxicological implication. Interdiscip Toxicol. 2008;1:29–31. doi: 10.2478/v10102-010-0029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckx C, Leivada E. Entangled parametric hierarchies: problems for an overspecified universal grammar. PloS One. 2013;8:e72357. doi: 10.1371/journal.pone.0072357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boque N, de la Iglesia R, de la Garza AL, Milagro FI, Olivares M, Banuelos O, Soria AC, Rodriguez-Sanchez S, Martinez JA, Campion J. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol Nutr Food Res. 2013;57:1473–8. doi: 10.1002/mnfr.201200686. [DOI] [PubMed] [Google Scholar]
- Brand D, Ratan RR. Epigenetics and the environment: in search of the “toleroasome” vital to execution of ischemic preconditioning. Transl Stroke Res. 2013;4:56–62. doi: 10.1007/s12975-012-0235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenseke B, Prater MR, Bahamonde J, Gutierrez JC. Current thoughts on maternal nutrition and fetal programming of the metabolic syndrome. J Pregnancy. 2013;2013:368461. doi: 10.1155/2013/368461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie TN, Heinzelmann M, Gill J. A framework to examine the role of epigenetics in health disparities among Native Americans. Nursing Res Pract. 2013;2013:410395. doi: 10.1155/2013/410395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS. The environment and susceptibility to schizophrenia. Progr Neurobiol. 2011;93:23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JX, Zhang HP, Du LX. [Influence of environmental factors on DNA methylation] Yi chuan. 2013;35:839–46. doi: 10.3724/sp.j.1005.2013.00839. [DOI] [PubMed] [Google Scholar]
- Castillo J, Amaral A, Oliva R. Sperm nuclear proteome and its epigenetic potential. Andrology. 2014;2:326–38. doi: 10.1111/j.2047-2927.2013.00170.x. [DOI] [PubMed] [Google Scholar]
- Cheng TF, Choudhuri S, Muldoon-Jacobs K. Epigenetic targets of some toxicologically relevant metals: a review of the literature. J Appl Toxicol. 2012;32:643–53. doi: 10.1002/jat.2717. [DOI] [PubMed] [Google Scholar]
- Choi SW, Claycombe KJ, Martinez JA, Friso S, Schalinske KL. Nutritional epigenomics: a portal to disease prevention. Adv Nutr. 2013;4:530–2. doi: 10.3945/an.113.004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysohoou C, Stefanadis C. Longevity and diet. Myth or pragmatism? Maturitas. 2013;76:303–7. doi: 10.1016/j.maturitas.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Combs-Orme T. Epigenetics and the social work imperative. Social Work. 2013;58:23–30. doi: 10.1093/sw/sws052. [DOI] [PubMed] [Google Scholar]
- Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol. 2012;28:159–65. doi: 10.1097/MOG.0b013e32834e7b4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D. Epigenetic modifications of brain and behavior: theory and practice. Hormones Behav. 2011;59:393–8. doi: 10.1016/j.yhbeh.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza V, Chavan-Gautam P, Joshi S. Counteracting oxidative stress in pregnancy through modulation of maternal micronutrients and omega-3 fatty acids. Curr Med Chem. 2013;20:4777–83. doi: 10.2174/09298673113209990160. [DOI] [PubMed] [Google Scholar]
- de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- DelCurto H, Wu G, Satterfield MC. Nutrition and reproduction: links to epigenetics and metabolic syndrome in offspring. Curr Opin Clin Nutr Metab Care. 2013;16:385–91. doi: 10.1097/MCO.0b013e328361f96d. [DOI] [PubMed] [Google Scholar]
- Dong K, Pelle E, Yarosh DB, Pernodet N. Sirtuin 4 identification in normal human epidermal keratinocytes and its relation to sirtuin 3 and energy metabolism under normal conditions and UVB-induced stress. Exp Dermatol. 2012;21:231–3. doi: 10.1111/j.1600-0625.2011.01439.x. [DOI] [PubMed] [Google Scholar]
- Duque-Guimaraes DE, Ozanne SE. Nutritional programming of insulin resistance: causes and consequences. Trends Endocrinol Metab. 2013;24:525–35. doi: 10.1016/j.tem.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan K, Sassone-Corsi P. Epigenetic regulation of the molecular clockwork. Prog Mol Biol Trans Sci. 2013;119:29–50. doi: 10.1016/B978-0-12-396971-2.00002-6. [DOI] [PubMed] [Google Scholar]
- Feng Y, Zhao LZ, Hong L, Shan C, Shi W, Cai W. Alteration in methylation pattern of GATA-4 promoter region in vitamin A-deficient offspring's heart. J Nutr Biochem. 2013;24:1373–80. doi: 10.1016/j.jnutbio.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Fiorito G, et al. B-vitamins intake, DNA-methylation of One Carbon Metabolism and homocysteine pathway genes and myocardial infarction risk: The EPICOR study. Nutr Metab Cardiovasc Dis. 2014;24:483–8. doi: 10.1016/j.numecd.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Fuso A, Scarpa S. One-carbon metabolism and Alzheimer's disease: is it all a methylation matter? Neurobiol Aging. 2011;32:1192–5. doi: 10.1016/j.neurobiolaging.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Gayacharan Joel., AJ Epigenetic responses to drought stress in rice (Oryza sativa L.) Physiol Mol Biol Plants. 2013;19:379–87. doi: 10.1007/s12298-013-0176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geison GL. Darwin and heredity: the evolution of his hypothesis of pangenesis. J Hist Med Allied Sci. 1969;24:375–411. doi: 10.1093/jhmas/xxiv.4.375. [DOI] [PubMed] [Google Scholar]
- Gueant JL, Namour F, Gueant-Rodriguez RM, Daval JL. Folate and fetal programming: a play in epigenomics? Trends Endocrinol Metab. 2013;24:279–89. doi: 10.1016/j.tem.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagna CM, Skinner MK. Environmental epigenetics and phytoestrogen/phytochemical exposures. J Steroid Biochem Mol Biol. 2014;139:270–6. doi: 10.1016/j.jsbmb.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty P, Hoad G, Horgan GW, Campbell DM. DNA methyltransferase candidate polymorphisms, imprinting methylation, and birth outcome. PloS One. 2013;8:e68896. doi: 10.1371/journal.pone.0068896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty P, McCallum H, McBain H, Andrews K, Duthie S, McNeill G, Templeton A, Haites N, Campbell D, Bhattacharya S. Effect of B vitamins and genetics on success of in-vitro fertilisation: prospective cohort study. Lancet. 2006;367:1513–9. doi: 10.1016/S0140-6736(06)68651-0. [DOI] [PubMed] [Google Scholar]
- Hamlin JC, Pauly M, Melnyk S, Pavliv O, Starrett W, Crook TA, James SJ. Dietary intake and plasma levels of choline and betaine in children with autism spectrum disorders. Autism Res Treat. 2013;2013:578429. doi: 10.1155/2013/578429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese P, et al. Alterations of homocysteine serum levels during alcohol withdrawal are influenced by folate and riboflavin: results from the German Investigation on Neurobiology in Alcoholism (GINA) Alcohol Alcohol. 2012;47:497–500. doi: 10.1093/alcalc/ags058. [DOI] [PubMed] [Google Scholar]
- Henning SM, Wang P, Carpenter CL, Heber D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics. 2013;5:729–41. doi: 10.2217/epi.13.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Plass C, Gerhauser C. Cancer chemoprevention by targeting the epigenome. Curr Drug Targets. 2011;12:1925–56. doi: 10.2174/138945011798184155. [DOI] [PubMed] [Google Scholar]
- James P. Marabou 2005: nutrition and human development. Nutr Rev. 2006;64:S1–11. doi: 10.1301/nr.2006.may.s1-s11. [DOI] [PubMed] [Google Scholar]
- James SJ. Autism and folate-dependent one-carbon metabolism: serendipity and critical branch-point decisions in science. Global Adv Health Med. 2013;2:48–51. doi: 10.7453/gahmj.2013.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch A. Oxygen, epigenetic signaling, and the evolution of early life. Trends Biochem Sci. 2013;38:172–6. doi: 10.1016/j.tibs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Ji C, Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World Journal Gastroenterol. 2004;10:1699–708. doi: 10.3748/wjg.v10.i12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan RL, Wilson JG, Schumacher HJ. Embryotoxicity of the folate antagonist methotrexate in rats and rabbits. Teratology. 1977;15:73–9. doi: 10.1002/tera.1420150110. [DOI] [PubMed] [Google Scholar]
- Kim KC, Friso S, Choi SW. DNA methylation, an epigenetic mechanism connecting folate to healthy embryonic development and aging. J Nutr Biochem. 2009;20:917–26. doi: 10.1016/j.jnutbio.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr. 2005;135:2703–9. doi: 10.1093/jn/135.11.2703. [DOI] [PubMed] [Google Scholar]
- Koletzko B, Brands B, Poston L, Godfrey K, Demmelmair H. Early nutrition programming of long-term health. Proc Nutr Soc. 2012;71:371–8. doi: 10.1017/S0029665112000596. [DOI] [PubMed] [Google Scholar]
- Krishnaveni GV, Veena SR, Karat SC, Yajnik CS, Fall CH. Association between maternal folate concentrations during pregnancy and insulin resistance in Indian children. Diabetologia. 2014;57:110–21. doi: 10.1007/s00125-013-3086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzystanek M, Palasz A, Krzystanek E, Krupka-Matuszczyk I, Wiaderkiewicz R, Skowronek R. [S-adenosyl L-methionine in CNS diseases] Psychiatria Polska. 2011;45:923–31. [PubMed] [Google Scholar]
- Kubota T, Miyake K, Hirasawa T. Epigenetic understanding of gene-environment interactions in psychiatric disorders: a new concept of clinical genetics. Clin Epigenet. 2012;4:1. doi: 10.1186/1868-7083-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujur A, Saxena MS, Bajaj D, Laxmi, Parida SK. Integrated genomics and molecular breeding approaches for dissecting the complex quantitative traits in crop plants. J Biosci. 2013;38:971–87. doi: 10.1007/s12038-013-9388-6. [DOI] [PubMed] [Google Scholar]
- Kussmann M, Van Bladeren PJ. The extended nutrigenomics - understanding the interplay between the genomes of food, gut microbes, and human host. Front Genet. 2011;2:21. doi: 10.3389/fgene.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasalle JM. Autism genes keep turning up chromatin. OA Autism. 2013;1:14. doi: 10.13172/2052-7810-1-2-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzel V, Allan E, Bortolini Silveira A, Colot V, Fischer M, Bossdorf O. Epigenetic diversity increases the productivity and stability of plant populations. Nat Commun. 2013;4:2875. doi: 10.1038/ncomms3875. [DOI] [PubMed] [Google Scholar]
- Li CC, et al. Maternal obesity and diabetes induces latent metabolic defects and widespread epigenetic changes in isogenic mice. Epigenetics. 2013a;8:602–11. doi: 10.4161/epi.24656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, et al. Major epigenetic development distinguishing neuronal and non-neuronal cells occurs postnatally in the murine hypothalamus. Human Mol Genet. 2013b;23:1579–60. doi: 10.1093/hmg/ddt548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Chen H. Silencing of Wnt5a during colon cancer metastasis involves histone modifications. Epigenetics. 2012;7:551–8. doi: 10.4161/epi.20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksmith GJ, Duff P. Preventing neural tube defects: the importance of periconceptional folic acid supplements. Obstet Gynecol. 1998;91:1027–34. doi: 10.1016/s0029-7844(98)00060-x. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Naninck EF, van Goudoever JB, Fitzsimons C, Joels M, Korosi A. Perinatal programming of adult hippocampal structure and function; emerging roles of stress, nutrition and epigenetics. Trends Neurosci. 2013;36:621–31. doi: 10.1016/j.tins.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Lukaszewski MA, Eberle D, Vieau D, Breton C. Nutritional manipulations in the perinatal period program adipose tissue in offspring. Am J Physiol Endocrinol Metab. 2013;305:E1195–207. doi: 10.1152/ajpendo.00231.2013. [DOI] [PubMed] [Google Scholar]
- Lumey LH. Decreased birthweights in infants after maternal in utero exposure to the Dutch famine of 1944-1945. Paediatr Perinatal Epidemiol. 1992;6:240–53. doi: 10.1111/j.1365-3016.1992.tb00764.x. [DOI] [PubMed] [Google Scholar]
- Maccani MA, Knopik VS. Cigarette smoke exposure-associated alterations to non-coding RNA. Front Genet. 2012;3:53. doi: 10.3389/fgene.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malireddy S, Kotha SR, Secor JD, Gurney TO, Abbott JL, Maulik G, Maddipati KR, Parinandi NL. Phytochemical antioxidants modulate mammalian cellular epigenome: implications in health and disease. Antioxid Redox Signal. 2012;17:327–39. doi: 10.1089/ars.2012.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SL, Hardy TM, Tollefsbol TO. Medicinal chemistry of the epigenetic diet and caloric restriction. Curr Med Chem. 2013;20:4050–9. doi: 10.2174/09298673113209990189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzio EA, Soliman KF. Basic concepts of epigenetics: impact of environmental signals on gene expression. Epigenetics. 2012;7:119–30. doi: 10.4161/epi.7.2.18764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci. 2007;1:7. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milagro FI, Campion J, Cordero P, Goyenechea E, Gomez-Uriz AM, Abete I, Zulet MA, Martinez JA. A dual epigenomic approach for the search of obesity biomarkers: DNA methylation in relation to diet-induced weight loss. FASEB J. 2011;25:1378–89. doi: 10.1096/fj.10-170365. [DOI] [PubMed] [Google Scholar]
- Miret JA, Munne-Bosch S. Plant amino acid-derived vitamins: biosynthesis and function. Amino Acids. 2013;46:809–24. doi: 10.1007/s00726-013-1653-3. [DOI] [PubMed] [Google Scholar]
- Mitteldorf JJ. How does the body know how old it is? Introducing the epigenetic clock hypothesis. Biochemistry (Mosc) 2013;78:1048–53. doi: 10.1134/S0006297913090113. [DOI] [PubMed] [Google Scholar]
- Miyake K, Hirasawa T, Koide T, Kubota T. Epigenetics in autism and other neurodevelopmental diseases. Adv Exp Med Biol. 2012;724:91–8. doi: 10.1007/978-1-4614-0653-2_7. [DOI] [PubMed] [Google Scholar]
- Moleres A, Campion J, Milagro FI, Marcos A, Campoy C, Garagorri JM, Gomez-Martinez S, Martinez JA, Azcona-Sanjulian MC, Marti A. Differential DNA methylation patterns between high and low responders to a weight loss intervention in overweight or obese adolescents: the EVASYON study. FASEB J. 2013;27:2504–12. doi: 10.1096/fj.12-215566. [DOI] [PubMed] [Google Scholar]
- Muskiet FA. The importance of (early) folate status to primary and secondary coronary artery disease prevention. Reprod Toxicol. 2005;20:403–10. doi: 10.1016/j.reprotox.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Oommen AM, Griffin JB, Sarath G, Zempleni J. Roles for nutrients in epigenetic events. J Nutr Biochem. 2005;16:74–7. doi: 10.1016/j.jnutbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Ouedraogo M, Charles C, Guissou IP, Stevigny C, Duez P. An overview of cancer chemopreventive potential and safety of proanthocyanidins. Nutr Cancer. 2011;63:1163–73. doi: 10.1080/01635581.2011.607549. [DOI] [PubMed] [Google Scholar]
- Owen CM, Goldstein EH, Clayton JA, Segars JH. Racial and ethnic health disparities in reproductive medicine: an evidence-based overview. Semin Reprod Med. 2013;31:317–24. doi: 10.1055/s-0033-1348889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palou A. [New challenges in basic and applied nutrition] Revista de medicina de la Universidad de Navarra. 2006;50:62–70. [PubMed] [Google Scholar]
- Papoutsis AJ, Selmin OI, Borg JL, Romagnolo DF. Gestational exposure to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin induces BRCA-1 promoter hypermethylation and reduces BRCA-1 expression in mammary tissue of rat offspring: Preventive effects of resveratrol. Mol Carcinog. 2013 doi: 10.1002/mc.22095. published online (doi:10.1002/mc.22095) [DOI] [PubMed] [Google Scholar]
- Park LK, Friso S, Choi SW. Nutritional influences on epigenetics and age-related disease. Proc Nutr Soc. 2012;71:75–83. doi: 10.1017/S0029665111003302. [DOI] [PubMed] [Google Scholar]
- Paternain L, Batlle MA, De la Garza AL, Milagro FI, Martinez JA, Campion J. Transcriptomic and epigenetic changes in the hypothalamus are involved in an increased susceptibility to a high-fat-sucrose diet in prenatally stressed female rats. Neuroendocrinology. 2012;96:249–60. doi: 10.1159/000341684. [DOI] [PubMed] [Google Scholar]
- Reinius LE, et al. DNA methylation in the Neuropeptide S Receptor 1 (NPSR1) promoter in relation to asthma and environmental factors. PloS One. 2013;8:e53877. doi: 10.1371/journal.pone.0053877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F, Olguin EJ, Diels L, De Philippis R. Microbial fixation of CO2 in water bodies and in drylands to combat climate change, soil loss and desertification. New Biotechnol. 2013 doi: 10.1016/j.nbt.2013.12.002. . 10.1016/j.nbt.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Rush EC, Katre P, Yajnik CS. Vitamin B12: one carbon metabolism, fetal growth and programming for chronic disease. Eur J Clin Nutr. 2014;68:2–7. doi: 10.1038/ejcn.2013.232. [DOI] [PubMed] [Google Scholar]
- Satake A, Iwasa Y. A stochastic model of chromatin modification: cell population coding of winter memory in plants. J Theor Biol. 2012;302:6–17. doi: 10.1016/j.jtbi.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Schneider E, et al. Widespread differences in cortex DNA methylation of the “language gene” between humans and chimpanzees. Epigenetics. 2014;9:533–45. doi: 10.4161/epi.27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo YJ, Muench L, Reid A, Chen J, Kang Y, Hooker JM, Volkow ND, Fowler JS, Kim SW. Radionuclide labeling and evaluation of candidate radioligands for PET imaging of histone deacetylase in the brain. Bioorg Med Chem Lett. 2013;23:6700–5. doi: 10.1016/j.bmcl.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MS, Davidson LA, Chapkin RS. Mechanistic insights into the role of microRNAs in cancer: influence of nutrient crosstalk. Front Genet. 2012;3:305. doi: 10.3389/fgene.2012.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar S, Kumar D, Srivastava RK. Epigenetic modifications by dietary phytochemicals: implications for personalized nutrition. Pharmacol Ther. 2013;138:1–17. doi: 10.1016/j.pharmthera.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Guerrero-Bosagna C. Environmental signals and transgenerational epigenetics. Epigenomics. 2009;1:111–7. doi: 10.2217/epi.09.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubry A, Hoyo C, Jirtle RL, Murphy SK. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. Bioessays. 2014;36:359–71. doi: 10.1002/bies.201300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers-Theunissen RP, Twigt J, Pestinger V, Sinclair KD. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update. 2013;19:640–55. doi: 10.1093/humupd/dmt041. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Prendergast BJ. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc Natl Acad SciUSA. 2013;110:16651–6. doi: 10.1073/pnas.1310643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk L, Bouwland-Both MI, van Mill NH, Verbiest MM, Eilers PH, Zhu H, Suarez L, Uitterlinden AG, Steegers-Theunissen RP. Epigenetic profiles in children with a neural tube defect; a case-control study in two populations. PloS One. 2013;8:e78462. doi: 10.1371/journal.pone.0078462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover PJ. Polymorphisms in 1-carbon metabolism, epigenetics and folate-related pathologies. J Nutrigenet Nutrigenomics. 2011;4:293–305. doi: 10.1159/000334586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakovsky RS, Zhang X, Zhou D, Pan YX. The regulation of hepatic Pon1 by a maternal high-fat diet is gender specific and may occur through promoter histone modifications in neonatal rats. J Nutr Biochem. 2014;25:170–6. doi: 10.1016/j.jnutbio.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden C. One-carbon metabolism in psychiatric illness. Nutr Res Rev. 2006;19:117–36. doi: 10.1079/NRR2006119. [DOI] [PubMed] [Google Scholar]
- Thayer ZM, Kuzawa CW. Biological memories of past environments: epigenetic pathways to health disparities. Epigenetics. 2011;6:798–803. doi: 10.4161/epi.6.7.16222. [DOI] [PubMed] [Google Scholar]
- Titus-Ernstoff L, et al. Offspring of women exposed in utero to diethylstilbestrol (DES): a preliminary report of benign and malignant pathology in the third generation. Epidemiology. 2008;19:251–7. doi: 10.1097/EDE.0b013e318163152a. [DOI] [PubMed] [Google Scholar]
- Tollefsbol TO. Dietary epigenetics in cancer and aging. Cancer Treat Res. 2014;159:257–67. doi: 10.1007/978-3-642-38007-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V, Fry RC, Niculescu MD, Rager JE, Saunders J, Paul DS, Zeisel SH, Waalkes MP, Styblo M, Drobna Z. The epigenetic effects of a high prenatal folate intake in male mouse fetuses exposed in utero to arsenic. Toxicol Appl Pharmacol. 2012;264:439–50. doi: 10.1016/j.taap.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegehuchte MB, Janssen CR. Epigenetics in an ecotoxicological context. Mutat Res. 2013;764–765:36–45. doi: 10.1016/j.mrgentox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Varela-Rey M, Woodhoo A, Martinez-Chantar ML, Mato JM, Lu SC. Alcohol, DNA methylation, and cancer. Alcohol Res Curr Rev. 2013;35:25–35. doi: 10.35946/arcr.v35.1.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Wellenius GA, Butler RA, Bartell SM, Fletcher T, Kelsey KT. Associations between serum perfluoroalkyl acids and LINE-1 DNA methylation. Environ Int. 2014;63:71–6. doi: 10.1016/j.envint.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Imhoff-Kunsch B, Girard AW. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinat Epidemiol. 2012;26(Suppl. 1):4–26. doi: 10.1111/j.1365-3016.2012.01291.x. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Gene response elements, genetic polymorphisms and epigenetics influence the human dietary requirement for choline. IUBMB Life. 2007;59:380–7. doi: 10.1080/15216540701468954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Genetic polymorphisms in methyl-group metabolism and epigenetics: lessons from humans and mouse models. Brain Res. 2008;1237:5–11. doi: 10.1016/j.brainres.2008.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen H. Genistein, an epigenome modifier during cancer prevention. Epigenetics. 2011;6:888–91. doi: 10.4161/epi.6.7.16315. [DOI] [PubMed] [Google Scholar]