Abstract
The optimal BP level in kidney transplant recipients remains uncertain. This post hoc analysis of the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) trial cohort assessed associations of BP with a pooled cardiovascular disease (CVD) outcome and with all-cause mortality. In 3474 prevalent kidney transplant patients, mean age was 52±9 years, 63% were men, 76% were white, 20% had a history of CVD, 40% had a history of diabetes mellitus, and the median time since transplant was 4.1 years (25th to 75th percentiles, 1.7–7.4); mean systolic BP was 136±20 mmHg and mean diastolic BP was 79±12 mmHg. There were 497 CVD events and 406 deaths. After adjustment for demographic and transplant characteristics and CVD risk factors, each 20-mmHg increase in baseline systolic BP associated with a 32% increase in subsequent CVD risk (hazard ratio [HR], 1.32; 95% confidence interval [95% CI], 1.19 to 1.46) and a 13% increase in mortality risk (HR, 1.13; 95% CI, 1.01 to 1.27). Similarly, after adjustment, at diastolic BP levels<70 mmHg, each 10-mmHg decrease in diastolic BP level associated with a 31% increase in CVD risk (HR, 1.31; 95% CI, 1.06 to 1.62) and a 31% increase in mortality risk (HR, 1.31; 95% CI, 1.03 to 1.66). However, at diastolic BP levels>70 mmHg, there was no significant relationship between diastolic BP and outcomes. Higher systolic BP strongly and independently associated with increased risk of CVD and all-cause mortality, without evidence of a J shape, whereas only lower levels of diastolic BP associated with increased risk of CVD and death in this trial.
Keywords: blood pressure, cardiovascular disease, mortality, kidney transplantation
Cardiovascular disease (CVD) is the leading cause of death among kidney transplant recipients, accounting for almost 30% of deaths among patients with a functioning graft.1 Hypertension is an independent risk factor for CVD in the general population, and controlling hypertension reduces the risk of incident and recurrent CVD and development and progression of CKD.2–6
Uncertainty remains over the optimal BP targets, particularly in high-risk populations, including kidney transplant recipients and patients with CKD.7–12 Several studies have shown a continuous relationship between BP and CVD outcomes, such that risk reduction is maintained even at very low BP levels, whereas some studies demonstrate a bimodal distribution, resulting in a J- or U-shaped curve, with higher event rates at both lower and higher BP values.13 This nonlinear risk pattern may be more common in populations with a high prevalence of comorbid conditions in which lower BPs may reflect significant coexistent illnesses such as impaired cardiac function or increased arterial stiffness.14–17 Critically, this J-shaped relationship is observed in studies of patients with CKD,18,19 particularly in hemodialysis patients, in which higher mortality risk is seen with postdialysis systolic BPs<140 mmHg and >180 mmHg.20 There are few observational studies and no randomized clinical trials of BP targets to inform the relationship between BP level and outcomes in kidney transplant recipients.
On the basis of limited data, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines21,22 suggest a target BP level of <130/80 mmHg in kidney transplant recipients. Given these uncertainties, we analyzed data from >3400 stable kidney transplant recipients enrolled the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) trial23–25 to examine the association between BP and CVD risk, specifically evaluating whether there was a threshold BP below which CVD risk appeared higher.
Results
Study Population
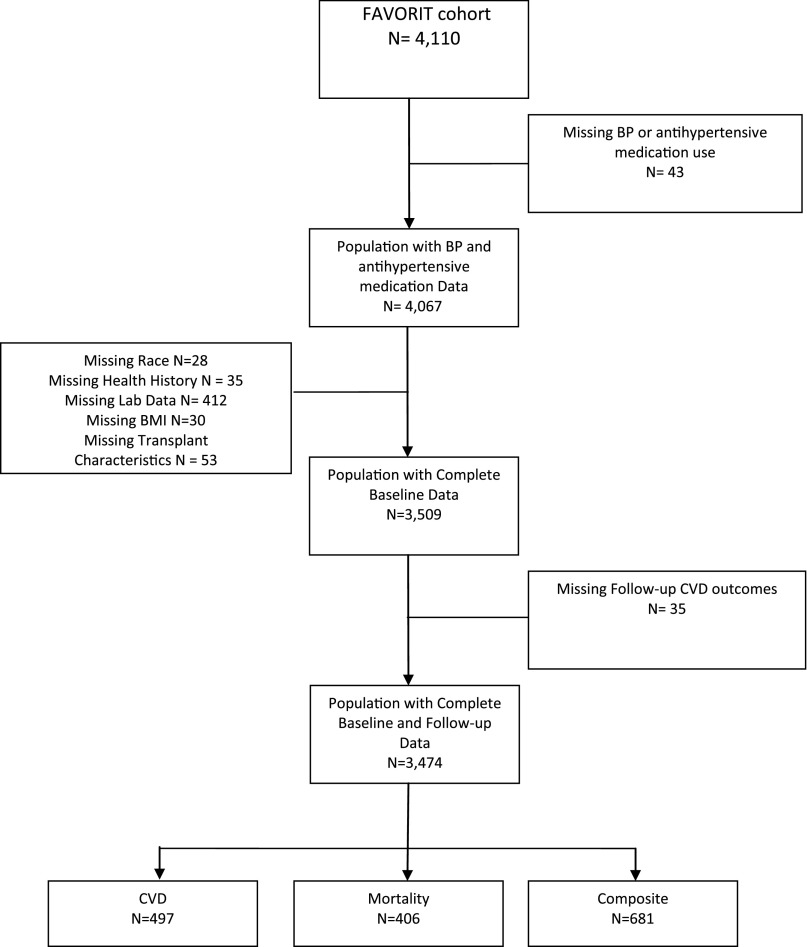
Of 4110 FAVORIT participants, 3474 (85%) had complete data and were included in the present analyses (Figure 1). The mean age of the study population was 52±9 years; 63% were men, 76% were white, 20% had a history of CVD, 40% had a history of diabetes mellitus, and the median time since transplant was 4.1 years (25th to 75th percentiles, 1.7–7.4) (Tables 1 and 2). The average systolic and diastolic BP levels at baseline were 136±20 mmHg and 79±12 mmHg, respectively. At baseline, 3072 participants (88%) were prescribed at least one antihypertensive medication (mean 2.1±0.9 medications among users). Individuals with higher levels of baseline systolic BP were significantly older, were more likely to be men, were of nonwhite race, had a history of diabetes, and had a higher body mass index (BMI), total cholesterol, and albumin to creatinine ratio (ACR) (Table 1). Individuals with higher levels of baseline diastolic BP were significantly more likely to be men, nonwhite race, current smokers, and have higher total cholesterol and ACR whereas those with lower diastolic BP were older and had higher prevalence of CVD and diabetes (Table 2).
Figure 1.
Flow chart for FAVORIT participants in the analysis dataset.
Table 1.
Baseline characteristics by systolic BP level
| Characteristic | Systolic BP (mmHg) | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n=3474) | 70–109 (n=222) | 110–119 (n=433) | 120–129 (n=738) | 130–139 (n=722) | 140–149 (n=582) | 150–159 (n=376) | 160–248 (n=401) | ||
| Systolic BP (mmHg) | 136.2±19.8 | 102.9±6.2 | 114.9±3.1 | 124.5±3.1 | 134.6±3.1 | 144.3±3.0 | 154.3±3.0 | 173.3±12.9 | <0.001 |
| Diastolic BP (mmHg) | 78.8±12.4 | 65.3±8.2 | 71.3±8.6 | 75.1±8.8 | 78.8±9.5 | 81.8±11.0 | 85.6±11.8 | 90.7±14.7 | <0.001 |
| Age (yr) | 51.8±9.4 | 50.7±9.1 | 50.3±9.4 | 50.8±9.4 | 51.6±9.4 | 52.4±9.4 | 53.2±9.4 | 53.8±8.9 | <0.001 |
| Women | 1297 (37) | 112 (50) | 180 (42) | 278 (38) | 246 (34) | 205 (35) | 118 (31) | 158 (39) | <0.001 |
| Nonwhite race | 817 (24) | 35 (16) | 93 (21) | 134 (18) | 174 (24) | 144 (25) | 114 (30) | 123 (31) | <0.001 |
| Country | |||||||||
| Brazil | 608 (18) | 20 (9) | 52 (12) | 87 (12) | 112 (16) | 107 (18) | 94 (25) | 136 (34) | <0.001 |
| Canada | 388 (11) | 21 (9) | 49 (11) | 102 (14) | 93 (13) | 60 (10) | 39 (10) | 24 (6) | |
| United States | 2478 (71) | 181 (82) | 332 (77) | 549 (74) | 517 (72) | 415 (71) | 243 (65) | 241 (60) | |
| Graft vintage (yr) | 4.1 (1.7, 7.4) | 4.2 (1.5, 8.8) | 3.7 (1.6, 8.0) | 4.3 (1.9, 7.6) | 3.9 (1.6, 7.6) | 4.0 (1.6, 7.0) | 4.0 (1.8, 6.9) | 3.8 (1.6, 6.8) | 0.38 |
| History of CVD | 700 (20) | 48 (22) | 65 (15) | 146 (20) | 142 (20) | 121 (21) | 79 (21) | 99 (25) | 0.04 |
| History of diabetes | 1392 (40) | 87 (39) | 158 (36) | 272 (37) | 264 (37) | 258 (44) | 150 (40) | 203 (51) | <0.001 |
| BP-lowering medication use | |||||||||
| ACE inhibitors | 1127 (32) | 65 (29) | 123 (28) | 223 (30) | 222 (31) | 197 (34) | 134 (36) | 163 (41) | 0.002 |
| Angiotensin receptor blockers | 464 (13) | 41 (18) | 58 (13) | 92 (12) | 80 (11) | 79 (14) | 59 (16) | 55 (14) | 0.10 |
| β-Blockers | 1958 (56) | 113 (51) | 231 (53) | 396 (54) | 392 (54) | 333 (57) | 220 (59) | 273 (68) | <0.001 |
| Calcium channel blockers | 1386 (40) | 71 (32) | 154 (36) | 295 (40) | 302 (42) | 247 (42) | 168 (45) | 149 (37) | 0.01 |
| Diuretics | 1309 (38) | 81 (36) | 159 (37) | 248 (34) | 271 (38) | 217 (37) | 159 (42) | 174 (43) | 0.02 |
| Any of the above | 3072 (88) | 187 (84) | 358 (83) | 633 (86) | 637 (88) | 525 (90) | 348 (93) | 384 (96) | <0.001 |
| No. of BP-lowering medications used | 1.8±1.1 | 1.7±1.2 | 1.7±1.2 | 1.7±1.1 | 1.8±1.1 | 1.9±1.1 | 2.0±1.1 | 2.1±1.0 | <0.001 |
| BMI (kg/m2) | 29.1±6.2 | 27.8±6.3 | 28.4±6.1 | 28.7±6.1 | 29.4±6.4 | 29.2±5.9 | 29.9±6.1 | 30.2±5.9 | <0.001 |
| Current smoker | 384 (11) | 29 (13) | 39 (9) | 88 (12) | 75 (10) | 64 (11) | 44 (12) | 45 (11) | 0.70 |
| Total cholesterol (mg/dl) | 185.0±44.1 | 179.3±42.2 | 179.1±39.8 | 182.8±42.2 | 183.1±41.8 | 186.1±42.6 | 189.7±50.2 | 195.7±50.7 | <0.001 |
| HDL cholesterol (mg/dl) | 46.2±13.9 | 46.4±15.4 | 45.9±14.1 | 46.0±13.4 | 46.1±14.0 | 46.4±14.1 | 45.2±13.4 | 47.8±14.0 | 0.27 |
| LDL cholesterol (mg/dl) | 101.3±34.5 | 96.5±32.5 | 96.7±31.2 | 99.7±33.3 | 100.4±33.2 | 102.6±33.7 | 105.0±38.6 | 107.9±39.1 | <0.001 |
| Triglycerides (mg/dl) | 199.3±188.4 | 189.4±139.5 | 192.4±128.5 | 194.5±125.3 | 194.1±132.5 | 197.5±144.8 | 225.9±416.7 | 208.4±157.6 | 0.10 |
| Urine ACR (mg/g) | |||||||||
| <10 | 1010 (29) | 88 (40) | 181 (42) | 253 (34) | 218 (30) | 145 (25) | 77 (20) | 48 (12) | <0.001 |
| 10–29 | 908 (26) | 64 (29) | 118 (27) | 209 (28) | 181 (25) | 159 (27) | 89 (24) | 88 (22) | |
| 30–299 | 1118 (32) | 56 (25) | 104 (24) | 216 (29) | 240 (33) | 193 (33) | 136 (36) | 173 (43) | |
| ≥300 | 438 (13) | 14 (6) | 30 (7) | 60 (8) | 83 (11) | 85 (15) | 74 (20) | 92 (23) | |
| eGFR (ml/min per 1.73 m2) | 48.9±17.6 | 49.1±18.7 | 50.8±18.5 | 48.9±17.3 | 49.0±16.9 | 48.8±17.4 | 49.1±17.8 | 46.9±17.2 | 0.10 |
| CKD stage (eGFR ml/min per 1.73 m2) | |||||||||
| 1T (≥90) | 96 (3) | 10 (5) | 14 (3) | 19 (3) | 19 (3) | 12 (2) | 9 (2) | 13 (3) | 0.17 |
| 2T (60–89) | 723 (21) | 43 (19) | 107 (25) | 152 (21) | 141 (20) | 129 (22) | 88 (23) | 63 (16) | |
| 3Ta (45–59)a | 1055 (30) | 63 (28) | 133 (31) | 224 (30) | 234 (32) | 165 (28) | 117 (31) | 119 (30) | |
| 3Tb (30–44) | 1163 (33) | 79 (36) | 128 (30) | 251 (34) | 244 (34) | 203 (35) | 111 (30) | 147 (37) | |
| 4T (15–29) | 428 (12) | 26 (12) | 50 (12) | 92 (12) | 83 (11) | 73 (13) | 48 (13) | 56 (14) | |
| 5T (<15) | 9 (0) | 1 (0) | 1 (0) | — (—) | 1 (0) | — (—) | 3 (1) | 3 (1) | |
Data reported as mean±SD, median (25th percentile, 75th percentile), or frequency (%). P value comparisons across BP categories are based on chi-square for categorical variables, Kruskal–Wallis test for median graft vintage, and ANOVA for other continuous variables. ACE, angiotensin enzyme-converting; T, transplant.
Terminology developed by Levey et al.40
Table 2.
Baseline characteristics by diastolic BP level
| Characteristic | Diastolic BP (mmHg) | P Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n=3474) | 35–59 (n=155) | 60–69 (n=591) | 70–79 (n=1129) | 80–89 (n=971) | 90–99 (n=450) | 100–139 (n=178) | ||
| Systolic BP (mmHg) | 136.2±19.8 | 119.6±19.1 | 124.8±16.6 | 131.6±16.1 | 139.1±15.9 | 150.3±16.4 | 166.2±21.5 | <0.001 |
| Diastolic BP (mmHg) | 78.8±12.4 | 55.0±4.3 | 65.1±3.0 | 74.6±3.0 | 83.6±2.9 | 93.8±2.9 | 107.6±7.6 | <0.001 |
| Age (yr) | 51.8±9.4 | 57.0±9.4 | 54.1±9.7 | 52.1±9.4 | 50.8±9.1 | 49.6±8.8 | 47.8±7.6 | <0.001 |
| Women | 1297 (37) | 71 (46) | 250 (42) | 394 (35) | 351 (36) | 160 (36) | 71 (40) | 0.01 |
| Nonwhite race | 817 (24) | 23 (15) | 109 (18) | 231 (20) | 258 (27) | 142 (32) | 54 (30) | <0.001 |
| County | ||||||||
| Brazil | 608 (18) | 2 (1) | 23 (4) | 87 (8) | 171 (18) | 176 (39) | 149 (84) | <0.001 |
| Canada | 388 (11) | 8 (5) | 56 (9) | 141 (12) | 135 (14) | 46 (10) | 2 (1) | |
| United States | 2478 (71) | 145 (94) | 512 (87) | 901 (80) | 665 (68) | 228 (51) | 27 (15) | |
| Graft vintage (yr) | 4.1 (1.7, 7.4) | 2.9 (1.2, 6.2) | 3.7 (1.6, 7.3) | 3.9 (1.6, 8.0) | 4.2 (1.8, 7.5) | 4.5 (2.1, 7.2) | 4.4 (1.9, 6.7) | 0.04 |
| History of CVD | 700 (20) | 56 (36) | 163 (28) | 208 (18) | 183 (19) | 64 (14) | 26 (15) | <0.001 |
| History of diabetes | 1392 (40) | 99 (64) | 304 (51) | 465 (41) | 361 (37) | 130 (29) | 33 (19) | <0.001 |
| BP-lowering medication use | ||||||||
| ACE inhibitors | 1127 (32) | 38 (25) | 190 (32) | 358 (32) | 306 (32) | 151 (34) | 84 (47) | <0.001 |
| Angiotensin receptor blockers | 464 (13) | 33 (21) | 95 (16) | 150 (13) | 125 (13) | 49 (11) | 12 (7) | <0.001 |
| β-Blockers | 1958 (56) | 106 (68) | 333 (56) | 600 (53) | 539 (56) | 271 (60) | 109 (61) | 0.002 |
| Calcium channel blockers | 1386 (40) | 66 (43) | 251 (42) | 498 (44) | 357 (37) | 162 (36) | 52 (29) | <0.001 |
| Diuretics | 1309 (38) | 77 (50) | 241 (41) | 413 (37) | 337 (35) | 168 (37) | 73 (41) | 0.004 |
| Any of the above | 3072 (88) | 141 (91) | 521 (88) | 996 (88) | 847 (87) | 402 (89) | 165 (93) | 0.31 |
| Number of BP-lowering medications used | 1.8±1.1 | 2.1±1.2 | 1.9±1.1 | 1.8±1.1 | 1.7±1.1 | 1.8±1.0 | 1.9±1.1 | <0.001 |
| BMI (kg/m2) | 29.1±6.2 | 29.4±7.3 | 29.1±6.2 | 29.2±6.2 | 29.3±6.2 | 29.0±5.8 | 28.4±5.2 | 0.51 |
| Current smoker | 384 (11) | 16 (10) | 52 (9) | 127 (11) | 101 (10) | 58 (13) | 30 (17) | 0.05 |
| Total cholesterol (mg/dl) | 185.0±44.1 | 176.9±40.3 | 176.1±41.7 | 181.6±44.1 | 188.0±42.0 | 193.8±43.4 | 204.1±56.6 | <0.001 |
| HDL cholesterol (mg/dl) | 46.2±13.9 | 44.1±14.0 | 47.0±14.4 | 46.1±13.8 | 46.3±13.9 | 46.0±13.6 | 46.7±14.2 | 0.32 |
| LDL cholesterol (mg/dl) | 101.3±34.5 | 93.5±33.2 | 93.6±32.4 | 98.6±33.3 | 103.3±33.1 | 109.6±34.4 | 118.5±45.6 | <0.001 |
| Triglycerides (mg/dl) | 199.3±188.4 | 215.1±167.5 | 183.6±112.5 | 203.0±271.7 | 201.8±133.8 | 199.2±126.5 | 201.7±140.2 | 0.32 |
| Urine ACR (mg/g) | ||||||||
| <10 | 1010 (29) | 56 (36) | 200 (34) | 364 (32) | 268 (28) | 93 (21) | 29 (16) | <0.001 |
| 10–29 | 908 (26) | 44 (28) | 181 (31) | 299 (26) | 243 (25) | 110 (24) | 31 (17) | |
| 30–299 | 1118 (32) | 35 (23) | 164 (28) | 350 (31) | 329 (34) | 164 (36) | 76 (43) | |
| ≥300 | 438 (13) | 20 (13) | 46 (8) | 116 (10) | 131 (13) | 83 (18) | 42 (24) | |
| eGFR (ml/min per 1.73 m2) | 48.9±17.6 | 46.5±18.1 | 47.9±17.3 | 49.2±17.3 | 49.2±17.8 | 50.2±18.1 | 47.8±16.8 | 0.14 |
| CKD stage (eGFR ml/min per 1.73 m2) | ||||||||
| 1T (≥90) | 96 (3) | 7 (5) | 15 (3) | 25 (2) | 29 (3) | 18 (4) | 2 (1) | 0.07 |
| 2T (60–89) | 723 (21) | 20 (13) | 120 (20) | 251 (22) | 204 (21) | 95 (21) | 33 (19) | |
| 3Ta (45–59)a | 1055 (30) | 42 (27) | 164 (28) | 353 (31) | 297 (31) | 135 (30) | 64 (36) | |
| 3Tb (30–44) | 1163 (33) | 59 (38) | 217 (37) | 356 (32) | 318 (33) | 159 (35) | 54 (30) | |
| 4T (15–29) | 428 (12) | 27 (17) | 74 (13) | 143 (13) | 118 (12) | 42 (9) | 24 (13) | |
| 5T (<15) | 9 (0) | 0 (0) | 1 (0) | 1 (0) | 5 (1) | 1 (0) | 1 (1) | |
Data reported as the mean±SD, median (25th percentile, 75th percentile), or frequency (%). P value comparisons across BP categories are based on the chi-squared test for categorical variables, Kruskal–Wallis test for median graft vintage, and ANOVA for other continuous variables. ACE, angiotensin enzyme-converting.
Terminology developed by Levey et al.40
CVD Outcomes
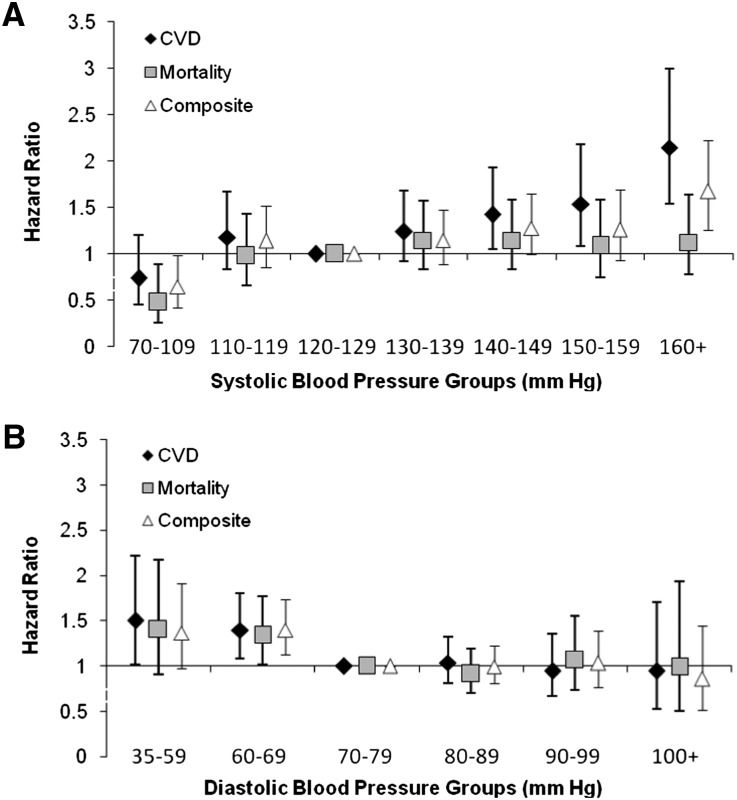
Over a mean follow-up of 4.0±1.5 years, there were 497 first CVD events (Table 3), including 125 nonfatal myocardial infarctions, 49 strokes, 7 resuscitated sudden death events, 89 cardiovascular deaths, and 227 cardiovascular-related procedures (coronary revascularization [n=131]; peripheral vascular [n=69], carotid [n=9], or renal artery [n=13] procedures; or thoracoabdominal aortic aneurysm repair [n=5]). In unadjusted models and partially adjusted models (including age, sex, race, diabetes, prior CVD, ACR, eGFR, treatment allocation, country of origin, and systolic and diastolic BP), each 20-mmHg higher systolic BP was associated with a 35% (hazard ratio [HR], 1.35; 95% confidence interval [95% CI], 1.24 to 1.46) and 31% (HR, 1.31; 95% CI, 1.18 to 1.45) higher risk for CVD outcomes, respectively. Notably, diastolic BP had a significant nonlinear shape, with CVD risk greatest at the lowest levels of diastolic BP (Figure 2). Although diastolic BP>70 mmHg was not associated with increased risk of CVD outcomes, each 10-mmHg lower diastolic BP at diastolic BP levels<70 mmHg was associated with a 51% (HR, 1.51; 95% CI, 1.23 to 1.86) and 34% (HR, 1.34; 95% CI, 1.09 to 1.65) higher risk of cardiovascular events in unadjusted and partially adjusted models, respectively. The fully adjusted model additionally incorporated smoking, LDL cholesterol, HDL cholesterol, triglycerides, BMI, graft donor type, graft vintage, and use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, dihydropyridine calcium channel blockers, nondihydropyridine calcium channel blockers, loop diuretics, and other diuretics. In the fully adjusted model, each 20-mmHg higher systolic BP was associated with a 32% (HR, 1.32; 95% CI, 1.19 to 1.46) higher risk for CVD outcomes, whereas each 10-mmHg lower diastolic BP<70 mmHg was associated with a 31% (HR, 1.31; 95% CI, 1.06 to 1.62) higher risk for CVD outcomes. There was no increased risk observed with diastolic BP values>70 mmHg. In models exploring combined systolic and diastolic BP groups, increased CVD risk associated with BP is dominated by elevated systolic BP but low diastolic pressure (<70 mmHg) attenuates the beneficial effect of moderate systolic BP (120–139 mmHg) (Table 4, Supplemental Figure 1).
Table 3.
Events and event rates stratified by BP groups
| BP Group | Total | CVD | Mortality | Composite | |||
|---|---|---|---|---|---|---|---|
| n | Rate | n | Rate | n | Rate | ||
| Systolic BP (mmHg) | |||||||
| <110 | 222 | 21 | 24.8 | 12 | 13.4 | 27 | 31.8 |
| 110–119 | 433 | 54 | 32.2 | 41 | 22.9 | 78 | 46.5 |
| 120–129 | 738 | 84 | 28.3 | 79 | 25.2 | 126 | 42.5 |
| 130–139 | 722 | 90 | 32.4 | 83 | 28.2 | 129 | 46.4 |
| 140–149 | 582 | 93 | 42.4 | 79 | 33.3 | 128 | 58.4 |
| 150–159 | 376 | 62 | 45.5 | 50 | 34.2 | 80 | 58.7 |
| ≥160 | 401 | 93 | 68.8 | 62 | 41.2 | 113 | 83.6 |
| Diastolic BP (mmHg) | |||||||
| <60 | 155 | 37 | 65.0 | 28 | 44.7 | 47 | 82.5 |
| 60–69 | 591 | 115 | 51.3 | 93 | 37.7 | 159 | 70.9 |
| 70–79 | 1129 | 151 | 33.3 | 133 | 27.5 | 213 | 47.0 |
| 80–89 | 971 | 129 | 34.7 | 96 | 24.3 | 170 | 45.7 |
| 90–99 | 450 | 48 | 30.5 | 43 | 25.7 | 71 | 45.1 |
| ≥100 | 178 | 17 | 31.8 | 13 | 23.5 | 21 | 39.2 |
Rates are per 1000 participant years.
Figure 2.
HRs for CVD and mortality outcomes by level of systolic BP (A) and diastolic BP (B). Models are adjusted for age, sex, race, diabetes, prior CVD, eGFR, treatment allocation, country of origin, smoking, LDL cholesterol, HDL cholesterol, triglycerides, ACR, BMI, donor type, graft vintage, medication classes, and systolic/diastolic BP.
Table 4.
Association of combined systolic and diastolic BP groups with outcomes
| Systolic BP and Diastolic BP Categorya | n | CVD | Mortality | Composite | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Low_Low | 324 | 1.19 (0.83 to 1.70) | 0.34 | 0.89 (0.59 to 1.35) | 0.58 | 1.13 (0.84 to 1.53) | 0.41 |
| Low_Mod | 325 | 1.13 (0.75 to 1.72) | 0.56 | 1.00 (0.63 to 1.58) | 1.00 | 1.14 (0.81 to 1.61) | 0.45 |
| Mod_Low | 298 | 1.57 (1.14 to 2.17) | 0.01 | 1.51 (1.08 to 2.11) | 0.02 | 1.56 (1.19 to 2.04) | 0.001 |
| Mod_Mod | 1034 | Reference | — | Reference | — | Reference | — |
| Mod_High | 128 | 0.62 (0.27 to 1.44) | 0.27 | 0.81 (0.37 to 1.78) | 0.60 | 0.77 (0.41 to 1.44) | 0.41 |
| High_Low | 124 | 1.82 (1.24 to 2.67) | 0.002 | 1.47 (0.97 to 2.24) | 0.07 | 1.65 (1.18 to 2.29) | 0.003 |
| High_Mod | 741 | 1.50 (1.16 to 1.95) | 0.002 | 1.02 (0.77 to 1.35) | 0.89 | 1.30 (1.04 to 1.62) | 0.02 |
| High_High | 494 | 1.65 (1.17 to 2.32) | 0.004 | 1.24 (0.86 to 1.79) | 0.25 | 1.48 (1.11 to 1.97) | 0.01 |
Models are adjusted for age, sex, race, diabetes, prior CVD, eGFR, treatment allocation, country of origin, smoking, LDL, HDL, triglycerides, ACR, BMI, donor type, graft vintage, BP medication use, and systolic/diastolic BP.
Systolic BP categories are as follows: low, <120 mmHg; moderate, 120–139 mmHg; and high, ≥140 mmHg. Diastolic BP categories are as follows: low, <70 mmHg; moderate, 70–89 mmHg; and high, ≥90 mmHg.
Mortality
There were 406 deaths in the study cohort (Table 3). In unadjusted, partially adjusted, and extended models, each 20 mmHg higher systolic BP was associated with a 27% (HR, 1.27; 95% CI, 1.16 to 1.40), 13% (HR, 1.13; 95% CI, 1.00 to 1.26), and 13% (HR, 1.13; 95% CI, 1.01–1.27) higher risk of all-cause death, respectively. Diastolic BP levels>70 mmHg were not associated with a higher risk of death, but each 10-mmHg lower diastolic BP<70 mmHg was associated with a 50% (HR, 1.50; 95% CI, 1.20 to 1.88), 27% (HR, 1.27; 95% CI, 1.01 to 1.61), and 31% (HR, 1.31; 95% CI, 1.03 to 1.66) higher risk of all-cause death in unadjusted, partially adjusted, and extended models, respectively. The lowest risk for death was noted in individuals with systolic BP<110 mmHg (HR, 0.48; 95% CI, 0.26 to 0.89), relative to systolic BP of 120–129 mmHg (Figure 2, Supplemental Table 1). When evaluating mortality outcomes across the range of systolic BP, risk generally increased with higher systolic BP. For diastolic BP, the highest risk occurred at levels <70 mmHg. When considering the joint distribution of systolic and diastolic BP (Table 4), the highest risk of death was evident in those with systolic BP>120 mmHg and diastolic BP<70 mmHg relative to those with moderate BP levels (120–139 mmHg systolic and 70–89 mmHg diastolic).
Secondary and Sensitivity Analyses
To assess for semicompeting risk factors, we examined the composite outcome of CVD and mortality (681 total outcomes). In univariate, partially adjusted, and extended models, each 20-mmHg higher systolic BP was associated with a 27% (HR, 1.27; 95% CI, 1.18 to 1.36), 20% (HR, 1.20; 95% CI, 1.10 to 1.32), and 22% (HR, 1.22; 95% CI, 1.11 to 1.33) higher risk in the composite outcome, respectively. For diastolic BP, in unadjusted, partially adjusted, and extended models, each 10-mmHg lower diastolic BP<70 mmHg was associated with a 47% (HR, 1.47; 95% CI, 1.23 to 1.75), 28% (HR, 1.28; 95% CI, 1.07 to 1.54), and 26% (HR, 1.26; 95% CI, 1.05 to 1.52) higher risk of the composite outcome, respectively. Interaction analyses revealed no significant effect modification by history of CVD, history of diabetes, level of albuminuria, statin use, or country on the relationship between BP and CVD, mortality, or composite outcomes (data not shown). Analyses restricted to participants in the United States had similar results (data not shown). In sensitivity analyses in which missing data were imputed for non-BP covariates thereby increasing the sample size to 4017 (98% of total randomized), the relationship of BP to CVD, mortality, and composite outcomes remained similar (data not shown). Findings remained similar when CVD events were defined as not including procedures, resulting in 320 events, with each 20-mmHg higher systolic BP associated with a 42% (HR, 1.42; 95% CI, 1.28 to 1.57), 30% (HR, 1.30; 95% CI, 1.14 to 1.48), and 29% (HR, 1.29; 95% CI, 1.14 to 1.47) increased risk in unadjusted, partially adjusted, and extended models, respectively, and each 10-mmHg lower diastolic BP<70 mmHg was associated with a 46% (HR, 1.46; 95% CI, 1.12 to 1.91), 32% (HR, 1.32; 95% CI, 1.01 to 1.73), and 32% (HR, 1.32; 95% CI, 1.00 to 1.74), respectively.
There was a nonlinear association of systolic BP and kidney failure (n=282). Risk of kidney failure increased by 48% (HR, 1.48; 95% CI, 1.26 to 1.74) with each 20-mmHg increase in systolic BP up to 160 mmHg, with no further increase in risk at levels >160 mmHg. Risk was attenuated but remained statistically significant in the extended model (HR, 1.24; 95% CI, 1.02 to 1.51). No association was evident between diastolic BP and kidney failure.
Discussion
Among stable kidney transplant recipients, higher levels of systolic BP are independently associated with a higher risk of CVD and all-cause mortality, without a lower threshold systolic BP value below which risk begins to rise. By contrast, lower levels of diastolic BP are associated with a higher risk of CVD and all-cause mortality. This pattern is similar to that seen in other high CVD risk populations, in which both higher systolic BP and lower diastolic BP are associated with worse outcomes. However, unlike these other populations (including individuals treated with maintenance dialysis), we did not observe higher risk associated with lower systolic BP alone.
To date, there are no adequately powered randomized clinical trials evaluating BP treatment targets in kidney transplant recipients. The Assessment of Lescol in Renal Transplantation study, which assessed the effects of lipid-lowering therapy on outcomes in transplant recipients, reported that cardiovascular events were more frequent among participants with diastolic BP<80 mmHg treated with placebo rather than fluvastatin and that individuals with systolic BP>155 mmHg had the highest unadjusted CVD event rates.26 Despite limited data, both the 2010 KDIGO Guidelines on Care of the Transplant Recipient and the 2012 KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease recommend a target BP of ≤130/80 mmHg in kidney transplant recipients, regardless of other risk factors.21,22
Hypertension is common in individuals with CKD and is a risk factor for CVD outcomes.27 The Action to Control Cardiovascular Risk in Diabetes study17 as well as a recent systematic review concluded that more intensive BP control was not associated with a reduction in the primary outcome of major cardiovascular events, although CVD risk reduction was not the primary outcome for these trials.11 In individuals with CVD in the Treating to New Targets Trial, the relationship between BP and CVD outcomes followed a more bimodal distribution, resulting in a J- or U-shaped relationship, with higher event rates at both very low and very high levels of BP.14 This may reflect an association between lower BP, particularly diastolic BP, and more severe underlying comorbid conditions, including arteriosclerotic vascular disease or a wider pulse pressure.28,29 Kovesdy and colleagues30 recently noted an increased mortality risk in nondialysis-dependent CKD patient with diastolic BP<70 mmHg and ideal systolic BP. Our results in stable kidney transplant recipients are consistent with this observation. A clearer understanding of the etiologic role of BP control among transplant recipients as well as optimal targets for control are needed because BP is a modifiable factor that contributes to the risk of CVD in these patients.
Kidney transplantation is the treatment of choice for many patients with kidney failure. The nature of the relationship among BP, kidney function, and cardiovascular and mortality outcomes is complicated by the rise in BP that may occur with progressive allograft failure or treatment with calcineurin inhibitors. However, because chronic allograft nephropathy may be, in part, mediated by renal ischemia,31 one could theorize that higher BP and thereby better kidney perfusion may be beneficial for graft survival. In one study of >29,000 recipients of cadaveric kidneys, a reduction in systolic BP from 1 to 3 years after transplantation to <140 mmHg was associated with improved graft and patient survival.5 Similarly, several prior studies have described associations between hypertension and the development of CVD in kidney transplant recipients.4,32–34 Our findings reinforce these conclusions in a population with detailed ascertainment of CVD, further demonstrating that there is no notable J-shaped association between systolic BP level and cardiovascular or mortality outcomes.
This study has several limitations. First, this analysis uses an observational design, introducing potential biases regarding the receipt of care based on antihypertensive medication use and follow-up. Critically though, FAVORIT participants comprise a treated population with specialized medical care, as evidenced by antihypertensive medication use in 88% of participants. Second, the study results are applicable to prevalent kidney transplant recipients, and no inferences can be made about care in the immediate post-transplant period. Finally, analyses did not incorporate levels of BP, eGFR, or antihypertensive medication use during follow-up. This study also has several strengths. It uses a large, multiracial and multinational cohort that is fairly generalizable to the wider kidney transplant population. In addition, there is thorough ascertainment of cardiovascular events and sufficient events to allow evaluation of the relationship between BP and CVD and mortality outcomes in extensively adjusted models.
In conclusion, we have demonstrated that higher levels of systolic BP are strongly and independently associated with an increased risk of CVD and all-cause mortality with no increased risk of adverse outcomes noted at even the lowest systolic BP values. By contrast, lower levels of diastolic BP are associated with increased rates of CVD and all-cause mortality. Clinical trials comparing treatment groups randomized to different levels of BP control will be required to confirm these relationships and to explore the interplay among systolic BP targets, diastolic BP targets, and adverse outcomes in kidney transplant recipients.
Concise Methods
Study Design, Patient Population, and Trial Overview
This is a post hoc analysis of FAVORIT trial, a multicenter, double-blind, randomized trial examining the effect of B-vitamin therapy on CVD and mortality.24,25 The protocol was approved by the human participants research entity with oversight at each center, and every patient provided written informed consent before participation. The trial was registered with ClinicalTrials.gov (NCT00064753) in July 2003. The main results demonstrated no significant difference between the randomized groups on the primary or main secondary outcomes. FAVORIT investigators at transplant centers in the United States (n=27), Canada (n=2) and Brazil (n=1) enrolled 4110 kidney transplant recipients who were at least 6 months post-transplant, had elevated total homocysteine levels, and met other eligibility criteria.25 Participants were randomized to receive either a standard multivitamin with high doses of folic acid (5 mg), vitamin B6 (pyridoxine; 50 mg), and vitamin B12 (cyanocobalamin; 1 mg) or a multivitamin containing low doses of vitamin B6 (1.4 mg) and vitamin B12 (2 µg) with no folic acid. Follow-up contacts occurred every six months through January 31, 2010, to obtain study related outcomes through June 24, 2009. Because the high-dose vitamin intervention had no effect on risk of CVD or all-cause death compared with the lose-dose vitamin,23 treatment groups are combined with a term retained for randomization allocation in all multivariate analyses for this report.
Baseline and Outcome Data
Seated BP was measured twice at 5- to 10-minute intervals at the randomization visit, with the mean value used for analyses. Prevalent CVD was defined as history of myocardial infarction, coronary artery revascularization, stroke, carotid arterial revascularization, abdominal or thoracic aortic aneurysm repair, lower extremity arterial revascularization, or lower extremity amputation above the ankle. BP control, including medication regimen, was managed by the participants’ physicians and was not specified by the FAVORIT protocol. Prescription BP medication use was assessed at baseline from medication containers brought to the clinic visit, review of current medical records, and patient report. Prior publications describe our laboratory methods.25,35–38 Because of its skewed distribution, the ACR was log transformed for use in modeling.
The primary outcome for this analysis was the occurrence of a major cardiovascular event, defined as CVD death, myocardial infarction, resuscitated sudden death, stroke, coronary revascularization, or peripheral, carotid, aortic, or renal artery procedures. The first four components of this primary outcome were centrally reviewed and adjudicated by the FAVORIT Clinical Endpoints Committee, whereas the others were identified through medical record abstraction. Secondary outcomes included all-cause mortality and dialysis-dependent kidney failure. To account for semicompeting risks, we also examined a composite outcome of major cardiac event and all-cause mortality. Participants were not censored at the time of return to dialysis or at retransplantation.
Statistical Analyses
The Kruskal–Wallis test (graft vintage), ANOVA (other continuous variables), and chi-squared tests (categorical variables) were used to compare baseline characteristics across systolic and diastolic BP groups. Cox proportional hazards regression was used to evaluate the association of baseline systolic and diastolic BP with time to primary and secondary study outcomes. Systolic and diastolic BP values were categorized into clinically relevant equal width intervals (10 mmHg) with the exception of the minimum and maximum groupings that collapsed the tails of the BP distributions to have sufficient numbers for analysis. To evaluate the joint distribution, eight BP categories were formed as all possible combinations of systolic and diastolic BP based on low (70–119), moderate (120–139), and high (140–248) systolic BP and low (35–69), moderate (70–89), and high (90–139) diastolic BP, omitting the ninth low systolic/high diastolic category due to an insufficient number for analysis. Reflecting a significant quadratic term for diastolic BP and examination of diastolic BP strata, diastolic BP was parameterized for a two-slope model to accommodate the different risk profile for patients with diastolic BP above and below 70 mmHg. Initial models were a priori adjusted for systolic or diastolic BP, age, sex, race, diabetes, prior CVD, ln ACR, and eGFR in a two-slope model accounting for levels above and below 45 ml/min per 1.73 m2 to accommodate nonlinearity,39 treatment allocation, and country of origin. Reflecting available data in the FAVORIT trial, extended models were a priori further adjusted for BP medications, albuminuria, smoking, BMI, LDL cholesterol, HDL cholesterol, triglyceride level, transplant vintage, and living versus deceased donor kidney. Nonviolation of the proportional hazards assumptions was evaluated for the primary analysis variables by examination of log-log plot of survival probability and the supremum test. Interaction terms were evaluated between BP variables and preexisting CVD and diabetes, as well as ACR, to evaluate for nonlinear relationships in higher-risk groups. Multivariate imputation utilizing a discriminant function method for categorical variables and regression method for continuous variables was performed to handle missing laboratory, BMI, race, and transplant data. Partial and extended models with imputed data were considered as a sensitivity analysis. Analyses were performed using SAS software, Inc. (versions 9.2 and 9.3).
Disclosures
M.R.W. has served as a scientific advisor for Amgen, Janssen, Otsuka, Relypsa, and Sanofi.
Supplementary Material
Acknowledgments
We are grateful for the many contributions of the participants, physicians, nurses, coordinators, and professional staffs at the clinical centers in Brazil, Canada, and the United States and at the data coordinating center at the University of North Carolina at Chapel Hill.
The FAVORIT Clinical Site Investigators are as follows: Deborah Adey (University of Vermont), Edward Alfrey (Southern Illinois University), Paul Bolin Jr. (East Carolina University), Andrew Bostom (Rhode Island Hospital), Daniel C. Brennan (Washington University—St. Louis), Barbara Bresnahan (Medical College of Wisconsin), Edward Cole (University of Toronto), David Conti (Albany Medical Center), Fernando Cosio (Mayo Clinic), Gabriel Danovitch (University of California—Los Angeles), Alfredo Fabrega (Banner Good Samaritan Transplant Services), Lorenzo Gallon (Northwestern University), Andrew House (London Health Sciences Center), Lawrence Hunsicker (University of Iowa), Bertram Kasiske (Hennepin County Medical Center), Clifton Kew (University of Alabama—Birmingham), Matthew Koch (private practice), Anil Kumar (Reata Pharmaceuticals), Mariana Markell (State University of New York Health Science Center), Arthur Matas (University of Minnesota), Douglas Norman (Oregon Health Sciences University), Akinlolu Ojo (University of Michigan), Alvaro Pacheco-Silva (Universidade Federal de Sao Paulo), Alice Peng (Cedars—Sinai Health System), Todd Pesavento (Ohio State University), John Pirsch (University of Wisconsin—Madison), Ajay Singh (Brigham and Women’s Hospital), Stephen Smith (Duke University), John Vella (Maine Medical Center), Matthew Weir (University of Maryland), and Muhammad Yaqub (Indiana University).
The FAVORIT trial is supported by cooperative agreement U01-DK61700 from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). J.W.K., an employee of the NIDDK, was involved in the study design, interpretation, and writing of the report. Support also provided by the National Institutes of Health Office of Dietary Supplements. PamLab, LLC of Covington, Louisiana, provided the high- and low-dose multivitamins.
Data included in this manuscript were presented in abstract form and during an oral presentation at the American Society of Nephrology 2012 Annual Meeting on November 3, 2012, in San Diego, California.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “BP Targets in Renal Transplant Recipients: Too High or Too Low?,” on pages 1371–1373.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013040435/-/DCSupplemental.
References
- 1.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L: United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 59: A7, e1–e420, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS, AIPRD Study Group : Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: A patient-level meta-analysis. Ann Intern Med 139: 244–252, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Anjum S, Shah R, Skogen J, Kandaswamy C, Danielson B, O’Shaughnessy EA, Dahl DC, Silkensen JR, Sahadevan M, Snyder JJ: Hypertension after kidney transplantation. Am J Kidney Dis 43: 1071–1081, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Mange KC, Feldman HI, Joffe MM, Fa K, Bloom RD: Blood pressure and the survival of renal allografts from living donors. J Am Soc Nephrol 15: 187–193, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Opelz G, Döhler B, Collaborative Transplant Study : Improved long-term outcomes after renal transplantation associated with blood pressure control. Am J Transplant 5: 2725–2731, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Sowers JR, Epstein M, Frohlich ED: Diabetes, hypertension, and cardiovascular disease: An update. Hypertension 37: 1053–1059, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ, Wright JT, Jr, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X, AASK Collaborative Research Group : Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronow WS: What should the optimal blood pressure goal be in patients with diabetes mellitus or chronic kidney disease? Arch Med Sci 8: 399–402, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang TI, Cheung AK, Chertow GM: Blood pressure control in type 2 diabetes mellitus. Am J Kidney Dis 56: 1029–1031, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS: The effect of a lower target blood pressure on the progression of kidney disease: Long-term follow-up of the Modification of Diet in Renal Disease study. Ann Intern Med 142: 342–351, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Upadhyay A, Earley A, Haynes SM, Uhlig K: Systematic review: Blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med 154: 541–548, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Upadhyay A, Uhlig K: Is the lower blood pressure target for patients with chronic kidney disease supported by evidence? Curr Opin Cardiol 27: 370–373, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Banach M, Aronow WS: Blood pressure j-curve: Current concepts. Curr Hypertens Rep 14: 556–566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, LaRosa JC, Treating to New Targets Steering Committee and Investigators : J-curve revisited: An analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J 31: 2897–2908, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, Drury PL, Esmatjes E, Hricik D, Pohl M, Raz I, Vanhille P, Wiegmann TB, Wolfe BM, Locatelli F, Goldhaber SZ, Lewis EJ, Collaborative Study Group : Impact of achieved blood pressure on cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial. J Am Soc Nephrol 16: 2170–2179, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, Kolloch R, Benetos A, Pepine CJ: Dogma disputed: Can aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med 144: 884–893, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F, Ismail-Beigi F, ACCORD Study Group : Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 362: 1575–1585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal R: Blood pressure components and the risk for end-stage renal disease and death in chronic kidney disease. Clin J Am Soc Nephrol 4: 830–837, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, Eisner G, Esmatjes E, Gilbert RE, Hunsicker LG, de Faria JB, Mangili R, Moore J, Jr, Reisin E, Ritz E, Schernthaner G, Spitalewitz S, Tindall H, Rodby RA, Lewis EJ: Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the Irbesartan Diabetic Nephropathy Trial: Clinical implications and limitations. J Am Soc Nephrol 16: 3027–3037, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P: “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 54: 561–569, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Kidney Disease Improving Global Outcomes (KDIGO) Blood Pressure Work Group : KDIGO clinical practice guidelines for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2: 337–414, 2012 [Google Scholar]
- 22.Kidney Disease Improving Global Outcomes (KDIGO) Transplant Work Group : KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9[Suppl 3]: S1–S155, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, Selhub J, Jacques PF, Cole E, Gravens-Mueller L, House AA, Kew C, McKenney JL, Pacheco-Silva A, Pesavento T, Pirsch J, Smith S, Solomon S, Weir M: Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: Primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation 123: 1763–1770, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Pfeffer MA, Levey AS, Jacques PF, McKenney J: Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J 152: 448.e1–448.e7, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS, McKenney JL, Mercier RY, Pfeffer MA, Selhub J, FAVORIT Study Investigators : Baseline characteristics of participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. Am J Kidney Dis 53: 121–128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jardine AG, Holdaas H, Fellström B, Cole E, Nyberg G, Grönhagen-Riska C, Madsen S, Neumayer HH, Maes B, Ambühl P, Olsson AG, Holme I, Fauchald P, Gimpelwicz C, Pedersen TR, ALERT Study Investigators : Fluvastatin prevents cardiac death and myocardial infarction in renal transplant recipients: Post-hoc subgroup analyses of the ALERT Study. Am J Transplant 4: 988–995, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, Drury PL, Esmatjes E, Hricik D, Parikh CR, Raz I, Vanhille P, Wiegmann TB, Wolfe BM, Locatelli F, Goldhaber SZ, Lewis EJ, Irbesartan Diabetic Nephropathy Trial. Collaborative Study Group : Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med 138: 542–549, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D: Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 103: 1245–1249, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Haider AW, Larson MG, Franklin SS, Levy D, Framingham Heart Study : Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med 138: 10–16, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kovesdy CP, Bleyer AJ, Molnar MZ, Ma JZ, Sim JJ, Cushman WC, Quarles LD, Kalantar-Zadeh K: Blood pressure and mortality in U.S. veterans with chronic kidney disease: A cohort study. Ann Intern Med 159: 233–242, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman JR: Clinical renal transplantation: Where are we now, what are our key challenges? Transplant Proc 42[Suppl]: S3–S6, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Cosio FG, Alamir A, Yim S, Pesavento TE, Falkenhain ME, Henry ML, Elkhammas EA, Davies EA, Bumgardner GL, Ferguson RM: Patient survival after renal transplantation: I. The impact of dialysis pre-transplant. Kidney Int 53: 767–772, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Lentine KL, Schnitzler MA, Abbott KC, Li L, Burroughs TE, Irish W, Brennan DC: De novo congestive heart failure after kidney transplantation: A common condition with poor prognostic implications. Am J Kidney Dis 46: 720–733, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Oliveras A, Roquer J, Puig JM, Rodríguez A, Mir M, Orfila MA, Masramon J, Lloveras J: Stroke in renal transplant recipients: Epidemiology, predictive risk factors and outcome. Clin Transplant 17: 1–8, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Carpenter MA, Weir MR, Adey DB, House AA, Bostom AG, Kusek JW: Inadequacy of cardiovascular risk factor management in chronic kidney transplantation-evidence from the FAVORIT study. Clin Transplant 26: E438–E446, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 37.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, Hamm LL, Lewis JB, Mauer M, Navis GJ, Steffes MW, Eggers PW, Coresh J, Levey AS: Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 56: 486–495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiner DE, Carpenter MA, Levey AS, Ivanova A, Cole EH, Hunsicker L, Kasiske BL, Kim SJ, Kusek JW, Bostom AG: Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: The FAVORIT trial. Am J Transplant 12: 2437–2445, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.