Abstract
Stimulation of renal proximal tubule (PT) transport by angiotensin II (Ang II) is critical for regulation of BP. Notably, in rats, mice, and rabbits, the regulation of PT sodium transport by Ang II is biphasic: transport is stimulated by picomolar to nanomolar concentrations of Ang II but inhibited by nanomolar to micromolar concentrations of Ang II. However, little is known about the effects of Ang II on human PT transport. By functional analysis with isolated PTs obtained from nephrectomy surgery, we found that Ang II induces a dose-dependent profound stimulation of human PT transport by type 1 Ang II receptor (AT1)-dependent phosphorylation of extracellular signal-regulated kinase (ERK). In PTs of wild-type mice, the nitric oxide (NO) /cGMP/cGMP-dependent kinase II (cGKII) pathway mediated the inhibitory effect of Ang II. In PTs of cGKII-deficient mice, the inhibitory effect of Ang II was lost, but activation of the NO/cGMP pathway failed to phosphorylate ERK. Conversely, in human PTs, the NO/cGMP pathway mediated the stimulatory effect of Ang II by phosphorylating ERK independently of cGKII. These contrasting responses to the NO/cGMP pathway may largely explain the different modes of PT transport regulation by Ang II, and the unopposed marked stimulation of PT transport by high intrarenal concentrations of Ang II may be an important factor in the pathogenesis of human hypertension. Additionally, the previously unrecognized stimulatory effect of the NO/cGMP pathway on PT transport may represent a human-specific therapeutic target in hypertension.
Among the several regulatory systems for BP, the renin-angiotensin system (RAS) plays a key role. Angiotensin II (Ang II) can regulate BP by type 1 (AT1) Ang II receptors in either the kidney or the extrarenal tissues. Using a kidney cross-transplantation strategy, Crowley et al.1 revealed that AT1A in the kidney is critical for the pathogenesis of Ang II-mediated hypertension and its cardiovascular complications. Crowley et al.1 also showed that genetic abrogation AT1A in renal proximal tubules (PTs) alone is sufficient to reduce BP and provide substantial protection against Ang II–induced hypertension in mice, suggesting that the stimulation of PT sodium transport by Ang II has a major impact on BP regulation.2
Paradoxically, however, the regulation of PT sodium transport by Ang II is biphasic: transport is stimulated by low (picomolar to nanomolar) concentrations of Ang II, whereas it is inhibited by high (nanomolar to micromolar) concentrations of Ang II.3,4 Notably, the concentrations of Ang II within the kidney are known to be much higher than the concentrations in plasma, although the measurement of urinary Ang II may be less valuable.5,6 Therefore, the inhibitory effect of high concentrations of Ang II could also have some physiologic relevance to the regulation of in vivo PT transport.5 Ang II regulates the major PT sodium transporters the apical Na+/H+ exchanger isoform 3 (NHE3), the basolateral Na+-HCO3− cotransporter (NBCe1), and the basolateral Na+/K+ ATPase in the biphasic manner.7–12 Although controversial results had been reported of the receptor subtype(s) mediating the biphasic effects of Ang II,13,14 the studies using isolated PTs obtained from AT1A knockout (KO) mice have established that AT1A mediates both the stimulatory and inhibitory effects of Ang II.11,15 The stimulation by Ang II has been attributed to the activation of protein kinase C and/or the decrease in the intracellular cAMP concentration, resulting in the activation of extracellular signal-regulated kinase (ERK) pathway.16–18 However, the inhibition by Ang II has been attributed to the activation of the phospholipase A2/arachidonic acid/5,6-epoxyeicosatrienoic acid (EET) pathway and/or the nitric oxide (NO)/guanosine 3′,5′-cyclic monophosphate (cGMP) pathway.7,12,17,19 Although the biphasic regulation of PT transport by Ang II has been reported in several species, such as rats, mice, and rabbits,3,4,15 whether Ang II has similar biphasic effects on human PT transport remains unknown. Moreover, the signaling mechanisms mediating the Ang II actions on human kidney have not been clarified at all.
To clarify these issues, we examined the effects of Ang II on isolated human PTs obtained from nephrectomy surgery. Unexpectedly, our findings revealed that Ang II, unlike in the other species, dose-dependently stimulates human PT transport. The different modes of transport regulation by Ang II can be largely explained by the contrasting roles of the NO/cGMP pathway, which as a downstream mediator of Ang II signaling in PTs, is stimulatory in human subjects but inhibitory in the other species. The unopposed marked stimulation of PT transport by the high local concentrations of Ang II may be an important factor in the pathogenesis of human hypertension. Furthermore, the stimulatory effect of the NO/cGMP pathway on human PT transport may represent a previously unrecognized therapeutic target of hypertension.
Results
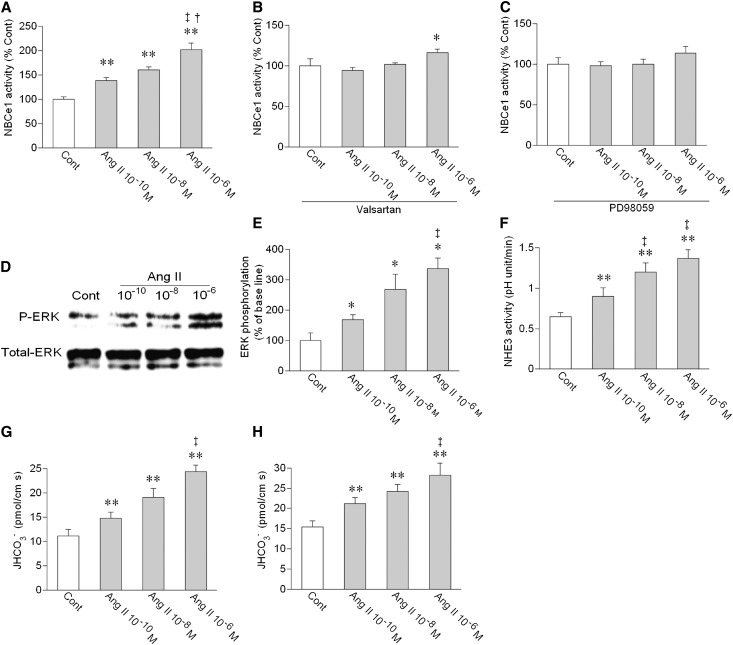
We first examined the effects of Ang II on NBCe1 activity in isolated luminally collapsed human PTs. This preparation is suitable for the identification of biphasic effects of Ang II on NBCe1 mediated through basolateral AT1 receptors.11,15,17,19 The basal NBCe1 activity (60.0±2.3 mM/min, n=70) determined from 22 persons ages 59.3±2.0 years was lower than the activity (82.2±2.2 mM/min, n=41) from 11 mice ages 9.1±0.8 weeks. Unlike Ang II in mouse or rabbit PTs,9–11,17 Ang II in human PTs induced a dose-dependent marked stimulation of the NBCe1 activity (Figure 1A). The stimulatory effect of 10−5 M Ang II (191.1±6.7%, n=9) was not different from the effect of 10−6 M Ang II, indicating that the stimulatory effect of Ang II was already saturated at 10−6 M. This dose–response is comparable with the kinetics for Ang II–induced cell calcium increase by AT1 receptors endogenously expressed in mouse PTs or heterologously expressed in Xenopus oocytes.11,20 The stimulation of NBCe1 by Ang II was largely suppressed by a specific AT1 inhibitor valsartan (Figure 1B) and completely suppressed by an mitogen-activated protein kinase/ERK (MEK) inhibitor PD98059 (Figure 1C), indicating that the AT1/MEK/ERK pathway mediates the stimulatory effect of Ang II. Indeed, Ang II dose-dependently stimulated ERK phosphorylation in human kidney cortex (Figure 1, D and E).
Figure 1.
Dose-dependent stimulatory effect of Ang II on human PT transport. (A) Effects of Ang II on NBCe1 activity. NBCe1 activity was measured before and 5 minutes after the addition of Ang II. n=11–13 for each concentration of Ang II; **P<0.01 versus control (Cont); †P<0.05 versus Ang II 10−8 M; ‡P<0.01 versus Ang II 10−10 M. (B) Effects of Ang II on NBCe1 in the presence of an AT1 inhibitor valsartan (2×10−7 M). n=7–10 for each concentration of Ang II; *P<0.05 versus Cont. (C) Effects of Ang II on NBCe1 in the presence of an MEK inhibitor PD98059 (10−5 M). n=4 for each concentration of Ang II. (D) Effects of Ang II on ERK phosphorylation in human kidney cortex samples. (E) Densitometric analysis of ERK phosphorylation by Ang II. The relative density of phospho-ERK (P-ERK) to total ERK is shown. Open bars indicate control values, and closed bars indicate values after Ang II. n=5 for each concentration of Ang II; *P<0.05 versus Cont; ‡P<0.01 versus Ang II 10−10 M. (F) Effects of Ang II on NHE3 activity. NHE3 activity was measured before and 5 minutes after the addition of Ang II. n=5 for each concentration of Ang II; **P<0.01 versus Cont; ‡P<0.01 versus Ang II 10−10 M. (G) Effects of bath Ang II on the rate of bicarbonate absorption (JHCO3−). Ang II was added to bath perfusate for 5 minutes. n=4–5 for each concentration of Ang II; **P<0.01 versus Cont; ‡P<0.01 versus Ang II 10−10 M. (H) Effects of luminal Ang II on JHCO3−. Ang II was added to luminal perfusate for 5 minutes. n=5 for each concentration of Ang II; **P<0.01 versus Cont; ‡P<0.01 versus Ang II 10−10 M.
Using luminally perfused human PTs, we next found that Ang II added to bath perfusate dose-dependently stimulated not only the apical NHE3 activity but also, the rate of sodium-dependent bicarbonate absorption (JHCO3−), as shown in Figure 1, F and G. Ang II added to luminal perfusate also dose-dependently stimulated JHCO3− (Figure 1H), suggesting that both basolateral and luminal AT1 receptors mediate the dose-dependent marked stimulatory effect of Ang II on net human PT transport.
To clarify the mechanism underlying the loss of inhibitory effect of Ang II in human PTs, we examined the role of phospholipase A2/arachidonic acid/5,6-EET pathway, which represents one of the inhibitory mediators of Ang II effects in the other species.7,17 Both arachidonic acid and 5,6-EET failed to inhibit the human NBCe1 activity (Supplemental Figure 1), although they did inhibit the mouse NBCe1 activity as reported.11,17
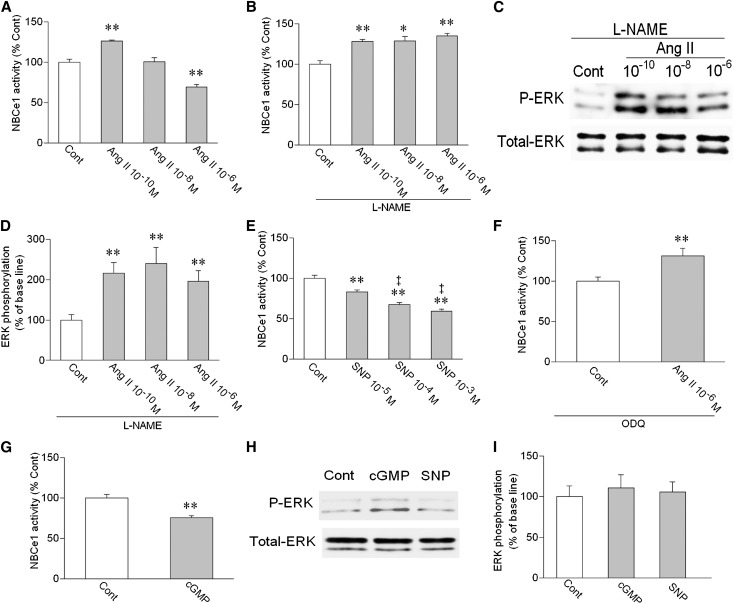
To further clarify the mechanism(s) underlying the different modes of PT transport regulation by Ang II, we next examined the role of the NO/cGMP pathway, which might also mediate the inhibitory effect of Ang II in the other species.12,18 As previously reported,11,17 Ang II had biphasic effects on NBCe1 activity in mouse PTs (Figure 2A). In the presence of an NO synthase (NOS) inhibitor l-nitro-arginine methyl ester (L-NAME), however, the inhibitory effect of Ang II was lost, and all of the concentrations of Ang II now induced the similar stimulation (Figure 2B). In the presence of L-NAME, all of the concentrations of Ang II also induced the similar magnitude of ERK phosphorylation (Figure 2, C and D). An NO donor sodium nitroprusside (SNP) dose-dependently inhibited the NBCe1 activity (Figure 2E). A soluble guanylyl cyclase (sGC) inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) converted the inhibition by 10−6 M Ang II to the stimulation (Figure 2F). Furthermore, a cell-permeable cGMP analog 8Br-cGMP inhibited the NBCe1 activity (Figure 2G). These results indicate that the NOS/NO/sGC/cGMP pathway mediates the inhibitory effect of Ang II in mouse PTs. Consistent with this view, 8Br-cGMP and SNP failed to stimulate ERK phosphorylation in mouse kidney cortex (Figure 2, H and I).
Figure 2.
Roles of NO/cGMP in mouse PTs. (A) Effects of Ang II on NBCe1 activity. **P<0.01 versus Cont; n=7–10 for each concentration of Ang II. (B) Effects of Ang II on NBCe1 in the presence of an NOS inhibitor L-NAME (2×10−4 M). n=7–10 for each concentration of Ang II; *P<0.05 versus Cont.; **P<0.01 versus Cont. (C) Effects of Ang II on ERK phosphorylation in the presence of L-NAME. (D) Densitometric analysis of ERK phosphorylation. n=5 for each concentration of Ang II; **P<0.01 versus Cont. (E) Effects of an NO donor SNP on NBCe1. n=6–8 for each concentration of SNP; **P<0.01 versus Cont; ‡P<0.01 versus SNP 10−5 M. (F) Effect of 10−6 M Ang II on NBCe1 in the presence of the sGC inhibitor ODQ (5×10−5 M). n=6; **P<0.01 versus Cont. (G) Effect of 2×10−5 M 8Br-cGMP (cGMP) on NBCe1. n=6; **P<0.01 versus Cont. (H) Effects of 8Br-cGMP (2×10−5 M) and SNP (10−3 M) on ERK phosphorylation in mouse kidney cortex. (I) Densitometric analysis of ERK phosphorylation. n=4 for both cGMP (2×10−5 M) and SNP (10−3 M).
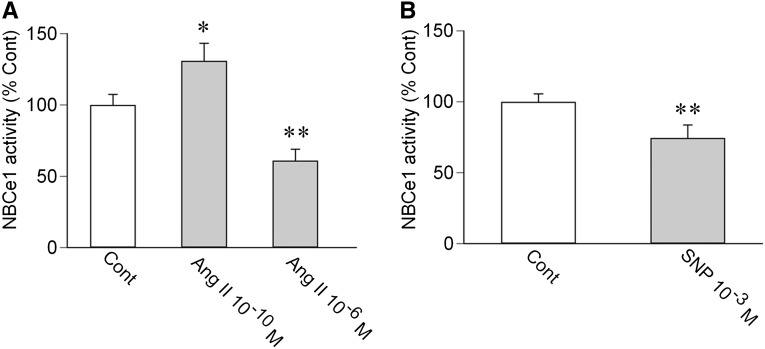
Although the NO/cGMP pathway is generally thought to inhibit PT transport,21,22 controversial results have been also reported.23 Therefore, we examined the effects of NO in rat PTs. As in mouse PTs, Ang II had biphasic effects on NBCe1 activity in rat PTs (Figure 3A). Furthermore, SNP significantly inhibited the NBCe1 activity (Figure 3B), indicating that NO is also inhibitory in rat PTs.
Figure 3.
Effects of the Ang II/NO pathway on NBCe1 in rat PTs. (A) Effects of Ang II on NBCe1. n=9 for each concentration of Ang II; *P<0.05 versus Cont; **P<0.01 versus Cont. (B) Effect of 10−3 M SNP on NBCe1. n=6; **P<0.01 versus Cont.
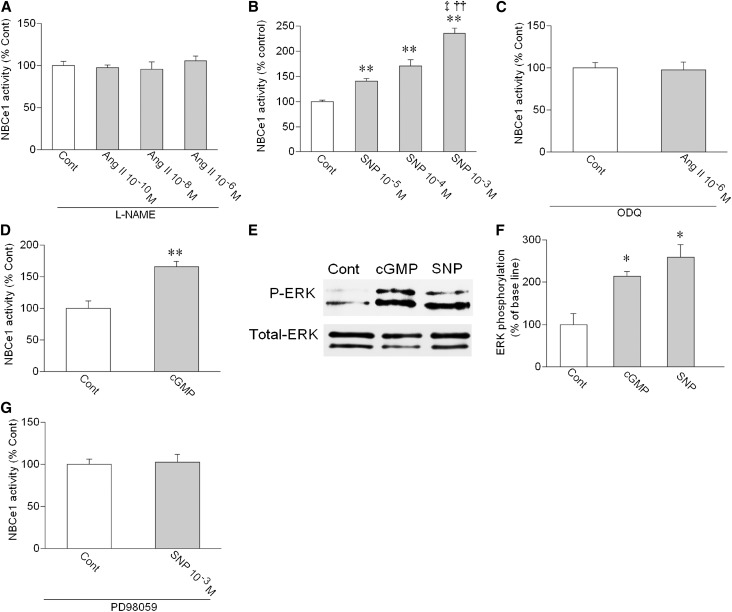
In contrast to the results obtained in mouse and rat PTs, we found that the NOS/NO/sGC/cGMP pathway mediates the stimulatory effect of Ang II in human PTs. Thus, L-NAME completely suppressed the stimulatory effect of Ang II (Figure 4A). SNP dose-dependently stimulated the NBCe1 activity (Figure 4B). Although ODQ totally suppressed the stimulation by 10−6 M Ang II (Figure 4C), 8Br-cGMP significantly stimulated the NBCe1 activity (Figure 4D). Consistent with the stimulatory role of the NO/cGMP pathway, 8Br-cGMP and SNP stimulated ERK phosphorylation in human kidney cortex (Figure 4, E and F). Furthermore, PD98059 completely suppressed the stimulation by 10−3 M SNP (Figure 4G). These results indicate that the ERK activation by the NOS/NO/sGC/cGMP pathway mediates the stimulatory effect of Ang II in human PTs.
Figure 4.
Roles of NO/cGMP in human PTs. (A) Effects of Ang II on NBCe1 in the presence of L-NAME (2×10−4 M). n=4–7 for each concentration of Ang II. (B) Effects of SNP on NBCe1. n=8 for each concentration of SNP; **P<0.01 versus Cont; ‡P<0.01 versus SNP 10−5 M; ††P<0.01 versus SNP 10−4 M. (C) Effect of 10−6 M Ang II on NBCe1 in the presence of ODQ (5×10−5 M). n=7. (D) Effect of 8Br-cGMP (2×10−5 M) on NBCe1. n=4; **P<0.01 versus Cont. (E) Effects of 8Br-cGMP (2×10−5 M) and SNP (10−3 M) on ERK phosphorylation in human kidney cortex samples. (F) Densitometric analysis of ERK phosphorylation. n=5 for both cGMP (2×10−5 M) and SNP (10−3 M); *P<0.05 versus Cont. (G) Effect of SNP on NBCe1 in the presence of PD89059 (10−5 M). n=4.
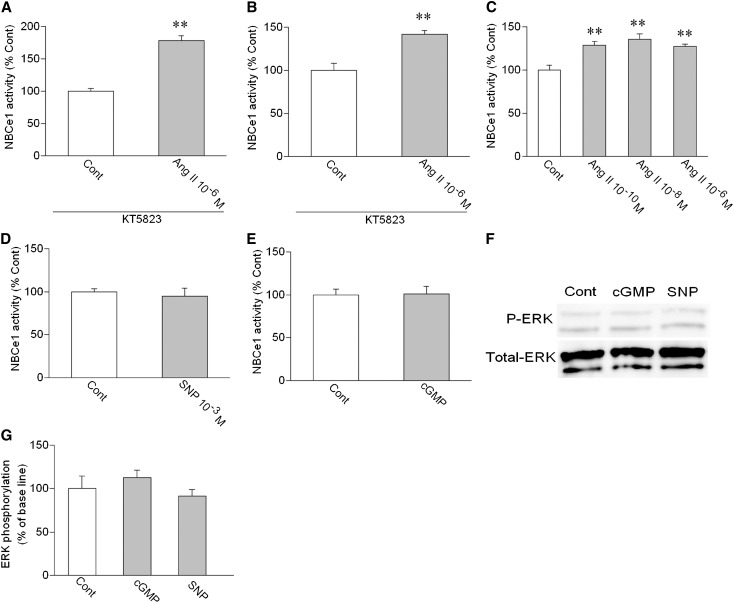
Among several potential signaling pathways, cGMP-dependent protein kinases may represent the principle mediator of cGMP signaling.24 Therefore, we examined the roles of cGKII, the only cGK isoform expressed in PTs.25 The analyses by RT-PCR and immunohistochemistry, indeed, confirmed the cGKII expression in PTs of human subjects and wild-type (WT) mice (Supplemental Figure 2). In PTs of cGKII KO mice,26 however, the cGKII expression was completely absent. A cGK inhibitor KT5823 failed to affect the stimulation by 10−6 M Ang II in human PTs (Figure 5A). However, KT5823 converted the inhibition by 10−6 M Ang II to the stimulation in WT mouse PTs (Figure 5B). Furthermore, the inhibitory effect of Ang II was lost, and all of the Ang II concentrations induced the similar stimulation in cGKII KO mouse PTs (Figure 5C). These results indicate that, although cGKII mediates the inhibitory effect of Ang II in mouse PTs, it is not involved in Ang II signaling in human PTs. The phosphorylation status of GSK-3β, the in vitro and in vivo substrate of cGKII,27 also supported the lack of cGKII activation by the Ang II/NO/cGMP pathway in human PTs (Supplemental Figure 3). Thus, Ang II dose-dependently stimulated GSK-3β phosphorylation in WT mouse kidney cortex as expected. However, Ang II failed to stimulate GSK-3β phosphorylation in human and cGKII KO mouse kidneys. Furthermore, 8Br-cGMP and SNP stimulated GSK-3β phosphorylation in WT mouse kidneys but not human and cGKII KO mouse kidneys.
Figure 5.
Roles of cGKII in Ang II signaling. (A) Effect of 10−6 M Ang II on NBCe1 in the presence of the cGK inhibitor KT5823 (10−6 M) in human PTs. n=5; **P<0.01 versus Cont. (B) Effect of 10−6 M Ang II on NBCe1 in the presence of KT5823 in WT mouse PTs. n=7; **P<0.01 versus Cont. (C) Effects of Ang II on NBCe1 in cGKII KO mouse PTs. n=5–7 for each concentration of Ang II; **P<0.01 versus Cont. (D) Effect of 10−3 M SNP on NBCe1 in cGKII KO mouse PTs. n=7. (E) Effect of 8Br-cGMP (2×10−5 M) on NBCe1 in cGKII KO mouse PTs. n=7. (F) Effects of 8Br-cGMP (2×10−5 M) and SNP (10−3 M) on ERK phosphorylation in cGKII KO mouse kidney cortex. (G) Densitometric analysis of ERK phosphorylation in cGKII KO mouse kidney cortex. n=4 for both cGMP (2×10−5 M) and SNP (10−3 M).
Unlike SNP and 8Br-cGMP in human PTs, SNP (Figure 5D) and 8Br-cGMP (Figure 5E) failed to stimulate the NBCe1 activity in cGKII KO mouse PTs. Moreover, 8Br-cGMP and SNP failed to stimulate ERK phosphorylation in cGKII KO mouse kidney cortex (Figure 5, F and G). These results indicate that the removal of cGKII alone from mouse PTs cannot reproduce the dose-dependent stimulatory effect of Ang II in human PTs that is based on the NO/cGMP-mediated ERK activation.
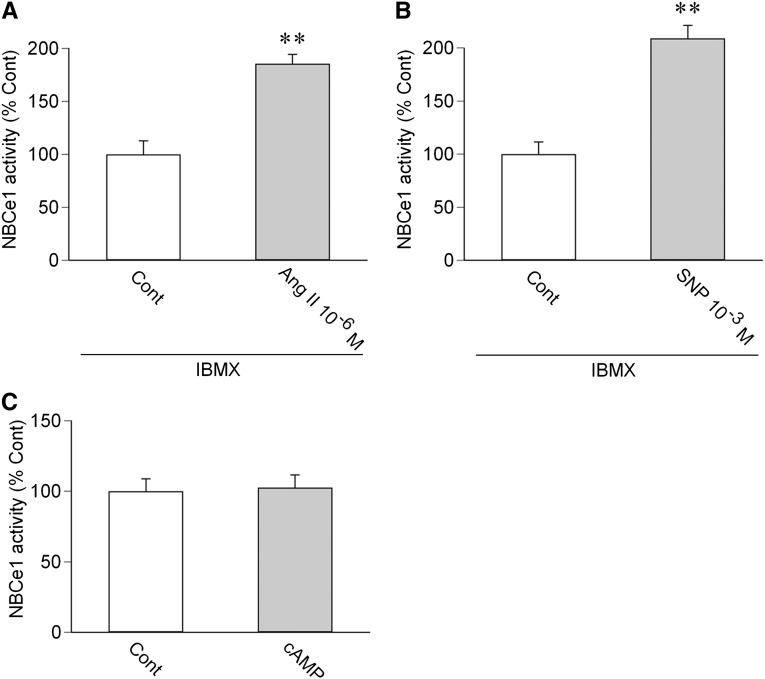
cGMP might also work through the cAMP/protein kinase A pathway through either crossactivation or its effects on cyclic nucleotide phosphodiesterases.24 In human PTs, however, a nonselective inhibitor of phosphodiesterases, isobutylmethylxanthine, did not affect the stimulatory effects of 10−6 M Ang II (Figure 6A) or 10−3 M SNP (Figure 6B). Moreover, dibutyryl cAMP at 2×10−5 M did not affect the NBCe1 activity (Figure 6C). These results do not support the view that the cAMP/protein kinase A pathway mediates Ang II/NO/cGMP-dependent stimulation in human PTs.
Figure 6.
Roles of cAMP/protein kinase A pathway in human PTs. (A) Effect of 10−6 M Ang II on NBCe1 in the presence of isobutylmethylxanthine (IBMX; 10−4 M). n=5; **P<0.01 versus Cont. (B) Effect of 10−3 M SNP on NBCe1 in the presence of IBMX. n=4; **P<0.01 versus Cont. (C) Effect of 2×10−5 M dibutyryl cAMP (cAMP) on NBCe1. n=6.
Finally, we examined Ang II concentration in the human kidney cortex. Mean Ang II concentration was 2.0±0.4 pmol/g tissue (n=8), confirming the high intrarenal Ang II concentration in human subjects.28
Discussion
This study revealed that Ang II has concentration-dependent marked stimulatory effect on human PT transport. The lack of inhibition by arachidonic acid and 5,6-EET may be at least partially involved in the loss of inhibitory effect of Ang II in human PTs. Remarkably, however, the responses to the NOS/NO/sGC/cGMP pathway differed even more sharply, being inhibitory through cGKII activation in mouse PTs but stimulatory through ERK activation in human PTs. These results indicate that the opposite responses to the NO/cGMP pathway may be largely responsible for the difference in PT transport regulation by Ang II among human and other species.
PTs are known to express endothelial NOS, inducible NOS, and neuronal NOS, and they can produce substantial amounts of NO in response to a variety of stimuli.21,22 NO is generally considered to be inhibitory on PT transport.21,22 Moreover, several studies reported that the NO/cGMP pathway mediates the inhibitory effect of Ang II on PT transport.12,18 In the present study, the NOS inhibitor L-NAME, the sGC inhibitor ODQ, and the cGK inhibitor KT5823 converted the inhibition by 10−6 M Ang II to the stimulation in WT mouse PTs. Furthermore, the NO donor SNP and 8Br-cGMP inhibited the NBCe1 activity in WT mouse PTs. SNP was also inhibitory in rat PTs. In cGKII KO mouse PTs, however, the inhibitory effects of SNP and 8Br-cGMP were lost, and all of the concentrations of Ang II induced the similar stimulation. These results indicate that the NO/cGMP/cGKII pathway mediates the inhibitory effect of Ang II in mouse PTs. The cGKII-dependent GSK-3β phosphorylation by Ang II, SNP, and 8Br-cGMP in mouse kidney cortex also supported this view.
In human PTs, by contrast, the ERK-dependent stimulatory effect of Ang II was completely suppressed by L-NAME and ODQ. Both SNP and 8Br-cGMP stimulated the NBCe1 activity, and the stimulatory effect of SNP was dependent on ERK activation. These results indicate that the NO/cGMP/ERK pathway mediates the dose-dependent stimulatory effect of Ang II in human PTs. Although human PTs did express cGKII, KT5823 failed to affect the stimulation by Ang II. Moreover, Ang II, SNP, and 8Br-cGMP stimulated ERK phosphorylation without inducing GSK-3β phosphorylation, suggesting that cGKII is not involved in the Ang II/NO/cGMP-mediated ERK activation in human PTs. The AT1/MEK/ERK pathway also works as a stimulatory signal in mouse PTs.17,19 However, the difference in kinetics for Ang II–mediated ERK phosphorylation shown in this study also supports a view that the mechanisms of Ang II–mediated ERK activation are different in mouse and human PTs.
Although the NO/cGMP pathway may work as either stimulatory or inhibitory on ERK depending on the cell types,29,30 a puzzling question remains unanswered as to why the NO/cGMP pathway, acting as the downstream mediator of Ang II signaling, exerts the contrasting effects on PT transport in humans and other species. However, several previous studies have suggested that such species differences may, indeed, exist in the roles of the NO/cGMP pathway in renal sodium handling. For example, chronic salt loading into normal rats and mice induces an enhanced renal NO synthesis, which facilitates sodium excretion and preservation of normal BP.31–33 NO deficiency may be causally linked to the development of hypertension in salt-sensitive Dahl rats on high-salt diet.34 In healthy human subjects, however, high sodium intake (200 mmol/d) for 5 days decreased plasma renin activity and aldosterone concentrations but did not change renal NO synthesis.35 Ultra-high sodium intake (400 mmol/d) for 3 days also failed to increase renal NO synthesis.36 Furthermore, the NO donor SNP induces marked diuretic and natriuretic responses in conscious rats.35 In human subjects, by contrast, SNP fails to induce significant natriuresis,37 and another SNP donor nitroglycerin even induces sodium retention and plasma volume expansion.38 Thus, the role of NO/cGMP in adaptive natriuretic response to salt loading has been consistently confirmed in experimental animals but not human subjects. It is tempting to speculate that the contrasting effects of NO/cGMP on PT transport revealed in the present study may be at least partially responsible for these conflicting results. It remains to be determined whether the similar species differences also exist in the effects of NO/cGMP on sodium transport in the other nephron segments, such as thick ascending limb and collecting ducts.39
In 1977, Harris and Young3 found that Ang II regulates rat PT transport in a biphasic way. Since that time, the biphasic effects of Ang II on PT transport have been confirmed in rabbits and mice as well.4,9–11,15 The major role of Ang II is thought to be the stimulation of PT transport.2 However, as we confirmed in this study, intrarenal concentration of Ang II (approximately 2 pmol/g tissue) is much higher than the concentration in plasma (approximately 20 fmol/ml) in human subjects.28,40 High intrarenal concentrations of Ang II were also found in mice (approximately 0.5 pmol/g tissue) and rats (approximately 0.1 pmol/g tissue).41,42 Moreover, the high-salt diet paradoxically enhances the luminal Ang II concentration in rat PTs.43 Therefore, the inhibitory effect of high concentrations of Ang II could also have some physiologic significance.5 For example, the inhibitory effect of Ang II may work to prevent unwarranted sequences, such as exaggerated volume expansion, cell hypertrophy, or cell damages. However, the unopposed stimulation of human PT transport by high local concentrations of Ang II suggests that the roles of Ang II in volume homeostasis and BP regulation may be even more important in human subjects than other species. Humans may evolutionally acquire this kind of atypical response to Ang II to adapt to life-threatening salt deprivation. The dose-dependent ERK activation by Ang II observed in human but not mouse PTs suggests that the effects of Ang II on cell survival or differentiation may be also somehow different in human subjects and other species. Interestingly, gene mutations in any components of RAS cause renal tubular dysgenesis characterized by absence or quasiabsence of differentiated PTs in human subjects, whereas inactivation of RAS genes in mice or rats does not induce renal tubular dysgenesis.44
In summary, we identified that, unlike in other species, Ang II in humans dose-dependently stimulates PT transport. This atypical mode of PT transport regulation by Ang II dependent on the NO/cGMP/ERK pathway may represent a human-specific therapeutic target in hypertension.
Concise Methods
Measurements of NBCe1 and NHE3 Activities
All animal procedures were in accordance with local institutional guidelines. Thin slices of kidney cortex were obtained from WT and cGKII KO mice, both of C57BL/6 background,26 or Wistar rats as described.45 Human kidney cortex tissues were obtained during the unilateral nephrectomy for renal carcinoma. The institutional review board of the University of Tokyo School of Medicine approved the study, and written informed consent was obtained from all subjects as described.45 For the analysis of NBCe1 activity, the PT (S2 segment) fragment was microdissected manually and transferred to a perfusion chamber mounted on an inverted microscopy. Both ends of the tubule were sucked into two holding pipettes. To avoid the influence of luminal transporters, the luminally collapsed tubule was used as described.11,17,45 The tubule was incubated with the acetoxymethylester form of a pH-sensitive fluorescence dye 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein (Dojindo), and cell pH was monitored with a photometry system (OSP-10; Olympus). Prewarmed (38°C) DMEM, equilibrated with 5% CO2/95% O2 gas and supplemented with 1 μM norepinephrine, which was shown to be essential for the long-term functional preservation of isolated proximal tubules,15,46 was used for the peritubular perfusate and perfused at 10 ml/min. The intracellular buffer capacity was determined by monitoring the cell pH changes to sudden alteration of bath CO2 tension. The rate of cell pH decrease to bath HCO3− reduction and the buffer capacity were used to calculate the NBCe1 activity as described.11,17,45 Bath HCO3− reduction (from 25 to 12.5 mM) was repeated two or three times during the control period, and only the PTs that show the constant cell pH responses were used for analysis. Valsartan, PD98059, L-NAME, ODQ, KT5823, and isobutylmethylxanthine were used at the concentrations that did not affect the basal NBCe1 activity.
For the analysis of NHE3 activity, cell pH was monitored in the microperfused PT segment as described.45,47 Initially, both luminal and bath sides were perfused with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Hepes)–buffered Ringer solution equilibrated with 100% O2 at approximately 80 nl/min and 10 ml/min, respectively. Thereafter, luminal perfusate was suddenly switched to Na+-free Hepes solution, which replaces Na+ in Hepes-buffered Ringer solution with N-methyl-d-glucamine. The resultant decrease in cell pH was shown to reflect the luminal NHE3 activity.45,47
Determination of Bicarbonate Absorption Rates (JHCO3−)
The rate of bicarbonate absorption (JHCO3−) was measured by the stop-flow microspectrofluorometric method as described.15,48 Isolated human PTs were microperfused with luminal solution containing 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein, 25 mM HCO3−, and 40 mM raffinose, and luminal pH was monitored by the OSP-10 system. To determine JHCO3−, the rapid (approximately 80 nl/min) luminal perfusion was abruptly stopped by reducing the perfusion pressure from approximately 18 to 0 cm H2O. The decay in luminal HCO3− concentration was calculated from the changes in luminal pH, and JHCO3− was calculated as described.15,48
Immunoblotting
Antibodies against total ERK, phospho (Thr202/Thr204) ERK, GSK-3β, and phospho (Ser9) GSK-3β were from Cell Signaling Technology. For detection of phosphorylation of ERK or GSK-3β, thin slices of kidney cortex were obtained. They were divided into pieces of small bundles, consisting mostly of PTs. To reduce the influence of locally produced Ang II, these samples were incubated at 37°C for 40 minutes in DMEM containing 10−7 M captoril under 5% CO2 as described.17,45 After the test agonists were added for 2 minutes, samples were homogenized in ice cold lysis buffer containing 25 mM Tris⋅HCl (pH 7.4), 10 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM EDTA, 10 mM EGTA, and 1 mM phenylmethylsulfonyl fluoride, and they were centrifuged at 1000g for 5 minutes. Equal amounts of protein samples were obtained from the cell lysates, separated by SDS-PAGE on acrylamide minigels, and transferred to a nitrocellulose membrane. After incubation in blocking buffer, the membrane was treated with one of the primary antibodies and then, their respective secondary antibodies. The signal was detected by an ECL Plus system (Amersham).
Immunohistochemical Analysis
To examine the intrarenal expression of cGKII, the kidney samples from humans and mice were frozen without fixation and cryosectioned at 10-μm thick; then, they were fixed with buffered 4% paraformaldehyde for 10 minutes. After being washed with PBS, sections were serially incubated with the anti-cGKII antibody (Santa Cruz Biotechnology) for 1 hour and the mixtures of Cy5-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories), Alexa Fluor 568 phalloidin (Molecular Probes), and SYBR Green I (Molecular Probes) for nuclei for 30 minutes at room temperature as described.45 The specimens were observed with a confocal laser scanning microscope (LSM510META; Carl Zeiss).
Detection of mRNA for cGKII
Total RNA in renal cortex tissues was extracted by ISOGEN (Nippon Gene), and RT-PCR was performed with the SuperScript III One-Step RT-PCR System (Invitrogen). The primers for cGKII were 5′-TGAGAAAAAGCTCATCACAGATGC-3′ (sense) and 5′-CGGCCAGCACAAAGATATGG-3′ (antisense). The primers for glyceraldehyde 3-phosphate dehydrogenase were 5′-ACCACAGTCCATGCCATCAC-3′ (sense) and 5′-TCCACCACCCTGTTGCTGTA-3′ (antisense).
Determination of Intrarenal Ang II Concentration
Ang II concentration in human kidney cortex was determined as previously described.42,49 The kidney samples were homogenized in 100% methanol, evaporated in a vacuum centrifuge, reconstituted in assay buffer, and assayed.
Statistical Analyses
Data are means±SEMs. Significant differences were determined by applying t test or ANOVA with Bonferroni’s adjustment as appropriate. Statistical significance was set at P<0.05.
Disclosures
This study was supported, in part, by a research grant from Novartis.
Supplementary Material
Acknowledgments
The authors thank Ms. Tomoko Miura (Department of Anatomy, Kyorin University School of Medicine) for technical assistance.
This study was supported, in part, by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013060596/-/DCSupplemental.
References
- 1.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM: Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM: AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab 13: 469–475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris PJ, Young JA: Dose-dependent stimulation and inhibition of proximal tubular sodium reabsorption by angiotensin II in the rat kidney. Pflugers Arch 367: 295–297, 1977 [DOI] [PubMed] [Google Scholar]
- 4.Schuster VL, Kokko JP, Jacobson HR: Angiotensin II directly stimulates sodium transport in rabbit proximal convoluted tubules. J Clin Invest 73: 507–515, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navar LG, Harrison-Bernard LM, Wang CT, Cervenka L, Mitchell KD: Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol 10[Suppl 11]: S189–S195, 1999 [PubMed] [Google Scholar]
- 6.Fukuchi S: Estimation of urinary angiotensin II by radioimmunoassay. Tohoku J Exp Med 114: 205–213, 1974 [DOI] [PubMed] [Google Scholar]
- 7.Houillier P, Chambrey R, Achard JM, Froissart M, Poggioli J, Paillard M: Signaling pathways in the biphasic effect of angiotensin II on apical Na/H antiport activity in proximal tubule. Kidney Int 50: 1496–1505, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Banday AA, Lokhandwala MF: Angiotensin II-mediated biphasic regulation of proximal tubular Na+/H+ exchanger 3 is impaired during oxidative stress. Am J Physiol Renal Physiol 301: F364–F370, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Coppola S, Frömter E: An electrophysiological study of angiotensin II regulation of Na-HCO3 cotransport and K conductance in renal proximal tubules. I. Effect of picomolar concentrations. Pflugers Arch 427: 143–150, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Coppola S, Frömter E: An electrophysiological study of angiotensin II regulation of Na-HCO3 cotransport and K conductance in renal proximal tubules. II. Effect of micromolar concentrations. Pflugers Arch 427: 151–156, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Horita S, Zheng Y, Hara C, Yamada H, Kunimi M, Taniguchi S, Uwatoko S, Sugaya T, Goto A, Fujita T, Seki G: Biphasic regulation of Na+-HCO3- cotransporter by angiotensin II type 1A receptor. Hypertension 40: 707–712, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Zhang C, Mayeux PR: NO/cGMP signaling modulates regulation of Na+-K+-ATPase activity by angiotensin II in rat proximal tubules. Am J Physiol Renal Physiol 280: F474–F479, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Poggioli J, Lazar G, Houillier P, Gardin JP, Achard JM, Paillard M: Effects of angiotensin II and nonpeptide receptor antagonists on transduction pathways in rat proximal tubule. Am J Physiol 263: C750–C758, 1992 [DOI] [PubMed] [Google Scholar]
- 14.Haithcock D, Jiao H, Cui XL, Hopfer U, Douglas JG: Renal proximal tubular AT2 receptor: Signaling and transport. J Am Soc Nephrol 10[Suppl 11]: S69–S74, 1999 [PubMed] [Google Scholar]
- 15.Zheng Y, Horita S, Hara C, Kunimi M, Yamada H, Sugaya T, Goto A, Fujita T, Seki G: Biphasic regulation of renal proximal bicarbonate absorption by luminal AT1A receptor. J Am Soc Nephrol 14: 1116–1122, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Liu FY, Cogan MG: Angiotensin II stimulates early proximal bicarbonate absorption in the rat by decreasing cyclic adenosine monophosphate. J Clin Invest 84: 83–91, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Yamada H, Kita Y, Kunimi M, Horita S, Suzuki M, Endo Y, Shimizu T, Seki G, Fujita T: Roles of ERK and cPLA2 in the angiotensin II-mediated biphasic regulation of Na+-HCO3− transport. J Am Soc Nephrol 19: 252–259, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banday AA, Lokhandwala MF: Loss of biphasic effect on Na/K-ATPase activity by angiotensin II involves defective angiotensin type 1 receptor-nitric oxide signaling. Hypertension 52: 1099–1105, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Yamada H, Kita Y, Suzuki M, Endo Y, Horita S, Yamazaki O, Shimizu T, Seki G, Fujita T: Arachidonic acid metabolites inhibit the stimulatory effect of angiotensin II in renal proximal tubules. Hypertens Res 31: 2155–2164, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Sandberg K, Ji H, Clark AJ, Shapira H, Catt KJ: Cloning and expression of a novel angiotensin II receptor subtype. J Biol Chem 267: 9455–9458, 1992 [PubMed] [Google Scholar]
- 21.Liang M, Knox FG: Production and functional roles of nitric oxide in the proximal tubule. Am J Physiol Regul Integr Comp Physiol 278: R1117–R1124, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Ortiz PA, Garvin JL: Cardiovascular and renal control in NOS-deficient mouse models. Am J Physiol Regul Integr Comp Physiol 284: R628–R638, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Wang T: Nitric oxide regulates HCO3- and Na+ transport by a cGMP-mediated mechanism in the kidney proximal tubule. Am J Physiol 272: F242–F248, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA: Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52: 375–414, 2000 [PubMed] [Google Scholar]
- 25.Gambaryan S, Häusler C, Markert T, Pöhler D, Jarchau T, Walter U, Haase W, Kurtz A, Lohmann SM: Expression of type II cGMP-dependent protein kinase in rat kidney is regulated by dehydration and correlated with renin gene expression. J Clin Invest 98: 662–670, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeifer A, Aszódi A, Seidler U, Ruth P, Hofmann F, Fässler R: Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science 274: 2082–2086, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki Y, Kugimiya F, Chikuda H, Kamekura S, Ikeda T, Kawamura N, Saito T, Shinoda Y, Higashikawa A, Yano F, Ogasawara T, Ogata N, Hoshi K, Hofmann F, Woodgett JR, Nakamura K, Chung UI, Kawaguchi H: Phosphorylation of GSK-3beta by cGMP-dependent protein kinase II promotes hypertrophic differentiation of murine chondrocytes. J Clin Invest 118: 2506–2515, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McPherson EA, Luo Z, Brown RA, LeBard LS, Corless CC, Speth RC, Bagby SP: Chymase-like angiotensin II-generating activity in end-stage human autosomal dominant polycystic kidney disease. J Am Soc Nephrol 15: 493–500, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Yu X, Brecher P: Nitric oxide and N-acetylcysteine inhibit the activation of mitogen-activated protein kinases by angiotensin II in rat cardiac fibroblasts. J Biol Chem 273: 33027–33034, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Rangaswami H, Marathe N, Zhuang S, Chen Y, Yeh JC, Frangos JA, Boss GR, Pilz RB: Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J Biol Chem 284: 14796–14808, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shultz PJ, Tolins JP: Adaptation to increased dietary salt intake in the rat. Role of endogenous nitric oxide. J Clin Invest 91: 642–650, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tolins JP, Shultz PJ: Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int 46: 230–236, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Jia Z, Zhang A, Zhang H, Dong Z, Yang T: Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res 99: 1243–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Kone BC, Baylis C: Biosynthesis and homeostatic roles of nitric oxide in the normal kidney. Am J Physiol 272: F561–F578, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Facchini FS, DoNascimento C, Reaven GM, Yip JW, Ni XP, Humphreys MH: Blood pressure, sodium intake, insulin resistance, and urinary nitrate excretion. Hypertension 33: 1008–1012, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Schmidt RJ, Beierwaltes WH, Baylis C: Effects of aging and alterations in dietary sodium intake on total nitric oxide production. Am J Kidney Dis 37: 900–908, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White WB, Halley SE: Comparative renal effects of intravenous administration of fenoldopam mesylate and sodium nitroprusside in patients with severe hypertension. Arch Intern Med 149: 870–874, 1989 [PubMed] [Google Scholar]
- 38.Parker JD, Farrell B, Fenton T, Cohanim M, Parker JO: Counter-regulatory responses to continuous and intermittent therapy with nitroglycerin. Circulation 84: 2336–2345, 1991 [DOI] [PubMed] [Google Scholar]
- 39.Ortiz PA, Garvin JL: Role of nitric oxide in the regulation of nephron transport. Am J Physiol Renal Physiol 282: F777–F784, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Mølstrøm S, Larsen NH, Simonsen JA, Washington R, Bie P: Normotensive sodium loading in normal man: Regulation of renin secretion during beta-receptor blockade. Am J Physiol Regul Integr Comp Physiol 296: R436–R445, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG: Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol 298: F150–F157, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox J, Guan S, Hymel AA, Navar LG: Dietary Na and ACE inhibition effects on renal tissue angiotensin I and II and ACE activity in rats. Am J Physiol 262: F902–F909, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Thomson SC, Deng A, Wead L, Richter K, Blantz RC, Vallon V: An unexpected role for angiotensin II in the link between dietary salt and proximal reabsorption. J Clin Invest 116: 1110–1116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gribouval O, Morinière V, Pawtowski A, Arrondel C, Sallinen SL, Saloranta C, Clericuzio C, Viot G, Tantau J, Blesson S, Cloarec S, Machet MC, Chitayat D, Thauvin C, Laurent N, Sampson JR, Bernstein JA, Clemenson A, Prieur F, Daniel L, Levy-Mozziconacci A, Lachlan K, Alessandri JL, Cartault F, Rivière JP, Picard N, Baumann C, Delezoide AL, Belar Ortega M, Chassaing N, Labrune P, Yu S, Firth H, Wellesley D, Bitzan M, Alfares A, Braverman N, Krogh L, Tolmie J, Gaspar H, Doray B, Majore S, Bonneau D, Triau S, Loirat C, David A, Bartholdi D, Peleg A, Brackman D, Stone R, DeBerardinis R, Corvol P, Michaud A, Antignac C, Gubler MC: Spectrum of mutations in the renin-angiotensin system genes in autosomal recessive renal tubular dysgenesis. Hum Mutat 33: 316–326, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Endo Y, Suzuki M, Yamada H, Horita S, Kunimi M, Yamazaki O, Shirai A, Nakamura M, Iso-O N, Li Y, Hara M, Tsukamoto K, Moriyama N, Kudo A, Kawakami H, Yamauchi T, Kubota N, Kadowaki T, Kume H, Enomoto Y, Homma Y, Seki G, Fujita T: Thiazolidinediones enhance sodium-coupled bicarbonate absorption from renal proximal tubules via PPARγ-dependent nongenomic signaling. Cell Metab 13: 550–561, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Müller-Berger S, Coppola S, Samarzija I, Seki G, Frömter E: Partial recovery of in vivo function by improved incubation conditions of isolated renal proximal tubule. I. Change of amiloride-inhibitable K+ conductance. Pflugers Arch 434: 373–382, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Yamada H, Seki G, Taniguchi S, Uwatoko S, Nosaka K, Suzuki K, Kurokawa K: Roles of Ca2+ and PKC in regulation of acid/base transport in isolated proximal tubules. Am J Physiol 271: F1068–F1076, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Müller-Berger S, Samarzija I, Kunimi M, Yamada H, Frömter E, Seki G: A stop-flow microperfusion technique for rapid determination of HCO3- absorption/H+ secretion by isolated renal tubules. Pflugers Arch 439: 208–215, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Nishiyama A, Seth DM, Navar LG: Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension 39: 129–134, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.