Abstract
Human B cells with immunoregulatory properties in vitro (Bregs) have been defined by the expression of IL-10 and are enriched in various B-cell subsets. However, proinflammatory cytokine expression in B-cell subsets is largely unexplored. We examined the cytokine profiles of human PBMCs and found that subsets of CD24hiCD38hi transitional B cells (TrBs), CD24hiCD27+ memory B cells, and naïve B cells express IL-10 and the proinflammatory cytokine TNF-α simultaneously. TrBs had the highest IL-10/TNF-α ratio and suppressed proinflammatory helper T cell 1 (Th1) cytokine expression by autologous T cells in vitro more potently than memory B cells did, despite similar IL-10 expression. Whereas neutralization of IL-10 significantly inhibited TrB-mediated suppression of autologous Th1 cytokine expression, blocking TNF-α increased the suppressive capacity of both memory and naïve B-cell subsets. Thus, the ratio of IL-10/TNF-α expression, a measure of cytokine polarization, may be a better indicator of regulatory function than IL-10 expression alone. Indeed, compared with TrB cells from patients with stable kidney graft function, TrBs from patients with graft rejection displayed similar IL-10 expression levels but increased TNF-α expression (i.e., reduced IL-10/TNF-α ratio), did not inhibit in vitro expression of Th1 cytokines by T cells, and abnormally suppressed expression of Th2 cytokines. In patients with graft dysfunction, a low IL-10/TNF-α ratio in TrBs associated with poor graft outcomes after 3 years of follow-up. In summary, these results indicate that B cell–mediated immune regulation is best characterized by the cytokine polarization profile, a finding that was confirmed in renal transplant patients.
Keywords: chronic allograft rejection, immunology, renal function decline
B lymphocytes have functions beyond the production of antibodies.1–9 An immunoregulatory role for B cells, first described in murine colitis,10 has since been supported by studies in a variety of autoimmune and alloimmune models.11–16 Regulatory B-cell (Breg) activity is characteristically IL-10 dependent. Murine B cells also express a variety of proinflammatory cytokines that can polarize T cells in vitro and markedly influence the immune response to pathogens in vivo.5,9 However, the phenotype of B cells expressing proinflammatory cytokines is largely unknown.
In humans, CD19+CD24hiCD38hi transitional B cells (TrBs) isolated from peripheral blood were shown to be enriched for IL-10 expression, and inhibit inflammatory cytokine expression by autologous CD4+ T cells in vitro.17 In patients with SLE, TrBs were defective in IL-10 expression and unable to suppress helper T cell 1 (Th1) responses in vitro.17 By contrast, another study showed that human Bregs, defined by IL-10 expression, were enriched in the CD19+CD24hiCD27+ “memory” subpopulation18 with increased B-cell IL-10 expression in patients with autoimmune disorders.
Although IL-10 expression by putative regulatory B-cell subsets has been studied, the concomitant expression of proinflammatory cytokines, possibly by the very same B cells, and their effect on regulatory activity in vivo or in vitro is unknown. Such insights may aid further description of Breg subsets and clarify discrepancies in the literature regarding the influence of B cells in inflammatory settings.
In this study, we characterize B cells in human peripheral blood based upon both proinflammatory (TNF-α) and anti-inflammatory (IL-10) cytokine expression and show that a simple ratio of IL-10 to TNF-α best characterizes B-cell immune regulatory function. Based on the IL-10/TNF-α ratio, we show that TrBs exhibit the most anti-inflammatory cytokine profile in vitro. Whereas TrBs in renal allograft recipients with stable function were similar to healthy controls, those from patients with rejection were quantitatively and qualitatively altered. Remarkably, a reduced TrB IL-10/TNF-α ratio was predictive of worse clinical outcome.
Results
Human (CD19+CD24hiCD38hi) TrBs Exhibit the Most Polarized Anti-Inflammatory Cytokine Profile Based on IL-10 and TNF-α Expression
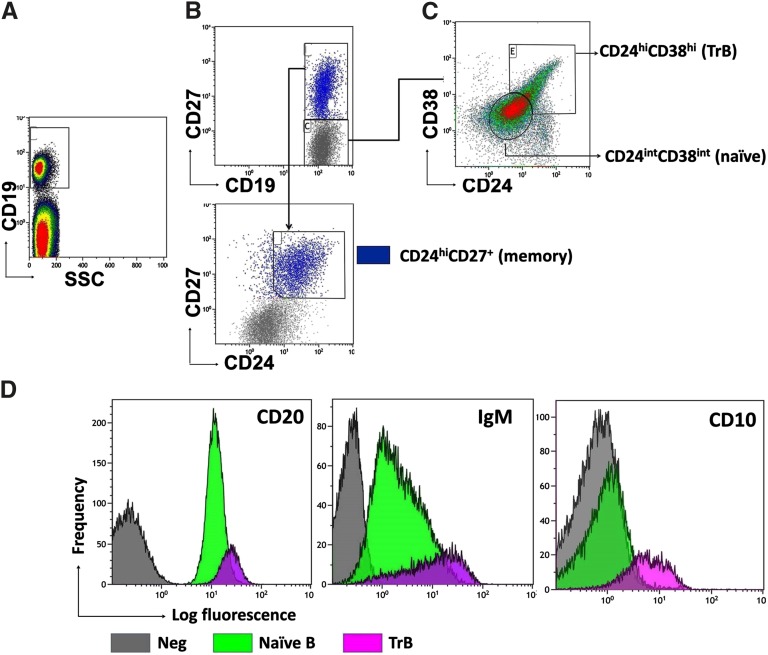
PBMCs from 15 healthy volunteers were analyzed and divided into three distinct B-cell (CD19+) subsets; CD27−CD24hiCD38hi TrBs, CD24hiCD27+ memory B cells, and CD27−CD24+CD38+ naïve B cells (Figure 1, A–C) as previously described.18,19 Further phenotypic characterization of both naïve and TrBs revealed that TrBs were CD20hi, CD10+, and IgMhi, whereas naïve B cells were CD20+, CD10−, and IgMint (Figure 1D), consistent with previous reports.19,20
Figure 1.
Characterization of transitional, memory, and naïve B subsets. (A–C) Definition of human B subsets including CD19+CD24hiCD38hiCD27− TrB cells, CD19+CD24+CD38+ CD27− naïve B cells, and CD19+CD24hiCD27+ memory B cells. (D) Representative overlay histograms comparing the strength of expression of CD20, CD10, and IgM between the TrB and mature naïve B cells (n=5).
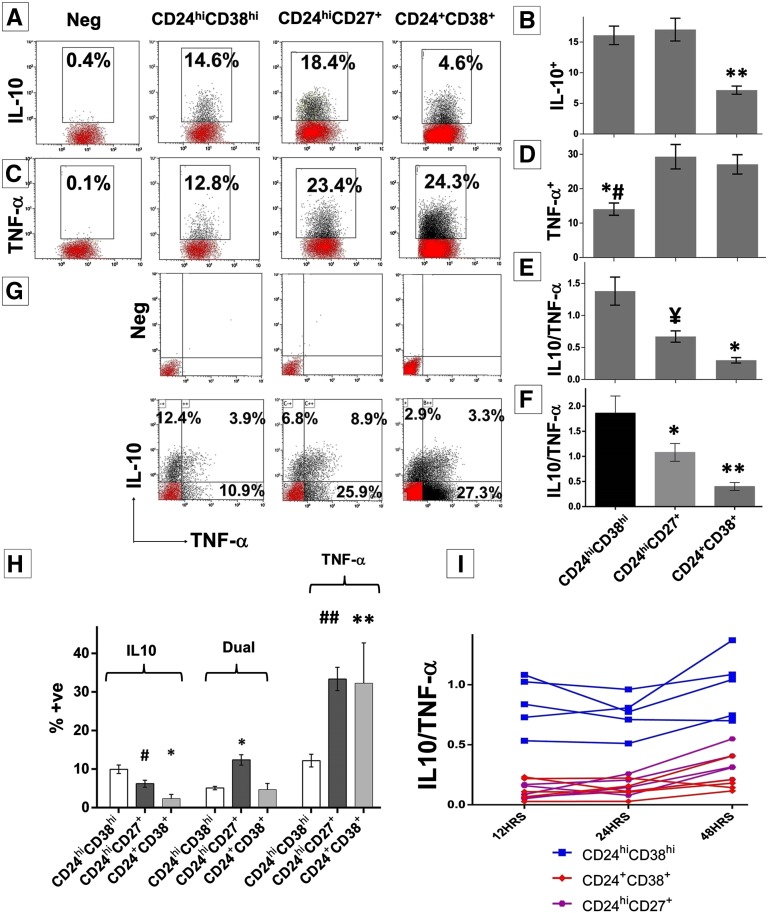
Next, magnetic bead–enriched (CD19+) B cells were stimulated with CpG and CD40L for 48 hours and analyzed for cytokine expression by intracellular staining, as previously described.18 Both memory and TrBs were enriched for IL-10 expression compared with naïve B cells17,18 (Figure 2, A and B). These B-cell subsets also expressed the proinflammatory cytokine, TNF-α, at a surprisingly high frequency, which approximated or even surpassed that of IL-10 expression. TrBs contained the fewest TNF-α+ B cells (Figure 2, C and D). Taken together, TrBs had the highest IL-10/TNF-α ratio, followed by memory B cells, whereas naïve B cells had the lowest (Figure 2E). Thus, CD24hiCD38hi TrBs exhibit the most anti-inflammatory cytokine profile. To address the possibility that in vitro stimulation could alter phenotype, B subsets were first purified and then cultured and cytokine expression was analyzed by ELISA. These results paralleled those obtained above, in which cells were assessed for phenotype after in vitro culture (Figure 2F).
Figure 2.
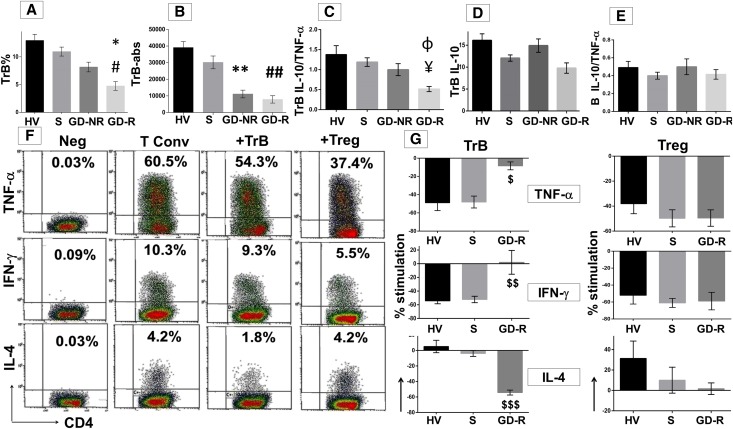
Analysis of IL-10 and TNF-α expression by B subsets. Magnetic bead–enriched CD19+ B cells are stimulated with CpG and CD40L for 48 hours plus phorbol ester, ionomycin, and brefeldin A (last 5 hours). (A and C) Each representative dot plot shows the frequencies of IL-10+ or TNF-α+ B cells within the respective B subsets. (B and D) Cumulative frequency (mean±SEM) of IL-10 (*P<0.001, TrB versus memory or naive) and TNF-α producing cells (*#P=0.001, TrB versus memory; P=0.01, TrB versus naïve) within each B cell subset in 15 healthy volunteers. (E) Analysis of the ratio of IL-10+/TNF-α+ cells within B cell subsets analyzed by flow cytometry (¥P=0.002, TrB versus memory; *P<0.001, TrB versus naïve). (F) Cumulative results expressed as mean±SEM for the IL-10/TNF-α ratio obtained from purified B cell subsets and analyzed by ELISA in 11 healthy volunteers (*P<0.001, TrB versus memory or naive). (G) Representative dot plot for the combined analysis of IL-10 and TNF-α by dual staining in the B subsets. (H) Cumulative results (mean±SEM) from six healthy volunteers of the distribution of either IL-10+ or TNF-α+ or mixed cytokine-positive B cells (*P<0.001; #P=0.01; **P=0.001; ##P<0.001, in comparison with TrBs). (I) Analysis of IL-10+ and TNF-α+ B subsets at 12, 24, and 48 hours in five independent controls after stimulation with CpG and CD40L. The graphs represent the IL-10/TNF-α ratio obtained for the respective B-cell subset at the three different time points in five healthy volunteers. Statistical analysis is performed by ANOVA using Tukey post hoc correction for multiple comparisons.
We next sought to determine whether individual B cells simultaneously express more than one cytokine, using dual-intracellular staining. This analysis revealed that a significant fraction of B cells coexpressed both IL-10 and TNF-α. Although TrBs had the highest frequency of B cells expressing IL-10 alone, memory B cells had the largest fraction of dual positive cells, and both memory and naïve B-cell populations were enriched for cells expressing TNF-α+ alone (Figure 2, G and H). The nature of cytokine polarization within these B-cell subsets remained stable when analyzed at 12, 24, and 48 hours (Figure 2I).
TrBs Selectively Inhibit Th1 Cytokine Expression in an IL-10–Dependent Fashion
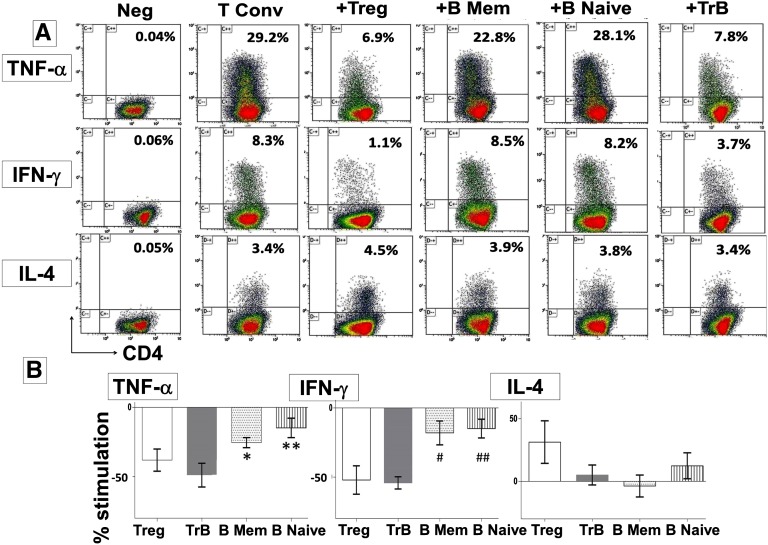
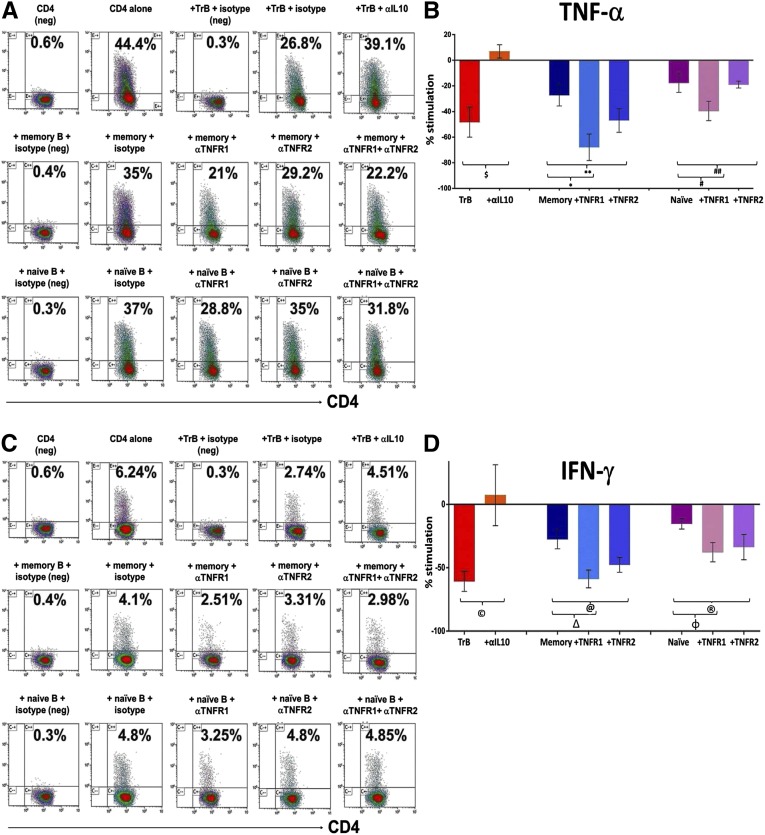
To determine the regulatory capacity of each B-cell subset, purified populations from healthy volunteers were examined for their ability to inhibit cytokine expression by anti-CD3 stimulated autologous CD4+CD25− conventional T cells (Tconvs). The regulatory activity of B-cell subpopulations was compared with that of an equal number of CD4+CD25hi regulatory T cells (Tregs) in the same assay (Figure 3). Sorting gates and purities of the cell subsets are shown in Supplemental Figure 1. TrBs were more potent at selectively suppressing TNF-α and IFN-γ expression by Tconvs with no significant effect on IL-4, compared with memory or naïve B cells, and similar in potency to Tregs (Figure 3). Thus, the strength of suppression in vitro correlates with and supports use of the IL-10/TNF-α ratio to define regulatory B cells (Bregs). The addition of neutralizing anti–IL-10 antibody to TrB cocultured with autologous Tconvs, restored both TNF-α (Figure 4, A and B) and IFN-γ expression (Figure 4, C and D) by CD4 cells to the baseline levels observed when Tconvs were cultured alone. Thus, regulatory activity of TrBs was IL-10 dependent. The influence of TNF-α on regulatory function of memory or naïve B cells was analyzed by adding neutralizing antibodies to its receptors, TNFR1 and TNFR2, during coculture with Tconvs (Figure 4). Neutralizing TNFR1 increased the ability of memory B cells to suppress TNF-α and IFN-γ expression to levels comparable with those obtained with TrBs. Neutralization of TNFR2 was somewhat less effective than neutralizing TNFR1. Neutralizing TNFR1 and TNFR2 on naïve B cells also increased suppression, but the effect was much smaller than the effect on memory B cells, perhaps as a result of lower IL-10 expression by the naïve subset. In summary, neutralizing IL-10 inhibited the regulatory effects of TrBs, whereas blocking TNF receptors significantly improved the suppressive capacity of both memory and naïve B cells. This highlights the significance of both IL-10 and TNF-α in regulation of CD4 T cells by B-cell subsets. Supplemental Figure 2 shows the effect of TrB on CD45RA+ and CD45RA− CD4 T cells.
Figure 3.
TrB cells with a high IL-10/TNF-α ratio selectively suppress inflammatory cytokines by conventional T cells. Magnetic bead–enriched Tconv cells (CD4+CD25−) are stimulated for 72 hours with plate-bound anti-human CD-3 (0.5 µg/ml) in the presence of TrB (CD24hiCD38hi) B cells, CD24hiCD27+ memory B cells, CD24+CD38+ naïve B cells, or CD4+CD25hi Treg cells. Phorbol ester, ionomycin, and brefeldin A are added for the last 6 hours of cell culture. Data are derived from six healthy volunteers. (A) Representative dot plots showing frequency of TNF-α, IFN-γ, and IL-4 expression among Tconvs in the respective cell cultures. (B) Cumulative percentage stimulation/inhibition of the frequency of TNF-α+, IFN-γ+, or IL-4+cells (mean±SEM) after culture with Tregs or the respective B subsets. (*P<0.05, TrB versus CD24hiCD27+; **P=0.03, TrB versus CD24+CD38+; #P=0.02, TrB versus CD24hiCD27+; ##P=0.01, TrB versus CD24+CD38+).
Figure 4.
The IL-10/TNF-α ratio predicts the capacity of the B subsets to suppress autologous Th1 cytokines. Magnetic bead-enriched Tconvs (CD4+CD25−) are stimulated for 72 hours with plate-bound anti-human CD-3 (0.5 µg/ml) in the presence of TrB (CD24hiCD38hi) B cells, CD24hiCD27+ memory B cells, CD24+CD38+ naïve B cells, or CD4+CD25hi Treg cells with either an isotype control or neutralizing antibodies to IL-10, TNFR1, TNFR2, or a combination of TNFR1 and TNFR2. Phorbol ester, ionomycin, and brefeldin A are added for the last 6 hours of cell culture. Data are derived from three healthy volunteers. (A and C) Representative scatter plots showing the detection of TNF-α and IFN-γ by intracellular staining when Tconvs are cultured alone or in the presence of TrB, memory, and naïve B cells with matched isotype controls or neutralizing antibodies to IL-10, TNFR1, or TNFR2. Appropriate isotype controls (negative controls) for cytokine staining labeled as “neg” are included for defining the cytokine positive gates for respective cell subsets. (B and D) Cumulative percentage stimulation/inhibition of the frequency of TNF-α+ ($P=0.01; *P=0.02; **P=0.10; #P=0.20; ##P=0.80) and IFN-γ+ (©P=0.03; ΔP=0.01; @P=0.04; φP=0.2; ®P=0.1) T cells (mean±SEM) after culture with the respective B subsets and neutralizing antibodies. Statistical analysis is performed by a paired t test.
Renal Transplant Recipients with Rejection Exhibit Qualitative and Quantitative Differences in TrBs
Having established the regulatory properties of TrBs, we investigated whether quantitative differences exist in renal transplant patients with stable allograft function versus rejection. TrBs from healthy volunteers (n=15) were compared with 88 renal allograft recipients (26–320 months after transplantation) who were predominantly nonsensitized recipients of first deceased donor transplants (79%) treated with basiliximab induction and maintained on calcineurin inhibitors and mycophenolate mofetil without maintenance steroids (Tables 1 and 2). Transplant recipients with stable function (n=41) were compared with 47 patients who underwent biopsies for graft dysfunction. On the basis of graft histology, this group was divided into recipients with graft dysfunction and no rejection (GD-NR; n=22) and recipients with graft dysfunction with rejection (GD-R; n=25). The mean time to PBMC sampling from the date of the biopsy was 15.8±9.3 days for the GD-NR group and 18.5±17.9 days for the GD-R group (P=NS). Clinical parameters for these participants are detailed in Table 2. Significant differences between the groups included a longer time since transplantation for GD groups and a higher usage of maintenance prednisolone as immunosuppression in the GD-R group, in part reflecting a change made at the time of diagnosis for the six patients with a concomitant diagnosis of T cell–mediated rejection and the nine patients who were taking long-term maintenance steroids. No other specific therapeutic interventions were initiated in these patients before blood sampling. Histologic classification of the GD-R group is detailed in Figure 5 and graft dysfunction in this group was largely attributable to chronic antibody-mediated rejection, although 25% of the patients also had a component of T cell–mediated rejection. Eighty percent of these patients (20 of 25) had a donor-specific antibody detected at the time of the biopsy.
Table 1.
Patient characteristics
| Clinical Parameter | Healthy Volunteers (n=15) | Patients with Stable Allograft Function (n=41) | Patients with GD-NR (n=22) | Patients with GD-R (n=25) | P Value |
|---|---|---|---|---|---|
| Age, yr (95% CI) | 45 (41 to 49) | 49 (44 to 53) | 51 (45 to 58) | 49 (43 to 55) | NS |
| Men | 61 | 66 | 64 | 64 | NS |
| White ethnicity | 68 | 91 | 92 | 95 | 0.03a |
| Primary renal diagnosis | NS | ||||
| GN | N/A | 15 | 45 | 28 | 0.01b |
| Hypertension/diabetes | N/A | 15 | 9 | 4 | |
| Inherited | N/A | 15 | 14 | 16 | |
| Othersc | N/A | 55 | 32 | 52 | |
| Transplantation details | |||||
| Graft number | N/A | 1.06 (1 to 1.15) | 1.26 (1.04 to 1.48) | 1.1 (0.95 to 1.26) | NS |
| Type of allograft donor | NS | ||||
| Donation after circulatory death | N/A | 12 | 14 | 12 | |
| Donation after brain death | N/A | 68 | 68 | 64 | |
| Live donor | N/A | 20 | 18 | 24 | |
| Donor cause of death | NS | ||||
| Trauma | N/A | 15 | 29 | 20 | |
| Vascular | N/A | 77 | 47 | 53 | |
| Other | N/A | 8 | 24 | 27 | |
| HLA mismatches | N/A | 2.6 (2.1 to 3.1) | 1.6 (1.0 to 2.3) | 2.9 (2.5 to 3.5) | 0.02d |
| Donor age, yr (95% CI) | N/A | 39 (33 to 45) | 47 (38 to 55) | 42 (35 to 49) | NS |
Data are presented as percentages or medians (interquartile ranges) unless otherwise indicated. All categorical variables were compared using the chi-squared test. All continuous variables are analyzed using ANOVA with Tukey post hoc test for multiple comparisons if normally distributed and the Kruskal–Wallis test for variables with skewed distribution. Adjusted P values for multiple comparisons after the post hoc tests are presented. P<0.05 is significant. N/A, not applicable.
Significant differences between healthy volunteers and the rest of the study population.
Significant difference between patients with stable allograft function and the GD-NR group.
Others include obstruction, chronic pyelonephritis, and unknown causes.
Significant difference between GD-NR and other groups (stable allograft function versus GD-NR, P=0.02; GD-NR versus GD-R, P=0.01).
Table 2.
Allograft characteristics at the time of biopsy
| Clinical Parameter | Healthy Volunteers | Patients with Stable Allograft Function | Patients with GD-NR | Patients with GD-R | P Value |
|---|---|---|---|---|---|
| Allograft function | |||||
| Age of the graft (mo)a | N/A | 56 (43 to 78) | 78 (61 to 128) | 83 (51 to 153) | 0.04b |
| Best eGFR achieved | N/A | 74 (69 to 79) | 59 (48 to 69) | 66 (56 to 76) | 0.01c |
| eGFR (ml/min) | N/A | 65 (61 to 70) | 31 (25 to 36) | 29 (22 to 36) | <0.001d |
| Proteinuria (g/L) | N/A | 0.17 (0.13 to 0.20) | 0.84 (0.51 to 1.18) | 1.47 (0.67 to 2.26) | 0.01d |
| Maintenance immunosuppression | |||||
| CNI | N/A | 97 | 92 | 83 | NS |
| MMF/azathioprine | N/A | 71 | 76 | 78 | NS |
| Prednisolone | N/A | 7 | 18 | 60 | 0.01e |
| DSA-HLA class-1% positive | N/A | 9 | 2.5 | 48 | <0.001e |
| DSA-HLA class-2% positive | n/a | 0 | 2.5 | 36 | <0.001e |
| Late cellular rejection | n/a | 0 | 23 | 25 | |
| Allograft pathology | N/A | N/A | |||
| Recurrent GN | N/A | N/A | 27 | ||
| Othersf | N/A | N/A | 32 | ||
| IFTA (scarring) | N/A | N/A | 23 | ||
| Viral infection | 9 | ||||
| CNI toxicity | N/A | N/A | 9 | ||
| Chronic antibody-mediated changes | |||||
| Glomerulitis | N/A | N/A | 0 | 44 | |
| Transplant glomerulopathyg | N/A | N/A | 0 | 64 | |
| Peritubular capillaritis | N/A | N/A | 0 | 36 | |
| Peritubular capillary C4d deposition | N/A | N/A | 0 | 48 |
Data are presented as percentages. All categorical variables were compared using the chi-squared test. All continuous variables are analyzed using ANOVA with the Tukey post hoc test for multiple comparisons if normally distributed and the Kruskal–Wallis test for variables with skewed distribution. Adjusted P values for multiple comparisons after the post hoc tests are presented. P<0.05 is significant. N/A, not applicable; CNI, calcineurin inhibitor (cyclosporine or tacrolimus); MMF, mycophenolate mofetil/mycophenolate sodium; DSA, donor-specific antibody; IFTA, interstitial fibrosis and tubular atrophy (no obvious cause identified).
Because graft age (vintage) is extremely skewed, median and interquartile ranges have been presented and data were analyzed by a nonparametric test.
Significant difference between stable allograft function and GD-R.
Significant difference between stable allograft function and GD-NR.
Significant difference between stable allograft function and GD-NR; stable allograft function and GD-R.
Significant differences between GD-R and the rest of the study population.
Other pathology includes urinary tract obstruction or vascular problems (two patients with definitive evidence of obstructive uropathy and one patient with renal artery stenosis were not biopsied).
Chronic transplant glomerulopathy with basement membrane multilayering.
Figure 5.
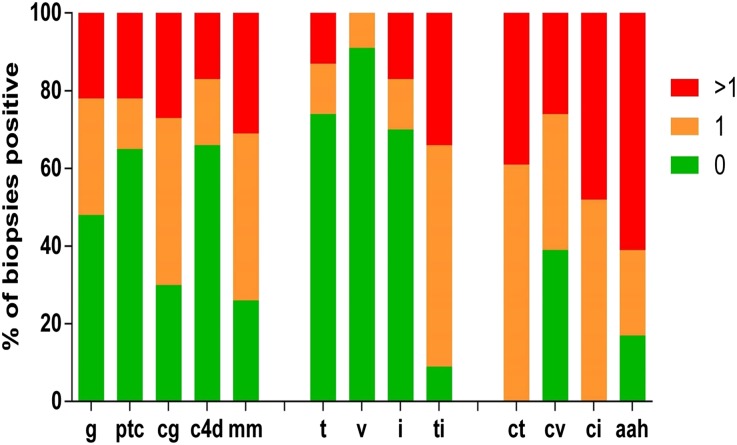
Details of the Banff classification of the biopsies for patients in the GD-R group. Classification is as follows: 0, absent; 1, mild; and >1, moderate to severe. These findings show that the biopsies have a significant degree of chronic damage as suggested by ct, cv, and ci scores. aah, arteriolar hyalinosis; c4d, C4d deposition in the peritubular capillaries; cg, chronic transplant glomerulopathy with basement membrane multilayering; ci, interstitial fibrosis; ct, tubular atrophy; cv, fibrointimal thickening; g, glomerulitis; i, interstitial inflammation; mm, mesangial expansion; ptc, peritubular capillaritis; t, tubulitis; ti, total inflammation; v, endothelitis.
TrBs from patients with stable allograft function were similar to those from healthy volunteers in terms of frequency (proportion of total B cells), absolute number, IL-10 expression, and IL-10/TNF-α ratio (Figure 6, A–D), although the mean value for each parameter was slightly reduced (not statistically significant). Whereas patients with GD-NR had a lower absolute number of TrBs compared with healthy volunteers and patients with stable allograft function, the trend toward a slightly lower IL-10/TNF-α ratio was not significant. By contrast, patients with rejection (GD-R) showed a significant decrease in the percentage and, importantly, in the IL-10/TNF-α ratio of TrBs compared with all other groups. The GD-R group had significantly higher TNF-α expression (13.4±1.3 for patients with stable allograft function versus 21.6±1.9 for patients with GD-R; P<0.001) and a relatively lower IL-10 expression (statistically not significant) that explains the significantly lower IL-10/TNF-α ratio (Figure 6, A–D). Thus, transplant patients with rejection display a quantitative decrease in Bregs and a change in their cytokine polarization, compared with those with stable graft function or GD-NR. Some patients with GD-R received corticosteroids for cellular rejection before having their blood sampled for this study. However, when only patients with GD-R who did not receive steroids are considered, they still have a significantly lower IL-10/TNF-α ratio compared with either healthy volunteers or patients with stable graft function (Supplemental Figure 3). Of note, despite the decrease in IL-10/TNF-α ratio in TrBs in patients with GD-R, the IL-10/TNF-α ratio for the total B-cell population is similar across all groups (Figure 6E).
Figure 6.
CD19+CD24hiCD38hi TrB cells are altered qualitatively and quantitatively in renal allograft rejection. Bar graphs (mean±SEM) comparing healthy volunteers and patient groups for the percentage (A) and absolute number (B) of CD24hiCD38hi TrB cells (#P=0.03, S versus GD-R; *P=0.05, GDR versus GD-NR; **P<0.001, S versus GD-NR; ##P<0.001, S versus GD-R). (C–E) The percentage of IL-10 for TrBs, IL-10/TNF-α ratio for TrBs, and whole B cells (¥P<0.001, S versus GD-R; ɸP=0.03, GD-NR versus GD-R). (F) Representative dot plots for one of five patients in the GD-R group for the TNF-α, IFN-γ, and IL-4 expression by Tconvs cultured alone or in the presence of TrBs or Tregs. Numbers in each plot indicate the frequency of Tconvs expressing each cytokine under respective culture conditions. (G) Graphical representation (mean±SEM) of the cumulative results for the suppressive/stimulatory effect of the TrB cells or Tregs on the Tconv cytokine expression in healthy volunteers (n=6), stable (n=7), and GD-R (n=5) groups ($P<0.001, S versus GD-R: TNF-α; $$P=0.002, IFN-γ; $$$P<0.001, IL-4,) Statistical analysis is performed by ANOVA using Tukey post hoc correction. GD-NR, patients with graft dysfunction-no rejection; GD-R, patients with graft dysfunction-rejection; HV, healthy volunteers; S, patients with stable graft function.
To determine whether the observed difference in the cytokine profile of TrBs between patient groups correlates with qualitative differences in regulatory function, we examined the ability of TrB to inhibit cytokine expression by autologous Tconvs in vitro. Purified TrBs from transplant patients with stable allograft function and those from healthy volunteers exhibited similar inhibition of IFN-γ and TNF-α expression by Tconvs, while sparing IL-4 expression (Figure 6, F and G). By contrast, TrBs from patients with GD-R exerted a minimal effect on TNF-α and IFN-γ expression by Tconvs, while significantly suppressing IL-4 expression. As a control, we examined Tregs from the same patients (Figure 6G). Unlike their B-cell counterparts, Tregs from patients with rejection were no different in their activity than those from patients with stable function or healthy controls. Because the same numbers of TrBs were added in each assay, the principal difference in TrBs from patients with GD-R and patients with stable allograft function was a decrease in their IL-10/TNF-α ratio. These results confirm the advantage of the IL-10/TNF-α ratio over IL-10 expression alone in predicting Breg activity.
Low IL-10/TNF-α Ratio Is Associated with Adverse Clinical Outcomes
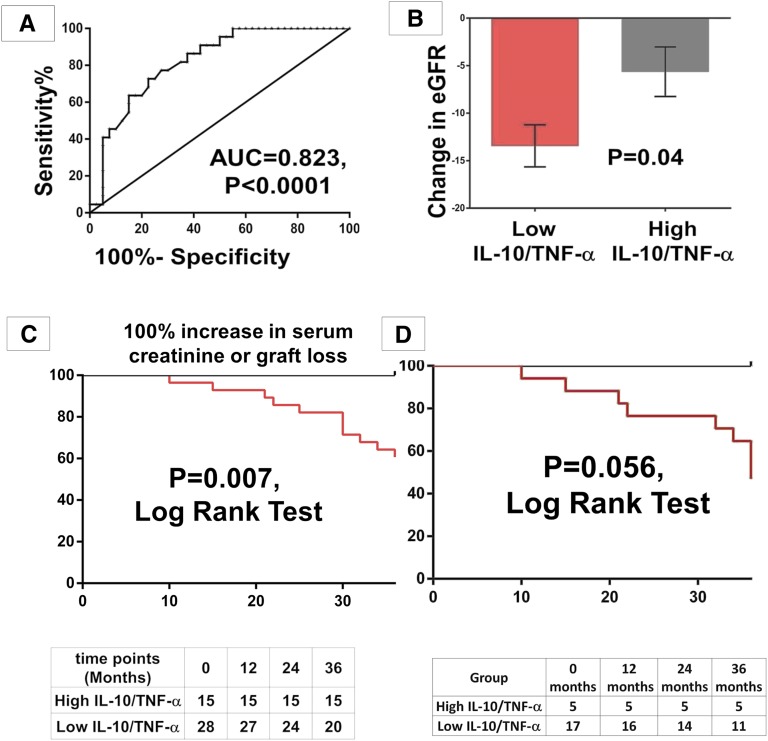
Finally, we analyzed the relationship between cytokine polarization within the TrB subset and graft outcomes. The IL-10/TNF-α ratio was reasonably strong in distinguishing patients with stable grafts from those with rejection by receiver operation characteristic curve analysis with an area under the curve of 0.823 (P<0.001) (Figure 7A). In patients with graft dysfunction, when the rate of deterioration of graft function was assessed over a 3-year follow-up from the time of the biopsy (n=44), patients with an IL-10/TNF-α ratio of TrBs below the group mean of 0.75 (low ratio) had significantly more graft deterioration (measured by ΔeGFR) compared with patients with a ratio above the group mean (high ratio) (Figure 7B). Moreover, a low IL-10/TNF-α ratio among TrBs was associated with a worse outcome (defined by either a doubling in serum creatinine from the time of the biopsy, or graft loss), with a trend toward poorer graft survival when this was analyzed for the GD-R group alone (Figure 7, C and D). A similar, although nonsignificant, trend with diverging curves was seen even when the analysis was repeated with graft loss as an end point (Supplemental Figure 4). Thus, functional variation within the TrBs was clinically significant and was associated with adverse graft outcomes.
Figure 7.
Clinical significance of TrB IL-10/TNF-α in patients with graft dysfunction. (A) Receiver operating characteristic curve showing the ability of TrB-IL-10/TNF-α to distinguish stable grafts and rejection. All patients who are biopsied are divided into those with a high or low IL-10/TNF-α based on the mean IL-10/TNF-α for the group. (B) Bar chart showing the change in eGFR (Δ eGFR) over 3 years from the time of the biopsy in patients with graft dysfunction (mean±SEM) based on their IL-10/TNF-α (TrB). (C) Kaplan–Meier survival analysis for the high and low IL-10/TNF-α (TrB) for 100% increase in serum creatinine or graft loss over 3 years in patients with graft dysfunction. (D) Kaplan–Meier survival analysis for the high and low IL-10/TNF-α (TrB) for a 100% increase in serum creatinine or graft loss over 3 years in the GD-R group. Statistical analysis is performed by a Mann–Whitney U test and survival analysis is performed by the Kaplan–Meier method. Groups are compared using the log-rank test.
Discussion
Bregs have been shown to play an important role in regulating immune responsiveness through IL-10 expression in a variety of animal models.10–12,14–16,21–25 Their role in human disease is less clear with studies showing that IL-10 is enriched in either TrB (CD24hiCD38hi) or memory B cells (CD24hiCD27+) and IL-10 expression may be unchanged, elevated, or reduced in patients with various autoimmune diseases.4,17,18,26,27
We demonstrate for the first time that Bregs in renal transplant recipients with rejection are both reduced in number and altered in function, compared with either healthy controls or immunosuppressed patients with stable allograft function. By directly comparing human TrB and memory B cells from healthy volunteers, which have been reported to be enriched for IL-10+ B cells,17,18 we have shown that the TrB subset is most potent in suppressing proinflammatory cytokine expression by Tconvs in vitro. Importantly, this is not due to a higher frequency of IL-10–expressing cells within the TrB population; rather, increased activity is associated with diminished expression of proinflammatory cytokines. Indeed, B cells within naïve, memory, and TrB subsets are not only capable of expressing IL-10, but also TNF-α and individual cells can express both cytokines concomitantly. Our data demonstrate that a high IL-10/TNF-α ratio is a better indicator of polarization toward anti-inflammatory cytokines and B-cell regulatory activity than IL-10 expression alone. This may help explain inconsistencies between earlier studies.
The validity of the IL-10/TNF-α ratio as a marker for Bregs is supported by its ability to predict the potency of the B-cell subsets with equal IL-10 expression to suppress proinflammatory cytokine expression by Tconvs in vitro. Importantly, whereas neutralizing IL-10 blocked the suppressive activity of TrBs on autologous Th1 cytokine expression, neutralizing the effects of TNF-α by blocking its receptors increased the in vitro suppressive capacity of both memory and naïve B cells. The value of the IL-10/TNF-α ratio is further substantiated by predicting suppressive activity of TrBs from renal transplant patients with different clinical status. Despite exhibiting the same frequency of IL-10 expression, TrBs from patients with GD-R displayed a significantly lower IL-10/TNF-α ratio than other groups, including patients with graft dysfunction but without rejection (GD-NR). In addition, TrBs from rejecting patients were unable to inhibit Th1 cytokines while inhibiting Th2 cytokine production, in direct contrast with TrBs from transplant recipients with stable function. This suggests that aberrant immune regulation by Bregs in the setting of allograft rejection and is consistent with murine models in which Th1 responses are deleterious and Th2 responses promote engraftment.28 Moreover, in patients with GD-R, the change in IL-10/TNF-α ratio was compounded by a decrease in the number of TrBs. Although causality cannot be established in this retrospective study, a reduced IL-10/TNF-α ratio within TrBs not only correlates with chronic antibody-mediated rejection (CAMR), but a low ratio is associated with adverse graft outcomes among patients with graft dysfunction. This suggests that the IL-10/TNF-α ratio of TrB cells may be a useful supplement to existing biomarkers of graft outcome, such as donor-specific antibodies, and warrants further prospective evaluation.29,30
There are several important consequences of this B cell functional heterogeneity. First, TrBs are not only more polarized toward an anti-inflammatory function as a subset, but individual cells within that subset are more likely to express IL-10 alone (without TNF-α coexpression) compared with either memory or naïve B cells. Thus, although the total number of naive and especially memory B cells expressing IL-10 is not substantially lower than TrBs, a greater proportion of the former express or coexpress inflammatory cytokines and appear less able to suppress T-cell responses. Moreover, in different clinical or pathologic states, the degree of polarization can change. These observations cast doubt over the ability of any phenotypic subset or single cytokine to adequately define human Bregs. Rather, our findings indicate that cytokine polarization is a better predictor of Breg activity.
Definitive murine studies have shown that the expression of proinflammatory cytokines by B cells can markedly affect immune responses, for example by promoting Th1 differentiation or protective antibody responses to infectious pathogens.5,8,31 It has been reported that B cells from patients with relapsing multiple sclerosis express significantly more proinflammatory cytokines than those from healthy controls, although cytokine expression was not actually correlated with disease activity nor was the phenotype of such B cells examined.32 However, B-cell depletion with rituximab reduced T-cell proliferation and expression of proinflammatory cytokines. Our study highlights the importance of identifying the balance of proinflammatory and anti-inflammatory cytokine expression within specific B-cell subsets.
Recent studies showed that renal transplant patients who were “operationally tolerant” exhibit higher numbers of TrBs and IL-10 expression than nontolerant patients with stable renal function.33,34 In this study, patients with stable allograft function (on immunosuppression) have a similar number of and IL-10 expression by TrBs as healthy volunteers. Moreover, we recently showed that stable renal transplant patients with superior graft function, irrespective of immunosuppressive regimen, have higher numbers of both total B cells and TrBs compared with those with reduced renal function.35 In this study, not only are TrBs reduced in the setting of rejection, but their cytokine profile and functional activity upon in vitro noncognate polyclonal stimulation are abnormal. Closer inspection reveals that most of the patients (80%) in the GD-R group exhibited microvascular inflammation and scarring with or without complement (C4d) deposition, and serologic evidence of circulating donor-specific HLA antibody—all features of CAMR. In 25% of these patients, a component of acute cellular rejection was superimposed. Whether Breg abnormalities precede the development of antibody-mediated or cell-mediated rejection (suggesting causality) or occur as a result of these processes must await prospective longitudinal study. This is important because CAMR is a major cause of chronic renal allograft loss.36–38
One limitation of this study is that six patients who exhibited an acute cellular component to their rejection on biopsies were initiated on treatment with corticosteroids before blood sampling. Thus, more patients with rejection were on maintenance corticosteroids. However, as noted above, patients with GD-R who did not receive corticosteroids still had a significantly lower IL-10/TNF-α ratio than other patient groups. Finally, it should be noted that the GD-NR group was heterogeneous and some patients had “immune-mediated” pathology such as recurrent GN. This may explain why this group exhibited some reduction in TrBs and IL-10/TNF-α ratio (not significant) compared with patients with stable allograft function and healthy volunteers, while remaining significantly higher than the GD-R group. In this study, although the in vitro suppressor assay utilized nonantigen-specific stimulation of T cells, it correlated well with IL-10/TNF-α ratio and clinical diagnosis and was predictive of 3-year outcomes. Validation of Ag-specific T- and B-cell responses in this in vitro assay will require further study.
Our study provides novel insights into the description of B cell–mediated immunoregulation in humans. Because a significant number of B cells, even in IL-10–enriched subsets, can express proinflammatory cytokines that can be modulated in the course of immune-mediated disease, we contend that any definition of the Breg phenotype should take this finding into account. Using such a strategy, we have shown that CD24hiCD38hi TrBs outperformed other B-cell subsets in their regulatory potential, and their cytokine polarization profile correlated with in vitro function and clinical status and was associated with clinical outcomes after biopsy in renal transplant recipients.
Concise Methods
Study Participants
Venous blood samples were collected from healthy volunteers (n=15) and renal transplant recipients (n=88) and PBMCs were isolated according to standard criteria by Ficoll-Paque (Cedar Lane, Burlington, ON, Canada) density gradient centrifugation. Written informed consent was obtained from all of the participants according to the Declaration of Helsinki. The study was approved by the York Regional Ethics Committee (reference no. 08/H1311/41). Serum samples from the transplant recipients were stored for the analysis of HLA-specific antibodies. All renal transplant recipients followed up in our out-patient clinic were screened for graft dysfunction based on serial serum creatinine and proteinuria assessments. Patients with proteinuria of >0.5g/d assessed by urine protein creatinine ratio on three consecutive random urine samples and/or deteriorating function as assessed by change in 1/creatinine over a year39 were offered a transplant biopsy. On the basis of the histology, they were grouped into patients with a histologic phenotype of rejection (GD-R, n=25) or patients with no evidence of rejection (GD-NR, n=22). This group was randomly selected based on the time elapsed since the date of the biopsy. Blood samples were collected over a period of 18 months. Patients who had a biopsy to blood sampling time of >3 months were not included in the study. The details of the Banff classification for the patients within the GD-R group are summarized in Figure 3. Blood samples from these patients were collected for analysis within 3 months of the biopsy when the patients attended for their clinic appointments. A random group of 41 patients with stable graft function defined by stable creatinine and absence of proteinuria were used for comparative analysis. This group of patients was not biopsied in light of their stable renal function. The GD-R group was created on the basis of graft dysfunction along with that of the presence of at least one of either glomerulitis, peritubular capillaritis, and transplant glomerulopathy with or without peritubular C4d deposition in the peritubular capillaries in the presence of interstitial fibrosis and tubular atrophy.40 All transplant recipients irrespective of their graft function were tested for the presence of HLA-specific antibodies. Table 1 summarizes the characteristics of patients and controls. None of the patients were commenced on any other therapy for rejection before blood sampling apart from the small subgroup of patients who received corticosteroids for the diagnosis of T cell–mediated rejection.
Antibodies, Flow Cytometry, Lymphocyte Isolation, and Cell Sorting
All experiments were performed on fresh cells on the same day of blood sampling. Anti-human mAbs included FITC-CD24 (ML5), PE-CD27 (M-T271), APC-CD27 (M-T271), PerCP-Cy5.5-CD38 (HIT2), PE-CD1d (UCHT2), FITC-CD5 (UCHT2), APC-H7-CD19 (SJ25C1), FITC-IgM (G20-127), PE-CD25(M-A251), PE-IL-10 (JES3-9D7), PE-TNF-α (MAb11), AlexaFluor 488-TNF-α (MAb11), PE-IL4 (8D4-8), AlexaFluor 488-IFN-γ (B27), functional grade CD3 (HIT3a), and neutralizing anti–IL-10 (JES3-19F1) from Beckton Dickinson. APC-CD4 (OKT4), PerCP-Cy5.5-CD20 (2H7), PE-CD24 (eBioSN3), APC-CD38 (HIT2), FITC-CD27 (LG.7F9), and PerCP-Cy5.5-TNF-α (MAb-11) were from eBioscience (San Diego, CA). Neutralizing TNFR1 (clone 16805) and TNFR2 (clone 22210) antibodies along with the isotype controls were from R&D Systems. Brilliant violet 421-CD24 (M1/69) and AlexaFluor 700-CD27 (O323) were from Biolegend.
Absolute lymphocyte numbers were calculated using BD-Trucount beads as per the manufacturer’s instructions. Red cells were lysed using BD-FACSlyse. Nonspecific FcR binding was blocked by 20% human normal serum. Cursors were set using isotype and fluorochrome matched negative controls for both surface and intracellular staining. Cells were then analyzed using a BD FACSCalibur flow cytometer (Beckton Dickinson).
In some experiments, B cells were isolated from PBMCs by positive selection using CD19 magnetic beads (Miltenyi Biotec). In this setting, B-cell purity was reexamined using anti-CD20. CD4+CD25− and CD4+CD25hi T cells were also enriched using MACS magnetic bead kits (Miltenyi Biotec). Various B-cell subsets were enriched by a Moflo cell sorter (Beckman Coulter) based on their surface expression of CD19, CD24, CD38, and CD27.
Cell Culture-Cytokine Detection
Magnetic bead-enriched B cells were suspended in RPMI 1640 (Life Sciences) supplemented with 10% FBS and 1% penicillin-streptomycin l-glutamine (Sigma-Aldrich) at 1×106 cells/ml and stimulated with 10 µg/ml CpG (CpG-ODN2006; Invivogen, San Diego, CA) and 1 µg/ml CD40L (Sigma-Aldrich). Next, 50 ng/ml PMA (Sigma-Aldrich) and 1 µg/ml ionomycin (Sigma-Aldrich) and 1 µl/ml Brefeldin A (Beckton Dickinson) were added for the last 5 hours of cell culture. Cultured cells were stained with surface markers, fixed, and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences) and then stained for intracellular cytokines. Alternatively, B-cell subsets were sort-purified and stimulated with anti-CD40 and CpG for 48 hours, and then cytokines were analyzed from the cell culture supernatants by bead-based ELISA (Invitrogen) using the manufacturer’s instructions. Because all experiments were conducted on fresh PBMCs within a few hours of blood sampling, the cell viability tested by trypan blue method was approximately 80%–85% even after 48 or 72 hours of cell culture.
B Functional Assays
Cell cultures of 1×105 magnetic bead–enriched CD4+CD25− T cells and FACS-sorted CD24hiCD38hi or CD24hiCD27+ or CD24+CD38+ B-cell subsets (1:1) were stimulated with 0.5 µg/ml of plate-bound anti-human CD3 in 96-well u-bottom tissue culture plates (Nunc). Brefeldin-A was added for the last 6 hours along with PMA and ionomycin. Cells were surface stained with CD4-APC and CD20-PerCp-Cy5.5 followed by fixation and permeabilization and staining for intracellular cytokines. Cultures of CD4+CD25− cells with CD4+CD25hi Tregs (1:1) were used as positive controls. To test the utility of either IL-10 or TNF-α, neutralizing antibodies to IL-10 or TNFR1 and TNR2 or isotype controls were added to the respective wells of the culture plate.
Statistical Analyses
Statistical analysis was performed by SPSS (version 20) and GraphPad Prism (version 6) for Windows. Categorical variables were compared using the chi-squared test and continuous variables using the paired t test. Analyses with multiple comparisons were completed using ANOVA with Tukey post hoc analysis to account for the multiple comparisons in the case of normally distributed variables or the Kruskal–Wallis test for variables with a skewed distribution. Analysis of allograft outcomes over a follow-up period was by the Kaplan–Meier method and the groups were compared by the log-rank test. A P value of <0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by educational grants from the Yorkshire Kidney Research Fund and the Leeds Teaching Hospitals Charitable Foundation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “B Cells and Kidney Transplantation: Beyond Antibodies,” on pages 1373–1374.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013080837/-/DCSupplemental.
References
- 1.Janeway CA, Jr, Ron J, Katz ME: The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol 138: 1051–1055, 1987 [PubMed] [Google Scholar]
- 2.Shlomchik MJ, Craft JE, Mamula MJ: From T to B and back again: Positive feedback in systemic autoimmune disease. Nat Rev Immunol 1: 147–153, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Ron Y, Sprent J: T cell priming in vivo: A major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol 138: 2848–2856, 1987 [PubMed] [Google Scholar]
- 4.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF: B-lymphocyte contributions to human autoimmune disease. Immunol Rev 223: 284–299, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Lund FE, Randall TD: Effector and regulatory B cells: Modulators of CD4+ T cell immunity. Nat Rev Immunol 10: 236–247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barr TA, Gray M, Gray D: B cells: Programmers of CD4 T cell responses. Infect Disord Drug Targets 12: 222–231, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Shlomchik MJ: Activating systemic autoimmunity: B’s, T’s, and tolls. Curr Opin Immunol 21: 626–633, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE: Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 1: 475–482, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Lund FE: Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol 20: 332–338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK: Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. J Exp Med 186: 1749–1756, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK: Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16: 219–230, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM: B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3: 944–950, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Mauri C, Gray D, Mushtaq N, Londei M: Prevention of arthritis by interleukin 10-producing B cells. J Exp Med 197: 489–501, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM: Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest 121: 3645–3656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF: A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28: 639–650, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Hussain S, Delovitch TL: Intravenous transfusion of BCR-activated B cells protects NOD mice from type 1 diabetes in an IL-10-dependent manner. J Immunol 179: 7225–7232, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C: CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 32: 129–140, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF: Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117: 530–541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, Looney RJ, Sanz I, Anolik JH: Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol 182: 5982–5993, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE: Identification and characterization of circulating human transitional B cells. Blood 105: 4390–4398, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C: Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol 178: 7868–7878, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Gray M, Miles K, Salter D, Gray D, Savill J: Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci U S A 104: 14080–14085, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN: B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol 178: 3447–3456, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF: Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest 118: 3420–3430, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizoguchi E, Mizoguchi A, Preffer FI, Bhan AK: Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int Immunol 12: 597–605, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A: Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 178: 6092–6099, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Correale J, Farez M, Razzitte G: Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol 64: 187–199, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Sho M, Yamada A, Najafian N, Salama AD, Harada H, Sandner SE, Sanchez-Fueyo A, Zheng XX, Strom TB, Sayegh MH: Physiological mechanisms of regulating alloimmunity: Cytokines, CTLA-4, CD25+ cells, and the alloreactive T cell clone size. J Immunol 169: 3744–3751, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Loupy A, Lefaucheur C, Vernerey D, Prugger C, van Huyen JP, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana JP, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW: Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 12: 1157–1167, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, Kusser K, Honjo T, Mohrs K, Mohrs M, Randall T, Lund FE: Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity 30: 421–433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, Calabresi PA, Waubant E, Hauser SL, Zhang J, Smith CH: Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol 67: 452–461, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, Burlingham WJ, Marks WH, Sanz I, Lechler RI, Hernandez-Fuentes MP, Turka LA, Seyfert-Margolis VL, Immune Tolerance Network ST507 Study Group : Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 120: 1836–1847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, Chapman S, Craciun L, Sergeant R, Brouard S, Rovis F, Jimenez E, Ballow A, Giral M, Rebollo-Mesa I, Le Moine A, Braudeau C, Hilton R, Gerstmayer B, Bourcier K, Sharif A, Krajewska M, Lord GM, Roberts I, Goldman M, Wood KJ, Newell K, Seyfert-Margolis V, Warrens AN, Janssen U, Volk HD, Soulillou JP, Hernandez-Fuentes MP, Lechler RI: Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 120: 1848–1861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cherukuri A, Salama AD, Carter C, Smalle N, McCurtin R, Hewitt EW, Hernandez-Fuentes M, Clark B, Baker RJ: An analysis of lymphocyte phenotype after steroid avoidance with either alemtuzumab or basiliximab induction in renal transplantation. Am J Transplant 12: 919–931, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Brouard S, Renaudin K, Soulillou JP: Revisiting the natural history of IF/TA in renal transplantation. Am J Transplant 11: 647–649, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, Grande J, Halloran P, Hunsicker L, Mannon R, Rush D, Matas AJ: Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation 90: 68–74, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Kieran N, Wang X, Perkins J, Davis C, Kendrick E, Bakthavatsalam R, Dunbar N, Warner P, Nelson K, Smith KD, Nicosia RF, Alpers CE, Leca N, Kowalewska J: Combination of peritubular c4d and transplant glomerulopathy predicts late renal allograft failure. J Am Soc Nephrol 20: 2260–2268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dudley C, Pohanka E, Riad H, Dedochova J, Wijngaard P, Sutter C, Silva HT, Jr, Mycophenolate Mofetil Creeping Creatinine Study Group : Mycophenolate mofetil substitution for cyclosporine a in renal transplant recipients with chronic progressive allograft dysfunction: The “creeping creatinine” study. Transplantation 79: 466–475, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, Farver CF, Rodriguez ER, Wallace WD, Glotz D, Banff Meeting Report Writing Committee : Banff 2011 meeting report: New concepts in antibody-mediated rejection. Am J Transplant 12: 563–570, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.