Abstract
Testosterone acts directly at androgen receptors and also exerts potent actions following 5α-reduction to dihydrotestosterone (DHT). Finasteride (type II 5α-reductase inhibitor) lowers DHT and is used to treat benign prostatic hyperplasia. However, it is unknown whether elevated DHT mediates either beneficial musculoskeletal effects or prostate enlargement resulting from higher-than-replacement doses of testosterone. Our purpose was to determine whether administration of testosterone plus finasteride to older hypogonadal men could produce musculoskeletal benefits without prostate enlargement. Sixty men aged ≥60 yr with a serum testosterone concentration of ≤300 ng/dl or bioavailable testosterone ≤70 ng/dl received 52 wk of treatment with testosterone enanthate (TE; 125 mg/wk) vs. vehicle, paired with finasteride (5 mg/day) vs. placebo using a 2 × 2 factorial design. Over the course of 12 mo, TE increased upper and lower body muscle strength by 8–14% (P = 0.015 to <0.001), fat-free mass 4.04 kg (P = 0.032), lumbar spine bone mineral density (BMD) 4.19% (P < 0.001), and total hip BMD 1.96% (P = 0.024) while reducing total body fat −3.87 kg (P < 0.001) and trunk fat −1.88 kg (P = 0.0051). In the first 3 mo, testosterone increased hematocrit 4.13% (P < 0.001). Coadministration of finasteride did not alter any of these effects. Over 12 mo, testosterone also increased prostate volume 11.4 cm3 (P = 0.0051), an effect that was completely prevented by finasteride (P = 0.0027). We conclude that a higher-than-replacement TE combined with finasteride significantly increases muscle strength and BMD and reduces body fat without causing prostate enlargement. These results demonstrate that elevated DHT mediates testosterone-induced prostate enlargement but is not required for benefits in musculoskeletal or adipose tissue.
Keywords: testosterone, hypogonadal, prostate enlargement
some studies of testosterone treatment in older, hypogonadal men report substantial increases in muscle strength and bone mineral density (BMD) (12, 21), whereas others report only modest improvements (31, 41). Studies documenting substantial effects typically employed intramuscular (im) doses of ≥100 mg/wk im injection of long-acting testosterone esters (12, 21). In contrast, lower doses of testosterone that result from transdermal patch or gel administration produce only modest myotrophic effects (31, 32, 41). Meta-analysis data indicate that im testosterone produces a 4% increase in lumbar spine BMD, while transdermal testosterone has no effect (39). Unfortunately, higher doses of testosterone also increase the risk of adverse events, including three that have been confirmed by meta-analysis: polycythemia, a small reduction in HDL-cholesterol, and increased incidence of combined prostate-related events (16, 20, 25). Currently, many question whether the risk-to-benefit ratio is favorable enough to recommend higher-than-replacement testosterone therapy for the ∼20% of men aged over 60 yr who are at least moderately hypogonadal (27).
Testosterone exerts direct effects at androgen receptors (ARs) but also undergoes 5α-reduction to dihydrotestosterone (DHT), which mediates many of the developmental (42) and androgenic effects of testosterone (44). Both finasteride (type II 5α-reductase inhibitor) and dutasteride (types I and II 5α-reductase inhibitor) are used clinically to treat benign prostatic hyperplasia (BPH) and act by significantly reducing endogenous DHT (3). However, it remains unclear whether elevated DHT mediates the beneficial and/or adverse effects of administered testosterone. A single preliminary report indicates that 200 mg im testosterone enanthate (TE) biweekly plus 5 mg finasteride/day improved total body fat-free mass and lumbar spine and hip BMD and lessened the prostate enlargement resulting from TE alone in older hypogonadal men. However, this combination of TE plus finasteride failed to improve lower-extremity maximal strength over 3 yr (33). Others have reported that dutasteride (2.5 mg/day) does not limit the dose-dependent TE-induced improvements in lean mass or muscle function in younger eugonadal men who were administered a GnRH agonist to suppress endogenous testosterone, although dutasteride did not prevent TE-induced prostate enlargement in this study (11). As such, questions remain regarding the safety and efficacy of concomitant testosterone and steroid 5α-reductase inhibitors.
The primary purpose of this study was to determine whether coadministration of higher-than-replacement TE (125 mg/wk) and Proscar (5 mg finasteride/day) increases muscle strength, fat-free mass, and hematocrit in older hypogonadal men while limiting prostate enlargement. Secondary purposes were to determine whether coadministration of higher-than-replacement TE and finasteride improves BMD and body composition without adversely affecting blood lipids.
METHODS
Study Design
This study was approved by the Institutional Review Board of the University of Florida (UF). All participants gave written informed consent. Potential participants underwent screening to determine eligibility, including structured medical history, blood acquisition (performed twice between 8:00 and 10:00 AM, separated by at least 30 min) to determine complete blood count, complete metabolic profile, luteinizing hormone, lipid panel, total and bioavailable testosterone (BioT), hematocrit (Hct), PSA, and a physical exam that included prostate digital rectal examination (DRE) and transrectal ultrasound sizing (TRUS), and American Urological Association International Prostate Symptom Score (AUA/IPSS) (5). Participants were men aged ≥60 yr, with a serum total testosterone ≤300 ng/dl or BioT ≤70 ng/dl. Two blood samples were obtained during screening, because the Endocrine Society recommends repeated measurements to confirm low testosterone prior to initiating testosterone treatment (9).
We excluded individuals who failed the Mini-Cog test (13) or who had a history of prostate or breast cancer, severe BPH, AUA/IPPS score ≥25, class 3 or 4 congestive heart failure, sleep apnea, Hct >49%, PSA ≥2.6 ng/ml, BMI >35, orthopedic limitations precluding one-repetition maximum (1-RM) strength testing, or who had received testosterone within 4 wk, finasteride/dutasteride within 6 mo, or who were taking Coumadin. Participants were advised to maintain their current level of physical activity.
A randomization table was prepared with RanPro release 1.1 (Applied Logic Associates, Houston, TX) to assign each qualifying participants to one of four treatment groups: vehicle-placebo, TE-placebo, vehicle-finasteride, or TE-finasteride (Fig. 1), using a 2 × 2 factorial design. Treatment lasted 12 mo and consisted of Proscar (5 mg/day po finasteride) or placebo and Delatestryl (125 mg/wk im TE) or vehicle. Proscar and matching placebo were donated by Merck, Delatestryl was donated by Novartis, and matching vehicle was prepared by WestLab Pharmacy (Gainesville, FL). Participants were not paid for participation other than receiving reimbursement for travel mileage. Study participants and investigators performing consenting, screening, drug administration, safety testing, and outcomes testing were blinded to treatment. The only unblinded team members were Research Pharmacy, the Laboratory Manager, who compared safety testing values to removal criteria, and the Study Physician, who assessed adverse events to determine participant removal criteria.
Fig. 1.
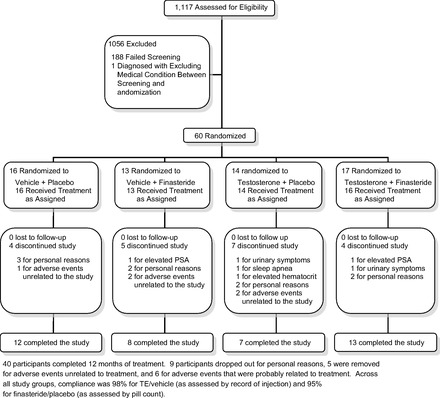
Flow of participants through the trial.
Protection from Risks
Participants underwent thorough health screenings every 3 mo throughout the study, including all baseline measures described above, with the exception of prostate TRUS (assessed every 6 mo). Participants were removed from the study if the following occurred: hematocrit ≥54%, serum PSA ≥4.0, increase in AUA/IPSS ≥4 points, or if gynecomastia or peripheral edema were noted at physical exam (Fig. 1).
Outcomes
Outcome measures were performed at baseline and at 3-mo intervals thereafter except dual X-ray absorptiometry (baseline and 12 mo only) and prostate TRUS (every 6 mo).
Prostate DRE and TRUS.
DRE and TRUS were performed by the Urology Service, Malcom Randall VA Medical Center (VAMC), Gainesville, FL. TRUS was performed using the General Electric LOGICQ P5 scanning system with the highest sector transrectal probe/transducer of 7 MHz. The GE LOGICQ has built-in software for electronically calculating prostate volume (height × length × width × 0.52). The same operator performed testing for all participants.
1-RM strength testing.
1-RM strength testing was performed on Cybex (Medway, MA) leg press, knee flexion, knee extension, chest press, and triceps extension selectorized resistance exercise machines. A 5-min warm-up preceded testing, and weights were gradually increased to 1-RM. Testing was repeated within 2–7 days, and the highest load completed was utilized.
Grip strength.
Grip strength of the right hand was assessed using a Jaymar hand-held dynamometer (Sammons Preston Roylan, Bolingbrook, IL).
Urinary symptoms.
Urinary symptoms were assessed using the AUA/IPPS questionnaire (5).
Hormone Assays
Serum samples were obtained 1 wk after TE/vehicle injection. Clinical laboratory values, including total testosterone, were assessed in the Clinical Laboratory, Malcom Randall VAMC. Testosterone was assayed by Cobas electrochemoluminescence immunoassay. Separate serum samples were stored at −80°C and analyzed in duplicate in a single assay for CTX-1 and osteocalcin by ELISA (Immunodiagnostic Systems, Fountain Hills, AZ). Estradiol (E2) was assessed by ELISA (American Laboratory Products, Salem NH). BioT and BioE2 were assessed by ammonium sulfate precipitation of samples spiked with [3H]testosterone or [3H]estradiol (40). DHT was assessed by LC-MS-MS at Laboratory Corp of America (Calabasas Hills, CA).
Body Composition and Lumbar Spine and Hip BMD
These were assessed using a fan-bean densitometer (Lunar Prodigy; GE Medical Systems, Little Chalfont, Buckinghamshire, UK), calibrated daily.
Statistical Methods
Descriptive statistics are given as means with standard deviations (SD) or as means with 95% confidence intervals (CI), as appropriate. P values < 0.05 (two-sided) were considered statistically significant. Repeated-measures dependent variables were analyzed as linear mixed models with participants as random effects, and time (3, 6, 9, or 12 mo), testosterone, finasteride, interaction, and baseline value of the dependent measures as fixed effects. We utilized an autoregressive correlational structure, allowing measures closer in time to have higher within-subject correlations than when farther apart. Missing at random was presumed for our analyses, and we chose not to use imputation, because it results in greater bias. For variables collected only at baseline and 12 mo, we used a fixed-effects ANOVA model with dependent variable as 12-mo minus baseline difference with the same treatment-related independent variables as above. This coding yields valid interpretable main effects even if interactions are present. The main effect of testosterone, for example, represents the average effect of testosterone under placebo and finasteride (weighted 50–50). In comparison, the interaction addresses whether or not the difference between the means for treatment factors (e.g., testosterone vs. vehicle) differ quantitatively according to the level of other treatment factors (e.g., finasteride vs. placebo). The changes from baseline to 12 mo are presented, but they estimate a different outcome than the mixed model, which measures an average effect over the times after baseline. The mixed model has two additional advantages over the simple 0- to 12-mo difference comparisons: 1) It uses all of the data, and 2) it is more sensitive than the 0- to 12-mo comparison for effects that increase early and decrease over time.
The study was powered around four end points: fat-free mass, 1-RM leg press, hematocrit, and prostate volume, although a wide-range of variables were evaluated due to the systemic nature of androgen-AR interactions and to ensure participant safety. We intended to obtain 12 completers in each of the four arms. Allowing for a 20% dropout rate, the goal was 60 randomized participants. Bhasin et al. (1) reported that older men experience a ≥2σ (σ = SD) increase in fat-free mass (4.2 ± 0.6 SE, n = 12) and hematocrit (0.07 ± 0.01 SE, n = 12) and 1.18α increase in 1-RM strength (28.0 ± 6.8 SE, n = 12) after 20 wk of 125 mg/wk TE, whereas Zitzmann et al. (46) reported that testosterone replacement increased prostate volume 1.85α per year (6.1 ± 3.3 SD). For each variable, a 2 × 2 factorial study has 80% power to detect a 0.87σ difference at P < 0.05 (two-sided), and 80% power to detect a 1.0σ difference at P = 0.0125 (0.05/4). Importantly, 1-RM strength, fat-free mass, and hematocrit were powered using data from changes occurring over a 20-wk period of testosterone administration, whereas our study duration was 52 wk. As such, we powered to achieve significance in the aforementioned outcomes at the 6-mo time point of our study, which protects against the adverse effects of an unexpectedly high participant dropout rate on statistical power.
RESULTS
Primary Outcomes
Baseline values are reported in Table 1 and treatment effects in Tables 2–4 and Fig. 2. TE progressively increased 1-RM strength over 12 mo (Table 2) by 12.9 kg for leg press, an 11.4% increase (P < 0.001; Table 2 and Fig. 2H), 6.00 kg for knee extension (8.1%, P = 0.012), 5.42 kg for knee flexion (12.5%, P = 0.0023), 6.47 kg for chest press (14.5%, P < 0.001), 5.32 kg for triceps extension (9.5%, P < 0.001), and 0.77 kg for grip (11.3%, P = 0.015). TE also increased total body fat-free mass 4.04 kg (P = 0.032; Table 3 and Fig. 2G) and HCT 4.13% (P < 0.001; Table 2 and Fig. 2C), with most of the Hct increase occurring in the first 3 mo of treatment. Finasteride did not significantly affect muscle strength, fat-free mass, or Hct. Additionally, TE increased prostate volume 5.33 cm3 (Table 2; main effect, P = 0.0051), and finasteride reduced prostate volume by −5.79cm3 (main effect, P = 0.0027). These changes resulted in progressive prostate enlargement of 11.4 cm3 (P = 0.0051) within the TE group, an increase that was fully prevented by finasteride coadministration (P = 0.0027; Fig. 2D). Prostate volume remained similar between TE-finasteride, vehicle-finasteride, and vehicle-placebo treatments.
Table 1.
Subjects' baseline characteristics
| Variable | Vehicle-Placebo | Vehicle-Finasteride | TE-Placebo | TE-Finasteride |
|---|---|---|---|---|
| Age, yr | 70.8 ± 9.7 | 69.5 ± 9.2 | 69.2 ± 8.0 | 64.2 ± 4.8 |
| BMI, kg/m2 | 30.4 ± 3.4 | 28.8 ± 3.9 | 29.4 ± 4.6 | 31.1 ± 2.5 |
| Weight, kg | 92.0 ± 13.2 | 86.6 ± 13.6 | 93.8 ± 17.7 | 96.2 ± 9.5 |
| Fat-free mass, kg | 56.1 ± 5.8 | 55.7 ± 6.1 | 58.0 ± 9.5 | 59.9 ± 6.6 |
| Hematocrit, % | 40.3 ± 3.9 | 41.2 ± 3.9 | 42.6 ± 3.0 | 42.0 ± 2.8 |
| Prostate volume, cm3 | 36.9 ± 15.6 | 29.7 ± 12.7 | 26.4 ± 8.4 | 37.1 ± 16.3 |
| PSA, ng/ml | 0.98 ± 0.53 | 1.1 ± 0.59 | 0.78 ± 0.64 | 1.4 ± 0.79 |
| AUA/IPPS score | 7.8 ± 5.6 | 7.7 ± 5.1 | 7.5 ± 5.4 | 8.1 ± 4.9 |
| Leg press 1-RM, kg | 118.8 ± 35.3 | 109.4 ± 28.4 | 129.1 ± 41.7 | 137.2 ± 23.9 |
| Knee extension 1-RM, kg | 60.6 ± 12.9 | 56.6 ± 13.6 | 63.7 ± 17.7 | 71.9 ± 14.0 |
| Knee flexion 1-RM, kg | 39.3 ± 10.4 | 32.0 ± 9.5 | 43.5 ± 11.0 | 43.2 ± 9.9 |
| Chest press 1-RM, kg | 47.6 ± 19.0 | 43.1 ± 14.1 | 55.5 ± 19.2 | 59.2 ± 14.1 |
| Triceps extension 1-RM, kg | 36.2 ± 8.5 | 32.8 ± 9.3 | 35.8 ± 10.1 | 38.8 ± 6.6 |
| Grip strength, kg | 17.4 ± 3.4 | 17.0 ± 3.7 | 16.9 ± 4.8 | 18.2 ± 2.1 |
| Testosterone, ng/dl | 264 ± 92 | 240 ± 110 | 245 ± 73 | 242 ± 147 |
| BioT, ng/dl | 46 ± 22 | 41 ± 17 | 46 ± 17 | 44 ± 20 |
| Estradiol, pg/ml | 12.7 ± 9.5 | 13.6 ± 7.4 | 25.8 ± 34.4 | 26.0 ± 30.2 |
| BioE2, pg/ml | 4.7 ± 3.1 | 4.7 ± 2.1 | 11.0 ± 17.2 | 9.8 ± 9.2 |
| DHT, ng/dl | 25.2 ± 15.1 | 18.9 ± 12.7 | 21.4 ± 6.3 | 21.9 ± 22.4 |
| Total body fat, kg | 30.8 ± 9.4 | 26.9 ± 9.6 | 29.9 ± 9.9 | 31.3 ± 4.6 |
| L2-L4 spine BMD, g/cm2 | 1.53 ± 0.36 | 1.4 ± 0.23 | 1.4 ± 0.36 | 1.2 ± 0.16 |
| Rt total hip BMD, g/cm2 | 1.07 ± 0.18 | 1.02 ± 0.15 | 1.03 ± 0.12 | 0.99 ± 0.069 |
Values are means ± SE.
TE, testosterone enanthate; 1-RM, one-repetition maximum; BioT, bioavailable testosterone; BioE2, bioavailable estradiol; DHT, dihydrotestosterone.
Table 2.
Treatment effects
| Variable | Group | 0–12 mo Change | Main Effect of T | Main Effect of F | Interaction |
|---|---|---|---|---|---|
| BMI, kg/m2 | Vehicle-Placebo | 0.75 (0.038 to 1.45) | 0.49 (−0.031 to 1.02) | −0.40 (−0.916 to 0.12) | −0.378 (−1.44 to 0.68) |
| Vehicle-Finasteride | −0.073 (−0.92 to 0.77) | P = 0.066 | P = 0.13 | P = 0.48 | |
| TE-Placebo | 1.03 (−0.54 to 2.56) | ||||
| TE-Finasteride | 1.56 (−0.81 to 3.92) | ||||
| Weight, kg | Vehicle-Placebo | 1.99 (−0.056 to 4.04) | 1.38 (−0.068 to 2.82) | −1.00 (−0.42 to 1.71) | −1.18 (−4.00 to 0.98) |
| Vehicle-Finasteride | 0.23 (−2.50 to 2.95) | P = 0.063 | P = 0.16 | P = 0.41 | |
| TE-Placebo | 2.272 (−2.51 to 6.97) | ||||
| TE-Finasteride | 0.65 (−1.85 to 3.14) | ||||
| Fat-free mass, kg | Vehicle-Placebo | 0.45 (−1.07 to 1.9) | 4.04 (0.43 to 7.64) | 0.13 (−3.47 to 3.74) | −1.58 (−8.80 to 5.63) |
| Vehicle-Finasteride | 0.086 (−1.76 to 1.93) | P = 0.032 | P = 0.94 | P = 0.66 | |
| TE-Placebo | 5.7 (0.26 to 11.1) | ||||
| TE-Finasteride | 3.21 (1.09 to 5.34) | ||||
| Hct, % | Vehicle-Placebo | −0.19 (−1.66 to 1.28) | 4.13 (3.07 to 5.18) | 0.15 (−0.88 to 1.18) | 0.14 (−1.93 to 2.21) |
| Vehicle-Finasteride | −1.18 (−3.50 to 1.15) | P < 0.001 | P = 0.77 | P = 0.89 | |
| TE-Placebo | 5.22 (3.01 to 7.42) | ||||
| TE-Finasteride | 4.17 (2.28 to 6.07) | ||||
| Prostate volume, cm3 | Vehicle-Placebo | −2.81 (−8.62 to 2.83) | 5.33 (1.73 to 8.94) | −5.79 (−9.40 to −2.17) | −6.27 (−13.8 to 1.25) |
| Vehicle-Finasteride | −4.93 (−12.7 to 2.86) | P = 0.0051 | P = 0.0027 | P = 0.10 | |
| TE-Placebo | 11.4 (1.40 to 21.4) | ||||
| TE-Finasteride | −1.72 (−5.26 to 1.80) | ||||
| PSA, ng/ml | Vehicle-Placebo | 0.123 (−0.24 to 0.49) | 0.61 (0.32 to 0.90) | −0.34 (−0.65 to 0.040) | 0.51 (−0.088 to 1.09) |
| Vehicle-Finasteride | −0.61 (−1.07 −0.14) | P < 0.001 | P = 0.028 | P = 0.090 | |
| TE-Placebo | 0.43 (−0.09 to 0.96) | ||||
| TE-Finasteride | 0.24 (−0.76 to 1.24) | ||||
| AUA/IPPS score | Vehicle-Placebo | −0.083 (−4.96 to 4.79) | 0.89 (−0.64 to 2.43) | 0.51 (−1.02 to 2.05) | 2.38 (−0.66 to 5.43) |
| Vehicle-Finasteride | −2.00 (−4.75 to 0.75) | P = 0.26 | P = 0.51 | P = 0.12 | |
| TE-Placebo | −2.00 (−4.66 to 0.66) | ||||
| TE-Finasteride | −0.08 (−2.51 to 2.35) | ||||
| Leg press 1-RM, kg | Vehicle-Placebo | 1.50 (−6.49 to 9.52) | 12.9 (7.86 to 18.0) | −1.54 (−6.40 to 3.32) | 2.00 (−7.70 to 11.7) |
| Vehicle-Finasteride | 0.00 (−6.42 to 6.42) | P < 0.001 | P = 0.53 | P = 0.68 | |
| TE-Placebo | 14.7 (−7.62 to 37.2) | ||||
| TE-Finasteride | 12.5 (2.95 to 22.0) | ||||
| Knee extension 1-RM, kg | Vehicle-Placebo | 0.227 (−4.22 to 4.67) | 6.00 (1.44 to 10.6 | 1.27 (−3.17 to 5.70) | 1.22 (−7.75 to 10.2) |
| Vehicle-Finasteride | 1.11 (−4.07 to 6.28) | P = 0.012) | P = 0.57 | P = 0.79 | |
| TE-Placebo | 5.17 (−14.5 to 24.8) | ||||
| TE-Finasteride | 9.44 (−1.24 to 20.1) | ||||
| Knee flexion 1-RM, kg | Vehicle-Placebo | −0.74 (−2.66 to 1.18) | 5.42 (2.12 to 8.72) | −0.86 (−4.02 to 2.29) | 1.12 (−5.17 to 7.42) |
| Vehicle-Finasteride | −3.79 (−8.99 to 1.40) | P = 0.0023 | P = 0.58 | P = 0.72 | |
| TE-Placebo | −2.88 (−15.3 to 9.51) | ||||
| TE-Finasteride | 3.89 (−3.25 to 11.0) | ||||
| Chest press 1-RM, kg | Vehicle-Placebo | 1.87 (−1.122 to 4.95) | 6.47 (3.63 to 9.32) | 0.29 (−2.40 to 2.99) | 0.50 (−4.92 to 5.91) |
| Vehicle-Finasteride | 0.68 (−2.31 to 3.67) | P < 0.001 | P = 0.83 | 0.85 | |
| TE-Placebo | 8.05 (3.53 to 12.5) | ||||
| TE-Finasteride | 8.54 (1.94 to 15.2) | ||||
| Triceps extension 1-RM, kg | Vehicle-Placebo | −1.71 (−4.34 to 0.93) | 5.32 (3.27 to 7.37) | 1.22 (−0.83 to 3.28) | −0.78 (−4.91 to 3.36) |
| Vehicle-Finasteride | 0.65 (−3.85 to 5.14) | P < 0.001 | P = 0.24 | 0.71 | |
| TE-Placebo | 3.41 (1.32 to 5.50) | ||||
| TE-Finasteride | 5.78 (2.08 to 9.49) | ||||
| Grip strength, kg | Vehicle-Placebo | 0.15 (−0.90 to 1.21) | 0.77 (0.16 to 1.31) | −0.094 (−0.70 to 0.51) | −1.06 (−1.68 to −0.44) |
| Vehicle-Finasteride | 1.47 (−0.84 to 2.12) | P = 0.015 | P = 0.75 | P = 0.088 | |
| TE-Placebo | 1.91 (0.75 to 3.10) | ||||
| TE-Finasteride | 1.17 (0.38 to 1.97) |
T, testosterone; F, finasteride.
Table 4.
Treatment effects
| Group | Baseline Value | 0–12 mo Change | Main Effect of T | Main Effect of F | Interaction |
|---|---|---|---|---|---|
| Vehicle-Placebo | 13.4 (12.8 to 14.2) [16] | 0.00 (−0.44 to 4.4) [12] | 1.13 (0.68 to 1.56) | 0.28 (0.07 to 0.49) | 0.31 (−0.15 to 1.14) |
| Vehicle-Finasteride | 13.8 (12.9 to 14.7) [13] | −0.28 (−0.95 to 0.40) [8] | P < 0.001 | P = 0.18 | P = 0.46 |
| TE-Placebo | 14.7 (13.9 to 15.5) [13] | 1.06 (−0.31 to 2.43) [5] | |||
| TE-Finasteride | 14.6 (14.1 to 15.1) [17] | 1.31 (0.45 to 2.16) [11] | |||
| Vehicle-Placebo | 2.30 (0.91 to 3.6) [16] | 0.062 (−0.12 to 0.24) [12] | 0.54 (0.34 to 0.75) | 0.12 (−0.08 to 0.32) | −0.10 (−0.50 to 0.30) |
| Vehicle-Finasteride | 2.45 (1.2 to 3.7) [13] | −0.030 (−0.56 to 0.50) [6] | P < 0.001 | P = 0.24 | 0.62 |
| TE-Placebo | 2.76 (1.2 to 4.3) [13] | 0.055 (−0.67 to 0.78) [6] | |||
| TE-Finasteride | 2.86 (1.2 to 4.5) [17] | 0.30 (−0.18 to 0.78) [10] | |||
| Vehicle-Placebo | 1.33 (−0.022 to 2.6) [16] | 0.056 (−0.19 to 0.30) [12] | 0.77 (0.51 to 1.03) | 0.034 (−0.22 to 0.29) | −0.17 (−0.67 to 0.33) |
| Vehicle-Finasteride | 1.39 (−0.040 to 2.8) [13] | −0.057 (−0.80 to 0.69) [6] | P < 0.001 | P = 0.79 | P = 0.51 |
| TE-Placebo | 1.75 (−0.21 to 3.7) [13] | 0.18 (−0.58 to 0.94) [6] | |||
| TE-Finasteride | 1.88 (0.018 to 3.7) [17] | 0.45 (−0.078 to 0.97) [10] | |||
| Vehicle-Placebo | 160.2 (145.2 to 175.3) [16] | 0.17 (−13.2 to 13.5) [12] | −0.81 (−12.0 to 10.4) | −4.9 (−16.1 to 6.30) | −20.5 (−42.9 to 1.89) |
| Vehicle-Finasteride | 160.5 (143.9 to 177.2) [13] | −9.25 (−35.0 to 16.5) [8] | P = 0.88 | P = 0.40 | P = 0.074 |
| TE-Placebo | 161.2 (140.0 to 182.2) [13] | 17.5 (−25.5 to 60.2) [6] | |||
| TE-Finasteride | 173.6 (149.3 to 198.0) [17] | −4.46 (−18.6 to 9.65) [11] | |||
| Vehicle-Placebo | 89.8 (75.2 to 104.3) [16] | −2.58 (−14.3 to 9.14) [12] | 10.5 (0.54 to 20.4) | −11.5 (−18.9 to −1.5) | −21.7 (−41.6 to −1.87) |
| Vehicle-Finasteride | 85.8 (72.6 to 99.0) [13] | −20.9 (−44.1 to 2.36) [8] | P = 0.041 | P = 0.026 | P = 0.037 |
| TE-Placebo | 84.5 (64.4 to 104.5) [13] | 25.3 (−22.2 to 72.8) [6] | |||
| TE-Finasteride | 94.1 (75.1 to 113.0) [17] | −0.82 (−9.87 to 8.23) [11] | |||
| Vehicle-Placebo | 144.0 (111.1 to 176.9) [16] | 4.33 (−18.7 to 27.4) [12] | −42.8 (−71.2 to −14.3) | 42.6 (14.1 to 71.0) | −17.1 (−74.0 to 39.9) |
| Vehicle-Finasteride | 148.1 (114.2 to 182.0) [13] | 46.4 (−7.4 to 100.1) [8] | P = 0.0051 | P = 0.0046 | P = 0.55 |
| TE-Placebo | 154.6 (100.8 to 208.4) [13] | −16.5 (−39.2 to 6.22) [6] | |||
| TE-Finasteride | 183.4 (135.1 to 231.8) [17] | −2.72 (−58.7 to 53.2) [11] | |||
| Vehicle-Placebo | 1.10 (0.97 to 1.26) [16] | −0.01 (−0.59 to 0.04) [12] | 0.072 (0.018 to 0.13) | 0.019 (−0.031 to 0.068) | −0.11 (−0.15 to 0.022) |
| Vehicle-Finasteride | 1.08 (0.84 to 1.32) [13] | 0.10 (−0.01 to 0.21) [8] | P = 0.010 | P = 0.45 | P = 0.034 |
| TE-Placebo | 0.97 (0.89 to 1.04) [13] | 0.083 (0.004 to 0.16) [6] | |||
| TE-Finasteride | 0.88 (0.77 to 1.01) [17] | 0.091 (0.004 to 0.18) [11] | |||
| Vehicle-Placebo | 24.9 (21.1 to 28.7) [16] | −0.50 (−4.62 to 3.62) [12] | 1.19 (−2.59 to 4.93) | 1.89 (−1.88 to 5.66) | −1.02 (−8.56 to 6.52) |
| Vehicle-Finasteride | 23.6 (17.1 to 30.1) [13] | 2.13 (−2.35 to 6.60) [8] | P = 0.53 | P = 0.32 | P = 0.78 |
| TE-Placebo | 24.9 (19.9 to 29.9) [13] | 1.33 (−1.53 to 4.20) [6] | |||
| TE-Finasteride | 46.8 (16.3 to 77.2) [17] | −9.82 (−28.2 to 8.56) [11] | |||
| Vehicle-Placebo | 21.9 (18.3 to 25.4) [16] | −0.67 (−4.55 to 3.22) [12] | 0.075 (−5.31 to 5.46) | 4.16 (−1.23 to 9.54) | 1.72 (−9.02 to 12.5) |
| Vehicle-Finasteride | 21.8 (15.6 to 28.1) [13] | 1.75 (−3.28 to 6.78) [8] | P = 0.98 | P = 0.13 | P = 0.75 |
| TE-Placebo | 23.3 (19.1 to 27.5) [13] | −2.67 (−8.40 to 3.07) [6] | |||
| TE-Finasteride | 58.0 (17.7 to 98.3) [17] | −11.2 (−35.8 to 13.4) [11] | |||
| Vehicle-Placebo | 67.1 (54.6 to 79.7) [16] | 3.33 (−1.38 to 8.05) [12] | −6.88 (−11.5 to 2.28) | 2.76 (−1.94 to 7.46) | 3.43 (−6.07 to 12.9) |
| Vehicle-Finasteride | 89.5 (63.5 to 115.6) [13] | 1.00 (−10.7 to 8.68) [8] | P = 0.0044 | P = 0.24 | P = 0.47 |
| TE-Placebo | 79.4 (62.4 to 96.3) [13] | −8.50 (−22.5 to 5.47) [6] | |||
| TE-Finasteride | 72.1 (60.2 to 83.9) [17] | −6.46 (−14.0 to 1.06) [11] |
Fig. 2.
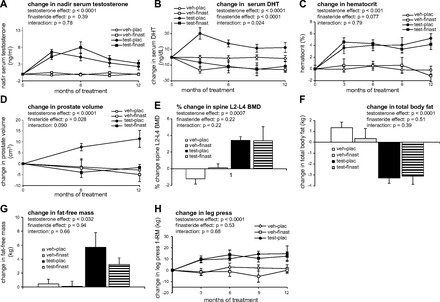
Changes occurring from baseline to 12 mo in testosterone, dihydrotestosterone (DHT), hematocrit, prostate volume, lumbar spine bone miberal density (BMD), total body fat, fat-free mass and one-repetition maximum (1-RM) leg press strength in participants receiving vehicle (veh)-placebo(plac), veh-finasteride(finast), testosterone enanthate (test)-plac or test-finast. A: significant increase shown in nadir serum testosterone levels following testosterone-enanthate (TE) administration. B: significant increase shown in serum DHT following TE administration and a significant decrease in DHT following finasteride administration. C: significant increase shown in hematocrit following TE administration. D: significant increase shown in prostate volume following TE administration and a significant decrease in prostate volume following finasteride administration. E: significant increase shown in lumbar spine BMD following TE administration. F: significant decrease shown in fat mass following TE administration. G: significant increase shown in fat-free mass following TE administration. H: significant increase shown in 1-RM leg press strength following TE administration. Values are means ± SE; n = 7–13 per group. Effect and interaction columns are derived from repeated-measures analysis for effects of T, F, and Interaction. These effects/interactions are indicative of the mean change occurring over each individual 3-, 6-, 9-, and 12-mo time point, as applicable, with Main Effects representing the average of testosterone under placebo and finasteride (weighted 50–50) or finasteride under vehicle and testosterone (weighted 50–50) and Interaction addressing whether or not the difference between the means for the first treatment factor (e.g., testosterone vs. vehicle) differ quantitatively based on the level of the second treatment factor (e.g., finasteride vs. placebo).
Table 3.
Treatment effects
| Variable | Group | 0–12 mo Change | Main Effect of T | Main Effect of F | Interaction |
|---|---|---|---|---|---|
| Testosterone, ng/dl | Vehicle-Placebo | 15 (−48 to 78) | 479 (374 to 584) | 45 (−59 to 152) | 28 (−182 to 238) |
| Vehicle-Finasteride | −3.7 (−59 to 52) | P < 0.001 | P = 0.39 | P = 0.79 | |
| TE-Placebo | 229 (−27 to 485) | ||||
| TE-Finasteride | 323 (106 to 541) | ||||
| BioT, ng/dl | Vehicle-Placebo | 7.3 (−11 to 25) | 148 (114 to 184) | 25 (−30 to 38) | −6.7 (−39 to 64) |
| Vehicle-Finasteride | 3.0 (−9.4 to 15) | P < 0.001 | P = 0.89 | P = 0.84 | |
| TE-Placebo | 65 (−33 to 164) | ||||
| TE-Finasteride | 70 (21 to 118) | ||||
| DHT, ng/dl | Vehicle-Placebo | −1.67 (−10.35 to 7.02) | 14.7 (8.80 to 20.6) | −23.3 (−29.2 to −17.4) | −13.8 (−23.6 to −1.94) |
| Vehicle-Finasteride | −15.7 (−24.8 to −6.61) | P < 0.001 | P < 0.001 | P = 0.024 | |
| TE-Placebo | 12.3 (−3.59 to 28.2) | ||||
| TE-Finasteride | −11.6 (−26.2 to 3.05) | ||||
| LH, U/l | Vehicle-Placebo | −0.29 (−2.41 to 1.83) [15] | −7.40 (−9.38 to −5.42) | 2.73 (0.74 to 4.72) | −0.47 (−4.52 to 3.58) |
| Vehicle-Finasteride | 0.15 (−2.23 to 2.52) [8] | P < 0.001 | P = 0.0083 | P = 0.81 | |
| TE-Placebo | −8.00 (−12.8 to −3.24) [6] | ||||
| TE-Finasteride | −5.89 (−12.12 to 0.38) [9] | ||||
| Osteocalcin, ng/ml | Vehicle-Placebo | −2.95 (−5.68 to −0.22) [12] | 1.28 (−0.92 to 2.38) | −0.64 (−2.82 to 1.58) | −5.60 (−7.87 to −3.42) |
| Vehicle-Finasteride | −0.005 (−5.54 to 5.12) [7] | P = 0.24 | P = 0.56 | P = 0.13 | |
| TE-Placebo | −0.934 (−6.87 to 5.00) [6] | ||||
| TE-Finasteride | −1.55 (−4.53 to 1.44) [11] | ||||
| CTX-1, pg/ml | Vehicle-Placebo | −0.12 (−0.067 to 0.043) [12] | −0.81 (−0.13 to 0–0.029) | −0.0055 (−0.057 to 0.046) | −0.0088 (−0.13 to 0.095) |
| Vehicle-Finasteride | 0.015 (−0.088 to 0.18) [7] | P = 0.0028 | P = 0.83 | P = 0.86 | |
| TE-Placebo | −0.065 (−0.19 to 0.057) [6] | ||||
| TE-Finasteride | −0.092 (−0.20 to 0.014) [11] |
Demographic and Blood Values
TE elevated nadir testosterone and BioT, representing 1.8-fold and 2.2-fold increases over baseline, respectively (Table 2 and Fig. 2A). Finasteride did not affect those increases. TE elevated serum DHT, and finasteride lowered DHT (Table 2 and Fig. 2B). TE reduced LH to near-zero concentrations, whereas finasteride modestly increased LH (Table 3). TE elevated E2 1.7-fold and BioE2 2.2-fold (P < 0.001), whereas finasteride had no effect (Table 4). TE elevated hemoglobin with a time course similar to that of Hct (Table 4), whereas finasteride had no significant effects on hemoglobin. TE lowered HDL-cholesterol and triglycerides while increasing LDL-cholesterol (Table 4). Finasteride significantly reduced LDL-cholesterol while increasing triglycerides (Table 4). Neither treatment altered total cholesterol. TE significantly decreased CTX-1 but did not affect osteocalcin (Table 4). All clinical values remained within normal reference ranges throughout the study (Table 4).
Body Composition
TE increased lumbar spine BMD 4.19% (P < 0.001; Table 3 and Fig. 2E), lumbar spine BMC 3.94% (P = 0.0032), and total hip BMD 1.96% (P = 0.024); BMC was not affected in other regions. TE also reduced total body fat mass 3.87 kg (P < 0.001; Table 3 and Fig. 2F), trunk fat 1.88 kg (P = 0.0051; data not shown), and android fat mass 0.42 kg (P < 0.001; data not shown). Finasteride did not affect the above measurements.
Prostate-Related Measures
TE elevated serum PSA, and finasteride reduced PSA (Table 2). The AUA/IPPS was not altered by treatment. Two participants received prostate biopsies, prompted by DRE findings (one each from the TE-placebo and TE-finasteride groups); both were negative.
DISCUSSION
Testosterone exerts direct effects at ARs and can also induce indirect effects at ARs and/or estrogen receptors (ERs) following the 5α-reduction to DHT (44) or the aromatization to E2 (28), respectively. However, the role of DHT in mediating beneficial and/or adverse effects of administered testosterone has received little focus in the literature. We report that 12-mo administration of higher-than-replacement TE (alone) or TE plus finasteride increased fat-free mass, muscle strength, Hct, and lumbar spine and hip BMD while reducing fat mass in older hypogonadal men. TE (alone) also elevated PSA and prostate volume, whereas coadministration of a clinically relevant dose of finasteride completely prevented TE-induced increases in PSA and prostate volume.
In young and older men, testosterone dose-dependently augments lean mass with high-dose TE (600 mg/wk for 10 wk) increasing quadriceps area 7% and muscle strength 10–12% (10). In contrast, low-dose testosterone (5 mg/day for 2 yr) increased femoral neck BMD but did not alter muscle strength in older men (31), suggesting that higher doses of testosterone may be required for improvements in muscle function (41). In support of this contention, moderate-dose TE (200 mg biweekly) improved lumbar spine and hip BMD, physical performance, and grip strength but did not increase leg strength over 3 yr of treatment (4, 33). Importantly, equivalent results were observed whether TE was administered alone or in combination with finasteride (14, 23), indicating that elevated DHT is not required for the beneficial skeletal responses to testosterone in adults. We report similar findings, in that TE plus finasteride markedly reduced DHT while increasing lumbar spine and hip BMD 2–4% (via antiresorptive actions indicated by reduced CTX-1) and total body fat-free mass >3 kg. Additionally, we observed reductions in total body fat mass of ∼10% over 12 mo. We expand upon previous findings (33) by demonstrating, for the first time, that coadministration of TE and finasteride improves upper- and lower-body maximal strength in older hypogonadal men. One difference between these studies is that Page et al. (33) measured isokinetic strength, whereas we measured 1-RM strength. Interestingly, type I 5α-reductase expression appears essential for normal musculoskeletal development, as demonstrated by male 5α-reductase type I knockout (srd5a1−/−) mice that exhibit reduced bone mass and muscle force despite normal circulating androgen concentrations (42). However, 5α-reductase type I activity is not essential for testosterone-induced myotrophic effects in adult men. As evidence, Bhasin et al. (11) reported that dutasteride did not inhibit TE-induced improvements in lean mass or muscle function in young eugonadal men administered GnRH agonists to suppress testosterone despite a near-complete ablation of circulating DHT. Preclinical findings also indicate that high-dose TE plus MK-434 (types I and II 5α-reductase inhibitor) (14, 15) and trenbolone [a highly potent non-5α-reducible and nonaromatizable testosterone analog (15, 30, 43)] protect against orchiectomy-induced muscle and bone loss, although no clinical study has examined the skeletal responses to TE plus dutasteride.
We also observed that TE treatment significantly elevated serum E2 and BioE2. In this regard, recent evidence indicates that the lipolytic (but not the muscular) effects of testosterone may be influenced by the aromatization of testosterone to E2 (22). Additionally, E2 is a more potent bone antiresorptive agent than testosterone in older men undergoing experimental sex steroid deficiency (at least when administered in doses that result in physiological serum concentrations) (19). As such, the possibility exists that the lipolytic and bone-protective effects that we observed were, in part, mediated by elevated E2. However, this certainly does not preclude the possibility that androgens influence bone and adipose tissue through direct AR-mediated mechanisms. In support of this contention, trenbolone (a highly potent nonaromatizable and non-5α-reducible testosterone analog) reduces adiposity and preserves bone mass in several animal models of sex steroid deficiency (30, 43). Additionally, human preadipocytes and mature adipocytes express ARs (35) and nonaromatizable androgens inhibit adipogenesis in human adipose tissue (17). Human osteoblasts also express ARs at sites of bone formation (1), and physiological testosterone replacement maintains bone formation in older men undergoing experimental sex steroid deficiency (19). Regardless of the mechanism, the biological significance of our findings is profound given that we have demonstrated that the desirable musculoskeletal and lipolytic effects of higher-than-replacement testosterone can be obtained without deleterious effects on prostate mass, which significantly improves the safety profile of this clinically relevant treatment.
DHT mediates many of the androgenic effects of testosterone, including prostate enlargement, and may worsen prostate cancer risk (29). Both finasteride and dutasteride are used clinically to treat BPH due to their ability to reduce circulating DHT (38). However, only two previous clinical studies (4, 11) have examined whether elevated DHT mediates prostate enlargement following testosterone administration, with somewhat conflicting results. Bhasin et al. (11) reported that graded doses of TE (ranging from 50 to 600 mg/wk) increased prostate volume ∼2.5cm3 over 20 wk in young men and that dutasteride did not prevent this enlargement. Conversely, Amory et al. (4) reported that TE (200 mg biweekly) increased prostate volume 14 cm3 over 3 yr in older hypogonadal men and that finasteride coadministration limited prostate enlargement to only 5 cm3. Others reported that men receiving testosterone by patch, gel, or injection experienced prostate volume increases of 4.9 cm3/yr of treatment (46) and that coadministration of transdermal testosterone (1% T gel) and dutasteride reduced prostate size in older men with BPH (34). Similarly, we report that the main effect of 125 mg/wk TE on prostate volume was 5.3 cm3 when administered to hypogonadal older men; however, this underestimates the actual 12-mo change in the TE-placebo group (11.4 cm3), because finasteride coadministration completely prevented TE-induced prostate enlargement.
Testosterone dose-dependently increases hemoglobin and hematocrit, possibly independently of erythropoietin, and this effect is greater in older men compared with younger (18). We observed a 4–5 point Hct increase following administration of either TE or TE plus finasteride; suggesting that elevated DHT does not mediate this change. The Hct increase that occurs in most older men receiving testosterone is not considered detrimental and may be beneficial (37), although this must be weighed against the risk of polycythemia (25) and associated cardiovascular risks (23).
Several meta-analyses (16, 25) have identified polycythemia, combined incidence of prostate events (including elevated PSA, increased urinary symptoms, number of prostate biopsies, and prostate cancer), and a modest reduction in HDL-cholesterol as the three proven adverse events resulting from testosterone administration. Rates of adverse events were similar between groups in our study, although we observed that TE lowered HDL by ∼10%, an effect not altered by finasteride. TE also increased LDL 29% and reduced triglycerides. Conversely, finasteride coadministration reduced LDL-cholesterol but did not alter triglycerides. The clinical ramifications of these blood lipid changes remains unknown (36). Additionally, several putative adverse events are associated with testosterone administration, including worsening of sleep apnea, edema, gynecomastia, increased incidence of cardiovascular events, acceleration of underlying prostate cancer, and liver-related side effects (6, 7, 25). In this regard, coadministration of testosterone plus finasteride has some advantages, including lessening of androgen-induced prostate enlargement, and some potential disadvantages compared with treatment with testosterone alone, including altering the clinical usefulness of PSA measurements (29). However, the value of PSA testing has come into question, especially in men aged ≥70 yr (8). Testosterone administration combined with 5α-reductase inhibition may also alter prostate cancer risk or severity, given that finasteride alone reduces the incidence of prostate cancer while increasing the number of prostate cancers with Gleason scores of 7–10 (38). Similarly, testosterone-stimulated prostate cancer growth in culture is ablated by dutasteride (2), perhaps through mechanisms involving upregulation of testosterone-sensitive tumor suppressors (24).
As with all studies, several limitations exist with our data set, including a relatively small sample size and a slightly higher than expected non-completion rate. In this regard, the 2 × 2 factorial design that we utilized is the most powerful study design to detect outcomes resulting from a combination pharmacological therapy and allows for adequate power with smaller sample sizes. To ensure an appropriate sample size, we powered to detect differences in three of four primary outcomes (i.e., 1-RM strength, fat-free mass, and Hct) at the 6-mo time point despite the 12-mo duration of our study. In this regard, we met our expected non-completion rate of 20% at 6 mo, whereas the 12-mo noncompletion rate in our study (i.e., 33%) was slightly higher than anticipated. Importantly, non-completion was similar among all groups (χ2 P value = 0.48, indicating no differences) and was comparable to the non-completion rates of 27–29% in other long-term testosterone studies (4, 11, 26). Additionally, our non-completion rate was not exceedingly high, given that once-weekly visits to the hospital were required for 52 consecutive weeks and that we observed a significant main effect for testosterone in each of our primary and secondary outcomes and a significant interaction for prostate volume (the only primary outcome powered on 12-mo data), as hypothesized. Indeed, we detected all a priori hypotheses for primary and secondary outcomes. However, it remains theoretically possible that we did not observe significant interactions (at the 12-mo time point) because our noncompletion rate resulted in a slightly lower than expected power to detect such outcomes. We believe this to be highly unlikely given that the 0- to 12-mo changes and point estimates (i.e., 95% CI) for the vast majority of our data were directionally similar and of a comparable magnitude in the 1) testosterone-placebo and testosterone-finasteride groups and 2) vehicle-placebo and vehicle-finasteride groups. Regardless, when one is interpreting our data, nonsignificant interactions should not be used to infer that an interaction was not present. In addition to the above mentioned limitations, some nonsignificant differences also existed between groups at baseline. To account for this random variability, baseline values were included in our statistical model as fixed effects. Additionally we examined the baseline characteristics of the randomized cohort (n = 60) and of the completers (n = 40) and observed no differences between cohorts, indicating that completers were representative of the overall randomized cohort. Irrespective of these limitations, we report significance in each of our primary and secondary outcomes, demonstrating the robustness of our data and that our study achieved appropriate power to detect all desired outcomes.
In conclusion, our results add to the growing number of preclinical (24–26) and clinical trials (4, 11, 33) reporting that musculoskeletal and lipolytic improvement can be obtained without prostate enlargement when higher-than-replacement testosterone is coadministered with finasteride (an inhibitor of the type II 5α-reductase enzyme). These findings indicate that elevated DHT is not required for the benefits of exogenous testosterone and support the contention that finasteride ablates prostate enlargement associated with higher-than-replacement TE administration. As such, our findings provide a rationale for larger and more comprehensive clinical trials examining the safety and efficacy of higher-than-replacement testosterone plus finasteride/dutasteride treatment in hypogonadal elderly men.
GRANTS
This study was upported by a Veteran's Health Administration Clinical Services R&D Merit Award to S. E. Borst, by Grant 1UL1 TR-000064 from the National Center for Advancing Translational Science, National Institutes of Health, and in part by the Merck-Investigator-Initiated Studies Program (Merck & Co.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.E.B., U.N., and M.M. conception and design of research; S.E.B., J.F.Y., C.F.C., U.N., J.R.M., J.A.L., R.W.B., D.T.B., J.S.M., M.M., S.R., L.A.B., S.C.M., and D.F.C. performed experiments; S.E.B., J.F.Y., C.F.C., U.N., J.A.L., R.W.B., D.T.B., J.S.M., L.A.B., D.F.C., and J.J.S. analyzed data; S.E.B., J.F.Y., C.F.C., U.N., R.W.B., D.T.B., J.S.M., L.A.B., and S.C.M. interpreted results of experiments; S.E.B. and J.F.Y. prepared figures; S.E.B., J.F.Y., R.W.B., D.T.B., J.S.M., M.M., L.A.B., S.C.M., D.F.C., and J.J.S. drafted manuscript; S.E.B., J.F.Y., C.F.C., U.N., J.R.M., J.A.L., R.W.B., D.T.B., J.S.M., M.M., S.R., L.A.B., S.C.M., D.F.C., and J.J.S. edited and revised manuscript; S.E.B., J.F.Y., C.F.C., U.N., J.R.M., J.A.L., R.W.B., D.T.B., J.S.M., M.M., S.R., L.A.B., S.C.M., D.F.C., and J.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Keith Kaufman at Merck for editorial comments; Warren Reed, Tom Cornwell, and the Urology Service from the Malcom Randall VAMC for assistance with urological exams; and Helen Dunn and Linda Sawka, The Living Well Center at the University of Florida, for allowing use of resistance exercise equipment. Trial registration: Clinical trials.gov identifier NCT00475501.
REFERENCES
- 1.Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE. The localization of androgen receptors in human bone. J Clin Endocrinol Metab 82: 3493–3497, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Alisky JM, Tang Y, Habermehl GK, Iczkowski KA. Dutasteride prevents the growth response to testosterone in benign and androgen-sensitive malignant prostate cells. Int J Clin Exp Med 3: 245–247, 2010 [PMC free article] [PubMed] [Google Scholar]
- 3.Amory JK, Wang C, Swerdloff RS. The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab 92: 1659–1665, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab 89: 503–510, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Barry MJ, Fowler FJ, Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, Cockett AT. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 148: 1549–1557, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med 363: 109–122, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basaria S, Davda MN, Travison TG, Ulloor J, Singh R, Bhasin S. Risk Factors Associated With Cardiovascular Events During Testosterone Administration in Older Men With Mobility Limitation. J Gerontol A Biol Sci Med Sci 68: 153–160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basch E, Oliver TK, Vickers A, Thompson I, Kantoff P, Parnes H, Loblaw DA, Roth B, Williams J, Nam RK. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology Provisional Clinical Opinion. J Clin Oncol 30: 3020–30255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhasin S, Cunningham GR, Hayes FJ. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 95: 2536–2559, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 335: 1–7, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Bhasin S, Travison TG, Storer TW. Effect of testosterone supplementation with and without a dual 5alpha-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. JAMA 307: 931–939, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 90: 678–688, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry 15: 1021–1027, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Borst SE, Conover CF, Carter CS, Gregory CM, Marzetti E, Leeuwenburgh C, Vandenborne K, Wronski TJ. Anabolic effects of testosterone are preserved during inhibition of 5α-reductase. Am J Physiol Endocrinol Metab 293: E507–E514, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Borst SE, Lee JH, Conover CF. Inhibition of 5α-reductase blocks prostate effects of testosterone without blocking anabolic effects. Am J Physiol Endocrinol Metab 288: E222–E227, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Calof OM, Singh AB, Lee ML, Lee ML, Kenny AM, Urban RJ, Tenover JL, Bhasin S. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 60: 1451–457, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids 78: 920–6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 93: 914–919, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106: 1553–60, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, Agrwal N, Elamin MB, Gallegos-Orozco JF, Wang AT, Erwin PJ, Bhasin S, Montori VM. Clinical review 1. Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 95: 2560–2575, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282: E601–E607, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 369: 1011–1022, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg G, Assali A, Vaknin-Assa H, Brosh D, Teplitsky I, Fuchs S, Battler A, Kornowski R, Lev EI. Hematocrit level as a marker of outcome in ST-segment elevation myocardial infarction. Am J Cardiol 105: 435–440, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Wang Y, Ramos-Garcia R, Shevrin D, Nelson JB, Wang Z. Inhibition of 5alpha-reductase enhances testosterone-induced expression of U19/Eaf2 tumor suppressor during the regrowth of LNCaP xenograft tumor in nude mice. Prostate 70: 1575–1585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, Sideras K, Uraga MV, Erwin PJ, Montori VM. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc 82: 29–39, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Idan A, Griffiths KA, Harwood DT, Seibel MJ, Turner L, Conway AJ, Handelsman DJ. Long-term effects of dihydrotestosterone treatment on prostate growth in healthy, middle-aged men without prostate disease. Ann Int Med 153: 621–632, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 26: 833–876, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lombardi G, Zarrilli S, Colao A, Paesano L, Di Somma C, Rossi F, De Rosa M. Estrogens and health in males. Mol Cell Endocrinol 178: 51–55, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Marks LS, Andriole GL, Fitzpatrick JM, Schulman CC, Roehrborn CG. The interpretation of serum prostate specific antigen in men receiving 5alpha-reductase inhibitors: a review and clinical recommendations. J Urol 176: 868–874, 2006 [DOI] [PubMed] [Google Scholar]
- 30.McCoy SC, Yarrow JF, Conover CF, Beggs LA, Goldberger BA, Borst SE. 17β-Hydroxyestra-4,9,11-trien-3-one (Trenbolone) preserves bone mineral density in skeletally mature orchiectomized rats without prostate enlargement. Bone 51: 667–673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ, 3rd, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355: 1647–1659, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA, Ostir GV. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc 54: 1666–1673, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page ST, Amory JK, Bowman FD. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 90: 1502–1510, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Page ST, Hirano L, Gilchriest J, Dighe M, Amory JK, Marck BY, Matsumoto AM. Dutasteride reduces prostate size and prostate specific antigen in older hypogonadal men with benign prostatic hyperplasia undergoing testosterone replacement therapy. J Urol 186: 191–197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen SB, Fuglsig S, Sjøgren P, Richelsen B. Identification of steroid receptors in human adipose tissue. Eur J Clin Invest 26: 1051–1056, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Rubinow KB, Vaisar T, Tang C, Matsumoto AM, Heinecke JW, Page ST. Testosterone replacement in hypogonadal men alters the HDL proteome but not HDL cholesterol efflux capacity. J Lipid Res 53: 1376–1383, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, Kawakubo M, Stewart Y, Yarasheski KE, Ulloor J, Colletti P, Roubenoff R, Azen SP. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab 94: 1991–2001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA., Jr The influence of finasteride on the development of prostate cancer. N Engl J Med 349: 215–224, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Tracz MJ, Sideras K, Bolona ER, Haddad RM, Kennedy CC, Uraga MV, Caples SM, Erwin PJ, Montori VM. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab 91: 2011–2016, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84: 3666–3672, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 85: 2839–2853, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Windahl SH, Andersson N, Borjesson AE, Swanson C, Svensson J, Movérare-Skrtic S, Sjögren K, Shao R, Lagerquist MK, Ohlsson C. Reduced bone mass and muscle strength in male 5-alpha-reductase type 1 inactivated mice. PLoS One 6: e21402, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yarrow JF, Conover CF, McCoy SC, Lipinska JA, Santillana CA, Hance JM, Cannady DF, VanPelt TD, Sanchez J, Conrad BP, Pingel JE, Wronski TJ, Borst SE. 17β-Hydroxyestra-4,9,11-trien-3-one (trenbolone) exhibits tissue-selective anabolic activity: effects on muscle, bone, adiposity, hemoglobin, and prostate. Am J Physiol Endocrinol Metab 300: E650–E660, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yarrow JF, McCoy SC, Borst SE. Intracrine and myotrophic roles of 5alpha-reductase and androgens: a review. Med Sci Sports Exerc 44: 818–826, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yarrow JF, McCoy SC, Borst SE. Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity. Steroids 75: 377–389, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E. Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic study. J Clin Endocrinol Metab 88: 2049–2054, 2003 [DOI] [PubMed] [Google Scholar]


