Abstract
Secretion of growth hormone (GH), and consequently that of insulin-like growth factor 1 (IGF-1), declines over time until only low levels can be detected in individuals aged ≥60 years. This phenomenon, which is known as the ‘somatopause’, has led to recombinant human GH being widely promoted and abused as an antiageing drug, despite lack of evidence of efficacy. By contrast, several mutations that decrease the tone of the GH/IGF-1 axis are associated with extended longevity in mice. In humans, corresponding or similar mutations have been identified, but whether these mutations alter longevity has yet to be established. The powerful effect of reduced GH activity on lifespan extension in mice has generated the hypothesis that pharmaceutically inhibiting, rather than increasing, GH action might delay ageing. Moreover, mice as well as humans with reduced activity of the GH/IGF-1 axis are protected from cancer and diabetes mellitus, two major ageing-related morbidities. Here, we review data on mouse strains with alterations in the GH/IGF-1 axis and their effects on lifespan. The outcome of corresponding or similar mutations in humans is described, as well as the potential mechanisms underlying increased longevity and the therapeutic benefits and risks of medical disruption of the GH/IGF-1 axis in humans.
Introduction
Growth hormone (GH), which is also known as somatotropin, and insulin-like growth factor 1 (IGF-1 or IGF-I) exert a myriad of pleiotropic effects via a number of similar and distinct intracellular signalling pathways. One of the main actions common to GH and IGF-1 is the promotion of growth (Figure 1). However, GH and IGF-1 act very differently on glucose and lipid metabolism; GH blocks insulin action, promotes lipolysis and impedes lipogenesis, whereas IGF-1 has opposing effects.1
Figure 1.
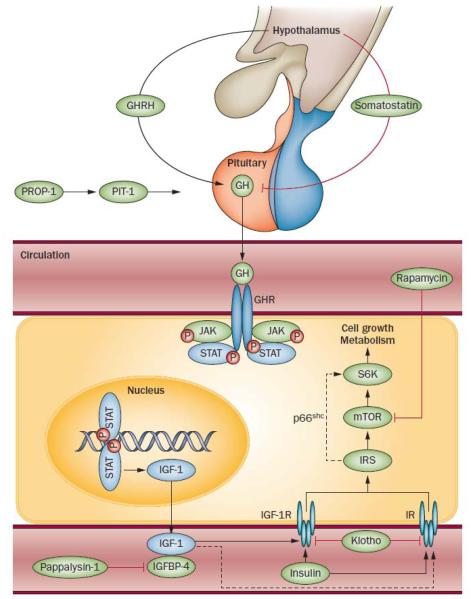
Factors of the GH/IGF-1 axis known to influence ageing. The embryonically expressed genes PROP1 (which encodes PROP-1) and POU1F1 (which encodes PIT-1) are involved in pituitary development, including differentiation of pituitary somatotrophic cells. GHRH and somatostatin are released by different populations of hypothalamic neurons and stimulate or inhibit, respectively, GH production and secretion by the somatotrophs in the anterior pituitary. GH binds to GH receptors, which activates the JAK–STAT pathway and induces the production of IGF-1, predominantly in the liver. In the circulation, much of IGF-1 is bound by IGFBPs, which act as carrier proteins and regulators of IGF-1 bioavailability. Pappalysin-1 is an IGFBP-4 protease that increases IGF-1 bioavailability. IGF-1 mediates its effects by binding to IGF-1R and, with less affinity, to IRs in many tissues. Importantly, not only endocrine but also autocrine and paracrine actions of IGF-1 have a crucial role in normal animal physiology. The protein klotho suppresses insulin and IGF-1 action, whereas IRS-1 and IRS-2 transduce signals from the IGF-1R and the IR, resulting in the activation of several pathways, including one that acts via S6K, a downstream effector of mTOR that is linked to IRS-1 via p66shc. Abbreviations: GH, growth hormone; GHR, GH receptor; GHRH, GH-releasing hormone; IGF-1, insulin-like growth factor 1; IGFBP, IGF-1 binding protein; IGF-1R, IGF-1 receptor; IR, insulin receptor; IRS, IR substrate; JAK, janus kinase; mTOR, mammalian target of rapamycin; PIT-1, pituitary-specific positive transcription factor 1; PROP-1, prophet of PIT-1; STAT, signal transducer and activator of transcription; S6K, S6 kinase.
After reaching adulthood, secretion of GH and IGF-1 declines continuously to very low levels in those aged ≥60 years.2 This phenomenon is known as ‘somatopause’ and is also seen in other mammals.3 In 1990, Rudman et al. proposed recombinant human (rh) GH as a potent antiageing therapeutic, with positive effects on body composition, BMD and skin thickness.4 The investigators postulated that the effects of 6 months of rhGH treatment on lean body mass and adipose tissue mass were equivalent in magnitude to the changes incurred over 10–20 years of ageing.4 This report started a hype around rhGH as an antiageing therapeutic, with some proponents proclaiming it as the ‘fountain of youth’. At the same time, however, data about adverse effects of GH were increasingly emerging. In 2007, a meta-analysis on rhGH use in the elderly (mean age 69 years) indicated that this agent has minor benefits on body composition but also has risks, such as the development of diabetes mellitus.5
By contrast, decreased GH/IGF-1 signalling has been shown to extend longevity in a wide variety of species including worms, fruit flies, yeast and mice.6 Caloric restriction, which is one of the most consistent means to increase lifespan in most species, also reduces the activity of this pathway (Box 1).6 These findings raise the question of whether lowered activity of the GH/ IGF-1 pathway might be beneficial for the extension of human lifespan.
Box 1. Caloric restriction.
Caloric restriction—a dietary regimen that reduces total energy intake without malnutrition—can extend lifespan and prevent ageing-related pathologies in a variety of species.114 The molecular mechanism underlying these effects is still debated, although reduced mTOR signalling has been implicated. However, caloric restriction also consistently reduces IGF-1 concentrations115 and so affects the GH/IGF-1 axis. In fact, mTOR signalling is reduced in specific cells and tissues in GH-deficient or GH-resistant mice, suggesting that caloric restriction and the GH/ IGF-1 axis might both act via altered mTOR signalling.39,104
Animals fed a calorie-restricted diet are protected against common age-related conditions such as obesity, neurodegenerative disorders, autoimmune disorders and neoplastic lesions.116 No lifespan data exists for humans on a calorie-restricted diet, but several reports support protection from age-related diseases.117,118 Given that feeding studies are difficult to conduct in humans, caloric restriction studies in nonhuman primates are valuable. Two studies in rhesus monkeys reveal disparate results; although one study shows that caloric restriction does extend lifespan,119 the second study found no effects of caloric restriction on longevity.120 Interestingly, these findings point to a central role for genetics and dietary composition, not merely a decrease in calories, in the efficacy of caloric restriction.
Will caloric restriction in humans result in life extension? The jury is still out. Perhaps the question should rather be whether humans are able to adhere to a radical lifestyle change, such as caloric restriction, for a long period of time for the purpose of health promotion. On the basis of our understanding of the lifestyle and dietary practices that contribute to obesity and the fact that rates of obesity continue to escalate, this hope seems doubtful. So the quest for a simpler route to extend human longevity, for example a calorie-restriction-mimetic drug, begins.
Abbreviations: GH, growth hormone; IGF-1, insulin-like growth factor 1; mTOR, mammalian target of rapamycin.
A number of mutations that alter the tone of the GH/ IGF-1 axis have been identified in mice and humans. In this Review, we discuss mutations in genes that regulate aspects of GH and IGF-1 action and their effects on ageing (Table 1). Additionally, we highlight phenotypic similarities and differences resulting from these mutations. Given that reductions in GH/IGF-1 activity at various points of this axis affect mouse longevity, the concept of pharmacologically inhibiting the GH/ IGF-1 axis in humans as an ‘antiageing’ approach will be discussed.
Table 1.
Mouse strains with altered GH/IGF-1 axis and effects on longevity
| Mouse model | Size (% of control) |
Lifespan (change in %) |
Lifespan (days) | Background strain |
Body fat |
Insulin sensitivity |
Tumour incidence |
Stress resistance |
|
|---|---|---|---|---|---|---|---|---|---|
| Mutant | Control | ||||||||
| Snell7,8,10–12,92 | 25–33% | f and m +42 | f and m 1,178±235 |
f and m 832±158 | C3H/HeJ × DW/J | ↑ | ↑ | ↓ | ↑ |
| Ames14,17,92,99,112 | 33% | f +68% m +49% |
f 1,206±32 m 1,076±56 |
f 718±45 m 723±54 |
ND | ↑ | ↑ | ↓ | ↑ |
| lit/lit 10,24,97,100 | 50–67% | f +25% m +23% |
f 1,070±127 m 1,093±186 |
f 857±169 m 886±148 |
C57BL/6J | ↑ | ND | ↓ | ↑ |
| Ghr −/−35-37,92,101 | <50% | f +21% m +40% |
f 921±41 m 917±55 |
f 759±41 m 656±67 |
Ola-BALB/cJ | ↑ | ↑ | ↓ | ↑ |
| Bovine GH transgenic mouse49,50 |
200% | m –45% | m 425±22 | m 773 | ND | ↓ | ↓ | ↑ | ND |
| GHA transgenic mouse35,36,46,47,102 |
70% | ns | f 839±25 m 790±41 |
f 771±26 m 758±40 |
C57BL/6 | ↑ | ↑ | ↓ | ND |
| LI-Igf1 −/−60,61 | 75–100% | f +16% | f 812±33 (26.7±1.1 months) |
f 700±21 (23.0±0.7 months) |
C57BL/6 | ↓ | ↓ | ND | ND |
| LID59,62,113 | 100% | ↓ | ND | ND | ND | ↑ | ↓ | ↓ | ND |
| Pappa−/−64,65,67 | 40% | f and m +38% | f and m 960±28 |
f and m 698±23 | C57BL/6 × 129SV/E |
ND | ↔ | ↓ | ND |
| Igf1r +/−68 | 90% | f +33% m ns |
f 756±46 m 679±80 |
f 568±49 m 585±69 |
129/J | ND | ↓ | ND | ↑ |
| Igf1r +/−69 | 90% | ns | f 923±21 m 983±21 |
f 967±29 m 939±24 |
C57BL/6 | ND | ↓ | ↔ | f ↑ m ↔ |
| Klotho transgenic mouse73 |
100% | f1&2 +19% m1 +20% m2 +31% |
f1 829±32 f2 830±29 m1 858±40 m2 936±47 |
f 697±45 m 715±44 |
C57BL/6 × C3H | ND | f ↔ m ↓ |
ND | ↑ |
| Irs1 −/−76–80 | 70% | f +17% m ns |
f 891±39 m 897±41 |
f 763±21 m 786±21 |
C57BL/6 | ↓ | ↓ | ND | ND |
| Irs2 +/−81,82 | 100% | f and m +17% | f and m 905±22 |
f and m 775±10 | C57BL/6 | ND | ↑ | ND | ND |
| Irs2 +/−76,79 | 100% | f and m ns | f and m 788±17 |
f and m 755±22 | C57BL/6 | ND | ↔ | ND | ND |
| Irs2 −/−79 | 90% | f –26% m –84% |
f 560±63 m 123±20 |
f 755±23 m 767±40 |
C57BL/6 | ND | ↓ | ND | ND |
| Brain-specific Irs2+/− and Irs2−/−81 |
100% | +/− +18% −/− +14% |
ND | ND | C57BL/6 | ND | ↓ | ND | ND |
| p66shc+/− and p66shc−/−84,85 | 100% | f and m (+/−) +7% f and m (−/−) +28% |
f and m (+/−) 815±37 f and m (−/−) 973±37 |
f and m 761±19 | 129/SvEv | ↓ | ↔ | ND | ↑ |
Two transgenic mouse strains (1 and 2) were investigated. Abbreviations: f, females; m, males; ND, no data available; ns, not significant.
GH and longevity
Multiple pituitary hormone deficiencies
Snell mice
In 1929, Snell reported a mouse strain with a small phenotype that was passed to offspring in a recessive manner.7 At birth, these mice are the same size as their unaffected siblings; however, after 2 weeks of age, growth is dramatically inhibited, which ultimately results in mice that weigh ~25–33% of a normal adult mouse.7,8 A mouse strain with a similar mutation was identified ~50 years later.8 These mutations were found to lie in Pou1f1, the gene encoding the pituitary transcription factor PIT-1.9
Given that PIT-1 is necessary for the differentiation of pituitary cells that produce GH, TSH and prolactin, Snell mice lack all three hormones.9 These hormone deficiencies cause obesity and, counterintuitively, increase life- span in these mice by 42% compared with unaffected siblings.10 Snell mice also have improved glucose metabolism, which is believed to contribute to the increased lifespan and is thought to be a result of the lack of GH.11 Treatment of Snell dwarfs using a combination of GH and levothyroxine from 4–15 weeks of age does not alter lifespan.12 However, only partial growth restoration is achieved with this treatment regimen; adult mice are still less than half the size of wild-type mice.12 Additional lifelong treatment with levothyroxine decreases the life- span to a level between that of wild-type and nontreated dwarf mice.12
Interestingly, tumour development is delayed in Snell dwarfs compared with nonaffected siblings; in one study, no cause of death could be determined for nearly 50% of Snell mice, compared with <10% of normal littermates.12 This finding suggests that these dwarf mice die more often of multiple abnormalities than of a single cause, which in most wild-type mice is cancer.12
Ames mice
Ames dwarf mice carry a mutation that was first described in 1961 at Iowa State University in the city of Ames.13 Mice homozygous for this mutation are phenotypically similar to Snell dwarf mice, but the mutation is in a different gene.14 In 1995, the mutated gene was reported to encode a protein that is necessary for the transcription of Pou1f1, the gene that encodes PIT-1.15 Hence, the protein was named PROP-1 for ‘homeobox protein prophet of PIT-1’.16 The hierarchical organization of PIT-1 and PROP-1 offers an explanation for the similarities between Ames and Snell mice. Female Ames mice live 68% and males live 49% longer than wild-type mice.17 When Ames dwarfs are subjected to caloric restriction, lifespan is increased even further.18 Treatment with GH over 6 weeks reduces lifespan in Ames dwarfs, as opposed to treatment with levothyroxine, which has no effect on longevity.19 Notably, the GH doses used in this study were twice as high as those used to treat Snell mice, and greater catch-up growth was achieved, which resulted in Ames mice reaching ~70% of the body weight of control mice.19
Krk cohort
A group of 25 patients with dwarfism has been studied on the island of Krk, Croatia.20 These individuals have short stature (106–152 cm) and are deficient in GH, TSH, prolactin, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), as a result of a homozygous PROP1 mutation.21 Of note, FSH and LH deficiency is not a feature of Ames mice even though their phenotype is caused by a mutation in the corresponding gene. Despite the presence of obesity, wrinkled skin and absence of secondary sex traits, the Krk patients do not die prematurely; in fact, some have lived until the age of 80–90 years. These individuals also exhibit a delay in the appearance of grey hair and do not develop diabetes mellitus.20,21
GH deficiency
lit/lit mice
The first ‘little’ mouse (lit/lit) was described in 1976.22 Similar to Ames and Snell dwarf mice, the ‘little’ phenotype is inherited in a recessive manner. The mice are born normal-sized, with the dwarf phenotype first manifesting after 2 weeks of age.22 The lit/lit mice are GH-deficient owing to a mutation in the Ghrhr gene, which encodes the GH-releasing hormone receptor.10,23 These animals reach ~50% of the size of unaffected siblings and show increased adiposity (Figure 2).24 The lifespan of lit/lit mice is significantly increased; females live 25% and males 23% longer than wild-type mice.10
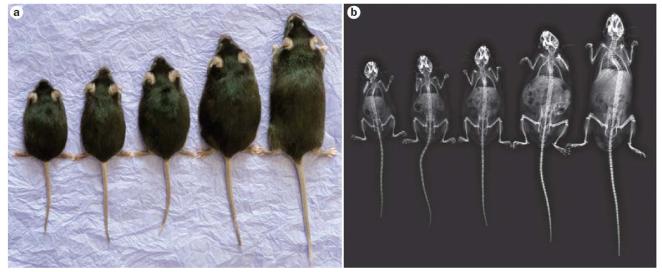
Figure 2.
Mouse strains with altered GH signalling. Ghr−/−, lit/lit, GHA, wild-type and bGH transgenic mouse strains (from left to right) were a | photographed and then b | scanned using a digital mammography unit. In this figure, Ghr−/−, GHA and wild-type mice were ~7 months old, the lit/lit mouse was ~5 months old, and the bGH transgenic mouse was ~4 months old. Weights of the mice were 14.4, 17.7, 24.9, 38.7 and 45.6 g, respectively. All except the Ghr−/− mouse are males. An average Ghr– /– male of this age weighs ~17 g. Bone structure is remarkably similar for the various mouse strains; however, kyphosis is typical for transgenic bGH mice. Abbreviations: bGH, bovine GH transgenic; GH, growth hormone; GHA, GH receptor antagonist; Ghr, GH receptor. Courtesy of J. Sattler, Ohio University Heritage College for Osteopathic Medicine, Athens, OH, USA
Itabaianinha cohort
More than 100 humans with dwarfism caused by homozygous GHRHR mutations have been identified in Itabaianinha, Brazil. Their adult heights vary from 105 cm to 135 cm, and they show central adipose tissue accumulation.25 According to investigators, these individuals have normal longevity; however, a reduced mean lifespan—47.7 years compared with 63.3 years for the unaffected population—has been reported.26 This reduction is a result of early deaths in young women, mostly from diarrhoeal diseases.26 One death from cancer was observed in a subset of nine patients with dwarf- ism, which implies that the number of cancer-related deaths in comparison to that in the general population is unchanged.26
Sindh cohort
A cohort of 18 patients with dwarfism from Sindh, Pakistan, carries homozygous mutations in the GHRHR gene.27,28 Their heights range from 113 cm to 135 cm.27 These individuals have mild obesity but do not display signs of impaired glucose metabolism.28 The oldest representative of this population is now 43 years old; thus, lifespan data are not yet available.
Swiss cohort
A retrospective study of a cohort of 11 Swiss patients with isolated GH deficiency showed that they carried a homozygous 6.7 kb deletion mutation that spanned the GH1 gene. Their lifespan was substantially shortened. Mean lifespan of affected women and men was 47.4 years and 57.4 years, respectively, whereas healthy sisters and brothers lived for a mean of 74.2 years and 70.9 years, respectively. Causes of death did not differ between groups.29
The genomic region of the gene deletion associated with this particular cohort includes possible regulatory elements of the neighbouring CD79B gene, which might have influenced the phenotype of this population. A CD79B mutation has previously been shown to result in impaired B-cell development and immunodeficiency.30
GH resistance
Ghr−/− mice
The Ghr−/− mouse, in which the GH receptor gene is disrupted,31 is also known as the Laron mouse, in reference to the similarities with human Laron syndrome (see below).32,33 Mice homozygous for this deletion are born normal-sized but reach less than 50% of the adult weight of wild-type littermates (Figure 2). Ghr−/− mice have higher than normal GH levels but very low IGF-1 levels (~20% of those of control mice).34 Also, their fasting glucose concentration and, even more drastically, their fasting insulin level are reduced throughout life.35 Despite being insulin-sensitive, Ghr−/− mice are obese, which is mostly due to highly increased accumulation of subcutaneous adipose tissue.36,37 Mean lifespans of female and male Ghr−/− mice are 21% and 40% longer, respectively, than those of wild-type control mice.35 In fact, one Ghr−/− mouse lived nearly 5 years and currently holds the record for the longest-lived laboratory mouse.38 Unlike wild-type control and Ames dwarf mice, caloric restriction does not further increase the lifespan of Ghr−/− mice, which suggests that the GH/IGF-1 axis and caloric restriction might share similar or overlapping mechanisms for lifespan extension.39
Laron syndrome
GH resistance or insensitivity, also known as Laron syndrome, is caused by homozygous mutations in GHR or GH-induced intracellular signalling molecules. A cohort of 60 Mediterranean patients has been studied since 1958 by Laron and colleagues. Patient heights range between 108 cm and 142 cm, and these individuals have obesity from early childhood onwards.40 Some patients show impaired glucose metabolism and even overt diabetes mellitus.41 Large-scale longevity data are not available for this cohort, given that many patients have been treated with rhIGF-1, and most patients are still alive.42
In 1990, another cohort of patients with Laron syndrome in Loja, Ecuador, was reported.43 As of 2011, the size of this cohort is 90 patients.44 Their adult heights vary from 106 cm to 122 cm,43 and they have obesity.44 No change in lifespan is observed relative to control individuals; however, only patients aged ≥10 years have been included in the lifespan analysis owing to a high mortality rate (from common childhood diseases) in affected children.44 Causes of death in adults differ between patients and control individuals; deaths from alcohol abuse or accidents are increased, whereas cancer-related deaths are reduced in patients with Laron syndrome.44 Remarkably, to date, only one case of (nonlethal) cancer has been reported in both Laron syndrome cohorts.44,45 Moreover, the Ecuadorian cohort seems to be protected from the development of diabetes mellitus, unlike the Mediterranean cohort.41,44
GH receptor antagonism
A single amino acid substitution in GH (Gly120Lys in human and Gly119Arg in bovine GH) generates a GH receptor antagonist (GHA). Such mutations have not been found to occur naturally. The GHA binds to the GH receptor with the same affinity as GH but does not induce intracellular signalling.46,47 Thus, in the presence of a GHA, endogenous GH binding is prevented and subsequent GH-induced action is decreased.
GHA transgenic mice that express bovine GH Gly119Arg have a dwarf phenotype (Figure 2).46 These mice are ~70% of the size of their wild-type littermates, and their IGF-1 levels are approximately ~50% of normal levels.47 Similar to Ghr−/− mice, GHA mice are obese owing to a large subcutaneous adipose tissue depot.36 However, GHA mice show only a slight improvement in glucose metabolism, and their lifespan is not notably increased.35
These differences are presumably the result of higher IGF-1 levels found in GHA compared with Ghr−/− mice. The effects of GH receptor antagonism in GHA transgenic mice were the basis for the development and FDA approval of the drug pegvisomant, which is used for the treatment of patients with acromegaly (see below).
GH excess
GH transgenic mice
The first GH transgenic mice were generated approximately three decades ago by appending rat GH cDNA to the murine metallothionein-1 promoter and enhancer.48 These and several other GH transgenic mice—expressing either a human, mouse, rat, bovine or ovine GH transgene in addition to endogenous GH—all exhibit high expression levels of the respective GH transgene and show a ‘giant’ phenotype (Figure 2). Given the anabolic, diabetogenic and lipolytic effects of GH, these mice are extremely lean. Also, whereas adiposity increases over the lifespan of wild-type mice, bovine-GH-expressing mice remain lean throughout life.49
Mice expressing bovine GH or human GH exhibit a shortened lifespan (~30–40% shorter than that of wild- type mice).36,50 Pathological changes that arise from GH excess include glomerulosclerosis (scarring within the renal glomeruli) and hepatocellularmegaly (increased hepatocyte size).51 The anti-insulin activity of GH leads to high insulin levels followed by insulin resistance in GH transgenic mice.50 Thus, the combination of insulin resistance and histopathological changes in several organs is the probable cause of the premature death of these transgenic mice.
Acromegaly
GH excess in humans, a condition known as acromegaly, is predominantly caused by a GH-secreting pituitary adenoma.52 If the disorder starts in childhood before closure of the epiphyseal growth plates and is left untreated, patients exhibit gigantism.53 In adult patients, excess growth is focused on the extremities (hands, feet, face) as well as internal organs, such as the heart.52 Patients with acromegaly are lean and have an increased amount of extracellular fluid.54 These individuals are often insulin-resistant and some have diabetes mellitus and heart problems.52 Whether cancer incidence in these patients is increased or decreased is a matter of some debate; however, an increased risk of developing colon cancer has been confirmed in several studies.55,56 The overall mortality rate is higher in untreated patients with acromegaly than in the normal population, but treatment that successfully normalizes IGF-1 levels, such as surgery, radiotherapy or pharmacological treatment with somatostatin analogues or the GH antagonist pegvisomant, reduces the mortality rate to that of the normal population.55,57
IGF-1 and longevity
Whereas mice lacking GH and the GH receptor are viable and long-lived, most IGF-1 receptor null mice (Ig f1r−/−) die at birth; however, depending on the mouse strain, 10–68% of Ig f1 null mice (Ig f1−/−) survive.58 Studies on the role of IGF-1 and IGF-1R in ageing have concentrated on GH-deficient and GH-resistant mice as well as mice with nonlethal genetic modifications in the IGF-1/IGF-1R axis (Table 1), which are reported below.
IGF-1 deficiency
Liver-specific Igf1-disrupted mice
Two mouse strains with liver-specific Igf1 disruption have been generated: inducible liver-specific Ig f1 null (LI-Igf1−/−) mice59 and liver-specific IGF-1-deficient (LID) mice.60 Both strains have a 75% decrease in serum IGF-1 levels, with high serum GH levels owing to the loss of the long-loop negative feedback inhibi- tion of IGF-1 on the pituitary and hypothalamus.59,60
Young LI-Igf1−/− mice do not show a difference in size relative to control mice. However, if Igf1 expression is abolished at 12 months of age, LI-Igf1−/− mice weigh 25% less than wild-type mice at 18 months of age as a result of decreased adiposity.61 Female LI-Igf1−/− mice in which the gene was inactivated at 1 month of age have a 16% longer mean lifespan than control mice, but the mean lifespan of male LI-Igf1−/− mice is not significantly increased.61 In LID mice, Igf1 gene disruption is driven by the albumin promoter and enhancer and is active during the first 2 postnatal weeks.60 Despite the 75% reduction in IGF-1 levels, LID mice exhibit similar growth profiles as wild-type control mice.60 A shortened lifespan has been reported for male LID mice, but actual longevity data are not yet available.62 However, cardiac ageing has been shown to be delayed in these mice.63 Differences in lifespan data between these two liver-specific IGF-1-deficient mouse strains might be due to the different developmental stages at which Igf1 expression is attenuated: at 1 month of age in LI-Igf1−/− mice compared with <2 weeks of age in LID mice.
Pappa−/− mice
Pappalysin-1 is a metalloproteinase that cleaves IGF-1-bound IGF-binding protein 4 (IGFBP-4) (Figure 1). Deletion of the encoding gene, Pappa, decreases bioavailability of IGF-1 due to an increased abundance of IGFBP-4. Pappa−/− mice are born small, approximately 60% of the size of wild-type mice, and reach 40% of the normal adult size.64 The mean lifespan is 38% longer in Pappa−/− mice than in wild-type control mice.65 Serum glucose, insulin, IGF-1 and GH levels of Pappa−/− mice do not differ from those of wild-type animals, which suggests that the effects of pappalysin-1 are mostly autocrine or para- crine.65,66 Pappa−/− mice have a lower incidence of tumours and degenerative lesions at 18, 24 and 30 months of age, showing an approximate 6-month delay in ageing-related pathologies compared with wild-type control mice.67
Disrupted IGF-1 or insulin signalling
Igf1r+/− mice
Mice that are heterozygous for a mutated allele of the IGF-1R (Ig f1r+/−) are reported to be ~10% smaller than wild-type mice, despite expressing ~50% of wild-type IGF-1R levels. Moreover, these mice exhibit a marked reduction in IGF-1-induced intracellular signalling and become insulin-resistant with age.68,69 Nevertheless, female Ig f1r+/− mice have a 33% longer mean lifespan than wild-type littermates. No significant change in lifespan was observed in male mice.68 These findings are controversial, given that the wild-type 129/J control mice used in this study had a 39% shorter median lifespan than previously reported, which suggests that their husbandry conditions might have been suboptimal.70 A study using mice in a C57BL/6 background could not detect differences in lifespan or end-of-life necropsy between Ig f1r+/− and wild-type mice.69
Human IGF1R variants
Two heterozygous IGF1R gene mutations, Ala37Thr and Arg407His, are more frequent in a cohort of Ashkenazi Jewish centenarians than in control individuals with the same ancestry but no family history of exceptional longevity.71 These mutations have been shown to suppress IGF-1 signalling and decrease transcription of target genes.71 Female carriers of these mutations have high IGF-1 levels and slightly short stature, which implies that they are IGF-1-resistant.72 These studies provide evidence that reduced IGF-1 signalling can positively affect human lifespan.
Klotho-modified mice
Klotho is a protein that inhibits insulin and IGF-1 signalling, presumably by interfering with the receptor– ligand interaction.73,74 The Kl gene was first identified in 1997 in a mutant mouse strain that exhibited accelerated ageing. These mice are normal-sized and healthy at birth but stop growing at 3–4 weeks and die at 8–9 weeks of age.75 In 2005, mice that express a mouse Kl transgene were generated. They are normal in size, and males, but not females, develop insulin resistance. Compared to wild-type litter-mates, mean lifespans of two female klotho transgenic strains were each increased by 19%, whereas the increases were 20% and 31% in the respective male mice.73
Irs1−/− mice
Insulin-receptor substrates (IRS) are important media- tors of both insulin and IGF-1 signalling. Irs1−/− mice are insulin-resistant with defects in insulin signalling mainly in skeletal muscle.76,77 From birth into adulthood, Irs1−/− mice are ~30% smaller than wild-type or Irs1+/− mice.78 Female Irs1−/− have a 17% increase in mean lifespan, but no significant difference is observed in male Irs1−/− mice compared with wild-type animals.79,80 Lifespan of Irs1+/− mice does not differ from that of wild-type mice.79
Irs2−/− mice
Irs2−/− mice are also insulin-resistant, but unlike Irs1−/− mice they become diabetic. These animals exhibit defects in insulin signalling in more tissues than Irs1−/− mice, mainly in adipose tissue and liver, as well as skeletal muscle.77 Irs2−/− mice are ~10% smaller than wild-type and Irs2+/− mice.76 Irs2+/− mice have normal to improved insulin sensitivity,76,81 and they have been reported to have a 17% longer mean lifespan than wild-type mice.80,81 However, these findings are controversial given the unusual survival profile of wild-type controls,82 especially as another study did not show an increased lifespan in Irs2+/− mice.79 Regardless, homozygous deletion of Irs2 results in a drastically shortened lifespan, especially in male mice, which live for ~4 months.79 Additionally, brain-specific Irs2+/− and Irs2−/− mice are insulin-resistant but live 18% and 14% longer than wild-type mice, respectively.81 This observation raises new questions about central control of pathways that might affect ageing.
Human IRS2 variant
Gly1057Asp is a common IRS2 gene variant that is present in ~34% of the population. The molecular effects of this mutation are not known. A study of an Italian population revealed that individuals heterozygous or homozygous for this polymorphism have a higher probability of reaching old age (96– 104 years) than do those with the wild-type allele,83 which suggests a relationship between ageing and IRS-2 function in humans.
p66shc−/− mice
The p66shc isoform of SHC-transforming protein 1 mediates signals from growth factor receptors, such as IGF-1R. It has been suggested that p66shc couples IRS-1 and the S6 kinase, a downstream effector of the growth regula- tor mTOR.84 The phenotype of p66shc−/− mice is normal, but these animals have an increased lifespan. Compared with wild-type mice, the median lifespan of p66shc−/− and p66shc+/− mice is 28% and 7% increased, respectively.85
Mechanisms of increased longevity
Improbable mechanisms
Size
An inverse correlation between size and lifespan has been observed in diverse species.86 However, klotho transgenic and p66shc−/− mice are not smaller than wild- type animals, yet live longer,73,85 whereas GHA mice do not live longer despite being dwarf.35 Also, GH and levo-thyroxine treatment of Snell dwarfs over 11 weeks (starting from 4 weeks of age) increases their size by almost 50% but does not shorten lifespan.12
In humans with dwarfism, no formal longevity studies have been performed. Patients with Laron syndrome exhibit dwarfism, have a decreased rate of cancer and often die from ageing-unrelated causes, such as accidents or alcohol abuse.44 Given that cancer is one of the primary causes of death in the general population, one could hypothesize that patients with Laron syndrome live longer than healthy individuals. Nevertheless, no perfect correlation between dwarfism and increased longevity exists in mammals.
Insulin sensitivity
High insulin sensitivity is often associated with improved health and prolonged lifespan. Many GH-deficient or GH-insensitive human and mouse populations seem to be protected from diabetes mellitus, although individuals with Laron syndrome (Mediterranean cohort) do not exhibit differences in rates of diabetes mellitus compared with the general population.41 However, some long-living mouse models, as described above, are insulin-resistant. Disruption of the genes Ig f1r, Irs1 or Irs2 as well as an increase in Kl expression induce some degree of insulin resistance, as the encoded proteins participate in insulin signalling.68,69,73,77 Among these different strains, only Irs2−/− mice become diabetic, and these mice have a shortened lifespan.79 Thus, the direct effect of insulin sensitivity or resistance as a function of ageing still needs to be resolved. Regardless, insulin resistance does not preclude an increased lifespan.
Adiposity
Mouse strains with reduced GH action, as well as humans with GH deficiency or Laron syndrome, have obesity because of the loss of the lipolytic and antilipogenic activities of GH. The long lifespan of obese mice with reduced GH action has initiated speculations that adipose tissue—especially subcutaneous adipose tissue—could protect these mice from the detrimental effects of ageing. Intra-abdominal adipose tissue is associated with the metabolic syndrome, whereas subcutaneous adipose tissue has been shown to have beneficial metabolic effects.87 However, the long-living Irs1−/− (females),79 p66shc– /–84 and LI-Ig f1−/− mice61 are leaner than their respective controls. Taken together, these findings indicate that neither obesity nor a lean phenotype are an absolute prerequisite for a long life.
Probable mechanisms
Stress resistance, cancer incidence and cellular senescence are three factors that can be used to determine the health status of an organism and could potentially be used to predict lifespan.88,89 Also, cellular senescence has been shown to be a cause of many age-related phenotypes.89 For example, klotho transgenic mice show a reduction in senescent cells in the kidney.90 Unfortunately, senescence has not been systematically characterized in any of the other aforementioned mouse strains.
Stress resistance
Increased resistance to stress has been observed in several animal species with increased longevity, including worms, fruit flies and mice.91,92 Cellular stress can be caused by a variety of extracellular or intracellular stimuli, such as heat, hypoxia, irradiation, glucose deprivation and accumulation of reactive oxygen species (ROS).93 To avoid organelle and DNA damage, as well as aggregation of unfolded proteins, the cell can respond to stress by activating survival mechanisms, such as autophagy, or by inducing apoptosis.93 Various stress stimuli inhibit mTOR, a central signal transducer that promotes cell growth, metabolism and survival in conditions of nutrient abundance.94 Long-term activation of mTOR can cause cellular stress through accumulation of unfolded proteins and ROS as well as stem-cell exhaustion.95 Inhibition of mTOR by rapamycin, caloric restriction or decreased GH/IGF-1 signalling stimulates autophagy and is believed to lead to an improved response to cellular stress and an increased lifespan (Box 1).6,93,94
The ‘oxidative stress theory of ageing’ hypothesizes that free radicals and ROS cause cumulative damage of macromolecules, which is responsible for the process of ageing.88 ROS are a by-product of normal metabolism, but ROS accumulation and oxidative damage increase with age, presumably owing to both increased ROS production and decreased cellular stress response.88 However, a large longevity study in mice, in which expression of antioxidant enzymes, such as glutathione peroxidases and superoxide dismutases, was altered, showed that only one out of 18 manipulations affected lifespan.96 Namely, disrupting Sod1, the gene encoding superoxide dismutase 1, which is one of the major enzymes that destroy free radicals in cells, substantially shortens lifespan.96
Stress resistance has been studied in several mouse strains with increased lifespan, by exposing either cultured fibroblasts (from Ames, Snell, Ghr−/−, Ig f1r+/− and p66shc mice) or mice (klotho, Ig f1r+/− and p66shc mice) to different stressors. Exact methods vary considerably between studies, but common markers of improved stress resistance include fewer DNA doublestrand breaks and increased survival.92,97 Ames and Snell dwarfs,92 lit/lit,97 Ghr−/−, Ig f1r+/−, klotho and p66shc mice92 have shown improved stress resistance. Furthermore, stress resistance of Ames dwarfs is decreased if they are treated with GH.19
Patients with Laron syndrome (Ecuadorian cohort) also show a reduction in stress-induced signalling. This finding was investigated by incubating human epithelial cells with serum from these patients or their unaffected relatives. The patient serum caused an increase in expression of SOD2 and a decrease in mTOR mRNA levels. Following treatment with H2O2, cells treated with the patient serum displayed less DNA breakage.44
Stress resistance could be caused by an increased expression of genes that are regulated by FOXO transcription factors, which are upregulated in the absence of IGF-1 signalling.44,92 More research is needed to elucidate the many functions of FOXO proteins, but they have been shown to induce expression of antioxidant genes and to act as tumour suppressors.6,92 Even though improved resistance to stress accompanies extended longevity in mice, further studies are necessary to establish a causative relationship.
Tumour resistance
Increased GH and IGF-1 levels have been associated with the development of several types of cancers, such as breast and colon cancer, in mammals.98 Thus, reduced levels of these hormones might lead to the development of fewer tumours. Tumour incidence has been investigated in Snell,12 Ames,99 lit/lit,100 Ghr−/−,101 GHA102 and Pappa−/−65 mice; the results show that reduced GH and/or IGF-1 action enhances tumour resistance. Human studies are in accordance with the findings in mice; tumours are nearly nonexistent in patients with Laron syndrome.44,45
On the other hand, GH transgenic mice have an increased tumour incidence.50 Also, individuals with acromegaly seem to have an increase in colon cancer incidence,55,56 although an overall increased tumour incidence has not been confirmed in these patients.
Inhibition of mTOR by rapamycin or by caloric restriction improves tumour resistance and increases lifespan across species (Box 1).6,94,103,116 In vitro, fibroblasts from Ames, Snell and Ghr−/− mice show reduced mTOR action under autophagy-inducing conditions.104 Inhibition of mTOR could, therefore, offer an explanation for the reduced incidence of tumours in the models discussed above.
Targeting the GH/IGF-1 axis in humans
Currently, rhGH therapy in adults is approved for normalization of IGF-1 levels—a common marker of successful treatment—in patients with GH deficiency as a result of hypopituitarism.105 A study investigating the effects of 15 years of rhGH therapy in patients with adult- onset GH deficiency showed that long-term rhGH treatment improved body composition and decreased cardiac risk factors, although effects on glucose metabolism are still unclear.106
Regardless of guidelines on clinical use, rhGH is widely misused as an antiageing therapeutic. A meta-analysis of 18 clinical trials on rhGH therapy in healthy elderly individuals (mean age 69 years) indicated that rhGH therapy has minor benefits on body composition but potential risks. Adipose tissue mass is decreased in both sexes, which is accompanied by an increase in lean mass in men.5 In one of these studies, more than half of the male participants developed glucose intolerance or diabetes mellitus during 26 weeks of rhGH therapy.107
On the other hand, local rhGH injections to the knee of older men (mean age 61 years) were shown to improve tendon collagen synthesis, which suggests that rhGH could be of future therapeutic value when administered locally rather than systemically.108 Whether the consequences of rhGH treatment in healthy as well as GH-deficient individuals are a result of direct effects of GH or mediated by IGF-1 remains to be established.
In 2009, the Growth Hormone Society stated that antiageing rhGH therapy cannot be recommended or encouraged before more basic studies have elucidated the mechanisms through which the GH/IGF-1 axis affects ageing.105 Moreover, clinical, long-term trials of rhGH treatment in the elderly must be performed.105 Given the data from long-living mouse models, the suggested role of GH and IGF-1 in many cancers,98 and the development of insulin resistance in previously healthy elderly individuals receiving rhGH treatment,107 it is apparent that rhGH therapy is not an option to delay ageing.
By contrast, the possibility of using the GH antagonist pegvisomant or somatostatin analogues to slow ageing could be envisioned. Somatostatin analogues inhibit GH secretion from the pituitary, whereas pegvisomant inhibits endogenous GH binding to the GH receptor. Both drugs are used to treat patients with acromegaly. Pegvisomant has been shown to be more efficient than somatostatin analogues in terms of normalizing IGF-1 levels in patients with acromegaly.109,110 No major safety issues have been reported when using pegvisomant, except a rise in the level of liver transaminases in ~2.5% of treated patients.109,110 The ongoing ACROSTUDY is currently investigating the efficac y and safety of long-term pegvisomant treatment in patients with acromegaly.109,110 Somatostatin analogues normalize IGF-1 levels in ~45% of patients but can inhibit insulin secretion, thus aggravating the insulin resistance and diabetes mellitus found in some patients with acromegaly. Also, somatostatin analogues have been shown to induce major adverse effects on the gastrointestinal tract.111 Given the current price of somatostatin analogues and pegvisomant, these agents are probably not cost-effective for treating ‘healthy’ individuals over extended periods of time. More importantly, the long-term consequences of agents that lower GH action need to be carefully studied before their use is indicated in an antiageing scenario. Thus, the future will dictate whether either of these treatment modalities will be useful to combat ageing.
Conclusions
Several long-lived mouse strains with reduced activity of the GH/IGF-1 axis have a dwarf phenotype, increased size of the subcutaneous adipose tissue depot and improved insulin sensitivity. However, other mouse strains are normal-sized, lean and even insulin-resistant but still display increased lifespan. What is required for a long life? According to mouse data, resistance to tumours as well as to stress are important factors. Human data seem to be consistent with this hypothesis, but no single factor correlates with longevity. Nevertheless, lowered activity of the GH/IGF-1 pathway, as seen in the somatopause, might not be something to combat but rather nature’s way of sustaining the ageing individual.
Key points.
∎ Growth hormone (GH) is a potent metabolic hormone that has been touted as a ‘fountain of youth’
∎ Recombinant human (rh) GH and insulin-like growth factor 1 (IGF-1) are approved therapeutics for patients with GH deficiency or primary IGF-1 deficiency, respectively; however, these drugs have been misused
∎ Currently available data do not suggest that rhGH treatment should be used to promote longevity
∎ Lack of GH action in mouse models is associated with extended longevity, but the mechanism underlying the increased lifespan has yet to be established
∎ Given the effects of reduced GH/IGF-1 signalling on lifespan in rodents, decreased GH action might be beneficial for humans, but clinical trials are needed to assess long-term outcome of GH/IGF-1 inhibition
Acknowledgements
The authors thank J. Sattler at Ohio University Heritage College for Osteopathic Medicine, Athens, OH, USA for taking the mouse photograph.
J. J. Kopchick is supported by the State of Ohio’s Eminent Scholar Program, which includes a gift from Milton and Lawrence Goll, by AMVETS, and by NIH (P01AG031736).
Footnotes
Review criteria A search for original articles was performed in PubMed from August to October 2012 using the search terms “GH”, “IGF-1”, “ageing”, “longevity”, “lifespan” and combinations thereof. We also searched the reference lists of relevant papers for original publications. All articles identified were English-language papers. Apart from two exceptions,22,48 we investigated only full-text papers.
Author contributions R. K. Junnila and J. W. Murrey researched the data for the article. All authors provided a substantial contribution to discussions of the content and reviewed and/or edited the manuscript before submission
Competing interests: J. J. Kopchick declares that he is an inventor of US patent 5350836 entitled ‘Growth hormone antagonists’. The other authors declare no competing interests.
References
- 1.Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009;30:152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- 2.Zadik Z, Chalew SA, McCarter RJ, Jr, Meistas M, Kowarski AA. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J. Clin. Endocrinol. Metab. 1985;60:513–516. doi: 10.1210/jcem-60-3-513. [DOI] [PubMed] [Google Scholar]
- 3.Bartke A. Growth hormone and aging: a challenging controversy. Clin. Interv. Aging. 2008;3:659–665. doi: 10.2147/cia.s3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudman D, et al. Effects of human growth hormone in men over 60 years old. N. Engl. J. Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, et al. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann. Intern. Med. 2007;146:104–115. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- 6.Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snell GD. Dwarf, a new Mendelian recessive character of the house mouse. Proc. Natl Acad. Sci. USA. 1929;15:733–734. doi: 10.1073/pnas.15.9.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eicher EM, Beamer WG. New mouse dw allele: genetic location and effects on lifespan and growth hormone levels. J. Hered. 1980;71:187–190. doi: 10.1093/oxfordjournals.jhered.a109344. [DOI] [PubMed] [Google Scholar]
- 9.Li S, et al. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- 10.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl Acad. Sci. USA. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks NL, et al. Low utilization of circulating glucose after food withdrawal in Snell dwarf mice. J. Biol. Chem. 2007;282:35069–35077. doi: 10.1074/jbc.M700484200. [DOI] [PubMed] [Google Scholar]
- 12.Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated snell dwarf mice regain fertility but remain long lived and disease resistant. J. Gerontol. A. Biol. Sci. Med. Sci. 2004;59:1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaible R, Gowen JW. A new dwarf mouse. Genetics. 1961;46:896. [Google Scholar]
- 14.Buckwalter MS, Katz RW, Camper SA. Localization of the panhypopituitary dwarf mutation (df) on mouse chromosome 11 in an intersubspecific backcross. Genomics. 1991;10:515–526. doi: 10.1016/0888-7543(91)90430-m. [DOI] [PubMed] [Google Scholar]
- 15.Andersen B, et al. The Ames dwarf gene is required for Pit-1 gene activation. Dev. Biol. 1995;172:495–503. doi: 10.1006/dbio.1995.8040. [DOI] [PubMed] [Google Scholar]
- 16.Sornson MW, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 17.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 18.Bartke A, et al. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 19.Panici JA, et al. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24:5073–5079. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krzisnik C, Grgurić S, Cvijović K, Laron Z. Longevity of the hypopituitary patients from the island Krk: a follow-up study. Pediatr. Endocrinol. Rev. 2010;7:357–362. [PubMed] [Google Scholar]
- 21.Krzisnik C, et al. The “little people” of the island of Krk—revisited. Etiology of hypopituitarism revealed. J. Endocr. Genet. 1999;1:9–19. [Google Scholar]
- 22.Eicher EM, Beamer WG. Inherited ateliotic dwarfism in mice. Characteristics of the mutation, little, on chromosome 6. J. Hered. 1976;67:87–91. doi: 10.1093/oxfordjournals.jhered.a108682. [DOI] [PubMed] [Google Scholar]
- 23.Godfrey P, et al. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat. Genet. 1993;4:227–232. doi: 10.1038/ng0793-227. [DOI] [PubMed] [Google Scholar]
- 24.Donahue LR, Beamer WG. Growth hormone deficiency in ‘little’ mice results in aberrant body composition, reduced insulin-like growth factor-I and insulin-like growth factor- binding protein-3 (IGFBP-3), but does not affect IGFBP-2, -1 or -4. J. Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- 25.Salvatori R, et al. Familial dwarfism due to a novel mutation of the growth hormone-releasing hormone receptor gene. J. Clin. Endocrinol. Metab. 1999;84:917–923. doi: 10.1210/jcem.84.3.5599. [DOI] [PubMed] [Google Scholar]
- 26.Aguiar-Oliveira MH, et al. Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene. J. Clin. Endocrinol. Metab. 2010;95:714–721. doi: 10.1210/jc.2009-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumann G, Maheshwari H. The Dwarfs of Sindh: severe growth hormone (GH) deficiency caused by a mutation in the GH-releasing hormone receptor gene. Acta Paediatr. Suppl. 1997;423:33–38. doi: 10.1111/j.1651-2227.1997.tb18366.x. [DOI] [PubMed] [Google Scholar]
- 28.Maheshwari HG, Silverman BL, Dupuis J, Baumann G. Phenotype and genetic analysis of a syndrome caused by an inactivating mutation in the growth hormone-releasing hormone receptor: dwarfism of Sindh. J. Clin. Endocrinol. Metab. 1998;83:4065–4074. doi: 10.1210/jcem.83.11.5226. [DOI] [PubMed] [Google Scholar]
- 29.Besson A, et al. Reduced longevity in untreated patients with isolated growth hormone deficiency. J. Clin. Endocrinol. Metab. 2003;88:3664–3667. doi: 10.1210/jc.2002-021938. [DOI] [PubMed] [Google Scholar]
- 30.Dobbs AK, et al. Cutting edge: a hypomorphic mutation in Igβ (CD79b) in a patient with immunodeficiency and a leaky defect in B cell development. J. Immunol. 2007;179:2055–2059. doi: 10.4049/jimmunol.179.4.2055. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc. Natl Acad. Sci. USA. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laron Z, Kopchick J, editors. Laron Syndrome —From Man to Mouse. Springer; 2011. [Google Scholar]
- 33.List EO, et al. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR−/−) mouse. Endocr. Rev. 2011;32:356–386. doi: 10.1210/er.2010-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene- disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 35.Coschigano KT, et al. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin and IGF-1 levels and increased lifespan. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 36.Berryman DE, et al. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm. IGF Res. 2004;14:309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Berryman DE, et al. Two-year body composition analyses of long-lived GHR null mice. J. Gerontol. A. Biol. Sci. Med. Sci. 2010;65:31–40. doi: 10.1093/gerona/glp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Methuselah Foundation Latest Mprize Winners [online] 2013 http://www.mprize.org/?pn=mj_mprize_record.
- 39.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc. Natl Acad. Sci. USA. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laron Z. Laron syndrome (primary growth hormone resistance or insensitivity): the personal experience 1958–2003. J. Clin. Endocrinol. Metab. 2004;89:1031–1044. doi: 10.1210/jc.2003-031033. [DOI] [PubMed] [Google Scholar]
- 41.Laron Z, Avitzur Y, Klinger B. Carbohydrate metabolism in primary growth hormone resistance (Laron syndrome) before and during insulin-like growth factor-I treatment. Metabolism. 1995;44(Suppl. 4):113–118. doi: 10.1016/0026-0495(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 42.Laron Z. The GH–IGF1 axis and longevity. The Paradigm of IGF1 deficiency. Hormones (Athens) 2008;7:24–27. doi: 10.14310/horm.2002.1111034. [DOI] [PubMed] [Google Scholar]
- 43.Rosenbloom AL, Guevara Aguirre J, Rosenfeld RG, Fielder PJ. The little women of Loja— growth hormone-receptor deficiency in an inbred population of southern Ecuador. N. Engl. J. Med. 1990;323:1367–1374. doi: 10.1056/NEJM199011153232002. [DOI] [PubMed] [Google Scholar]
- 44.Guevara-Aguirre J, et al. Growth hormone receptor deficiency is associated with a major reduction In pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur. J. Endocrinol. 2011;164:485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- 46.Chen WY, Wight DC, Mehta BV, Wagner TE, Kopchick JJ. Glycine 119 of bovine growth hormone is critical for growth- promoting activity. Mol. Endocrinol. 1991;5:1845–1852. doi: 10.1210/mend-5-12-1845. [DOI] [PubMed] [Google Scholar]
- 47.Chen WY, White ME, Wagner TE, Kopchick JJ. Functional antagonism between endogenous mouse growth hormone (GH) and a GH analog results in dwarf transgenic mice. Endocrinology. 1991;129:1402–1408. doi: 10.1210/endo-129-3-1402. [DOI] [PubMed] [Google Scholar]
- 48.Palmiter RD, et al. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982;300:611–615. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmer AJ, et al. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology. 2009;150:1353–1360. doi: 10.1210/en.2008-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210–216. doi: 10.1159/000073704. [DOI] [PubMed] [Google Scholar]
- 51.Quaife CJ, et al. Histopathology associated with elevated levels of growth hormone and insulin-like growth factor I in transgenic mice. Endocrinology. 1989;124:40–48. doi: 10.1210/endo-124-1-40. [DOI] [PubMed] [Google Scholar]
- 52.Chanson P, Salenave S. Acromegaly. Orphanet J. Rare Dis. 2008;3:17. doi: 10.1186/1750-1172-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eugster EA, Pescovitz OH. Gigantism. J. Clin. Endocrinol. Metab. 1999;84:4379–4384. doi: 10.1210/jcem.84.12.6222. [DOI] [PubMed] [Google Scholar]
- 54.Katznelson L. Alterations in body composition in acromegaly. Pituitary. 2008;12:136–142. doi: 10.1007/s11102-008-0104-8. [DOI] [PubMed] [Google Scholar]
- 55.Ayuk J, Sheppard MC. Does acromegaly enhance mortality? Rev. Endocr. Metab. Disord. 2008;9:33–39. doi: 10.1007/s11154-007-9067-8. [DOI] [PubMed] [Google Scholar]
- 56.Melmed S. Acromegaly pathogenesis and treatment. J. Clin. Invest. 2009;119:3189–3202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holdaway IM, Bolland MJ, Gamble GD. A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur. J. Endocrinol. 2008;159:89–95. doi: 10.1530/EJE-08-0267. [DOI] [PubMed] [Google Scholar]
- 58.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 59.Sjögren K, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc. Natl Acad. Sci. USA. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yakar S, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl Acad. Sci. USA. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Svensson J, et al. Liver-derived IGF-I regulates mean life span in mice. PLoS ONE. 2011;6:e22640. doi: 10.1371/journal.pone.0022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Novosyadlyy R, Leroith D. Insulin-like growth factors and insulin: at the crossroad between tumor development and longevity. J. Gerontol. A. Biol. Sci. Med. Sci. 2012;67:640–651. doi: 10.1093/gerona/gls065. [DOI] [PubMed] [Google Scholar]
- 63.Li Q, Ceylan-Isik AF, Li J, Ren J. Deficiency of insulin-like growth factor 1 reduces sensitivity to aging-associated cardiomyocyte dysfunction. Rejuvenation Res. 2008;11:725–733. doi: 10.1089/rej.2008.0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conover CA, et al. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131:1187–1194. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- 65.Conover CA, Bale LK. Loss of pregnancy- associated plasma protein A extends lifespan in mice. Aging Cell. 2007;6:727–729. doi: 10.1111/j.1474-9726.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- 66.Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol. Metab. 2012;23:242–249. doi: 10.1016/j.tem.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conover CA, et al. Longevity and age-related pathology of mice deficient in pregnancy- associated plasma protein-A. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:590–599. doi: 10.1093/gerona/glq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 69.Bokov AF, et al. Does reduced IGF-1R signaling in Igf1r+/− mice alter aging? PLoS ONE. 2011;6:e26891. doi: 10.1371/journal.pone.0026891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ladiges W, et al. Lifespan extension in genetically modified mice. Aging Cell. 2009;8:346–352. doi: 10.1111/j.1474-9726.2009.00491.x. [DOI] [PubMed] [Google Scholar]
- 71.Tazearslan C, Huang J, Barzilai N, Suh Y. Impaired IGF1R signaling in cells expressing longevity-associated human IGF1R alleles. Aging Cell. 2011;10:551–554. doi: 10.1111/j.1474-9726.2011.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suh Y, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc. Natl Acad. Sci. USA. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurosu H, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolf I, et al. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–7105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 75.Kuro-o M, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 76.Withers DJ, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 77.Previs SF, Withers DJ, Ren JM, White MF, Shulman GI. Contrasting effects of IRS-1 versus IRS-2 gene disruption on carbohydrate and lipid metabolism in vivo. J. Biol. Chem. 2000;275:38990–38994. doi: 10.1074/jbc.M006490200. [DOI] [PubMed] [Google Scholar]
- 78.Tamemoto H, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- 79.Selman C, et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- 80.Selman C, Partridge L, Withers DJ. Replication of extended lifespan phenotype in mice with deletion of insulin receptor substrate 1. PLoS ONE. 2011;6:e16144. doi: 10.1371/journal.pone.0016144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 82.Selman C, Lingard S, Gems D, Partridge L, Withers DJ. Comment on “Brain IRS2 signaling coordinates life span and nutrient homeostasis”. Science. 2008;320:1012. doi: 10.1126/science.1152366. [DOI] [PubMed] [Google Scholar]
- 83.Barbieri M, et al. The IRS2 Gly1057Asp variant is associated with human longevity. J. Gerontol. A. Biol. Sci. Med. Sci. 2010;65:282–286. doi: 10.1093/gerona/glp154. [DOI] [PubMed] [Google Scholar]
- 84.Ranieri SC, et al. Mammalian life-span determinant p66shcA mediates obesity-induced insulin resistance. Proc. Natl Acad. Sci. USA. 2010;107:13420–13425. doi: 10.1073/pnas.1008647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Migliaccio E, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 86.Bartke A. Healthy aging: is smaller better?—a mini-review. Gerontology. 2012;58:337–343. doi: 10.1159/000335166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 89.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haruna Y, et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc. Natl Acad. Sci. USA. 2007;104:2331–2336. doi: 10.1073/pnas.0611079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harper JM, Durkee SJ, Dysko RC, Austad SN, Miller RA. Genetic modulation of hormone levels and life span in hybrids between laboratory and wild-derived mice. J. Gerontol. A. Biol. Sci. Med. Sci. 2006;61:1019–1029. doi: 10.1093/gerona/61.10.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murakami S. Stress resistance in long-lived mouse models. Exp. Gerontol. 2006;41:1014–1019. doi: 10.1016/j.exger.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 93.Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. Int. J. Cell Biol. 2010;2010:214074. doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 95.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pérez VI, et al. Is the oxidative stress theory of aging dead? Biochim. Biophys. Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amador-Noguez D, et al. Alterations in xenobiotic metabolism in the long-lived Little mice. Aging Cell. 2007;6:453–470. doi: 10.1111/j.1474-9726.2007.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chhabra Y, Waters MJ, Brooks AJ. Role of the growth hormone-IGF-1 axis in cancer. Expert Rev. Endocrinol. Metab. 2011;6:71–84. doi: 10.1586/eem.10.73. [DOI] [PubMed] [Google Scholar]
- 99.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J. Gerontol. A. Biol. Sci. Med. Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 100.Majeed N, et al. A germ line mutation that delays prostate cancer progression and prolongs survival in a murine prostate cancer model. Oncogene. 2005;24:4736–4740. doi: 10.1038/sj.onc.1208572. [DOI] [PubMed] [Google Scholar]
- 101.Ikeno Y, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J. Gerontol. A. Biol. Sci. Med. Sci. 2009;64:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pollak M, Blouin MJ, Zhang JC, Kopchick JJ. Reduced mammary gland carcinogenesis in transgenic mice expressing a growth hormone antagonist. Br. J. Cancer. 2001;85:428–430. doi: 10.1054/bjoc.2001.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang M, Miller RA. Augmented autophagy pathways and MTOR modulation in fibroblasts from long-lived mutant mice. Autophagy. 2012;8 doi: 10.4161/auto.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thorner MO. Statement by the Growth Hormone Research Society on the GH/IGF-I axis in extending health span. J. Gerontol. A. Biol. Sci. Med. Sci. 2009;64:1039–1044. doi: 10.1093/gerona/glp091. [DOI] [PubMed] [Google Scholar]
- 106.Elbornsson M, et al. Fifteen years of growth hormone (GH) replacement improves body composition and cardiovascular risk factors. Eur. J. Endocrinol. doi: 10.1530/EJE-12-1083. http://dx.doi.org/10.1530/EJE-12-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blackman MR, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–2292. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 108.Vestergaard P, et al. Local administration of growth hormone stimulates tendon collagen synthesis in elderly men. J. Appl. Physiol. 2012;113:1432–1438. doi: 10.1152/japplphysiol.00816.2012. [DOI] [PubMed] [Google Scholar]
- 109.Trainer PJ. ACROSTUDY: the first 5 years. Eur. J. Endocrinol. 2009;161(Suppl. 1):S19–S24. doi: 10.1530/EJE-09-0322. [DOI] [PubMed] [Google Scholar]
- 110.van der Lely AJ, et al. Long-term safety of pegvisomant in patients with acromegaly: comprehensive review of 1288 subjects in ACROSTUDY. J. Clin. Endocrinol. Metab. 2012;97:1589–1597. doi: 10.1210/jc.2011-2508. [DOI] [PubMed] [Google Scholar]
- 111.Parkinson C, et al. A comparison of the effects of pegvisomant and octreotide on glucose, insulin, gastrin, cholecystokinin, and pancreatic polypeptide responses to oral glucose and a standard mixed meal. J. Clin. Endocrinol. Metab. 2002;4:1797–1804. doi: 10.1210/jcem.87.4.8432. [DOI] [PubMed] [Google Scholar]
- 112.Liang H, et al. Genetic mouse models of extended lifespan. Exp. Gerontol. 2003;38:1353–1364. doi: 10.1016/j.exger.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 113.Yakar S, et al. Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes. 2001;50:1110–1118. doi: 10.2337/diabetes.50.5.1110. [DOI] [PubMed] [Google Scholar]
- 114.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of the life span and upon the ultimate body size. 1935. Nutrition. 1989;5:155–171. [PubMed] [Google Scholar]
- 115.Katic M, Kahn CR. The role of insulin and IGF-1 signaling in longevity. Cell. Mol. Life Sci. 2005;62:320–343. doi: 10.1007/s00018-004-4297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Omodei D, Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011;585:1537–1542. doi: 10.1016/j.febslet.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Larson-Meyer DE, et al. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 2008;16:1355–1362. doi: 10.1038/oby.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heilbronn LK, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mattison JA, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]