Abstract
Essential protectors against infection and injury, macrophages can also contribute to many common and fatal diseases. Here we discuss the mechanisms that control different types of macrophage activities in mice. We follow the cells’ maturational pathways over time and space, and elaborate on events that influence the type of macrophage eventually settling a particular destination. The nature of the precursor cells, developmental niches, tissues, environmental cues, and other connecting processes appear to contribute to the identity of macrophage type. Together, the spatial and developmental relationships of macrophages comprise a topo-ontogenic map that can guide our understanding of their biology.
Keywords: macrophage, monocyte, hematopoesis
Introduction
The discovery that macrophages are among the immune system’s most important defenders dates back to 1882, when the biologist Ellie Metchnikoff undertook light microscopic studies in starfish larvae and identified wandering cells that could accumulate in sites of infection and eliminate incoming foreign material1. Macrophages were soon identified in other species, including humans and mice, and were shown to protect the host by controlling infection and by mediating the repair and healing of injured tissues. We now know that macrophages can also be harmful by contributing to the development and progression of many deadly diseases. Macrophages are therefore being studied in considerable detail to clarify how protective and deleterious responses occur in vivo.
One line of investigation consists of revealing macrophage ontogeny and the spatiotemporal dynamics of the responses they mediate. This information allows not only to delineate more precisely where, when and how defined types of macrophage responses unfold at the organismal level but also to identify new vantage points for therapy. Here we discuss the diverse origins and developmental progression of tissue macrophages. By following these cells along their maturational pathway, we review what determines the type of macrophage response that eventually reaches the destination tissue. We present mechanistic information on the spatiotemporal dynamics of macrophage and macrophage precursor development, primarily in the context of inflammatory conditions; elaborate on the role of extramedullary monocytopoiesis as an additional pathway that generates macrophages; identify research areas that require further exploration; and illustrate the capacity to translate some of the new findings into therapeutic options. The molecular tools currently available for studying2 and imaging3 macrophages and their progenitors are reviewed elsewhere.
Macrophages and their progenitors: terminology and relationships
Macrophages are large white blood cells that inhabit most tissues of the body. Macrophages can identify either pathogens or cellular stress using pattern recognition receptors;4 engulf solid particles (e.g., foreign material, dying cells) in a process called phagocytosis;5,6 and control inflammatory responses and tissue homeostasis by producing cytokines, growth factors, proteases, and other products7. Transcriptional profiling of various tissue macrophages has revealed common features, such as the expression of MerTK and CD64.8 Although macrophages differ from each other in the expression of other markers (F4/80, for example is expressed at high levels in peritoneal macrophages but not in microglia), they are nevertheless, as a group, distinct from dendritic cells (DC). Compared to macrophages, DC are, perhaps simplistically, specialized mononuclear phagocytes that prime CD4+ and CD8+ T cells and/or produce antiviral compounds such as type-I interferons.9–11 Recently, a cluster of exciting studies have been deciphering how macrophages and DC differ from each other developmentally, phenotypically, and functionally.12–17 In this review we will focus mainly on macrophages, as defined below.
Macrophages found in resting adult tissues derive either from circulating monocytes (e.g. macrophages in the gut and splenic marginal zone)18–20 or are established prior to birth (e.g. brain microglia, liver Kupffer cells, cardiac macrophages, and other macrophages that reside in the lungs, spleen, bone marrow, and peritoneal cavity) and are then maintained independently of monocytes.18,19,21–28 The latter macrophages derive from either primitive yolk-sac macrophages or embryonic fetal liver monocytes and are maintained during adult life at least in part through local proliferation. An examination of macrophage ontogeny using mice deficient in the transcription factors PU.1 and Myb provided a molecular rationale for the dual origin of tissue macrophages. Myb, which is essential for HSC expansion in the fetal liver,29 is dispensable to the production of many tissue macrophages, revealing Myb-independent macrophages as a lineage separate from HSCs.23 Future studies will need to carefully evaluate the relative contribution of fetal liver monocytes and yolk sac-derived macrophages in populating macrophages in various adult tissues (although microglia are yolk sac-derived, while Langerhans cells and alveolar macrophages arise from fetal monocytes, the origin of macrophages in other tissues has not yet been entirely solved). In diseased tissues, macrophage number often expands dramatically. Disruption of tissue macrophage homeostasis can be triggered by local amplification of resident macrophages30–32 but often involves recruitment of newly made circulating monocytes.24,28,32–36
To highlight how inflammatory triggers deregulate macrophage ontogenic pathways, we consider not only circulating monocytes but also their hematopoietic stem cell (HSC) precursors. HSCs are pluripotent cells found in specialized niches of the bone marrow where they produce discrete intermediate progenitor populations (Fig. 1). The known intermediates of HSC-derived macrophages—common myeloid progenitors (CMPs),37 granulocyte and macrophage progenitors (GMPs),38 macrophage and dendritic cell progenitors (MDPs)39, 40 and common monocyte progenitor (CMoPs)41—progressively lose self-renewing capacity and become increasingly restricted to the mononuclear phagocyte lineage. When combined operationally, HSCs and hematopoietic progenitor cells are often referred to as HSPCs (hematopoietic stem and progenitor cells).
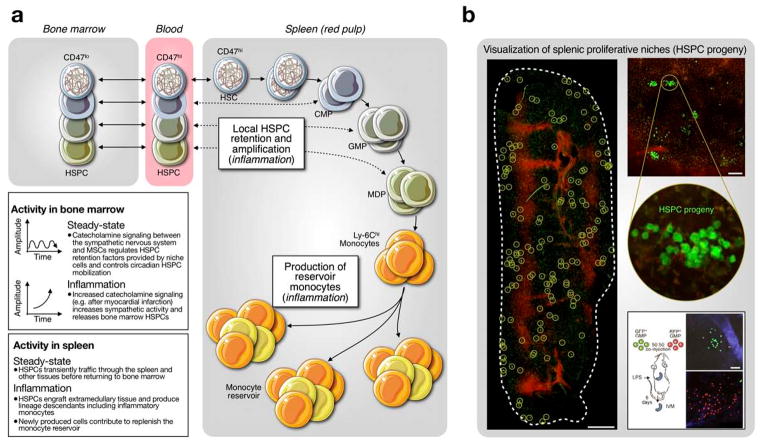
Fig. 1.
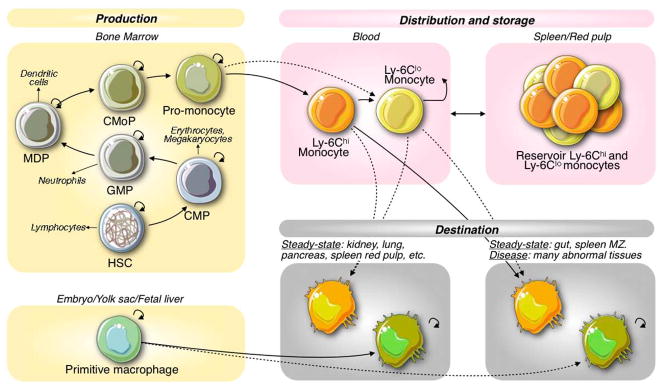
Topo-ontogenic map of macrophages and their progenitors in steady state. HSCs in bone marrow produce discrete intermediate progenitor populations, which increasingly lose self-renewing capacity as they commit to a given lineage. HSC-derived monocytes are found in circulation and in a splenic reservoir and can renew macrophages upon tissue extravasation. Some tissue macrophages develop in the embryo (yolk sac) before the appearance of HSCs and are maintained independently from the bone marrow.
Production of HSC-derived monocytes in bone marrow
The bone marrow niche
The bone marrow, protected by bone and comprised of various cell types, is the primary hematopoietic site in the adult (Fig. 2). Within the bone marrow, HSCs and their progeny inhabit specialized microenvironments, or niches. Supporting cells include nestin+ mesenchymal stem cells (MSCs), osteoblasts, CXCL12-abundant reticular (CAR) cells, CD169+ macrophages, neurons, T regulatory lymphocytes, endothelial and perivascular cells, among others. Aside from providing structural support, these accessory cells release factors and express cell surface proteins that regulate HSC’s retention and maintenance. Instructive cytokines and growth factors guide HSCs along their differentiation paths (reviewed in Refs. 42–44), whereas final cell fate decisions are orchestrated by transcription factors.45–48 More detailed information on the bone marrow niche and its constituents is discussed elsewhere.42,49–52
Fig. 2.
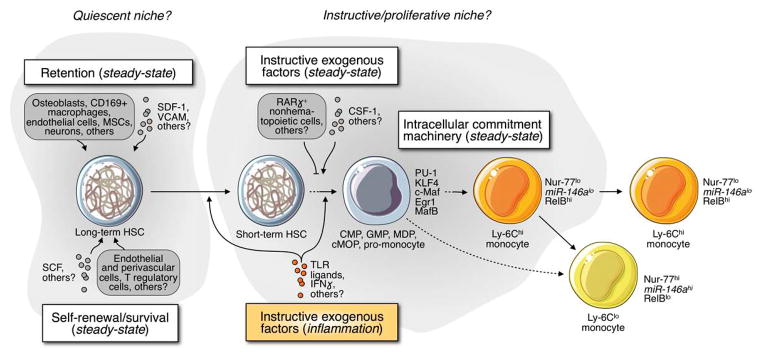
Monocyte production in bone marrow. Working model describing how long-term HSCs may produce monocytes in vivo. Several exogenous cells and soluble factors promote the retention and self-renewal/survival of HSCs (presumably in a quiescent niche) but also instruct commitment toward the myeloid lineage (presumably in an instructive/proliferative niche). The cartoon describes how monocyte production is controlled in steady state (white inserts) and in response to inflammatory cues (orange insert), which can induce HSCs to proliferate and skew them to the myeloid lineage.
Monocyte production in steady state
Histological studies indicate that early myeloid progenitors appear in a defined anatomical region of the bone marrow that is near the surface of bony trabeculae and small blood vessels53. Genetic mouse studies report that myeloid commitment and monocyte production require the growth factor CSF-1 and its receptor (CSF-1R, CD115),54,55 and elegant in vivo fate mapping approaches have described a HSC → GMP → MDP → monocyte relationship.39,40,56 A longitudinal in vitro bio-imaging approach further indicated that CSF-1 instructs, rather than permits, commitment of GMPs to the mononuclear phagocyte lineage.43 More recently it was found that CSF-1 directly induces PU.1 in HSCs, which instructs these cells to become myeloid cells independently of survival or proliferation57 (Fig. 2). Additionally, because CSF-1-mediated activation of the intracellular lineage commitment machinery is antagonized by the transcription factor MafB,44 conditions that repress MafB expression are expected to promote monocytopoiesis. Subsequent decisions between cell types, such as neutrophils and monocytes, also depend on graded expression of transcription factors.58 Yet, more studies are needed to fully characterize the complexity of niche factors that may either permit or instruct monocyte commitment in vivo. Receptors such as CX3CR1 and CCR2 (in addition to CSF-1R), which are expressed by monocytes and their most immediate precursors, may also be involved in this process.
Monocyte production in disease
Bone marrow niches continuously produce monocytes in the steady-state, but are dynamic microenvironments that can further adapt in response to specific demand imposed by particular states of inflammation. In vivo studies are now starting to elucidate how niches change during disease and, in turn, how these changes affect HSPC responses and monocyte production. For example, IFN-γ produced in response to chronic infection can induce long-term HSCs to proliferate in bone marrow and, by doing so, amplify a hematological response.59 Toll-like receptors (TLRs) are expressed by HSCs, and TLR ligation has been proposed to induce HSC proliferation and skew their differentiation toward the myeloid lineage.60 Since the bone marrow is often far away from an affected organ, long-range signals that stimulate monocyte production must exist. The sympathetic nervous system (SNS) was recently identified as a source of signals that control CXCL12 secretion by mesenchymal stem cells (MSCs) in bone marrow and HSC retention in steady-state61 and disease.62 Mesenchymal cells interconnect with connexin gap junctions, which may serve to propagate SNS-derived information beyond contacts of innervation.63 Other regulatory components may include soluble factors, microvesicles, and cells that can access specific niche microenvironments to deliver information.
The importance in understanding the mechanisms that regulate bone marrow cell production in disease is reinforced by recent observations that diseases may cause dysfunctional bone marrow hematopoietic activity, and inversely that niche dysfunction might predispose an organism to disease. For instance, diabetes can alter the composition of stromal niche component and impair G-CSF-mediated progenitor cell mobilization.64 While the system-wide impact of these effects still needs to be elucidated, it is conceivable that they influence disease progression and promote the manifold inflammatory complications of diabetes. Additionally, mice that lack the retinoic acid receptor gamma selectively in nonhematopoietic cells have increased bone marrow GMPs and develop myeloproliferative syndromes.65 Though the nonhematopoietic components involved are not fully defined, these findings suggest that regulation of myeloid cell production may not necessarily be intrinsic to hematopoietic cells.
Monocyte heterogeneity
Monocytes produced by the bone marrow and found in the circulation are phenotypically and functionally heterogenous and can be separated according to Ly-6C expression in mice. Development of the two monocyte subsets involves conversion of Ly-6Chi to Ly-6Clo cells, a process that was first proposed in 200466, and subsequently substantiated.19 Nur77, a transcription factor without which Ly-6Clo monocytes do not develop,67 and miR-146a, a microRNA expressed at higher levels in Ly-6Clo monocytes,68 may be involved in subset fate decisions (Fig 2).
In the steady-state, Ly-6Clo monocytes patrol the resting vasculature69, 70 and participate in clearing damaged endothelial cells,71 whereas Ly-6Chi monocytes circulate, survey tissues in the steady state without differentiating, transport antigen to lymph nodes,72 and convert into Ly-6Clo monocytes.19 During inflammation, Ly-6Chi monocytes differentiate to macrophages or dendritic cells in tissue.24,32,35,36,73–77 A large population of both subsets also remains undifferentiated in the splenic red pulp parenchyma, is organized in clusters along the perimeter of the organ and constitutes a monocyte reservoir78, 79 (Fig. 1). These cells are called monocytes, rather than macrophages or DCs, because they resemble blood monocytes morphologically, phenotypically, and functionally, and can be mobilized during inflammation to produce macrophages in distant tissues. During inflammation, Ly-6Chi monocytes, and to a lesser extent Ly-6Clo monocytes, can be efficiently mobilized to sites of inflammation. The human spleen also likely contains an important monocyte reservoir that can be mobilized in various disease settings.62,74,80 Additionally, the spleen may serve to clear senescent monocytes.
Alternative to bone marrow for monocyte production
HSPCs circulate
In 1968 van Furth and Cohn proposed that macrophages arise in tissue from bone marrow-derived circulating monocytes in the healthy adult.81 This macrophage development model has dominated our thinking over the last 40 years and implies that the key differentiation steps (progenitor to monocyte and monocyte to macrophage) are fixed in specific environments, such as bone marrow and peripheral tissue. We have also known since 1962 that HSCs circulate82 (Fig 3A). Using various methods, Goodman and Hodgson showed that peripheral blood contains cells which differentiate into lymphocytes, granulocytes and erythrocytes upon transplantation in lethally irradiated hosts. The presence of hematopoietic progenitors in the blood suggested that the linear model proposed by van Furth and Cohn, though useful, was probably an oversimplification.
Fig. 3.
Monocyte production in extramedullary tissue. (A) A small fraction of HSPCs constitutively exit the bone marrow. Under inflammatory conditions, extramedullary HSPCs can be retained and amplified in the spleen, where they produce and replenish reservoir monocytes. Bottom left inserts provide additional information on how bone marrow and splenic activities differ in steady-state and inflammation. (B) Intravital micrographs depict the formation of splenic proliferative niches upon adoptive transfer of green GMPs. Cell clusters are highlighted in circles, venous sinuses in red. Bottom right: co-transfer of green and red GMPs identifies the formation of distinct green and red colonies. Images reproduced from refs74, 95.
Fate of extramedullary HSPCs
The bone marrow niche is a protected environment containing various components that shield HSPCs from danger.42,83,84 The extramedullary environment is more hostile because it contains populations of scavenging macrophages that can eliminate circulating HSPCs. Mobilized HSPCs prepare for such an environment by upregulating CD47,85 a “don’t eat me” signal that protects them from phagocytosis. Elegant studies in 2001 showed that circulating HSCs are long-lived and can re-engraft bone marrow niches.86 The finding was consistent with the idea that recirculation maintains hematopoietic hemostasis by ensuring that medullary niches remain occupied after HSC death or differentiation. HSPC release from the bone marrow depends in part on circadian oscillations61 (Fig 3A) and bone-remodeling activity is highest when HSC numbers in peripheral blood are high.87 Thus, reconstruction of bone marrow niches may require temporal HSC evacuation.88 The migratory pool of HSPCs has also been proposed to function as a cell population that can be immediately recruited for extramedullary hematopoiesis “after catastrophic blood loss”.86 Circulating HSPCs express Toll-like receptors and can presumably respond to danger signals before the information has spread to the bone marrow.89 A subset of HSPCs also expresses CCR2, which can guide the cells to sites of inflammation.90 While the evidence supporting the rapid differentiation of HSPC to macrophages in inflamed tissue requires substantiation, a fraction of HSPC-derived cells seeding non-lymphoid tissue nevertheless amplify the monocytic/granulocytic marker Gr-1.
Extramedullary monocytopoiesis
Circulating HSPCs can produce macrophage precursors outside of the bone marrow. The existence of extramedullary hematopoiesis has been recognized for a long time. In the developing mouse embryo, definitive hematopoiesis begins in the para-aortic splanchnopleura and the aorto-gonado-mesonephros regions before it switches to the liver and finally the bone marrow,91 whereas in adults, the spleen supports hematopoiesis under specific conditions in mice and humans.92, 93 Recent work shows that extramedullary hematopoiesis in the adult is a feature of inflammation (Fig. 3A and 3B). Very few HSPCs inhabit the spleen in steady-state, but during inflammation, the spleen becomes myelopoietic. It is likely that exogenous cues at least partially reprogram HSPCs to promote extramedullary hematopoiesis. For instance, sensing of the peptide hormone angiotensin (Ang) II by angiotensis receptor I (AT-1) on the surface of HSCPs downregulates sphingosine-1 phosphate receptor 1 (S1P1) expression in these cells. This process increases HSPC retention in the spleen, presumably in part because it impairs HSPC migration toward S1P1 ligand S1P, which is found in high concentrations in peripheral blood. Splenic monocytopoiesis mediated by Ang II depends on S1P1 signaling because HSCs that constitutively express S1P1 fail to produce monocytes in the extramedullary tissue.94
In mouse models of atherosclerosis,95,96 myocardial infarction,62,97 and cancer,74,98,99 for example, HSPCs progressively populate the splenic red pulp and generate monocytes. Newly made cells may not only supplement the local monocyte reservoir but also exit and infiltrate distant sites (growing atheromata, ischemic myocardium, and tumor, respectively) where they contribute large numbers of macrophages.
Functions of monocytes produced in extramedullary tissue
Because spleen-derived monocytes are predominantly a product of inflammation, and thus develop in a milieu distinct from the steady state, it stands to reason that they differ from bone marrow-derived monocytes produced in the steady state. A recent study indicates that myocardial infarction liberates bone marrow HSCs, which seed the spleen to produce Ly-6Chi monocytes. Splenic monocytes then relocate to atherosclerotic plaques, differentiate to lesional macrophages, and accelerate atherosclerosis.62 Transcriptional profiling of splenic and medullary monocytes in atherosclerotic mice after myocardial infarction suggested several differences. Among them, expression of the inflammatory cytokine IL-1β, as well as other members of the IL-1 superfamily (IL-1a, IL-18), was higher in monocytes of splenic origin compared to those retrieved from the bone marrow62. Accordingly, Ly-6Chi monocytes recruited to atherosclerotic plaques after myocardial infarction showed increased expression of these genes. Recent studies have also shown that monocytes of splenic origin accumulate in the heart and induce long-term left ventricular dilatation, dysfunction, and fibrosis.100 In other conditions, monocytic cells produced in the spleen during tumor progression selectively tolerize memory CD8+ T cells to tumor antigens.98 These data suggest that inflammation is not merely a heightened steady-state: it can transform the spleen towards a site of hematopoiesis, producing monocytes with unique functions. Monocyte functional heterogeneity, it therefore appears, depends on the generative environment. A precise understanding of the distinctions that exist between medullary and extramedullary monocytes requires further study. The existence of similar extramedullary activity in humans62, 74 is discussed in more details elsewhere.79
Triggers of extramedullary monocyte production
Inflammation changes the spleen to favor seeding of HSPCs for hematopoiesis. Several factors that promote such activity have been identified. HSPCs express the common beta chain of the granulocyte-macrophage colony stimulating factor (GM-CSF) and IL-3 receptor (CD131), and the IL-1β receptor, among others. During atherosclerosis, GM-CSF and IL-3–expressing cells appear in the spleen. Injection of antibodies against the growth factors reduces extramedullary monocytopoiesis and blood monocytosis.95 After myocardial infarction, GM-CSF in the spleen remains unchanged, but IL-1β levels rise.97 Accordingly, adoptively transferred HSPCs lacking the IL-1 receptor are impaired in generating extramedullary monocytes compared to their wild type controls.97 These approaches suggest the existence of a specific splenic niche, rich in GM-CSF, IL-3 or IL-1β. Characterization of the functional relevance of this niche will benefit from the development of tools that can target candidate niche components exclusively in the spleen (i.e., development of Cre-expression under spleen-specific promoters to delete a floxed factor of choice from the spleen specifically).
Monocyte mobilization to destination tissue
The mobilization of monocytes from the bone marrow or spleen is a controlled process. In order to leave the bone marrow, Ly-6Chi monocytes require the chemokine receptor CCR2,101,102 which recognizes MCP-1 and MCP-3, two chemokines that are produced by medullary MSCs and CAR cells and secreted close to (and perhaps into) the lumen.103 Mobilization of monocytes from extramedullary sites such as the spleen, however, is independent of CCR2 and instead relies on the peptide hormone angiotensin II (Fig. 4). These observations suggest that spleen does not only make distinct monocytic cells, but is also sensitive to different cues for monocyte mobilization. The hormone releases splenic Ly-6Chi and Ly-6Clo monocytes indiscriminately.78 Because the inflammatory response is coordinated by a large range of compounds that form complex regulatory networks, it should be useful to identify more comprehensively the molecular mechanisms that orchestrate monocyte release from either bone marrow or spleen and then assess whether these mechanisms can be prevented or promoted therapeutically. Once mobilized, circulating monocytes can enter peripheral tissue. To reach their target, circulating cells extravasate in a complex process that includes capture, rolling, arrest, adhesion, crawling, and transendothelial migration.35,104 The destination tissue faces a dynamically shifting and heterogeneous repertoire of circulating cells, and controls cell entry.
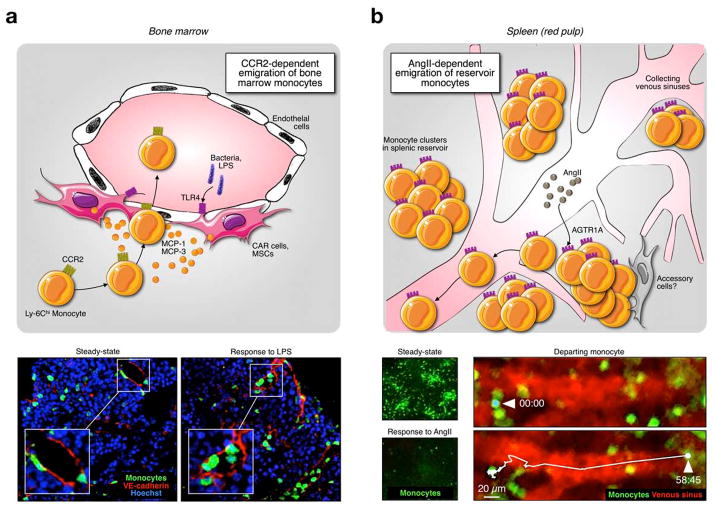
Fig. 4.
Distinct mechanisms of monocyte mobilization from medullary and extramedullary tissue. The mobilization of monocytes from the bone marrow or spleen is a controlled process and is reviewed in detail elsewhere.35 In order to leave the bone marrow, Ly-6Chi monocytes require the chemokine receptor CCR2, which recognizes MCP-1 and MCP-3, two chemokines produced by medullary MSCs and CAR cells and secreted close to (and perhaps into) the lumen, and increase monocyte movement and intravasation. This process can be activated through sensing of bacterial products by TLRs expressed on MSCs and CAR cells. Panel A shows bone marrow sections illustrating emigration of monocytes in response to LPS, a process that depends on CCR2 signaling. Mobilization of monocytes from extramedullary sites such as the spleen, however, is independent of CCR2 and instead can be triggered by the peptide hormone angiotensin II (panel B). Whether accessory cells are involved in this process remains unknown. Increased concentrations of angiotensin II induce AGTR1A receptor dimerization on splenic monocytes, an event that increases monocyte motility and promotes intravasation (tracking of a departing monocyte shows time in min:sec). Images are reproduced from Refs 103 and 78.
Regulation of monocyte recruitment
Monocyte subsets express chemokine receptors differentially: Ly-6Chi cells are CCR2hi CX3CR1lo CCR5+ whereas Ly-6Clo cells are CCR2− CX3CR1hi CCR5+. Destination tissue that expresses MCP-1, the ligand for CCR2, thus attracts Ly-6Chi monocytes, whereas tissue expressing the ligand for CX3CR1, fractalkine, attracts Ly-6Clo monocytes. In atherosclerosis, Ly-6Chi monocytes infiltrate lesions preferentially,75,105 and inhibition of CCR2, CX3CR1, and CCR5 almost abolishes atherosclerosis, presumably by interfering with monocyte infiltration.106,107 Primary and metastatic tumor sites also recruit Ly-6Chi monocytes. Such recruitment depends largely on MCP-1/CCR2 signaling.74,108 Accordingly, MCP-1 over-expression accelerates macrophage recruitment and tumor progression in animal models and associates with poor prognosis in many human cancer types.109
The number of circulating inflammatory Ly-6Chi (but not Ly-6Clo) monocytes, as well as the recruitment of these cells to tissues, is also controlled by the circadian clock gene Bmal1. In Ly-6Chi monocytes, Bmal1 binds rhythmically to the promoter regions of MCP-1 and MCP-2 and silences these loci by recruiting the histone methyltransferase enhancer of zest homolog 2 (EZH2). The circadian oscillation selectively increases the capacity of monocytes to infiltrate tissues during the animal’s active phase, which may be useful in anticipation of environmental challenges.110 Enhanced sensitivity to detect and respond to pathogens during the active phase has also been shown for splenic macrophages.111,112
Fates of recruited monocytes
Ly-6Chi monocytes accumulate in inflammatory sites where they often differentiate to M1-like macrophages characterized by the expression of IL-12, TNF-α, NOS2, proteases and MHC class II. Accordingly, acute responses mediated by these cells control intracellular pathogens,35 digest damaged tissue113 and activate adaptive immunity in lymph nodes,77 and accelerate inflammatory diseases as described above. As the tissue changes, so does the fate of recruited monocytes. For example, Ly-6Chi monocytes recruited to sites of infection with Toxoplasma gondii acquire an immunosuppressive phenotype associated with production of the lipid mediator prostrangladin E2 and inhibit neutrophil activation.114 However, Ly-6Chi monocytes that seed the intestinal lamina propria give rise to distinct types of macrophages depending on the tissue’s inflammatory status: whereas in a healthy host these cells contribute to gut homeostasis, in an inflamed environment the same cells become responsive to bacterial products and differentiate into pro-inflammatory effector cells.76 There is also evidence that Ly-6Chi monocytes can be reprogrammed in tissue to acquire proangiogenic capabilities.115 The fate of Ly-6Clo monocytes is less well understood. On the one hand, Ly-6Clo monocytes have been suggested to participate in the resolution of inflammation,113 but accumulating evidence suggests that this subset functions principally as a blood macrophage.71 The molecular determinants of macrophage diversity are reviewed in detail elsewhere.116,117
Macrophage proliferation
Studies conducted almost 30 years ago concluded that in steady-state one half of splenic macrophages is continuously replenished by the circulation, whereas the other half is maintained locally.118 Interestingly, at least some macrophages and DCs can self renew, for example, in the skin,119,120 brain1,21 and atherosclerotic lesion,122,123 but circulating monocytes can also renew macrophages and DCs in these sites.36, 75, 124 Evidence that macrophage proliferation can dominate macrophage renewal has recently received considerable attention. The observation that pleural macrophages increase in number through proliferation rather than monocyte recruitment in response to the helminth Litomosoides sigmodontis30 (Fig. 5) challenged the long-held belief in an inverse relationship between cell maturation/differentiation and cell proliferation/renewal125. Macrophage proliferation in tissue is not exclusive to monocyte-independent, tissue macrophages, however. Although it has been appreciated for many years that macrophages in atherosclerotic lesions proliferate, possibly by recognizing oxidized lipoproteins,126 a recent study using fate-mapping approaches has concluded that renewal of monocyte-derived lesional macrophages is a major mechanism in established disease.32 An increasing number of studies have concluded that local macrophage proliferation is a major mechanism of macrophage renewal, regardless of initial orgin (i.e., hematopoietic or embryonic).28,33,34,127
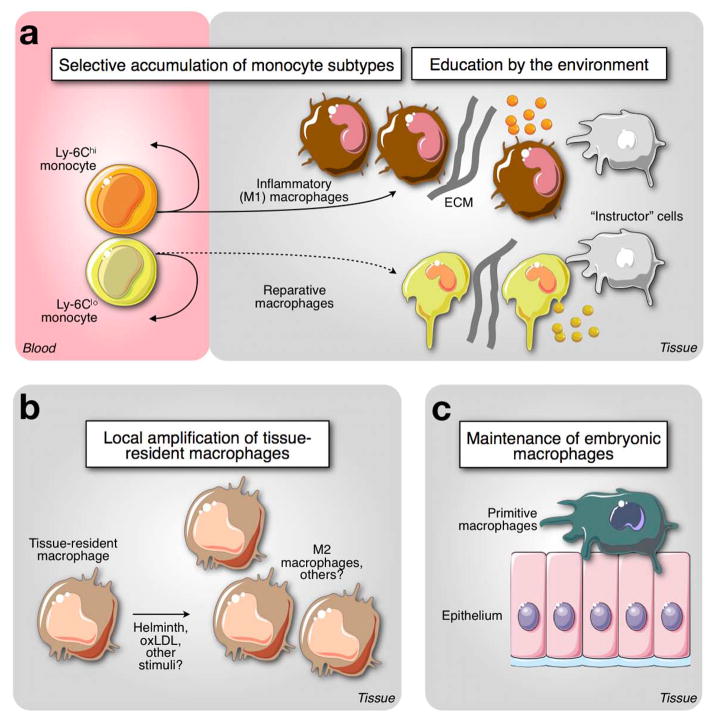
Fig. 5.
Expanding macrophage diversity in tissue. The cartoon illustrates three different origins of tissue macrophages (A) Selective accumulation of monocyte subsets committed with separate functions contributes different types of macrophages. For instance, CCR2-dependent recruitment of Ly-6Chi monocytes mediates tissue inflammation and proteolysis. This response is triggered upon intracellular pathogen infection or tissue damage. Ly-6Chi monocytes made both in medullary and extramedullary sites express CCR2 and response to MCP-1 for recruitment to tissues; however these cells might be equipped with divergent effector functions. Also, CX3CR1-dependent recruitment of Ly-6Clo monocytes may be associated with resolution of inflammation and mediation of tissue remodeling and healing. The recruited cells are also educated and polarized by their environment through cell-cell communication, sensing of soluble factors and interactions with extracellular matrix. (B) Local proliferation of tissue-resident macrophages. Some macrophages may proliferate at very low levels in steady-state conditions to maintain cell repertoires locally. Abnormal conditions, such as helminth infection and cardiovascular disease can induce potent macrophage proliferation in pleural cavity and atherosclerotic lesions, respectively. Such amplification can an occur in presence or absence of Ly-6Chi monocytes, and be promoted by locally produced factors including IL-4. (C) Maintenance of primitive macrophages. A lineage independent of HSCs is seeded during embryonic life and generates a repertoire of macrophages in various tissues, that is maintained after birth. These cells are often associated with epithelial structures, are thought to be unable to exit their tissue of residence, and may be poor antigen-presenting cells. These cells might serve in part as structural components that protect the tissue by sequestering foreign material and/or be involved in specific (non-immune) tissue functions.
Macrophage functional diversity: the immune system and beyond
The biological activities of monocyte-derived macrophages are relatively well characterized, e.g. with Ly-6Chi cells being recognized as the main drivers of classical inflammation as discussed above. The relevance of other macrophage types requires further study, although recent findings indicate that their functions are distinct from monocyte-derived macrophages.
Type-2 inflammation mediated by resident macrophages
IL-4 is a prototypical Th2 cytokine that has been used extensively in vitro to polarize macrophages into an alternative (type 2) phenotype. Recent evidence indicates that this cytokine controls the macrophage response in vivo in an unexpected manner. Specifically, in a model of helminth infection (L. sigmodontis), IL-4 drives a type 2 macrophage response in the pleural cavity30. The response requires macrophage-intrinsic IL-4R signaling31 and involves local amplification of tissue-resident macrophages. This process can occur without recruitment of Ly-6Chi monocytes. Consequently, this finding illustrates that the respective involvement of tissue-residence macrophages or infiltrating monocytes can trigger divergent responses in tissue. It is possible that the absence of Ly-6Chi monocytes, which would exhibit potentially damaging (type 1) effector functions, is required for efficient (type 2) parasite sequestration.
Tissue-specific functions supported by resident macrophages
Macrophages that inhabit organs throughout life may participate in tissue-specific functions. In favor of this hypothesis, a study indicates that alternatively activated macrophages are central regulators of adaptive thermogenesis in adipose tissue128. The study unexpectedly shows that exposure to cold promotes alternative activation of adipose tissue macrophages (but not other macrophages in the body) and that this process is necessary to maintain body temperature. The macrophage response, which depends on IL-4 signaling, involves thermogenic gene expression in brown adipose tissue and lipolysis in white adipose tissue. Considering the kinetics of the response, adaptive thermogenesis is mediated by tissue-resident macrophages and does not depend on recruitment of circulating monocytes. Recent studies have also shown that obesity activates a program of lysosomal biogenesis associated with lipid catabolism but not classic macrophage activation129. Other evidence suggests that macrophages and IL-4 regulate learning and memory in the meninges130. The source of IL-4 and the precise origin of these macrophages (HSC-derived versus HSC-independent tissue-resident macrophages) remain unknown.
New therapeutic targets in macrophage pathways
The growing appreciation that macrophages are heterogeneous functionally and developmentally has therapeutic implications. Because macrophages are central immune protectors, it is not feasible to entirely deplete them. However, depleting particular populations that cause harm may be a strategy for combatting diseases such as atherosclerosis and cancer.131 These diseases in particular claim >25 million deaths and cost the global economy nearly 2 trillion dollars each year. Thus, ongoing efforts aim to define clinically beneficial therapies that target macrophages and associated mononuclear phagocytes.
Below we present selected examples of drugs that can tailor or “re-program” macrophage responses and thereby improve outcome in mice. These drugs attack cells at different developmental stages, from HSC to macrophage (Fig. 6). The motivation for targeting HSCs and macrophage progenitors—rather than macrophages themselves—is based on evidence that inflammatory macrophages in diseased tissue are often short lived and continuously replaced by circulating precursors.74, 95, 97, 132, 133 Drugs that control macrophage precursors at their source—i.e., upstream of powerful amplification programs—may represent relevant anti-inflammatory therapeutics, provided that they do not produce undesirable side effects on non-macrophage lineage descendants or other cell types. Alternatively, drugs may also control tissue resident macrophages that are maintained, e.g. through local proliferation.
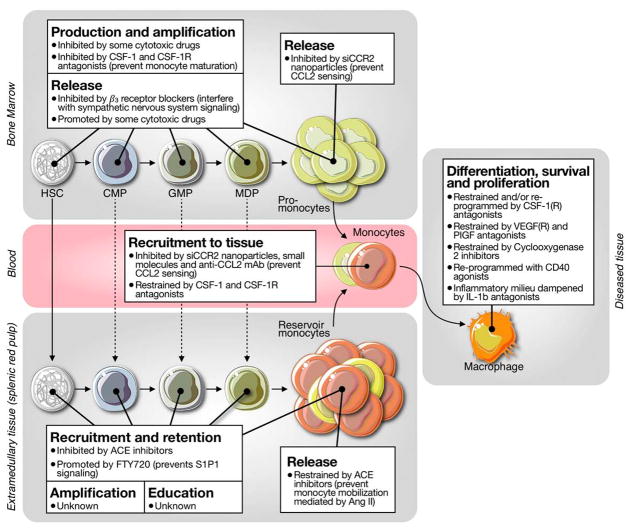
Fig. 6.
New targets in macrophage pathways? Drugs can attack cells at different developmental stages, from HSC to macrophage. The cartoon illustrates drugs that can interfere with macrophage differentiation survival and proliferation, monocyte recruitment to tissue, monocyte release from bone marrow or spleen, and production, amplification and release of bone marrow HSPCs.
Interfering with tissue macrophages
Researchers are evaluating many drugs’ potential to reduce macrophage activity within diseased tissue (Fig. 6). For example, CSF-1R inhibitors134 and agonist CD40 antibodies135 may re-program tissue macrophages in ways that enhance their killing activity; COX-2 inhibitors may suppress enzymatic reactions that trigger inflammation mediated by macrophages,136 anti-IL-4 treatment may suppress alternative macrophage activation and type-2 inflammation; anti-VEGF and anti-PIGF therapies may inhibit angiogenesis, a process driven at least in part by macrophages;137 and antagonist IL-1b antibodies may dampen inflammatory reactions contributed by macrophages.138 Studies are also required to determine how interfering with macrophage proliferation affects disease progression. For example, delivery of the anti-proliferative agent 5-FU attenuates monocyte production, and macrophage proliferation, and decreases atherosclerosis.32 Other candidates include interfering with IL-4,30, 31 SR-A,32 or CSF-1.139
Interfering with monocyte recruitment to tissue
Genetic studies have demonstrated that recruitment of Ly-6Chi monocytes into inflamed tissue frequently involves CCR2 signaling,101,140 while Ly-6Clo monocyte trafficking depends on other molecules. Targeting CCR2 or its ligand MCP-1 may therefore selectively control inflammatory monocytes. Different strategies are being considered to achieve this goal. They include the use of small molecule inhibitors,141 antibodies,108 and short interfering RNA (siRNA) encapsulated in nanoparticles142 (Fig. 6). For example, intravenously injecting nanoparticles that incorporate siCCR2 can systemically silence CCR2 expression in Ly-6Chi monocytes. Such treatment limits monocytes’ capacity to accumulate in CCL2-producing tissues (e.g., ischemic myocardium, atherosclerotic plaque, tumor stroma) and delays disease progression.74, 142 The ability to deliver siRNA to immune cells in vivo should also open the possibility of “surgically” silencing multiple target proteins that drive disease-promoting responses.
Interfering with monocyte mobilization from (extra)medullary tissue
Angiotensin II is a hormone that induces monocyte migration by binding to the AGTR1A receptor. Angiotensin II/AGTR1A signaling regulates inflammation by controlling the deployment of splenic (but not bone marrow) monocytes.78 Conversely, preventing angiotensin II production using an angiotensin converting enzyme inhibitor (ACEi) is sufficient to arrest the release of monocytes from the splenic reservoir and, in turn, to improve healing after myocardial infarction143 (Fig. 6). Because angiotensin II receptor blockers (ARB) typically antagonize angiotensin II/AGTR1A signaling, their capacity to prevent the mobilization of reservoir monocytes should also be considered. Although both ACEi and ARB are used clinically to treat high blood pressure, the newly described effect of angiotensin II on monocyte mobilization indicates that these drugs may also be useful in treating other diseases. CCR2 signaling promotes the exit of bone marrow (but not splenic) Ly-6Chi monocytes.101 Drugs that interfere with CCR2 signaling (see above) are expected to prevent not only the recruitment of circulating monocytes to MCP-1-producing tissues but also the mobilization of bone marrow monocytes into circulation.
Interfering with monocyte production
The cytokine CSF-1 regulates the amplification and differentiation of myeloid progenitors in bone marrow.139 Pharmacologic blockade of CSF-1 signaling is being tested both in animal models and human patients with cancer and other inflammatory diseases. Such research considers several classes of agents, including CSF-1R protein tyrosine kinase activity inhibitors (GW2580, Gleevec, PLX3397) and CSF-1/CSF1-R interaction antagonists144 (Fig 6). Recent studies indicate that targeting CSF-1 signaling restrains the tumor-associated macrophage response and consequently delays metastatic progression of breast cancer.145 Unfortunately, suppressing CSF-1 signaling can have deleterious effects. For example, treatment with anti-CSF-1R antibodies in a transplantation model reduces the pool of macrophages and precipitates graft-versus-host disease (GVHD). In contrast, exogenous administration of CSF-1 expands macrophages and inhibits GVHD.146 Collectively, these observations indicate that therapeutic inhibition or activation of CSF-1R signaling can efficiently modulate macrophage responses in vivo; however, the possible benefit of such treatments will remain context-dependent. CSF-1 antagonists may also alter macrophage activation and survival in tissue. Treatment with ACEi has also been shown to maintain high S1P1 levels on the surface of HSPCs; this is associated with a decreased ability of these cells to accumulate in extramedullary tissues and to produce monocyte progeny94. Thus ACEi may strongly inhibit inflammatory macrophage amplification in tissue by interfering with both monocyte production and (see above) from extramedullary tissue.
Interfering with HSPC mobilization
Catecholamine signaling between the sympathetic nervous system and mesenchymal stem cells controls HSPC liberation from the bone marrow niche.61, 147 Conversely, blocking catecholamine signaling by administering a β3 receptor antagonist (SR 59230A) prevents the liberation of bone marrow HSPC after myocardial infarction and consequently decreases both extramedullary monocyte production and macrophage accumulation in atherosclerotic plaques62 (Fig. 5). β-Blockers are used widely in managing cardiovascular disease in humans, but these agents typically have higher affinity for the β1 or β2 receptors. Future studies should define what role (if any) different β receptors exert on the hematopoietic response and whether β3 receptor antagonists may more potently restrain inflammatory macrophage responses in cardiovascular and other diseases.
Impact of cytotoxic drugs
Chemotherapeutic agents were originally designed as anti-proliferative agents against carcinoma cells. Some of them also act as myeloablative agents by interfering with proliferating HSCs and myeloid progenitors in bone marrow (Fig. 6). Conceivably, this side effect should reduce the number of lineage-descendant tissue macrophages. A recent study showed, however, that administering the mitotic inhibitor paclitaxel increases the number of tumor-associated macrophages in both genetic mouse models and human patients with cancer.145 The drug-induced macrophages likely promotes tumor progression because blocking macrophage recruitment with a CSF-1R antagonist (in combination with paclitaxel) ameliorates treatment efficacy. The mechanisms behind this enhanced macrophage activity are currently unknown. Yet, some cytotoxic drugs—including paclitaxel—mobilize bone marrow HSPCs which then remain functionally active in the periphery148. It is plausible that drug-induced circulating HSPCs produce cell progeny in extramedullary tissue and consequently propagate the macrophage response in diseased tissue. Future studies should ascertain drugs’ effects on individual cell types and in discrete locations in vivo. Such findings may serve to redefine widely applicable therapeutic approaches since cytotoxic drugs remain the most commonly used treatment modalities for cancer patients.
Conclusion
Recent studies have shown that macrophage precursors can be produced in and outside the bone marrow; that cells produced in medullary and extramedullary tissue differ; that diseases can co-opt macrophage production at different stages; and that pathologic progenitor responses may be targeted therapeutically. Yet, some fundamental questions remain unanswered. For example, the diversity of accessory cells and the number of possible interactions between HSPCs and their immediate microenvironment suggest that bone marrow niches can be heterogenous and transformable already in steady-state. However the in vivo microenvironments that produce monocytes require identification. We also know remarkably little about how bone marrow niches change during disease and, in turn, how these changes affect HSPC responses and monocyte production. Similarly, extramedullary niches assemble only during inflammation and are likely compositionally divergent from bone marrow niches, but their precise composition remains unknown. Recent studies also indicate that disease causes niche dysfunction64 and, inversely, that niche dysfunction may predispose to disease.65 Yet, the mode of communication between diseased sites and macrophage production sites remain unclear. Unless the disease affects the bone marrow and spleen, these production sites are away from an affected organ. Long-range signals that stimulate monocyte production must therefore exist. Candidates include the sympathetic nervous system (SNS)61,62 or any regulatory components (soluble factors, microvesicles and cells) that can access niche microenvironments to deliver information. Additionally, diseased tissues continuously replace large populations of macrophages. These cells conceivably die locally or leave, yet both options remain largely unstudied. Remnants of dead macrophages might be removed by other phagocytes and “recycled” within the tissue, whereas live cells might relocate to die elsewhere or to transfer information to distant sites. In addition, some circulating myeloid cells can migrate back and forth across the endothelial and pericyte layers149,150 and therefore enter and leave tissue without differentiating. The consequences for such cell behavior are unknown and, again, may involve transfer of information between tissues. An increasingly sophisticated understanding of macrophage biology will serve as the foundation for the development of efficient treatment strategies likely employing drug combinations that target multiple pathways. The next decade will certainly see our increasing knowledge on macrophage biology as well as the identification of more agents that reprogram deleterious macrophage responses without compromising the cells’ protective actions.
Acknowledgments
This work was supported in part by US National Institutes of Health (NIH) grants R01-AI084880, P50-CA086355 and U54-CA126515 (to M.J.P.), R01-HL096576, R01-HL095629 (to M.N.) and R01-HL095612, R56-AI104695 (to F.K.S.).
References
- 1.Metschnikoff E. Der Kampf der Phagocyten gegen Krankeitserreger. Virchows Archiv. 1884;96 [Google Scholar]
- 2.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011;11:788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 3.Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nat Mater. 2014;13:125–138. doi: 10.1038/nmat3780. [DOI] [PubMed] [Google Scholar]
- 4.Janeway CAJ, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 5.Stuart LM, Ezekowitz RA. Phagocytosis and comparative innate immunity: learning on the fly. Nat Rev Immunol. 2008;8:131–141. doi: 10.1038/nri2240. [DOI] [PubMed] [Google Scholar]
- 6.Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol. 2012;12:492–502. doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 8.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilliams M, Henri S, Tamoutounour S, Ardouin L, Schwartz-Cornil I, Dalod M, Malissen B. From skin dendritic cells to a simplified classification of human and mouse dendritic cell subsets. Eur J Immunol. 2010;40:2089–2094. doi: 10.1002/eji.201040498. [DOI] [PubMed] [Google Scholar]
- 13.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014 doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- 14.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, Vanhoutte L, Neyt K, Killeen N, Malissen B, Hammad H, Lambrecht BN. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, Hoeffel G, Malleret B, Heiseke A, Chew S, Jardine L, Purvis HA, Hilkens CM, Tam J, Poidinger M, Stanley ER, Krug AB, Renia L, Sivasankar B, Ng LG, Collin M, Ricciardi-Castagnoli P, Honda K, Haniffa M, Ginhoux F. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, Bain CC, Mowat AM, Reis e Sousa C, Poulin LF, Malissen B, Guilliams M. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012;42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 17.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, Pollet E, Ardouin L, Luche H, Sanchez C, Dalod M, Malissen B, Henri S. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A-Gonzalez N, Guillen JA, Gallardo G, Diaz M, de la Rosa JV, Hernandez IH, Casanova-Acebes M, Lopez F, Tabraue C, Beceiro S, Hong C, Lara PC, Andujar M, Arai S, Miyazaki T, Li S, Corbi AL, Tontonoz P, Hidalgo A, Castrillo A. The nuclear receptor LXRalpha controls the functional specialization of splenic macrophages. Nat Immunol. 2013;14:831–839. doi: 10.1038/ni.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, Pierce RH, Crispe IN. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy J, Summer R, Wilson AA, Kotton DN, Fine A. The prolonged life-span of alveolar macrophages. Am J Respir Cell Mol Biol. 2008;38:380–385. doi: 10.1165/rcmb.2007-0224RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A Lineage of Myeloid Cells Independent of Myb and Hematopoietic Stem Cells. Science. 2012 doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 24.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M, Ginhoux F. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumner R, Crawford A, Mucenski M, Frampton J. Initiation of adult myelopoiesis can occur in the absence of c-Myb whereas subsequent development is strictly dependent on the transcription factor. Oncogene. 2000;19:3335–3342. doi: 10.1038/sj.onc.1203660. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkins SJ, Ruckerl D, Thomas GD, Hewitson JP, Duncan S, Brombacher F, Maizels RM, Hume DA, Allen JE. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J Exp Med. 2013 doi: 10.1084/jem.20121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, Fraser DJ, Allen JE, Jones SA, Taylor PR. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun. 2013;4:1886. doi: 10.1038/ncomms2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974. doi: 10.1126/science.1242974. [DOI] [PubMed] [Google Scholar]
- 35.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 37.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 38.Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci U S A. 2002;99:11872–11877. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 40.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- 42.Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2012;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rieger MA, Hoppe PS, Smejkal BM, Eitelhuber AC, Schroeder T. Hematopoietic cytokines can instruct lineage choice. Science. 2009;325:217–218. doi: 10.1126/science.1171461. [DOI] [PubMed] [Google Scholar]
- 44.Sarrazin S, Mossadegh-Keller N, Fukao T, Aziz A, Mourcin F, Vanhille L, Kelly Modis L, Kastner P, Chan S, Duprez E, Otto C, Sieweke MH. MafB restricts M-CSF-dependent myeloid commitment divisions of hematopoietic stem cells. Cell. 2009;138:300–313. doi: 10.1016/j.cell.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 45.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 46.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pietras EM, Warr MR, Passegue E. Cell cycle regulation in hematopoietic stem cells. J Cell Biol. 2011;195:709–720. doi: 10.1083/jcb.201102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarrazin S, Sieweke M. Integration of cytokine and transcription factor signals in hematopoietic stem cell commitment. Semin Immunol. 2011;23:326–334. doi: 10.1016/j.smim.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 50.Lo Celso C, Scadden DT. The haematopoietic stem cell niche at a glance. J Cell Sci. 2011;124:3529–3535. doi: 10.1242/jcs.074112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 52.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilkins BS. Histology of normal haemopoiesis: bone marrow histology. I. J Clin Pathol. 1992;45:645–649. doi: 10.1136/jcp.45.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiktor-Jedrzejczak WW, Ahmed A, Szczylik C, Skelly RR. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982;156:1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 56.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, Molina T, Charo I, Hume DA, Cumano A, Lauvau G, Geissmann F. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mossadegh-Keller N, Sarrazin S, Kandalla PK, Espinosa L, Stanley ER, Nutt SL, Moore J, Sieweke MH. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature. 2013;497:239–243. doi: 10.1038/nature12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeKoter RP, Singh H. Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science. 2000;288:1439–1441. doi: 10.1126/science.288.5470.1439. [DOI] [PubMed] [Google Scholar]
- 59.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 62.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schajnovitz A, Itkin T, D’Uva G, Kalinkovich A, Golan K, Ludin A, Cohen D, Shulman Z, Avigdor A, Nagler A, Kollet O, Seger R, Lapidot T. CXCL12 secretion by bone marrow stromal cells is dependent on cell contact and mediated by connexin-43 and connexin-45 gap junctions. Nat Immunol. 2011;12:391–398. doi: 10.1038/ni.2017. [DOI] [PubMed] [Google Scholar]
- 64.Ferraro F, Lymperi S, Mendez-Ferrer S, Saez B, Spencer JA, Yeap BY, Masselli E, Graiani G, Prezioso L, Rizzini EL, Mangoni M, Rizzoli V, Sykes SM, Lin CP, Frenette PS, Quaini F, Scadden DT. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, Westmoreland SV, Chambon P, Scadden DT, Purton LE. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 67.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Etzrodt M, Cortez-Retamozo V, Newton A, Zhao J, Ng A, Wildgruber M, Romero P, Wurdinger T, Xavier R, Geissmann F, Meylan E, Nahrendorf M, Swirski FK, Baltimore D, Weissleder R, Pittet MJ. Regulation of monocyte functional heterogeneity by miR-146a and Relb. Cell Rep. 2012;1:317–324. doi: 10.1016/j.celrep.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 70.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 71.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, Ryan RJ, Iwamoto Y, Marinelli B, Gorbatov R, Forghani R, Novobrantseva TI, Koteliansky V, Figueiredo JL, Chen JW, Anderson DG, Nahrendorf M, Swirski FK, Weissleder R, Pittet MJ. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, Shakhar G, Halpern Z, Jung S. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 77.Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, Koh H, Rodriguez A, Idoyaga J, Pack M, Velinzon K, Park CG, Steinman RM. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–818. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Laan AM, Ter Horst EN, Delewi R, Begieneman MP, Krijnen PA, Hirsch A, Lavaei M, Nahrendorf M, Horrevoets AJ, Niessen HW, Piek JJ. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goodman JW, Hodgson GS. Evidence for stem cells in the peripheral blood of mice. Blood. 1962;19:702–714. [PubMed] [Google Scholar]
- 83.Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H, Sato T, Cote D, Sykes M, Strom TB, Scadden DT, Lin CP. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 87.Simmons DJ, Nichols GJ. Diurnal periodicity in the metabolic activity of bone tissue. Am J Physiol. 1966;210:411–418. doi: 10.1152/ajplegacy.1966.210.2.411. [DOI] [PubMed] [Google Scholar]
- 88.Lymperi S, Ferraro F, Scadden DT. The HSC niche concept has turned 31. Has our knowledge matured? Ann N Y Acad Sci. 2010;1192:12–18. doi: 10.1111/j.1749-6632.2009.05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Si Y, Tsou CL, Croft K, Charo IF. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest. 2010;120:1192–1203. doi: 10.1172/JCI40310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 92.Freedman MH, Saunders EF. Hematopoiesis in the human spleen. Am J Hematol. 1981;11:271–275. doi: 10.1002/ajh.2830110307. [DOI] [PubMed] [Google Scholar]
- 93.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 94.Cortez-Retamozo V, Etzrodt M, Newton A, Ryan R, Pucci F, Sio SW, Kuswanto W, Rauch PJ, Chudnovskiy A, Iwamoto Y, Kohler R, Marinelli B, Gorbatov R, Wojtkiewicz G, Panizzi P, Mino-Kenudson M, Forghani R, Figueiredo JL, Chen JW, Xavier R, Swirski FK, Nahrendorf M, Weissleder R, Pittet MJ. Angiotensin II drives the production of tumor-promoting macrophages. Immunity. 2013;38:296–308. doi: 10.1016/j.immuni.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012;125:364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, Bronte V. Immune tolerance to tumor antigens occurs in a specialized environment in the spleen. Cell Rep. 2012;2:628–639. doi: 10.1016/j.celrep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 99.Pittet MJ, Weissleder R. Intravital imaging. Cell. 2011;147:983–991. doi: 10.1016/j.cell.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–282. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 102.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 105.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 107.Saederup N, Chan L, Lira SA, Charo IF. Fractalkine deficiency markedly reduces macrophage accumulation and atherosclerotic lesion formation in CCR2−/− mice: evidence for independent chemokine functions in atherogenesis. Circulation. 2008;117:1642–1648. doi: 10.1161/CIRCULATIONAHA.107.743872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, Koo LY, Brenchley JM, Fraser ID, Belkaid Y. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med. 2013;19:713–721. doi: 10.1038/nm.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Avraham-Davidi I, Yona S, Grunewald M, Landsman L, Cochain C, Silvestre JS, Mizrahi H, Faroja M, Strauss-Ayali D, Mack M, Jung S, Keshet E. On-site education of VEGF-recruited monocytes improves their performance as angiogenic and arteriogenic accessory cells. J Exp Med. 2013 doi: 10.1084/jem.20120690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 117.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 118.van Furth R, Diesselhoff-den Dulk MM. Dual origin of mouse spleen macrophages. J Exp Med. 1984;160:1273–1283. doi: 10.1084/jem.160.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chorro L, Sarde A, Li M, Woollard KJ, Chambon P, Malissen B, Kissenpfennig A, Barbaroux JB, Groves R, Geissmann F. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med. 2009;206:3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 122.Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, Shiomi M, Schoen FJ, Libby P. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 123.Rosenfeld ME, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of WHHL and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1990;10:680–687. doi: 10.1161/01.atv.10.5.680. [DOI] [PubMed] [Google Scholar]
- 124.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aziz A, Soucie E, Sarrazin S, Sieweke MH. MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science. 2009;326:867–871. doi: 10.1126/science.1176056. [DOI] [PubMed] [Google Scholar]
- 126.Hamilton JA, Myers D, Jessup W, Cochrane F, Byrne R, Whitty G, Moss S. Oxidized LDL can induce macrophage survival, DNA synthesis, and enhanced proliferative response to CSF-1 and GM-CSF. Arterioscler Thromb Vasc Biol. 1999;19:98–105. doi: 10.1161/01.atv.19.1.98. [DOI] [PubMed] [Google Scholar]
- 127.Swirski FK, Hilgendorf I, Robbins CS. From proliferation to proliferation: monocyte lineage comes full circle. Semin Immunopathol. 2014 doi: 10.1007/s00281-013-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AWJ. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pittet MJ, Swirski FK. Monocytes link atherosclerosis and cancer. Eur J Immunol. 2011;41:2519–2522. doi: 10.1002/eji.201141727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, Matsushima K. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–5466. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 133.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 134.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N, Giacca M, Stassen JM, Dewerchin M, Collen D, Carmeliet P. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 138.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 139.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. 2006;18:39–48. doi: 10.1016/j.coi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 140.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 141.Brodmerkel CM, Huber R, Covington M, Diamond S, Hall L, Collins R, Leffet L, Gallagher K, Feldman P, Collier P, Stow M, Gu X, Baribaud F, Shin N, Thomas B, Burn T, Hollis G, Yeleswaram S, Solomon K, Friedman S, Wang A, Xue CB, Newton RC, Scherle P, Vaddi K. Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J Immunol. 2005;175:5370–5378. doi: 10.4049/jimmunol.175.8.5370. [DOI] [PubMed] [Google Scholar]
- 142.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, Chudnovskiy A, Figueiredo JL, Sosnovik DE, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–1373. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–1820. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- 145.Denardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hashimoto D, Chow A, Greter M, Saenger Y, Kwan WH, Leboeuf M, Ginhoux F, Ochando JC, Kunisaki Y, van Rooijen N, Liu C, Teshima T, Heeger PS, Stanley ER, Frenette PS, Merad M. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J Exp Med. 2011;208:1069–1082. doi: 10.1084/jem.20101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Richman CM, Weiner RS, Yankee RA. Increase in circulating stem cells following chemotherapy in man. Blood. 1976;47:1031–1039. [PubMed] [Google Scholar]
- 149.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Voisin MB, Woodfin A, Nourshargh S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arterioscler Thromb Vasc Biol. 2009;29:1193–1199. doi: 10.1161/ATVBAHA.109.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]