Abstract
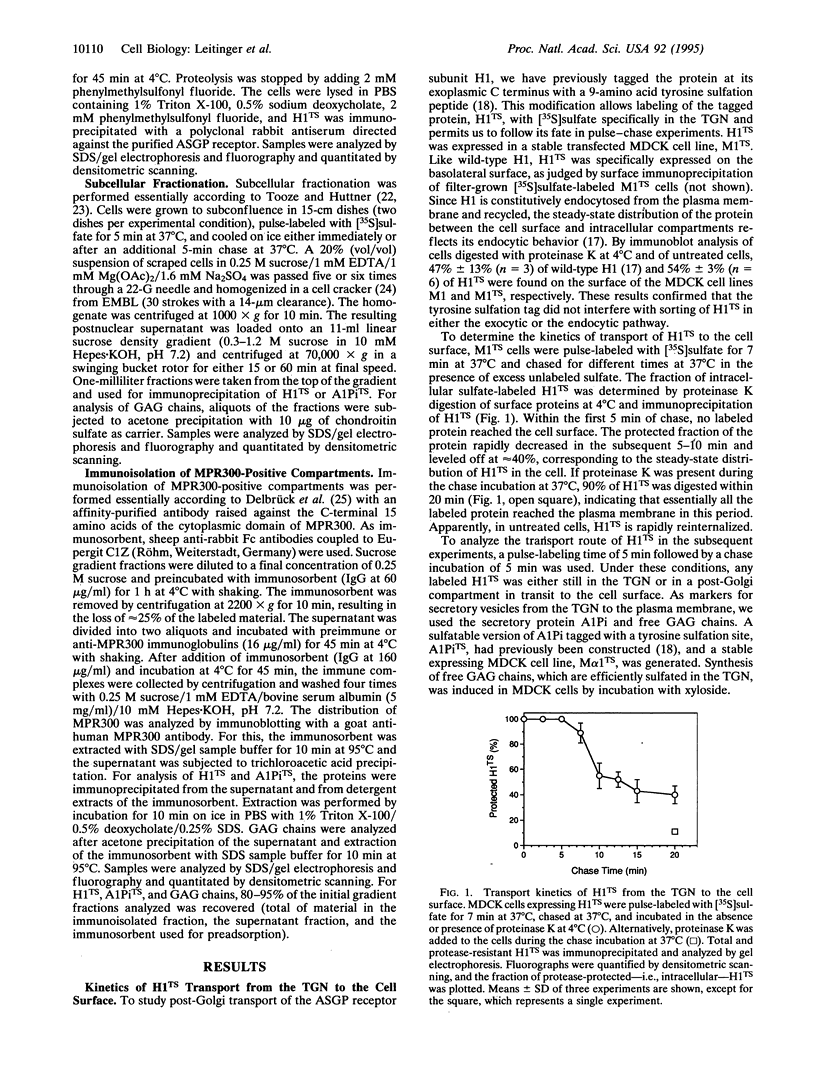
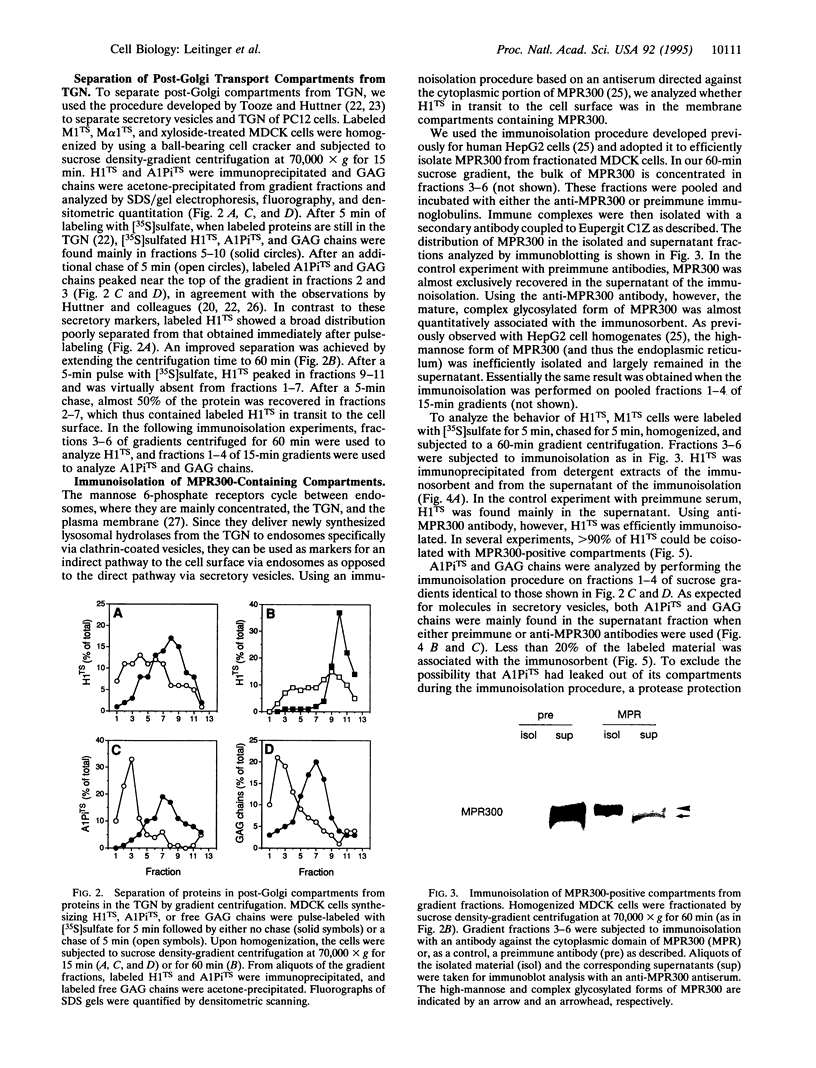
Signals for endocytosis and for basolateral and lysosomal sorting are closely related in a number of membrane proteins, suggesting similar sorting mechanisms at the plasma membrane and in the trans-Golgi network (TGN). We tested the hypothesis that basolateral membrane proteins are transported to the cell surface via endosomes for the asialoglycoprotein receptor H1. This protein was tagged with a tyrosine sulfation site (H1TS) to allow specific labeling with [35S]sulfate in the TGN. Madin-Darby canine kidney cells expressing H1TS were pulse-labeled and chased for a period of time insufficient for labeled H1TS to reach the cell surface. Upon homogenization and gradient centrifugation, fractions devoid of TGN were subjected to immunoisolation of compartments containing mannose 6-phosphate receptor, which served as an endosomal marker. H1TS in transit to the cell surface was efficiently coisolated, whereas a labeled secretory protein and free glycosaminoglycan chains were not. This indicates an indirect pathway for the asialoglycoprotein receptor to the plasma membrane via endosomes and has important implications for protein sorting in the TGN and endosomes.
Full text
PDF
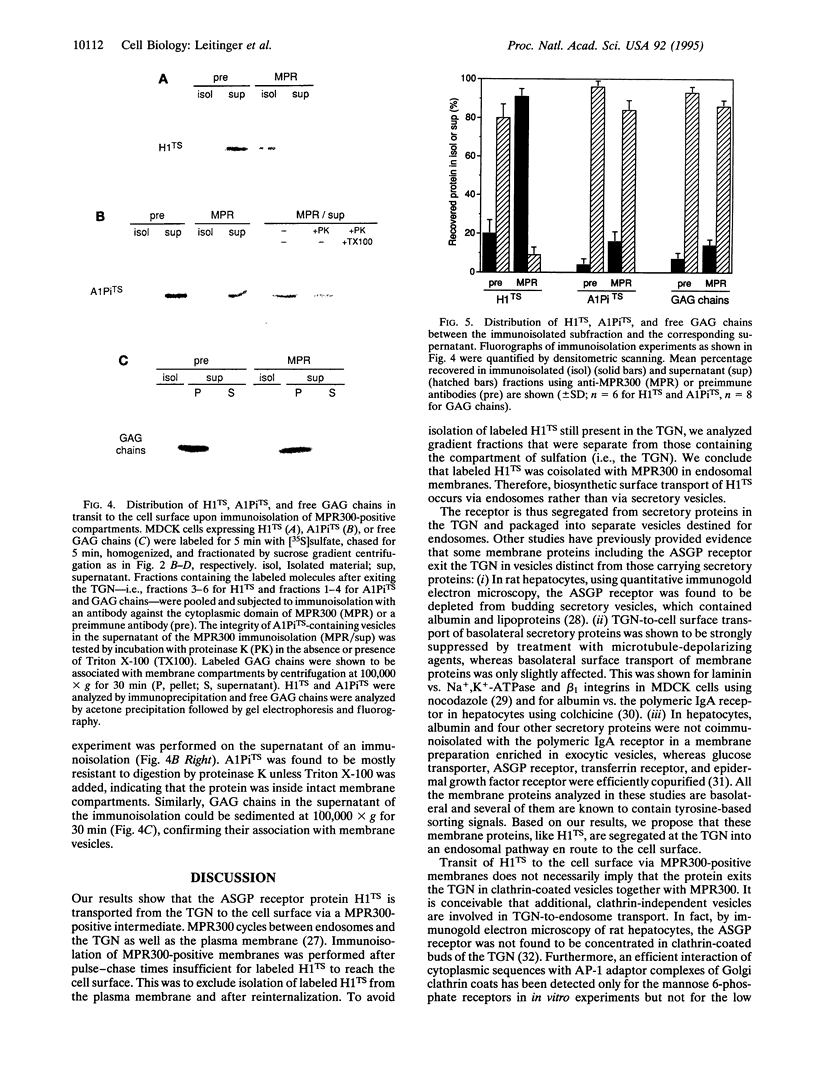

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aroeti B., Mostov K. E. Polarized sorting of the polymeric immunoglobulin receptor in the exocytotic and endocytotic pathways is controlled by the same amino acids. EMBO J. 1994 May 15;13(10):2297–2304. doi: 10.1002/j.1460-2075.1994.tb06513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Rothman J. E. Characterization of protein transport between successive compartments of the Golgi apparatus: asymmetric properties of donor and acceptor activities in a cell-free system. Arch Biochem Biophys. 1985 Jul;240(1):413–425. doi: 10.1016/0003-9861(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Boll W., Partin J. S., Katz A. I., Caplan M. J., Jamieson J. D. Distinct pathways for basolateral targeting of membrane and secretory proteins in polarized epithelial cells. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8592–8596. doi: 10.1073/pnas.88.19.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer C. B., Roth M. G. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991 Aug;114(3):413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
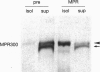
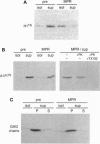
- Delbrück R., Desel C., von Figura K., Hille-Rehfeld A. Proteolytic processing of cathepsin D in prelysosomal organelles. Eur J Cell Biol. 1994 Jun;64(1):7–14. [PubMed] [Google Scholar]
- Fuhrer C., Geffen I., Spiess M. Endocytosis of the ASGP receptor H1 is reduced by mutation of tyrosine-5 but still occurs via coated pits. J Cell Biol. 1991 Aug;114(3):423–431. doi: 10.1083/jcb.114.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter C. E., Connolly C. N., Cutler D. F., Hopkins C. R. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995 May 5;270(18):10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- Geffen I., Fuhrer C., Leitinger B., Weiss M., Huggel K., Griffiths G., Spiess M. Related signals for endocytosis and basolateral sorting of the asialoglycoprotein receptor. J Biol Chem. 1993 Oct 5;268(28):20772–20777. [PubMed] [Google Scholar]
- Geffen I., Wessels H. P., Roth J., Shia M. A., Spiess M. Endocytosis and recycling of subunit H1 of the asialoglycoprotein receptor is independent of oligomerization with H2. EMBO J. 1989 Oct;8(10):2855–2861. doi: 10.1002/j.1460-2075.1989.tb08433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Schwartz A. L. Membranes of sorting organelles display lateral heterogeneity in receptor distribution. J Cell Biol. 1987 Jun;104(6):1715–1723. doi: 10.1083/jcb.104.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J. N., Conibear E., Pearse B. M. Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J. 1989 Apr;8(4):1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 1994 Jul 1;13(13):2963–2969. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Harter C., Matter K., Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991 Sep 6;66(5):907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- Johnson K. F., Kornfeld S. A His-Leu-Leu sequence near the carboxyl terminus of the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor is necessary for the lysosomal enzyme sorting function. J Biol Chem. 1992 Aug 25;267(24):17110–17115. [PubMed] [Google Scholar]
- Johnson K. F., Kornfeld S. The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J Cell Biol. 1992 Oct;119(2):249–257. doi: 10.1083/jcb.119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Kuroda S., Tanizawa K., Sakamoto Y., Tanaka H., Soda K. Alanine dehydrogenases from two Bacillus species with distinct thermostabilities: molecular cloning, DNA and protein sequence determination, and structural comparison with other NAD(P)(+)-dependent dehydrogenases. Biochemistry. 1990 Jan 30;29(4):1009–1015. doi: 10.1021/bi00456a025. [DOI] [PubMed] [Google Scholar]
- Leitinger B., Brown J. L., Spiess M. Tagging secretory and membrane proteins with a tyrosine sulfation site. Tyrosine sulfation precedes galactosylation and sialylation in COS-7 cells. J Biol Chem. 1994 Mar 18;269(11):8115–8121. [PubMed] [Google Scholar]
- Letourneur F., Klausner R. D. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992 Jun 26;69(7):1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- Matter K., Hunziker W., Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992 Nov 27;71(5):741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Matter K., Whitney J. A., Yamamoto E. M., Mellman I. Common signals control low density lipoprotein receptor sorting in endosomes and the Golgi complex of MDCK cells. Cell. 1993 Sep 24;74(6):1053–1064. doi: 10.1016/0092-8674(93)90727-8. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Moore H. P. Movement from trans-Golgi network to cell surface in semiintact cells. Methods Enzymol. 1992;219:234–248. doi: 10.1016/0076-6879(92)19025-2. [DOI] [PubMed] [Google Scholar]
- Odorizzi C. G., Trowbridge I. S., Xue L., Hopkins C. R., Davis C. D., Collawn J. F. Sorting signals in the MHC class II invariant chain cytoplasmic tail and transmembrane region determine trafficking to an endocytic processing compartment. J Cell Biol. 1994 Jul;126(2):317–330. doi: 10.1083/jcb.126.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata S., Fukuda M. Lysosomal targeting of Limp II membrane glycoprotein requires a novel Leu-Ile motif at a particular position in its cytoplasmic tail. J Biol Chem. 1994 Feb 18;269(7):5210–5217. [PubMed] [Google Scholar]
- Prill V., Lehmann L., von Figura K., Peters C. The cytoplasmic tail of lysosomal acid phosphatase contains overlapping but distinct signals for basolateral sorting and rapid internalization in polarized MDCK cells. EMBO J. 1993 May;12(5):2181–2193. doi: 10.1002/j.1460-2075.1993.tb05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P., Barr F. A., Stinchcombe J. C., Binacchi C., Huttner W. B. Brefeldin A inhibits the formation of constitutive secretory vesicles and immature secretory granules from the trans-Golgi network. Eur J Cell Biol. 1992 Dec;59(2):265–274. [PubMed] [Google Scholar]
- Régnier-Vigouroux A., Tooze S. A., Huttner W. B. Newly synthesized synaptophysin is transported to synaptic-like microvesicles via constitutive secretory vesicles and the plasma membrane. EMBO J. 1991 Dec;10(12):3589–3601. doi: 10.1002/j.1460-2075.1991.tb04925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval I. V., Arredondo J. J., Alcalde J., Gonzalez Noriega A., Vandekerckhove J., Jimenez M. A., Rico M. The residues Leu(Ile)475-Ile(Leu, Val, Ala)476, contained in the extended carboxyl cytoplasmic tail, are critical for targeting of the resident lysosomal membrane protein LIMP II to lysosomes. J Biol Chem. 1994 Mar 4;269(9):6622–6631. [PubMed] [Google Scholar]
- Saucan L., Palade G. E. Differential colchicine effects on the transport of membrane and secretory proteins in rat hepatocytes in vivo: bipolar secretion of albumin. Hepatology. 1992 Apr;15(4):714–721. doi: 10.1002/hep.1840150427. [DOI] [PubMed] [Google Scholar]
- Saucan L., Palade G. E. Membrane and secretory proteins are transported from the Golgi complex to the sinusoidal plasmalemma of hepatocytes by distinct vesicular carriers. J Cell Biol. 1994 May;125(4):733–741. doi: 10.1083/jcb.125.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shia M. A., Lodish H. F. The two subunits of the human asialoglycoprotein receptor have different fates when expressed alone in fibroblasts. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1158–1162. doi: 10.1073/pnas.86.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa M. A., Schmidt B., von Figura K., Hille-Rehfeld A. In vitro binding of plasma membrane-coated vesicle adaptors to the cytoplasmic domain of lysosomal acid phosphatase. J Biol Chem. 1993 Jun 15;268(17):12537–12543. [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free formation of immature secretory granules and constitutive secretory vesicles from trans-Golgi network. Methods Enzymol. 1992;219:81–93. doi: 10.1016/0076-6879(92)19012-u. [DOI] [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990 Mar 9;60(5):837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Collawn J. F., Hopkins C. R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]