Abstract
OBJECTIVE:
To examine the relation between the neurobehavior of very preterm infants and the level of NICU quality of developmental care.
METHODS:
The neurobehavior of 178 very preterm infants (gestational age ≤29 weeks and/or birth weight ≤1500 g) from 25 NICUs participating in a large multicenter, longitudinal study (Neonatal Adequate Care for Quality of Life, NEO-ACQUA) was examined with a standardized neurobehavioral assessment, the NICU Network Neurobehavioral Scale (NNNS). A questionnaire, the NEO-ACQUA Quality of Care Checklist was used to evaluate the level of developmental care in each of the NICUs. A factor analyses applied to NEO-ACQUA Quality of Care Checklist produced 2 main factors: (1) the infant-centered care (ICC) index, which measures parents’ involvement in the care of their infant and other developmentally oriented care interventions, and (2) the infant pain management (IPM) index, which measures the NICU approach to and the procedures used for reducing infant pain. The relations between NNNS neurobehavioral scores and the 2 indexes were evaluated.
RESULTS:
Infants from NICUs with high scores on the ICC evidenced higher attention and regulation, less excitability and hypotonicity, and lower stress/abstinence NNNS scores than infants from low-care units. Infants from NICUs with high scores on the IPM evidenced higher attention and arousal, lower lethargy and nonoptimal reflexes NNNS scores than preterm infants from low-scoring NICUs.
CONCLUSIONS:
Very preterm infant neurobehavior was associated with higher levels of developmental care both in ICC and in IPM, suggesting that these practices support better neurobehavioral stability.
KEY WORDS: preterm infant, very low birth weight, developmental care, pain management, neurobehavioral examination, NNNS
What's Known on This Subject:
Although developmental care in NICUs reduces the stress experienced by preterm infants, the actual level of developmental care may vary and little is known about how the level of developmental care relates to preterm infants’ neurobehavioral performance.
What This Study Adds:
The study demonstrates the relationship between variations in developmental care in NICUs and the neurobehavior of preterm infants. Infants from NICUs with high-quality developmental care compared with infants from units with low quality of care evidenced a better neurobehavioral profile.
Preterm infants in NICUs are exposed to numerous stressors, including painful stimuli, disruption of sleep, excessive noise and light levels, frequent handling associated with medical or nursing procedures, and maternal separation and disrupted parenting.1–3 In an effort to improve developmental outcomes, management has shifted toward neuroprotective strategies and early neurodevelopmental support.4 Several studies suggest that modifications of the care practices that reduce the stress and pain experienced by infants improve their clinical and neurobehavioral functioning.5–7 However, not all studies have demonstrated beneficial effects of developmental care.8–10 One possible reason for a lack of consistent findings is that, even though a NICU ascribes to carrying out developmental care, the actual level of developmental care may vary among different NICUs or over time.11,12
The seminal work of Als13 led to the development of the her Neonatal Individualized Developmental Care and Assessment Program (NIDCAP). NIDCAP was designed to create a NICU environment that minimized the stress experienced by the infant by utilizing naturalistic observations of the infant before, during, and after caregiving procedures, such as control of external stimuli (eg, vestibular, auditory, visual, tactile), clustering of nursery care activities, and positioning or swaddling of the preterm infant. Over the years, the concept of developmental care has been elaborated. Recently, 5 core measure sets for evidence-based developmental care were identified: protected sleep, pain and stress assessment and management, developmental activities of daily living, family-centered care, and a healing environment.14 Consequently, apart from a specific developmental care program (eg, NIDCAP), NICUs might apply different aspects of so-called developmental care in their routine management of their infants. To our knowledge, differences in the actual level of quality of developmental care incorporated in a NICU’s standard care has seldom been evaluated and the relation between level of care and infant neurobehavior has not been investigated.
The goal of this study was to evaluate the relations of variation of the quality of NICU developmental care “routinely” carried out in 25 NICUs to the neurobehavioral functioning of a large cohort of very preterm infants. Given that there is no generally accepted definition of developmental care,14 we developed a questionnaire, the Neonatal Adequate Care for Quality of Life (NEO-ACQUA) Quality of Care Checklist (QCC) to evaluate the level of developmental care in NICUs, including parental involvement and pain management. We compared infants from NICUs with low and high levels of developmental care practices. We expected that infants from NICUs with high-quality neonatal developmental care compared with infants from units with low-quality care would evidence better neurobehavioral performance.
Methods
The research was conducted as part of a large multicenter, longitudinal study in a collaborative of 25 regional tertiary-level Italian NICUs, named the Neonatal Adequate Care for Quality of Life (NEO-ACQUA). One hundred seventy-eight healthy very preterm infants (90 female, 50.6%) were recruited consecutively between January 2006 and December 2007. Inclusion criteria were gestational age ≤29 weeks and/or birth weight ≤1500 g; no documented neurologic pathologies as shown by cerebral ultrasound, intraventricular hemorrhage up to stage 1 or 2; no sensory deficits; and no malformation syndromes and/or major malformations. Mothers were included if they were aged >18 years, had no manifest psychiatric or cognitive pathologies and no drug addiction, and were not a single parent. The study was approved by the hospitals’ institutional review boards, and written informed consent was obtained from all infants’ parents.
Perinatal Data Collection
Perinatal variables collected included gender, gestational age, birth weight, multiple birth, Apgar scores, type of delivery, intrauterine growth status classified as appropriate for gestational age or small for gestational age.15
Socioeconomic Status
Socioeconomic status (SES) was assessed by using the Hollingshead 4-factor index of SES (A. B. Hollingshead, unpublished observations, 1975). The more prestigious occupational level of either parents was considered as family SES score ranging from 0 to 90: lower scores reflect lower SES.
Neonatal Risk Adjustment Score
Neonatal clinical condition was assessed with the Vermont Oxford Network Risk Adjustment index (VON-RA).16 The VON-RA index considers clinical and demographical variables, such as gestational age, presence of congenital anomaly, multiple gestation, Apgar score at 1 minute, gender, delivery typology (vaginal or caesarean), and out-born status. Low scores indicate less serious of preterm clinical outcomes.
Neurobehavioral Evaluation
Infant neurobehavior was evaluated by using the NICU Network Neurobehavioral Scale (NNNS). The NNNS is a well-standardized test to evaluate the neurobehavioral status of high-risk infants.17 The scale has 45 individual neurologic and neurobehavioral items that are clustered into state-dependent “packages”; in addition, there are 21 individual summary items. Individual scores are summarized by 13 summary scales (see Table 1).18 The NNNS was administered by certified, blinded research assistants at NICUs when infants were clinically stable (postconceptional age range, 35–43 weeks).
TABLE 1.
Short Definitions and Score Ranges of NNNS Summary Scales
| 1 | Habituation | Response decrement to repeated auditory and visual stimuli during sleep (range 1–9) |
| 2 | Attention | Ability to localize and track animate and inanimate auditory and visual stimuli (range 1–9) |
| 3 | Arousal | Level of arousal maintained during the examination, including state and motor (range 1–9) |
| 4 | Regulation | Capacity to modulate arousal and organize motor activity, physiology, and state in response to stimulation (range 1–9) |
| 5 | Handling | Extent to which handling strategies were used during attention sequence to maintain alert state (range 0–1) |
| 6 | Quality of movement | Attributes of motor control, including smoothness, maturity, and lack of startles and tremors (range 1–9) |
| 7 | Excitability | High levels of motor, state, and physiologic reactivity (range 0–15) |
| 8 | Lethargy | Low levels of motor, state, and physiologic reactivity (range 0–15) |
| 9 | Asymmetric reflexes | Number of asymmetric responses to elicited reflexes (range 0–16) |
| 10 | Nonoptimal reflexes | Number of poor scores to elicited reflexes (range 0–15) |
| 11 | Hypertonicity | Hypertonic responses in arms, legs, trunk, or general tone (range 0–10) |
| 12 | Hypotonicity | Hypotonic response in arms, legs, trunk, or general tone (range 0–10) |
| 13 | Stress/abstinence | Number of stress and abstinence signs observed during the examination (range 0–1) |
Scores on the summary scales indicate “higher/more” or “lower/less” of the behavior, not a “better” or “worse” performance.
Measurement of Developmental Care
The NEO-ACQUA QCC assesses a variety of procedures and practices of developmental care used in NICUs.14,19 The QCC covers the following areas: developmental care practices, policies toward parents, control of environment, and infant pain management. Furthermore, general information about the unit is obtained, such as number of beds and admissions per year. For each NICU, a neonatologist with at least 5 years of clinical experience who was not involved in the direct care of the infants filled out the QCC. A factor analyses applied to QCC responses revealed 3 main factors*; however, given the goal of this study, 2 indices of developmental care were used. (1) The infant centered care (ICC) index accounted for 20% of the variance. The ICC index included 4 items. The first 3 items assessed the parent’s involvement, such as the possibility for parents to spend the night in the unit; use of parental kangaroo care as a routine procedure; the average duration per day of kangaroo care; and 1 item assessed the presence of nursing interventions to support infant development by decreasing infant energy expenditure and promoting stability, such as infant containment, postural maneuvers, and reduction of disturbing tactile stimulations. (2) The infant pain management (IPM) index accounted for 9% of the variance. The IPM index included 5 items. Two items measured the number of pharmacologic and nonpharmacologic procedures used for reducing pain during invasive medical procedures (eg, intravenous lines, drainage tubes, and endotracheal tubes), 1 item measured the use of pharmacologic analgesia or sedation during continuous mechanical ventilation, 1 item measured the kind of blood collection procedure (ie, heel stick), and the last item measured the use of a clinical evaluation scale of newborn pain and/or a protocol written for the management of newborn pain. Based on factor weightings, a composite score for the ICC and IPM indices was computed for each NICU. The ICC index ranged from 0 to 8, with higher scores indicating higher levels of ICC. The IPM index ranged from 0 to 10, with higher scores indicating higher levels of IPM. To distinguish the quality of care level, each NICU was assessed as being a low-care group or high-care group based on median splits for the ICC and IPM. This approach had the advantage of avoiding bias from extreme scores. For the ICC (median = 7.00, mean = 6.65, SD = 2.12): 12 NICUs had low-quality care (97 infants) and 13 NICUs had high-quality care (81 infants). For the IPM (median = 6.00; mean = 5.74, SD = 2.38): 14 NICUs had low-quality care (91 infants) and 11 NICUs had high-quality care (87 infants).
Statistical Analysis
Preliminarily statistical analyses evaluated the following: (1) general characteristics of the units (eg, number of beds, total admissions per year), (2) perinatal data, and (3) sociodemographic variables. Categorical variables were examined by using χ2 tests. Continuous variables were evaluated with separate analyses of variance (ANOVAs) by using a 2 (ICC index: low and high level of care) × 2 (IPM index: low and high level of care). To determine if the care quality level was related to preterm infants’ neurobehavioral performance, separate analyses of covariance (ANCOVAs) were applied to 13 NNNS scales with a 2 ICC × 2 IPM factorial design, with the VON-RA to estimate any confounding effect of the neonatal clinical conditions. For covariate analysis, the regression coefficients (B) were given to better describe the effect. Significant effects were evaluated in pairwise comparison post hoc tests with the P value for significance adjusted with Bonferroni correction. Effect size was evaluated by using the partial η squared (η2p). All analyses were performed at a significance level P ≤ .05.
Results
NICU Features and Infants' Characteristics
Descriptive statistics, separately subdivided for quality of care level, are presented for each variable in Tables 2 and 3. No differences were found in general characteristics of the units, perinatal data, and sociodemographic, with the exception of the VON-RA score, F(1, 173) = 5.34, P = .02, η2p = 0.03. Preterm infants from high ICC NICUs had a higher VON-RA score than those from units with low ICC.
TABLE 2.
Summary of General Characteristics of the NICUs, Perinatal and Sociodemographic Variables, Separately Subdivided on the Basis of the Developmental Care Quality Level (Mean and SDs)
| ICC Index | IPM Index | |||||||
|---|---|---|---|---|---|---|---|---|
| Low (NICU = 13) (n = 97, 47 F) | High (NICU = 12) (n = 81, 43 F) | Low (NICU = 11) (n = 91, 44 F) | High (NICU = 14) (n = 87, 46 F) | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Total admissions per year | 406.58 | 224.45 | 675.46 | 475.50 | 471.36 | 226.00 | 641.91 | 536.22 |
| Number of beds | 19.08 | 9.27 | 22.92 | 12.96 | 20.79 | 10.06 | 21.46 | 13.17 |
| Birth weight, g | 1161.56 | 229.36 | 1091.75 | 258.16 | 1129.08 | 249.15 | 1130.54 | 241.36 |
| Gestational age at birth, wk | 29.12 | 1.96 | 28.81 | 2.41 | 28.97 | 2.02 | 29.00 | 2.35 |
| Length of hospitalization, d | 59.88 | 20.38 | 61.32 | 24.76 | 60.69 | 23.01 | 60.37 | 21.92 |
| VON-RA score (range, 0.01 ÷ 0.99) | 0.05 | 0.07 | 0.09 | 0.15 | 0.07 | 0.11 | 0.07 | 0.12 |
| Postmenstrual age at test, wk | 37.12 | 1.39 | 37.21 | 1.64 | 37.04 | 1.30 | 37.30 | 1.69 |
| Chronological age at NNNS, d | 52.94 | 16.69 | 55.70 | 19.40 | 53.32 | 16.78 | 55.11 | 19.19 |
| Mother's age, y | 33.31 | 5.02 | 33.92 | 4.61 | 33.27 | 4.95 | 33.92 | 4.72 |
| Father's age, y | 35.82 | 5.21 | 36.60 | 6.33 | 35.75 | 5.36 | 36.59 | 6.09 |
| Education mother, y | 12.42 | 3.90 | 12.97 | 3.73 | 12.36 | 4.14 | 12.98 | 3.47 |
| Education father, y | 11.39 | 3.38 | 11.72 | 3.62 | 11.42 | 3.53 | 11.66 | 3.46 |
| SES score | 50.83 | 23.47 | 53.33 | 18.44 | 49.89 | 23.26 | 54.19 | 18.88 |
F, female.
TABLE 3.
Summary of General Characteristics of the NICUs, Perinatal and Sociodemographic Variables, Separately Subdivided on the Basis of the Developmental Care Quality Level (Number and Percentage)
| ICC Index | IPM Index | |||||||
|---|---|---|---|---|---|---|---|---|
| Low (NICU = 13) (n = 97, 47 F) | High (NICU = 12) (n = 81, 43 F) | Low (NICU = 11) (n = 91, 44 F) | High (NICU = 14) (n = 87, 46 F) | |||||
| n | % | n | % | n | % | n | % | |
| Birth weight ≤1000 g | 27 | 27.8 | 33 | 40.7 | 31 | 34.1 | 29 | 33.3 |
| Gestational age ≤29 wk | 55 | 56.7 | 48 | 59.3 | 54 | 59.3 | 49 | 56.3 |
| Multiple birth | 0 | 0.0 | 3 | 3.7 | 1 | 1.1 | 2 | 2.3 |
| Small for gestational age | 18 | 18.6 | 21 | 25.9 | 18 | 19.8 | 21 | 24.1 |
| Antenatal corticosteroids | 72 | 75.0 | 68 | 86.1 | 65 | 73.0 | 75 | 87.2 |
| Patent ductus arteriosus | 28 | 28.9 | 24 | 30.0 | 28 | 31.1 | 24 | 27.6 |
| Conventional ventilation | 64 | 66.0 | 40 | 50.0 | 58 | 64.4 | 46 | 52.9 |
| High-frequency ventilation | 10 | 10.3 | 8 | 10.0 | 9 | 10.0 | 9 | 10.3 |
| Oxygen dependency at 36 wk | 5 | 6.0 | 11 | 16.9 | 6 | 7.8 | 10 | 13.9 |
| Proven or suspected necrotizing enterocolitis | 1 | 1.0 | 0 | 0.0 | 1 | 1.1 | 0 | 0.0 |
| IVH (grade 1 or 2) | 14 | 14.4 | 13 | 16.0 | 17 | 18.7 | 10 | 11.5 |
| Sepsis | 8 | 8.2 | 2 | 2.5 | 10 | 11.0 | 0 | 0.0 |
F, female; IVH, intraventricular hemorrhage.
Level of Quality of Developmental Care and Neurobehavioral Profile
Table 4 shows the mean scores, the SDs, and the results of ANCOVAs for the NNNS scales, stratified for ICC and IPM indexes. Significant ICC effects were observed for the 5 scales: infants from high ICC NICUs exhibited higher attention and regulation, less excitability, and lower scores on the hypotonicity and stress/abstinence than infants from low-care units. Significant IPM effects emerged for 4 scales: infants from high IMP NICUs showed higher attention and arousal, and had lower scores on the lethargy and nonoptimal reflexes than preterm infants from low-care units. ANCOVA revealed an effect of VON-RA score for the following scales: attention, nonoptimal reflexes, asymmetric reflexes, and hypotonicity. These findings suggest that a higher VON-RA score was associated with a decreased capacity to attend to visual and auditory stimuli, more suboptimal reflexes, and higher abnormal muscle tone. No interactions emerged between the 2 main factors (ICC and IPM). Furthermore, no significant interactions emerged between VON-RA and 2 main factors suggesting that the differences on NNNS scales among the levels of care quality did not vary as a function of the neonatal clinical conditions. The η2p effect sizes, in general, were small (range, 1%–5.9%), with the exception of the stress/abstinence scale, where the ICC effect was of medium size (range, 6.0–13.9%)
TABLE 4.
Descriptive Statistics of the NNNS Scales Across the NICUs Stratified for Quality Care Level and ANOVAs Results
| NNNS Scales | ICC Index | IPM Index | Main Effects | Covariatea | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (NICU = 12) | High (NICU = 13) | Low (NICU = 14) | High (NICU = 11) | ICC | IPM | VON-RA index | ||||||||||||
| Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | F | η2p | F | η2p | B | η2p | |
| Habituation | 7.51 | 1.63 | 62 | 7.33 | 1.83 | 52 | 7.69 | 1.44 | 54 | 7.20 | 1.92 | 60 | 0.45 | 0.00 | 3.05 | 0.03 | 2.61 | 0.03 |
| Attention | 5.59 | 1.20 | 92 | 5.97 | 1.41 | 76 | 5.46 | 1.25 | 86 | 6.08 | 1.31 | 82 | 4.42b | 0.03 | 10.44d | 0.06 | −2.00b | 0.03 |
| Arousal | 3.89 | 0.66 | 95 | 3.74 | 0.55 | 76 | 3.73 | 0.64 | 88 | 3.92 | 0.58 | 83 | 3.20 | 0.02 | 4.41b | 0.03 | 0.02 | 0.00 |
| Regulation | 5.65 | 0.84 | 94 | 5.96 | 0.66 | 76 | 5.76 | 0.90 | 87 | 5.82 | 0.62 | 83 | 7.06c | 0.04 | 0.03 | 0.00 | −0.56 | 0.01 |
| Handling | 0.47 | 0.26 | 90 | 0.44 | 0.23 | 74 | 0.46 | 0.26 | 83 | 0.45 | 0.24 | 81 | 1.18 | 0.01 | 0.03 | 0.00 | 0.16 | 0.01 |
| Quality of movement | 4.52 | 0.58 | 93 | 4.64 | 0.73 | 74 | 4.62 | 0.66 | 86 | 4.54 | 0.65 | 81 | 1.63 | 0.01 | 1.14 | 0.01 | −0.17 | 0.00 |
| Excitability | 2.51 | 1.79 | 96 | 1.88 | 1.59 | 77 | 2.11 | 1.76 | 89 | 2.36 | 1.70 | 84 | 6.23b | 0.04 | 1.52 | 0.01 | 0.21 | 0.00 |
| Lethargy | 3.73 | 2.28 | 97 | 3.60 | 1.98 | 81 | 4.00 | 2.39 | 91 | 3.33 | 1.80 | 87 | 0.18 | 0.00 | 4.24b | 0.02 | 0.95 | 0.00 |
| Nonoptimal reflexes | 5.05 | 2.48 | 97 | 4.35 | 2.59 | 81 | 5.20 | 2.72 | 91 | 4.24 | 2.27 | 87 | 3.21 | 0.02 | 6.37c | 0.04 | 3.30b | 0.02 |
| Asymmetric reflexes | 1.21 | 1.71 | 97 | 0.86 | 1.56 | 81 | 1.04 | 1.79 | 91 | 1.06 | 1.50 | 87 | 2.92 | 0.02 | 0.03 | 0.00 | 2.42b | 0.03 |
| Hypertonicity | 0.03 | 0.18 | 95 | 0.01 | 0.11 | 77 | 0.05 | 0.21 | 88 | 0.00 | 0.00 | 84 | 0.49 | 0.00 | 3.35 | 0.02 | 0.06 | 0.00 |
| Hypotonicity | 0.18 | 0.56 | 95 | 0.06 | 0.25 | 77 | 0.10 | 0.48 | 88 | 0.15 | 0.42 | 84 | 5.04b | 0.03 | 0.79 | 0.00 | 0.82c | 0.04 |
| Stress/abstinence | 0.18 | 0.07 | 96 | 0.13 | 0.08 | 77 | 0.16 | 0.08 | 89 | 0.16 | 0.08 | 84 | 21.31d | 0.11 | 0.30 | 0.00 | −0.02 | 0.00 |
For covariate analysis, values are reported for the regression coefficients (B) that allow an easier data interpretation of effect direction.
P ≤ .05.
P ≤ .01.
P ≤ .001.
Neurobehavior Related to the Different Combinations of ICC and IPM
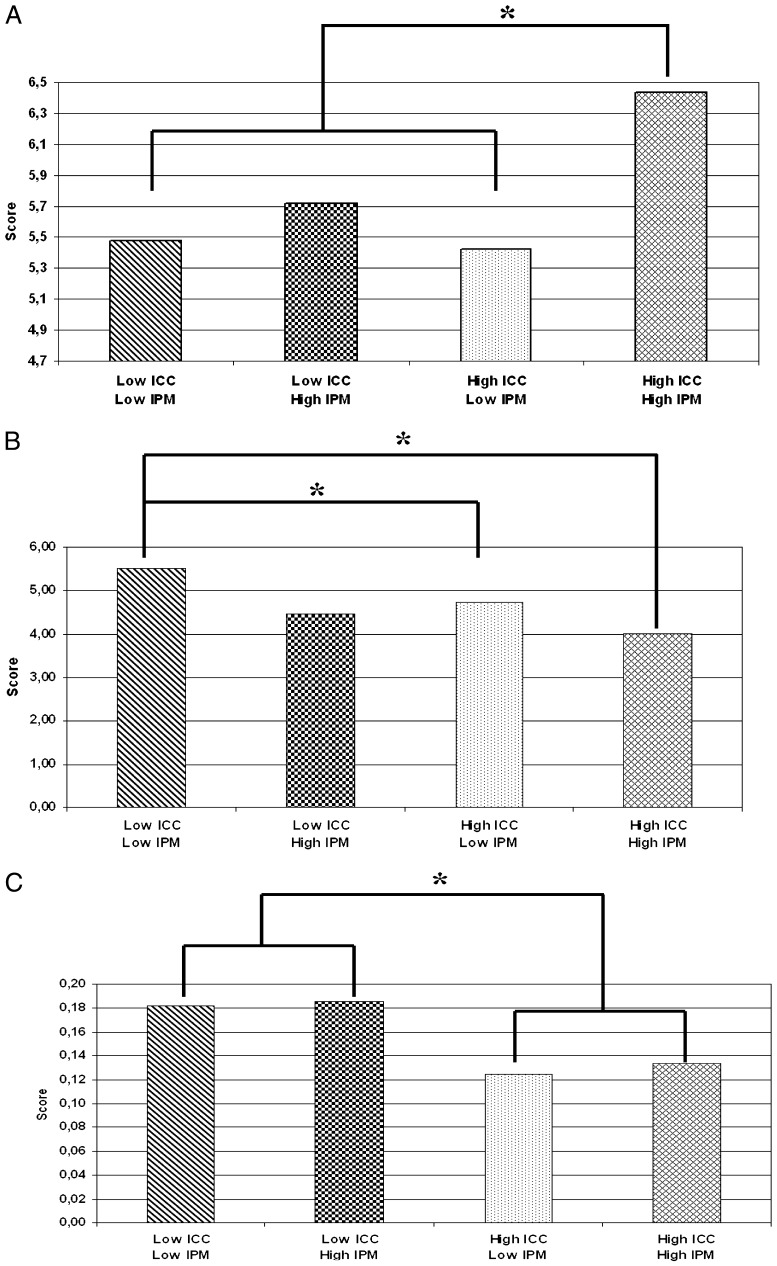
Given these findings, we asked if different combinations of levels of ICC and IPM might be related to a preterm infant’s neurobehavioral performance. We expected, for example, that low scores on both the ICC and IPM would be associated with poorer neurobehavioral performance in comparison with infants from high ICC- and IPM-scoring NICUs, and that NICUs with 1 high and 1 low score would not differ from each other but might differ from the high-high and low-low NICUs. To analyze what may be thought of as cumulative effects of ICC and IPM, we defined 4 groups of NICUs representing combinations of high and low ICC index and high and low IMP index: low ICC-low IPM (NICU = 7; 54 infants); low ICC-high IPM (NICU = 5; 43 infants) or high ICC-low IPM (NICU = 7; 37 infants); and high ICC-high IPM (NICU = 6; 44 infants). Univariate ANOVAs were applied to 13 NNNS scales with the 4 groups of NICUs as between-subjects variables.† Significant effects were found for 3 scales of the NNNS: attention, F(3, 164) = 5.61, P = .000, η2p = 0.09; nonoptimal reflexes, F(3, 174) = 3.11, P = .03, η2p = 0.05; and stress/abstinence, F(3, 169) = 7.63, P = .000, η2p = 0.12. The Bonferroni post hoc test showed that preterm infants from high ICC-high IPM NICUs exhibited a significantly higher attention score than infants from all other types of units. Preterm infants from low ICC-low IPM NICUs exhibited more nonoptimal reflexes than those from high ICC-low IPM and high ICC-high IPM NICUs. Preterm infants from low-quality-of-care NICUs for ICC, both with low and high IPM, had higher scores on stress/abstinence than infants from high-quality-of-care NICUs for ICC, both with low and high IPM (see Fig 1). The size effects were small to moderate.
FIGURE 1.
Mean of the scores for attention (A), nonoptimal reflexes (B), and stress/abstinence (C) and their relation to the different combinations of ICC and IPM. * P ≤ .05.
Discussion
The main objective of this study was to investigate whether the level of quality of developmental care found in a large number of NICUs was associated with neurobehavioral performance of very preterm infants. The overall quality of developmental care was measured by 2 indices of a broad range of care practices “routinely” used in NICUs. The findings indicate that, after controlling for neonatal clinical conditions, the ICC and IPM have specific relations to neurobehavioral performance. Regardless of the level of pain management, a low level of ICC was associated with decreased capacity of regulation and increased signs of stress, less attention, higher excitability, and abnormal muscle tone, indicating that some infant-centered developmental care interventions were critical to moderating infant distress and scaffolding their infants’ regulatory and motor capacities. By contrast, regardless of the level of ICC, a low level of IPM was associated with less attention and arousal, more lethargy, and more suboptimal reflexes, suggesting that the pain control is crucial to ameliorating some aspects of the neurologic immaturity. Interestingly, the NICU features and infants characteristics were homogeneously distributed across units, with the exception of the VON-RA clinical status score. In particular, infants from high ICC NICUs had higher VON-RA scores, indicating more challenging clinical conditions. It should be noted that, regardless of the level of quality of care, the neonatal clinical conditions affected the infants’ performance (primarily, neurologic items). Nevertheless, the lack of significant interactions between the VON-RA scores and the ICC index make it unlikely that the differences in neurobehavioral profile between infants from high ICC NICUs and those from units with low ICC can be explained by the neonatal clinical conditions. Furthermore, given that there were no differences in the time of exposure to the developmental care (ie, the length of hospitalization and the chronological age at time of assessment), it may be that the high score of VON-RA for infants from high ICC NICUs is a spurious finding.
In NICUs with a higher ICC score, infants had better attention and self-regulation, were less excitable, had less hypotonia, and were less stressed, suggesting that these infants had greater physiologic and behavioral stability. A higher score of the ICC index identifies several practices used by a NICU, including parents being allowed to spend the night in the unit whenever they chose, even when an infant’s condition is critical; parents being allowed to practice kangaroo care as a routine procedure as well as being encouraged to hold their infants, both of which they do more of the time than in NICUs with a low ICC index; and, in addition, the high ICC index NICUs have implemented more nursing interventions to decrease infant energy expenditure and promote stability. Previous research has documented that infants of parents involved in the care of their infant and preterm infants who received kangaroo care showed improvements in neurobehavioral functioning.20–22 Furthermore, positioning and containing (eg, use of blanket rolls) have positive effects by reducing physiologic distress and increasing motor organization and self-regulatory ability.23,24 The ICC is a global index, and it is not possible to identify which specific practices are related to the neurobehavioral performance of infants. Nonetheless, taken together, the findings from the ICC corroborate previous findings suggesting that a greater use of developmental care practices lead to better neurobehavioral stability and neuromaturation.8,25
The findings also indicate that IPM is related to the neurobehavioral performance of preterm infants. The IPM index denotes the number of pharmacologic and nonpharmacologic treatments used to alleviate pain associated with procedures, the presence and use of a protocol for measuring infant pain, and guidelines for preventing or treating neonatal pain. In the NICUs with a lower IPM score, infants had reduced levels of attention and arousal, were more lethargic, and had more nonoptimal reflexes. These findings are consistent with previous research. They suggest that invasive procedures affect preterm infants’ arousal levels and likely induce physiologic and behavioral changes, energy expenditure, and instability.26 Moreover, invasive procedures expose preterm infants to numerous nociceptive events, and several lines of evidence suggest that repeated and prolonged pain exposure has detrimental consequences.27–29 Although we did not directly assess infant pain reactivity to invasive procedures (eg, observing facial expressions), our findings suggest that, during the neonatal period, less protection from repetitive painful experiences alters neurobehavioral functioning. These findings have important implications for the control of pain during the infants’ NICU experience, because the effects of early exposure to negative environments may be at least partially ameliorated by minimizing of the number of painful procedures performed and by using treatments that can alleviate procedural pain in neonates.30 Thus, in agreement with others, these findings suggest it is important to prevent and minimize the number and intensity of painful events for preterm infants,31 and they support the use of evidence-based guidelines for preventing or treating neonatal pain and its adverse consequences.32
The findings highlight that ICC and IPM can act together in relation to preterm infants’ neurobehavioral functioning. Better attentional performance was related to NICUs that scored higher on both indices. Fewer nonoptimal reflexes were seen in NICUs that scored high on ICC than in NICUs with low scores in both indexes. Preterm infants from low -quality-of-care NICUs for ICC, both with low and high IPM, had more signs of stress than preterm infants from NICUs with high ICC scores, both with low and high IPM. It seems likely that different aspects of quality of care may contribute to specific adverse neurobehavioral performance both separately and cumulatively.
Although the primary goal of this study was not to develop or evaluate the QCC, the findings support its usefulness. The factor analysis revealed the robustness of the instrument. The range of scores on the ICC and IPM suggest that QCC is sensitive to differences in the extent of developmental quality of care even in a group of NICUs that ascribe to using similar levels of care. Although additional research is needed, the relation of the 2 indexes to neurobehavioral performance provides evidence to support the sensitivity and specificity of the QCC and its usefulness in discriminating differences in quality of developmental care level among NICUs. The QCC appears to be useful for evaluating how different levels of developmental care relate to infant outcomes (eg, motor development, stress reactivity). The QCC would also be useful not only for tracking changes, but also for maintaining and improving the quality of care within a NICU over time.
The study has limitations. The 25 NICUs sampled self-selected themselves into the NEO-ACQUA study and cannot be considered representative of developmental care quality in the ∼120 Italian NICUs. This limitation applies to its application to NICUs in other countries. The indexes emerged from factor analyses of the information gathered on developmental care, but not all aspects of development care were examined, and other aspects of care may have influenced the infants’ performance. It also would be useful to evaluate the relations of the QCC to a broader array of clinical variables. Furthermore, because the indexes aggregated several aspects of care practices, it is not possible establish which particular aspects affected the infants’ functioning. However, with more experience in the use of the QCC, it would be possible to disaggregate the variables. The use of a self-administered questionnaire is an additional limitation, and it is not possible to rule out evaluation biases by those who filled out the QCC. The relation of the QCC to observed actual practices in a large number of units would be valuable, but difficult to carry out. Nonetheless, it is important to note that compilation of QCC was done independently from the NNNS assessment, which was administrated by research assistants blind to all other information gathered by QCC. Furthermore, because the care level of each NICU was established on a statistical basis, both the informant who filled out the QCC and research assistant who administered the NNNS were unaware of the care level assigned to their unit.
Conclusions
The view that developmental care and pain control have potential beneficial effects on infant neurodevelopment is generally accepted.7,20 However, although tremendous advances in the care offered to high-risk preterm infants have been made over the past decades, variability in the practice of developmental care remains a constant concern, and the effects of developmental care need further exploration. The findings reported here suggest that incorporating more developmental care practices and more pain control practices into a NICU’s conventional care may promote neuromaturation of preterm infants, including their capacity for regulation and resilience.33
Acknowledgments
Neonatal Adequate Care for Quality of Life (NEO-ACQUA) Study Group collaborators are as follows: Fabio Mosca, MD; Odoardo Picciolini, MD (NICU, Department of Maternal and Pediatric Sciences, University of Milan Fondazione IRCCS Ca’ Granda); Stefano Visentin, MD; Nadia Battajon, MD (Neonatology and NICU, Ca' Foncello Hospital); Maria Lucia Di Nunzio, MD; Fiorina Ramacciato, MD (NICU Cardarelli Hospital); Laura Barberis, MD; Emmanuele Mastretta, MD (Division of Neonatology and NICU, S. Anna Hospital); Rinaldo Zanini, MD (NICU, Manzoni Hospital); Giovanna Carli, MD; Michela Alfiero Bordigato, MD (NICU, Hospital of Camposampiero); Valeria Chiandotto, MD; Cristiana Boiti, MD (Department of Neonatology, University Hospital S. M. M.); Rosangela Litta, MD; Giovanna Minelli, MD (Division of Neonatology and NICU, Ospedali Riuniti); Marcello Napolitano, MD (NICU, Evangelic Hospital Villa Betania); Alessandro Arco, MD (NICU, University Hospital G. Martino); Palma Mammoliti, MD (NICU, Ospedale degli Infermi); Cinzia Fortini, MD (NICU, Pediatric University Hospital); Paolo Tagliabue, MD (Neonatology, San Gerardo Hospital); Lorenzo Quartulli, MD (NICU, Perrino Hospital); Giuliana Motta, MD (NICU, Niguarda Hospital Ca' Granda); Paola Introvini, MD (NICU, Buzzi Hospital); Rosetta Grigorio, MD (NICU Umberto I Hospital); Paola Mussini, MD (NICU, C. Poma Hospital); Giulia Pomero, MD (NICU, Santa Croce e Carle Hospital); Carlo Poggiani, MD (NICU, Istituti Ospitalieri); Ananda Bauchiero, MD (Department of Neonatology, S. Anna University Hospital).
We thank Massimo Agosti, Guido Calciolari, and Maria Caterina Cavallo of the NEO-ACQUA study Advisory Board. We thank Julie Hofherimer (University of North Carolina at Chapel Hill) and Barry Lester (Brown University Medical Center) for their expertise and support during the NNNS training. Thanks go to MediData Studi e Ricerche staff in Modena for the organizational, technical, and scientific support. We thank the staff of all of the 25 NICUs that participated in the survey. Finally, we thank the participating infants and their parents.
Glossary
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- ICC
infant-centered care
- IPM
infant pain management
- NEO-ACQUA study
Neonatal Adequate Care for Quality of Life study
- NIDCAP
Neonatal Individualized Developmental Care and Assessment Program
- NNNS
NICU Network Neurobehavioral Scale
- QCC
quality-of care checklist
- SES
socioeconomic status
- VON-RA
Vermont Oxford Network Risk Adjustment
Footnotes
Dr Montirosso was one of the principal investigator and responsible for study design, data collection, analysis, and interpretation of data, and manuscript preparation; Dr Del Prete, one of the principal investigators, made substantial contributions to conception and design, provided supervision with all aspects of the research including study design, and data collection, performed examinations on infants hospitalized in NICU, made substantial contributions to interpretation of results, and critically revised the manuscript, final approval of the version to be published; Dr Bellù was one of the principal investigators, made substantial contributions to study design, data analysis, and interpretation of results, and critically revised the manuscript, and gave final approval of the version to be published; Dr Tronick, one of the principal investigators, provided assistance with the manuscript preparation, including review and critique of each version of the manuscript, and critically revised the manuscript; and Dr Borgatti was one of the principal investigators, made substantial contributions to study design, provided assistance with this manuscript preparation, obtained funding, and gave final approval of the version to be published. NEO-ACQUA Study Group: Dr Mosca made substantial contributions to acquisition of data, critically revised the manuscript, and gave final approval of the version to be published; Drs Picciolini, Visentin, Battajon, Di Nunzio, Ramacciato, Barberis, and Mastretta made substantial contributions to acquisition of data, performed examinations on infants hospitalized in NICU, critically revised the manuscript, and gave final approval of the version to be published; Dr Zanini made substantial contributions to conception and design, obtained funding, critically revised the manuscript, and gave final approval of the version to be published; Drs Carli, Bordigato, Chiandotto, Boiti, Litta, Minelli Napolitano, Arco, Mammoliti, Fortini, Tagliabue, Quartulli, Motta, Introvini, Grigorio, Mussini, Pomero, Poggiani, and Bauchiero made substantial contributions to acquisition of data, performed examinations on infants hospitalized in NICU, critically revised the manuscript, and gave final approval of the version to be published.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The NEO-ACQUA project was supported by an unrestricted educational grant from Chiesi Farmaceutici S.p.A. Dr Tronick was supported by a grant from National Institute Child Health & Human Development (R01HD37138; ET, PI) Standardization of the NRN-Neurobehavioral Scale.
COMPANION PAPER: A companion to this article can be found on page e1322, online at www.pediatrics.org/cgi/doi/10.1542/peds.2012-0511.
The third principal factor, labeled "nursing staffing,” which accounted for 12% of the variance, included items such as number of physicians per bed, number of graduate students, fellows, or consultants per bed, nurse chiefs per bed, nurses per bed, and nursery nurse, assistants per bed.
Given the lack of interaction between the covariate and the main factors (ICC and IPM), the VON-RA scores did not enter into this analysis.
References
- 1.Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108(6):1339–1348 [DOI] [PubMed] [Google Scholar]
- 2.Aucott S, Donohue PK, Atkins E, Allen MC. Neurodevelopmental care in the NICU. Ment Retard Dev Disabil Res Rev. 2002;8(4):298–308 [DOI] [PubMed] [Google Scholar]
- 3.Newnham CA, Inder TE, Milgrom J. Measuring preterm cumulative stressors within the NICU: the Neonatal Infant Stressor Scale. Early Hum Dev. 2009;85(9):549–555 [DOI] [PubMed] [Google Scholar]
- 4.Byers JF. Components of developmental care and the evidence for their use in the NICU. MCN Am J Matern Child Nurs. 2003;28(3):174–180, quiz 181–182 [DOI] [PubMed] [Google Scholar]
- 5.Slevin M, Farrington N, Duffy G, Daly L, Murphy JF. Altering the NICU and measuring infants’ responses. Acta Paediatr. 2000;89(5):577–581 [DOI] [PubMed] [Google Scholar]
- 6.Als H, Duffy FH, McAnulty GB, et al. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–857 [DOI] [PubMed] [Google Scholar]
- 7.Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate. 2000;77(2):69–82 [DOI] [PubMed] [Google Scholar]
- 8.Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database Syst Rev. 2006;(2):CD001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westrup B, Kleberg A, von Eichwald K, Stjernqvist K, Lagercrantz H. A randomized, controlled trial to evaluate the effects of the newborn individualized developmental care and assessment program in a Swedish setting. Pediatrics. 2000;105(1 pt 1):66–72 [DOI] [PubMed] [Google Scholar]
- 10.Wielenga JM, Smit BJ, Merkus MP, Wolf MJ, van Sonderen L, Kok JH. Development and growth in very preterm infants in relation to NIDCAP in a Dutch NICU: two years of follow-up. Acta Paediatr. 2009;98(2):291–297 [DOI] [PubMed] [Google Scholar]
- 11.Stevens B, Petryshen P, Hawkins J, Smith B, Taylor P. Developmental versus conventional care: a comparison of clinical outcomes for very low birth weight infants. Can J Nurs Res. 1996;28(4):97–113 [PubMed] [Google Scholar]
- 12.Ashpaugh A, Leick-Rude MK, Kilbride HW. Developmental care teams in the neonatal intensive care unit: Survey on current status. Am J Perinatol. 1999;19(1):48–52 [DOI] [PubMed] [Google Scholar]
- 13.Als H. Developmental care in the newborn intensive care unit. Curr Opin Pediatr. 1998;10(2):138–142 [DOI] [PubMed] [Google Scholar]
- 14.Coughlin M, Gibbins S, Hoath S. Core measures for developmentally supportive care in neonatal intensive care units: theory, precedence and practice. J Adv Nurs. 2009;65(10):2239–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parazzini F, Cortinovis I, Bortolus R, Fedele L. Standards of birth weight in Italy [in Italian]. Ann Ostet Ginecol Med Perinat. 1991;112(4):203–246 [PubMed] [Google Scholar]
- 16.Zupancic JA, Richardson DK, Horbar JD, Carpenter JH, Lee SK, Escobar GJ, Vermont Oxford Network SNAP Pilot Project Participants . Revalidation of the score for neonatal acute physiology in the Vermont Oxford Network. Pediatrics. 2007;119(1) Available at: www.pediatrics.org/cgi/content/full/119/1/e156 [DOI] [PubMed] [Google Scholar]
- 17.Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;113(3 pt 2):641–667 [PubMed] [Google Scholar]
- 18.Lester BM, Tronick EZ, La Gasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Lu J. Summary statistics of neonatal intensive care unit network neurobehavioral scale scores from the maternal lifestyle study: A quasinormative sample. Pediatrics. 2004;113(3 pt 2):668–675 [PubMed] [Google Scholar]
- 19.Greisen G, Mirante N, Haumont D, et al. ESF Network . Parents, siblings and grandparents in the Neonatal Intensive Care Unit. A survey of policies in eight European countries. Acta Paediatr. 2009;98(11):1744–1750 [DOI] [PubMed] [Google Scholar]
- 20.Als H, Gilkerson L. The role of relationship-based developmentally supportive newborn intensive care in strengthening outcome of preterm infants. Semin Perinatol. 1997;21(3):178–189 [DOI] [PubMed] [Google Scholar]
- 21.Feldman R, Eidelman AI. Skin-to-skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev Med Child Neurol. 2003;45(4):274–281 [DOI] [PubMed] [Google Scholar]
- 22.Ohgi S, Fukuda M, Moriuchi H, et al. Comparison of kangaroo care and standard care: behavioral ganization, development, and temperament in healthy, low-birth-weight infants through 1 year. J Perinatol. 2002;22(5):374–379 [DOI] [PubMed] [Google Scholar]
- 23.Grenier IR, Bigsby R, Vergara ER, Lester BM. Comparison of motor self-regulatory and stress behaviors of preterm infants across body positions. Am J Occup Ther. 2003;57(3):289–297 [DOI] [PubMed] [Google Scholar]
- 24.Short MA, Brooks-Brunn JA, Reeves DS, Yeager J, Thorpe JA. The effects of swaddling versus standard positioning on neuromuscular development in very low birth weight infants. Neonatal Netw. 1996;15(4):25–31 [PubMed] [Google Scholar]
- 25.Feldman R, Eidelman AI, Sirota L, Weller A. Comparison of skin-to-skin (kangaroo) and traditional care: parenting outcomes and preterm infant development. Pediatrics. 2002;110(1 pt 1):16–26 [DOI] [PubMed] [Google Scholar]
- 26.Grunau RE. Self-regulation and behavior in preterm children: Effects of early pain. In: McGrath PJ, Finley GA, eds. Pediatric Pain: Biological and Social Context, Progress in Pain Research and Management. Vol 26 Seattle, WA: IASP Press; 2003 [Google Scholar]
- 27.Grunau RE. Self-regulation and behavior in preterm children: effects of early pain. In: McGrath PJ, Finley GA, eds. Pediatric Pain: Biological and Social Context, Progress in Pain Research and Management. Seattle, WA:IASP Press; 2003;26:23–55 [Google Scholar]
- 28.Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Lee SK. Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 weeks’ postconceptional age. Pediatrics. 2001;107(1):105–112 [DOI] [PubMed] [Google Scholar]
- 29.Johnston CC, Stevens BJ. Experience in a neonatal intensive care unit affects pain response. Pediatrics. 1996;98(5):925–930 [PubMed] [Google Scholar]
- 30.Axelin A, Salanterä S, Kirjavainen J, Lehtonen L. Oral glucose and parental holding preferable to opioid in pain management in preterm infants. Clin J Pain. 2009;25(2):138–145 [DOI] [PubMed] [Google Scholar]
- 31.Anand KJS, International Evidence-Based Group for Neonatal Pain . Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155(2):173–180 [DOI] [PubMed] [Google Scholar]
- 32.Batton DG, Barrington KJ, Wallman C, American Academy of Pediatrics Committee on Fetus and Newborn; American Academy of Pediatrics Section on Surgery; Canadian Paediatric Society Fetus and Newborn Committee . Prevention and management of pain in the neonate: an update. Pediatrics. 2006;118(5):2231–2241 [DOI] [PubMed] [Google Scholar]
- 33.DiCorcia JA, Tronick E. Quotidian resilience: exploring mechanisms that drive resilience from a perspective of everyday stress and coping. Neurosci Biobehav Rev. 2011;35(7):1593–1602 [DOI] [PubMed] [Google Scholar]