Abstract
BACKGROUND AND OBJECTIVE:
Controversy remains concerning the optimal treatment approach for cryptorchidism. The objective of this study was to assess effectiveness of hormone therapy or surgery for cryptorchidism.
METHODS:
We searched Medline and other databases from 1980 to February 2012. Two reviewers independently assessed studies against predetermined criteria. Two reviewers independently extracted data and assigned overall quality and strength of evidence ratings using predetermined criteria.
RESULTS:
Fourteen studies addressed effectiveness of hormonal treatments, and 26 studies addressed surgical intervention outcomes. Hormonal treatment is associated with testicular descent in some children, but rates generally do not exceed those seen with placebo by >10%. Surgical treatment is associated with success rates of testicular descent ranging from 33% to 100%, depending on surgery. Weighted success averages were 78.7% for 1-stage Fowler-Stephens (FS), 86% for 2-stage FS, and 96.4% for primary orchiopexy. Descent rates were similar among studies comparing laparoscopic and open surgeries. Reported harms of hormonal treatments were mild and transient. Adverse effects specifically associated with surgical repair were rare.
CONCLUSIONS:
The body of the reviewed literature comprises primarily fair- and poor-quality studies, limiting our ability to draw definitive conclusions. Hormonal treatment is marginally effective relative to placebo but is successful in some children and with minimal harms, suggesting that it may be an appropriate trial of care for some patients. Surgical options are effective, with high rates of testicular descent (moderate strength of evidence for FS procedures, high for primary orchiopexy). Comparable outcomes occur with laparoscopic and open approaches.
Keywords: cryptorchidism, undescended testicle, systematic review
Cryptorchidism is a congenital condition in which 1 or both testicles are not appropriately positioned in the scrotum at birth and cannot be moved into the proper position manually. It affects an estimated 3% of full-term male neonates and up to 30% of premature infants, making it the most common male genital anomaly identified at birth.1,2 Although about 70% of cryptorchid testicles spontaneously descend within the first year of life (most occurring in the first 3 months), the number of boys whose condition persists remains constant at ∼1%.1,2 Longer-term consequences of cryptorchidism can include testicular malignancy and infertility/subfertility. Once cryptorchidism is diagnosed, treatment choices may include watchful waiting, hormonal treatment, or surgery.
In clinical practice, the choice of initial therapy is often selected on the basis of age at presentation and the location of the cryptorchid testicle.3,4 Watchful waiting may be used in boys <1 year of age with lower-lying testis in whom spontaneous descent is still a realistic possibility. Hormonal and surgical options are primarily selected on the basis of location and appearance of the undescended testicle. Hormonal treatment with luteinizing hormone releasing hormone (LHRH) analogs and/or human chorionic gonadotropin (hCG) could theoretically increase circulating androgens that may, in turn, promote testicular descent.
Surgical options include various forms of orchiopexy or orchiectomy. Primary orchiopexy (surgical mobilization of the testicle with placement and fixation in the scrotum) is usually performed for palpable cryptorchid testicles that are of relatively normal size and appearance that are located in the inguinal canal.3 In cases in which the testicle is found to be atrophic with little or no viable germ cell tissue remaining, orchiectomy may be performed. For nonpalpable testicles located just inside the internal inguinal ring or in the abdomen, surgical management is more complicated and is dependent on location in the abdomen and the length of the gonadal vessels. If the testicle is of normal size and appearance and if the vessels are of adequate length, primary orchiopexy is usually performed.3,4 If the vessels are so short as to prohibit tension-free placement of the testicle in the scrotum, a Fowler-Stephens (FS) orchiopexy is performed. This procedure entails ligating the testicular vessels. The testicular blood supply then depends on collateral circulation from the deferential artery and the cremasteric system.3
This procedure can be performed as a single-stage operation, in which the vessels are ligated and the testicle is then placed into the proper position in the scrotum, or as a 2-stage procedure. In a 2-stage procedure, the vessels are ligated in the first operation, the testicle is allowed to develop presumably better collateral circulation in its abdominal position and is then moved to the proper position in the scrotum during a second procedure, usually 3 to 6 months later. Both primary orchiopexy and the FS procedure can be performed using laparoscopic or open surgical technique.
The immediate goal of most interventions for cryptorchidism is to reposition the undescended gonad in a “normal” position in the scrotum. Intermediate outcomes include psychological benefits in terms of body image, and long-term goals include preservation of fertility and prevention of testicular malignancy
As part of a larger systematic review of evaluation methods and treatments for cryptorchidism funded by the Agency for Healthcare Research and Quality,5 we assessed the effectiveness of hormone therapy and surgical approaches for treating cryptorchidism on outcomes including testicular descent, function of testicles, further surgical intervention, infertility/subfertility, and development of testicular malignancy. We present these findings here; the full report and review protocol are available from the Agency for Healthcare Research and Quality Effective Health Care Web site (http://effectivehealthcare.ahrq.gov).
Methods
Search Strategy
We searched Medline via the PubMed interface, the Cumulative Index to Nursing and Allied Health Literature, and Embase from 1980 to February 2012 by using controlled vocabulary terms and key terms related to cryptorchidism. We also hand-searched the reference lists of all included studies and of recent reviews related to cryptorchidism treatment to identify additional references.
Study Selection
Study inclusion and exclusion criteria were developed in conjunction with an expert panel of clinicians and researchers involved in treating cryptorchidism. Studies were limited to those whose participants were prepubescent boys with cryptorchidism. Studies that evaluated hormonal or surgical treatments had to include at least 1 comparison group and provide data on the position of the testicles after treatment. Two investigators independently reviewed each study against the inclusion criteria (Table 1) with disagreements resolved through adjudication by a senior investigator.
TABLE 1.
Study Inclusion Criteria: Population, Intervention, Comparator, Outcomes, Timing, Setting
| Category | Criteria |
|---|---|
| Study population | Prepubescent males presenting with cryptorchidism or suspected cryptorchidism |
| Interventions | Hormones, including hCG or gonadotropin-releasing hormone, surgical therapy, and specific surgical techniques (ie, 1-stage versus 2-stage orchiopexy, laparoscopic versus open approach) |
| Comparators | Nontreatment, later treatment, hormones, and different surgical techniques |
| Outcomes | • Immediate (within 6 wk of therapy) and short-term (6 wk to 2 y of therapy) outcomes: |
| o Testicular size and appearance | |
| o Testicular position | |
| o Pain | |
| o Parent/patient satisfaction | |
| o Need for further surgical intervention | |
| o Emotional/psychosocial response | |
| o Adverse effects, including but not limited to pain, infection, hematoma, and edema | |
| • Long-term (>2 y after therapy) outcomes: | |
| o Testicular size and appearance | |
| o Testicular position | |
| o Endocrine function | |
| o Body image | |
| o Parent/patient satisfaction | |
| o Infertility/subfertility | |
| o Torsion | |
| o Testicular malignancy and cancer | |
| o Hernia | |
| o Emotional/psychosocial response | |
| Timing | Time frame for reporting of outcomes was not restricted. |
| Setting | All settings were considered, including hospitals and university or academic medical centers. |
hCG, human chorionic gonadotropin.
Data Extraction
Investigators extracted data using a standardized form. Data were verified by a second investigator. We collected data on study design, study population characteristics, baseline and follow-up data on testicular position and other outcomes as available, and harms.
Study Quality Assessment
Two investigators independently assessed each study using the Cochrane Risk of Bias tool6 for randomized controlled trials (RCTs) and the Newcastle-Ottawa Quality Assessment Scale7 for cohort studies. Results were adjudicated when necessary. The domains used to assess quality for RCTs included sequence generation, allocation concealment, blinding, completeness of outcome data, and selective reporting bias. For the cohort studies, the criteria included selection of study groups, comparability of study groups, and ascertainment of exposure or outcome of interest. The scores for each study were converted into a rating of “good,” “fair,” or “poor” quality. Conversion details are provided in the full report.
The strength of evidence (SOE) reflects an assessment of the overall body of literature, and specifically reflects our confidence that the observed effect is close to the actual effect and unlikely to change with further research. We assessed SOE for the primary outcomes of treatment based on 4 major domains, including risk of bias (low, medium, or high), consistency of findings (inconsistency not present or present or unknown), directness (whether the outcome measured was the direct health outcome of interest), and precision (precise or imprecise).8 The overall SOE was graded “high,” indicating high confidence that evidence reflects true effect; “moderate,” indicating moderate confidence that evidence reflects the true effect and further research may change our confidence in the estimate of effect and may change the estimate; “low,” indicating low confidence that the evidence reflects the true effect and further research is likely to change our confidence in the estimate of effect and is likely to change the estimate; or “insufficient,” indicating that evidence is either unavailable or does not permit estimation of an effect.
Data Synthesis
Data on the hormonal treatments were analyzed qualitatively with the development of evidence tables. For the surgical interventions, data on proportions achieving testicular descent were pooled and the weighted proportions (sum of all successful testicles/total number of testicles in studies) were calculated for each treatment type. Similarly, weighted testicular atrophy rates were derived for each of the surgical techniques.
Results
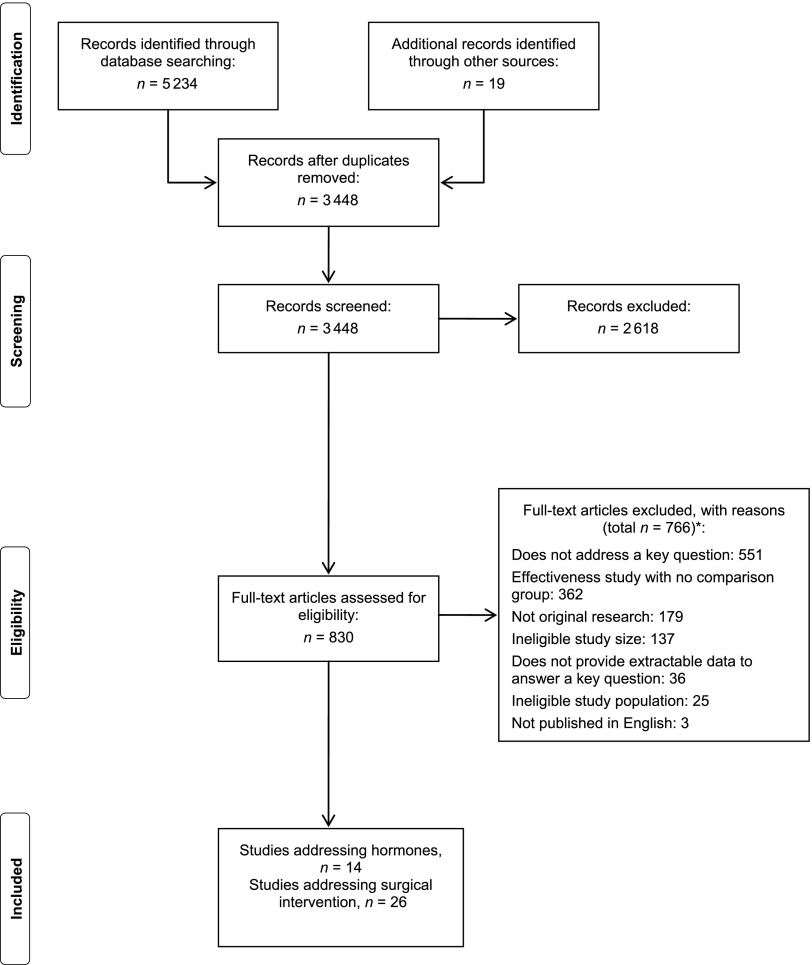
Figure 1 outlines the flow of studies identified for the review. We identified 3448 unique abstracts. Of these, 14 studies met our inclusion/exclusion criteria and addressed the effectiveness of hormonal treatments, and 26 addressed outcomes of surgical interventions. Information on modifiers of hormonal and surgical treatments was available in 23 studies, and 11 studies included data on harms.
FIGURE 1.
Disposition of studies identified. *Articles may be excluded for multiple reasons.
Effectiveness of Hormones for Achieving Testicular Descent
Fourteen studies in 19 publications assessed the effectiveness of hormonal therapy as a treatment of cryptorchidism. Individual studies often included multiple arms. Six studies compared LHRH with placebo, 1 compared hCG with placebo, 4 compared LHRH with hCG, and 6 compared various doses or regimens of the same agent. Of the 14 studies, 11 were RCTs,9–22 2 were prospective cohort studies,23,24 and 1 was a retrospective cohort study.25,26 Three studies were of good quality,17,23,25,26 2 were of fair quality,14,15 and 9 were of poor quality.9–13,16,18–22,24
Six studies9–16 specifically compared successful testicular descent rates after administration of LHRH versus placebo (2 fair quality14,15 and 4 poor quality9–13,16). Five of 6 studies concluded that LHRH was more effective than placebo in inducing testicular descent with variable reported effect sizes across studies, whereas 1 study was equivocal (see Table 2).12–16 In the 5 studies that appeared to show a benefit to LHRH therapy, 4 did not assess statistical significance at all, whereas 1 failed to document statistical significance likely because of inadequate sample size. No harms of hormonal treatment were reported.
TABLE 2.
Short-Term Testicular Descent in Randomized, Placebo-Controlled Studies of LHRH
| Study n Length of Follow-up Quality | LHRH Dose | LHRH Frequency | LHRH Duration | LHRH Descent, % | Placebo Descent, % |
|---|---|---|---|---|---|
| Statistical Significance | |||||
| Olsen et al 199215 | 400 µg | 3 times daily | 4 wk | 9.7 | 1.6 |
| n = 123 | |||||
| 4 wk | |||||
| Fair | |||||
| P = .12 (95% CI: 0.1%–16.6%) | |||||
| Christiansen et al 198814 | 200 µg | 3 times daily | 4 wk | 9 (bilateral cryptorchidism) | 0 (bilateral cryptorchidism) |
| n = 220 | 0 (unilateral cryptorchidism) | 0 (unilateral cryptorchidism) | |||
| 4 wk | |||||
| Fair | |||||
| NS | |||||
| De Muinck Keizer-Schrama and Hazebroek et al 1986–19879–11 | 200 µg | 3 times daily | 4 wk | 9.0 | 8.0 |
| n = 237 | |||||
| 8 wk | |||||
| Poor | |||||
| NR | |||||
| Hagberg and Westphal, 198212 | 100 µg | 3 times daily | 28 d | 62.0 | 3.0 |
| n = 50 | |||||
| 4 wk | |||||
| Poor | |||||
| NR | |||||
| Karpe et al 198313 | 100 µg | 6 times daily | 28 d | 20.0 | 12.0 |
| n = 50 | |||||
| 6 mo | |||||
| Poor | |||||
| NR | |||||
| Wit et al 198616 | 400 µg | 3 times daily | 28 d | 37 | 18 |
| n = 49 | |||||
| 8 wk | |||||
| Poor | |||||
| NR | |||||
CI, confidence interval; LHRH, luteinizing-hormone-releasing hormone; NR, not reported; NS, not significant.
One of the studies comparing LHRH to placebo also included a third hCG arm.14 As noted previously, results comparing LHRH to placebo were equivocal, with LHRH being more effective in achieving testicular descent than placebo in patients with bilateral cryptorchidism, but no better than placebo in patients with unilateral cryptorchidism. In this study, hCG was better than placebo at achieving testicular descent in both bilateral and unilateral patients, but the SOE was considered low (Table 3).
TABLE 3.
Strength of Evidence of Hormonal Treatments for Cryptorchidism
| No. of Studies; Total Subjects; Testes Treated | Risk of Bias | Consistency | Directness | Precision | Strength of Evidence and Magnitude of Effect |
|---|---|---|---|---|---|
| Testicular descent | |||||
| LHRH versus placebo 6; 752; 935 | RCTs/Moderate | Consistent | Direct | Imprecise | Moderate LHRH: 9%–62% |
| Placebo: 0%–18% | |||||
| hCG versus placebo 1; 243; 280 | RCT/Moderate | Unknown | Direct | Unknown | Low Bilateral: 23% vs 0% |
| Unilateral: 15% vs 0% | |||||
| LHRH versus hCG 3; 431; 465 | RCT/Low | Inconsistent | Direct | Imprecise | Low RCT: |
| LHRH: 0%–18.8% | |||||
| hCG: 5.9%–23.0% | |||||
| LHRH versus hCG 1; 324; 198 | Cohort/High | Consistent | Direct | Imprecise | Cohort: |
| LHRH: 29.4% | |||||
| hCG: 34.5% |
hCG human chorionic gonadotropin; LHRH Luteinizing-hormone-releasing hormone; RCT randomized controlled trial.
Four studies provided data on LHRH compared with hCG, with no clear indication of either being better than the other. The studies that compared doses and dosing schedules within hormone type were of poor quality and too heterogeneous to permit drawing useful conclusions.
We assessed the SOE for our primary outcome of testicular descent. There is moderate SOE for increased testicular descent with LHRH compared with placebo, low SOE for increased testicular descent with hCG compared with placebo, and low SOE for equivalence between LHRH and hCG.
No studies provided cancer or fertility outcomes for the comparisons listed, so the SOE is insufficient for these outcomes.
Effectiveness of Surgical Procedures
We identified 26 studies, including 5 RCTs and 1 prospective and 20 retrospective cohort studies, that evaluated surgical treatments.27–53 Four studies were judged good quality,27,45,49,52 1 fair quality,32 and the remainder poor quality.28–31,33–40,42–44,46–48,50,51,53
Eleven studies reported outcomes after either 1-stage FS orchiopexy, 2-stage FS orchiopexy, or primary orchiopexy.36–40,42–45,47,48 Nine of these studies, all retrospective cohorts, provided success rates by surgical procedure, although the choice of surgical method is made clinically and not with the intent of comparative effectiveness. Only 1 study controlled for starting testicle location.28
Surgical treatment of cryptorchidism was associated with success rates of testicular descent that ranged from 33% to 100% (Tables 4, 5, and 6), depending on type of surgery. Each surgical approach was assessed independently for ability to achieve testicular descent because, as described in the report, each approach is used under different clinical circumstances, and thus it is inappropriate to compare them with one another. No studies compared hormonal therapy alone to surgery. Only 1 study assessing testicular descent was rated as good quality.45 This study had a testicular descent rate of 63% for 1-stage FS, 67.6% for 2-stage FS, and 89.1% for primary orchiopexy, slightly lower in all types of surgery than the pooled estimate.
TABLE 4.
Success Rates After 1-Stage FS
| Author and Country | Quality | Total Participants | Total Testicles | % Success (n Testicles Treated) |
|---|---|---|---|---|
| Stec et al 200945 United States | Good | 136 | 156 | 63 (27) |
| Baker et al 200148 United States | Poor | 226 | 263 | 74.1 (27) |
| Chang et al 200144 United States | Poor | 80 | 92 | 84 (19) |
| Chang et al 200847 United States | Poor | 48 | 48 | 94.3 (35) |
| Comploj et al 201136 Austria | Poor | 41 | 50 | 79 (33) |
| Denes et al 200843 Brazil | Poor | 46 | 54 | 33 (3) |
| Kim et al 201038 South Koreaa | Poor | 67 | 86 | 82 (11) |
| Pooled % | Total: 644 | Total: 749 | 78.7 |
All studies were retrospective cohorts.
Controlled for location.
TABLE 5.
Success Rates After 2-Stage FS
| Author and Country | Quality | Total Participants | Total Testicles | % Success (n Testicles) |
|---|---|---|---|---|
| Stec et al 200945 United States | Good | 136 | 156 | 67.6 (37) |
| Baker et al 200148 United States | Poor | 226 | 263 | 87.9 (58) |
| Chang et al 200144 United States | Poor | 80 | 92 | 86 (7) |
| Chang et al 200847 United States | Poor | 48 | 48 | 80 (10) |
| Comploj et al 201136 Austria | Poor | 41 | 50 | 82 (17) |
| Denes et al 200843 Brazil | Poor | 46 | 54 | 88 (25) |
| Dhanani et al 200442 United States | Poor | 74 | 83 | 98 (49) |
| Kim et al 201038 South Koreaa | Poor | 67 | 86 | 67 (3) |
| Moursy et al 201137 Egypt | Poor | 66 | 76 | 88.8 (36) |
| Pooled % | Total: 784 | Total: 908 | 86.0 |
All studies were retrospective cohorts.
Controlled for location.
TABLE 6.
Success Rates After Primary Orchiopexy
| Author and Country | Quality | Total Participants | Total Testicles | % Success (n Testicles Treated) |
|---|---|---|---|---|
| Stec et al 200945 United States | Good | 136 | 156 | 89.1 (92) |
| Baker et al 200148 United States | Poor | 226 | 263 | 97.2 (178) |
| Chang et al 200144 United States | Poor | 80 | 92 | 100 (66) |
| Denes et al 200843 Brazil | Poor | 46 | 54 | 96 (26) |
| Dhanani et al 200442 United States | Poor | 74 | 83 | 100 (28) |
| Kim et al 201038 South Koreaa | Poor | 67 | 86 | 98 (49) |
| Moursy et al 201137 Egypt | Poor | 66 | 76 | 100 (28) |
| Pooled % | Total: 695 | Total: 810 | 96.4 |
All studies were retrospective cohorts.
Controlled for location.
The weighted success rate for all 3 approaches exceeds 75%. The overall success rate for 1-stage FS is 78.7% (Table 4). The overall success rate for 2-stage FS is 86% (Table 5). The overall success rate of primary orchiopexy is 96.4% (Table 6).
Atrophy rates were reported in 5 studies and pooled results were 1.83% for primary orchiopexy (range 0%–4%, 5 studies),37,39,40,43,48 28.1% for 1-stage FS (range 22%–67%, 3 studies),40,43,48 and 8.2% for 2-stage FS (range 0%–12%, 5 studies).37,39,40,43,48
We assessed the SOE as our confidence in the weighted average of successful testicular descent associated with each surgical approach separately (Table 7). Although retrospective studies typically had high risk of bias because of lack of a control group, in grading the overall SOE, we used an implicit comparator group given the known natural history of disease. Given the low rate of spontaneous testicular descent, despite the high risk of bias of retrospective studies, SOE might be considered high because of the high magnitude of effect when compared with an implicit control.
TABLE 7.
Strength of Evidence of Surgical Treatments for Cryptorchidism
| No. of Studies; Total Subjects; Treated Testicles | Risk of Bias | Consistency | Directness | Precision | Strength of Evidence and Magnitude of Effecta |
|---|---|---|---|---|---|
| Testicular descent | |||||
| 1-stage FS 7; 644; 155 | Retrospective cohorts/ High | Consistent | Direct | Imprecise | Moderate |
| 78.7% (range: 33%–94.3%) | |||||
| 2-stage FS 9; 784; 242 | Retrospective cohorts/ High | Consistent | Direct | Imprecise | Moderate |
| 86.0% (range: 67%–98%) | |||||
| Primary orchiopexy 7; 695; 467 | Retrospective cohorts/ High | Consistent | Direct | Precise | High |
| 96.4% (range: 89.1%–100%) | |||||
| Open versus laparoscopic repair 1; 75; 75 | RCT High | Unknown | Direct | Unknown | Low RCT: No difference in postoperative testicular position |
| Open versus laparoscopic repair 2; 96; 110 | Cohorts/High | Consistent | Direct | Imprecise | Cohorts: No difference in postoperative testicular position |
| Atrophy | |||||
| 1-stage FS 3; 320; 32 | Retrospective cohorts/ High | Consistent | Direct | Imprecise | Low |
| 28.1% (range: 22%–67%) | |||||
| 2-stage FS 5; 470; 158 | Retrospective cohorts/ High | Consistent | Direct | Precise | Low |
| 8.2% (range: 0%–12%) | |||||
| Primary orchiopexy 5; 470; 273 | Retrospective cohorts/ High | Consistent | Direct | Precise | Moderate |
| 1.83% (range: 0%–4%) | |||||
| Open versus laparoscopic repairb 1; 75; 75 | RCT High | Unknown | Direct | Unknown | Low Laparoscopy: 10% |
| Open: 19% |
Pooled proportion (range).
Atrophy rates for second-stage orchiopexy; no atrophy reported with primary orchiopexy.
For the outcome of testicular descent, SOE was moderate for 1- and 2-stage orchiopexy and high for primary orchiopexy. All studies were retrospective cohort studies, and thus had high risk of bias, but we deemed these to be an appropriate study design for the question of ability of orchiopexy to achieve testicular descent and considered the relative challenges of this design to be outweighed by the magnitude of effect. Primary orchiopexy had higher SOE than 1-stage and 2-stage procedures based on the higher number of testicles (outcomes) reported in the literature.
We also assessed SOE for the outcome of testicular atrophy, and on the same methodological basis as was used for testicular descent, found the SOE to be low for a 28.10% atrophy rate with 1-stage FS, low for an 8.20% atrophy rate with 2-stage FS, and moderate for a 1.83% atrophy rate for primary orchiopexy.
Effectiveness of Surgical Approach (Open Versus Laparoscopic)
Five studies compared an open versus laparoscopic approach for the same procedure (1 good,27 1 fair,32 and 3 poor quality28,50,51). Two studies noted success rates for laparoscopic surgeries similar to those of open surgeries.27,50 One fair-quality cohort study reported that participants undergoing a laparoscopic approach “had less pain when compared to the open technique in 80% of cases” using a visual analog scale, but how this comparison was made is unclear. Similarly, although the authors note that all patients had “satisfactory results in relation to size and location of testicle,” details regarding these outcomes are lacking.32 Another poor-quality cohort study that included both palpable and nonpalpable testicles, failed to control for the location of the testicle in the analysis, and grouped both primary and FS orchiopexies into two heterogeneous groups based on whether an open or laparoscopic approach was used, making it difficult to draw meaningful conclusions in terms of postoperative testicular position or viability.51
Finally, 1 poor-quality RCT compared outcomes after various types of laparoscopic or open orchiopexies for nonpalpable testicles.28 This study is one of the few reports in the literature that controlled for location of the testicle within the abdomen, allowing for comparisons between procedures and minimizing the possibility of confounding by indication. If a testicle was noted via laparoscopic evaluation to be high in the abdomen, the patient underwent a laparoscopic 1-stage FS procedure (laparoscopic clipping of the testicular vessels). Patients were then randomized to receive either open or laparoscopic 2-stage FS orchiopexy.
Perioperative outcomes between participants undergoing laparoscopic or open second procedures were compared, with patients undergoing laparoscopic 2-stage FS orchiopexy noted to have statistically significantly shorter operative times (P = .000), time to oral feeding (P = .004), hospital stays (P = .008), and return to normal activities (P = .000). Although all testicles in both groups were noted to have satisfactory scrotal position after surgery, 2 (10%) of the 20 testicles in the laparoscopic arm and 3 (19%) of the 16 testicles in the open arm had atrophied after 1 year of follow-up. Patients in this study who had viable testicles located in the lower portion of the abdomen (close to the inguinal ring) were randomized to undergo either laparoscopic or open primary orchiopexy. Like the high abdominal group, patients randomized to laparoscopic orchiopexy had statistically superior perioperative outcomes. Of the 21 testicles randomized to laparoscopic orchiopexy and the 18 randomized to open orchiopexy, all were satisfactorily placed in the scrotum and no cases of atrophic testicles were noted after 1 year of follow-up.
We assessed the SOE for equivalence of laparoscopic and open approaches for achieving testicular descent to be low with only 1 RCT28 of poor quality and 2 cohort studies of fair32 and poor51 quality, although the individual studies report that success rates are similar with both approaches. Similarly, SOE was low for the effect of the approach on atrophy (Table 7).
There are few studies comparing the effectiveness of interventions on future fertility associated with treatment of cryptorchidism. Furthermore, in those studies (where the participants are adults who had cryptorchidism in childhood), the primary outcome is usually semen analysis parameters, which is at best a proxy for fertility. One study examined ability to father children and focused on the addition of hormonal therapy to surgery, finding no advantage to the combination of hormones and surgery compared with surgery alone. No studies compared paternity rates between surgery and hormonal therapy in isolation. To this end, no data are available to assess whether 1 approach is superior for fertility outcomes, although it is accepted that untreated cryptorchidism is associated negatively with later fertility.
Harms of Treatments
Eleven studies of hormonal and surgical interventions included harms; 2 studies were of good quality,17,52 2 were of fair quality,14,15 and 7 were of poor quality.12,13,16,18,24,37,48 Eight12–18,24 of 14 hormonal studies reported harms. The most common outcomes were virilizing effects (eg, hair, increase in penis size, and erections), and behavioral changes (eg, aggression). Of the 8 hormonal studies reporting harms, 2 did not segregate data by study arm, and thus harms could have presented in either a treatment or placebo arm.17,24 One study reported that 74% of 116 boys receiving hCG had virilizing effects, compared with 5.1% of boys receiving only LHRH, but 1 of the hCG arms also included LHRH and another included human menopausal gonadotropin.18 All side effects had receded by the 6-month follow-up. No other study reported side effects to be as common as virilization. Reported harms of hormonal treatments were mild and transient and had receded by 6 months.
Three studies reported harms associated with laparoscopic surgery.37,48,52 Rare cases of intestinal injury due to Veress needle puncture (1 case),48 postoperative laparoscopic port site reducible (3 cases),52 and incarcerated (2 cases) hernia37 were noted with laparoscopy. They are not specific to cryptorchidism repair and can occur with any type of laparoscopy. Overall, adverse effects specifically associated with surgical repair for cryptorchidism were rare.
Discussion
The goal of any intervention for cryptorchidism is to move the undescended testicle to a normal position in the scrotum, in as safe and least invasive way possible. Although there are a number of therapeutic options available to parents and providers, our study found that the current literature on comparative effectiveness may not provide definitive answers for those seeking to provide guidance in clinical decision-making. Specifically, only 7 studies identified in our analysis were of good quality17,23,25–27,45,49,52 and this is reflected in the SOE, which was generally low to moderate for any intervention and outcome. This, in turn, underscores the need for further research.
In the case of hormonal treatment, most studies were of poor quality, precluding definitive conclusions as to a specific expected effect rate for any hormone or combination thereof. Acknowledging this, studies report slightly higher rates of testicular descent for LHRH and hCG compared with placebo and seem to suggest that lower initial location of the testicle may be associated with a greater likelihood of success (although none of the studies was sufficiently powered for this assessment). In addition, some harms of treatment were also noted. Specifically, some studies reported more frequent temporary virilizing side effects, including increased penile length, erections, and testicular enlargement, although all side effects were transitory.
With regard to the surgical treatment of cryptorchidism, all approaches were associated with success rates of 75% or higher. When surgically treating boys with cryptorchidism, providers must select both a specific procedure (primary orchiopexy, 1- or 2-stage FS orchiopexy, or orchiectomy) and approach (open versus laparoscopic). In the case of specific procedures, although the fairly substantive observational literature reviewed here reports outcomes after various types of orchiopexies, the fact that the choice of procedure is based primarily on the initial location of the testicle makes comparing the results of studies difficult. Specifically, primary orchiopexy is more likely to be used in cryptorchid testes found to be in lower positions, closer to the scrotum, increasing the likelihood of success. Conversely, FS orchiopexies (whether 1 or 2 stage) tend to be reserved for higher-located testicles, which, by their nature, are more difficult to treat and more likely to retract back to an abnormal position after surgery. To this end, the observation that primary orchiopexy is associated with higher success rates when compared with the FS approach is more likely due to underlying baseline differences in patients undergoing this procedure than true differences in the effectiveness of the technique. That being said, SOE was high only for the effect of primary orchiopexy on testicular descent, for which the most data are available.
There is increased use of laparoscopic techniques throughout pediatric surgery, primarily because of technological advances coupled with the commonly accepted belief that it is less invasive and, therefore, better tolerated by patients. The literature comparing open to laparoscopic surgery in cryptorchidism appears to indicate that success rates are at least comparable between the 2 approaches, although it is worth noting that the SOE was low. There appeared to be some evidence that patients undergoing laparoscopy reported shorter convalescence and less postoperative pain; however, certain studies reported rare harms that were unique to laparoscopic surgery, such as hernia at the port site or Veress needle injury.
Despite the low SOE associated with the literature on laparoscopy in cryptorchidism, it is clear that this approach plays an important role in the diagnosis and treatment of cryptorchidism. In 2013, diagnostic laparoscopy is almost always the approach of choice when attempting to localize a nonpalpable cryptorchid testicle thought to be potentially located in the abdomen. As evidence, all but 1 of the studies in our review published in the past 5 years that included assessment of the abdomen for a nonpalpable testicle28,32,36–38,43,47,51 used laparoscopy for this part of the procedure, even if they used an open technique to repair the cryptorchidism.
Clearly, the existing evidence leaves many questions regarding the optimal approach to the treatment of cryptorchidism unanswered. In the case of hormonal therapy, most studies have focused primarily on LHRH and its agonists because it is easily administered intranasally. A wide range of success rates is seen across studies, possibly owing to heterogeneity in the study populations or potentially owing to variability in drug absorption through the intranasal route. Some literature suggests that differences may be because of initial location of the testicle, but this is an area warranting more study, including conducting additional studies in which patients are carefully selected to assess efficacy by testicle location, or analyses carefully controlled for this effect. Given that any side effects from hormonal therapy are temporary and not life-threatening, it would be of some value to be able to accurately inform parents of what the possibility of success is with this treatment, as even a small likelihood of success coupled with the avoidance of surgery may be appealing.
Because most reviewed studies of surgery were observational, the potential for confounding and effect measure modification in this literature to obscure true effects is significant. Studies intended to address comparative effectiveness of treatment in this condition, including 1-stage versus 2-stage FS orchiopexy for nonpalpable abdominal testicles should either use a randomized design or carefully control for covariates, such as testicular location, size and appearance, ectopia, and unilateral or bilateral disease. This is particularly important for initial testicular location, which may be both a modifier of effectiveness and a factor used to choose the surgical procedure. Finally, these studies must include follow-up for at least 6 to 12 months to observe for delayed atrophy of the testicle.
The literature available to assess treatment of cryptorchidism also is characterized by a lack of standardization of outcomes. Studies routinely use the term “success rates” but fail to define a successful outcome. In some cases, the authors report success rates as proper placement of the testicle in the scrotum in the early postoperative period and then report ≥6-month atrophy rates separately. Given that the goal of the procedure is usually to place the testicle in the scrotum and to maximize long-term endocrine function and fertility, the definition of success should always reflect both of these important end points (testicular location and size), and we encourage researchers to report both.
Conclusions
Hormonal treatment is marginally effective relative to placebo, with moderate SOE, but is successful in some children and with minimal side effects, suggesting that it may be an appropriate trial of care for some patients. If successful, these patients should continue to be monitored for late re-ascent, as most of the studies on this issue did not include long-term follow-up. Surgical options appear effective, with rates of normal postoperative scrotal position >75%. Our ability to draw definitive conclusions regarding the comparative effectiveness of the surgical approaches is limited by confounding by indication in the individual studies, which also affects the quality of the literature. The strength of the evidence for the effects of either 1-stage or 2-stage FS procedures on testicular descent is moderate (low for atrophy) and high for primary orchiopexy (moderate for atrophy). Comparable outcomes have been seen with laparoscopic and open approaches to surgical repair (low SOE for testicular descent and atrophy in studies comparing these approaches).
Acknowledgments
We thank Tanya Surawicz and Nila Sathe for their assistance with this article. We also thank our Technical Expert Panel and Agency for Healthcare Research and Quality Task Order Officer for their input on the full review.
Glossary
- FS
Fowler-Stephens procedure
- hCG
human chorionic gonadotropin
- LHRH
luteinizing hormone releasing hormone
- RCT
randomized controlled trial
- SOE
strength of evidence
Footnotes
All authors helped to conceptualize the study, extract and analyze data, draft the manuscript, revise it critically for important intellectual content, and gave final approval of the manuscript submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Agency for Healthcare Research and Quality (contract number: HHSA 290 2007 10065 I).
COMPANION PAPER: A companion to this article can be found on page e1908, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2013-0073.
References
- 1.Miller DC, Saigal CS, Litwin MS. The demographic burden of urologic diseases in America. Urol Clin North Am. 2009;36(1):11–27, v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthold JS, González R. The epidemiology of congenital cryptorchidism, testicular ascent and orchiopexy. J Urol. 2003;170(6 pt 1):2396–2401 [DOI] [PubMed] [Google Scholar]
- 3.Campbell MF, Wein AJ, Kavoussi LR. Campbell-Walsh Urology. 9th ed. Philadelphia, PA: W.B. Saunders; 2007 [Google Scholar]
- 4.White S. Undescended testes (cryptorchidism). In: Gomella LG, ed. The 5-Minute Urology Consult. Philadelphia, PA: Wolters Kluwer Health; 2010:464–465 [Google Scholar]
- 5.Penson DF, Krishnaswami S, Jules A, Seroogy JC, McPheeters ML. Evaluation and treatment of cryptorchidism. Comparative Effectiveness Review No. 88. (Prepared by the Vanderbilt Evidence-based Practice Center under Contract No. 290-2007-10065-I.) AHRQ Publication No. 13-EHC001-EF. Rockville, MD: Agency for Healthcare Research and Quality. November 2012. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Accessed March 25, 2013
- 6.Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. Accessed March 25, 2013
- 7.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://wwwohrica/programs/clinical_epidemiology/oxfordasp. Accessed January 24, 2012
- 8.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2 Group . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536 [DOI] [PubMed] [Google Scholar]
- 9.De Muinck Keizer-Schrama SM, Hazebroek FW, Drop SL, Molenaar JC, Visser HK. LH-RH nasal spray treatment for cryptorchidism. A double-blind, placebo-controlled study. Eur J Pediatr. 1987;146(suppl 2):S35–S37 [DOI] [PubMed] [Google Scholar]
- 10.deMuinck Keizer-Schrama SM, Hazebroek FW, Matroos AW, Drop SL, Molenaar JC, Visser HK. Double-blind, placebo-controlled study of luteinising-hormone-releasing-hormone nasal spray in treatment of undescended testes. Lancet. 1986;1(8486):876–880 [DOI] [PubMed] [Google Scholar]
- 11.Hazebroek FW, de Muinck Keizer-Schrama SM, van Maarschalkerweerd M, Visser HK, Molenaar JC. Why luteinizing-hormone-releasing-hormone nasal spray will not replace orchiopexy in the treatment of boys with undescended testes. J Pediatr Surg. 1987;22(12):1177–1182 [DOI] [PubMed] [Google Scholar]
- 12.Hagberg S, Westphal O. Treatment of undescended testes with intranasal application of synthetic LH-RH. Eur J Pediatr. 1982;139(4):285–288 [DOI] [PubMed] [Google Scholar]
- 13.Karpe B, Eneroth P, Ritzén EM. LHRH treatment in unilateral cryptorchidism: effect on testicular descent and hormonal response. J Pediatr. 1983;103(6):892–897 [DOI] [PubMed] [Google Scholar]
- 14.Christiansen P, Müller J, Buhl S, et al. Treatment of cryptorchidism with human chorionic gonadotropin or gonadotropin releasing hormone. A double-blind controlled study of 243 boys. Horm Res. 1988;30(4-5):187–192 [DOI] [PubMed] [Google Scholar]
- 15.Olsen LH, Genster HG, Mosegaard A, et al. Management of the non-descended testis: doubtful value of luteinizing-hormone-releasing-hormone (LHRH). A double-blind, placebo-controlled multicentre study. Int J Androl. 1992;15(2):135–143 [DOI] [PubMed] [Google Scholar]
- 16.Wit JM, Delemarre-Van de Waal HA, Bax NM, Van den Brande JL. Effect of LHRH treatment on testicular descent and hormonal response in cryptorchidism. Clin Endocrinol (Oxf). 1986;24(5):539–548 [DOI] [PubMed] [Google Scholar]
- 17.Rajfer J, Handelsman DJ, Swerdloff RS, et al. Hormonal therapy of cryptorchidism. A randomized, double-blind study comparing human chorionic gonadotropin and gonadotropin-releasing hormone. N Engl J Med. 1986;314(8):466–470 [DOI] [PubMed] [Google Scholar]
- 18.Bertelloni S, Baroncelli GI, Ghirri P, Spinelli C, Saggese G. Hormonal treatment for unilateral inguinal testis: comparison of four different treatments. Horm Res. 2001;55(5):236–239 [DOI] [PubMed] [Google Scholar]
- 19.Forest MG, David M, David L, Chatelain PG, Francois R, Bertrand J. Undescended testis: comparison of two protocols of treatment with human chorionic gonadotropin. Effect on testicular descent and hormonal response. Horm Res. 1988;30(4-5):198–205; discussion 205–196 [DOI] [PubMed]
- 20.Hesse V, Fischer G. Three injections of human chorionic gonadotropin are as effective as ten injections in the treatment of cryptorchidism. Horm Res. 1988;30(4-5):193–197 [DOI] [PubMed] [Google Scholar]
- 21.Bica DT, Hadziselimovic F. Buserelin treatment of cryptorchidism: a randomized, double-blind, placebo-controlled study. J Urol. 1992;148(2 pt 2):617–621 [DOI] [PubMed] [Google Scholar]
- 22.Bica DT, Hadziselimovic F. The behavior of epididymis, processus vaginalis and testicular descent in cryptorchid boys treated with buserelin. Eur J Pediatr. 1993;152(suppl 2):S38–S42 [DOI] [PubMed] [Google Scholar]
- 23.Aycan Z, Ustünsalih-Inan Y, Cetinkaya E, Vidinlisan S, Ornek A. Evaluation of low-dose hCG treatment for cryptorchidism. Turk J Pediatr. 2006;48(3):228–231 [PubMed] [Google Scholar]
- 24.Esposito C, De Lucia A, Palmieri A, et al. Comparison of five different hormonal treatment protocols for children with cryptorchidism. Scand J Urol Nephrol. 2003;37(3):246–249 [DOI] [PubMed] [Google Scholar]
- 25.Hadziselimovic F. Successful treatment of unilateral cryptorchid boys risking infertility with LH-RH analogue. Int Braz J Urol. 2008;34(3):319–326; discussion 327–318 [DOI] [PubMed]
- 26.Hadziselimović F, Herzog B. Treatment with a luteinizing hormone-releasing hormone analogue after successful orchiopexy markedly improves the chance of fertility later in life. J Urol. 1997;158(3 pt 2):1193–1195 [DOI] [PubMed] [Google Scholar]
- 27.Ferro F, Spagnoli A, Zaccara A, De Vico A, La Sala E. Is preoperative laparoscopy useful for impalpable testis? J Urol. 1999;162(3 pt 2):995–996, discussion 997 [DOI] [PubMed] [Google Scholar]
- 28.Abolyosr A. Laparoscopic versus open orchiopexy in the management of abdominal testis: a descriptive study. Int J Urol. 2006;13(11):1421–1424 [DOI] [PubMed] [Google Scholar]
- 29.Arda IS, Ersoy E. The place of the technique of narrowing neck of the dartos pouch on the ascent of testis after surgery. Scand J Urol Nephrol. 2001;35(6):505–508 [DOI] [PubMed] [Google Scholar]
- 30.Na SW, Kim SO, Hwang EC, et al. Single scrotal incision orchiopexy for children with palpable low-lying undescended testis: early outcome of a prospective randomized controlled study. Korean J Urol. 2011;52(9):637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nazem M, Hosseinpour M, Shahbandari M. Evaluation of orchidopexy with or without opening the external oblique fascia in children with superficial inguinal undescended testis. Eur J Pediatr Surg. 2011;21(4):255–257 [DOI] [PubMed] [Google Scholar]
- 32.Escarcega-Fujigaki P, Rezk GHP, Huerta-Murrieta E, et al. Orchiopexy-laparoscopy or traditional surgical technique in patients with an undescended palpable testicle. J Laparoendosc Adv Surg Tech A. 2011;21(2):185–187 [DOI] [PubMed] [Google Scholar]
- 33.Yavetz H, Harash B, Paz G, et al. Cryptorchidism: incidence and sperm quality in infertile men. Andrologia. 1992;24(5):293–297 [DOI] [PubMed] [Google Scholar]
- 34.Okuyama A, Nonomura N, Nakamura M, et al. Surgical management of undescended testis: retrospective study of potential fertility in 274 cases. J Urol. 1989;142(3):749–751 [DOI] [PubMed] [Google Scholar]
- 35.Gilhooly PE, Meyers F, Lattimer JK. Fertility prospects for children with cryptorchidism. Am J Dis Child. 1984;138(10):940–943 [DOI] [PubMed] [Google Scholar]
- 36.Comploj E, Mian M, Koen M, Berger C, Becker T, Riccabona M. Single- vs. two-stage Fowler-Stephens orchidopexy: are two operations better than one? A retrospective, single-institution critical analysis. Curr Urol. 2011;5(1):12–17 [Google Scholar]
- 37.Moursy EE, Gamal W, Hussein MM. Laparoscopic orchiopexy for non-palpable testes: outcome of two techniques. J Pediatr Urol. 2011;7(2):178–181 [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Min GE, Kim KS. Laparoscopic orchiopexy for a nonpalpable testis. Korean J Urol. 2010;51(2):106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radmayr C, Oswald J, Schwentner C, Neururer R, Peschel R, Bartsch G. Long-term outcome of laparoscopically managed nonpalpable testes. J Urol. 2003;170(6 pt 1):2409–2411 [DOI] [PubMed] [Google Scholar]
- 40.Humphrey GM, Najmaldin AS, Thomas DF. Laparoscopy in the management of the impalpable undescended testis. Br J Surg. 1998;85(7):983–985 [DOI] [PubMed] [Google Scholar]
- 41.Anderson JB, Cooper MJ, Thomas WE, Williamson RC. Impaired spermatogenesis in testes at risk of torsion. Br J Surg. 1986;73(10):847–849 [DOI] [PubMed] [Google Scholar]
- 42.Dhanani NN, Cornelius D, Gunes A, Ritchey ML. Successful outpatient management of the nonpalpable intra-abdominal testis with staged Fowler-Stephens orchiopexy. J Urol. 2004;172(6 pt 1):2399–2401 [DOI] [PubMed] [Google Scholar]
- 43.Denes FT, Saito FJ, Silva FA, Giron AM, Machado M, Srougi M. Laparoscopic diagnosis and treatment of nonpalpable testis. Int Braz J Urol. 2008;34(3):329–334, discussion 335 [DOI] [PubMed] [Google Scholar]
- 44.Chang B, Palmer LS, Franco I. Laparoscopic orchidopexy: a review of a large clinical series. BJU Int. 2001;87(6):490–493 [DOI] [PubMed] [Google Scholar]
- 45.Stec AA, Tanaka ST, Adams MC, Pope JC, IV, Thomas JC, Brock JW, III. Orchiopexy for intra-abdominal testes: factors predicting success. J Urol. 2009;182(suppl 4):1917–1920 [DOI] [PubMed] [Google Scholar]
- 46.Cloutier J, Moore K, Nadeau G, Bolduc S. Modified scrotal (Bianchi) mid raphe single incision orchiopexy for low palpable undescended testis: early outcomes. J Urol. 2011;185(3):1088–1092 [DOI] [PubMed] [Google Scholar]
- 47.Chang M, Franco I. Laparoscopic Fowler-Stephens orchiopexy: the Westchester Medical Center experience. J Endourol. 2008;22(6):1315–1319 [DOI] [PubMed] [Google Scholar]
- 48.Baker LA, Docimo SG, Surer I, et al. A multi-institutional analysis of laparoscopic orchidopexy. BJU Int. 2001;87(6):484–489 [DOI] [PubMed] [Google Scholar]
- 49.Anoussakis C, Liakakos D, Kiburis J, Papaspyrou P, Doulas NL. Effect of surgical repair of cryptorchidism on endocrine testicular function. J Pediatr. 1983;103(6):919–921 [DOI] [PubMed] [Google Scholar]
- 50.Chandrasekharam VV. Laparoscopy vs inguinal exploration for nonpalpable undescended testis. Indian J Pediatr. 2005;72(12):1021–1023 [DOI] [PubMed] [Google Scholar]
- 51.Lintula H, Kokki H, Eskelinen M, Vanamo K. Laparoscopic versus open orchidopexy in children with intra-abdominal testes. J Laparoendosc Adv Surg Tech A. 2008;18(3):449–456 [DOI] [PubMed] [Google Scholar]
- 52.Al-Mandil M, Khoury AE, El-Hout Y, Kogon M, Dave S, Farhat WA. Potential complications with the prescrotal approach for the palpable undescended testis? A comparison of single prescrotal incision to the traditional inguinal approach. J Urol. 2008;180(2):686–689 [DOI] [PubMed] [Google Scholar]
- 53.Gheiler EL, Barthold JS, González R. Benefits of laparoscopy and the Jones technique for the nonpalpable testis. J Urol. 1997;158(5):1948–1951 [DOI] [PubMed] [Google Scholar]