Abstract
Declines in neuromuscular function, including measures of mobility, muscle strength, steadiness, and patterns of muscle activation, accompany advancing age and are often associated with reduced quality of life and mortality. Paradoxically, older adults are less fatigable than young adults in some tasks. The purpose of this study was to determine the influence of age on fatigability of the dorsiflexors and to evaluate the ecological validity of this test by comparing it to motor function subdomains known to decline with advancing age. The community-dwelling older adults (n = 52, 75.2 ± 6.0 years) were more fatigable than young adults (n = 26, 22.2 ± 3.7 years), as assessed by endurance time for supporting a submaximal load (20% of one-repetition maximum; 1-RM) with an isometric contraction of the dorsiflexor muscles (8.9 ± 0.6 min and 15.5 ± 0.9 min, p < 0.001), including participants matched for 1-RM load and sex (Y: 13.3 ± 4.0 min, O: 8.5 ± 6.1 min, n = 11 pairs, 6 women, p < 0.05). When the older adults were separated into two groups (65-75 and 76-90 yrs), however, only endurance time for the oldest group was less than that for the other two groups (p < 0.01). All measures of motor function were significantly correlated (all p < 0.05) with dorsiflexor endurance time for the older adults, and multiple regression analysis revealed that the variance in endurance time was most closely associated with age, steadiness, and knee flexor strength (R2 = 0.50, p < 0.001). These findings indicate that dorsiflexor fatigability provides a valid biomarker of motor function in older adults.
Keywords: aging, fatigability, endurance, muscle strength, steadiness, EMG
1. Introduction
Age-associated declines in motor function–the ability to perform physical tasks [Reuben et al. 2013]–are tightly coupled with quality of life [Cooper et al., 2011; Manini et al., 2007], independent-living status [Bischoff et al., 2003], disability [Guralnik et al., 1995; Rantanen et al., 1999], and mortality [Buchman et al., 2007; Rantanen et al., 2012; Stanaway et al., 2011]. Such associations are expected given that muscle strength [Forrest et al. 2007; Vandervoort 2002], walking endurance [Rikli and Jones 1999], sit-to-stand times [Bohannon 2006], and fine motor skills [Marmon et al. 2011, Enoka et al. 2003] all decline with advancing age. In contrast, fatigability [Kluger et al. 2013], the rate of decline in objective measures of motor performance, can be less [Hunter et al. 2005; Ditor and Hicks 2000; Griffith et al. 2010; Kent-Braun et al. 2002; Lanza et al. 2004] or greater [McNeil and Rice 2007] in older adults than young adults.
Differences in fatigability between young and older adults are attributable to several factors [Christie et al. 2011]. For example, average power produced by the dorsiflexor muscles during 25 dynamic contractions performed at maximal velocity declined more rapidly for the oldest men relative to young men [McNeil and Rice 2007]. In contrast, endurance time for a sustained submaximal isometric contraction with the dorsiflexor muscles was greater for older adults (men and women) than for strength-matched young adults [Griffith et al. 2010]; and meta-analysis suggested that the type of fatiguing contraction used to compare performance (isometric versus dynamic) might underlie these divergent results. However, the age of the older participants also appears to influence the outcome of such comparisons, although there are too few studies that have included older adults in multiple decades of life for this association to be assessed quantitatively [Christie et al. 2011]. Nonetheless, one study did find that the fatigability exhibited by men aged 60-69 years did not differ from that for younger (22-33 years) and older (80-90 years) men, whereas there was a significant difference between the young and oldest men [McNeil and Rice 2007]. The primary aim of the current investigation was to determine the association between age and fatigability of the dorsiflexor muscles in young and older adults (65-90 years), including a subset of strength-matched young and older adults, when supporting a submaximal inertial load for as long as possible. Based on the report of McNeil and Rice [2007], we hypothesized that endurance time would be briefest in the oldest adults when the task required supporting a submaximal load for as long as possible.
To assess the ecological validity of the fatigability test, a secondary aim of the current study was to examine the association between dorsiflexor endurance time and other measures of motor function that typically decline with advancing age. The comparisons included measures of mobility, muscle strength, steadiness, and patterns of muscle activation [Justice et al. 2014a]. Because maintaining the position of a limb is a fundamental element of many activities of daily living, we hypothesized that endurance time for the fatiguing contraction would be significantly associated with other measures of motor function, especially in older adults. The unique features of the study included a relatively large sample of older adults covering a broad range of ages (65-90 years), a subset of strength-matched young and older adults, the assessment of fatigability with a functionally relevant task, and comparison of the fatigability measurement with clinically relevant tasks used to characterize age-related declines in motor function.
2. Materials and Methods
2.1 Human Subjects
Fliers and local advertisements were used to recruit 52 older (65-90 years) and 26 young (19 - 30 years) participants from Boulder, Colorado. Subjects were free of neurological disorders, chronic pain, diabetes, advanced chronic disease states, and any other condition that might limit safe participation. Prior to the tests of motor function, physicians at the Clinical Translational Research Center (CTRC) located at the University of Colorado Boulder obtained medical histories and performed physical examinations for all older participants to ensure subject safety and ability to perform the rigorous physical tests. Older participants were also administered a modified activity questionnaire during this screening session. Young subjects were screened for physical activity status (light and moderate activity level included) for comparison with the estimated activity levels for older adults. All procedures were approved by the Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
2.2 Motor Function Testing Overview
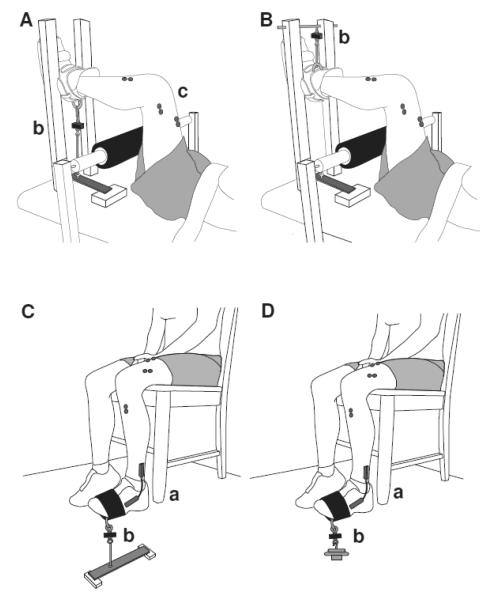
Motor function was assessed with measures of mobility, muscle strength, steadiness, and fatigability during a single, ~3-hour session in the Neurophysiology of Human Movement Laboratory on University of Colorado Boulder campus. Table 1 provides an overview of the motor function testing session and outcomes. Subjects, especially those aged 80-90 years, were allowed ample rest time between physical tests to ensure safety and adequate recovery from the previous task; the breaks times listed in Table 1 reflect minimal rest times after each test. The experimental apparatus used to measure strength, steadiness, and fatigability are shown in Figure 1.
Table 1.
Overview of motor function tests.
| Subdomain | Test | Equipment | Trials | Breaks | Variables | Outcome |
|---|---|---|---|---|---|---|
| Mobility | ||||||
| 500 m walk | Indoor track | 1 | 10 | Time | Endurance walk time | |
| Five sit-to-stand | Standard height chair | 1 | 15 | Time | Sit-to-stand time | |
|
| ||||||
| Strength | ||||||
| Dorsiflexor 1-RM | Weights | 6-8 | 5 | Mass lifted (kg) | 1-RM for load determination | |
| Knee Extensor MVC | Force transducer | 3-5 | 5 | Torque per mass | Knee extensor strength | |
| Knee flexor MVC | Force transducer | 3-5 | 5 | Torque per mass | Knee flexor strength | |
| Dorsiflexor MVC | Force transducer | 3-5 | 5 | Torque per mass | Dorsiflexor strength | |
| -- | -- | -- | -- | Average torque per mass | Composite lower body strength | |
|
| ||||||
| Steadiness | ||||||
| 60 s dorsiflexor isometric contraction @ 5% 1-RM |
Force transducer | 1 | 10 | SD and CV for force | Steadiness @ 5% 1-RM | |
| EMG | -- | -- | EMG amplitude (TA and MG) | Coactivation ratio | ||
| 60 s dorsiflexor isometric contraction @ 20% 1-RM |
Force transducer | 1 | 20 | SD and CV for force | Steadiness @ 20% 1-RM | |
| EMG | -- | -- | EMG amplitude (TA and MG) | Coactivation ratio | ||
|
| ||||||
| Fatigability | ||||||
| Dorsiflexor isometric contraction for as long as possible @ 20% 1-RM |
Weights | 1 | -- | Time | Endurance time | |
| EMG | -- | -- | EMG amplitude (TA, MG, KE) | EMG amplitude (% initial) | ||
| Force transducer | -- | -- | SD and CV for force | SD and CV for force | ||
| 0-10 scale | -- | -- | RPE | RPE | ||
Tests are listed in order of presentation. Rest intervals between strength trials were 60 s. Breaks are reported as minimal rest times after each test (min). 1-RM, one-repetition maximum; CV, coefficient of variation; EMG, electromyogram; KE, knee extensors; MVC, maximal voluntary contraction; MG, medial gastrocnemius; RPE, rating of perceived exertion; SD, standard deviation; TA, tibialis anterior
Figure 1.
Schematic drawings of the experimental setup for knee extensor (A), knee flexor (B), and dorsiflexor (C) maximal voluntary contractions (MVCs), and dorsiflexor one-repetition maximum (1 RM), steadiness, and fatigability tests (D). The foot and lower leg were placed in an orthosis and fixed to a rigid restraint for the measurement of knee extensor (A) and flexor (B) strength. In the dorsiflexor tasks, maximal force was exerted against a rigid restraint (C), or weights equivalent to 5% or 20% 1-RM load were suspended from the foot at the same point of contact (D). The ankle joint was maintained at a neutral position (1.61 ± 0.08 rad), as measured with an electrogoniometer (a). A force transducer (b) was placed in series with the restraint or weight for each task. Electromyography (EMG) was measured during each isometric contraction with surface electrodes (c) placed over selected muscles.
2.3 Electrical and Mechanical recordings
The force exerted by the limb during the strength, steadiness, and fatigability tasks was measured with a strain-gauge transducer (MLP-300, Transducer Techniques, Ternecula, CA) (Figure 1). The force signal was low-pass filtered (0-50 Hz; Coulbourn Instruments, Allentown, PA), recorded on a computer, and digitized at 1,000 samples/s. In addition, muscle activity (EMG) was recorded with surface electrodes (Ag–AgCl, 8-mm diameter, 20-mm distance between electrodes) placed over the tibialis anterior, medial gastrocnemius, rectus femoris, and vastus lateralis, as described previously [Rudroff et al. 2010]. The EMG signals were amplified (x 2,000), band-pass filtered 12-1,000 Hz; (Coulbourn Instruments, Allentown, PA), recorded on a computer, and digitized at 2,000 samples/s. Absolute and relative fluctuations in force and EMG were quantified in 10-s epochs at absolute and relative time points during steadiness and fatigability tasks via a custom MATLAB program (version 7.2, R2006a, MathWorks, Natick, MA). Coactivation of agonist and antagonist muscles was calculated during the steadiness and fatigability tasks as an assay of the neural control strategy used to maintain limb position during isometric contractions [Baudry et al. 2010]. Coactivation was quantified as the ratio of EMG amplitude for medial gastrocnemius relative to the average amplitude for tibialis anterior and medial gastrocnemius during the dorsiflexor contractions [Falconer and Winter 1985].
2.4 Mobility
Mobility was quantified with measures of endurance walk and sit-to-stand times. Mobility has been identified by the International Classification of Functioning, Disability, and Health as essential in classification of activity limitation [WHO 2001], and was measured in this investigation to determine the extent to which fatigability during a single joint task may be related to tasks required for everyday activity. Walking was assessed as the time to walk 500 m. Subjects walked at a brisk pace for 3 laps around an indoor track [Justice et al. 2014b]; the instructions given to each subject immediately before testing were: “Please walk 3 laps at a brisk, but comfortable pace. Walk as quickly as you would if you were at an airport, and you were a few minutes late for your plane. This should be a brisk walk, but please, no running.”. Sit-to-stand times were measured as the time to stand up and sit down five times as quickly as possible from a standard-height chair [Bohannon 2007, 2012].
2.5 Strength
Strength was quantified as the peak torque (N·m)–recorded force (N) multiplied by the measured moment arm for each subject–achieved during 3-5 maximal isometric contractions normalized to body mass for the dorsiflexors, knee extensors, knee flexors. An average of the three maximal voluntary contractions (MVCs) was determined and is reported as composite lower body strength. Strength for these three lower body muscles are essential for mobility [Bohannon 1997; Eriksrud and Bohannon 2003], and were measured as indices of functional ability. The knee extensor and knee flexor contractions were performed in a supine posture at the same hip- and knee-joint angles (1.57 rad, Figure 1A, 1B), as described previously [Rudroff et al. 2010]. The dorsiflexor contraction was performed in a seated posture with hip- and knee-joint angles of 1.57 rad and the ankle at a standing angle (1.60 ± 0.082 rad) (Figure 1C). A strain-gauge transducer was used to measure the force exerted by the limb during each MVC. The MVC task comprised a 3-s increase in force from zero to maximum with the maximal force held for 2–3 s, while subjects were verbally encouraged to achieve maximal force. There was a 60- to 90-s rest between trials. The subject was provided visual feedback of the recorded force (Spike2 V6, Cambridge Electronic Design, Cambridge, England) and the visual gain was adjusted between each MVC trial. When the peak forces achieved in two of the three trials differed by >5%, additional MVCs were performed until this criterion was met. The greatest force achieved by each subject was taken as the MVC force, which was subsequently multiplied by the relevant moment arm to derive peak torque. All strength measures are reported as peak torque normalized to body mass. The peak amplitude of the surface EMG signal for the primary agonist muscle was measured during MVCs and quantified with a MATLAB program as the maximal average rectified EMG during a 0.5 s interval.
Subjects also performed a dorsiflexor one-repetition maximum (1-RM) task to determine the loads for the steadiness and fatigability trials. The 1-RM load corresponded to the maximal mass the subject could lift to a neutral ankle angle (1.57 rad, Figure 1D), and is reported as torque. The protocol began with a moderate load and increased progressively up to maximum, with at least 60 s of rest between attempts. When a load was reached that could not be lifted to a neutral ankle angle, the last load successfully lifted was recorded as the 1-RM load. The 1-RM load was usually obtained within 6-8 trials, with only two to three of those trials at near-maximal loads [Tracy et al. 2004].
2.6 Steadiness
Steadiness was assessed during 60-s isometric contractions performed with the dorsiflexor muscles at loads equivalent to 5 and 20% 1-RM (Figure 1C). The force exerted by a limb fluctuates about an average value during steady contractions [Christou and Carlton 2002, Tracy and Enoka 2002], and the magnitude of these fluctuations is associated with performance of some motor tasks [Marmon et al. 2011]. Steadiness was measured in the current study to assess its association with dorsiflexor fatigability. Subjects maintained a neutral foot position based on the measurement of ankle angle with an electrogoniometer (SG110 and K800, Biometrics Ltd, Cwmfelinfach, Gwent, UK). Subjects received visual feedback (0.017 rad/cm) of the actual and target (standing neutral position) angles during the steady contraction using a customized Labview program (Version 8.2, National Instruments, Austin, TX). All subjects were able to maintain ankle position for the entire 60-s duration. Steadiness was quantified as the absolute and relative force fluctuations (standard deviation and coefficient of variation, respectively). Force was measured via the strain-gauge transducer placed in series with the inertial load. The force fluctuations and the normalized average EMG were quantified during 10-s epochs centered about 10, 20, 30, 40, and 50 s of the steadiness contractions.
2.7 Fatigability
Fatigability was assessed as the endurance time for a submaximal isometric contraction with the dorsiflexor muscles. The task involved maintaining foot position while supporting a load equivalent to 20% of 1-RM for as long as possible (Figure 1D) [Griffith et al. 2010]. The task was terminated when the ankle joint angle declined by 0.21 rad from neutral, despite strong verbal encouragement. Adjustments during the fatiguing contraction were characterized by measuring the absolute and relative force fluctuations (steadiness), the normalized average EMG of the tibialis anterior, medial gastrocnemius, and knee extensors (average of vastus lateralis and rectus femoris EMG), and the rating of perceived exertion (RPE; modified Borg Scale). Steadiness and EMG measures were quantified via a custom MATLAB program and analyzed both as 5-s non-overlapping sliding windows and as 10-s epochs relative to task duration: start, 20%, 40%, 60%, 80%, and endurance time. RPE was reported at 30-s intervals. The force fluctuations, EMG measures, and RPE were further quantified as the slope of an exponential or linear fit to the data for individual trials.
2.8 Statistical Analysis
Prior to primary analysis, normality of each variable was assessed with the Kolmogorov-Smirnov test. No outliers (>3 SD) were identified. Differences between young and older adults were assessed by independent t-test for all subject characteristics and motor function outcomes; a Bonferroni correction was applied to test the seven hypothesized differences in motor function between young and older adults (p < 0.007). Performance during the fatiguing contraction was examined with repeated-measures ANOVAs (age x time). The dependent variables were aEMG, aEMG normalized to initial aEMG, absolute (SD) and relative (CV) force fluctuations, and RPE. Greenhouse-Geisser corrections were applied when the assumption of sphericity (Mauchly’s test of sphericity) was violated (SD and CV of force). Homogeneity of variance between age groups was examined for each measure with Levene’s test. Post-hoc analyses (Tukey) examined differences among time intervals when appropriate. The repeated-measures analyses were performed in the young and older group at large, and for the subset of 1-RM-matched young and older participants. A stepwise, linear regression equation was performed to examine the contribution of the independent variables obtained during the fatiguing contraction (rates of increase and value at start of task for aEMG activity of the tibialis anterior, medial gastrocnemius and knee extensors, coefficient of variation for force, and RPE) to endurance time.
The associations between endurance time and other outcome variables were determined by Pearson correlation coefficients (r); linearity was verified by visual assessment of each scatterplot. Pearson correlation coefficients were also determined for all measures of motor function in the two age groups independently. The relation between age and endurance time was examined with simple linear and power regression models. Linear regression equations also described the associations between primary motor outcomes (mobility, strength, steadiness, coactivation), age, and sex with endurance time for the dorsiflexor fatiguing contraction. Race and comorbidities were not considered because only three study participants were not Caucasian and few reported existing comorbidities. The variables included in the final multivariate analysis were identified with a backward regression model. Subsequently, a stepwise, multiple-regression model was performed to explain the variance (coefficient of determination; R2) in fatigability. An absence of multicollinearity for the explanatory variables was verified by variance inflation factor (VIF) and tolerance. The α-level for all statistical analyses was set at 0.05, except when modified by the Bonferroni correction, with minimum accepted power at 80%. All data are presented as mean ± SD in the text and tables and mean ±SEM in the figures. The statistical procedures were performed with SPSS Statistics (version 16.0.1; SPSS, Inc., Chicago, IL).
3. Results
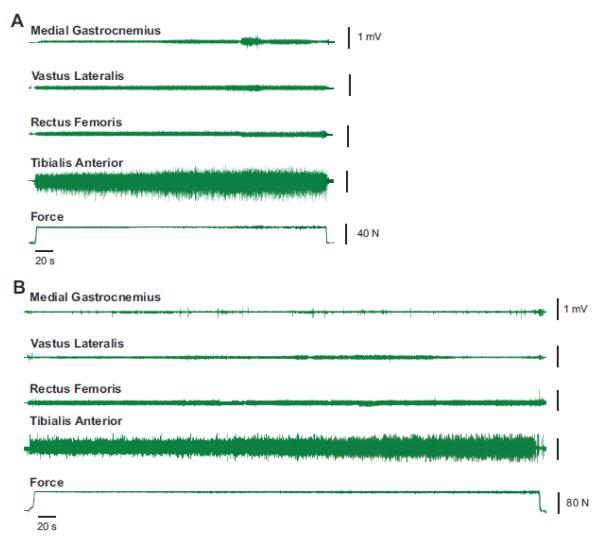
Sixty-nine older individuals (65-90 years) volunteered for the study, 52 (27 women) of whom were enrolled and completed the testing session. The performance of the older adults was compared with 26 young subjects (19-30 years; 14 women). Representative force and EMG signals from one young and one older participant during the fatigability task are shown in Figure 2.
Figure 2.
Representative force and EMG recordings during the dorsiflexion endurance task for one older (A) and one younger (B) subject. The amplitude of the interference EMG was greatest for tibialis anterior (fourth trace) and was substantially less for the knee extensors (vastus lateralis and recuts femoris, second and third traces) and medial gastrocnemius (top trace). The average force applied to the load (bottom trace) remained relatively constant until the endurance task approached failure.
3.1 Fatigability
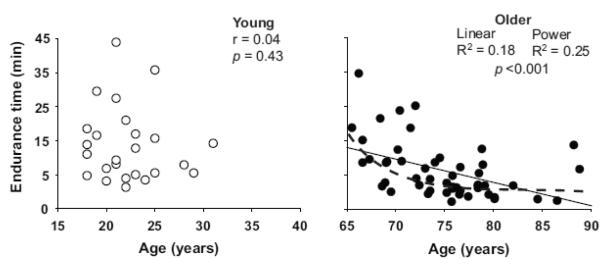
Endurance time for the submaximal dorsiflexor contraction was longer for the young (15.5 ± 0.9 min) than the old adults (8.9 ± 0.6 min, p < 0.001; Table 2), despite the two groups (Y= young; O = older) experiencing the same decline in MVC torque after the task (Y: 22.4 ± 13.3 % and O: 24.5 ± 22.2 % p = 0.69), and the young group exerting a greater absolute torque during the fatiguing contraction (Y: 44.1 ± 19.1 N·m and O: 27.5 ± 8.9 N·m, p < 0.001). When the older adults were separated into two age groups (65-75 and 76-90 years), however, the difference in endurance time was only significant for the oldest group (76-90 yrs) compared with the other two groups (young and 65-75 yrs, p < 0.01). Further, endurance time was significantly correlated with age for the older adults (r = −0.42, linear, p < 0.001), but not the young adults (R2 = 0.18, p = 0.43) (Figure 3), with the largest decline in endurance time seen in the oldest adults. Regression models indicated that the curvilinear power model was a better fit than linear regression model to explain the influence of age on endurance time (R2 = 0.25 vs R2 = 0.18, p < 0.001 both). Endurance time did not differ significantly between men and women (598 ± 373 s and 716 ± 521 s, respectively, p = 0.27).
Table 2.
Subject Characteristics
| Young | Older | |||
|---|---|---|---|---|
|
| ||||
| 19-30 yrs n = 26 |
65-75 yrs n = 27 |
76-90 yrs n = 25 |
65-90 yrs n = 52 |
|
| Women | 14 | 12 | 15 | 27 |
| Age (yrs) | 22.2 ± 3.7 | 70.3 ± 2.7* | 80.0 ± 4.1*† | 75.2 ± 6.0* |
| Body mass (kg) | 66.7 ± 10.6 | 77.4 ± 11.0* | 73.5 ± 14.4 | 75.3 ± 12.7 |
| Height (m) | 1.70 ± 0.08 | 1.70 ± 0.09 | 1.66 ± 0.08 | 1.68 ± 0.08 |
| Endurance walk time (s) | 249 ± 30 | 301 ± 61* | 324 ± 57* | 314± 59* |
| Sit-to-stand time (s) | 7.0 ± 1.4 | 11.2 ± 3.3* | 11.6 ± 2.6* | 11.5 ± 2.9* |
|
Composite lower body
strength (N•m/kg) |
1.34 ± 0.3 | 0.81 ± 0.20* | 0.67 ± 0.23* | 0.73± 0.2* |
|
Dorsiflexor 1-RM
torque (N•m) |
44.1 ± 18.1 | 29.5 ± 8.7* | 26.7 ± 8.5* | 27.5± 8.9* |
|
Steadiness 5% 1-RM
(%) |
1.81 ± 0.87 | 1.78± 0.98 | 1.92 ± 1.03 | 1.88 ± 0.98 |
|
Steadiness 20% 1-RM
(%) |
1.20 ± 0.62 | 1.12 ± 0.75 | 1.74 ± 0.69*† | 1.49 ± 0.78 |
| Fatigability (min) | 15.5 ± 0.9 | 12.1 ± 6.4 | 6.1 ± 3.6*† | 8.9 ± 0.6* |
p < 0.01 between young and older adults;
p < 0.01 between 65-75 yrs and 76-90 yrs groups. Composite lower body strength is the average of knee extensor, knee flexor, and dorsiflexor MVC torques relative to body mass.
Figure 3.
The association between age and endurance time for an isometric contraction in which the dorsiflexors supported a submaximal inertial load equivalent to 20% 1-RM load in young (open circles) and older adults (filled circles). The R2 of curvilinear power model was greater than that of the linear model, suggesting a non-linear relation between age and dorsiflexor fatigability in old adults.
Despite a longer endurance time, aEMG (% MVC value) for the young adults was less for tibialis anterior (Y: 21.9 ± 5.5% and O: 40.4 ± 13.0%, p < 0.001), medial gastrocnemius (Y: 12.7 ± 10.6% and O: 20.1 ± 14.9%, p < 0.001), and knee extensors (Y: 11.2 ± 12.7% and O: 16.2 ± 11.8%, p < 0.001), even at the start of task (tibialis anterior: Y: 19.1 ± 5.3% and O: 35.9 ± 12.2%, p < 0.001). When normalized relative to aEMG amplitudes at the beginning of the fatiguing contraction (% initial), aEMG increased for tibialis anterior (Y: 121%, O: 120% p < 0.001 both) and knee extensors (137% for both groups, p < 0.001 both), but not for medial gastrocnemius (Y: 112%, O: 114%, p = 0.19). There was no time x age interaction during the fatiguing contraction for either measure of aEMG, reflecting similar rates of change in aEMG across the task for the two groups of participants. Moreover, the amount of coactivation was not significantly different between young and older adults during the fatiguing contraction (Y: 0.56 ± 0.34 and O: 0.53 ± 0.43, p = 0.70).
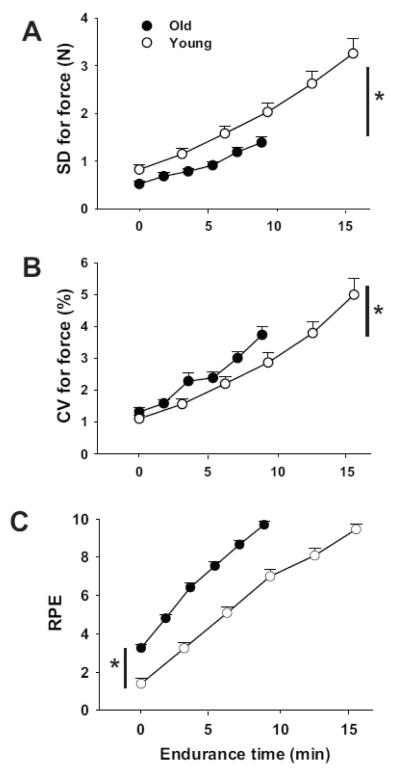
The absolute (standard deviation, SD) and relative (coefficient of variation, CV) amplitude of the vertical fluctuations in force increased during the fatiguing contraction for both groups (p < 0.001, Figure 4A, 4B). The magnitude of the fluctuations was similar at the start of the task for young (SD 0.828 ± 0.48 N, CV 1.13 ± 0.58 %) and older (SD 0.470 ± 0.375 N, CV 1.23 ± 0.81 %, p > 0.05) adults, and increased at a similar rate. Because endurance time was longer for the young adults, however, their force fluctuations were greater at the end of the fatiguing contraction (Y: SD 3.19 ± 1.48 N, CV 4.88 ± 2.5 %; O: SD 1.38 ± 0.79 N, CV 3.73 ± 1.86 %, p < 0.05). The RPE increased during the fatiguing contraction (Figure 4C) from initial values of 1.4 ± 1.2 and 3.3 ± 1.1 for young and old adults, respectively (p < 0.05), and ended at 9.5 ± 1.3 and 9.7 ± 1.3 (p > 0.05). The average RPE was greater for old (6.7 ± 1.0) than young adults (5.7 ± 1.2, p < 0.001), and the rate of increase in perceived exertion was also greater (p < 0.05) for the older adults due to a briefer endurance time. The average RPE did not differ for men and women (6.4 ± 1.2 and 6.3 ± 1.2, respectively, p = 0.70).
Figure 4.
Mean ± SEM for standard deviation (A) and coefficient of variation (CV, B) for the force (%) applied to the load (Fig 1) and rating of perceived exertion (RPE, C) during the dorsiflexor fatiguing contraction for young and old adults. Open symbols represent young adults, and filled symbols denote old adults. * p < 0.05 group differences between final (A and B) and initial (C) values between young and older adults.
Stepwise, multiple-regression analysis was used to predict the dorsiflexor endurance time from the initial values and rates of increase in aEMG for tibialis anterior, medial gastrocnemius and knee flexors, coefficient of variation for force, and RPE. The variance in endurance time across all participants was best explained by the rate of increase in RPE (partial r = −0.49) and aEMG for tibialis anterior averaged across the task (partial r = −0.47; model: 9 variables, R2 = 0.46, power 99.9%, maximum VIF = 1.117, minimum tolerance = 0.90, p < 0.001).
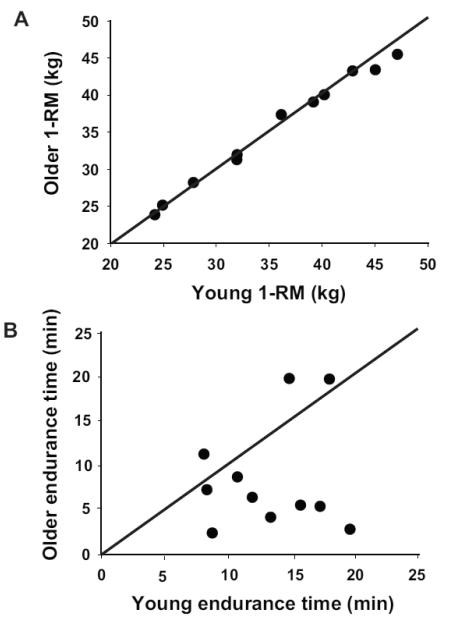
3.2 Young and Old Adults Matched for 1-RM Load
Endurance time for a subset of young and older adults who were matched for sex, 1-RM load, and absolute load supported during the fatiguing contraction (n = 11 pairs, 6 women) was briefer for the older adults (Y: 13.3 ± 4.0 min, O: 8.5 ± 6.1 min, p < 0.05, Figure 5). There were no significant differences in the changes in aEMG, force fluctuations, or RPE between the matched group and the entire sample. Increases in aEMG amplitude were observed during the fatiguing contraction in tibialis anterior (p < 0.001) and knee extensors (p < 0.01), but not medial gastrocnemius (p = 0.10). The absolute and relative force fluctuations were similar at the start of the task (p = 0.75 and p = 0.28, respectively), and increased at a similar rate (p = 0.25 and p = 0.98). In contrast to the entire sample, however, the force fluctuations at the end of the fatiguing contraction were similar for the young (SD 2.75 ± 0.97 N, CV 3.84 ± 1.62 %) and older (SD 1.95 ± 0.88 N, CV 4.25 ± 1.68 %) adults in the matched group (p = 0.07 and 0.57). Also, RPE at the start of the fatiguing contraction was greater for the older adults (3.0 ± 1.2) than the young adults (1.3 ± 1.4) in the matched group, but progressed to the same final value (Y: 9.6 ± 1.2, and O: 9.8 ± 3.0, p = 0.45), with older adults exhibiting a greater rate of increase in RPE (p < 0.05).
Figure 5.
Dorsiflexor one-repetition maximum load (1-RM, A) and endurance time (B) for young (n = 11) and old (n = 11) adults who were matched for sex (n = 6 women) and 1-RM load within 5% 1-RM load. The comparison of endurance time for these matched participants indicates that average endurance time was longer for the young adults (8 of 11 data points lie below the line of identity).
3.3 Associations Among Motor Tasks and Prediction of Fatigability
Multiple significant Pearson correlations were observed between various measures of motor function for the older subjects, but not young subjects (Table 3). Notably, significant correlations or trends were observed between endurance time during the dorsiflexor fatiguing contraction and all the other tests of motor function for the older adults.
Table 3.
Pearson correlations between pairs of outcome variables presented separately for young and old adults.
| Chair rise time |
Strength | Steadiness 5% |
Steadiness 20% |
Coactivation ratio |
Fatigability | |
|---|---|---|---|---|---|---|
|
| ||||||
| Y/ O | Y / O | Y / O | Y / O | Y / O | Y / O | |
| Endurance walk | 0.08 / 0.55 | −0.10 /−0.49c | −0.10 / −0.06 | −0.31 / 0.23 | −0.09 / 0.21 | −0.03 / −0.31 |
| Sit-to-stand | -- | 0.09 / −0.54c | −0.30 / 0.19 | 0.02/−0.12 | −0.01 / 0.55 | −0.23 / −0.29 |
| Strength | -- | 0.10/−0.24c | 0.11/0.10c | 0.26/−0.33kf | −0.08 / 0.39kf | |
| Steadiness | ||||||
| 5% 1-RM | -- | 0.66 / 0.44 | −0.11 / −0.30 | −0.09 / −0.29 | ||
| 20% 1-RM | -- | 0.08 / 0.26 | −0.21 / −0.47 | |||
| Coactivation ratio | -- | −0.01 / 0.34 | ||||
Significant (p < 0.05) Pearson correlations (r) are shown in bold, and trends (0.5 < p < 0.6) in italicized bold. Y = young adults; O = old adults. Strength corresponds to composite lower body strength (c) or knee flexor strength (kf) normalized to body mass. Coactivation ratio is an index of concurrent muscle activity during the steady contraction at 20% 1-RM. Fatigability refers to endurance time for the dorsiflexor fatiguing contraction.
Multiple regression models were developed to explain the variance in endurance time for the dorsiflexor fatiguing contraction by the values for walking endurance, strength, steadiness, and coactivation ratio, with age and sex included as covariates (Table 4). Models were developed with young and older participants combined, and for older adults only. The variance in endurance time for the young subjects could not be explained by age, sex, or any of the measures of motor function (p > 0.05). In the model that included all participants (young and older model), backward regression identified age, coefficient of variation for force when supporting the 20% 1-RM load, endurance walk time, chair-rise time, knee flexor strength, dorsiflexor MVC force, and 1-RM load normalized to body mass as significant explanatory variables (9 variables: R2 = 0.41, p < 0.01, power = 99.9%, maximum VIF = 3.5, minimum tolerance = 0.29). Verification with stepwise analysis excluded all variables except age and steadiness at 20% 1-RM (R2 = 0.27, p < 0.001, power = 99.8%, maximum VIF = 1.1, minimum tolerance = 0.92).
Table 4.
Regression models that predict endurance time for young and older adults.
| Beta | Standard Error | p-value | Partial Correlations | |
|---|---|---|---|---|
| Model 1: Young and Old | ||||
| Age | −7.49 | 2.38 | 0.003 | −0.39 |
| Steadiness 20% 1-RM | −179 | 84.0 | 0.038 | −0.28 |
|
| ||||
| Model 2: Old Adults Only | ||||
| Age | −20.4 | 8.98 | 0.029 | −0.35 |
| Steadiness 20% 1-RM | −200 | 62.7 | 0.003 | −0.47 |
| Knee flexor strength (N•m/kg) | 593 | 207 | 0.007 | 0.43 |
Model 1: Young and Old. Coefficient of determination: R2 = 0.27 (p < 0.001); intercept = 1348. In addition to the listed variables, endurance walk time (r = −0.16), chair rise time (r = −0.02), knee flexor strength (r = 0.04), dorsiflexor strength (r = 0.12), dorsiflexor 1-RM load (r = −0.07), and sex (r = −0.10) were entered into the final stepwise model, but were statistically excluded.
Model 2: Old adults only. Coefficient of determination: R2 = 0.50 (p < 0.001); intercept = 1770. In addition to the listed variables, dorsiflexor 1-RM load (r = −0.31) and knee extensor strength (r = −0.7) were entered into the model, but statistically excluded.
Similarly, backwards regression for the model with older adults only identified age, steadiness with the 20% 1-RM load, knee flexor strength, knee extensor strength, and dorsiflexor 1-RM load relative to body mass (9 variables: R2 = 0.75, p < 0.01, power = 1.00%, maximum VIF = 2.29, minimum tolerance = 0.44), but only age, steadiness with the 20% 1-RM load, and normalized knee flexor were entered in the stepwise model (R2 = 0.50, p < 0.001, power = 99.9%, maximum VIF = 1.21, minimum tolerance = 0.82).
4. Discussion
The current study characterizes the associations between measures of motor function known to decline with age and one (fatigability) for which the findings are less consistent. The primary findings of this study were that endurance time, as indexed by the time a submaximal isometric contraction could be sustained with the dorsiflexor muscles, was briefer in older adults than young adults despite similar declines in net torque following the fatiguing contraction. Moreover, the same association was observed in a subset of 11 pairs of participants matched for sex and 1-RM load. When the older participants were separated into two groups, however, only the oldest adults (76-90 yrs) exhibited a briefer endurance time compared with the other two groups (young and 65-90 yrs). Moreover, the measure of fatigability (endurance time) was significantly correlated with all other measurements of motor function, but for the older adults only. The variance in endurance time was most strongly associated with age and steadiness during a submaximal contraction with the dorsiflexor muscles, as well as the strength of the knee flexors in older adults.
4.1 Age and Fatigability
The main finding of this study was that the time older adults could support a submaximal inertial load with an isometric contraction of the dorsiflexor muscles declined nonlinearly with age (Figure 3). Furthermore, the greater fatigability exhibited by older adults was even evident in a subset of participants who were matched for 1-RM load and sex. In contrast, previous work found that older adults often exhibit longer endurance times than young adults for submaximal isometric contractions [Allman and Rice 2002; Griffith et al. 2010], but not dynamic contractions [Baudry et al. 2007; Christie et al. 2011; McNeil and Rice 2007]. Our results, however, indicate that the age of the older participants is likely a more important determinant than contraction mode when comparing the fatigability of the dorsiflexors. Consistent with this conclusion, McNeil and Rice [2007] found that dorsiflexor fatigability during dynamic contractions was greatest for oldest adults (80-90 yrs) relative to young (22-33 yrs) and older adults (60-69 yrs). Similarly, we found that fatigability for a sustained isometric contraction with the dorsiflexors was greater for the oldest participants (76-90 yrs) relative to two younger groups (19-30 yrs and 65-75 yrs).
In the subset of participants matched for 1-RM load and sex, the older adults were sampled across the age-range (ages 65.5 – 84.5 years). We observed group differences in endurance time between the young and older adults consistent with the entire sample. Three older adults, aged 65.5 – 69.5 years, had a longer endurance time than their matched young adults, indicating a relative preservation for this index of fatigability in the younger members of the group of older adults. Considered jointly, these findings suggest that the fatigability of the dorsiflexors when supporting a submaximal inertial load may remain relatively constant, or be slightly improved [Griffith et al. 2010], through the early 70s before reductions in endurance begin to emerge. Therefore, the age of the older adult is critical when comparing the fatigability of young and older adults.
4.2 Associations among motor functions and fatigability in young and old adults
The second aim of the current study was to determine the extent to which the fatigability task is related to performance on motor tasks known to reflect the functional status of older adults. In contrast to significant associations among multiple measures of motor functions in older adults, there were few significant correlations for the young adults (Table 3). Although the variance in performance was much less for the young adults, the group difference in associations between the tests of motor function suggests that adaptations in the neuromuscular system with advancing age influence multiple subdomains of motor function. Significantly, fatigability (endurance time) was the only measurement correlated with all of the other measures of motor function examined in the current study, which indicates that fatigability during sustained isometric contractions when supporting a submaximal load may represent a biomarker of motor function in older adults. Significant associations were also observed between coactivation of agonist and antagonist muscles during a steady contraction and several functional domains, including mobility, muscle strength, and steadiness. Heightened coactivation is often observed in older adults [Burnett et al. 2000, Hortobágyi et al. 2011] and likely increases the energetic cost of performing muscular work [Hortobágyi et al. 2011], at least during walking tasks [Larsen et al. 2008; Marques et al. 2013; Peterson and Martin 2010]. Although we observed no statistical difference in coactivation between young and old adults during either steadiness or fatigability tasks, the association of coactivation with multiple measures of motor function in older adults (Table 3) suggests that the amount of concurrent activity in agonist and antagonist muscle may limit functional performance in older adults.
The current study is the first to demonstrate an association between steadiness during a brief isometric contraction and endurance time for a sustained isometric contraction. A decrease in steadiness has also been shown to be associated with reductions in the ability to perform functionally relevant actions [Christou 2011]. For example, reduced steadiness during isometric contractions with the plantar flexors predicted the variability in the center of pressure during a quiet standing task in older persons [Kouzaki and Shinohara 2010], and declines in steadiness of the index finger abduction force was the strongest predictor of age-associated differences in manual dexterity [Marmon et al. 2011]. Force fluctuations during brief submaximal isometric contraction are likely caused by low-frequency oscillations in motor unit discharge rates [Negro et al. 2009], but it is not yet known if these increase during fatiguing contractions and the extent to which they contribute to declines in motor function with advancing age.
In addition to age and steadiness, the variability in dorsiflexor endurance time was also explained by knee flexor strength. Subjects had to hold the knee angle constant at ~1.57 rad during the fatiguing dorsiflexion contraction (Figure 1D), which required activation of the accessory knee flexor and extensor muscles. Previous reports have indicated that briefer endurance times during such position-holding tasks are associated with greater rates of increase in accessory muscle activity [Rudroff et al. 2005], and coactivation of the biceps femoris relative to vastus lateralis is greater in older adults than young adults [Hortobábyi et al. 2005; Hortobábyi and DeVita 2006]. Further, knee flexor strength has been previously identified as a strong predictor of health-related quality of life, including physical and social functioning, vitality and mental health, in older persons [Samuel et al. 2012]. Thus, knee flexor strength may be related to overall motor function status, as well as to fatigability.
4.3 Muscle activity, force fluctuations, and perceived exertion during the fatiguing contraction
The differences in fatigability between young and older adults were accompanied by greater aEMG amplitude for older adults in each muscle group, even for the subset of participants matched for 1-RM load and sex, although the change from initial EMG amplitude across the task was similar for young and old adults. Greater aEMG amplitude has been observed in older adults previously when performing the same relative task [Baudry et al. 2010, 2012, Hortobágyi et al. 2011; Larsen et al. 2008]. In a previous study in our laboratory, Baudry et al. [2010] found aEMG to be greater in older adults during brief isometric contractions when supporting an inertial load with the wrist extensor muscles, and this was accompanied by less presynaptic modulation of the Ia afferent input to the motor neuron pool and increased agonist and antagonist coactivation. Baudry et al. [2010] concluded that older adults rely more on feedforward control rather than feedback control to perform these types of contractions, which may limit endurance time. Consistent with this interpretation, linear regression models identified tibialis anterior aEMG at the start of the fatiguing contraction as one of two predictors for dorsiflexor endurance time.
Older adults reported greater ratings of perceived exertion than young adults during the fatiguing contraction. Although perceptions of fatigue can be unrelated to measures of fatigability [Kluger et al. 2013; Zwarts et al. 2008], perceived exertion during fatiguing activities likely limits exercise performance [Jones and Killian 2000]. Several studies have identified RPE as a significant predictor of endurance time during isometric contractions [Rudroff et al. 2011], although the association may depend on the task and age of the participants [Allman and Rice 2003; Aminoff et al. 1997; Taylor et al. 1991]. The current RPE results are similar to those of Allman and Rice [2003] who found greater RPE in old men (84 ± 1 years) than young men (25 ± 1 years) at the start of a fatigue protocol. However, RPE during fatiguing isometric contractions is not consistently greater for older adults [Hunter et al. 2005], even in the same muscle group [Griffith et al. 2010]. Moreover, RPEs in the current study did not differ for some older adults (65-75 yrs) relative to young adults (p > 0.05), whereas it did for the oldest adults (75-90 yrs, p < 0.01), which again underscores the importance of age when studying motor function in older adults.
4.4 Limitations and Future Directions
Although the current study suggests that the fatigability of the dorsiflexor muscles during a submaximal isometric contraction may represent a biomarker of motor function in older adults, additional factors likely modulate these associations. A meta-analysis suggests that potential confounding factors include contraction mode (dynamic or isometric) and the muscle group involved in the fatiguing contraction [Christie et al. 2011]. The current study was limited to an isometric contraction, albeit one that is relevant to functional tasks, and it focused exclusively on the dorsiflexor muscles. It remains to be determined if similar associations between fatigability and motor function can be observed in tasks with differing load compliance [Griffith et al. 2010] or in leg muscles that produce most of the power during walking, namely the knee extensor and plantar flexor muscles. Also, lifetime levels of physical activity undoubtedly modulate the preservation of motor function across the lifespan [Aagaard et al. 2007, 2010, Power et al. 2012] and future studies should account for this confounding influence. In the current study, we assessed physical activity in our older adults and enrolled young adults with estimated comparable levels of physical activity. Future studies of the association between age and fatigability should not only include a broad range of old ages, but should also carefully characterize contraction mode, muscle group, and lifetime patterns of physical activity. Also, we tested only relatively healthy community-dwelling older adults capable of continuously walking a brisk 500 m course and our findings should not be extrapolated beyond this cohort, especially to those individuals who are frail.
5. Conclusion
The duration that a submaximal isometric contraction could be sustained with the dorsiflexor muscles was briefer for older adults, even in a subset of men and women who were matched for sex and a measure of muscle strength (1-RM load). However, the group difference in fatigability (endurance time) seems to have been driven by the oldest participants in the study. The briefer endurance times for the older adults were accompanied by similar changes in muscle activity (aEMG) and force fluctuations for young and older adults, but greater ratings of perceived exertion for the older adults. Among the older adults, the variance in endurance time was best predicted by age, force fluctuations during a steady isometric contraction, and knee flexor strength. Significantly, the greater fatigability of older adults was associated with declines in performance on subdomains of motor function that reflect decreases in mobility, muscle strength, fine motor skills (steadiness), and muscle activation. The fatigability of the dorsiflexor muscles when supporting a submaximal load with an isometric contraction, therefore, represents a significant biomarker of motor function in older adults.
HIGHLIGHTS.
Dorsiflexor endurance time was briefer for older than young adults.
Endurance times were not influenced by strength or the sex of the participant.
The briefest endurance times were observed in the oldest adults (76-90 years).
Endurance times were associated with motor function in older adults only.
Endurance times were predicted by age, steadiness, and knee flexor strength.
Acknowledgements
The authors thank Mike Pont Carpentry, LLC (www.mikepontcarpentry.com) for developing customized experimental apparatus, the physicians and staff of the University of Colorado CTRC, Dr. Stéphane Baudry, and Dr. Matthew McQueen. This work was supported by CTRC Grant 1UL1 RR025780. An NIH T32 award (AG000279) to Dr. Schwartz supported JNJ.
ABBREVIATIONS
- MVC
maximal voluntary contraction
- 1-RM
one-repetition maximum
- EMG
electromyography
- aEMG
average electromyography amplitude
- SD
standard deviation
- CV
coefficient of variation
- RPE
rating of perceived exertion
- R2
coefficient of determination
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest The authors report no conflict of interest.
References
- Aagaard P, Magnusson SP, Larsson B, Kjær M, Krustrup P. Mechanical muscle function, morphology and fiber type in lifelong trained elderly. Med Sci Sports Exerc. 2007;39(11):1989–1996. doi: 10.1249/mss.0b013e31814fb402. [DOI] [PubMed] [Google Scholar]
- Aagaard P, Suetta C, Caserotti C, Magnusson SP, Kjær M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20:49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- Allman BL, Rice CL. Perceived exertion is elevated in old age during an isometric task. Eur J Appl Physiol. 2003;89:191–197. doi: 10.1007/s00421-002-0780-4. [DOI] [PubMed] [Google Scholar]
- Aminoff T, Smolander J, Korhonen O, Louhevaara V. Cardiorespiratory and subjective responses to prolonged arm and leg exercise in healthy young and older men. Eur J Appl Physiol. 1997;73:363–368. doi: 10.1007/s004210050173. [DOI] [PubMed] [Google Scholar]
- Baudry S, Klass M, Pasquet B, Duchateau J. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol. 2007;100:515–525. doi: 10.1007/s00421-006-0206-9. [DOI] [PubMed] [Google Scholar]
- Baudry S, Lecoeuvre G, Duchateau J. Age-related changes in the behavior of the muscle-tendon unit of the gastrocnemius medialis during upright stance. J Appl Physiol. 2012;112:296–304. doi: 10.1152/japplphysiol.00913.2011. [DOI] [PubMed] [Google Scholar]
- Baudry S, Maerz AH, Enoka RM. Presynaptic modulation of Ia afferents in young and old adults when performing force and position control. J Neurophysiol. 2010;103:623–631. doi: 10.1152/jn.00839.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff HA, Stähelin HB, Monsch AU, Iversen MD, Weyh A, von Dechend M, Akos R, Conzelmann M, Dick W, Theiler R. Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalized elderly women. Age Ageing. 2003;32:315–320. doi: 10.1093/ageing/32.3.315. [DOI] [PubMed] [Google Scholar]
- Bohannon RW. Reference values for the extremity muscle strength obtained by hand-held dynamometry from adults aged 20-79 years. Arch Phys Med Rehab. 1997;78:26–32. doi: 10.1016/s0003-9993(97)90005-8. [DOI] [PubMed] [Google Scholar]
- Bohannon RW. Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. Percept Mot Skills. 2006;103(1):215–222. doi: 10.2466/pms.103.1.215-222. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Shove ME, Barreca SR, Masters LM, Sigouin CS. Five-repetition sit-to-stand performance by community-dwelling adults: A preliminary investigation of times, determinants, and relationship with self-reported physical performance. Isokinet Exerc Sci. 2007;15:77–81. [Google Scholar]
- Bohannon RW. Measurement of sit-to-stand among older adults. Top Geriatr Rehabil. 2012;28(1):11–16. [Google Scholar]
- Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennet DA. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. 2007;29:66–73. doi: 10.1159/000109498. [DOI] [PubMed] [Google Scholar]
- Burnett RA, Laidlaw DH, Enoka RM. Coactivation of the antagonist muscle does not covary with steadiness in old adults. J Appl Physiol. 2000;89:61–71. doi: 10.1152/jappl.2000.89.1.61. [DOI] [PubMed] [Google Scholar]
- Christie A, Snook EM, Kent-Braun JA. Systematic review and meta-analysis of skeletal muscle fatigue in old age. Med Sci Sports Exerc. 2011;43(4):568–577. doi: 10.1249/MSS.0b013e3181f9b1c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou EA, Carlton LG. Age and contraction type influence motor output variability in rapid discrete tasks. J Appl Physiol. 2002;92:489–498. doi: 10.1152/japplphysiol.00335.2001. [DOI] [PubMed] [Google Scholar]
- Christou EA. Aging and variability of voluntary contractions. Exerc Sport Sci Rev. 2011;39(2):77–84. doi: 10.1097/JES.0b013e31820b85ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Kuh D, Cooper C, Gale CR, Lawlor DA, Matthews F, Hardy R. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40:14–23. doi: 10.1093/ageing/afq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditor DS, Hicks AL. The effect of age and gender on the relative fatigability of the human adductor pollicis muscle. Can J Physiol Pharmacol. 2000;78(10):781–790. [PubMed] [Google Scholar]
- Enoka RM. Neuromechanics of Human Movement. Human Kinetics; Champaign, IL: 2008. [Google Scholar]
- Enoka RM. Muscle fatigue – from motor units to clinical symptoms. J Biomech. 2012;45:427–4333. doi: 10.1016/j.jbiomech.2011.11.047. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kines. 2003;13:1–12. doi: 10.1016/s1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- Eriksrud O, Bohannon RW. Relationship of knee extension force to independence in sitto-stand performance. Phys Ther. 2003;83(6):544–551. [PubMed] [Google Scholar]
- Falconer K, Winter DA. Quantitative assessment of co-contraction at the ankle joint in walking. Electromyogr Clin Neurophysiol. 1985;25:135–149. [PubMed] [Google Scholar]
- Forrest KYZ, Zmuda JM, Cauley JA. Patterns and correlates of muscle strength loss in older women. Gerontology. 2007;53:140–147. doi: 10.1159/000097979. [DOI] [PubMed] [Google Scholar]
- Griffith EE, Yoon T, SK Age and load compliance alter time to task failure for a submaximal fatiguing contraction with the lower leg. J Appl Physiol. 2010;108:1510–1519. doi: 10.1152/japplphysiol.01396.2009. 2010. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Walace RB. Lower extremity function in persons over the age of 70 as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobágyi T, Westerkamp L, Beam S, Moody J, Garry J, Holbert D, DeVita P. Altered hamstring-quadriceps muscle balance in patients with knee osteoarthritis. Clin Biomech. 2005;20:97–104. doi: 10.1016/j.clinbiomech.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, DeVita P. Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc Sport Sci Rev. 2006;34(1):29–35. doi: 10.1097/00003677-200601000-00007. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, Finch A, Solnik S, Rider P, DeVita P. Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol A Biol Sci Med Sci. 2011;66A(5):541–547. doi: 10.1093/gerona/glr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Enoka RM. Muscle endurance is greater for old men compared with strength-matched young men. J Appl Physiol. 2005;99(3):890–897. doi: 10.1152/japplphysiol.00243.2005. [DOI] [PubMed] [Google Scholar]
- Jones NL, Killian KJ. Exercise limitation in health and disease. N Engl J Med. 2000;343:632–641. doi: 10.1056/NEJM200008313430907. [DOI] [PubMed] [Google Scholar]
- Justice JN, Carter CS, Beck HJ, Gioscia-Ryan RA, McQueen M, Enoka RM, Seals DR. Battery of behavioral tests in mice that models age-associated changes in human motor function. AGE. 2014a doi: 10.1007/s11357-013-9589-9. DOI 10.1007/s11357-013-9589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Pierpoint LA, Mani D, Schwartz RS, Enoka RM. Motor function is associated with 1,25(OH)2D and indices of insulin-glucose dynamics in non-diabetic older adults. Aging-Clin Exp Res. 2014b doi: 10.1007/s40520-013-0166-y. DOI 10.1007/s40520-013-0166-y b. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol. 2002;93(5):1813–1823. doi: 10.1152/japplphysiol.00091.2002. [DOI] [PubMed] [Google Scholar]
- Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80:409–416. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzaki M, Shinohara M. Steadiness in plantar flexor muscles and its relation to postural sway in young and elderly adults. Muscle Nerve. 2010;42:78–87. doi: 10.1002/mus.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Russ DW, Kent-Braun JA. Age-related enhancement of fatigue resistance is evident in men during both isometric and dynamic tasks. J Appl Physiol. 2004;97(3):967–975. doi: 10.1152/japplphysiol.01351.2003. [DOI] [PubMed] [Google Scholar]
- Larsen AH, Puggaard L, Hämäläinen U, Aagaard P. Comparison of ground reaction forces and antagonist muscle coactivation during stair walking with ageing. J Electromyogr Kinesiol. 2008;18:568–580. doi: 10.1016/j.jelekin.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Manini TM, Visser M, Won-Park S, Patel KV, Strotmeyer ES, Chen H, Goodpaster B, De Rekeneire N, Newman AB, Simonsick EM, Kritchevsky SB, Ryder K, Schwartz AV, Harris TB. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55:451–457. doi: 10.1111/j.1532-5415.2007.01087.x. [DOI] [PubMed] [Google Scholar]
- Marmon AR, Pascoe MA, Schwartz RS, Enoka RM. Associations among strength, steadiness, and hand function across the adult life span. Med Sci Sports Exerc. 2011;43(4):560–567. doi: 10.1249/MSS.0b013e3181f3f3ab. [DOI] [PubMed] [Google Scholar]
- Marques NR, LaRoche DP, Hallal CZ, Crozara LF, Morcelli MH, Karuka AH, Navega MT, Gonçalves M. Association between energy cost of walking, muscle activation, and biomechanical parameters in older female fallers and non-fallers. Clin Biomech. 2013;28:330–336. doi: 10.1016/j.clinbiomech.2013.01.004. [DOI] [PubMed] [Google Scholar]
- McNeil CH, Rice CL. Fatigability is increased with age during velocity-dependent contractions of the dorsiflexors. J Gerontol A Med Sci Biol Sci. 2007;62A(6):624–629. doi: 10.1093/gerona/62.6.624. [DOI] [PubMed] [Google Scholar]
- Negro F, Holobar A, Farina D. Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J Physiol. 2009;587:5925–5938. doi: 10.1113/jphysiol.2009.178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DS, Martin PE. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture. 2010;31:355–359. doi: 10.1016/j.gaitpost.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Power GA, Dalton BH, Behm DG, Doherty TJ, Vandervoort AA, Rice CL. Motor unit survival in lifelong runners is muscle dependent. Med Sci Sports Exerc. 2012;44(7):1235–1242. doi: 10.1249/MSS.0b013e318249953c. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Masaki K, He Q, Ross GW, Willcox BJ, White L. Midlife muscle strength and human longevity up to age 100 years: a 44-year prospective study among a decedent cohort. AGE. 2012;34:563–570. doi: 10.1007/s11357-011-9256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben DB, McCreath HE, Bohannon RW, Want YC, Bubela DJ, Beaumont J, Rine RM, Lai JS, Gershon RC. Motor assessment using the NIH Toolbox. Neurology. 2013;80(11 S3):S65–S75. doi: 10.1212/WNL.0b013e3182872e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129–161. [Google Scholar]
- Rudroff T, Poston B, Shin IS, Bosjen-Møller J, Enoka RM. Net excitation of the motor unit pool varies with load type during fatiguing contractions. Muscle Nerve. 2005;31:78–87. doi: 10.1002/mus.20241. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Justice JN, Matthews S, Zuo R, Enoka RM. Muscle activity differs with load compliance during fatiguing contractions with the knee extensor muscles. Exp Brain Res. 2010;203:307–316. doi: 10.1007/s00221-010-2233-3. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Justice JN, Holmes MR, Matthews SD, Enoka RM. Muscle activity and time to task failure differ with load compliance and target force for the elbow flexor muscles. J Appl Physiol. 2011;110:125–136. doi: 10.1152/japplphysiol.00605.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel D, Rowe P, Hood V, Nicol A. The relationships between muscle strength, biomechanical functional moments and health-related quality of life in non-elite older adults. Age Ageing. 2012;41:224–230. doi: 10.1093/ageing/afr156. [DOI] [PubMed] [Google Scholar]
- Stanaway FF, Gnjidic D, Blyth FM, Le Coueur DG, Naganathan V, Waite L, Seibel JM, Handelsman DJ, Sambrook PN, Cumming RG. How fast does the Grim Reaper walk? Receiver operating characteristics curve analysis in healthy men aged 70 and over. BMJ. 2011;343:1–4. doi: 10.1136/bmj.d7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Hand GA, Johnson DG, Seals DR. Sympathoadrenal-circulatory regulation during sustained isometric exercise in young and older men. Am J Physiol. 1991;261:R1061–R1069. doi: 10.1152/ajpregu.1991.261.5.R1061. [DOI] [PubMed] [Google Scholar]
- Tracy BL, Byrnes WC, Enoka RM. Strength training reduces force fluctuations during anisometric contractions of the quadriceps femoris muscles in old adults. J Appl Physiol. 2004;96:1530–1540. doi: 10.1152/japplphysiol.00861.2003. [DOI] [PubMed] [Google Scholar]
- Tracy BL, Enoka RM. Older adults are less steady during submaximal isometric contractions with the knee extensor muscles. J Appl Physiol. 2002;92:1004–1012. doi: 10.1152/japplphysiol.00954.2001. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- World Health Organization . International Classification of Functioning, Disability, and Health (Short Version) World Health Organization; Geneva Switzerland: 2001. [Google Scholar]
- Zwarts MJ, Bleigenberg G, van Engelen BGM. Clinical neurophysiology of fatigue. Clin Neurophysiol. 2008;119:2–10. doi: 10.1016/j.clinph.2007.09.126. [DOI] [PubMed] [Google Scholar]