ABSTRACT
Chromatin insulators are DNA–protein complexes that are situated throughout the genome that are proposed to contribute to higher-order organization and demarcation into distinct transcriptional domains. Mounting evidence in different species implicates RNA and RNA-binding proteins as regulators of chromatin insulator activities. Here, we identify the Drosophila hnRNP M homolog Rumpelstiltskin (Rump) as an antagonist of gypsy chromatin insulator enhancer-blocking and barrier activities. Despite ubiquitous expression of Rump, decreasing Rump levels leads to improvement of barrier activity only in tissues outside of the central nervous system (CNS). Furthermore, rump mutants restore insulator body localization in an insulator mutant background only in non-CNS tissues. Rump associates physically with core gypsy insulator proteins, and chromatin immunoprecipitation and sequencing analysis of Rump demonstrates extensive colocalization with a subset of insulator sites across the genome. The genome-wide binding profile and tissue specificity of Rump contrast with that of Shep, a recently identified RNA-binding protein that antagonizes gypsy insulator activity primarily in the CNS. Our findings indicate parallel roles for RNA-binding proteins in mediating tissue-specific regulation of chromatin insulator activity.
KEY WORDS: Chromatin, Insulator, Nuclear organization
INTRODUCTION
Chromatin insulators are DNA–protein complexes that can influence chromatin topology and gene expression. Insulators are capable of inhibiting the interaction of an enhancer with a promoter when placed between the two elements or of preventing the spread of silent chromatin over a transcriptionally active domain. These properties are termed enhancer-blocking or barrier activities, respectively (for review see Matzat and Lei, 2014). The concept that insulators alter the topology of chromatin by promoting specific looping interactions throughout the genome is gaining widespread acceptance. Functionally conserved from Drosophila to human, insulator complexes are generally classified based on the presence of a sequence-specific DNA-binding protein. In mammals, only a single insulator protein, CCCTC-binding Factor (CTCF), has thus far been identified, whereas Drosophila harbors the largest known variety of insulator complexes.
The Drosophila gypsy insulator complex [also known as Su(Hw) insulator complex] is a well-studied class made up of three core proteins that are required for its activity. These proteins include Suppressor of Hairy wing [Su(Hw)], the 2.2 kb isoform of Modifier of mdg4 [Mod(mdg4)2.2 also known as Mod(mdg4)67.2] and Centrosomal Protein 190 (CP190). The zinc-finger protein Su(Hw) confers specificity by binding a particular DNA motif, which is present in the 5′ UTR of the gypsy retrotransposon and also found at thousands of sites throughout the genome (Bushey et al., 2009; Nègre et al., 2010; Spana et al., 1988). Mod(mdg4)2.2 and CP190 both contain a broad complex–tramtrack–bric-a-brac (BTB) dimerization domain that might mediate long-range interactions between insulator complexes (Ghosh et al., 2001; Pai et al., 2004).
In diploid interphase nuclei, gypsy insulator proteins coalesce into large foci that are termed insulator bodies. These concentrations of proteins might serve as focal points of insulator complex interactions, creating discrete, higher-order transcriptional domains (Byrd and Corces, 2003; Gerasimova et al., 2000; Gerasimova and Corces, 1998). However, it has also been suggested that insulator bodies represent storage sites of insulator proteins (Golovnin et al., 2008) or form as a result of certain stress conditions (Schoborg et al., 2013). Although the presence of insulator bodies is not sufficient for insulator activity (Gerasimova et al., 2007; Golovnin et al., 2008), all mutations that are known to alter the integrity of insulator bodies also affect insulator function (Capelson and Corces, 2005; Capelson and Corces, 2006; Gerasimova and Corces, 1998; Ghosh et al., 2001; Golovnin et al., 2012; Lei and Corces, 2006; Matzat et al., 2013; Matzat et al., 2012; Pai et al., 2004). These results suggest that monitoring these structures serves as a useful phenotypic indicator.
The core gypsy insulator components associate with a variety of accessory factors that modulate their function. In both Drosophila and mammals, genome-wide binding profiles of insulator proteins are largely invariant across cell types (Bushey et al., 2009; Kim et al., 2007), suggesting that the particular higher-order chromatin configurations mediated by insulator proteins might be regulated in different cell types. Recent work has shown that RNA and RNA-binding proteins can modulate the activities of the gypsy insulator (Lei and Corces, 2006; Matzat et al., 2013; Matzat et al., 2012), the Drosophila CTCF insulator complex (Lim et al., 2013; Moshkovich et al., 2011), and mammalian CTCF and its partner cohesin (Sun et al., 2013; Yao et al., 2010). In the case of the RNA-binding protein Alan Shepard (Shep), gypsy insulator activity and insulator body localization is antagonized in a tissue-specific manner (Matzat et al., 2012). The basis of this specificity appears to be mainly due to the high expression of Shep in the CNS but not other tissues. We speculated that other RNA-binding proteins exist that might fulfill an analogous function to Shep and sought to test this hypothesis.
Rumpelstiltskin (Rump, also known as Hrp59) is a Drosophila homolog of mammalian heterogeneous ribonuclear protein M (hnRNP M). Similar to Shep (Matzat et al., 2012; Ray et al., 2013), Rump contains three highly conserved RNA recognition motifs and binds directly to RNA (Jain and Gavis, 2008). Like human hnRNP M (Gattoni et al., 1996), Rump affects the splicing of various mRNAs, including its own (Hase et al., 2006; Kiesler et al., 2005). Rump is best characterized with respect to its role in localization of the nanos (nos) and oskar (osk) mRNAs to the posterior pole of the developing embryo (Jain and Gavis, 2008; Sinsimer et al., 2011). Based on this function, a lack of maternal Rump expression results in defects in early posterior patterning of the embryo. Recent work shows that Rump is also required for nos transcript localization in sensory neuron dendrites (Xu et al., 2013). In fact, Rump is expressed throughout all stages of development and in all cell lines tested (Cherbas et al., 2011; Chintapalli et al., 2007), suggesting that it might have yet undiscovered roles.
In this study, we identify Rump as a novel tissue-specific antagonist of the gypsy chromatin insulator. A reduction of Rump levels improves insulator enhancer-blocking and barrier activities in certain tissues. Interestingly, the specificity of Rump function contrasts with that of Shep, in that Rump is expressed highly in all tissues but only antagonizes insulator activity in tissues outside of the CNS. Furthermore, loss of Rump restores insulator body localization in a compromised genetic background only in non-CNS tissues. Rump co-immunoprecipitates with core gypsy insulator proteins, and chromatin immunoprecipitation and sequencing (ChIP-seq) analysis reveals that the vast majority of Rump binding sites colocalize with gypsy insulator proteins at a subset of insulator sites within the genome. We propose that Rump regulates higher-order chromatin organization that is defined by chromatin insulators within certain tissues of the developing fly.
RESULTS
Rump antagonizes gypsy enhancer blocking at ct6
In order to test whether Rump affects gypsy insulator function, we examined the effect of the rump1 mutant on the phenotype of the well-characterized gypsy-insulator-dependent ct6 and y2 alleles. The rump1 null mutation corresponds to a deletion of the promoter and 5′ end of the rump gene, resulting in complete loss of RNA and protein (Jain and Gavis, 2008). The ct6 and y2 loss-of-function alleles correspond to an insertion of a gypsy retrotransposon between the promoter and particular enhancers of the respective gene (Gdula et al., 1996). By blocking communication between these enhancers and the promoter, these insertions result in disruption of the wing margin or loss of abdominal pigmentation for ct6 or y2, respectively. In order to detect either positive or negative effects on insulator function, analysis of ct6 and y2 was performed in the sensitized mod(mdg4)u1 null mutant genetic background, in which enhancer blocking at y2 and ct6 is disrupted and enhancer–promoter communication is partially restored.
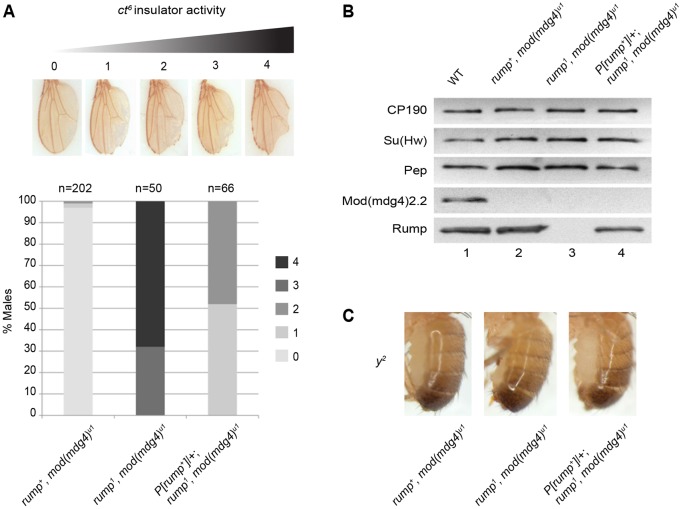
We found that rump1 mutants displayed an increase in enhancer-blocking activity at ct6. Flies were scored on a 0–4 scale, corresponding to the degree of wing notching, with greater numbers corresponding to higher insulator activity. Whereas control mod(mdg4)u1 flies showed almost no notching, double mutant rump1, mod(mdg4)u1 flies exhibited extensive disruption of the wing margin, indicating increased enhancer blocking when Rump is absent (Fig. 1A). Importantly, the enhancer-blocking phenotype was substantially rescued when a genomic copy of rump was expressed in rump1, mod(mdg4)u1 double mutants. Similar results were obtained with rump1 in combination with a loss-of-function allele of mod(mdg4)2.2 [mod(mdg4)T6] (supplementary material Fig. S1A). Furthermore, we observed dominant effects of the rump1 mutation in that rump1, mod(mdg4)u1/+, mod(mdg4)u1 flies show an increase in enhancer-blocking activity compared to mod(mdg4)u1 flies (supplementary material Fig. S1B). These phenotypic effects are specific to the gypsy-insulator-dependent ct6 allele and are not observed for the insulator-independent ctn loss-of-function allele (supplementary material Fig. S1C). Western blotting, using previously characterized antibodies, confirmed loss of Mod(mdg4)2.2 in mod(mdg4)u1, loss of both Rump and Mod(mdg4)2.2 in the double mutant, and restoration of Rump levels in the genomic rescue (Fig. 1B). Furthermore, we observed in rump1, mod(mdg4)+ flies no changes in Mod(mdg4)2.2 levels when Rump is absent (supplementary material Fig. S1D).
Fig. 1.
Null mutation of rump improves gypsy insulator enhancer-blocking activity at ct6. (A) All flies were homozygous for mod(mdg4)u1 and were rump+, rump1, or contained a genomic rescue P[rump+]/+; rump1 as indicated. Flies were scored on a scale of 0–4. 0, no notching; 1, slight notching in tip; 2, mild notching throughout posterior wing; 3, extensive notching in anterior and posterior wing; and 4, severe notching including proximal to longitudinal vein L5. (B) Western blotting of anterior third-instar larval extracts from for CP190, Su(Hw), Pep, Mod(mdg4)2.2 and Rump from wild type (lane 1); mod(mdg4)u1 (lane 2); rump1 mod(mdg4)u1 (lane 3) and P[rump+]/+; rump1 mod(mdg4)u1 (lane 4). Pep is used as a loading control. (C) Abdominal pigmentation due to y2 expression in male flies that were homozygous for mod(mdg4)u1 with rump genotype as indicated.
The effect of rump1, is specific for ct6 but not for other enhancer-blocking reporters. No effect on the y2 phenotype was observed comparing rump1, mod(mdg4)u1 flies with respective controls (Fig. 1C). Furthermore, no phenotypic difference between rump1 or rump1, mod(mdg4)u1 flies, and the respective controls, was observed for ombP1-D11, another gypsy-dependent allele indicative of silencer blocking in the eye (Tsai et al., 1997) (M.R.K. and E.P.L., unpublished). These results indicate that Rump antagonizes gypsy enhancer-blocking activity but also that it perhaps only functions at a subset of gypsy insulators.
Rump is an antagonist of gypsy barrier activity in non-CNS tissues
Given its negative effect on enhancer blocking, specifically at ct6, we tested the effect of Rump depletion on gypsy insulator barrier activity in various tissues. We employed a recently developed quantifiable luciferase-based assay that can be performed in specific tissues of interest (Matzat et al., 2012). In this assay, an upstream activator sequence (UAS)-luciferase reporter, either insulated by Su(Hw) binding sites or non-insulated, is inserted into the same genomic position. Next, a Gal4-inducible double-stranded (ds)RNA hairpin results in depletion of a protein of interest in order to test its effect on barrier activity. Finally, a tissue-specific Gal4 driver promotes expression of the reporter and knockdown. In control lines driving no hairpin, luciferase activity is considerably higher in insulated, compared with non-insulated, lines due to protection from local repressive activity.
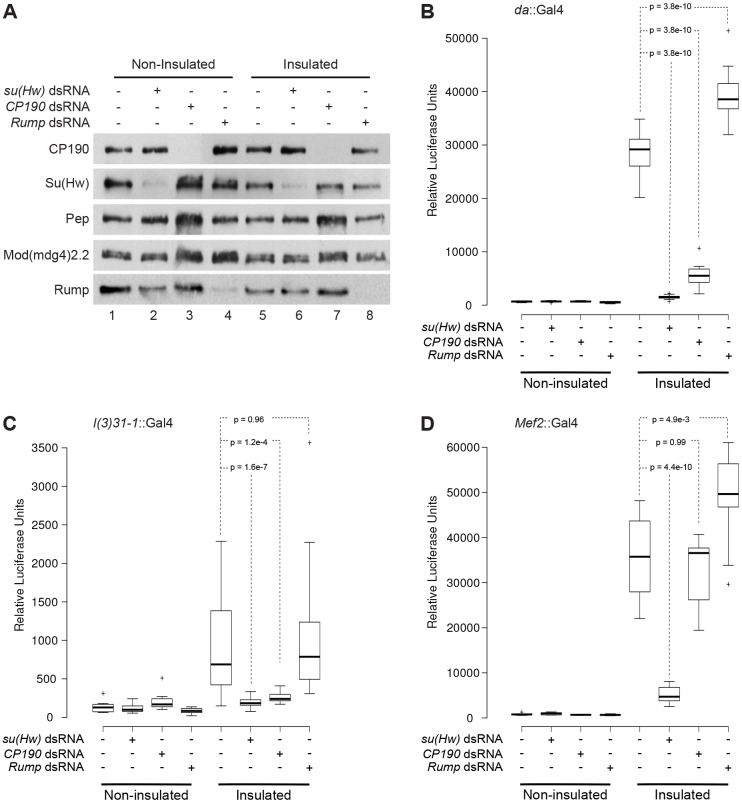
We first examined the effect of Rump depletion on barrier activity throughout the entire larva using the ubiquitous da::Gal4 driver. As positive controls, we knocked down su(Hw) or Cp190, which results in substantial reduction of the respective target protein in both insulated and non-insulated lines (Fig. 2A). As expected, either knockdown caused a dramatic reduction in luciferase signal from the insulated lines, validating the requirement of these proteins in barrier activity (Fig. 2B; supplementary material Fig. S2A). Low luciferase expression was observed in non-insulated lines, regardless of RNA interference (RNAi) hairpin expression (Fig. 2B). We verified that da::Gal4 also drives the efficient reduction of Rump by using western blotting (Fig. 2A). However, in contrast with insulator proteins, Rump depletion resulted in a substantial increase in luciferase signal, demonstrating a negative role for Rump in modulating gypsy-dependent barrier activity (Fig. 2B).
Fig. 2.
Knockdown of rump improves gypsy insulator barrier activity in muscle but not in CNS tissue. (A) Western blotting of anterior third-instar larval extracts for CP190, Su(Hw), Pep, Mod(mdg4)2.2 and Rump in non-insulated (lanes 1–4) and insulated (lanes 5–8) lines. UAS-dsRNA hairpins against su(Hw) (lanes 2 and 6), Cp190 (lanes 3 and 7) and rump (lanes 4 and 8) were driven using da::Gal4. (B–D) Box and whisker plots of the relative luciferase output for a population of larvae expressing indicated dsRNA constructs driven by (B) da::Gal4, (C) l(3)31-1::Gal4, or (D) mef2::Gal4. Populations were compared by using one-way ANOVA followed by a Tukey HSD post-hoc test to obtain P-values for pairwise multiple comparisons (see Materials and Methods). The box of the plots represent the 25th and 75th percentiles, the line represents the median and the whiskers represent a maximum of 1.5-fold interquartile range away from the box. The P-values for pairwise comparisons between the control and RNAi lines within the insulated group are shown within dashed lines. See supplementary material Fig. S2 for complete tables of P-values.
We next examined two additional drivers to test the modulation of barrier activity by Rump in two specific tissues. The l(3)31-1::Gal4 and mef2::Gal4 drivers are highly expressed in the CNS and muscle, respectively. Using l(3)31-1::Gal4, knockdown of su(Hw) and Cp190 caused a significant reduction in luciferase signal only for the insulated lines (Fig. 2C; supplementary material Fig. S2B). However, no significant change in signal was observed when Rump levels were reduced, suggesting that Rump is nonfunctional with respect to insulator activity in the CNS. A considerable reduction of Rump and CP190 levels, as a result of knockdown in brain tissue, was verified by western blotting (supplementary material Fig. S2D), as well as by immunofluorescence (E.P.L., unpublished). In contrast with a lack of effect with the l(3)31-1::Gal4 driver, muscle-specific mef2::Gal4 knockdown of rump caused a significant increase in luciferase signal only in insulated lines (Fig. 2D; supplementary material Fig. S2C), similar to ubiquitous knockdown with da::Gal4. As previously shown, knockdown of su(Hw) with mef2::Gal4 resulted in a decrease of luciferase signal, whereas knockdown of Cp190 produced no significant change in signal in the insulated lines. In summary, these findings demonstrate that Rump antagonizes barrier activity in the muscle but not the CNS.
Loss of Rump alters nuclear localization of gypsy insulators in tissues outside of the CNS
To gain further insight into the tissue-specific regulation of gypsy insulator function by Rump, we examined the effect of rump1 on the nuclear localization of gypsy insulator complexes in various diploid tissues from third-instar larvae. In wild type, indirect immunofluorescence using antibodies against CP190 revealed approximately one insulator body per focal plane in the eye disc, leg disc or optic lobe of the brain (Fig. 3A). In the mod(mdg4)u1 mutant, multiple smaller insulator bodies were observed per focal plane in all tissues tested. By contrast, in the rump1, mod(mdg4)u1 double mutant, insulator bodies were restored to a wild-type appearance in both eye and leg tissue. Significant differences in the number of insulator bodies per nucleus were observed between mod(mdg4)u1 and either wild type or rump1, mod(mdg4)u1 double mutants within the eye and leg (Fig. 3B). However, in the brain of rump1, mod(mdg4)u1 double mutants, no such recovery to wildtype nuclear localization is observed (Fig. 3A). In the brain, statistically significant differences were observed between wild type and either mod(mdg4)u1 or rump1, mod(mdg4)u1 double mutants (Fig. 3B). The rescue of insulator body localization in rump1 mod(mdg4)u1 double mutants in eye and leg, but not brain tissue, is consistent with the antagonistic effect of Rump on barrier activity in non-CNS tissues.
Fig. 3.
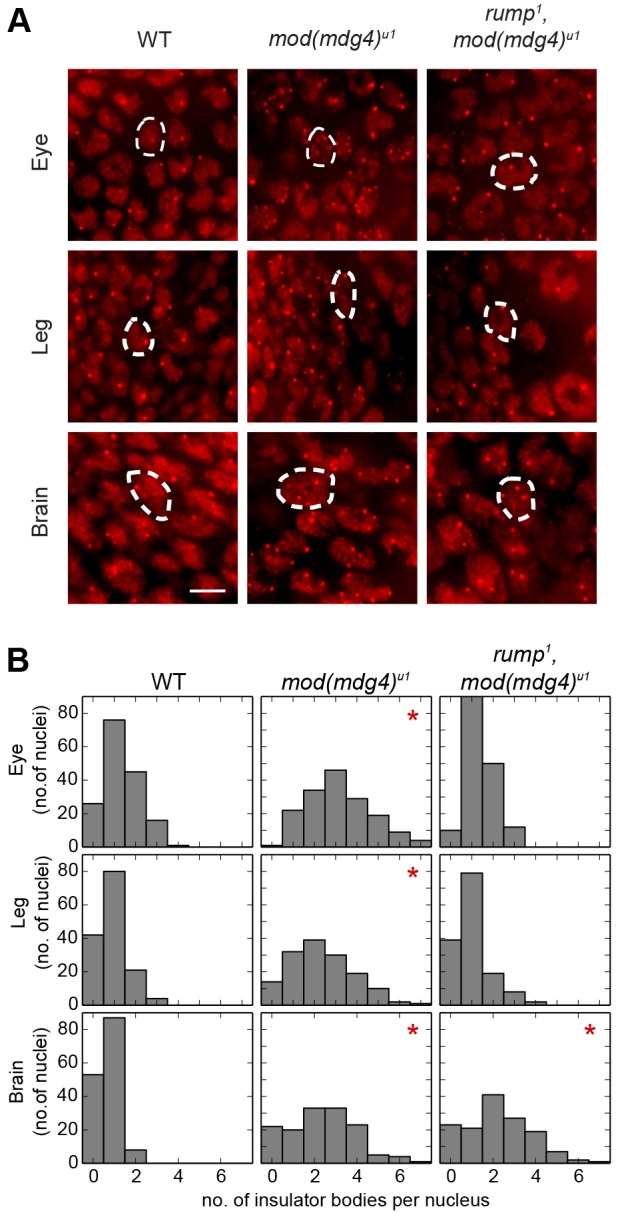
Mutation of rump affects insulator body localization in tissues outside the CNS. (A) Indirect immunofluorescence of insulator bodies using rabbit serum against CP190 and a secondary antibody against rabbit IgG conjugated to Alexa Fluor 594 in the eye (top row) and leg (center row) imaginal discs, as well as the optic lobe of the brain (bottom row) of wild-type (WT, left column), mod(mdg4)u1 (middle column) and rump1 mod(mdg4)u1 (right column) third-instar larvae. Example nuclei are outlined with a dashed line. (B) Histograms of the number of nuclei (y-axis, n>140) that contained the indicated number of insulator bodies per nucleus (x-axis) for a single representative experiment, as in A. *P<1×10−10 compared with wild type, Kruskal–Wallis test. For mod(mdg4)u1 compared with rump1 mod(mdg4)u1, a statistically significant difference was detected in eye and leg, but not brain, tissue. Scale bar: ∼5 µm.
Rump is ubiquitously expressed and localized to the nucleoplasm
Because Rump affects gypsy insulator activity in a tissue-specific manner, we examined the expression profile of Rump in various tissues. Western blotting of Rump in tissues that had been dissected from third-instar larvae showed relatively uniform expression across all of the tissues that were examined, similar to insulator proteins (Fig. 4A). On a subcellular level, Rump was exclusively nuclear in larval tissues and displayed a sponge-like distribution (Fig. 4B). Consistent with antagonistic activity, higher concentrations of Rump did not correspond to insulator bodies, as detected using antibodies against CP190. Both Rump and CP190 were absent from the DAPI-dense region of the nucleus. The ubiquitous expression of Rump throughout the developing fly indicates that spatial restriction of its expression within the organism does not govern the tissue-specific effects of Rump on insulator activity.
Fig. 4.
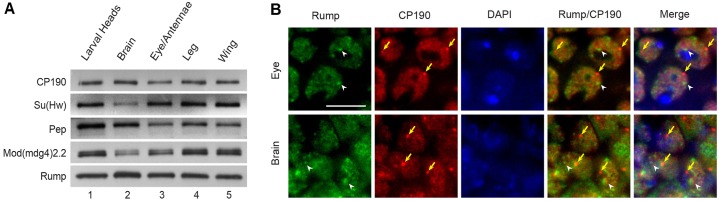
Rump is ubiquitously expressed and localized to the nucleus. (A) Western blotting of wild-type anterior third-instar larval extracts (lane 1), brains (lane 2), eye and antenna discs (lane 3), leg discs (lane 4) and wing discs (lane 5) for CP190, Su(Hw), Pep, Mod(mdg4)2.2 and Rump. (B) Indirect immunofluorescence of wild-type eye imaginal disc (top) or brain optic lobe (bottom) tissue using serum against Rump (green) and CP190 (red), detected by a secondary antibody against mouse IgG conjugated to Alexa Fluor 488 (green) and against rabbit IgG conjugated to Alexa Fluor 594 (red) and stained with DAPI (blue). White arrowheads indicate concentrated areas of Rump staining, and yellow arrows indicate insulator bodies. Scale bar: ∼5 µm.
Rump associates physically with gypsy insulator proteins
Given the antagonistic effects of Rump on gypsy insulator function and insulator body localization, we tested whether Rump interacts physically with gypsy insulator proteins in vivo. To this end, we immunoprecipitated Rump complexes from embryonic nuclear extracts using an antibody against Rump that has been previously used to isolate Rump complexes from the ovary (Jain and Gavis, 2008). Rump was purified with the three core gypsy insulator proteins CP190, Su(Hw) and Mod(mdg4)2.2 (Fig. 5, lane 4). However, the non-gypsy insulator protein CTCF was not detected in the bound fraction (M.R.K. and E.P.L., unpublished). Furthermore, control mouse IgG did not purify Rump or insulator proteins (lane 5). These results indicate that Rump associates physically, either directly or indirectly, specifically with the gypsy class of insulator proteins.
Fig. 5.
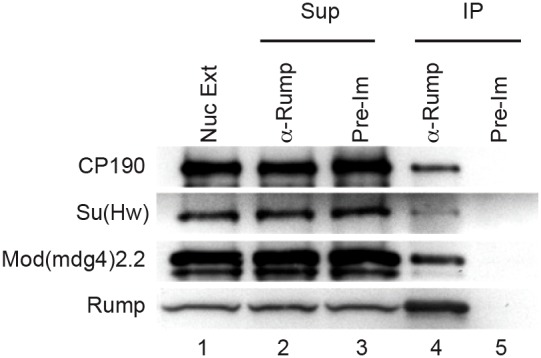
Coimmunoprecipitation of core gypsy insulator proteins with Rump. Nuclear extracts from mixed stage embryos (lane 1) were immunoprecipitated (IP) with either serum against Rump (lanes 2 and 4) or pre-immune serum (lanes 3 and 5). Unbound (lanes 2–3) and bound fractions (lanes 4–5) are shown. All of the indicated proteins were detected by western blotting. Approximately 2.4% of the total Rump and 0.2% of each total insulator protein was immunoprecipitated.
ChIP-seq of Rump reveals extensive colocalization with a subset of gypsy insulator complexes
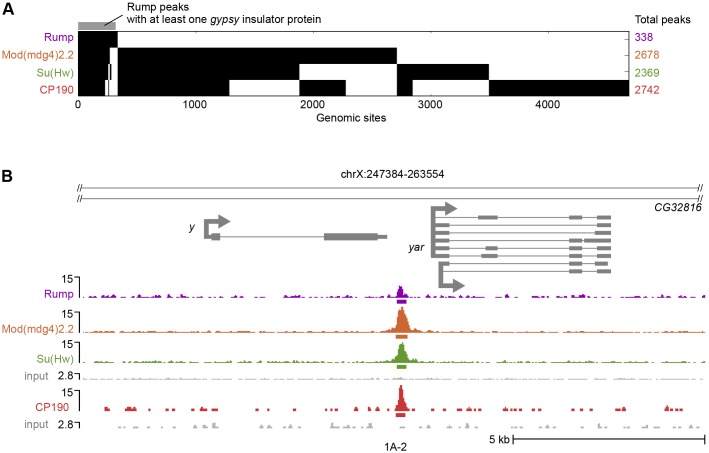
We next performed ChIP-seq of Rump in order to determine its genome-wide chromatin occupancy profile. We used two independent well-characterized monoclonal antibodies against Rump (Jain and Gavis, 2008) to purify associated chromatin from Kc167 cells, which are derived from embryonic hemocyte cells. We used the SPP peak calling algorithm (Kharchenko et al., 2008) at 1% false discovery rate (FDR) to identify 210 and 354 high-specificity binding sites for each respective antibody, which overlapped at 144 sites (supplementary material Fig. S3). Instead of arbitrarily choosing a single antibody for further analysis, we adopted an approach recommended by the Encyclopedia of DNA Elements (ENCODE) consortium (Landt et al., 2012) to calculate the irreproducible discovery rate (IDR), which is based on signal strength and consistency between two antibodies (Li et al., 2011). A chosen IDR threshold is then applied to the pooled signal in order to identify peaks with strong signal that were consistent between both samples. Using a 2% IDR threshold, we identified 338 peaks that correlated with the binding of Rump.
For comparison, we also profiled core gypsy insulator proteins and Shep in Kc167 cells. As with Rump, where two independent antibodies were available, we used SPP and IDR analysis for peak calling, otherwise SPP with a 1% FDR was used. Similar to previous studies, Su(Hw), Mod(mdg4)2.2 and CP190 associated with thousands of sites that, predominantly, overlapped with one another (Fig. 6A). Strikingly, 86% of Rump peaks coincided with those of Su(Hw), Mod(mdg4)2.2 or CP190, with all four factors present at 69% of sites. One of these overlapping sites includes the well-characterized 1A-2 endogenous Su(Hw)-binding site, which is located between y and the yar noncoding RNA gene (Fig. 6B) (Golovnin et al., 2003; Parnell et al., 2003). Interestingly, a parallel attempt to profile Rump by ChIP-seq in CNS-derived BG3 cells failed to detect peaks of chromatin association using the SPP algorithm (R.K.D. and E.P.L., unpublished), suggesting that Rump does not associate with chromatin in this cell type.
Fig. 6.
ChIP-seq of Rump reveals genome-wide colocalization with a subset of core gypsy insulator sites. (A) A binary heat map of Rump, Mod(mdg4)2.2, Su(Hw) and CP190 binding sites in Kc167 cells, ordered by supervised hierarchical clustering. Each row represents a single genomic location, and a mark in a column represents the presence of a particular factor. (B) Screenshot of Rump, Mod(mdg4)2.2, Su(Hw) and CP190 ChIP-seq signals at the 1A-2 endogenous Su(Hw) binding site in reads per million mapped reads. The bars below the signal tracks indicate called peaks. The bottom of each scale bar corresponds to 0. The input shown below each track, or set of tracks, corresponds to the respective immunoprecipitation or set of immunoprecipitations.
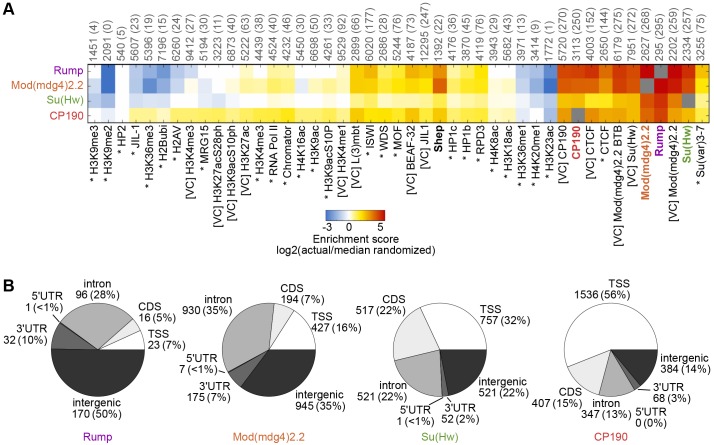
In order to assess the extent of overlap between Rump and insulator proteins, in comparison with other factors, we compared the profile of Rump binding sites with that of other factors that have been profiled previously by using ChIP-chip or ChIP-seq within the same Kc167 cell type, such as RNA polymerase II, various histone modifications and an assortment of chromatin associated proteins (Kellner et al., 2012; Kharchenko et al., 2011; Van Bortle et al., 2012; Wood et al., 2011). We calculated pairwise enrichment scores to evaluate the extent of overlap between all factors and observed high correspondence between Rump, Su(Hw), Mod(mdg4)2.2 and CP190 (Fig. 7A). Substantial enrichment of Rump binding was also observed at CTCF sites, with approximately half of Rump sites colocalizing with those of CTCF. However, only 22% of Rump sites overlapped with the BEAF-32 insulator protein, indicating the specificity of Rump colocalization with certain insulator classes. Moreover, we found that merely 7% of Rump sites overlapped with Shep. Rump shows substantially lower than expected overlap with several factors – including methylated histones, specifically H3K9me2 and H3K36me3, which are marks that are associated with heterochromatin and active transcription over gene bodies, respectively. Unsupervised hierarchical clustering revealed a high similarity between Rump and gypsy insulator protein profiles relative to all other factors, whereas Shep was excluded from this cluster (supplementary material Fig. S4).
Fig. 7.
Comparison of Rump chromatin association to other factors and gene features. (A) Heat map of log2 enrichment scores for pairwise comparisons of binding sites for Rump, Mod(mdg4)2.2, Su(Hw) and CP190 with additional data sets. The color scale corresponding to the enrichment value is indicated (bottom). Positive values indicate significant enrichment, whereas negative values indicate significant negative correlation of enrichment. Self–self comparisons are indicated in gray, and pairwise comparisons that are not statistically significant (P>0.001) are indicated in white. The numbers along top of each column indicate the total number of euchromatic features in each data set, and the number of sites overlapping with Rump are indicated in parentheses. Data from modENCODE (Kharchenko et al., 2011) are indicated by an asterisk, data from other studies (Kellner et al., 2012; Van Bortle et al., 2012; Wood et al., 2011) are indicated by [VC] and those generated in this study are in bold. The full heat map with hierarchical clustering is shown in supplementary material Fig. S4. (B) Classification of Rump, Mod(mdg4)2.2, Su(Hw) and CP190 binding sites. The number of sites and the percentage of the total (shown in parentheses) corresponding to TSS, transcription start site; CDS, coding sequence; 5′ UTR, 5′ untranslated region; 3′ UTR, 3′ untranslated region. See Materials and Methods for the hierarchy of overlapping categories.
We further characterized Rump binding sites by examining their distribution relative to gene features and specificity for particular sequences. Consistent with the finding that Rump associates extensively with gypsy insulator proteins, we found that half of Rump binding sites occurred at intergenic regions, and approximately a third of sites corresponded to intron regions (Fig. 7B). This profile is similar to the overall distribution of Mod(mdg4)2.2 and Su(Hw). Not surprisingly, motif analysis using MEME-ChIP (Machanick and Bailey, 2011) of Rump binding sites, using either only intergenic or intronic sites, returned the canonical Su(Hw)-binding motif, as reported previously (Nègre et al., 2010). These results support the notion that Rump associates specifically with a subset of gypsy insulator sites through an interaction with insulator proteins.
DISCUSSION
Our work demonstrates a newly identified nuclear role for Rump in the tissue-specific regulation of the gypsy chromatin insulator. Rump affects insulator activity and insulator body localization in all tissues tested that are located outside of the CNS, despite Rump being ubiquitously expressed. In the developing fly, Rump is localized to the nucleus and co-immunoprecipitates with core gypsy insulator proteins. Consistent with this observation, ChIP-seq analysis of Rump revealed extensive co-localization with core gypsy insulator proteins. Intriguingly, the tissue-specific activity of Rump contrasts with that of Shep, which antagonizes gypsy insulator activity and insulator body localization specifically in the CNS. Taken together, these findings suggest independent, but parallel, mechanisms to modulate gypsy insulator activity in distinct tissues using different RNA-binding proteins.
Rump is a multifunctional protein
This study provides the first evidence of a direct role for Rump in mediating chromatin function. Previous work has shown that Rump localizes to the nucleus of S2 cells, derived from embryonic hemocytes, as well as in both somatic and germline cells of the ovary (Hase et al., 2006; Jain and Gavis, 2008; Kiesler et al., 2005). Nuclear localization of Rump is consistent with its role in the regulation of splicing of its own transcript and, potentially, that of other mRNAs (Hase et al., 2006; Kiesler et al., 2005). Importantly, we found that insulator protein levels were not affected in rump mutants, suggesting that alteration of insulator activity is not likely to be due to changes in splicing and overall levels of insulator protein transcripts. The Chironomus tentans homolog of Rump, Ct-Hrp59, was shown to associate with a subset of actively transcribed regions on polytene chromosomes, dependent on the presence of RNA, suggesting that chromatin association might be due to the binding to nascent transcripts (Kiesler et al., 2005). Rump has also been recently identified by mass spectrometry in the chromatin-associated fraction of the dosage compensation complex (Wang et al., 2013). Because our high resolution ChIP-seq profiling of Rump was performed in a female cell line, the sites we observed cannot be due to interaction with the dosage compensation complex. Furthermore, the majority of the several hundred sites that we observed are coincident with gypsy insulator proteins and are also enriched for the canonical Su(Hw) binding motif. This binding profile suggests a more likely mode of recruitment by insulator proteins instead of an association with nascent transcripts.
The role of Rump in posterior mRNA localization in the early embryo also appears to be unrelated to its function in the regulation of chromatin insulator function. Rump is required for the posterior localization of the nos and osk mRNAs, which are involved in the patterning of the early embryo (Jain and Gavis, 2008), and it is the maternal levels of Rump that are crucial for this activity. In our insulator assays, we observed changes in adult flies that lacked zygotic Rump expression but that received wild-type levels of maternally deposited Rump. It is also interesting to note that the RNA-binding protein Aubergine (aub) interacts physically with Rump in an RNA-dependent manner in the ovary and also plays a role in posterior localization of nos mRNA (Becalska et al., 2011). However, loss-of-function zygotic mutants for aub actually disrupt gypsy insulator activity (Lei and Corces, 2006), which is the opposite effect to that observed in rump mutants. Rump is expressed ubiquitously and at fairly high levels throughout development, and Rump localizes to the nucleus in most tissues but is predominantly cytoplasmic in the early embryo and in the presumptive germ cells. These observations suggest that Rump engages in multiple distinct processes throughout development.
Rump antagonizes gypsy insulator activity in a tissue-specific manner
We observed that a decrease in Rump levels improved gypsy enhancer-blocking and barrier activities, but this effect was only observed in certain tissues outside of the CNS. Shep, the first identified tissue-specific regulator of insulator activity, displays a contrasting pattern of activity in that it appears to mainly function within CNS tissue (Matzat et al., 2012). The tissue-specificity of Shep function generally correlates with its substantially higher expression in the CNS. Although depletion of Shep in the muscle has no effect on barrier activity, its overexpression in this tissue results in reduced barrier activity. This result suggests that a certain threshold of Shep is sufficient to reduce insulator activity. By contrast, Rump is ubiquitously expressed and is also known to function in nos localization in sensory neurons (Xu et al., 2013); therefore, an alternative mechanism must exist to impart the specificity of its regulatory activity on insulator function.
We found that Rump associates with a subset of gypsy insulator sites in Kc167 cells but, in CNS-derived BG3 cells, little or no chromatin association was observed, suggesting that the interaction between Rump and insulator proteins is dependent on cell type. One possible explanation for its tissue-specific effect is that Rump can only act in concert with an, as yet, unknown factor in order to antagonize insulator activity, and that this factor itself is expressed in a tissue-specific manner. Alternatively, tissue-specific post-translational modification of Rump and/or gypsy insulator proteins, which affects their ability to interact, could be responsible for the differential effects that are observed.
Our luciferase reporter results showed, for the first time, that CP190 promotes gypsy insulator barrier activity. This result is not surprising considering that, like other core components, CP190 is required for enhancer-blocking activity and, also, that CP190 binding sites have been observed previously to preferentially localize at the borders of H3K27me3 islands (Bartkuhn et al., 2009; Schwartz et al., 2012; Van Bortle et al., 2012). Our luciferase assay probably assesses barrier activity against the spread of Polycomb Group (PcG) repression (Matzat et al., 2012). Although Su(Hw) is required for barrier activity for this reporter in all tissues tested to date, unexpectedly, CP190 knockdown has no effect in muscle tissue. This observation raises the possibility that not all core gypsy insulator components are required for insulator activity in all tissues. Overall, the binding profiles of core gypsy insulator proteins are largely invariant across cell types; however, a small fraction of sites do show cell type specificity (Bushey et al., 2009). Whether these differences reflect actual variation in occupancy or differential looping states, as a result of cell-type-specific chromatin configurations, is not yet clear.
Functional relevance of Rump in the regulation of chromatin insulator function
Our results are consistent with the possibility that Rump antagonizes gypsy insulator function in certain tissues by altering its ability to form higher-order chromatin structures. Loss of Rump restores wild-type insulator body localization in a compromised genetic background in which Mod(mdg4)2.2 is absent. Because Rump associates physically with insulator proteins and colocalizes to a small subset of insulator binding sites within the genome, we suggest that the association of Rump interferes with insulator–insulator interactions, specifically at these sites. Because Rump colocalizes with all of the core insulator proteins, it is more likely that Rump competes with insulator–insulator interactions. Future work will help to determine whether Rump regulates higher-order gypsy insulator interactions on a genome-wide scale.
Regulation of the gypsy chromatin insulator by mRNA-binding proteins as a general mechanism
Rump can now be added to the growing list of RNA-binding proteins that regulate chromatin insulator activity. It has been shown recently that certain mRNAs are stably associated with, and functional components of, the gypsy insulator (Matzat et al., 2013). Because none of the core insulator proteins harbors an obvious RNA-binding domain, it is probable that RNA-binding proteins act as mediators for these interactions. Ectopic expression of untranslatable versions of the gypsy-associated transcripts su(Hw) and Cp190 improve enhancer-blocking activity and alter insulator body localization. However, neither Shep nor Rump is a likely candidate to mediate these particular interactions because both factors antagonize insulator activity. Identification of a panel of RNAs that are associated with the gypsy insulator predicts the existence of additional RNA-binding protein adapters. Rump first came to our attention because it associates with a transcript that is currently annotated as CR18854 in S2 cells (Kiesler et al., 2005), and this transcript is also enriched in gypsy insulator complex purifications (Matzat et al., 2013). Given its role in mRNA localization, one possibility is that Rump acts as an antagonist of gypsy insulator function by sequestering mRNAs, which would otherwise promote insulator activity, into inactive structures.
Usage of a variety of mRNA binding adapters would increase the repertoire of RNAs that could be bound by the gypsy insulator. These putative adapters could be expressed either ubiquitously or in certain tissues, and they could either promote or repress insulator activity. The combinatorial use of both tissue-specific adapters and transcripts could provide context-dependent positive or negative regulation of insulator activity. Future work will provide insight into the precise mechanisms of how mRNAs and RNA-binding proteins work in concert to regulate chromatin insulator activities.
MATERIALS AND METHODS
Drosophila strains
Fly stocks were maintained at 25°C on standard cornmeal medium. P[rump+] and rump1 lines (Jain and Gavis, 2008) were kindly provided by Elizabeth Gavis (Princeton University, Princeton, NJ). The da::Gal4, mef2::Gal4 and l(3)31-1::Gal4 driver lines were obtained from the Bloomington Stock Center. Lines expressing dsRNA to su(Hw) (10724 GD), Cp190 (35078 GD) and rump (44659 GD) were obtained from the Vienna Drosophila RNAi Center (Vienna, Austria). The flies were aged for ct6 and y2 scoring as described previously (Matzat et al., 2012). The rump1 and mod(mdg4)u1 mutations were recombined onto the same chromosome in order to analyze homozygous mutant combinations. Due to low viability of rump1 flies under our growth conditions, mothers heterozygous for mod(mdg4)u1/+, mod(mdg4)T6/+ and/or rump1/+ were used to generate rump1 homozygous progeny and the respective controls. For tests of the dominant effects of rump1/+, along with the respective controls, mod(mdg4)u1 mothers were used to generate progeny. Thus, the same levels of maternal deposit of Mod(mdg4)2.2 and Rump were present per comparison. Flies of the genotype ctn; rump1, mod(mdg4)u1 were not viable and could not be scored. Larvae for the luciferase assay were raised at 25°C. Larvae for western blotting and whole-mount immunofluorescence were raised at 18°C. Oregon R was used as the wild-type strain.
Antibodies
Monoclonal supernatants against Rump (5G4 and 10C3) (Jain and Gavis, 2008) were prepared according to the Development Studies Hybridoma Bank (DSHB) protocol with cells obtained from the DSHB (Iowa City, IA). For western blotting, mouse tissue culture supernatant against Rump 5G4 was used at 1∶5000, guinea pig serum against CP190 (Matzat et al., 2012) was used at 1∶10,000, guinea pig serum against Mod(mdg4)2.2 (Moshkovich and Lei, 2010) was used at 1∶1000, guinea pig serum against Su(Hw) (Moshkovich and Lei, 2010) was used at 1∶7500, and purified serum against Pep (Amero et al., 1991) was used at 1∶1000. For immunofluorescence analysis, rabbit serum against CP190 (Pai et al., 2004) was used at 1∶30,000 and Rump 5G4 (Jain and Gavis, 2008) tissue culture supernatant was used at 1∶50. For immunoprecipitation, Rump 5G4 tissue culture supernatant was used. For ChIP, 5G4 and 10C3 tissue culture supernatants against Rump (Jain and Gavis, 2008), guinea pig serum against Mod(mdg4)2.2, rabbit serum against Mod(mdg4)2.2 (Van Bortle et al., 2012), rabbit serum against Su(Hw) (Bushey et al., 2009), rabbit serum against CP190 (Moshkovich et al., 2011), rabbit serum against Shep (Matzat et al., 2012) and guinea pig serum against Shep (Matzat et al., 2012) were used.
Luciferase insulator assay
Luciferase measurements were carried out as described previously (Matzat et al., 2012) with the exception that da::Gal4-knockdown larvae were homogenized in 60 µl Glo Lysis buffer (Promega, Madison, WI), all other larvae were prepared in 30 µl. Luciferase signal from one whole individual third-instar larva was measured, and a population (n≥10) of a single genotype was aggregated into a box and whisker plot. Populations are compared with one-way ANOVA followed by a Tukey HSD post-hoc test to obtain P-values for each pairwise comparison. The lowest P-value in each experiment is limited due to the relatively low resolution (four significant figures) in the code underlying the TukeyHSD function in R.
Immunofluorescence
Imaginal discs and brains were dissected from at least six larvae of each genotype and subjected to whole-mount staining, as previously described (Moshkovich et al., 2011), using ProLong Gold (Life Technologies, Carlsbad, CA) mounting media. For insulator body localization experiments, six out of seven independent experiments achieved similar results.
Cell lines
Kc167 cells were grown in CCM3 media (Thermo Scientific HyClone, Logan, UT). The cells were maintained in monolayer at 25°C.
Co-immunoprecipitation
Nuclei were isolated from 40 g of mixed stage (0–24 h) embryos as described previously (Lei and Corces, 2006), and nuclear extracts were prepared as indicated previously (Matzat et al., 2012). The extract was split equally between Protein G Sepharose beads that had been pre-bound with normal mouse serum, or 5G4 tissue culture supernatant against Rump, and allowed to bind for 1 h at 4°C. Unbound supernatant was removed, and the beads were washed three times in HBSM (50 mM HEPES, pH 6.7; 150 mM NaCl; 5 mM KCl; 2.5 mM MgCl2) that had been supplemented with 0.3% Triton X-100 and two times in HBSM. The bound protein was eluted in sample buffer by boiling, separated by using SDS-PAGE, transferred to nitrocellulose in 10 mM CAPS, pH 11 and detected by western blotting.
Chromatin immunoprecipitation
ChIP was performed as described previously (Matzat et al., 2012).
Computational analyses
ChIP-seq
ChIP-seq data are available at GSE51462. Analyses were performed in a similar manner to that previously described (Matzat et al., 2012) with the following version and setting updates: bowtie v0.12.8, with params –best –strata –sam -m1 -n2 –tryhard -k1 and picard v1.77.
IDR
For ChIP-seq experiments that were performed with two different antibodies [Rump, Mod(mdg4)2.2, and Shep], we considered the two independent antibodies as replicates. We then applied the irreproducible discovery rate (IDR) method (Landt et al., 2012; Li et al., 2011) to choose the conservative set of peaks that are consistent across replicates. Briefly, the SPP algorithm was used with relaxed parameters, and the IDR method, using a code downloaded from https://sites.google.com/site/anshulkundaje/projects/idr, was applied to identify the number of peaks, N, that were consistent among the replicates. After calling peaks on the pooled input and immunoprecipitation, and sorting them by signal intensity, the top N peaks were retained as the final set. For ChIP-seq experiments with only one replicate (Su(Hw) and CP190), we called peaks with SPP as previously described (Matzat et al., 2012), using a threshold FDR of 0.01 for the final peaks.
Downstream analyses
Analyses were performed using similar methods to those described previously (Matzat et al., 2012) with the following version and setting updates: pybedtools v0.6.2 and BEDTools v2.17.0.
Heat maps
Analyses were performed using similar methods to those described previously (Moshkovich et al., 2011).
Pie charts
Analyses were performed in a manner similar to those described previously (Matzat et al., 2012) using FlyBase version 5.47. Because a ChIP-seq peak can be categorized in to more than one class, we classified a peak by its highest priority annotation class, where the priorities from highest to lowest are transcription start site (TSS), coding sequence (CDS), intron, 5′ UTR, 3′ UTR and intergenic.
Motif analysis
Sequences corresponding to Rump peaks (either intergenic or intronic) were submitted to the MEME-ChIP web server (Machanick and Bailey, 2011) for de novo motif finding, using default parameters.
Acknowledgments
We thank Elizabeth Gavis (Princeton University, Princeton, NJ) for strains, Ann Beyer (University of Virginia School of Medicine, Charlottesville, VA) for serum against Pep, and Judith Kassis (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD) and members of the Lei laboratory for critical reading of the manuscript.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
M.R.K., L.H.M., S.L. and E.P.L. performed experiments; R.K.D. performed computational analyses; M.R.K. and E.P.L. designed the experiments and wrote the paper with input from L.H.M. and R.K.D.
Funding
This work was funded by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health [grant number DK015602-07 to E.P.L.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.151126/-/DC1
References
- Amero S. A., Elgin S. C., Beyer A. L. (1991). A unique zinc finger protein is associated preferentially with active ecdysone-responsive loci in Drosophila. Genes Dev. 5, 188–200 10.1101/gad.5.2.188 [DOI] [PubMed] [Google Scholar]
- Bartkuhn M., Straub T., Herold M., Herrmann M., Rathke C., Saumweber H., Gilfillan G. D., Becker P. B., Renkawitz R. (2009). Active promoters and insulators are marked by the centrosomal protein 190. EMBO J. 28, 877–888 10.1038/emboj.2009.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becalska A. N., Kim Y. R., Belletier N. G., Lerit D. A., Sinsimer K. S., Gavis E. R. (2011). Aubergine is a component of a nanos mRNA localization complex. Dev. Biol. 349, 46–52 10.1016/j.ydbio.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey A. M., Ramos E., Corces V. G. (2009). Three subclasses of a Drosophila insulator show distinct and cell type-specific genomic distributions. Genes Dev. 23, 1338–1350 10.1101/gad.1798209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd K., Corces V. G. (2003). Visualization of chromatin domains created by the gypsy insulator of Drosophila. J. Cell Biol. 162, 565–574 10.1083/jcb.200305013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelson M., Corces V. G. (2005). The ubiquitin ligase dTopors directs the nuclear organization of a chromatin insulator. Mol. Cell 20, 105–116 10.1016/j.molcel.2005.08.031 [DOI] [PubMed] [Google Scholar]
- Capelson M., Corces V. G. (2006). SUMO conjugation attenuates the activity of the gypsy chromatin insulator. EMBO J. 25, 1906–1914 10.1038/sj.emboj.7601068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L., Willingham A., Zhang D., Yang L., Zou Y., Eads B. D., Carlson J. W., Landolin J. M., Kapranov P., Dumais J. et al. (2011). The transcriptional diversity of 25 Drosophila cell lines. Genome Res. 21, 301–314 10.1101/gr.112961.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A. (2007). Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39, 715–720 10.1038/ng2049 [DOI] [PubMed] [Google Scholar]
- Gattoni R., Mahé D., Mähl P., Fischer N., Mattei M. G., Stévenin J., Fuchs J. P. (1996). The human hnRNP-M proteins: structure and relation with early heat shock-induced splicing arrest and chromosome mapping. Nucleic Acids Res. 24, 2535–2542 10.1093/nar/24.13.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdula D. A., Gerasimova T. I., Corces V. G. (1996). Genetic and molecular analysis of the gypsy chromatin insulator of Drosophila. Proc. Natl. Acad. Sci. USA 93, 9378–9383 10.1073/pnas.93.18.9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimova T. I., Corces V. G. (1998). Polycomb and trithorax group proteins mediate the function of a chromatin insulator. Cell 92, 511–521 10.1016/S0092--8674(00)80944--7 [DOI] [PubMed] [Google Scholar]
- Gerasimova T. I., Byrd K., Corces V. G. (2000). A chromatin insulator determines the nuclear localization of DNA. Mol. Cell 6, 1025–1035 10.1016/S1097--2765(00)00101--5 [DOI] [PubMed] [Google Scholar]
- Gerasimova T. I., Lei E. P., Bushey A. M., Corces V. G. (2007). Coordinated control of dCTCF and gypsy chromatin insulators in Drosophila. Mol. Cell 28, 761–772 10.1016/j.molcel.2007.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D., Gerasimova T. I., Corces V. G. (2001). Interactions between the Su(Hw) and Mod(mdg4) proteins required for gypsy insulator function. EMBO J. 20, 2518–2527 10.1093/emboj/20.10.2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovnin A., Biryukova I., Romanova O., Silicheva M., Parshikov A., Savitskaya E., Pirrotta V., Georgiev P. (2003). An endogenous Su(Hw) insulator separates the yellow gene from the Achaete-scute gene complex in Drosophila. Development 130, 3249–3258 10.1242/dev.00543 [DOI] [PubMed] [Google Scholar]
- Golovnin A., Melnikova L., Volkov I., Kostuchenko M., Galkin A. V., Georgiev P. (2008). ‘Insulator bodies’ are aggregates of proteins but not of insulators. EMBO Rep. 9, 440–445 10.1038/embor.2008.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovnin A., Volkov I., Georgiev P. (2012). SUMO conjugation is required for the assembly of Drosophila Su(Hw) and Mod(mdg4) into insulator bodies that facilitate insulator complex formation. J. Cell Sci. 125, 2064–2074 10.1242/jcs.100172 [DOI] [PubMed] [Google Scholar]
- Hase M. E., Yalamanchili P., Visa N. (2006). The Drosophila heterogeneous nuclear ribonucleoprotein M protein, HRP59, regulates alternative splicing and controls the production of its own mRNA. J. Biol. Chem. 281, 39135–39141 10.1074/jbc.M604235200 [DOI] [PubMed] [Google Scholar]
- Jain R. A., Gavis E. R. (2008). The Drosophila hnRNP M homolog Rumpelstiltskin regulates nanos mRNA localization. Development 135, 973–982 10.1242/dev.015438 [DOI] [PubMed] [Google Scholar]
- Kellner W. A., Ramos E., Van Bortle K., Takenaka N., Corces V. G. (2012). Genome-wide phosphoacetylation of histone H3 at Drosophila enhancers and promoters. Genome Res. 22, 1081–1088 10.1101/gr.136929.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharchenko P. V., Tolstorukov M. Y., Park P. J. (2008). Design and analysis of ChIP-seq experiments for DNA-binding proteins. Nat. Biotechnol. 26, 1351–1359 10.1038/nbt.1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharchenko P. V., Alekseyenko A. A., Schwartz Y. B., Minoda A., Riddle N. C., Ernst J., Sabo P. J., Larschan E., Gorchakov A. A., Gu T. et al. (2011). Comprehensive analysis of the chromatin landscape in Drosophila melanogaster. Nature 471, 480–485 10.1038/nature09725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesler E., Hase M. E., Brodin D., Visa N. (2005). Hrp59, an hnRNP M protein in Chironomus and Drosophila, binds to exonic splicing enhancers and is required for expression of a subset of mRNAs. J. Cell Biol. 168, 1013–1025 10.1083/jcb.200407173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. H., Abdullaev Z. K., Smith A. D., Ching K. A., Loukinov D. I., Green R. D., Zhang M. Q., Lobanenkov V. V., Ren B. (2007). Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell 128, 1231–1245 10.1016/j.cell.2006.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt S. G., Marinov G. K., Kundaje A., Kheradpour P., Pauli F., Batzoglou S., Bernstein B. E., Bickel P., Brown J. B., Cayting P. et al. (2012). ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 22, 1813–1831 10.1101/gr.136184.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei E. P., Corces V. G. (2006). RNA interference machinery influences the nuclear organization of a chromatin insulator. Nat. Genet. 38, 936–941 10.1038/ng1850 [DOI] [PubMed] [Google Scholar]
- Li Q. H., Brown J. B., Huang H. Y., Bickel P. J. (2011). Measuring reproducibility of high-throughput experiments. Ann. Appl. Stat. 5, 1752–1779 10.1214/11--AOAS466 [DOI] [Google Scholar]
- Lim S. J., Boyle P. J., Chinen M., Dale R. K., Lei E. P. (2013). Genome-wide localization of exosome components to active promoters and chromatin insulators in Drosophila. Nucleic Acids Res. 41, 2963–2980 10.1093/nar/gkt037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machanick P., Bailey T. L. (2011). MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27, 1696–1697 10.1093/bioinformatics/btr189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzat L. H., Lei E. P. (2014). Surviving an identity crisis: A revised view of chromatin insulators in the genomics era. Biochim. Biophys. Acta. 1839, 203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzat L. H., Dale R. K., Moshkovich N., Lei E. P. (2012). Tissue-specific regulation of chromatin insulator function. PLoS Genet. 8, e1003069 10.1371/journal.pgen.1003069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzat L. H., Dale R. K., Lei E. P. (2013). Messenger RNA is a functional component of a chromatin insulator complex. EMBO Rep. 14, 916–922 10.1038/embor.2013.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshkovich N., Lei E. P. (2010). HP1 recruitment in the absence of argonaute proteins in Drosophila. PLoS Genet. 6, e1000880 10.1371/journal.pgen.1000880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshkovich N., Nisha P., Boyle P. J., Thompson B. A., Dale R. K., Lei E. P. (2011). RNAi-independent role for Argonaute2 in CTCF/CP190 chromatin insulator function. Genes Dev. 25, 1686–1701 10.1101/gad.16651211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nègre N., Brown C. D., Shah P. K., Kheradpour P., Morrison C. A., Henikoff J. G., Feng X., Ahmad K., Russell S., White R. A. et al. (2010). A comprehensive map of insulator elements for the Drosophila genome. PLoS Genet. 6, e1000814 10.1371/journal.pgen.1000814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. Y., Lei E. P., Ghosh D., Corces V. G. (2004). The centrosomal protein CP190 is a component of the gypsy chromatin insulator. Mol. Cell 16, 737–748 10.1016/j.molcel.2004.11.004 [DOI] [PubMed] [Google Scholar]
- Parnell T. J., Viering M. M., Skjesol A., Helou C., Kuhn E. J., Geyer P. K. (2003). An endogenous suppressor of hairy-wing insulator separates regulatory domains in Drosophila. Proc. Natl. Acad. Sci. USA 100, 13436–13441 10.1073/pnas.2333111100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D., Kazan H., Cook K. B., Weirauch M. T., Najafabadi H. S., Li X., Gueroussov S., Albu M., Zheng H., Yang A. et al. (2013). A compendium of RNA-binding motifs for decoding gene regulation. Nature 499, 172–177 10.1038/nature12311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoborg T., Rickels R., Barrios J., Labrador M. (2013). Chromatin insulator bodies are nuclear structures that form in response to osmotic stress and cell death. J. Cell Biol. 202, 261–276 10.1083/jcb.201304181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Y. B., Linder-Basso D., Kharchenko P. V., Tolstorukov M. Y., Kim M., Li H. B., Gorchakov A. A., Minoda A., Shanower G., Alekseyenko A. A. et al. (2012). Nature and function of insulator protein binding sites in the Drosophila genome. Genome Res. 22, 2188–2198 10.1101/gr.138156.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsimer K. S., Jain R. A., Chatterjee S., Gavis E. R. (2011). A late phase of germ plasm accumulation during Drosophila oogenesis requires lost and rumpelstiltskin. Development 138, 3431–3440 10.1242/dev.065029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spana C., Harrison D. A., Corces V. G. (1988). The Drosophila melanogaster suppressor of Hairy-wing protein binds to specific sequences of the gypsy retrotransposon. Genes Dev. 2, 1414–1423 10.1101/gad.2.11.1414 [DOI] [PubMed] [Google Scholar]
- Sun S., Del Rosario B. C., Szanto A., Ogawa Y., Jeon Y., Lee J. T. (2013). Jpx RNA activates Xist by evicting CTCF. Cell 153, 1537–1551 10.1016/j.cell.2013.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Jang C. C., Prikhod'ko G. G., Bessarab D. A., Tang C. Y., Pflugfelder G. O., Sun Y. H. (1997). Gypsy retrotransposon as a tool for the in vivo analysis of the regulatory region of the optomotor-blind gene in Drosophila. Proc. Natl. Acad. Sci. USA 94, 3837–3841 10.1073/pnas.94.8.3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bortle K., Ramos E., Takenaka N., Yang J., Wahi J. E., Corces V. G. (2012). Drosophila CTCF tandemly aligns with other insulator proteins at the borders of H3K27me3 domains. Genome Res. 22, 2176–2187 10.1101/gr.136788.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. I., Alekseyenko A. A., LeRoy G., Elia A. E., Gorchakov A. A., Britton L. M., Elledge S. J., Kharchenko P. V., Garcia B. A., Kuroda M. I. (2013). Chromatin proteins captured by ChIP-mass spectrometry are linked to dosage compensation in Drosophila. Nat. Struct. Mol. Biol. 20, 202–209 10.1038/nsmb.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. M., Van Bortle K., Ramos E., Takenaka N., Rohrbaugh M., Jones B. C., Jones K. C., Corces V. G. (2011). Regulation of chromatin organization and inducible gene expression by a Drosophila insulator. Mol. Cell 44, 29–38 10.1016/j.molcel.2011.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Brechbiel J. L., Gavis E. R. (2013). Dynein-dependent transport of nanos RNA in Drosophila sensory neurons requires Rumpelstiltskin and the germ plasm organizer Oskar. J. Neurosci. 33, 14791–14800 10.1523/JNEUROSCI.5864--12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Brick K., Evrard Y., Xiao T., Camerini-Otero R. D., Felsenfeld G. (2010). Mediation of CTCF transcriptional insulation by DEAD-box RNA-binding protein p68 and steroid receptor RNA activator SRA. Genes Dev. 24, 2543–2555 10.1101/gad.1967810 [DOI] [PMC free article] [PubMed] [Google Scholar]