Abstract
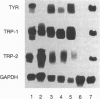
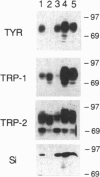
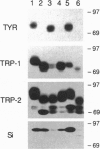
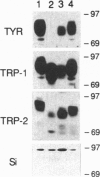
Cutaneous melanomas of Tyr-SV40E transgenic mice (mice whose transgene consists of the tyrosinase promoter fused to the coding regions of simian virus 40 early genes) strikingly resemble human melanomas in their development and progression. Unlike human melanomas, the mouse tumors all arise in genetically identical individuals, thereby better enabling expression of specific genes to be characterized in relation to advancing malignancy. The products of pigment genes are of particular interest because peptides derived from these proteins have been reported to function as autoantigens with immunotherapeutic potential in some melanoma patients. However, the diminished pigmentation characteristic of many advanced melanomas raises the possibility that some of the relevant products may no longer be expressed in the most malignant cells. We have therefore investigated the contributions of several pigment genes in melanotic vs. relatively amelanotic components of primary and metastatic mouse melanomas. The analyses reveal marked differences within and among tumors in levels of mRNAs and proteins encoded by the wild-type alleles at the albino, brown, slaty, and silver loci. Tyrosinase (the protein encoded by the albino locus) was most often either absent or undetectable as melanization declined. The protein encoded by the slaty locus (tyrosinase-related protein 2) was the only one of those tested that was clearly present in all the tumor samples. These results suggest that sole reliance on targeting tyrosinase-based antigens might selectively favor survival of more malignant cells, whereas targeting the ensemble of the antigens tested might contribute toward a more inclusive and effective antimelanoma strategy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anichini A., Mazzocchi A., Fossati G., Parmiani G. Cytotoxic T lymphocyte clones from peripheral blood and from tumor site detect intratumor heterogeneity of melanoma cells. Analysis of specificity and mechanisms of interaction. J Immunol. 1989 May 15;142(10):3692–3701. [PubMed] [Google Scholar]
- Aroca P., Urabe K., Kobayashi T., Tsukamoto K., Hearing V. J. Melanin biosynthesis patterns following hormonal stimulation. J Biol Chem. 1993 Dec 5;268(34):25650–25655. [PubMed] [Google Scholar]
- Bakker A. B., Schreurs M. W., de Boer A. J., Kawakami Y., Rosenberg S. A., Adema G. J., Figdor C. G. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994 Mar 1;179(3):1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M., Klein-Szanto A., Porter S., Mintz B. Malignant melanoma in transgenic mice. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):164–168. doi: 10.1073/pnas.88.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichard V., Van Pel A., Wölfel T., Wölfel C., De Plaen E., Lethé B., Coulie P., Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993 Aug 1;178(2):489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli C., Storkus W. J., Maeurer M. J., Martin D. M., Huang E. C., Pramanik B. N., Nagabhushan T. L., Parmiani G., Lotze M. T. Mass spectrometric identification of a naturally processed melanoma peptide recognized by CD8+ cytotoxic T lymphocytes. J Exp Med. 1995 Jan 1;181(1):363–368. doi: 10.1084/jem.181.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. H., Jr, Elder D. E., Guerry D., 4th, Epstein M. N., Greene M. H., Van Horn M. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984 Dec;15(12):1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- Cox A. L., Skipper J., Chen Y., Henderson R. A., Darrow T. L., Shabanowitz J., Engelhard V. H., Hunt D. F., Slingluff C. L., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994 Apr 29;264(5159):716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- Germain R. N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994 Jan 28;76(2):287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Ekel T. M. Mammalian tyrosinase. A comparison of tyrosine hydroxylation and melanin formation. Biochem J. 1976 Sep 1;157(3):549–557. doi: 10.1042/bj1570549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing V. J., Jr Mammalian monophenol monooxygenase (tyrosinase): purification, properties, and reactions catalyzed. Methods Enzymol. 1987;142:154–165. doi: 10.1016/s0076-6879(87)42024-7. [DOI] [PubMed] [Google Scholar]
- Houghton A. N., Real F. X., Davis L. J., Cordon-Cardo C., Old L. J. Phenotypic heterogeneity of melanoma. Relation to the differentiation program of melanoma cells. J Exp Med. 1987 Mar 1;165(3):812–829. doi: 10.1084/jem.165.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton A. N., Vijayasaradhi S., Bouchard B., Naftzger C., Hara I., Chapman P. B. Recognition of autoantigens by patients with melanoma. Ann N Y Acad Sci. 1993 Aug 12;690:59–68. doi: 10.1111/j.1749-6632.1993.tb43996.x. [DOI] [PubMed] [Google Scholar]
- Jackson I. J., Chambers D. M., Budd P. S., Johnson R. The tyrosinase-related protein-1 gene has a structure and promoter sequence very different from tyrosinase. Nucleic Acids Res. 1991 Jul 25;19(14):3799–3804. doi: 10.1093/nar/19.14.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J., Chambers D. M., Tsukamoto K., Copeland N. G., Gilbert D. J., Jenkins N. A., Hearing V. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J. 1992 Feb;11(2):527–535. doi: 10.1002/j.1460-2075.1992.tb05083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez M., Tsukamoto K., Hearing V. J. Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J Biol Chem. 1991 Jan 15;266(2):1147–1156. [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C. H., Robbins P. F., Sakaguchi K., Appella E., Yannelli J. R., Adema G. J., Miki T., Rosenberg S. A. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Szanto A. J., Silvers W. K., Mintz B. Ultraviolet radiation-induced malignant skin melanoma in melanoma-susceptible transgenic mice. Cancer Res. 1994 Sep 1;54(17):4569–4572. [PubMed] [Google Scholar]
- Klein-Szanto A., Bradl M., Porter S., Mintz B. Melanosis and associated tumors in transgenic mice. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):169–173. doi: 10.1073/pnas.88.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Urabe K., Orlow S. J., Higashi K., Imokawa G., Kwon B. S., Potterf B., Hearing V. J. The Pmel 17/silver locus protein. Characterization and investigation of its melanogenic function. J Biol Chem. 1994 Nov 18;269(46):29198–29205. [PubMed] [Google Scholar]
- Kwon B. S., Haq A. K., Pomerantz S. H., Halaban R. Isolation and sequence of a cDNA clone for human tyrosinase that maps at the mouse c-albino locus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7473–7477. doi: 10.1073/pnas.84.21.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner A. M., Pawelek J. Dopachrome conversion: a possible control point in melanin biosynthesis. J Invest Dermatol. 1980 Aug;75(2):192–195. doi: 10.1111/1523-1747.ep12522650. [DOI] [PubMed] [Google Scholar]
- Larue L., Dougherty N., Bradl M., Mintz B. Melanocyte culture lines from Tyr-SV40E transgenic mice: models for the molecular genetic evolution of malignant melanoma. Oncogene. 1993 Mar;8(3):523–531. [PubMed] [Google Scholar]
- Larue L., Dougherty N., Mintz B. Genetic predisposition of transgenic mouse melanocytes to melanoma results in malignant melanoma after exposure to a low ultraviolet B intensity nontumorigenic for normal melanocytes. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9534–9538. doi: 10.1073/pnas.89.20.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Klein-Szanto A. J. Malignancy of eye melanomas originating in the retinal pigment epithelium of transgenic mice after genetic ablation of choroidal melanocytes. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11421–11425. doi: 10.1073/pnas.89.23.11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Silvers W. K., Klein-Szanto A. J. Histopathogenesis of malignant skin melanoma induced in genetically susceptible transgenic mice. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8822–8826. doi: 10.1073/pnas.90.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Silvers W. K. Transgenic mouse model of malignant skin melanoma. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8817–8821. doi: 10.1073/pnas.90.19.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlow S. J., Boissy R. E., Moran D. J., Pifko-Hirst S. Subcellular distribution of tyrosinase and tyrosinase-related protein-1: implications for melanosomal biogenesis. J Invest Dermatol. 1993 Jan;100(1):55–64. doi: 10.1111/1523-1747.ep12354138. [DOI] [PubMed] [Google Scholar]
- Palumbo A., d'Ischia M., Misuraca G., Prota G. Effect of metal ions on the rearrangement of dopachrome. Biochim Biophys Acta. 1987 Aug 13;925(2):203–209. doi: 10.1016/0304-4165(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Robbins P. F., el-Gamil M., Kawakami Y., Stevens E., Yannelli J. R., Rosenberg S. A. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994 Jun 15;54(12):3124–3126. [PubMed] [Google Scholar]
- Steel K. P., Davidson D. R., Jackson I. J. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development. 1992 Aug;115(4):1111–1119. doi: 10.1242/dev.115.4.1111. [DOI] [PubMed] [Google Scholar]
- Terao M., Tabe L., Garattini E., Sartori D., Studer M., Mintz B. Isolation and characterization of variant cDNAs encoding mouse tyrosinase. Biochem Biophys Res Commun. 1989 Mar 15;159(2):848–853. doi: 10.1016/0006-291x(89)90072-7. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K., Jackson I. J., Urabe K., Montague P. M., Hearing V. J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992 Feb;11(2):519–526. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. F., Robbins P. F., Kawakami Y., Kang X. Q., Rosenberg S. A. Identification of a gene encoding a melanoma tumor antigen recognized by HLA-A31-restricted tumor-infiltrating lymphocytes. J Exp Med. 1995 Feb 1;181(2):799–804. doi: 10.1084/jem.181.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder A., Kobayashi T., Tsukamoto K., Urabe K., Aroca P., Kameyama K., Hearing V. J. The tyrosinase gene family--interactions of melanogenic proteins to regulate melanogenesis. Cell Mol Biol Res. 1994;40(7-8):613–626. [PubMed] [Google Scholar]
- Wölfel T., Van Pel A., Brichard V., Schneider J., Seliger B., Meyer zum Büschenfelde K. H., Boon T. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994 Mar;24(3):759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Yasumoto K., Suzuki H., Shibahara S. Cloning of the human DOPAchrome tautomerase/tyrosinase-related protein 2 gene and identification of two regulatory regions required for its pigment cell-specific expression. J Biol Chem. 1994 Oct 28;269(43):27080–27087. [PubMed] [Google Scholar]
- Zhou B. K., Kobayashi T., Donatien P. D., Bennett D. C., Hearing V. J., Orlow S. J. Identification of a melanosomal matrix protein encoded by the murine si (silver) locus using "organelle scanning". Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7076–7080. doi: 10.1073/pnas.91.15.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]