Abstract
To determine how responses evoked by natural odorant mixtures compare to responses evoked by individual odorant chemicals, we mapped 2-deoxyglucose uptake during exposures to vapors arising from a variety of odor objects that may be important to rodents in the wild. We studied 21 distinct natural odor stimuli ranging from possible food sources such as fruits, vegetables, and meats to environmental odor objects such as grass, herbs, and tree leaves. The natural odor objects evoked robust and surprisingly focal patterns of 2-deoxyglucose uptake involving clusters of neighboring glomeruli, thereby resembling patterns evoked by pure chemicals. Overall, the patterns were significantly related to patterns evoked by monomolecular odorant components that had been studied previously. Object patterns also were significantly related to the molecular features present in the mixture components. Despite these overall relationships, there were individual examples of object patterns that were simpler than might have been predicted given the multiplicity of components present in the vapors. In these cases, the object patterns lacked certain responses evoked by their major odorant mixture components. These data suggest the possibility of mixture response interactions and provide a foundation for understanding the neural coding of natural odor stimuli.
INDEXING TERMS: 2-deoxyglucose, odors, imaging techniques, mapping
Spatial patterns of glomerular activity in the rat and mouse olfactory bulb are related systematically to the chemical structures of monomolecular odorant stimuli (Mori et al., 2006; Johnson and Leon, 2007; Johnson et al., 2009). Responses to odorants that share particular sets of molecular features involving functional groups, hydrocarbon structure, and/or overall chemical properties evoke activity in overlapping clusters of glomeruli. These systematic overlaps can be rendered across the bulb as domains of responsiveness to molecular features (Johnson and Leon, 2007).
Understanding the relationships between spatial patterns of activity and odorant chemistry has involved mapping responses to hundreds of isolated odorant chemicals across the entire olfactory bulb (Johnson and Leon, 2007). The normal operation of the sense of smell, however, involves the detection and perception of odorant mixtures that are emitted by natural odor objects. Thus, it is important both to appreciate the nature of these mixtures and to determine the relationships between responses to natural odor stimuli and responses to the relevant pure odorant components.
Models of olfactory coding are influenced by implicit assumptions regarding the nature of the biologically relevant olfactory stimuli. One such assumption is that there are particular components of many natural mixtures that interact specifically with a very limited set of odorant receptors, so that the odor of a natural object is determined additively by only a few molecular components detected with high affinity and specificity (Lin et al., 2006; Bargmann, 2006). This notion is apparently supported by findings from optical imaging of the dorsal surface of the rodent olfactory bulb, where sparse responses to only a few identified volatile components of natural odor objects replicated the response to the object itself in a simple additive manner (Lin et al., 2006). A nearly opposite, but perhaps as well-regarded assumption, is that natural odor objects emit such complex mixtures of equally relevant and unrelated odorant molecules that the responses of individual receptors cannot account for the perceived odor. Rather, the combined responses of a large population of receptors and their associated downstream neurons is considered to be required to convey the pertinent information as a “spatiotemporal” code (Laurent, 1997; Riffell et al., 2009). This notion may be supported by the large number of odorous peaks that can be detected with gas chromatography/mass spectrometry of volatile components emitted by natural odor objects. A third notion is that while natural odor objects may indeed emit mixtures of numerous chemicals, the most abundant odorant components may resemble one another chemically, creating what would be an effectively higher concentration of shared molecular features such as those that have been associated with particular response domains in the glomerular layer (Johnson and Leon, 2007). This possibility might be referred to as a feature-averaging code for odorant mixtures. Although these different coding mechanisms may not be entirely exclusive of one another, and although different mechanisms may operate for different species or different odor objects, there have been few attempts to collect the kind of empirical data that would be needed to move beyond these assumptions.
Animal species differ in the size of their odorant receptor genomes, as well as in the variety and complexity of the odor objects with which they naturally interact (Bargmann, 2006; Leon and Johnson, 2009). Most of our own experiments have focused on the Norway rat, an omnivorous rodent with a large odorant receptor genome and an opportunistic diet that is adapted to life in diverse environments ranging from cities and farms to remote islands with extreme climates (Schein and Orgain, 1953; Calhoun, 1963; Twigg, 1975; Pye and Bonner, 1980; Moors, 1985; Taylor and Thomas, 1993; Drever and Harestad, 1998; Zhang et al., 2007). Given the large number of potentially relevant natural stimuli, and the likelihood that different objects will differ in the rules of the composition of their volatiles, any real understanding of biologically relevant stimuli and their associated responses in the rat will require the investigation of a large number of odor objects.
As a step toward understanding the response of the rat olfactory bulb to natural odor objects, we selected 21 different stimuli (banana, pineapple, apple, orange, strawberry, tomato, beef, fish, garlic, celery, grass, wheat, cedarwood, pine needles, eucalyptus leaves, spearmint, cinnamon, roasted peanuts, mushroom, carrot, and bean sprouts) that sample part of the spectrum of foods and environmental cues to which rats may be exposed in the variety of natural environments in which they live. We then exposed rats to the volatile mixtures emitted by these objects and we monitored the responses of all olfactory glomeruli by mapping uptake of [14C]2-deoxyglucose (2-DG) into anatomically standardized matrices. We wished to determine if the evoked responses: 1) occurred in only a few glomeruli such as would suit a specialized receptor code; 2) involved multiple widely scattered glomeruli to a similar extent such as would suit a distributed population code; or 3) involved larger clusters of multiple glomeruli such as would suit a feature-averaging code. We then collected information from the literature regarding the volatile components emitted by these natural stimuli to determine whether the components tend to be unrelated or whether they bear structural similarities to one another. Finally, we compared the patterns evoked by these natural odor objects to our archive of responses involving over 350 unique isolated chemicals. We wished to determine to what extent the pattern evoked by the natural odor object can be related to the patterns evoked by identified components that are in our archive as well as to patterns evoked by other odorants that share molecular features with identified natural odorant mixture components.
MATERIAL AND METHODS
Preparation and presentation of odors
This study was comprised of six separate experiments involving four or five natural odor objects each, as well as two additional experiments in which bananas were the only natural odor object. On a given experimental day, one rat was exposed to a nitrogen/air mixture to serve as a vehicle blank, and four or five littermates were exposed to different odor objects, the order of which was varied on each day. Four rats were exposed to vapors from each object. In general, objects were chopped into small pieces or blended with deionized water immediately prior to use, as detailed online under Supporting Methods. Photographs of the prepared odor objects are shown in the figures displaying the components and evoked patterns.
In all cases, preparations were placed into 500-mL glass gas-washing bottles and research-grade nitrogen gas was flushed through the bottle at a flow rate of 100 mL/min. The effluent was combined with ultrazero-grade pure air at a dilution of 1/10 for a final flow rate of 1 L/min. All components except for the exposure chamber were equilibrated for at least 15 minutes before exposing rats.
Exposures of rats to odors were conducted according to our usual procedure, which was approved by the University of California, Irvine Institutional Animal Care and Use Committee. Rat pups (17–20 days of age) were injected subcutaneously at the back of the neck with [14C]2-DG (Sigma, St. Louis, MO; 54 mCi/mmol, 0.1 mCi/ml in 0.9% saline, 0.08 mL for a 50-g rat) just before being placed in an odorized chamber, which was a 2-L glass Mason jar with ports bored in the lid for odorant entry and exhaust. Exposures lasted 45 minutes, after which the rat was decapitated. The brain was removed and frozen in 2-methylbutane at about −45°C. Developmental studies have indicated that odorant-evoked patterns of 2-DG uptake change very little between postnatal day 21 and adulthood (Astic and Saucier, 1981).
Mapping
Sectioning of bulb tissue, staining, and autoradiography were performed as previously described and measurements of relative glomerular 2-DG uptake were collected into anatomically standardized matrices by using our semi-automated methods (Johnson et al., 1999, 2004). Data from the two bulbs of a given rat were averaged. Within each of the five separate experiments, uptake in matrices from all vehicle-exposed rats was averaged and this average matrix was subtracted from each matrix of a rat exposed to an odor. Values then were converted to z-scores relative to the mean and standard deviation of uptake over the entire glomerular layer of the same bulb. Matrices from different rats exposed to the same odorant condition in a given experiment were then averaged before generating contour charts or calculating correlation coefficients.
To quantitatively compare patterns evoked by banana to patterns evoked by individual odorants in our archive, we first averaged the matrices from three separate experiments involving banana and then used the average matrix to calculate correlation coefficients. To compare patterns evoked by apple, we first averaged matrices from two presentations of Fuji apples together with individual presentations of three other apple cultivars after determining that there were no significant differences between the patterns evoked by the different cultivars.
Compilation of archived data for analysis of correlations
In our analysis of correlation data, we eliminated archived patterns that were thought to represent blank exposures, usually due to low vapor phase concentrations for odorants that were either not very volatile or that were diluted excessively before use (Johnson et al., 2007b, 2009). For odorants that were present multiple times in our database, we calculated the average correlation of the individual patterns with the odor object. For these odorants we also averaged the separate response matrices and plotted the result as a contour chart for display in our figures.
Compilation of literature data on volatile compositions
We used PubMed, ISI Web of Knowledge, and Google to search for reports on the volatile composition of the odor objects we had used as stimuli. Search terms were combinations of the object’s name with “volatile composition,” “volatile components,” or “headspace.” We attempted to collect five or six articles on each object. If database searches failed to return a sufficient number of reports, we also pursued references in the few articles that we did locate to find older reports. Two book compilations of volatile composition also were used for this purpose (Maarse, 1991; Leung and Foster, 1996). For wheat bran, peanut butter, cedarwood, and eucalyptus, the components are based on only four studies. If we found fewer than four studies on volatile composition (raw beef, raw fish, and mung bean sprouts), we chose to not report any information on the composition. We also chose to not report the composition of carrots or shitake mushrooms, as we judged these patterns to resemble vehicle blanks too closely.
To be used in our analysis, we required that articles give quantitative data on the entire range of volatile components, although in a few cases we estimated relative chromatographic peak areas from figures. In most cases the reports used gas chromatography and mass spectrometry (GC/MS) to identify the components. When information was abundant for a given object we prioritized recent publications and headspace analyses involving freshly homogenized material over extraction procedures and analysis of essential oils. We also favored publications to which we had online access through our institutional subscriptions.
Our primary objective in surveying the literature was to make certain that the major mixture components were represented in our analysis. Because of the wide variance in reported composition for most objects, some reports did not even note the presence of components that other reports considered among the most abundant. Therefore, for each distinct type of object analyzed in each report we listed the 10 most abundant components and assigned them ranks according to their relative abundance. To determine the order of presentation of these odorants we used the average rank across the different studies, ignoring reports in which that component was either not mentioned or not among the 10 most abundant. With exceptions described below, we are only reporting components that were reported in at least two different studies. The highest-ranked component as calculated in this way is listed in the upper left corner of panels C of our various composition figures (Supporting Figs. 1–16), and components of decreasing average rank are read from left to right, and then top to bottom in these panels.
Because we wanted to compare the 2-DG uptake patterns evoked by objects to 2-DG uptake patterns in our archive, we did not include methanol or ethanol in our lists of components, as we have been unable to evoke 2-DG uptake with these compounds in our previous studies. Sometimes articles reported multiple cultivars or species, in which case we treated each occurrence separately. Some reports also considered different maturational stages of the same cultivar, different analysis methods, different times of storage, and so forth, in which case we took an average if the same components were identified, or we treated the results separately if distinct components were identified.
Again, there was considerable variation across the different studies in the reported components and in their relative quantities for most of the odor objects that we investigated. Accordingly, beyond deciding the relative order of presentation in our figures, we only made further attempts to analyze or communicate the relative prevalence of components when the different reports showed unusual agreement. Differences in techniques for extraction and analysis of the chemicals have in many cases been shown to change the measured quantities, probably because of differences in recovery and/or degradation of components after collection. Furthermore, there is widespread evidence for changes in volatile composition with such parameters as time of year, maturation, time and condition of storage, method of preparation of the sample, difference in cultivars, and growing conditions. The different sources of variability provide an explanation for the disagreements in composition across different studies, and they also call for caution in any attempts to compare our data quantitatively with data on composition from the literature.
In some cases the variability in reported composition was so great that we made some exceptions to the rules given above for selecting components. There was very poor agreement between different studies on grass and wheat volatile components, and in order to achieve a reasonable number of components for these two objects we included any compound that was among the top four components in any study, even if that component was not reported in any other study. In the case of eucalyptus, one very extensive study (He et al., 2000) detected myrcene, trans-ocimene, alpha-terpinene, and terpinolene in multiple varieties of eucalyptus. We included these compounds in our figures even though they were not reported in the other three studies on eucalyptus. Similarly, a very extensive study of pineapple volatiles (Elss et al., 2005) detected methyl caproate and methyl butyrate in multiple varieties of the fruit, so that we included these compounds in our list even though they were not reported in the four other studies on pineapple. Finally, for peanut butter, only one study reported explicitly on the presence of 2,5-dimethylpyrazine (Buckholz et al., 1980), while another study did not distinguish between the 2,6- and 2,5-dimethylpyrazine isomers (Burroni et al., 1997). We decided to include 2,5-dimethylpyrazine as a component of peanut butter in our figure.
RESULTS
Overall appearance of object-evoked activity patterns
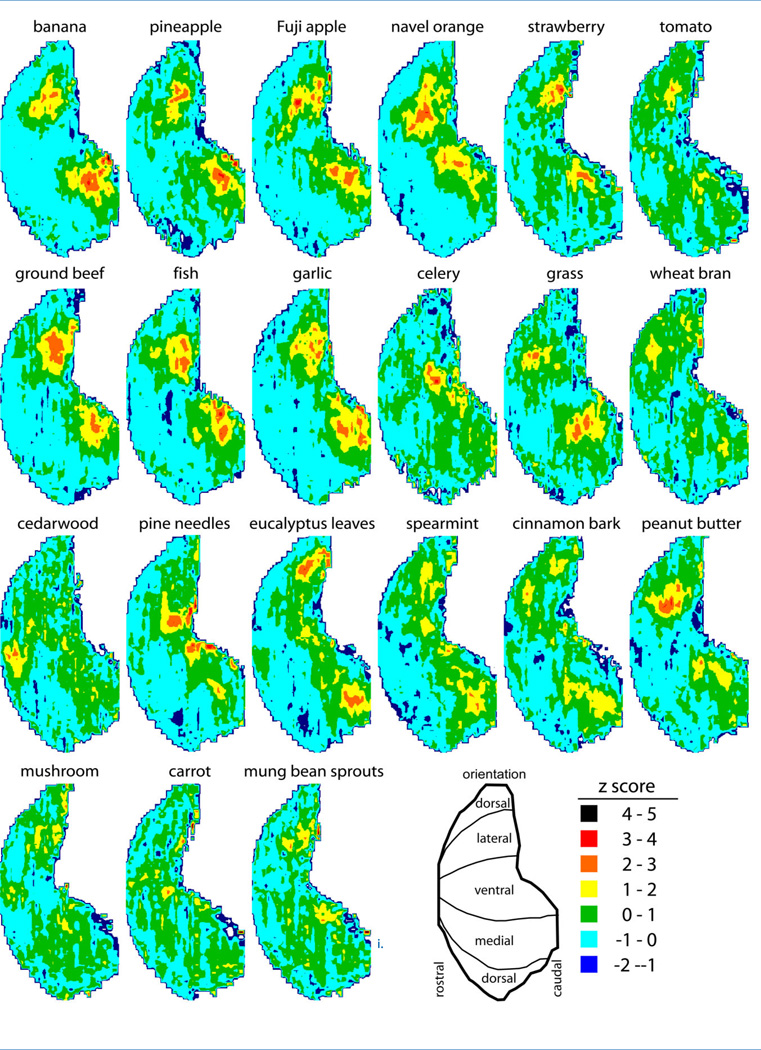
In the great majority of cases (18 of 21), vapors emitted by natural odor objects evoked robust patterns of 2-DG uptake that were clearly distinguishable from patterns obtained in the presence of nonodorized air (Fig. 1). Thus, the 2-DG method can represent levels of odorant stimulation that are naturally relevant. Only the exposures to fragments of shitake mushrooms, carrot shavings, and pieces of mung bean sprouts failed to stimulate appreciable levels of uptake, and the odors arising from these preparations also were only very faintly detectable by the investigators.
Figure 1.
Patterns of average glomerular 2-DG uptake evoked by odor objects are shown as ventral-centered color contour charts of z-score-standardized values across the entire surface of the olfactory bulb. Each chart represents the average z-score pattern for both bulbs of up to four separate rats exposed to each object. The charts can be thought of as rolled-out maps of the bulbar surface after cutting along the dorsal extremity. Warmer colors indicate higher uptake, and the boundary between green and blue occurs at locations displaying the average 2-DG uptake across the entire glomerular layer. The patterns involved high levels of uptake in clusters of neighboring glomeruli rather than similar levels of uptake across widely distributed glomeruli.
The patterns of activity in most cases involved pairs of lateral and medial glomerular domains, which resembles the paired responses observed for most pure chemical odorant stimuli (Johnson and Leon, 2007). The paired structure of the response likely reflects the stimulation of sensory neurons expressing the same odorant receptor gene, but located in different compartments of the epithelium that project separately to the lateral and medial aspects of the bulb (Johnson et al., 1998). Instead of paired responses, celery and cedarwood mostly stimulated single domains along the ventral midline.
The patterns of activity were similar for different animals exposed to the same odor object, but, as expected, differed greatly between natural odor objects. For example, banana stimulated a midlateral cluster of glomeruli and an associated medial response located more posterior in the bulb. In contrast, eucalyptus stimulated glomeruli positioned more dorsally and caudally than the banana response, while grass evoked paired responses more ventral and anterior, and, as mentioned above, celery and cedar-wood stimulated extremely ventral glomeruli at unique rostral-caudal positions in the bulb.
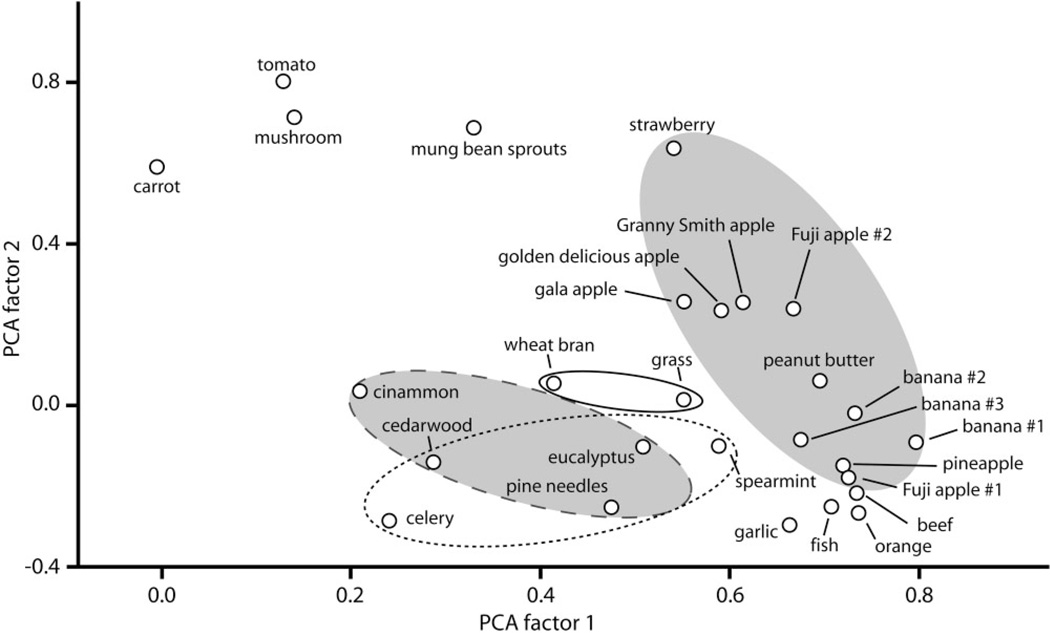
Although each pattern was unique, there were certain similarities within object categories (e.g., fruits, trees, grasses) that may suggest a means for odor generalization as well as discrimination. To characterize the relative pattern similarities between the different odor objects, we performed a principal components analysis using as input the Pearson correlation coefficients obtained by comparing each pair of average 2-DG uptake matrices in the study (Fig. 2). This type of analysis reduces the complexity of the data to only a few dimensions that are more easily illustrated. In Figure 2, each pattern is represented as a single point, with more similar patterns being clustered together. A single ellipse can encompass all of the patterns evoked by noncitrus fruit (gray ellipse without border), while another ellipse can encompass all of the patterns evoked by wood or leaves from trees (gray ellipse with long-dashed border). Wheat bran and fescue leaves, both derived from grasses, also are located near one another in this plot (plain ellipse with solid border). The ellipse with the dotted border encloses patterns from odor objects possessing primarily terpene components, as will be discussed in a later section.
Figure 2.
Principal components analysis of 2-DG uptake patterns indicates clustering of odor objects belonging to similar general classes. Patterns evoked by all the objects of Figure 1 in addition to patterns evoked during replications of the banana exposure and exposures to different cultivars of apple (Supporting Figs. 1, 2) were compared pairwise using cell-by-cell Pearson correlations of uptake matrices. The resulting correlations matrix was used as input data for a principal components analysis and loadings on the first two extracted factors are displayed as a scatterplot to communicate more simply the multidimensional relationships that exist in the data. Each pattern is represented as a symbol, and symbols plotted nearer one another indicate more similar patterns. Ellipses enclose clusters of symbols involving related objects. The gray ellipse without border encloses noncitrus fruits. The gray ellipse with dashed border encloses objects derived from tree leaves or wood. The plain ellipse with solid border encloses two objects that are derived from grasses. The plain ellipse with dotted border indicates objects containing primarily terpene components.
Complexity of activity patterns
The activity patterns did not appear to represent the nearly equal stimulation of numerous glomeruli widely dispersed across the glomerular layer such as would require a distributed population model for encoding. The patterns also did not represent a highly restricted activation of only several glomeruli in each bulb such as would suit a model in which information about the odor of an object would be carried by only a few receptors highly tuned to detect low concentrations of only a few key odorant chemicals. Rather, the patterns involved the stimulation of clusters of nearby glomeruli, not unlike what we have observed when using isolated monomolecular odorant chemicals at relatively high concentrations as stimuli.
Reproducibility of activity patterns
Banana was used as an odor object in three separate studies involving different litters of rats at different times of the year. The average activity patterns evoked in each of these studies are plotted separately in Figure 2, where they clustered fairly tightly within the fruit group, indicating the quantitative similarity of the three banana patterns in comparison to the differences that are present between different odor objects. Supporting Figure 1B shows the average patterns from the three separate studies on banana, and although differences can be detected among the response patterns, the overall similarity across studies is evident.
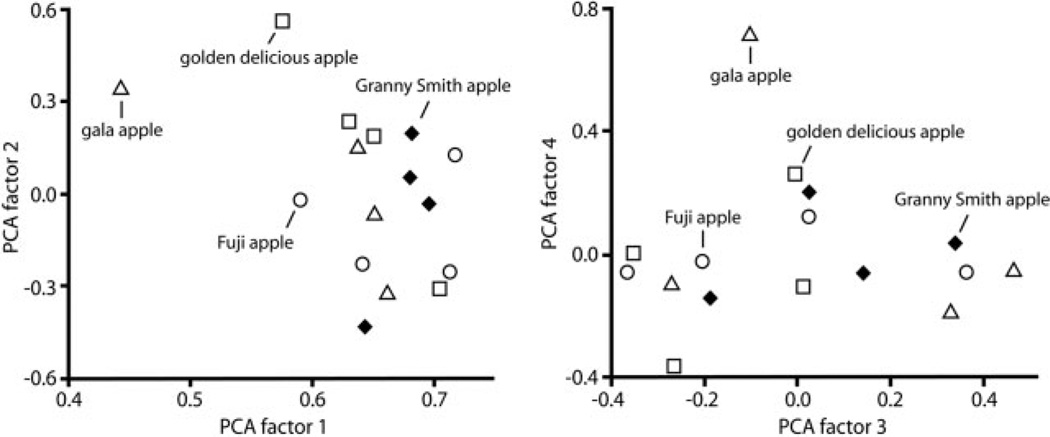
We also wished to determine whether differences in pattern could be detected for different varieties of a single type of object. For this purpose, we repeated our experiment using Fuji apples as stimuli, and we also exposed littermates to pieces of apples from three other cultivars (Golden Delicious, Gala, and Granny Smith). Matrices from these four additional exposures were included in the principal components analysis of Figure 2, where the four cultivars from the same experiment formed a cluster, even though the pattern obtained for the second Fuji apple exposure was more distant from the first exposure to Fuji apples. Supporting Figure 2B shows charts of the average patterns from these five exposures.
For the single experiment involving all four cultivars, we also performed a principal components analysis in which patterns from individual rats were compared to each other. Figure 3 shows plots of the first four factors from this analysis, in which data from the four different cultivars were intermingled and did not separate into distinct clusters of points. Indeed, analysis of variance (ANOVA) on factor loadings indicated no significant differences between cultivars (factor 1, F = 1.0, P = 0.4; factor 2, F = 0.7, P = 0.6; factor 3, F = 0.8, P = 0.5; factor 4, F = 0.2, P = 0.9). This finding contrasts with the fact that we commonly find significant differences between isolated odorants differing incrementally along various chemical dimensions using these methods (Farahbod et al., 2006; Johnson et al., 2006, 2007a).
Figure 3.
Principal components analysis shows that different cultivars of apple evoke indistinguishable patterns of 2-DG uptake. Patterns from individual rats exposed to different types of apples (four rats for each cultivar) were compared by calculating Pearson correlation coefficients for each pair of z-score-transformed uptake matrices. The resulting correlation matrix was used as input for principal components analysis. Loadings on the first four extracted factors are displayed as scatterplots, with individual symbols representing individual animals. Open circles indicate rats exposed to Fuji apple pieces, open triangles indicate rats exposed to Gala apples, open squares indicate rats exposed to Golden Delicious apples, and closed diamonds indicate rats exposed to Granny Smith apples. The patterns involving the different cultivars are interspersed in these plots, and were found not to differ significantly along any factor in ANOVA tests.
Characterization of volatile components
Our first step toward understanding the basis of the activity patterns evoked by natural odor objects was to systematically collect information from the literature regarding the compositions of the mixtures of volatile chemicals that are emitted by the odor objects in our study. As detailed in Materials and Methods, these reports vary considerably in the identity and relative quantities of the chemicals that are present, so we have prioritized those compounds that are abundant and reproducibly detected. Drawings of the chemical structures of the identified components are included in the C panels of each of Supporting Figures 1–16.
The majority of the odor objects emitted mixtures that were dominated by components that resembled one another by sharing molecular features such as functional groups or hydrocarbon structures. The principal types of compounds emitted by each object are summarized in Table 1. More than 50% of the major components belonged to a single, easily recognizable chemical class for 11 of the 16 objects for which reliable composition data could be collected. Components of three of the objects were fairly evenly divided into two distinct classes of chemicals, and only two of the 16 objects were more difficult to classify into groups of related components (Table 1). The relationships among different components associated with each of the objects are discussed in detail in Supporting Results.
TABLE 1.
Shared Functional Groups or Hydrocarbon Features among the Multiple Volatile Components Emitted from Each Odor Object
| Odor object | Class | Frequency | Secondary defining characteristic |
|---|---|---|---|
| Primarily one class of compound | |||
| Banana | Esters | 7/12 (58%) | Branched chain in alcohol portion |
| Apple | Esters | 16/19 (84%) | Straight chain in alcohol portion |
| Strawberry | Esters | 9/15 (60%) | Double bonds, short alcohol portion |
| Pineapple | Esters | 11/15 (73%) | Multiple functional groups |
| Garlic | Sulfides | 11/11 (100%) | |
| Cinnamon | Aromatics | 10/14 (71%) | |
| Eucalyptus | Terpenes | 12/12 (100%) | Primarily hydrocarbons |
| Pine needles | Terpenes | 14/14 (100%) | Primarily hydrocarbons |
| Cedarwood | Terpenes | 6/6 (100%) | Polycyclic |
| Celery | Terpenes | 10/12 (83%) | Primarily hydrocarbons |
| Spearmint | Terpenes | 14/14 (100%) | Oxygenic functional groups |
| Two distinct classes of compounds | |||
| Orange | Terpenes | 7/15 (47%) | |
| Alcohols | 6/15 (40%) | ||
| Peanut butter | Aldehydes | 4/10 (40%) | |
| Methylpyrazines | 4/10 (40%) | ||
| Wheat bran | Aldehydes | 4/9 (44%) | |
| Alcohols | 3/9 (33%) | ||
| Many unrelated compounds | |||
| Tomato | Aldehydes | 4/16 (25%) | Seven of these aliphatic components have double bonds in their alkyl moiety |
| Alcohols | 4/16 (25%) | ||
| Ketones | 3/16 (19%) | ||
| Sulfides | 3/16 (19%) | ||
| Aromatic | 1/16 (6%) | ||
| Nitrosyl | 1/16 (6%) | ||
| Grass | Alcohols | 3/10 (30%) | The top three components share a cis-2-hexenyl moiety |
| Aldehydes | 2/10 (20%) | ||
| Esters | 2/10 (20%) | ||
| Ketones | 2/10 (20%) | ||
| Terpene | 1/10 (10%) |
Overall relationship between patterns evoked by odor objects and patterns evoked by individual components
Over the past decade we have mapped patterns of 2-DG uptake in response to over 350 different isolated odorant chemicals, and we have archived these patterns for quantitative comparisons (Johnson and Leon, 2007). Some of the chemicals in our archive are among those identified as components of the volatile mixtures emitted by the odor objects in this study. If there were a relationship between patterns evoked by individual components of the mixtures and the overall patterns evoked by the natural odorant mixtures, then the patterns evoked by the components should be more closely related to the patterns evoked by the corresponding odor object than would be expected by chance. To test this prediction, we determined the similarity between each object-evoked pattern and each odorant-evoked pattern in our archive, expressing the similarity as a correlation coefficient. We then asked what fraction of the identified components evoked patterns that were among the top 30 correlations calculated for the 323 patterns currently in the archive. By chance, we would expect to find 9.3% (30/323 × 100) of the components in the top 30 correlations. As shown in Table 2, the actual fraction of identified components was greater than the fraction expected by chance for every object in the study for which there were corresponding components in our archive. The difference between the average proportion (38.3%) and the 9.3% expected by chance was statistically significant (one-way t-test, T = 4.19, 14 degrees of freedom, P < 0.0005). As we have detailed for each individual object in Supporting Results, the proportion of components yielding high correlations ranged from 100% for celery and garlic to only 14% for spearmint (Table 2).
TABLE 2.
Each Activity Pattern Evoked by a Natural Odor Object was Compared to a Total of 323 Patterns Previously Evoked by Unique, Nonblank, Pure Chemical Odorants
| Object | Components present in archive |
Components giving top 30 matches |
Fraction |
|---|---|---|---|
| Celery | 4 | 4 | 1.000 |
| Garlic | 1 | 1 | 1.000 |
| Pine needles | 4 | 2 | 0.500 |
| Peanut butter | 5 | 2 | 0.400 |
| Banana | 8 | 3 | 0.375 |
| Apple | 11 | 4 | 0.364 |
| Grass | 3 | 1 | 0.333 |
| Cinnamon bark | 6 | 2 | 0.333 |
| Wheat bran | 4 | 1 | 0.250 |
| Eucalyptus | 4 | 1 | 0.250 |
| Orange | 10 | 2 | 0.200 |
| Strawberry | 10 | 2 | 0.200 |
| Pineapple | 5 | 1 | 0.200 |
| Tomato | 5 | 1 | 0.200 |
| Spearmint | 7 | 1 | 0.143 |
| Cedarwood | 0 | 0 | — |
| Average | 0.383 | ||
| Expected by chance | 0.093 |
This table shows the number of the identified major volatile components that were previously used to evoke patterns of 2-DG uptake, and the fraction of those odorants that gave correlations ranking among the top 30 for each object.
As we further describe in Supporting Results, there were important examples of components that evoked patterns that did not correlate well overall with the patterns evoked by the corresponding objects (orange and cinnamon), but that evoked more focal responses closely overlapping with only a part of the object-evoked pattern, while other components accounted for the remaining pattern. Thus, the relationship between object patterns and component patterns is even more intimate than the correlations analysis suggests.
Overall relationship between patterns evoked by odor objects and patterns evoked by odorants having the same features as volatile components
If there were a relationship between the overlapping molecular features of the multiple components emitted from an odor object and the activity pattern that is evoked by that object, then odorants that possess that same feature should evoke patterns that are well correlated with the object pattern, even if those particular odorants were not actually components of the naturally emitted mixture. To test this prediction, we categorized odorants into a number of classes based on their chemical structure. For each natural odor object we determined the fraction of the identified components that possessed that feature. We also determined how frequently that feature was present in the odorants from our archive that yielded the top 15 correlations with that object’s evoked pattern. We then used linear regression to determine whether these two fractions were significantly correlated with one another across the 16 objects for which we had composition data.
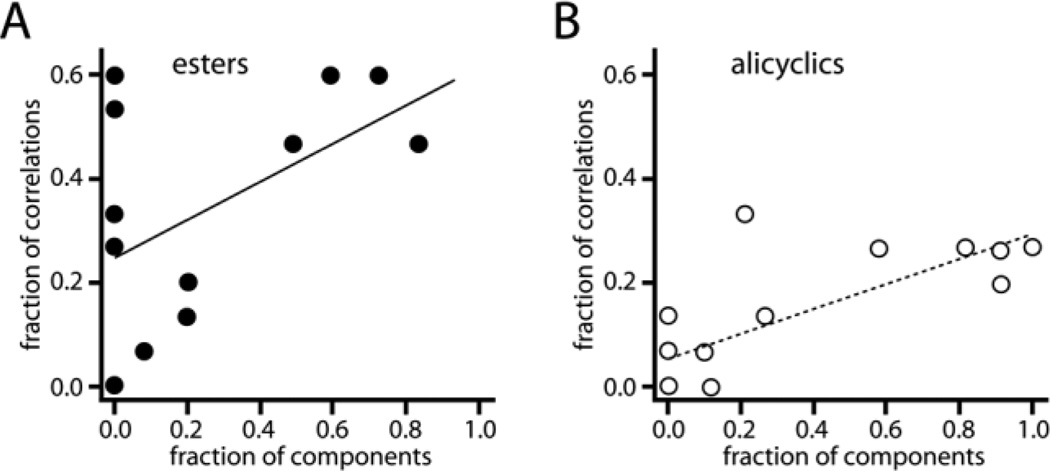
We found significant correlations for alicyclic structures, terpenes (any type), polycyclic structures, hydrocarbons (no element besides C and H), and ester functional groups (Table 3). As should be clear from our discussion of the individual objects in Supporting Results, most of the odorants among the top 15 correlations that possessed the features in question were not components themselves, suggesting that the patterns evoked by the objects are informative about the molecular features of the component odorants as well as being informative about the components themselves. The linear regression and scatter of data for esters and alicyclic odorants are shown in Figure 4.
TABLE 3.
Correlations between the Proportion of the Identified Components That Contain a Given Molecular Feature and the Fraction of the 15 Most Similar Patterns in Our Archive That Are Evoked by Odorants Possessing That Same Feature
| Feature | R | F1,14 | P |
|---|---|---|---|
| Alicyclic | 0.78 | 21.1 | 0.0004 |
| Terpene | 0.66 | 11.1 | 0.0050 |
| Polycyclic | 0.65 | 10.1 | 0.0068 |
| Hydrocarbon only | 0.60 | 8.0 | 0.0132 |
| Ester group | 0.53 | 5.6 | 0.0334 |
| Aromatic | 0.42 | 3.0 | 0.1054 |
| Aldehyde group | 0.35 | 1.9 | 0.1875 |
| Open-chain terpene | 0.30 | 1.4 | 0.2538 |
| Alcohol group | 0.26 | 1.0 | 0.3320 |
Figure 4.
Scatterplots illustrating the importance of chemical features in components to the pattern evoked by an object. A: We plotted on the x-axis the fraction of components in each odor object that possessed an ester group. We also calculated the correlation between the uptake pattern evoked each object and each of the patterns in our archive that were evoked by monomolecular odorants. Along the y-axis we plotted the fraction of the top 15 such correlations that involved odorants containing an ester group. B: The same analysis was done for the odorant feature involving alicyclic hydrocarbon structures. The ester and alicyclic features were among several that showed a significant correlation (Table 3). Lines are fit by least-squares regression.
Individual objects: compositions, correlations, and relative patterns evoked by isolated components
Despite the statistical evidence for the relevance of component-and feature-evoked activity patterns to the overall patterns evoked by odor objects, there were clear cases of major components evoking activity patterns that were quite distinct from the patterns evoked by the object emitting those components. Indeed, some object patterns (e.g., spearmint, strawberry, wheat bran, and tomato) were lacking parts of the activity patterns evoked by known components, which suggests the possibility of pattern simplification through averaging or active response suppression. However, in other cases, such as peanut butter, eucalyptus, and garlic, the overall pattern appeared to be particularly well described by the major component(s). In Supporting Results, we consider each odor object in turn, drawing attention to these interesting and important exceptions as well as to the equally interesting individual manifestations of the overall relationships between objects and components that emerged from the statistical analyses.
DISCUSSION
Chemical characteristics of natural odor stimuli
Our exploration of the composition literature indicated that most objects emit numerous volatile chemicals, but that the mixture generally can be classified into one or two sets of compounds that resemble one another in structure. Each of the individual chemical components would produce an individual peak during GC/MS which would suggest a complex sensory stimulus. However, the number of peaks belies the simplicity of there being a high concentration of a single molecular feature that is produced by the overlap in chemical structure within the components.
The similarity in chemical structure is predictable from the biochemistry of the odor objects. The different odorant components can represent distinct stages of biosynthesis along a single pathway, biosynthesis along a single pathway using distinct but related substrates, biosynthesis along distinct pathways starting from the same substrate, and/or stages in the spontaneous or enzymatic degradation that either occurs gradually during cellular storage, or is initiated upon injury to the cells during cutting and mixing of cellular compartments not naturally in contact with one another (Dixon and Hewett, 2000; Gershenzon et al., 2000; Olofsson et al., 2003).
Data on glomerular activity patterns (Johnson et al., 1998; 2002; Mori et al., 2006; Johnson and Leon, 2007) as well as data on the molecular specificity of various individually expressed receptors (Malnic, 1999; Araneda et al., 2000; Katada et al., 2005) indicate that the specificity of a receptor or glomerulus is better defined by a consideration of the chemical features shared by numerous effective ligands than it is by a single specific ligand. Some of the features we have identified as being associated with glomerular response domains (e.g., ester bonds and polycyclic hydrocarbons) are the same features that overlap naturally in the odor objects analyzed in the present study. Because glomeruli responding to odorants with similar features are clustered together in the bulb (Leon and Johnson, 2003; Johnson and Leon, 2007), interneuron-mediated lateral inhibition involving neighboring bulbar projection neurons of related specificity is likely to occur during responses to natural odor mixtures. Lateral inhibition has been proposed to tune responses to individual odorants so that fewer projection neurons respond to each individual chemical than would be the case without lateral inhibition (Yokoi et al., 1995; Johnson et al., 1999; Johnson and Leon, 2007). However, natural olfactory stimuli are rarely, if ever, isolated chemicals. Given the frequent co-occurrence of related chemicals in natural mixtures, the spatial clusters of similar specificity instead may normally play a different role related to generalization and/or discrimination of natural odor objects.
General features of responses to natural odor objects
Maps of glomerular 2-DG uptake in rat bulbs in the present study suggest that the response to odor objects is not well characterized as an independent and equal stimulation of a wide range of randomly interconnected response elements, but rather typically involves the stimulation of sets of spatially clustered glomeruli. Similar results have been obtained by others using techniques that can access the relative levels of stimulation across the entire main olfactory bulb, such as 2-DG uptake and mapping of c-fos expression, in response to natural objects including cage odors, plant extracts, and urine (Stewart et al., 1979; Astic and Saucier, 1981; Coopersmith et al., 1986; Guthrie et al., 1993; Schaefer et al., 2001). These results are fully consistent with the notion that the natural mixtures contain sets of related chemical components that would stimulate neighboring glomeruli. Therefore, the activation of glomerular clusters is not simply an artifact of the use of isolated odorant chemicals as stimuli, but rather also reflects natural responses to environmentally relevant stimuli.
Instead of finding responses involving very few glomeruli, such as were reported during optical imaging of the dorsal surface of the bulb (Lin et al., 2006), we found that odor objects tended to stimulate larger portions of the glomerular layer. The fact that 2-DG can resolve the stimulation of individual glomeruli when rigid monomolecular odorants are used as stimuli (Johnson and Leon, 2007) argues against the possibility that the larger areas of stimulation in our study simply reflect poor resolution of the 2-DG technique. We think that it is more likely that the dorsal responses are not characteristic of responses occurring elsewhere in the bulb. Indeed, we have reported that the dorsal aspect of the bulb rarely shows the kind of chemotopic organization that is found elsewhere in the glomerular layer (reviewed in Johnson and Leon, 2007), a finding recently confirmed using optical imaging (Soucy et al., 2009).
How many components should be considered?
In addition to different implicit assumptions about the relative composition of odorant mixtures, different investigators also seem to have different assumptions regarding the number of components that one would need to study to explain the response to a natural odor object. One way to test the number of components involved would be to recombine components and test for an animal’s generalization between the artificial mixture and the actual object. Using such an approach, a set of only nine chemicals was found to mimic the overall specialized behavioral response of Manduca moths to Datura flowers (Riffell et al., 2009). We are not aware of such odor reconstitution experiments having been done on rats.
To human evaluators, individual odorants can have odors very similar to the odors of the objects that emit them as major components (Dravnieks, 1985; Arctander, 1994), which may either mean that the neural coding of the object stimulus is mimicked by the response of the single compound or that over their lifetime of olfactory experience the subjects have learned to match a distinctive fragment of the naturally encountered pattern with the perceived odor of the complete stimulus (Wilson and Stevenson, 2003). On the other hand, there are examples of undesired and unpleasant off-odors caused by minor components (such as amines, sulfur compounds, and geosmin) that can dominate odor perception despite being nearly undetectable by chromatographic techniques (Lunden et al., 2002; Dreher et al., 2003; Plutowska and Wardenck, 2008). Such examples may help fuel any opinion that one needs to consider dozens or even hundreds of components to explain the odors of some compounds. However, it is not clear how representative the impact of low-concentration components might be on the perception or activity patterns evoked by natural odor objects in general.
The number of mixture components needed to replicate a natural odor likely will depend on the behavioral assay used to assess odor generalization, because it is clear that animals can be induced to discriminate between even very closely related stimuli after extensive training (Uchida and Mainen, 2003; Abraham et al., 2004). It also is likely that the number of components necessary will depend on the species of animal investigated and the odor object under consideration. For some objects (e.g., spearmint and orange in this study), a single component can greatly predominate in the emitted mixture, and the relative concentrations of additional components can fall off rapidly into undetectability, whereas for other objects there are many components that have similar relative concentrations and that may be more likely to contribute to the overall odor perception of the mixture.
In our analysis we restricted ourselves to the 10 most abundant components in each of several studies, mostly because there is even less agreement in the composition literature for the more minor components than for the major components, for which there already was considerable variance. We found that the spatial glomerular activity pattern in response to some odor objects might be well described by the overlapping responses to only a few major components (e.g., garlic and peanut butter), or by the summation of responses to a few subsets of odorants (e.g., cinnamon and orange), while other patterns bear less resemblance to the patterns evoked by their major components. There would seem to be no substitute for the systematic empirical investigation of a large number of odor objects in order to determine the relative frequencies of each type of response, if the question of how natural odor mixtures are encoded is to receive a meaningful general answer.
Generalizations between patterns evoked by similar objects
We found that different objects of a similar class (e.g., fruits, trees, or grasses) evoked similar activity patterns. These overlaps likely reflect the similarity in the chemical structures of the components (e.g., esters for fruits, terpenes for trees, and alcohols and aldehydes for grasses). There may be an advantage to the relatively large overlap in the responses to odor objects of a similar class. For example, once one fruit is recognized as food, there may be an advantage to considering another fruit as likely to be food even if there had been no previous contact with that particular food.
Generalization is likely to be as important in olfaction as discrimination. For example, because different fruits of the same kind will likely differ in their exact volatile compositions, animals need to be able to respond to the object in spite of these differences in order to benefit from learning that that fruit can serve as food. Likewise, animals need to respond appropriately to relevant stimuli despite the presence of various background odorants. By collecting data from all parts of the olfactory bulb, studies of 2-DG uptake are particularly well suited for analyzing the potential for generalization. Also, it has been repeatedly demonstrated that similarities between 2-DG patterns are correlated with the behavioral generalization of pairs of odorants that are related to one another along various chemical dimensions (Johnson and Leon, 2007).
Interactions between responses in natural mixtures
Despite the statistical correlations between patterns evoked by components and patterns evoked by objects in the present study, there were very remarkable cases where all or a part of a pattern evoked by a major component showed no evidence of contributing to the pattern evoked by the corresponding whole object. Therefore, feature averaging alone cannot explain the coding of all natural odor stimuli. One example was the absence of dorsal responses to L-carvone in the pattern evoked by spearmint, despite L-carvone being its dominant component. Dorsal responses to the major component benzaldehyde also were missing from the cinnamon bark pattern, as were dorsal responses to the major odorant components ethyl and methyl esters from the strawberry pattern. Responses to the potent major odorant component hexanal similarly were missing from the patterns evoked by wheat bran and tomato.
The complete absence of responses to these components is suggestive of an active suppression of activity as a consequence of the component being present in a mixture compared to the response when the same odorant is experienced in isolation. Examples of suppression of responses to one component in the presence of another can be found at many levels in the olfactory systems of many species (Kang and Caprio, 1997; Derby, 2000; Silbering and Galizia, 2007). In rats, competitive antagonists can affect the binding of odorant ligands to odorant receptors (Araneda, 2000; Oka et al., 2004; Sanz et al., 2005), and distinct odorants can excite and inhibit the same sensory neurons (Sanhueza et al., 2000; Duchamp-Viret et al., 2003). Indeed, previous work using 2-DG uptake has documented a peripheral suppression of the response to limonene in a binary mixture with propionic acid (Bell et al., 1987).
Future directions for experiments on natural odor objects
Although we think the present study is a reasonable first step toward understanding responses to natural odor stimuli, there are several issues that arise from the use of literature data to characterize volatile odorant compositions that might affect the conclusiveness of these data. As we have mentioned, there is considerable variance in the reported compositions, some of which can be attributed to different analysis techniques (Yu et al., 2004), different cultivars (Moshonas and Shaw, 1994; Telci et al., 2004), different stages of maturation (Kazeniac and Hall, 1970; Mazza et al., 1992; Echeverria et al., 2004), the possibility of contamination or infestation (Zini et al., 2001; Mumm et al., 2004; Bianchi et al., 2009), different methods of preparation of the material (Deruaz et al., 1994; Mumm et al., 2004), different times of analysis after preparation (Kazeniac and Hall, 1970; Olofsson et al., 2003), and different conditions of storage (Sjvall et al., 2000; Echeverria et al., 2004). It therefore remains possible that the sample of the object we used for our odor stimulus might differ in its volatile composition from the consensus set of components we have derived from the literature.
Our conclusions also may have been affected by the absence of critical odorant components from our archive of previously mapped responses. In no case had we mapped responses to all of the major identified volatile components emitted from an odor object. In addition, the concentrations of the odorants during our mapping probably did not correspond to the concentrations at which they are emitted from the object. Because odorants likely differ in the efficacy with which they stimulate the corresponding receptors, their relative vapor phase concentrations may not be an indication of the magnitude of their contribution to the mixture pattern. Also, although many odorants evoke a similar pattern at different concentrations, some odorants evoke strikingly different patterns when their concentrations are increased (Johnson and Leon, 2000).
More definitive conclusions regarding the contribution of mixture components to the pattern evoked by a natural odor object would come from studies where the volatile components of the actual object used in the mapping would be analyzed quantitatively, with proper corrections for recovery, and where responses to the individual components would be determined at the concentrations measured for that object. Stepwise reconstitution of the mixture and analyses of both the evoked patterns and the perceived odors could then be used to assess the types of mixture interactions as well as the completeness of the reconstitution. These experiments, even for a single odor object, would be intensive and expensive, but understanding the normal operation of the sense of smell requires such a commitment. Convenient shortcuts such as monitoring activity either in only a small part of the glomerular layer, or only in a small subset of neurons, would run the risk of obtaining results that are completely irrelevant to the actual coding of information about that odor object. Our current data suggest that one may have considerable success in relating natural odors to individual odorant chemicals, as well as in identifying meaningful interactions involving mixture components that actually coexist in nature.
Supplementary Material
Acknowledgments
Grant sponsor: United States Public Health Service; Grant numbers: DC03545, DC006391, and DC006516.
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- Arctander S. Perfume and flavor chemicals (aroma chemicals) Carol Stream, IL: Allured Publishing; 1994. [Google Scholar]
- Astic L, Saucier D. Metabolic mapping of functional activity in the olfactory projections of the rat: ontogenetic study. Brain Res. 1981;254:141–156. doi: 10.1016/0165-3806(81)90065-1. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. doi: 10.1038/nature05402. [DOI] [PubMed] [Google Scholar]
- Bell GA, Laing DG, Panhuber H. Odour mixture suppression: Evidence for a peripheral mechanism in human and rat. Brain Res. 1987;426:8–18. doi: 10.1016/0006-8993(87)90419-7. [DOI] [PubMed] [Google Scholar]
- Bianchi F, Careri M, Mangia A, Mattarozzi M, Musci M, Concina I, Falasconi M, Gobbi E, Pardo M, Sberveglieri G. Differentiation of the volatile profile of microbiologically contaminated canned tomatoes by dynamic headspace extraction followed by gas chromatography-mass spectrometry analysis. Talanta. 2009;77:962–970. doi: 10.1016/j.talanta.2008.07.061. [DOI] [PubMed] [Google Scholar]
- Buckholz LL, Withycombe DA, Daun H. Application and characteristics of polymer adsorption method used to analyze flavor volatiles from peanuts. J Agric Food Chem. 1980;28:760–765. [Google Scholar]
- Burroni LV, Grosso NR, Guzman CA. Principal volatile components of raw, roasted, and fried Argentinian peanut flavors. J Agric Food Chem. 1997;45:3190–3192. [Google Scholar]
- Calhoun JB. The ecology and sociology of the Norway rat. Bethesda, MD: US Dept of Health, Education, and Welfare, Public Health Service; 1963. [Google Scholar]
- Coopersmith, Henderson SR, Leon M. Odor specificity of the enhanced neural response following early odor experience in rats. Dev Brain Res. 1986;27:191–197. doi: 10.1016/0165-3806(86)90245-2. [DOI] [PubMed] [Google Scholar]
- Derby CD. Learning from spiny lobsters about chemosensory coding of mixtures. Physiol Behav. 2000;69:203–209. doi: 10.1016/s0031-9384(00)00202-x. [DOI] [PubMed] [Google Scholar]
- Deruaz D, Soussan-Marchal F, Desage M, Bannier A, Brazier JL. Analytical strategy by coupling headspace gas chromatography, atomic emission spectrometric detection and mass spectrometry Application to sulfur compounds from garlic. J Chromatogr A. 1994;677:345–354. [Google Scholar]
- Dixon J, Hewett EW. Factors affecting apple aroma/flavour volatile concentration: a review. N Z J Corp Hort Sci. 2000;28:155–173. [Google Scholar]
- Dravnieks A. Atlas of odor character profiles. Philadelphia: ASTM Data Series DS 61; 1985. [Google Scholar]
- Dreher JG, Rouseff RL, Naim M. GC-olfactometric characterization of aroma volatiles from the thermal degradation of thiamin in model orange juice. J Agric Food Chem. 2003;51:3097–3102. doi: 10.1021/jf034023j. [DOI] [PubMed] [Google Scholar]
- Drever MC, Harestad AS. Diets of Norway rats, Rattus norvegicus on Langara Island, Queen Charlotte Islands, British Columbia: implications for conservation of breeding seabirds. Can Field-Nat. 1998;112:676–683. [Google Scholar]
- Duchamp-Viret P, Duchamp A, Chaput MA. Single olfactory sensory neurons simultaneously integrate the components of an odour mixture. Eur J Neurosci. 2003;18:2690–2696. doi: 10.1111/j.1460-9568.2003.03001.x. [DOI] [PubMed] [Google Scholar]
- Echeverria G, Graell J, Lopez ML, Lara I. Volatile production, quality and aroma-related enzyme activities during maturation of ‘Fuji’ apples. Postharvest Biol Technol. 2004;31:217–227. [Google Scholar]
- Elss S, Preston C, Hertzig C, Heckel F, Richling E, Schreir P. Aroma profiles of pineapple fruit (Ananas comosus [L.] Merr.) and pineapple products. LWT Food Sci Technol. 38:263–274. [Google Scholar]
- Farahbod H, Johnson BA, Minami SS, Leon M. Chemotopic representations of aromatic odorants in the rat olfactory bulb. J Comp Neurol. 2006;496:350–366. doi: 10.1002/cne.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenzon J, McConkey ME, Croteau RB. Regulation of monoterpene accumulation in leaves of peppermint. Plant Physiol. 2000;122:205–213. doi: 10.1104/pp.122.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie KM, Anderson AJ, Leon M, Gall C. Odor-induced increases in c-fos mRNA expression reveal an anatomical 𠇜unit” for odor processing in olfactory bulb. Proc Natl Acad Sci U S A. 1993:903329–90333. doi: 10.1073/pnas.90.8.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Murray F, Lyons T. Monoterpene and isoprene emissions from 15 Eucalyptus species in Australia. Atmos Environ. 2000;34:645–655. [Google Scholar]
- Johnson BA, Leon M. Modular glomerular representations of odorants in the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Chemotopic odorant coding in a mammalian olfactory system. J Comp Neurol. 2007;503:1–34. doi: 10.1002/cne.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Leon M. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J Comp Neurol. 1998;393:457–471. doi: 10.1002/(sici)1096-9861(19980420)393:4<457::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Hingco EE, Pham KL, Leon M. Multidimensional chemotopic responses to n-aliphatic acid odorants in the rat olfactory bulb. J Comp Neurol. 1999;409:529–548. [PubMed] [Google Scholar]
- Johnson BA, Ho SL, Xu Z, Yihan JS, Yip S, Hingco EE, Leon M. Functional mapping of the rat olfactory bulb using diverse odorants reveals modular responses to functional groups and hydrocarbon structural features. J Comp Neurol. 2002;449:180–194. doi: 10.1002/cne.10284. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Farahbod H, Xu Z, Saber S, Leon M. Local and global chemotopic organization: general features of the glomerular representations of aliphatic odorants differing in carbon number. J Comp Neurol. 2004;480:234–249. doi: 10.1002/cne.20335. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Xu Z, Kwok J, Pancoast P, Ong J, Leon M. Differential specificity in the glomerular response profiles for alicyclic, bicyclic and heterocyclic odorants. J Comp Neurol. 2006;499:1–16. doi: 10.1002/cne.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ong J, Lee K, Ho SL, Arguello S, Leon M. Effects of double and triple bonds on the spatial representations of odorants in the rat olfactory bulb. J Comp Neurol. 2007a;500:720–733. doi: 10.1002/cne.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Arguello S, Leon M. Odorants with multiple oxygen-containing functional groups and other odorants with high water solubility preferentially activate posterior olfactory bulb glomeruli. J Comp Neurol. 2007b;502:468–482. doi: 10.1002/cne.21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Xu Z, Ali SS, Leon M. Spatial representations of odorants in olfactory bulbs of rats and mice: similarities and differences in chemotopic organization. J Comp Neurol. 2009;514:658–673. doi: 10.1002/cne.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Caprio J. In vivo responses of single olfactory receptor neurons of channel catfish to binary mixtures of amino acids. J Neurophysiol. 1997;77:1–8. doi: 10.1152/jn.1997.77.1.1a. [DOI] [PubMed] [Google Scholar]
- Katada S, Hirokawa T, Oka Y, Suwa M, Touhara K. Structural basis for a broad but selective ligand spectrum of a mouse olfactory receptor: mapping the odorant-binding site. J Neurosci. 2005;25:1806–1815. doi: 10.1523/JNEUROSCI.4723-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazeniac SJ, Hall RM. Flavor chemistry of tomato volatiles. J Food Sci. 1970;35:519–530. [Google Scholar]
- Laurent G. Olfactory processing: maps, time and codes. Curr Opin Neurobiol. 1997;7:547–553. doi: 10.1016/s0959-4388(97)80035-9. [DOI] [PubMed] [Google Scholar]
- Leon M, Johnson BA. Olfactory coding in the mammalian olfactory bulb. Brain Res Rev. 2003;42:23–32. doi: 10.1016/s0165-0173(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Leon M, Johnson BA. Is there a space-time continuum in olfaction? Cell Mol Life Sci. 2009;66:2135–2150. doi: 10.1007/s00018-009-0011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AY, Foster S. Encyclopedia of common natural ingredients used in food, drugs, and cosmetics. New York: John Wiley & Sons; 1996. [Google Scholar]
- Lin da Y, Shea SD, Katz LC. Representation of natural stimuli in the rodent main olfactory bulb. Neuron. 2006;50:937–949. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Lunden A, Gustafsson V, Imhof M, Gauch R, Bosset J-O. High trimethylamine concentration in milk from cows on standard diets is expressed as fishy off-flavor. J Dairy Res. 2002;69:383–390. doi: 10.1017/s002202990200568x. [DOI] [PubMed] [Google Scholar]
- Maarse H. Volatile compounds in foods and beverages. New York: Marcel Dekker; 1991. [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Mazza G, Ciaravolo S, Chiricosta G, Celli S. Volatile flavour components from ripening and mature garlic bulbs. Flav Fragr J. 1992;7:111–116. [Google Scholar]
- Moors PJ. Norway rats (Rattus norvegicus) on the Noises and Motukawao Islands, Hauraki Gulf, New Zealand. N Z J Ecol. 1985;8:37–54. [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Moshonas MG, Shaw PE. Quantitative determination of 46 volatile constituents in fresh, unpasteurized orange juices using dynamic headspace gas chromatography. J Agric Food Chem. 1994;42:1525–1528. [Google Scholar]
- Mumm R, Tiemann T, Schulz S, Hilker M. Analysis of volatiles from black pine (Pinus nigra): significance of wounding and egg deposition by a herbivorous sawfly. Phytochemistry. 2004;65:3221–3230. doi: 10.1016/j.phytochem.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Oka Y, Nakamura A, Watanabe H, Touhara K. An odorant derivative as an antagonist for an olfactory receptor. Chem Senses. 2004;29:815–822. doi: 10.1093/chemse/bjh247. [DOI] [PubMed] [Google Scholar]
- Olofsson M, Ek-Olausson B, Lungstrom E, Langer S. Flux of organic compounds from grass measured by relaxed eddy accumulation technique. J Environ Monit. 2003;5:963–970. doi: 10.1039/b303329e. [DOI] [PubMed] [Google Scholar]
- Plutowska B, Wardenck W. Application of gas chromatography-olfactometry (GC-O) in analysis and quality assessment of alcoholic beverages — a review. Food Chem. 2008;107:449–463. [Google Scholar]
- Pye T, Bonner WN. Feral brown rats, Rattus norvegicus in South Georgia (South Atlantic Ocean) J Zool Lond. 1980;192:237–255. [Google Scholar]
- Riffell JA, Lei H, Christensen TA, Hildebrand JG. Characterization and coding of behaviorally significant odor mixtures. Curr Biol. 2009;19:335–340. doi: 10.1016/j.cub.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M, Schmachtenberg O, Bacigalupo J. Excitation, inhibition, and suppression by odors in isolated toad and rat olfactory receptor neurons. Am J Physiol Cell Physiol. 2000;279:C31–C39. doi: 10.1152/ajpcell.2000.279.1.C31. [DOI] [PubMed] [Google Scholar]
- Sanz G, Schlegel C, Pernollet JC, Briand L. Comparison of odorant specificity of two human olfactory receptors from different phylogenetic classes and evidence for antagonism. Chem Senses. 2005;30:69–80. doi: 10.1093/chemse/bji002. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Young DA, Restrepo D. Olfactory fingerprints for major histocompatibility complex-determined body odors. J Neurosci. 2001;21:2481–2487. doi: 10.1523/JNEUROSCI.21-07-02481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein MW, Orgain H. A preliminary analysis of garbage as food for the Norway rat. Am J Trop Med Hyg. 1953;2:1117–1130. doi: 10.4269/ajtmh.1953.2.1117. [DOI] [PubMed] [Google Scholar]
- Silbering AF, Galizia CG. Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J Neurosci. 2007;27:11966–11977. doi: 10.1523/JNEUROSCI.3099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjvall O, Virtalaine T, Lapvetelinen, Kallio H. Development of rancidity in wheat germ analyzed by headspace gas chromatography and sensory analysis. J Agric Food Chem. 2000;48:3522–3527. doi: 10.1021/jf981309t. [DOI] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Stewart WB, Kauer JS, Shepherd GM. Functional organization of rat olfactory bulb analysed by the 2-deoxyglucose method. J Comp Neurol. 1979;185:715–734. doi: 10.1002/cne.901850407. [DOI] [PubMed] [Google Scholar]
- Taylor RH, Thomas BW. Rats eradicated from rugged Breaksea Island (170 ha), Fiordland, New Zealand. Biol Conserv. 1993;65:191–198. [Google Scholar]
- Telci I, Sahbaz NI, Yilmaz G, Tugay ME. Agronomical and chemical characterization of spearmint (Mentha spicata L.) originating in Turkey. Econ Botany. 2004;58:721–728. [Google Scholar]
- Twigg G. The brown rat. North Pomfret, VT: David and Charles; 1975. [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Stevenson RJ. Olfactory perceptual learning: the critical role of memory in odor discrimination. Neurosci Biobehav Rev. 2003;27:307–328. doi: 10.1016/s0149-7634(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci U S A. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu EJ, Kim TH, Kim KH, Lee HJ. Aroma-active compounds of Pinus densiflora (red pine) needles. Flav Fragr J. 2004;19:532–537. [Google Scholar]
- Zhang X, Zhang X, Firestein S. Comparative genomics of odorant and pheromone receptor genes in rodents. Genomics. 2007;89:441–450. doi: 10.1016/j.ygeno.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini CA, Augusto F, Christensen E, Smith BP, Caramao EB, Pawliszyn J. Monitoring biogenic volatile compounds emitted by Eucalyptus citriodora using SPME. Anal Chem. 2001;73:4729–4735. doi: 10.1021/ac0103219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.