Abstract
OBJECTIVE:
To test the effectiveness of combining conventional antineoplastic drugs (cisplatin and etoposide) with metformin in the treatment of non-small cell lung cancer in the NCI-H460 cell line, in order to develop new therapeutic options with high efficacy and low toxicity.
METHODS:
We used the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and calculated the combination index for the drugs studied.
RESULTS:
We found that the use of metformin as monotherapy reduced the metabolic viability of the cell line studied. Combining metformin with cisplatin or etoposide produced a synergistic effect and was more effective than was the use of cisplatin or etoposide as monotherapy.
CONCLUSIONS:
Metformin, due to its independent effects on liver kinase B1, had antiproliferative effects on the NCI-H460 cell line. When metformin was combined with cisplatin or etoposide, the cell death rate was even higher.
Keywords: Carcinoma, non-small-cell lung; Drug therapy, combination; Metformin
Introduction
Lung cancer (LC) is the most common and most deadly cancer worldwide.(1) In Brazil, LC is one of the five most common cancer types, according to estimates for 2012, and it is the cancer type that took the most lives in 2010.(2) With this data, it is evident that LC is a health challenge in Brazil and worldwide. It is known that 85% of all cases of LC are classified as non-small cell LC (NSCLC), a group composed of cancer types that are different in histology, such as adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, but that have similar clinical and pathological characteristics.(3,4)
Chemotherapy is used in patients staged as III or IV, in order to extend survival, control the disease, and improve quality of life.(5) In general, platinum derivatives, such as cisplatin and carboplatin, fulfill this role when combined with third-generation antineoplastic agents, such as etoposide.(6) Unquestionably, combination therapy is advantageous in cancer treatment, because it makes it possible to attack multiple molecular targets, with an increase in therapeutic efficacy, a reduction in dosage, and a consequent reduction in toxicity, as well as with a reduction or delay in resistance phenotype acquisition.(7)
In this scenario, there is metformin, a biguanide that is widely used as the first-line treatment for patients with type 2 diabetes mellitus.(8) However, since the 1970s, Dilman has suggested the use of antidiabetic biguanides as anti-aging and antineoplastic agents.(9)
In this context, the objective of the present study was to determine whether there was summation, synergism, or antagonism between antineoplastic agents used in the treatment of NSCLC and metformin, in the NCI-H460 cell line, in order to evaluate new therapeutic options for the treatment of NSCLC.
Methods
Initially, the NSCLC cell line NCI-H460, which is a representative of the histologic group of large cell carcinoma, was grown to subconfluence at 37°C and 5% CO2 in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% (v/v) bovine fetal serum (Gibco/Invitrogen, Grand Island, NY, USA) and a stabilized solution of penicillin (100 IU/mL), streptomycin (100 µg/mL), and 0.5% (w/v) amphotericin B (Gibco/Invitrogen). Provided through donations from collaborators, cisplatin ((Incel, 1 mg/mL injectable solution; Laboratório Darrow, Areal, Brazil), etoposide (Posidon, 20 mg/mL injectable solution; Piére, Buenos Aires, Argentina), and metformin hydrochloride (Pharma Nostra, Rio de Janeiro, Brazil) were diluted in 1× PBS.
In the in vitro cell metabolic viability test, cells were grown at a density of 7.5 x 104 cells/well in 96-well plates, and, after 24 h, they were treated with each drug, in a dose-dependent manner, for 24 h. Subsequently, the medium was removed, 15 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) were added to 5 mg/mL in each well, and the plates were incubated for 4 h. Finally, 100 µL of analytical grade DMSO (Vetec, Rio de Janeiro, Brazil) were added in order to dissolve the formazan crystals. Therefore, the production of formazan by mitochondrial succinate dehydrogenase activity reflects the proportion of live cells undergoing oxidative stress caused by the drugs under investigation.(10) In this experiment, the absorbance values obtained from the wells of untreated cells, i.e., cells exposed only to RPMI 1640 medium, represent 100% cell viability. In vitro cytotoxicity was determined by the estimate calculation of the values for inhibitory concentration of 50% for cell proliferation (IC50) on the basis of the dose-response curve for each drug, after calculation of metabolic viability, evaluated with the MTT assay (absorbance at 570 nm), by using a spectrophotometer. It is of note that the experiments to obtain the dose-response curves for each drug as monotherapy and estimate the IC50 values were performed in quadruplicate in three independent experiments, whereas the experiments to determine the combination index were performed in triplicate in three independent experiments. In addition, drug interaction was assessed by analysis of the combination index, the value of which was calculated by the classical isobologram equation described by Chou & Talalay, with the use of the CompuSyn software program, version 1.0 (ComboSyn, Paragon, NJ, USA).(11) In this context, combination index values greater than 1.1 indicate antagonistic interactions, values between 0.9 and 1.1 indicate additive interactions, and values lower than 0.9 indicate synergistic interactions. Regarding the statistical analysis, the mean and standard deviation of the absorbances were calculated on the basis of the results of the MTT assay, whereas the estimated IC50 values were calculated by the Prism software program, version 5 (GraphPad Inc., San Diego, CA, USA). All data on combination therapy are expressed as mean ± SD and were analyzed by one-way ANOVA followed by the Bonferroni test, with the use of the same software program.
Results
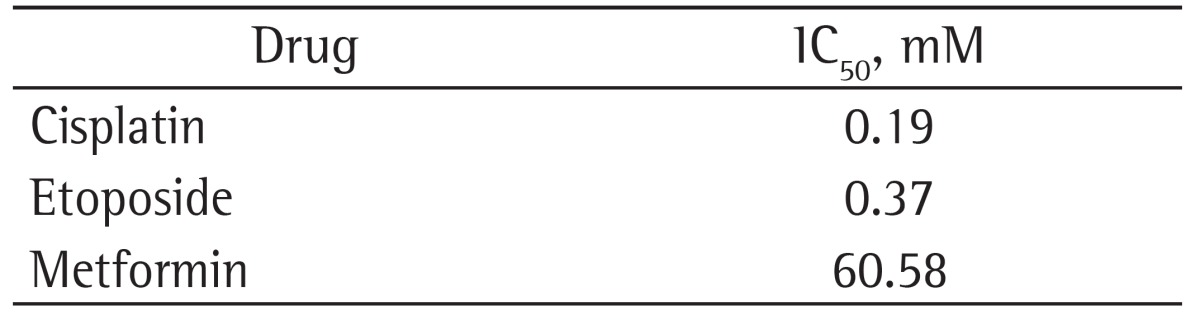
Initially, we performed the cell viability experiments to determine the IC50 concentrations for metformin, cisplatin, and etoposide in the large cell carcinoma cell line NCI-H460, which are shown in Table 1.
Table 1. Inhibitory concentration of viability at 50% (IC50) values in the NCI-H460 cell line with the drugs tested.
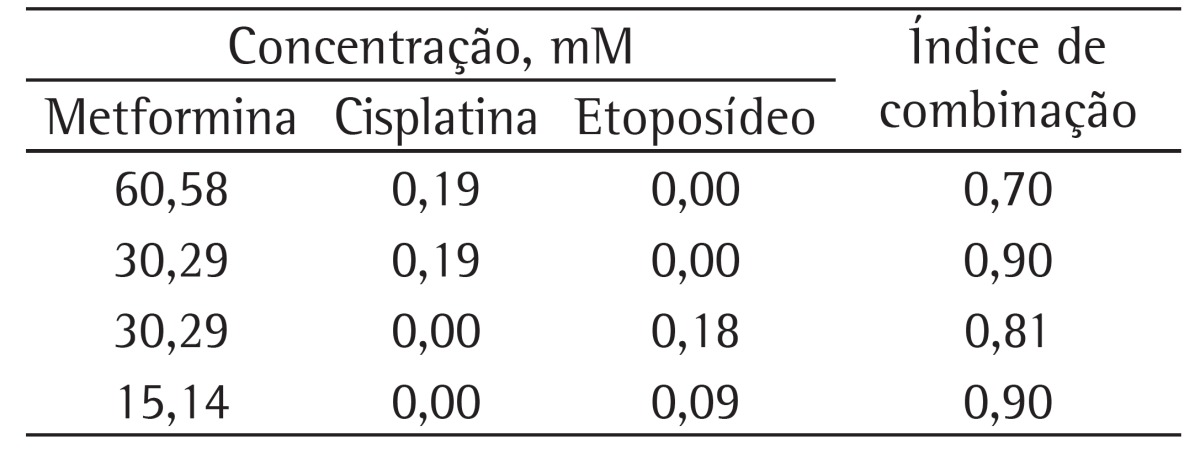
Subsequently, once these data were obtained, each drug was diluted to achieve concentrations in the IC50 range, as well as below and above this range, and in vitro experiments combining metformin with cisplatin and combining metformin with etoposide were performed to allow the construction of dose-response plots with the software program. However, only the most relevant data will be discussed. The data from the plots were statistically analyzed and are shown in Figures 1 and 2. Finally, the effects of the combinations were used to calculate the combination index, the values of which are shown in Table 2.
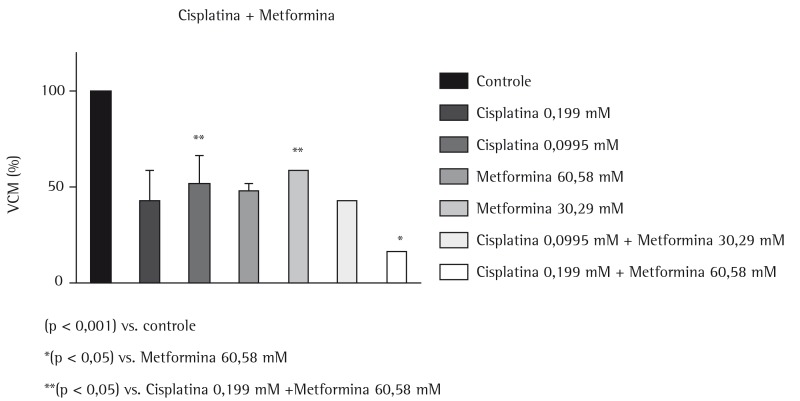
Figure 1. Evaluation of the effects of metformin and cisplatin (used as monotherapy or in combination therapy, at different concentrations) on the reduction in metabolic cell viability (MCV) in the NCI-H460 cell line.

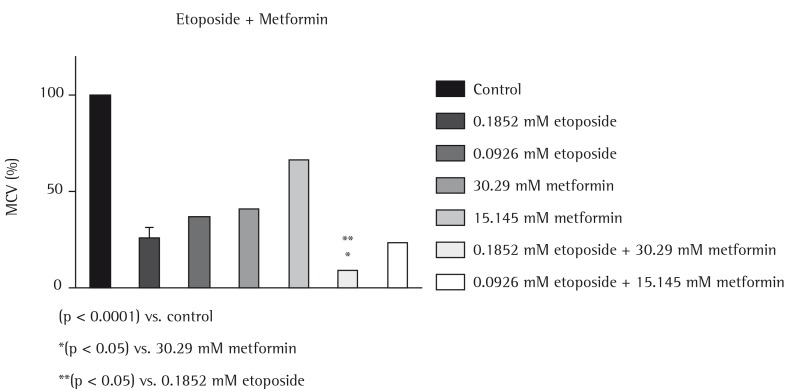
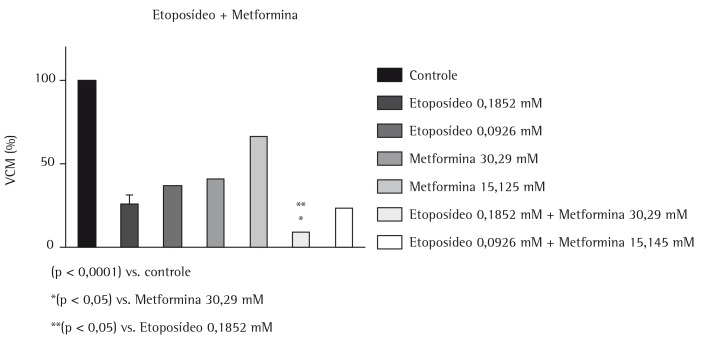
Figure 2. Evaluation of the effects of metformin and etoposide (used as monotherapy or in combination therapy, at different concentrations) on the reduction in metabolic cell viability (MCV) in the NCI-H460 cell line.
Table 2. Combination index for metformin plus cisplatin or etoposide in the NCI-H460 cell line.
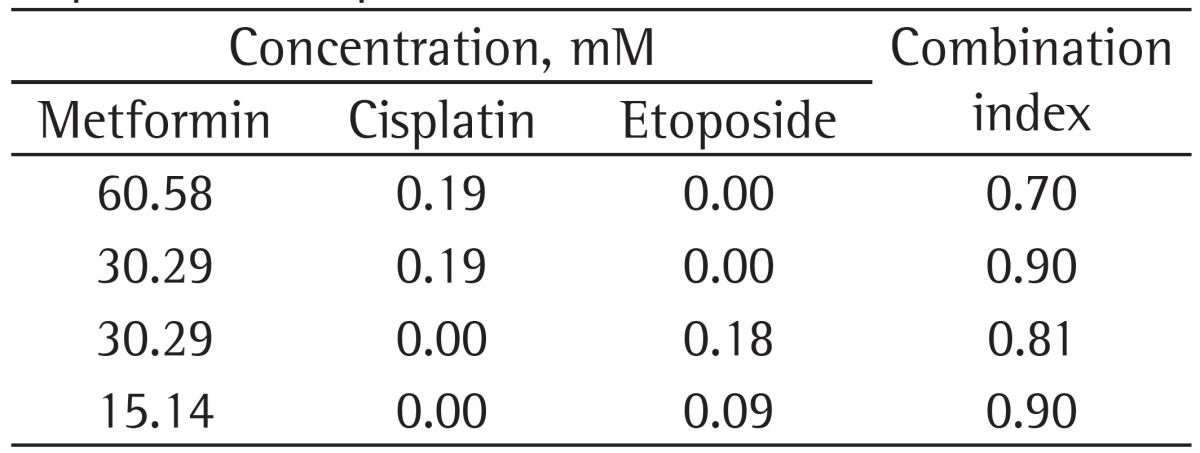
The results show that combining metformin (60.58 mM) with cisplatin (0.19 mM) produced greater antiproliferative effects than did the use of metformin (60.58 and 30.29 mM) or cisplatin (0.09 mM) as monotherapy. In addition, combining metformin (60.58 and 30.29 mM) with cisplatin (0.19 mM) produced synergy. Combining metformin (30.39 mM) with etoposide (0.18 mM) was more effective in reducing the metabolic viability of the cell line tested than was the use of etoposide (0.18 and 0.09 mM) or metformin (30.29 and 15.14 mM) as monotherapy. Furthermore, combining metformin with etoposide (30.39 mM and 0.18 mM, respectively, and 15.14 mM and 0.09 mM, respectively) produced a synergistic effect.
Discussion
Various epidemiological studies have reported the effects of metformin on the prevention and treatment of cancer.(12-15) There have also been studies reporting that metformin can improve treatment and thus survival in patients with NSCLC and diabetes who undergo chemotherapy.(16) Because the safety and use of metformin has been well established since it first underwent a clinical trial in 1957, its use as a potential anticancer agent is certainly an interesting strategy.(17)
Although in the present study the cytotoxic activity of metformin was found to be up to 200 times lower than that of etoposide or cisplatin, as determined by the IC50 values, the use of metformin as monotherapy reduced the metabolic viability of large cell carcinoma cells. However, in polytherapy regimens, metformin (60.58 and 30.29 mM) combined with cisplatin at 0.19 mM produced a synergistic effect and was more effective than was metformin combined with half the IC50 value for cisplatin (0.09 mM). Combining metformin (30.39 mM) with etoposide (0.18 mM) produced a synergistic effect and was more effective in reducing the metabolic viability of the NCI-H460 cell line than was the use of etoposide (0.18 and 0.09 mM) or metformin (30.29 and 15.14 mM) as monotherapy. In line with our findings, preclinical studies have reported that oral administration of metformin combined with chemotherapy can block tumor growth and prevent recurrence, as well as making it possible to reduce the doxorubicin doses used in the treatment.(18)
Although the use of metformin is well established in the treatment of diabetes, the mechanism through which metformin can act in tumor suppression still needs to be further elucidated. The major mechanism of action of biguanides might be related to their capacity to inhibit mitochondrial complex I and consequently cause energy imbalance, triggering liver kinase B1 (LKB1)-dependent phosphorylation of the enzyme AMP kinase (AMPK).(19,20) Under conditions of energy stress, LKB1 is the primary regulator of AMPK, which, under conditions of reduction of intracellular ATP, modulates cell growth and metabolism.(21,22)
Allelic loss at LKB1 occurs in various types of cancer-such as NSCLCs-especially in lung adenocarcinoma. In addition, it is believed that most mutations in LKB1 in NSCLC lead to loss of function of the encoded protein.(23,24) The large cell carcinoma cell line used in the present study (NCI-H460) has a nonsense mutation in LKB1, which results in lack expression of its protein product.(24) It is therefore possible to attest that the mechanisms of action of metformin in these cells are independent of LKB1 activity. Shackelford et al. showed that phenformin, a member of the biguanide class of drugs, can be used as an anticancer agent in LKB1-deficient tumors because it induces apoptosis possibly in response to metabolic stress.(25) It has thus been suggested that a possible mechanism of action of metformin and other biguanides in LKB1-defficient tumor cells is mitochondrial complex I inhibition, which induces the production of reactive oxygen species and consequent apoptosis, because these species cannot be effectively neutralized.(26)
Another plausible reason for the antiproliferative and synergistic effects of metformin is its preferential activity against tumor stem cells, a group of cells within the tumor mass that can be related to tumor formation, maintenance of the tumor mass, recurrence, and metastasis. Because of these characteristics, drugs that selectively target these cells are extremely promising, since, in combination with conventional chemotherapy, they could make treatment more effective and prevent disease recurrence.(27) Hirsch et al. found that, in xenographic models, metformin preferentially inhibits proliferation of tumor cells expressing a stem cell phenotype.(26)
In summary, the present study has shown for the first time that metformin has antiproliferative effects in a large cell carcinoma cell line (NCI-H460) and that, when combined with cisplatin or etoposide, it acts synergistically, increasing the cell death rate. However, larger studies are still needed to elucidate further the mechanisms involved.
Footnotes
Study carried out in the Laboratory of Cellular and Molecular Biology of Human Cancer, Universidade Federal do Espírito Santo - UFES, Federal University of Espírito Santo - Vitória, Brazil
Financial support: This study received financial support from the Fundação de Amparo à Pesquisa do Espírito Santo (FAPES, Espírito Santo Research Foundation) and the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council for Scientific and Technological Development).
Contributor Information
Sarah Fernandes Teixeira, Universidade Federal do Espírito Santo - UFES, Federal University of Espírito Santo - Vitória, Brazil.
Isabella dos Santos Guimarães, Graduate Program in Biotechnology, Universidade Federal do Espírito Santo - UFES, Federal University of Espírito Santo - Vitória, Brazil.
Klesia Pirola Madeira, Graduate Program of the Biotechnology Network of Northeastern Brazil, Universidade Federal do Espírito Santo - UFES, Federal University of Espírito Santo - Vitória, Brazil.
Renata Dalmaschio Daltoé, Agricultural Sciences Center, Universidade Federal do Espírito Santo - UFES, Federal University of Espírito Santo - Vitória, Brazil.
Ian Victor Silva, Health Sciences Center, Universidade Federal do Espírito Santo - UFES, Federal University of Espírito Santo - and Head, Laboratory of Cell Biology of Aging, Vitória, Brazil.
Leticia Batista Azevedo Rangel, Health Sciences Center, Universidade Federal do Espírito Santo - UFES, Federal University of Espírito Santo - and Head. Laboratory of Cellular and Molecular Biology of Human Cancer, Vitória, Brazil.
References
- 1.Globocan . [homepage on the Internet] Lyon: International Agency for Research on Cancer; Globocan 2008 Fast Stats; 2008. [.[updated 2008; cited 2013 May 26]]. Available from: Available from: http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=900#BOTH. [Google Scholar]
- 2.INCA . [homepage on the Internet] Rio de Janeiro: Ministério da Saúde; 2012. [[updated 2012; cited 2013 May 5]]. Tipo de câncer - Pulmão. Available from: Available from: http://www2.inca.gov.br/wps/wcm/connect/tiposdecancer/site/home/pulmao/definicao. [Google Scholar]
- 3.SEER - Surveillance, Epidemiology, and End Results Program . [homepage on the Internet] Bethesda: National Cancer Institute; SEER Cancer Statistics Review; 2007. [[updated 2009; cited 2013 Jun 30]]. - Available from: Available from: http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 4.IARC - International Agency for Research on Cancer . [homepage on the Internet] Lyon: International Agency for Research on Cancer; 2004. [[updated 2004; cited 2013 Jul 01]]. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. [Adobe Acrobat document, 344p.]. Available from: Available from: http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb10/BB10.pdf. [Google Scholar]
- 5.NCBI - National Center for Biotechnology Information . [homepage on the Internet] Bethesda: National Center for Biotechnology Information; The Diagnosis and Treatment of Lung Cancer (Update). [Adobe Acrobat document; 2011. [[updated 2011; cited 2013 Jul 05]]. 198p. Available from: Available from: http://www.ncbi.nlm.nih.gov/books/NBK99021/pdf/TOC.pdf. [Google Scholar]
- 6.Ettinger DS, Bepler G, Bueno R, Chang A, Chang JY, Chirieac LR, et al. Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4(6):548–582. doi: 10.6004/jnccn.2006.0046. [DOI] [PubMed] [Google Scholar]
- 7.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–681. doi: 10.1124/pr.58.3.10. http://dx.doi.org/10.1124/pr.58.3.10 Erratum in: Pharmacol Rev. 2007;59(1):124. [DOI] [PubMed] [Google Scholar]
- 8.IDF - International Diabetes Federation . [homepage on the Internet] Brussels: International Diabetes Federation; [[updated 2005; cited 2013 Apr 02]]. 2005. Global Guideline for Type 2 Diabetes. [Adobe Acrobat document, 82p.]. Available from: Available from: http://www.idf.org/webdata/docs/IDF%20GGT2D.pdf. [Google Scholar]
- 9.Dilman VM. Age-associated elevation of hypothalamic, threshold to feedback control, and its role in development, ageing, and disease. Lancet. 1971;1(7711):1211–1219. doi: 10.1016/s0140-6736(71)91721-1. http://dx.doi.org/10.1016/S0140-6736(71)91721-1 [DOI] [PubMed] [Google Scholar]
- 10.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. http://dx.doi.org/10.1016/0022-1759(83)90303-4 [DOI] [PubMed] [Google Scholar]
- 11.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. http://dx.doi.org/10.1016/0065-2571(84)90007-4 [DOI] [PubMed] [Google Scholar]
- 12.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–1305. doi: 10.1136/bmj.38415.708634.F7. http://dx.doi.org/10.1136/bmj.38415.708634.F7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766–1777. doi: 10.1007/s00125-009-1440-6. http://dx.doi.org/10.1007/s00125-009-1440-6 [DOI] [PubMed] [Google Scholar]
- 14.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27(20):3297–3302. doi: 10.1200/JCO.2009.19.6410. http://dx.doi.org/10.1200/JCO.2009.19.6410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121(4):856–862. doi: 10.1002/ijc.22717. http://dx.doi.org/10.1002/ijc.22717 [DOI] [PubMed] [Google Scholar]
- 16.Tan BX, Yao WX, Ge J, Peng XC, Du XB, Zhang R, et al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer. 2011;117(22):5103–5111. doi: 10.1002/cncr.26151. http://dx.doi.org/10.1002/cncr.26151 [DOI] [PubMed] [Google Scholar]
- 17.Sterne J. Du Nouveau dans les antidiabétiques. La NN diméthylamino guanyl guanidine (N.N.D.G.) . Maroc Med. 1957;36:1295–1296. (Fre). [Google Scholar]
- 18.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71(9):3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. http://dx.doi.org/10.1158/0008-5472.CAN-10-3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275(1):223–228. doi: 10.1074/jbc.275.1.223. http://dx.doi.org/10.1074/jbc.275.1.223 [DOI] [PubMed] [Google Scholar]
- 20.Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11(6):554–565. doi: 10.1016/j.cmet.2010.04.001. http://dx.doi.org/10.1016/j.cmet.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. http://dx.doi.org/10.1038/nrm3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. http://dx.doi.org/10.1016/S0092-8674(03)00929-2 [DOI] [PubMed] [Google Scholar]
- 23.Avizienyte E, Loukola A, Roth S, Hemminki A, Tarkkanen M, Salovaara R, et al. LKB1 somatic mutations in sporadic tumors. Am J Pathol. 1999;154(3):677–681. doi: 10.1016/S0002-9440(10)65314-X. http://dx.doi.org/10.1016/S0002-9440(10)65314-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carretero J, Medina PP, Pio R, Montuenga LM, Sanchez-Cespedes M. Novel and natural knockout lung cancer cell lines for the LKB1/STK11 tumor suppressor gene. Oncogene. 2004;23(22):4037–4040. doi: 10.1038/sj.onc.1207502. http://dx.doi.org/10.1038/sj.onc.1207502 [DOI] [PubMed] [Google Scholar]
- 25.Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23(2):143–158. doi: 10.1016/j.ccr.2012.12.008. http://dx.doi.org/10.1016/j.ccr.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69(19):7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. http://dx.doi.org/10.1158/0008-5472.CAN-09-2994 Erratum in: Cancer Res. 2009;69(22):8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. http://dx.doi.org/10.1038/nrc2620 [DOI] [PubMed] [Google Scholar]