Abstract
Sperm motility is essential for achieving fertilization. In animals with external fertilization as amphibians, spermatozoa are stored in a quiescent state in the testis. Spermiation to hypotonic fertilization media triggers activation of sperm motility. Bufo arenarum sperm are immotile in artificial seminal plasma (ASP) but acquire in situ flagellar beating upon dilution. In addition to the effect of low osmolarity on sperm motility activation, we report that diffusible factors of the egg jelly coat (EW) regulate motility patterns, switching from in situ to progressive movement. The signal transduction pathway involved in amphibian sperm motility activation is mostly unknown. In the present study, we show a correlation between motility activation triggered by low osmotic pressure and activation of protein kinase A (PKA). Moreover, this is the first study to present strong evidences that point toward a role of a transmembrane adenyl-cyclase (tmAC) in the regulation of amphibian sperm motility through PKA activation.
Keywords: Amphibia, sperm, motility activation, cAMP dependent kinase, adenylyl cyclase
Introduction
For fertilization to be successful the sperm must reach, bind and penetrate the egg vestments. For all these processes activation of sperm motility is essential. Initiation of sperm motility is uniquely regulated in different species and this regulation is dependent on the environment in which fertilization occurs. Several factors have been reported to regulate sperm motility; for example, in mammals, bicarbonate and calcium present in seminal plasma are essential to regulate this process (Morisawa, 1994; Okamura et al., 1985). In external fertilizers such as salmonids, higher potassium concentration in sea water results in the initiation of sperm motility upon spawning (Morisawa et al., 1983a). On the other hand, hypotonic media triggers sperm motility in freshwater fish species (Morisawa et al., 1983b). In amphibians, low osmolarity has been shown to initiate flagellar movement both in Anura (Inoda and Morisawa, 1987) and Urodele (Hardy and Dent, 1986) sperm. However, little is know about the molecular mechanism involved in this process.
The first interaction between amphibian gametes occurs when sperm contact molecules from the jelly coat (JC) that surrounds the oocyte. This coat is essential for successful fertilization (Katagiri, 1966; Shivers and James, 1970). Immediately after oocytes have been spawned, molecules of the JC are released from the jelly matrix resulting in a hypotonic fertilization conditioned media known as egg water (EW). In the toad Bufo arenarum, EW was reported to “activate” homologous free spermatozoa before they penetrate into the JC (Barbieri and Cabada, 1969). The passage of sperm through the jelly has been regarded as an important step in fertilization and proposed to be a sperm “capacitating” requisite, in analogy to the concept developed in mammals (Shivers and James, 1970). Consistent with this analogy, our laboratory has recently reported that EW factors drive physiological modifications in B. arenarum sperm similar to the ones described for mammalian capacitation (e.g. rise in protein tyrosine phosphorylation and cholesterol efflux) (Krapf et al., 2007). Also reminiscent of mammalian sperm capacitation is the observation that sperm incubation in the presence of EW prepare the sperm for the acrosome reaction and is essential for acquisition of their fertilizing capacity (Krapf et al., 2009; Krapf et al., 2007).
Regulation of sperm motility has been linked to cyclic adenosine monophosphate (cAMP) signaling pathways in several animal species, including mammals (Okamura et al., 1985; Tash and Bracho, 1994) salmonid fishes (Morisawa and Okuno, 1982), sparid seawater teleosts (Zilli et al., 2008) and even invertebrates like sea urchins (Bracho et al., 1998). While the contribution of the cAMP-dependent protein kinase (PKA) as a regulator of sperm motility is better known in some animal species, there is still scarce information on the signaling pathways associated with the molitity activation of amphibian sperm.
Two types of adenylyl cyclases (ACs) have been characterized; transmembrane AC (tmAC) and soluble AC (sAC or SACY) (Braun and Dods, 1975; Buck et al., 1999). TmACs are regulated by heterotrimeric G-proteins and stimulated by forskolin (Hanoune and Defer, 2001), whereas SACY is stimulated by bicarbonate (Chen et al., 2000) and Ca2+ (Jaiswal and Conti, 2003). In mammalian sperm, SACY is responsible for cAMP synthesis and its regulation by Ca2+ and HCO3− is essential to induce cAMP-dependent physiological changes related to sperm capacitation (Esposito et al., 2004; Hess et al., 2005). On the other hand, the presence of tmAC in mammalian sperm is still controversial and their exact role in sperm is not well understood (Baxendale and Fraser, 2003; Hildebrandt et al., 1985).
In the present study, we evaluated the signaling pathways involved in the low osmolarity-induced activation of Bufo sperm motility. Our results indicate that this process is mediated by a cAMP-dependent pathway involving the activation of PKA. This work also provides evidence that G protein αs is present in Bufo sperm and that the cAMP required to activate motility is synthesized by tmACs and not by SACY. In addition to the effect of low osmolarity, our data suggest that diffusible factors of the egg jelly coat (EW) regulate motility patterns, switching from in situ to progressive movement.
Materials and Methods
Reagents
H-89 (N-[2-(pbromocinnamylamino)ethyl]-5-isoquinolinesulfonamide), N6, O′-2-dibutyryladenosine 3′:5′-cyclic monophosphate (db-cAMP), 8-Bromoadenosine 3′,5′-cyclic monophosphate (8Br-cAMP), 3-Isobutyl-1-methylxanthine (IBMX), β-glycerophosphate, sodium vanadate, p-Nitrophenyl phosphate (pNPP), N-(cis-2-phenyl-cyclopentyl) azacyclotridecan- 2-imine-hydrochloride (MDL), and cocktail of protease inhibitors were obtained from Sigma-Aldrich (St. Louis, MO). E-2-(1H-Benzo[d]imidazol-2-ylthio)-N′-(5-bromo-2-hydroxybenzylidene) propanehydrazide (KH7) was obtained from Cayman Chemicals (Ann Arbor, MI) and forskolin (FK) was purchased from Calbiochem (San Diego, CA). Anti-phospho-PKA substrate antibodies (#9621) (anti-pPKAs) were obtained from Cell Signaling (Danvers, MA), anti-Actin (I-19) from Santa Cruz Biotecnology (SCB; Santa Cruz, CA) and anti-Gαs from Millipore (06-237) (Temecula, CA). ChromPure Normal Rabbit IgG was obtained from Jackson ImmunoResearch Labs, Inc. (West Grove, PA, USA). All other chemicals were of reagent grade. Stock solutions of chemicals were prepared in distilled water or dimethyl sulfoxide (DMSO) according to manufacture’s instructions. The concentration of DMSO in the incubation media was kept constant between treatments and never exceeded 1% (v/v), a condition that did not affect sperm motility.
Egg water
EW was obtained as described (Diaz Fontdevila, 1991). Briefly, strings of B. arenarum oocytes were removed from ovisacs after hormonal stimulated ovulation, and incubated for 10 min in distilled water. The resultant solution, named EW, had a final protein concentration of 70 μg/ml and pH 8.2. Changing the pH of the control isotonic media from 7.4 to 8.2 did not significantly alter motility parameters (data not shown).
Composition of media used
To properly evaluate motility activation of toad sperm, we designed an Artificial Seminal Plasma (ASP) medium in which these cells remained completely immotile (292 mOsm/Kg). ASP is a modified Ringer’s solution (Raisman and Pisano, 1970) and was prepared according to sodium and potassium concentrations measured in male testis plasma (see Table I). Ten percent of ASP corresponds to a 1/10 dilution of ASP. EW ASP was prepared with 80% EW in an ASP background. When the effect of NaHCO3 was analyzed, a modified ASP media was used (75 mM NaCl instead of 105 mM, 25 mM HEPES, pH 7.4) to maintain constant osmolality. Addition of NaHCO3 to this solution did not significantly change the pH.
Table 1.
Composition of the solutions
| ASP | 10% ASP | EW ASP | EW | |
|---|---|---|---|---|
| Na+ (mM) | 105* | 10.5 | 108.76 | 4.7* |
| K+ (mM) | 40* | 4 | 40.67 | 0.84* |
| Ca2+ (mM) | 1.4 | 0.14 | 1.62 | 0.28* |
| protein cc. (ug/ml) | 0 | 0 | 54.4 | 68* |
| pH | Tris 10mM 7.4 | Tris 10mM 7.4 | Tris 10mM 7.4 | 8.2* |
| osmolarity (Osm/Kg) | 292 | 30 | 302 | 13 |
Sodium, Potassium and Calcium concentration, proteins concentration and pH and osmolarity values. Artificial seminal plasma (ASP) was prepared according to the measured sodium and potassium concentrations of male testis plasma. 10% ASP is a 1:10 dilution of ASP at the same pH value. EW ASP was prepared with 80% of EW instead distiller. EW was obteined incubating egg strings with distillated water during 10 min. It contains diffusible substaces of the jelly coat. Asterisks correspond to measured concentration of cations and pH from seminal plasma and EW.
Animals and preparation of gametes
Bufo arenarum sexually mature specimens (150 g) were collected in the neighborhood of Rosario, Argentina, and maintained in a dark moist chamber between 15°C and 17°C until used. Experiments were performed according to the guide for care and use of laboratory animals of Facultad de Ciencias Biológicas y Farmacéuticas, Universidad Nacional de Rosario. Sperm suspensions were obtained as described elsewhere (Valz-Gianinet et al., 1991). After washing, spermatozoa were suspended in ice cold ASP to a final concentration of 1–1.4 × 108 cells/ml and used within 3 h.
Immunodetections
Sperm suspensions were diluted in the appropriate medium depending on the experiment. Live-dead staining (2% of eosin in ASP) was performed after every treatment in order to analyze possible sperm toxicity. Sperm protein extracts were performed in Triton X-100 lysis buffer (1% Triton X-100, 5 mM EDTA, 1% cocktail of proteases inhibitors, 1 mM sodium vanadate, 100 nM okadaic acid, 30 mM β-glycerophosphate, 5 mM pNPP, 150 mM NaCl, 10 mM Tris pH 7.6). The supernatants were mixed with sample buffer containing 50 mM DTT, incubated 10 min at 70 °C and subjected to 10% SDS–PAGE (Laemmli, 1970). Each lane was loaded with 7×106 cells. Proteins were transferred to nitrocellulose membranes (Hybond-ECL, Amersham Biosciences, UK) at 250 mA (constant) for 2 h at 4 °C. Immunoblotting was performed using a dilution 1/1,000 anti-phospho-PKA substrate (anti-pPKAs) antibodies following manufacturer’s directions and a 1/5,000 dilution of a secondary HRP labeled antibody provided with the enhanced chemiluminescence detection kit (Thermo Scientific SuperSignal West Femto Substrate). Western blot against Gαs proteins was performed following manufacturer’s directions using a 1/1,000 dilution of anti-Gαs. All membranes were stripped and western blot for actin detection were performed as loading controls. Immunolocalization in fixed cells was performed as described (Martinez and Cabada, 1996) using a 1/100 dilution of anti-phospho-PKA substrate (anti-pPKAs) antibodies. To visualize the sperm nucleus, Hoechst (33258) staining was performed. Samples were analyzed with a Nikon Eclipse TE-2000-E2 confocal microscope (Natick, MA).
Motility analysis
One volume of sperm was diluted with 25 volumes of specific medium and the suspension placed on a standard count 20 micron (Spectrum Technologies, Heldsburg, CA) chamber slides to assess sperm motility. Trajectories were recorded using an Olympus BH-2 microscope connected to a Nikon DS-Fi1 camera (Natick, MA) at 200X magnification. The percentage of motile sperm was assessed from the video recording. The movies were converted to image sequences (virtualdub.org) and analyzed with ImageJ software (http://rsb.info.nih.gov/ij). Motility index were classified as immotile, in situ (sperm flagella beating without midpiece movement) and progressive movement. Sperm were counted as “motile” when they either exhibited progressive movement or flagellar beatings. At least three independent experiments analyzing more than 100 sperm were performed for each condition tested.
Statistical analysis
Data were analyzed with paired student’s t test for comparing mean values, and with Mann – Whitney test to compare medians. Analysis of variance (ANOVA) was used for comparing multiple groups. Models were further tested according to Nagarsenker (1984), and Shapiro and Wilk (1965). Statistical significances are indicated in the text.
Results
Hypotonic media triggers sperm motility activation
Isotonic solutions have been widely used for preparation and handling of Anuran sperm suspensions (Cabada, 1975). Isotonicity helps maintain both acrosome integrity and sperm viability (Arranz and Cabada, 2000; Cabada, 1975). Dilution into hypotonic environments at either spawning or gamete handling activates flagellar motility rendering sperm capable of reaching the egg (Raisman, 1980). EW, a complex solution composed by oviduct derived ions and glucolipoproteic factors, mimics natural hypotonic medium for Bufo arenarum fertilization, where sperm acquire the capacity to undergo agonist stimulated acrosome reaction (Krapf et al., 2009) and fertilize the egg (Krapf et al., 2007). In order to independently analyze the contribution of hypotonicity and organic components in supporting fertilizing capacity, sperm motility was studied in hypotonic (10% ASP) and isotonic (ASP) media, either in the presence or absence of EW (see Table 1 for media compositions). In both isotonic solutions (Fig. 1A), ASP and EW supplemented with ASP, (EW ASP), sperm were mostly immotile. Noteworthy, the presence of EW in isotonic media promoted in situ movement in 20% of the sperm (not statistically significant). When Bufo sperm were incubated in hypotonic media; 10% ASP or EW (Fig. 1B, most of the sperm were motile, regardless the presence of EW. However, a significant higher rate of progressive movement was observed in the presence of EW (p<0.001), further substantiating the role of EW in the regulation of sperm motility.
Fig. 1. Toad sperm motility activation.
Motility analysis of sperm incubated in isotonic solutions (A) (ASP and EW ASP), and in hypotonic solutions (B) (10% ASP and EW) during 5 min at 20°C. Sperm motility patterns were identified as immotile, in situ (indicates sperm flagella beating) and progressive movement (indicates sperm swimming). Data represent mean ± SD; n=10. Asterisks indicate significant differences (* p<0.001).
Phosphorylation signaling associated with motility activation
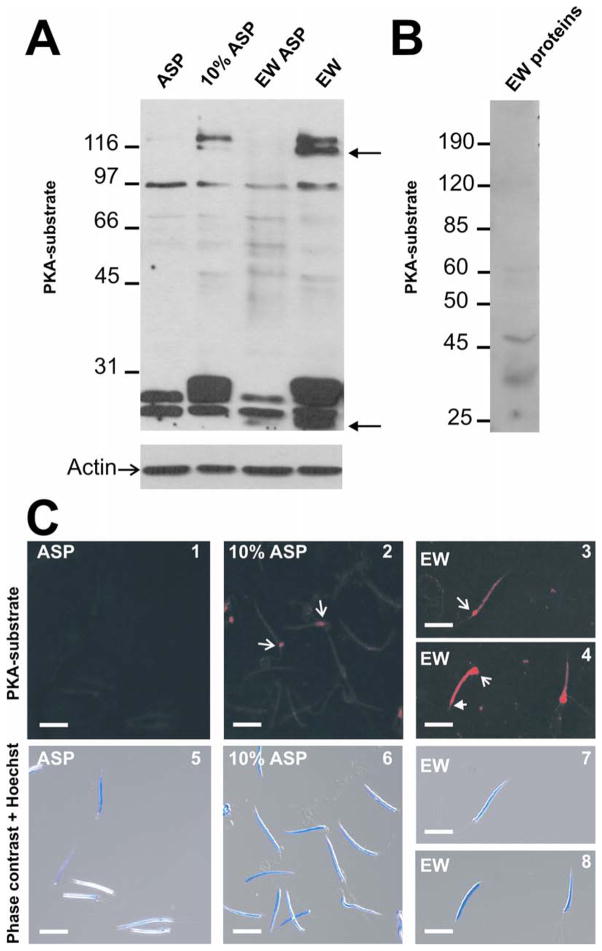
Sperm motility from different species is known to be modulated by cAMP-dependent pathways (Morisawa, 1994; Tash and Bracho, 1994). One of the major downstream targets of cAMP in sperm cells is PKA. To investigate whether PKA phosphorylation plays a role in toad sperm motility activation, Triton soluble sperm fractions were obtained after incubation in conditions that either support or not sperm motility (Table I). These protein extracts were analyzed by Western blot using anti-phospho-PKA substrate (anti-pPKAs) antibodies. These antibodies detect peptides containing the phosphorylated motif RRXpS/pT (corresponding to a PKA consensus phosphorylation sequence) and have been previously used in sperm from different species (Krapf et al., 2010; O’Flaherty et al., 2004). After sperm incubation in ASP, three major phophoproteins were detected (Fig. 2A). The presence of EW in isotonic solution (EW ASP) did not substantially modify the phosphorylation status of sperm proteins. However, when sperm were incubated in either 10% ASP or EW, other proteins (~120 kDa and 28 kDa) were detected suggesting activation of PKA by hypotonicity. Moreover, in hypotonic media, EW induced phosphorylation of two additional proteins of ~20 kDa and 111 kDa (see arrows in Fig. 2A). As a control, only low molecular weight proteins were detected in EW medium (no sperm) when analyzed with anti-pPKAs antibodies (Fig. 2B), indicating that phosphoproteins shown in Fig. 2A were from sperm origin.
Fig. 2. Hypotonic media induces PKA substrate phosphorylation and motility activation.
A. Sperm suspensions were incubated in ASP, 10% ASP, EW ASP or EW during 5 min at 20°C. Each line contains 1% Tritón X-100 soluble proteins of 7 × 106 spermatozoa analyzed by 10% SDS/PAGE and Western blot using a PKA substrate-specific antibody (anti-pPKAs). Western blot for actin was performed as loading control. B. EW (34 μg) assayed with anti-pPKAs. Molecular weight standards are indicated at the left of each blot (Mr × 103). C. Bufo sperm were incubated in ASP (panel 1 and 5), 10% ASP (panel 2 and 6) and EW (panel 3, 4, 7 and 8), fixed, permeabilized and stained with anti-pPKAs. Spermatozoa incubated in EW were washed twice. Arrows indicate the middle piece and head-arrows indicate the post-acrosomal section of the toad sperm. Barr represents 10 um.
In order to examine the subcellular localization of PKA substrate phosphoproteins, sperm were incubated in ASP, 10% ASP or EW and immunostained using anti-pPKAs antibodies. After treatment with 10% ASP, fluorescence signal was mainly localized along the head and a low percentage of these cells (~20%) showed a stronger fluorescence signal localized in the mitochondrial region (arrows in Fig. 2C panels 2 and 4). This signal was completely absent when sperm were incubated in isotonic conditions. Interestingly, after sperm incubation in EW, fluorescence was stronger than in 10% ASP, and distributed along the head excluding the acrosome region (head-arrows in Fig. 2C) and in the middle piece of the sperm (arrows in Fig. 2C panels 3 and 4, pattern seen in ~65% of sperm). Bottom panels show phase contrast merged with Hoechst staining (Fig. 2C, panels 5 to 8).
Role of cAMP and PKA on motility activation induced by hypotonic media
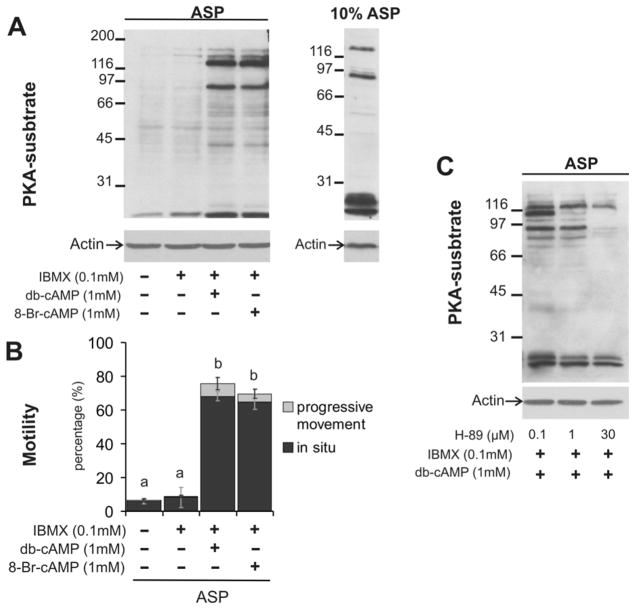
The onset of PKA-induced phosphorylation correlated with hypotonic induced motility suggesting a key role of PKA in sperm motility activation. To further investigate PKA-role in sperm motility, Bufo sperm were incubated in hypotonic media containing the PKA inhibitor H-89. As expected, in the presence of H-89, phosphorylation of PKA substrates was impaired (Fig. 3A). Most interestingly, this PKA inhibitor significantly reduced sperm progressive motility in 10% ASP (p< 0.002) and EW (p<0.0001) both with inhibitor (Fig. 3B).
Fig. 3. Effect of PKA antagonist on PKA substrate phosphorylation and sperm motility.
A. Sperm were incubated in ASP containing H-89 (30 μM) for 15 min. at 20 °C before exposure to 10% ASP or EW with inhibitor. Phosphoproteins were detected as in Figure 2A. Western blot for actin was performed to check equal loading. Molecular weight standards are indicated at the left of each blot (Mr × 103). B. Motility analysis of sperm incubated in 10% ASP or EW in the presence of H-89 (30 μM). Data represent mean ± standard deviations; n=5. Asterisks indicate a significant inhibition effect with respect to the control (* Mann – Whitney test to compare progressive movement medians with p< 0.002 and ** t-test to compare progressive movement means with p<0.0001).
These results suggest that hypotonicity triggers an intracellular cAMP rise which in turn activates PKA. To evaluate this hypothesis, cAMP agonists were added to isotonic ASP medium. As shown in figure 4A, the presence of dibutyryl cAMP or 8-Br-cAMP and IBMX (a phosphodiesterase inhibitor) stimulated PKA-induced phosphorylation. Interestingly, the proteins detected by anti-pPKAs antibodies are of same apparent MW as the ones detected in sperm incubated in hypotonic medium. Moreover, cAMP analogues triggered amphibian sperm in situ motility in isotonic media (Fig. 4B), indicating that cAMP is involved in Bufo sperm motility. Note that progressive movement is not achieved as in the case of EW, indicating the presence of alternative pathways governing sperm motility. The onset of PKA substrate phosphorylation promoted by cAMP analogues was impaired by the presence of H-89, further demonstrating that this phosphorylation pathway involves PKA activation (Fig. 4C).
Fig. 4. Effect of PKA agonists on PKA substrate phosphorylation and sperm motility.
A. Sperm (7 × 106 cells) were incubated in ASP in the presence of db-cAMP or 8-Br-cAMP (1mM each) and 0.1 mM IBMX for 15 min at 20°C. Sperm were incubated in 10% ASP (right panel). Phosphoproteins were detected as in Figure 2A. Western blot for actin was performed as loading control. Molecular weight standards are indicated at the left of each blot (Mr × 103). B. Motility analysis of sperm incubated in ASP in the presence of db-cAMP or 8-Br-cAMP (1mM each) and IBMX (0.1mM). The means of groups that have different letters differ significantly (mean ± standard deviations; n=5; p<0.0001). C. Sperm were incubated in ASP containing db-cAMP and H-89 (0.1, 1 and 30 μM) for 15 min at 20°C. Phosphoproteins were detected as in Fig. 2A.
Involvement of transmembrane adenylyl cyclase(s) in sperm motility
Above results indicate that Bufo sperm motility is activated by a cAMP/PKA-dependent pathway. Adenylyl cyclases (ACs) are responsible for the intracellular production of cAMP. Two different AC isoform categories have been identified so far in mammalian testes: 1) a membrane-associated ACs, regulated by G protein receptors (tmACs) (Baxendale and Fraser, 2003; Defer et al., 1998), and 2) a Mn2+ dependent isoform modulated by bicarbonate and calcium, and insensitive to G protein modifiers (SACY) (Braun and Dods, 1975). In order to analyze the role of tmACs in Bufo sperm motility, sperm were incubated in the presence of forskolin (FK), a broad activator of tmAC that do not stimulate SACY activity. When sperm suspensions were preincubated during 15 min in ASP containing FK, an increase in PKA-dependent phosphorylation was observed (Fig. 5A). Noteworthy, the phophoproteins detected after FK incubation are of similar molecular weight to those observed upon hypotonic stimulation (see Fig 2A and right panel 10% ASP of Fig. 5A) and to the ones induced in the presence of cAMP agonists (Fig. 4A). Phosphorylation was effectively blocked by 30 μM H-89 (Fig. 5B), suggesting that FK action is mediated by PKA. Furthermore, FK was able, as in the case of cAMP permeable analogs, to stimulate sperm motility in isotonic solutions. The percentage of total motile sperm (in situ and progressive movement) increased significantly in a media containing 50 μM FK (Fig. 5C). Although, motility increased in the presence of FK, the percentages of in situ and progressive movement are not the same than seen in hypotonic medium.
Fig. 5. Stimulation of tmAC triggered PKA substrate phosphorylation and sperm motility.
1% Tritón X-100 soluble proteins of 7 × 106 spermatozoa were separated in 10% SDS/PAGE and transferred to a nitrocellulose membrane. Western blot were performed using anti-pPKAs as in Fig. 2A (A and B), and polyclonal anti-Gαs protein antibodies (D). A. Sperm were incubated in ASP containing or not forskolin (FK) for 15 min at 20°C or in 10% ASP (right panel). B. Sperm were incubated in ASP containing FK and H-89. C. Motility analysis of sperm incubated in ASP in the absence or presence of foskolin (10 or 50 μM). Data represent mean ± standard deviations; n=3. Asterisk indicate a significant inhibition effect with respect to the control (* Mann – Whitney test to compare medians with p< 0.03). D. Sperm were incubated in ASP. Black arrows indicate the large (52–48 kDa) and small (45–43 kDa) Gα isoforms. Molecular weight standards are indicated at the left of each blot (Mr × 103). Normal rabbit IgG were used as a negative control.
Since tmACs are regulated by heterotrimeric G-proteins, the presence of Gαs was assessed in toad sperm extracts using heterologous polyclonal anti-Gαs antibodies. These antibodies recognize a C-terminal highly conserved region of Gαs (RMHLRQYELL), found in mammals and Xenopus (Olate et al., 1988). Western blot experiments revealed the Bufo arenarum sperm are endowed with a large (~50 kDa) and small (~43 kDa) Gsα isoforms (Fig. 5D) in accordance with the expected molecular weights.
A pharmacological approach using AC inhibitors was conducted in order to further substantiate the origin of cAMP in Bufo sperm. Cells were incubated in 10% ASP containing the non-nucleoside AC inhibitor MDL12330A (Lippe and Ardizzone, 1991). This inhibitor has been proposed to be specific for tmAC (Nunes et al., 2009). As shown in figure 6A, a dose dependant inhibition of PKA-dependent phosphorylation was detected. However, no inhibition was observed when the SACY selective inhibitor, KH7, was used (Fig. 6A). The effect of MDL12330A was also observed when sperm tmACs were directly stimulated with FK in isotonic ASP media (Fig. 6B). Finally, sperm motility was also affected upon incubation of sperm in 10% ASP media containing MDL12330A (Fig. 6C) but not when KH7 was tested (data not shown).
Fig. 6. Effect of AC inhibitors on PKA substrate phosphorylation and sperm motility.
A. Sperm were incubated in ASP containing MDL or KH7 for 15 min at 20 °C before exposure to 10% ASP with inhibitor. B. Sperm were incubated in ASP containing FK and MDL for 15 min. at 20°C. Phosphoproteins were detected as in Fig. 2A. Western blot for actin was performed to check equal loading. Molecular weight standards are indicated at the left of each blot (Mr × 103). C. Motility analysis of sperm incubated in 10% ASP in the presence of MDL tmAC inhibitor. The means of groups that have different letters differ significantly (mean± standard deviations; n=3; p<0.05).
Discussion
Mammalian sperm remain immotile during residence in the cauda epididymis, but are quickly activated once diluted into physiological media. Similarly, sperm from external fertilizers, which do not posses epidydimis, remain in a quiescent state in the testis. However, these sperm generally become motile upon osmotic change of the surrounding media. In both cases, little is known about molecular pathways leading to sperm motility. Activation of motility in Amphibian sperm results from the sudden drop in osmotic strength upon dilution into pond water where fertilization normally occurs. Bufo sperm remain quiescent in the testis due to seminal plasma osmotarity of 292 mOsm/kg, similar to Xenopus laevis (Inoda and Morisawa, 1987). No activation of motility was observed when suspensions were prepared in artificial seminal plasma (ASP, see Table I). However, almost the whole population became motile when the sperm suspension was diluted to 10% ASP. This medium, which resembles osmolarity of natural fertilization environments, promoted an in situ motility pattern. Noteworthy, EW medium shifts this in situ pattern to progressive sperm motility, even though its osmolarity resembles that of 10% ASP. A similar motility enhancement has been reported in the frog Crinia Georgiana (Simmons et al., 2009).
Several components of egg jelly coat have been shown to influence sperm motility of external fertilizers (Burnett et al., 2008; Hansbrough and Garbers, 1981; Ward et al., 1985; Yoshida et al., 2002). Despite some interesting advances on physiological responses to extracellular components that modulate sperm motility in sea urchin (Darszon et al., 2008), signaling events leading to acquisition of sperm motility have remained largely unknown in vertebrates.
Increasing evidence suggests that a network of kinases and phosphatases regulate flagellar beating. Cyclic AMP appears as a central regulator of mammalian sperm function. It plays roles in the regulation of different aspects of capacitation and acrosome reaction (Salicioni et al., 2007). In the present manuscript, it is shown that the presence of cAMP analogs induced Bufo sperm motility even when incubated in isosmotic conditions. Since one of the main cAMP targets is PKA, we aimed to study PKA involvement in Bufo sperm motility. These experiments demonstrated that hypotonic shock promotes phosphorylation by PKA in a similar way to that observed upon sperm stimulation with permeable cAMP analogs, indicating that hypotonic shock promotes intracellular cAMP rise. Moreover, both hypotonic induced PKA susbtrate phosphorylation and motility were abrogated in the presence of the PKA inhibitor H-89. When sperm were incubated in EW medium, a more intense phosphorylation was observed, as well as phosphorylation of extra proteins. This phosphorylation was mainly localized to the midpiece, where mitochondria are placed. The identity of these proteins remains to be elucidated. Noteworthy, a cAMP dependent phosphorylation of axonemal proteins has been reported in salmonid fish (Morisawa and Okuno, 1982), sea urchin (Bracho et al., 1998), ascidian (Nomura et al., 2000) and mammals (Tash and Bracho, 1998).
The canonical cAMP/PKA pathway characterized in mammalian sperm involves synthesis of cAMP through the atypical cyclase SACY. Sperm from SACY null mice are morphologically normal but immotile (Esposito et al., 2004). This cyclase responds to direct stimulation by HCO3−. Interestingly, 30 mM HCO3− promoted the onset of tyr phosphorylation in Bufo sperm, in an event reminiscent of mammalian sperm capacitation (Krapf et al., 2007). Unexpectedly, HCO3− did not promote PKA susbtrate phosphorylation or motility activation when added to isotonic media, further substantiating the presence of a mechanism independent of SACY governing PKA activation and motility in Bufo sperm (Table 2). It has been reported that extracellular calcium regulates the motility activation in several species (Cosson, 1989; Krasznai et al., 2000; Tash and Bracho, 1994). Interestingly, the presence of EGTA did not impair phosphorylation of PKA substrates even though sperm motility is not achieved, indicating that extracellular Ca2+ influx is not required in PKA activation (Table 2). Our results suggest that calcium regulates Bufo flagella activation downstream of PKA substrate-phosphorylation.
Table 2.
Effect of calcium and bicarbonate on motility and PKA substrate phosphorylation
| solution | motility | PKA substrate phophorylation |
|---|---|---|
| isotonic | — | — |
| isotonic with bicarbonate | — | — |
| hypotonic | ↑ | ↑ |
| hypotonic with EGTA | ↓ | ↑ |
Motility activation and PKA substrate phosphorylation were tested in isosmotic solution (modified ASP) containing 30 mM of NaHCO3 during 5 or 15 minutes and in a hypotonic solution (10% ASP) with 10 mM of EGTA. Increase of parameter is indicated by ↑, decrease with ↓ and no motility or non-phosphorylation changes by—.
Nine distinct genes encode a family of G-protein-regulated transmembrane adenylyl cyclases (tmACs), all independent of HCO3− and stimulated by Gαs. Forskolin, a well known tmAC agonist promotes dimerization of Gαs with tmAC with the consequent activation of the cyclase (Dessauer et al., 1997). Interestingly, motility was activated when Bufo sperm were incubated in the presence of 50 μM forskolin, in a similar extent to that observed with permeable cAMP analogs. The presence of tmAC has been addressed in human (Spehr et al., 2003), mouse (Baxendale and Fraser, 2003) and sea urchin (Beltran et al., 2007). Moreover, is has been suggested that a pathway involving tmAC in mammalian spermatozoa might function in chemotaxis (Spehr et al., 2003). However, other authors have not been able to detect Gs proteins in sperm (Hildebrandt et al., 1985) and the presence of tmACs and their role in mammalian sperm motility remain controversial. Our results present strong functional evidence that point toward a role of PKA as a downstream effector of tmAC in Bufo sperm motility: 1) both cAMP analogs and forskolin promoted a similar phosphorylation pattern of PKA substrates; this phosphorylation pattern is also similar to the one detected in sperm incubated in hypotonic media. 2) In all these cases, the increase in PKA substrate phosphorylation was blocked by H-89, at similar concentrations. 3) Gαs, the tmACs stimulatory protein, was detected in Bufo sperm. 4) The cell-permeant non-P-site inhibitor of tmAC MDL-12330A diminished agonist-evoked phosphorylation of PKA substrates as well as flagellar beating at a concentration of 100 μM, a concentration consistent with the reported IC50 of 250 μM (Lippe and Ardizzone, 1991). The same concentration of this inhibitor did not affect the increased of mouse sperm flagella beat frequency upon agonist stimulation. Consistent with this result, mouse sperm motility is known to depend only in SACY, which is unaffected by 100 μM MDL-12330A (Schuh et al., 2006). 5) KH7, a SACY specific inhibitor, did not block forskolin or hypotonicity-dependent increase in PKA substrate phosphorylation and did not affect Bufo sperm motility. Alltogether, these results indicate that tmAC(s) and not SACY is involved in Bufo sperm motility.
How toad sperm tmAC is activated upon hypotonic stress? Despite the presence of Gαs in Bufo sperm, an alternative hypothesis involves a direct stimulation of tmAC by hyperpolarization. It was shown almost twenty years ago (Schultz et al., 1992) that adenylyl cyclase from Paramecium was stimulated by membrane hyperpolarization. The purified adenylyl cyclase presented properties of a voltage-independent K+ channel. Four years later, Beltrán et al (1996) found that sea urchin sperm AC responded to membrane hyperpolarization. Our findings suggest that cAMP-dependent phosphorylation increase in response of a hypo-osmotic shock likely due to activation of tmAC activity. Interestingly, Bufo sperm showed a hyperpolarized state after dilution to 10% ASP (data not shown). Another possibility is an upstream activation of stretch-sensitive or osmo-sensitive proteins that triggers tmAC stimulation. Transient receptor potential channels (TRP) are well candidates to transmit osmotic-changes in the external environment within the membrane. Members of several TRP subfamilies have been shown to be activated in response to hypo-osmolality in somatic cells (Hoffmann et al., 2009). Specifically, some TRPs were immunolocalizated in head and fagellum of mouse (Jungnickel et al., 2001; Trevino et al., 2001) and human (De Blas et al., 2009) sperm suggesting physiological roles in sperm motility.
To sum up we propose a model for sperm motility activation in Bufo arenarum, which is depicted in Figure 7. Hypo-osmotic shock activates stretch-sensitive or osmo-sensitive protein witch triggers downstream tmAC activation and consequent cAMP increase. The latter determines the activation of the cAMP-signaling pathway, causing the phosphorylation of proteins by PKA and flagellar activation. This model may provide the basis for future studies to elucidate the upstream mechanisms that couple the osmotic shock with tmAC activation and the onset of sperm motility.
Fig. 7. A model for the modulation of Bufo sperm motility by transmembrane adenylyl cyclase.

(1) An osmo-sensing protein is activated by EW (due to its hypotonicity and/or specific components) which (2) stimulates a transmembrane adenylyl cyclase, (3) increasing intracellular cAMP. (4) Activation of Protein Kinase A by cAMP promotes phosphorylation of PKA substrates, involved in modulation of sperm motility. EW: egg water. OSM: Osmo-sensing protein. γ, β, and α means proteins Gγ, Gβ, and Gα.
Research highlights.
Amphibian sperm motility activation results from the drop in osmotic strength upon dilution
Hypotonic media stimulates a transmembrane adenylyl cyclase increasing intracellular cAMP
Activation of Protein Kinase A by cAMP, promotes phosphorylation of PKA substrates and causes flagellar activation
Acknowledgments
We thank Dr. Sonia Scarpeci for her help with the confocal microscopy studies and Sebastian Graziati for toad husbandry and technical help. This study was supported by NIH HD38082 and HD44044 (to PEV), grants from ANPCyT PICT15-31660 and CONICET PIP6428 (to MOC and SEA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arranz SE, Cabada MO. Diffusible highly glycosylated protein from Bufo arenarum egg-jelly coat: biological activity. Mol Reprod Dev. 2000;56:392–400. doi: 10.1002/1098-2795(200007)56:3<392::AID-MRD10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Barbieri FD, Cabada M. The role of the diffusible factor released by the egg jelly in fertilization of the toad egg. Experientia. 1969;25:1312–1313. doi: 10.1007/BF01897520. [DOI] [PubMed] [Google Scholar]
- Baxendale RW, Fraser LR. Evidence for multiple distinctly localized adenylyl cyclase isoforms in mammalian spermatozoa. Mol Reprod Dev. 2003;66:181–189. doi: 10.1002/mrd.10344. [DOI] [PubMed] [Google Scholar]
- Beltran C, Vacquier VD, Moy G, Chen Y, Buck J, Levin LR, Darszon A. Particulate and soluble adenylyl cyclases participate in the sperm acrosome reaction. Biochem Biophys Res Commun. 2007;358:1128–1135. doi: 10.1016/j.bbrc.2007.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran C, Zapata O, Darszon A. Membrane potential regulates sea urchin sperm adenylylcyclase. Biochemistry. 1996;35:7591–7598. doi: 10.1021/bi952806v. [DOI] [PubMed] [Google Scholar]
- Bracho GE, Fritch JJ, Tash JS. Identification of flagellar proteins that initiate the activation of sperm motility in vivo. Biochem Biophys Res Commun. 1998;242:231–237. doi: 10.1006/bbrc.1997.7937. [DOI] [PubMed] [Google Scholar]
- Braun T, Dods RF. Development of a Mn-2+-sensitive, “soluble” adenylate cyclase in rat testis. Proc Natl Acad Sci U S A. 1975;72:1097–1101. doi: 10.1073/pnas.72.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci US A. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett LA, Boyles S, Spencer C, Bieber AL, Chandler DE. Xenopus tropicalis allurin: expression, purification, and characterization of a sperm chemoattractant that exhibits cross-species activity. Dev Biol. 2008;316:408–416. doi: 10.1016/j.ydbio.2008.01.046. [DOI] [PubMed] [Google Scholar]
- Cabada MO. Sperm concentration and fertilization rate in Bufo arenarum (Amphibia: Anura) J Exp Biol. 1975;62:481–486. doi: 10.1242/jeb.62.2.481. [DOI] [PubMed] [Google Scholar]
- Cosson MBR, Letellier L. Rise of internal Ca2+ accompanies the initiation of trout sperm motility. Cell Motility and the Cytoskeleton. 1989;14:424–434. [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- Darszon A, Guerrero A, Galindo BE, Nishigaki T, Wood CD. Sperm-activating peptides in the regulation of ion fluxes, signal transduction and motility. Int J Dev Biol. 2008;52:595–606. doi: 10.1387/ijdb.072550ad. [DOI] [PubMed] [Google Scholar]
- De Blas GA, Darszon A, Ocampo AY, Serrano CJ, Castellano LE, Hernandez-Gonzalez EO, Chirinos M, Larrea F, Beltran C, Trevino CL. TRPM8, a versatile channel in human sperm. PLoS One. 2009;4:e6095. doi: 10.1371/journal.pone.0006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defer N, Marinx O, Poyard M, Lienard MO, Jegou B, Hanoune J. The olfactory adenylyl cyclase type 3 is expressed in male germ cells. FEBS Lett. 1998;424:216–220. doi: 10.1016/s0014-5793(98)00178-1. [DOI] [PubMed] [Google Scholar]
- Dessauer CW, Scully TT, Gilman AG. Interactions of forskolin and ATP with the cytosolic domains of mammalian adenylyl cyclase. J Biol Chem. 1997;272:22272–22277. doi: 10.1074/jbc.272.35.22272. [DOI] [PubMed] [Google Scholar]
- Diaz Fontdevila MF, Bloj B, Cabada MO. Effect of egg water from Bufo arenarum on the fertilizing capacity of homologous spermatozoa. J Exp Zool. 1991;257:408–414. [Google Scholar]
- Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, Gossen JA. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci U S A. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- Hansbrough JR, Garbers DL. Speract. Purification and characterization of a peptide associated with eggs that activates spermatozoa. J Biol Chem. 1981;256:1447–1452. [PubMed] [Google Scholar]
- Hardy MP, Dent JN. Regulation of motility in sperm of the red-spotted newt. J Exp Zool. 1986;240:385–396. doi: 10.1002/jez.1402400313. [DOI] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, Levin LR, Williams CJ, Buck J, Moss SB. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt JD, Codina J, Tash JS, Kirchick HJ, Lipschultz L, Sekura RD, Birnbaumer L. The membrane-bound spermatozoal adenylyl cyclase system does not share coupling characteristics with somatic cell adenylyl cyclases. Endocrinology. 1985;116:1357–1366. doi: 10.1210/endo-116-4-1357. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- Inoda T, Morisawa M. Effect of osmolality on the initiation of sperm motility in Xenopus laevis. Comp Biochem Physiol A. 1987;88:539–542. doi: 10.1016/0300-9629(87)90077-6. [DOI] [PubMed] [Google Scholar]
- Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci U S A. 2003;100:10676–10681. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel MK, Marrero H, Birnbaumer L, Lemos JR, Florman HM. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- Katagiri C. Fertilization of dejellied uterine toad eggs in various experimental conditions. Embryologia (Nagoya) 1966;9:159–169. doi: 10.1111/j.1440-169x.1966.tb00221.x. [DOI] [PubMed] [Google Scholar]
- Krapf D, Arcelay E, Wertheimer EV, Sanjay A, Pilder SH, Salicioni AM, Visconti PE. Inhibition of Ser/Thr phosphatases induces capacitation-associated signaling in the presence of Src kinase inhibitors. J Biol Chem. 2010;285:7977–7985. doi: 10.1074/jbc.M109.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapf D, O’Brien ED, Cabada MO, Visconti PE, Arranz SE. Egg water from the amphibian Bufo arenarum modulates the ability of homologous sperm to undergo the acrosome reaction in the presence of the vitelline envelope. Biol Reprod. 2009;80:311–319. doi: 10.1095/biolreprod.108.071076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapf D, Visconti PE, Arranz SE, Cabada MO. Egg water from the amphibian Bufo arenarum induces capacitation-like changes in homologous spermatozoa. Dev Biol. 2007;306:516–524. doi: 10.1016/j.ydbio.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasznai Z, Marian T, Izumi H, Damjanovich S, Balkay L, Tron L, Morisawa M. Membrane hyperpolarization removes inactivation of Ca2+ channels, leading to Ca2+ influx and subsequent initiation of sperm motility in the common carp. Proc Natl Acad Sci US A. 2000;97:2052–2057. doi: 10.1073/pnas.040558097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lippe C, Ardizzone C. Actions of vasopressin and isoprenaline on the ionic transport across the isolated frog skin in the presence and the absence of adenyl cyclase inhibitors MDL12330A and SQ22536. Comp Biochem Physiol C. 1991;99:209–211. doi: 10.1016/0742-8413(91)90101-x. [DOI] [PubMed] [Google Scholar]
- Martinez ML, Cabada MO. Assessment of the acrosome reaction in Bufo arenarum spermatozoa by immunostaining: comparison with other methods. Zygote. 1996;4:181–190. doi: 10.1017/s0967199400003099. [DOI] [PubMed] [Google Scholar]
- Morisawa M. Cell signaling mechanisms for sperm motility. Zoolog Sci. 1994;11:647–662. [PubMed] [Google Scholar]
- Morisawa M, Okuno M. Cyclic AMP induces maturation of trout sperm axoneme to initiate motility. Nature. 1982;295:703–704. doi: 10.1038/295703a0. [DOI] [PubMed] [Google Scholar]
- Morisawa M, Suzuki K, Morisawa S. Effects of potassium and osmolality on spermatozoan motility of salmonid fishes. J Exp Biol. 1983a;107:105–113. doi: 10.1242/jeb.107.1.105. [DOI] [PubMed] [Google Scholar]
- Morisawa M, Suzuki K, Shimizu H, Morisawa S, Yasuda K. Effects of osmolality and potassium on motility of spermatozoa from freshwater cyprinid fishes. J Exp Biol. 1983b;107:95–103. doi: 10.1242/jeb.107.1.95. [DOI] [PubMed] [Google Scholar]
- Nomura M, Inaba K, Morisawa M. Phosphorylation of axonemal 21 kDa and 26 kDa proteins modulates activation of sperm motility in the ascidian, Ciona intestinalis. Zygote. 2000;8(Suppl 1):S59–60. [PubMed] [Google Scholar]
- Nunes AR, Monteiro EC, Johnson SM, Gauda EB. Bicarbonate-regulated soluble adenylyl cyclase (sAC) mRNA expression and activity in peripheral chemoreceptors. Adv Exp Med Biol. 2009;648:235–241. doi: 10.1007/978-90-481-2259-2_27. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, de Lamirande E, Gagnon C. Phosphorylation of the Arginine-X-X-(Serine/Threonine) motif in human sperm proteins during capacitation: modulation and protein kinase A dependency. Mol Hum Reprod. 2004;10:355–363. doi: 10.1093/molehr/gah046. [DOI] [PubMed] [Google Scholar]
- Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem. 1985;260:9699–9705. [PubMed] [Google Scholar]
- Olate J, Mattera R, Codina J, Birnbaumer L. Reticulocyte lysates synthesize an active alpha subunit of the stimulatory G protein Gs. J Biol Chem. 1988;263:10394–10400. [PubMed] [Google Scholar]
- Raisman J, Pisano A. Fertilization of jelly-less Bufo arenarum oocytes in the presence of high sperm concentrations. Acta Embryol Exp (Palermo) 1970;1:3–11. [PubMed] [Google Scholar]
- Raisman JDC, RW, Cabada MO, Del Pino EJ, Mariano MI. Acrosome breakdown in Leptodactylus chaquensis (Amphibia: anura) spermatozoa. Develop Growth Differ. 1980;22:289–297. doi: 10.1111/j.1440-169X.1980.00289.x. [DOI] [PubMed] [Google Scholar]
- Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, Sosnik J, Visconti PE. Signalling pathways involved in sperm capacitation. Soc Reprod Fertil Suppl. 2007;65:245–259. [PubMed] [Google Scholar]
- Schuh SM, Carlson AE, McKnight GS, Conti M, Hille B, Babcock DF. Signaling pathways for modulation of mouse sperm motility by adenosine and catecholamine agonists. Biol Reprod. 2006;74:492–500. doi: 10.1095/biolreprod.105.047837. [DOI] [PubMed] [Google Scholar]
- Schultz JE, Klumpp S, Benz R, Schurhoff-Goeters WJ, Schmid A. Regulation of adenylyl cyclase from Paramecium by an intrinsic potassium conductance. Science. 1992;255:600–603. doi: 10.1126/science.1371017. [DOI] [PubMed] [Google Scholar]
- Shivers CA, James JM. Capacitation of frog sperm. Nature. 1970;227:183–184. doi: 10.1038/227183a0. [DOI] [PubMed] [Google Scholar]
- Simmons LW, Roberts JD, Dziminski MA. Egg jelly influences sperm motility in the externally fertilizing frog, Crinia georgiana. J Evol Biol. 2009;22:225–229. doi: 10.1111/j.1420-9101.2008.01628.x. [DOI] [PubMed] [Google Scholar]
- Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- Tash JS, Bracho GE. Regulation of sperm motility: emerging evidence for a major role for protein phosphatases. J Androl. 1994;15:505–509. [PubMed] [Google Scholar]
- Tash JS, Bracho GE. Identification of phosphoproteins coupled to initiation of motility in live epididymal mouse sperm. Biochem Biophys Res Commun. 1998;251:557–563. doi: 10.1006/bbrc.1998.9516. [DOI] [PubMed] [Google Scholar]
- Trevino CL, Serrano CJ, Beltran C, Felix R, Darszon A. Identification of mouse trp homologs and lipid rafts from spermatogenic cells and sperm. FEBS Lett. 2001;509:119–125. doi: 10.1016/s0014-5793(01)03134-9. [DOI] [PubMed] [Google Scholar]
- Valz-Gianinet JN, del Pino EJ, Cabada MO. Glycoproteins from Bufo arenarum vitelline envelope with fertility-impairing effect on homologous spermatozoa. Dev Biol. 1991;146:416–422. doi: 10.1016/0012-1606(91)90243-v. [DOI] [PubMed] [Google Scholar]
- Ward GE, Brokaw CJ, Garbers DL, Vacquier VD. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J Cell Biol. 1985;101:2324–2329. doi: 10.1083/jcb.101.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Murata M, Inaba K, Morisawa M. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proc Natl Acad Sci U S A. 2002;99:14831–14836. doi: 10.1073/pnas.242470599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilli L, Schiavone R, Storelli C, Vilella S. Molecular mechanisms determining sperm motility initiation in two sparids (Sparus aurata and Lithognathus mormyrus) Biol Reprod. 2008;79:356–366. doi: 10.1095/biolreprod.108.068296. [DOI] [PubMed] [Google Scholar]