Abstract
Late INa is an integral part of the sodium current, which persists long after the fast-inactivating component. The magnitude of the late INa is relatively small in all species and in all types of cardiomyocytes as compared with the amplitude of the fast sodium current, but it contributes significantly to the shape and duration of the action potential. This late component had been shown to increase in several acquired or congenital conditions, including hypoxia, oxidative stress, and heart failure, or due to mutations in SCN5A, which encodes the α-subunit of the sodium channel, as well as in channel-interacting proteins, including multiple β subunits and anchoring proteins. Patients with enhanced late INa exhibit the type-3 long QT syndrome (LQT3) characterized by high propensity for the life-threatening ventricular arrhythmias, such as Torsade de Pointes (TdP), as well as for atrial fibrillation. There are several distinct mechanisms of arrhythmogenesis due to abnormal late INa, including abnormal automaticity, early and delayed afterdepolarization-induced triggered activity, and dramatic increase of ventricular dispersion of repolarization. Many local anesthetic and antiarrhythmic agents have a higher potency to block late INa as compared with fast INa. Several novel compounds, including ranolazine, GS-458967, and F15845, appear to be the most selective inhibitors of cardiac late INa reported to date. Selective inhibition of late INa is expected to be an effective strategy for correcting these acquired and congenital channelopathies.
Keywords: Ion channel currents, Electrophysiology, Long QT syndrome, Sudden cardiac death, Cardiac arrhythmias
Recent years have witnessed a resurgence of interest in the late component of the sodium current (late INa), particularly its role in development of cardiac arrhythmias and as a pharmacologic target for the prevention of life-threatening arrhythmias and sudden cardiac death. In this review, our principal aim is to discuss the molecular basis for late INa, its cellular distinctions, and its contribution to the electrophysio-logical function of the heart in health and disease. We refer the readers to recent reviews dealing with the characteristics of the cardiac late INa (Belardinelli et al. 2006; Noble and Noble 2006; Saint 2006, 2008; Zaza et al. 2008; Undrovinas and Maltsev 2008a; Maier 2009; Antzelevitch et al. 2011; Shryock et al. 2013) as well as reviews dealing with cardiac sodium channelopathies associated with a gain of function of late INa and its role in arrhythmogenesis (Zimmer and Surber 2008; Ruan et al. 2009; Amin et al. 2010; Rook et al. 2012).
1 Late INa and Its Relationship to Peak INa
Voltage-gated sodium channels mediate excitability of heart, nerve, endocrine, and skeletal muscle tissues. When membrane depolarization achieves threshold potential, the Na+ channels open, thus giving rise to the “peak” INa and the rapid upstroke (phase 0) of the action potential (AP).
The sodium channel activates during phase 0 of the AP and largely inactivates within 1 ms at body temperature. Between 0.1 % and 1 % the current inactivates more slowly during the plateau of the action potential (AP) (Patlak and Ortiz 1985) with the time constant being between 75 and 450 ms in humans (Undrovinas et al. 2002; Maltsev and Undrovinas 2006). Amplitude of the late component being small compared with the peak INa is large as compared with other ionic currents during AP plateau, e.g., around 0.5 pA/pF in normal human and canine ventricular myocytes (Maltsev and Undrovinas 2006; Undrovinas et al. 2006). Late INa amplitude varies depending on cell type, species, and conditions of measurement (e.g., holding and test potentials, temperature, duration of test pulse, and intracellular Na+ concentration). Late INa amplitude is reported to be around 0.1 % of peak INa in rat (Patlak and Ortiz 1985) and guinea pig (Kiyosue and Arita 1989), but can reach 1 % in human (Maltsev and Undrovinas 2006) ventricular myocytes. It had been shown that all components of INa inactivation are due to different modes of gating of the same cardiac variant (NaV1.5) of the sodium channel (Maltsev et al. 2009).
The amplitude of late INa is largest in M cells (Eddlestone et al. 1996) and in Purkinje fibers and much smaller in epicardial or endocardial cells in the canine heart. The more prominent late INa contributes to the longer AP and greater rate dependence of AP duration in M cells and Purkinje fibers (Eddlestone et al. 1996; Zygmunt et al. 2001) and thus to transmural dispersion of repolarization. Tetrodotoxin (TTX) is reported to inhibit late INa and abbreviate APD more in M cells and Purkinje fibers than in epicardial cells in the dog heart (Zygmunt et al. 2001; Coraboeuf et al. 1979), thus reducing the transmural dispersion of repolarization. In the guinea pig heart, however, late INa has been reported to be smaller in mid-myocardial than in epicardial and endocardial cells (Sakmann et al. 2000). The difference appears to be due to methodological considerations. Experiments involving isolated tissues indicate that the guinea pig heart is similar to that of the dog, containing M and transitional cells in the midmyocardium and cells with much briefer APD, showing little response to IKr block, in the endocardial and epicardial layers (Sicouri et al. 1996). However, unlike the dog, dissociation of myocytes from smaller hearts is fraught with problems because epicardial and endocardial cells are under-represented (Antzelevitch et al. 1999). Indeed, studies involving dissociation of myocytes from guinea pig hearts have reported cells with electrophysiological and pharmacological profiles of M and transitional cells, but not of endocardial or epicardial cells (Bryant et al. 1998). Rather than lacking M cells, the studies reported by Sakmann et al. (2000) may be lacking in epicardial and endocardial cells.
At the single channel level, late INa is predominantly due to sodium channel reopenings during the plateau of the AP (Maltsev and Undrovinas 2006), which allow a relatively small but persistent influx of Na+ into the cell. Such activity may be the result of single or bursts of openings of Na+ channels (Fig. 1) (Patlak and Ortiz 1985; Undrovinas et al. 2002; Kiyosue and Arita 1989; Liu et al. 1992; Saint et al. 1992; Maltsev et al. 1998). The magnitude of the late INa is increased when the channel fails to enter the inactivated state after the initial opening. Sodium channel toxins including aconitine, veratridine, and sea anemone toxin II (ATX-II) prevent the sodium channel transition into the inactivated state, thus increasing late reopening of the channel, measured in excised or cell-attached patches during long voltage-clamp pulses (>200 ms).
Fig. 1.
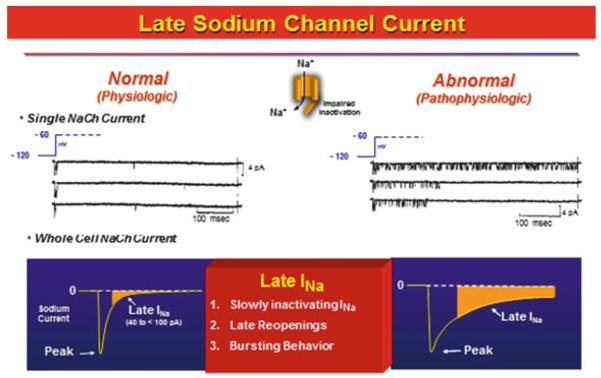
Late sodium channel current is the current flowing during the plateau of the cardiac action potential and is comprised of slowly inactivating sodium channel current, late re-openings, and bursting behavior of the sodium channels. Modified from Belardinelli et al. (2004), with permission
2 Causes of an Enhanced Late INa
The magnitude of late INa in cardiac myocytes increases in several acquired or congenital conditions such as heart failure (Maltsev et al. 2007; Valdivia et al. 2005), hypoxia (Hammarstrom and Gage 2002), inflammation (Ward et al. 2006), and hyperthyroxinemia (Harris et al. 1991), or due to mutations in SCN5A, which encodes the α-subunit of the sodium channel (Rivolta et al. 2001), as well as in channel-interacting proteins, including multiple β subunits and anchoring proteins. Such an increase of late INa can be reproduced experimentally by application of natural and synthetic toxins [see (Honerjager 1982) for review] such as ATX-II, veratridine, and aconitine that bind to various sites on the Na+ channel (Denac et al. 2000). It is important to make the distinction between toxins that bind near the local anesthetic binding sites (veratridine and aconitine) and those that bind to the sodium channel at unrelated sites (e.g., ATX-II). It had been shown that these compounds can cause arrhythmias when applied to intact or isolated heart preparations. For example, local application of aconitine causes atrial ectopic activity (Scherf et al. 1948) and ventricular tachycardia and fibrillation (Lu and De 1993). Bursting behavior, which results in a larger late INa, is reported to be increased as a consequence of some LQT3 mutations including ΔKPQ (Hartmann et al. 1994) and Y1795C (Rivolta et al. 2001). Table 1 lists the conditions and agents that are known to increase cardiac late INa.
Table 1.
Conditions and agents that have been demonstrated to increase cardiac late INa
| Conditions/endogenous agents | Drugs and toxins |
| Activation of CaMKII | Aconitine |
| Activation of Fyn tyrosine kinase | ATX-II |
| Activation of PKC | Batrachotoxin |
| Angiotensin II | DPI 201–106 and analogs |
| Carbon monoxide | KB130015 |
| 2,3-Diphosphoglycerate | Ouabain (indirectly) |
| Hydrogen peroxide (H2O2) | Pyrethroids (e.g., tefluthrin) |
| Hypoxia, ischemia Hyperthyroxinemia |
Veratridine |
| Lysophosphatidylcholine | |
| Nitric oxide (NO) | |
| Palmitoyl-l-carnitine |
Modified from Shryock et al. (2013), with permission
3 Acquired Causes of an Enhanced Late INa
Ischemia is the most common pathology associated with enhanced late INa. An increase in late INa during ischemia contributes to the intracellular Na+ loading observed in the ischemic heart (Xiao and Allen 1999; Liu et al. 2007; Tang et al. 2012). Simulated-demand ischemia was found to increase oxidative stress, late INa, and cytosolic Ca2+ levels in rabbit isolated myocytes; the rise of intracellular Ca2+ was reduced by inhibitors of late INa and Na+/Ca2+ exchanger (NCX) and by the free radical scavenger Tiron (Zhang et al. 2008). Activity of NCX is markedly increased by reactive oxygen species during re-oxygenation (Eigel et al. 2004). Hydrogen peroxide, nitric oxide, and thrombin are all reported to increase cardiac late INa (Ward and Giles 1997; Song et al. 2006; Ahern et al. 2000; Pinet et al. 2008). Nitric oxide (Ahern et al. 2000), hydrogen peroxide (Song et al. 2006), hypoxia (Ju et al. 1996), glycolytic metabolites (Kohlhardt et al. 1989), lysophosphatidylcholine (Undrovinas et al. 1992), and heart failure (Maltsev and Undrovinas 2006) also increase the incidence of bursts of Na+ channel late openings that contribute to late INa. Protein kinases including CaMKII, PKC, AMPK, and PKA are activated during ischemia. Calcium and reactive oxygen species activate CaMKII and PKC (Erickson et al. 2011; Barnett et al. 2007), lysophosphatidylcholine activates PKC and tyrosine kinase (Murray et al. 1997), loss of ATP and elevation of AMP stimulate AMP-activated protein kinase, and norepinephrine release from cardiac nerve terminals leads to activation of PKA and increased L-type Ca2+ current. Each of these kinases, as well as intracellular Ca2+, can modulate Na+ channel function or expression.
Late INa is increased in myocytes isolated from failing human and dog hearts (Undrovinas and Maltsev 2008a; Undrovinas et al. 2002; Maltsev et al. 1998, 2007; Valdivia et al. 2005; Undrovinas et al. 1999), although peak INa is decreased (Undrovinas et al. 2002; Zicha et al. 2004). INa inactivates more slowly in cells isolated from failing hearts and the magnitude of late INa is 2–5 fold greater (Maltsev et al. 2007; Valdivia et al. 2005). Most NaV1.5 channels in ventricular myocytes are localized near the intercalated discs where they interact with βIV-spectrin (Hund et al. 2010) and the rest are in the T-tubules and other areas of the plasma membrane. It is not known whether Na+ channels located in these different regions of the cell are differentially regulated in terms of their late current in normal and/or diseased hearts.
The final common pathways leading to an increase of late INa in the failing heart, as in ischemia, may be ROS-induced oxidation and Ca2+-induced kinase activation and phosphorylation of the Na+ channel and/or channel-interacting proteins (Maltsev et al. 2008; Xie et al. 2009; Gautier et al. 2008). In the hypertrophied and/or failing heart, reduction of repolarizing current contributes to prolongation of AP duration in a nonuniform manner (Keung and Aronson 1981) and to alternans of Ca2+ transients and AP duration (Wilson et al. 2009), thus promoting an arrhythmogenic substrate.
4 Congenital Causes of Late INa
The long QT syndrome type-3 (LQT3) is caused by inherited “gain of function” mutations in the SCN5A gene [for reviews, see (Zimmer and Surber 2008; Ruan et al. 2009; Moreno and Clancy 2012; Blaufox et al. 2012)] and by mutations in Na+ channel-interacting proteins that lead to an increase of late INa (Abriel 2010). The first description of an inherited LQT3 mutation, a deletion of amino acids 1,505–1,507 (ΔKPQ) in a patient with a prolonged QT interval, was presented in 1995 (Wang et al. 1995). The mutated SCN5A channel was heterologously expressed in HEK293 cells and demonstrated to cause an increase of late INa and of the duration of the action potential (Bennett et al. 1995; Wang et al. 1996). More than 80 different SCN5A mutations that increase cardiac (NaV1.5) late INa have since been described in patients with LQT3. Most LQT3 mutations are missense mutations that cause late current by increasing the probability that the Na+ channel will either fail to inactivate quickly or will reopen more readily from the closed state. Some of these mutations occur at sites that are also known targets for phosphorylation by protein kinases (Ahern et al. 2005). Sodium channel mutations such as ΔKPQ and Y1795C cause increased bursting activity of the Na+ channel (Chandra et al. 1998; Clancy and Rudy 1999; Clancy et al. 2002) as do mutations in the IFM motif of the inactivation gate (West et al. 1992).
Four β subunits have been shown to play a critical role in cell surface expression, subcellular localization, as well as biophysical function of the Na+ channels (Abriel 2010; Maier et al. 2004; Meadows and Isom 2005). Mutations in the Na+ channel β subunits Navβ (β1–β4) are reported to be a cause of congenital LQT syndrome, atrial fibrillation, Brugada syndrome, and conduction slowing [reviewed by Abriel (2010)]. Loss of β1 expression in mice results in increases of both peak and late INa and a prolonged QT interval (Lopez-Santiago et al. 2007). Late INa is increased in cells expressing SCN5A with the β1, but not with the β2, subunit compared to cells expressing SCN5A alone (Maltsev et al. 2009). Mutations in both β3 and β4 subunits have been identified in LQT3 and/or sudden infant death syndrome (SIDS) patients and found to cause an increased late INa when expressed with SCN5A in HEK293 cells (Medeiros-Domingo et al. 2007; Tan et al. 2010).
Numerous proteins are known to interact with the cardiac sodium channel (Rook et al. 2012; Abriel 2010; Vatta et al. 2006; Shao et al. 2009). Mutations in scaffolding and/or cytoskeletal “channel interaction proteins” (ChIPs) are recognized causes of LQT syndrome (Ackerman and Mohler 2010). Mutations in caveolin-3 (Vatta et al. 2006), alpha-1 syntrophin (Ueda et al. 2008; Wu et al. 2008a), and βIV spectrin (Hund et al. 2010; Wu et al. 2008a; Sarhan et al. 2009; Vatta and Faulkner 2006) are associated with increased magnitude of late INa and with LQT and/or SIDS. Proteins such as F-actin, telethonin, α-actinin-2, and Z-band alternatively spliced PDZ-motif (ZASP) that are anchored at the Z-line may participate in the trafficking of ion channels to the T-tubule membrane (Vatta and Faulkner 2006). A mutation in telethonin is reported to increase Na+ window current (Mazzone et al. 2008), and a missense mutation in ZASP shifts the voltage dependence of Na+ channel activation (Li et al. 2010). Reduced interaction of the Na+ channel with ChIPs such as the intercalated disc-associated proteins ankyrin-G and SAP97 may lead to a Na+ channel loss-of-function phenotype such as conduction slowing or Brugada syndrome (Mohler et al. 2004; Scherer et al. 2008). Silencing of expression of SAP97 reduced peak INa in rat cardiomyocytes (Petitprez et al. 2011). The last three residues of the NaV1.5 C-terminus associate with dystrophin protein complexes in the lateral membranes of cardiomyocytes, and a deficiency of dystrophin leads to decreased NaV1.5 expression and myocardial Na+ current (Petitprez et al. 2011; Gavillet et al. 2006).
5 Late INa--Mediated Arrhythmias
Patients with LQT3 syndrome are at a high risk not only for Torsade de Pointes (TdP) ventricular arrhythmias but also for atrial fibrillation (Benito et al. 2008; Darbar et al. 2008; Zellerhoff et al. 2009). In studies of isolated hearts and myocytes, enhancement of late INa using ATX-II, veratridine, or aconitine is reported to cause arrhythmic activity in both atrial and ventricular tissues. In both inherited and acquired sodium channelopathies, late INa may be increased while peak INa is decreased (Maltsev et al. 2007; Valdivia et al. 2005; Zicha et al. 2004; Makita et al. 2008; Remme et al. 2006) and both can contribute to arrhythmogenesis.
Reduction in repolarization reserve can amplify the effect of an increase in late INa to delay repolarization, thus enabling the development of arrhythmias (Wu et al. 2004, 2006, 2008b, 2011)
6 Mechanisms Underlying Late INa-Induced Arrhythmogenesis
6.1 Late INa and Diastolic Depolarization: Abnormal Automaticity
Spontaneous diastolic depolarization responsible for pacemaking in sinoatrial and compact atrioventricular node cells is driven by ion currents including L- and T-type Ca2+ channel currents, “funny” current (HCN channels), and NCX [for reviews see (Chandler et al. 2009; Hoeker et al. 2009)]. Diastolic depolarization of myocytes in atrial and ventricular tissues is rare in the normal intact heart but is often observed in atrial cells and tissues excised from diseased human (Gelband et al. 1972; Escande et al. 1986; Mary-Rabine et al. 1980; Trautwein et al. 1962) and animal (Chen et al. 2000; Cheung 1981; Hogan and Davis 1968; Wit and Cranefield 1977) hearts.
A critical role for late INa in pacemaking was highlighted by recent studies demonstrating that atrial automaticity can be modulated by late INa enhancers and inhibitors (Song et al. 2009). Late INa was found to be present in atrial myocytes that undergo diastolic depolarization (Song et al. 2009). Sea anemone toxin II (ATX-II), a specific enhancer of late INa (Isenberg and Ravens 1984), accelerates diastolic depolarization and induces rapid firing of APs by atrial myocytes (Song et al. 2009). Reactive oxygen species H2O2 increases late INa and causes diastolic depolarization and rapid AP firing of atrial myocytes, which can be suppressed by block of late INa using ranolazine (1–5 μmol/L) or TTX (1 μmol/L) (Song et al. 2009). A slowly inactivating TTX-sensitive current, similar to late INa, was reported to contribute to diastolic depolarization of cardiac Purkinje cells (Carmeliet 1987a; Rota and Vassalle 2003) and of sinoatrial node cells (Baruscotti et al. 2000). In non-pacemaking ventricular myocytes, late INa was shown to be present at voltages as negative as −70 mV (Sakmann et al. 2000; Saint et al. 1992), well within the voltage range at which spontaneous diastolic depolarization can be observed in these cells (Escande et al. 1986; Mary-Rabine et al. 1980; Trautwein et al. 1962). Sicouri et al. (2012a) recently demonstrated an effect of ranolazine to suppress phase 4 depolarization in superior vena cava sleeves isolated from the canine right atria. These findings suggest that enhancement of late INa may be a potential cause of atrial arrhythmogenesis.
6.2 Late INa-Induced Triggered Activity
Early and delayed afterdepolarization (EAD and DAD) are important mechanisms of arrhythmic activity whose occurrence is facilitated when late INa is enhanced.
Late INa contributes to AP prolongation (Kiyosue and Arita 1989; Liu et al. 1992; Colatsky 1982). Because the magnitude of late INa is greater at slow heart rates (Wu et al. 2011; Jia et al. 2011) and the effect of late INa to increase AP duration is greater in mid-myocardial than in epi- or endocardial myocytes (Zygmunt et al. 2001; Sicouri et al. 1997a; Antzelevitch and Belardinelli 2006), the role of late INa to increase dispersion of repolarization and EAD formation is facilitated by heart rate slowing. A role for late INa in EAD formation is supported by findings that enhancers of late INa such as ATX-II and anthopleurin-A cause EADs and TdP (Isenberg and Ravens 1984; Ben et al. 2008; Boutjdir and El-Sherif 1991; Song et al. 2004; Ueda et al. 2004; Spencer and Sham 2005; Auerbach et al. 2011). Moreover, inhibitors of late INa reduce occurrences of EADs and TdP induced by IKr blockers (Abrahamsson et al. 1996; Shimizu and Antzelevitch 1997a; Orth et al. 2006; Wu et al. 2009a), heart failure (Maltsev et al. 2007; Undrovinas et al. 1999), or left ventricular hypertrophy (Guo et al. 2010).
While a modest increase in late INa may not cause significant prolongation of AP duration in the normal heart, it can facilitate APD prolongation and EADs induction by IKr and IKs blockers. Consistent with this observation, individual susceptibility to drug-induced long QT syndromes has been shown to be linked to SCN5A mutations (e.g., L1825P or Y1102) that augment late INa (Makita et al. 2002; Splawski et al. 2002). Patients with these Na+ channel gene mutations have normal QT intervals prior to exposure to the drugs, but develop long QT intervals and TdP when given agents such as cisapride or amiodarone (Makita et al. 2002; Splawski et al. 2002). Experimental studies involving isolated hearts, tissues, or myocytes have shown that a small increase of late INa may facilitate the proarrhythmic effects of IKr and IKs blockers (Wu et al. 2006; Song et al. 2004), drugs such as cisapride, amiodarone (Wu et al. 2008c), and quinidine (Wu et al. 2008b), as well as “low-risk” drugs such as moxifloxacin and ziprasidone (Wu et al. 2006). As expected, inhibition of late INa by ranolazine attenuates the increase of AP duration and EAD induction caused by the combination of ATX-II and IKr-blocking drugs (Song et al. 2004, 2008). These observations suggest that enhanced late INa is a major risk factor predisposing cardiac myocytes to development of EADs under both acquired and inherited long QT conditions.
Delayed afterdepolarizations (DADs) have been recognized as a mechanism of digitalis-induced arrhythmogenesis distinct from diastolic phase 4 depolarization, for over 40 years (Ferrier et al. 1973; Wit and Rosen 1983). DADs are observed under conditions in which myocytes are overloaded with Ca2+, causing spontaneous Ca2+ release from sarcoplasmic reticulum and Ca2+ waves during diastole, leading to aftercontractions (Kass et al. 1978; Kort et al. 1985; Marban et al. 1986; Capogrossi et al. 1987; Stern et al. 1988; Schlotthauer and Bers 2000; Tweedie et al. 2000; Fujiwara et al. 2008). The transient inward current ITI generated by electrogenic Na+/Ca2+ exchange is responsible for these DADs (Kass et al. 1978; Schlotthauer and Bers 2000; Fedida et al. 1987).
Enhancement of late INa, similarly to inhibition of the sodium–potassium pump by cardiac glycosides, causes Na+ loading of myocytes. Late INa-mediated Na+ loading reduces the driving force for Ca2+ efflux from the cell via NCX (Noble and Noble 2006; Shattock and Bers 1989), thereby increasing the diastolic Ca2+ concentration and Ca2+ uptake by sarcoplasmic reticulum, and reducing the rate and extent of diastolic relaxation (Fig. 2) (Undrovinas et al. 2010; Sossalla et al. 2008). In the normal heart, Na+ influx during the AP plateau is estimated to account for 30 % of total Na+ influx at a heart rate of 60/min (Makielski and Farley 2006). Na+ influx can be increased several-fold when late INa is enhanced by ischemia, lysophosphatidylcholine, H2O2, or SCN5A mutations (Makielski and Farley 2006) thus increasing the incidence of DADs (Song et al. 2008; Wu and Corr 1995). Similarly, in myocytes from failing hearts the late INa is increased by 50 % or more (Valdivia et al. 2005; Undrovinas et al. 2010; Undrovinas and Maltsev 2008b).
Fig. 2.
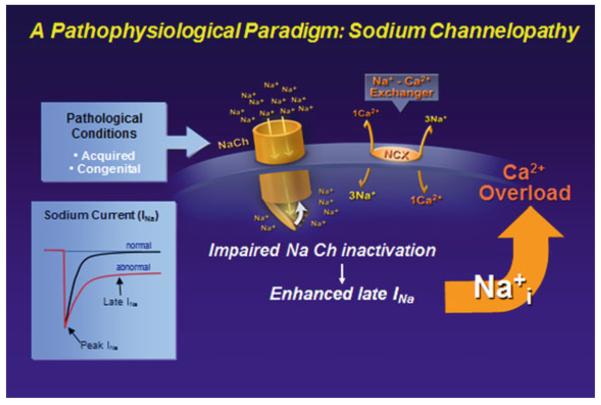
The pathophysiological paradigm for enhanced late INa. In both acquired and congenital syndromes, impaired inactivation of the sodium channel leads to enhanced late INa, causing a rise in intracellular Na concentration, which leads to calcium overload conditions. Modified from Belardinelli et al. (2006), with permission
DADs induced by cardiac glycosides or other interventions are suppressed by inhibitors of late INa, including TTX, lidocaine, mexiletine, propafenone, R56865, and ranolazine (Song et al. 2008; Rosen and Danilo 1980; Zeiler et al. 1984; Vollmer et al. 1987; Sawanobori et al. 1987; Inomata and Ishihara 1988; Tsuchida and Otomo 1990; Damiano et al. 1991). Reduction of late INa by ranolazine and GS-458967, a selective and potent inhibitor of late INa, has also been shown to reduce the incidence of DADs in experimental studies of pulmonary vein and superior vena cava sleeves (Sicouri et al. 2012a, b, 2013) Blocking late INa using R 56865, ranolazine, or TTX has been shown to reduce Na+-dependent Ca2+ loading of cardiac myocytes from normal and failing hearts (Song et al. 2006; Undrovinas et al. 2010; Sossalla et al. 2008; Haigney et al. 1994; Fraser et al. 2006). These findings implicate increased Na+ entry into myocytes via Na+ channels as a cause of Na+ and Ca2+ loading of myocytes, and arrhythmogenic DADs, while inhibition of late INa can be used as a means of reducing occurrences of DADs.
6.3 Role of CaMKII Activation in Augmentation of Late INa
Positive feedback loops have been identified between increases in late INa and increased expression and activation of CaMKII. These feedback loops appear to contribute to the pathology of Na+/Ca2+ overload in the failing and/or ischemic heart, where late INa (Saint 2006; Maltsev et al. 2007; Valdivia et al. 2005; Ju et al. 1996; Undrovinas et al. 1999; Le Grand et al. 1995) and expression and activity of CaMKII (Kirchhefer et al. 1999; Hoch et al. 1999) are increased. CaMKII phosphorylates phospholamban to increase Ca2+ uptake by sarcoplasmic reticulum (Ji et al. 2003) and RyR2 (Rodriguez et al. 2003) to increase the sensitivity of calcium release channels to Ca2+-induced opening. These two events can lead to increased leak of Ca2+ from the sarcoplasmic reticulum during diastole (Maier et al. 2003; Ai et al. 2005; Guo et al. 2006; Sag et al. 2009; Sossalla et al. 2010; Neef et al. 2010) that may elicit regenerative, spontaneous waves of Ca2+ release causing aftercontractions, transient inward current (ITi), and DADs (Fujiwara et al. 2008; Curran et al. 2010). CaMKII expression and activity is increased in the failing heart (Kirchhefer et al. 1999; Hoch et al. 1999; Ai et al. 2005; Sossalla et al. 2010; Anderson et al. 2011). Inhibition of CaMKII activity was shown to abolish isoproterenol-induced spontaneous Ca2+ waves and DADs in ventricular myocytes isolated from failing rabbit (Curran et al. 2010) and mouse (Sag et al. 2009) hearts, to decrease IKr-block-induced EADs in rabbit heart (Anderson et al. 1998), and to improve contractile function and reduce the leak of Ca2+ from the sarcoplasmic reticulum in myocytes from failing human hearts (Sossalla et al. 2010). Atrial fibrillation has been associated with increases of both late INa and CaMKII activity (Benito et al. 2008; Neef et al. 2010; Hove-Madsen et al. 2004).
The positive feedback loop between late INa and CaMKII activation is completed by a CaMKII-mediated increase of late INa (Maltsev et al. 2008; Wagner et al. 2006, 2011; Aiba et al. 2010; Ma et al. 2012). CaMKII associates with and phosphorylates the Na+ channel (Hund et al. 2010; Wagner et al. 2006). Inhibition of CaMKII has been shown to reduce both contractile dysfunction and late INa in guinea pig isolated hearts and myocytes exposed to ouabain (Hoyer et al. 2011), and inhibition of late INa was shown to reduce arrhythmic activity and a rapid pacing-induced increase of diastolic tension in papillary muscles isolated from mice overexpressing CaMKIIδC (Sossalla et al. 2011). The late INa inhibitor ranolazine decreases phosphorylation of CaMKII, RyR2, and phospholamban in N1325S mouse hearts (Yao et al. 2011). Thus, the feedback loop between the amplitude of late INa and CaMKII activity can be interrupted using inhibitors of CaMKII or late INa, either of which can reduce EADs and DAD incidence, dispersion of repolarization, Ca2+ alternans, and diastolic contracture. Finally, during the development of cardiac hypertrophy and failure, CaMKII appears to have an important role in the regulation of cardiac gene transcription (Zhang et al. 2007; Backs et al. 2009). Sodium channel expression is decreased in the failing heart (Undrovinas et al. 2002; Zicha et al. 2004), but evidence linking transcriptional control of SCN5A by CaMKII is lacking.
6.4 Role of Late INa in Dispersion of Repolarization and Related Arrhythmias
Reentrant arrhythmias generally involve unidirectional block and conduction around a circuit long enough to enable recovery of excitability at each point in the circuit before the circus wave of excitation returns (Mines 1914). The length of the circuit must be greater than the distance that an impulse can travel (i.e., the wavelength, a product of conduction velocity, and refractory period) before reaching the same point again. The establishment of unidirectional block is facilitated by an increase in dispersion of repolarization associated with both acquired or congenital conditions that prolong or abbreviate AP duration and the QT interval, thus promoting the substrate for reentry (Di Diego and Antzelevitch 1993; Antzelevitch 2007, 2008; Patel and Antzelevitch 2008a; Galinier et al. 1998; Yan et al. 2001; Sicouri et al. 2010; Benoist et al. 2012). Computational modeling studies also indicate that increased AP dispersion and weaker cell-to-cell coupling is associated with the susceptibility to reentrant arrhythmic activity (Ghanem et al. 2001; Burnes et al. 2001).
Preferential abbreviation of AP duration and refractoriness in epicardium vs. endocardium under short QT and Brugada syndrome conditions can also provide the substrate for reentry, both in atria and ventricles (Antzelevitch 2008; Sicouri et al. 2010; Antzelevitch and Sicouri 2012; Nof et al. 2010; Fish and Antzelevitch 2008; Patel and Antzelevitch 2008b). Acute ischemia also contributes to induction of reentry by causing electrical heterogeneity and conduction block (Vermeulen et al. 1996; Sidorov et al. 2011).
In normal hearts, late INa is greater in Purkinje fibers and M cells than in endo- or epicardial cells, thereby contributing to the longer duration of the AP in these cells (Zygmunt et al. 2001; Coraboeuf et al. 1979) and to spatial dispersion of AP duration and refractoriness. Block of late INa reduces AP duration in Purkinje fibers and M cells and is associated with reduction of the transmural dispersion of AP duration. (Sicouri et al. 1997a, b; Shimizu and Antzelevitch 1997b; Antzelevitch and Oliva 2006; Antzelevitch et al. 2006) Augmented late INa likely contributes to arrhythmogenesis in failing hearts due to increase in dispersion of repolarization and repolarization variability secondary to the increase in late INa (Maltsev et al. 2007). Reduction of late INa is effective in abbreviating APD and repolarization variability (Undrovinas et al. 2006). Late INa enhancers, including anthopleurin-A and veratridine increase the dispersion of AP duration in intact isolated guinea pig and rabbit hearts, respectively (Restivo et al. 2004; Milberg et al. 2005). Augmentation of late INa with ATX-II has been shown to dramatically increase transmural dispersion of repolarization and refractoriness in canine left ventricular wedge preparations, giving rise to TdP (Shimizu and Antzelevitch 1997a, b).
Inhibition of late INa whether by mexiletine, ranolazine, or other agents reverses the effect of the late INa enhancers, effectively reducing spatial dispersion of repolarization and refractoriness, thus suppressing TdP (Wu et al. 2004; Shimizu and Antzelevitch 1997b, 1998, 1999a, b; Wu et al. 2003). Figure 3 illustrates the effect of IKr and IKs blockers as well as a late INa agonist to promote transmural dispersion of repolarization (TDR) and prolonged Tpeak–Tend intervals in the ECG by producing a preferential prolongation of the M cell action potential duration. Block of late INa with mexiletine reduces TDR in these experimental models of long QT 1, 2, and 3. Reduction of late INa can also reduce spatial dispersion of repolarization and beat-to-beat variability of repolarization caused by treatment of rabbit isolated hearts with the IKr blocker, E-4031 (Wu et al. 2009b). Taken together, these results support the view that either reduction of repolarizing K+ current or enhancement of late INa results in increased dispersion of repolarization that may lead to arrhythmias and that reduction of late INa is effective is reversing these proarrhythmic effects (Fig. 4).
Fig. 3.
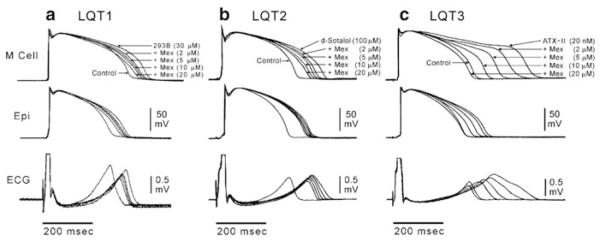
IKr and IKs blockers and Late INa agonist promote transmural dispersion (TDR) of repolarization and prolonged Tpeak–Tend intervals in the ECG by producing a preferential prolongation of the M cell action potential duration. Block of late INa with mexiletine reduces TDR in these experimental models of the long QT syndrome. Each panel shows transmembrane action potentials recorded from M and epicardial (Epi) sites in canine left ventricular wedge preparations together with a transmural ECG recorded across the bath (BCL of 2,000 ms). Traces are recorded in the presence of the IKs blocker, chromanol 293B (LQT1), IKr blocker d-sotalol (LQT2), and late INa agonist, ATX-II (LQT3), plus increasing concentrations of mexiletine. Mexiletine produced a greater abbreviation of the M cell vs. epicardial action potential at every concentration, resulting in a reduction in transmural dispersion of repolarization in all three LQTS models. Modified from (Shimizu and Antzelevitch 1997b, 1998) with permission
Fig. 4.
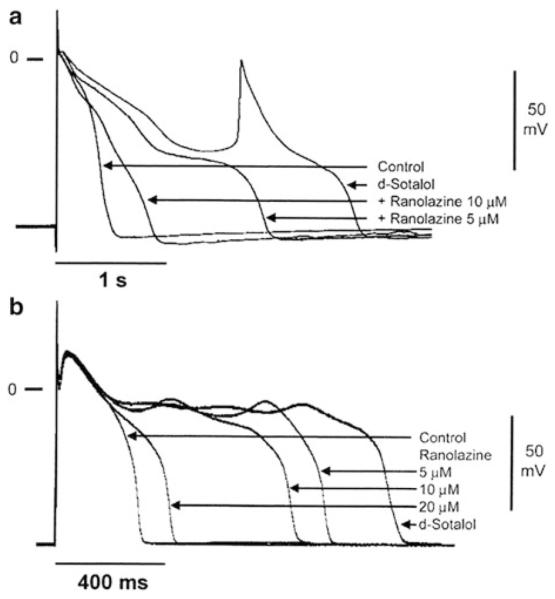
Effect of ranolazine to suppress d-sotalol-induced action potential prolongation and early afterdepolarizations in Purkinje fiber (a) and M cell preparations (b). Modified from Antzelevitch et al. (2004)
6.5 Role of Late INa in Cardiomyopathy
As discussed above, acquired and congenital defects that augment late INa do so by varied molecular mechanisms, including sodium channel phosphorylation, mutations of a specific amino acid residue(s) in the α or β subunits, or by alteration of channel-interacting proteins, which may promote varied phenotypes. In addition to congenital and acquired LQTS, an increase in late INa can result in mechanical dysfunction of the heart. Dilated cardiomyopathy has been reported in patients (McNair et al. 2004) as well as in mice (Zhang et al. 2011) with mutations in SCN5A associated with a gain of function in late INa. Mechanical dysfunction can result from an increase of intracellular Na+ to decrease the electrochemical gradient for Ca2+ extrusion by Na+–Ca2+ exchange (Belardinelli et al. 2006; Noble and Noble 2006; Sossalla et al. 2008). Slowing of Ca2+ extrusion could lead to slowing of diastolic relaxation. It is noteworthy that patients expressing the LQT3-ΔKPQ mutant Na+ channel are reported to have an impaired diastolic left ventricular relaxation and AP prolongation that were improved after administration of ranolazine (Moss et al. 2008).
Hypertrophic cardiomyopathy (HCM) is the most common monogenic cardiac disorder encountered in the clinic. Recent studies have identified the presence of cells with M cell characteristics in the septum of the human heart, (Barajas-Martinez et al. 2013; Coppini et al. 2013) as has previously been described in the canine heart (Glass et al. 2007; Sicouri et al. 1994) displaying higher levels of late INa. An ameliorative effect of ranolazine was shown to reduce the augmented late INa and thus to reduce the prolonged APD in the setting of HCM.
Coppini et al. (2013) showed that in cardiomyocytes isolated from HCM, enhanced CaMKII activity slows ICa inactivation and increases late INa, thus contributing to APD prolongation and related arrhythmias. Their data also suggested that by altering the function of EC-coupling proteins, CaMKII might also contribute to the altered Ca2+-transient kinetics and elevation of diastolic [Ca2+]i, which are responsible for the development of delayed afterdepolarizations (DAD). Therapeutic concentrations of ranolazine partially reversed the HCM-related cellular abnormalities via inhibition of late INa, with negligible effects in myocytes isolated from control hearts.
7 Drugs That Inhibit Cardiac Late INa
Local anesthetic and antiarrhythmic agents, including mexiletine, lidocaine, ranolazine, amiodarone, propranolol, verapamil, pentobarbital, quinidine, and flecainide, as well as antiepileptic drugs such as phenytoin and riluzole, are known to inhibit late INa in cardiac myocytes. These drugs lack selectivity for inhibition of late INa relative to other currents or receptor targets (Table 2). TTX is a selective Na+ channel blocker (Narahashi 2008) that inhibits late INa in the heart at concentrations 5–10-fold lower than it inhibits peak INa (Carmeliet 1987a; Wu et al. 2009a; Le Grand et al. 1995; Josephson and Sperelakis 1989) and it is commonly used in experiments to validate the effects of other late INa blockers. However, TTX blocks neuronal Na+ channels at much lower concentrations than those at which cardiac NaV1.5 channels are blocked, accounting for its high toxicity.
Table 2.
IC50 values for drug-induced block of peak and late INa as well as IKr by INa blockers
| IC50 value for tonic block (μM) |
|||||
|---|---|---|---|---|---|
| Drug | MW | Late INa | Peak INa | hERG (IKr) | References |
| Amiodarone (acute) | 645 | 3.0, 6.7 | 178, 87 | ~ 1 | Wu et al. (2008c), Maltsev et al. (2001) |
|
| |||||
| Flecainide | 414 | 1.4 | 10–15 | 2.1, 3.9 | Liu et al. (2003), Heath et al. (2011) |
|
| |||||
| F15845 | 376 | ~1 | 23 % at 10 μM | 15 % at 10 μM | Vacher et al. (2009), Pignier et al. (2010) |
|
| |||||
| GS458967 | 347 | 0.2 | >10 | >10 | Sicouri et al. (2012c) |
|
| |||||
| KC12291 | 413 | ≤10 | ~15 | No inhibition? | Tamareille et al. (2002), John et al. (2004) |
|
| |||||
| Lidocaine | 236 | ~25 | ~300 | No inhibition | Bean et al. (1983), Grant et al. (1989), Starmer et al. (1991) |
|
| |||||
| Mexiletine | 179 | 3–5 | 28–253 | No inhibition | Yatani and Akaike (1985), Sunami et al. (1993) |
|
| |||||
| Propafenone | 341 | <1 | ≥1 | 0.4–0.8 | Schreibmayer and Lindner (1992), Edrich et al. (2005), Witchel et al. (2004) |
|
| |||||
| Propranolol | 259 | ~3 | 22–28 | No inhibition | Wang et al. (2008, 2010) |
|
| |||||
| Quinidine | 324 | 12 | 11 | ~1 | Colatsky (1982), Grant et al. (1982) |
|
| |||||
| R56865 | 413 | 0.2 | ~5 | Probable inhibition | Wilhelm et al. (1991), Verdonck et al. (1991) |
|
| |||||
| Ranolazine | 428 | 7 | 428 | 12–14 | Antzelevitch et al. (2004), Zygmunt et al. (2011), Undrovinas et al. (2004) |
|
| |||||
| Riluzole | 234 | 2.7–3 | 100–150 | Song et al. (1997), Weiss et al. (2010) | |
|
| |||||
| Tetrodotoxin | 319 | 0.53 | 6.0 | No inhibition | Josephson and Sperelakis (1989), Carmeliet (1987b) |
|
| |||||
| Vernakalant | 349 | ~30 | 107 | 7–21 | Orth et al. (2006), Fedida (2007) |
The antianginal drug ranolazine (Antzelevitch et al. 2011), GS-458967 (Belardinelli et al. 2013), and the Pierre Fabre experimental compound F15845 (Vacher et al. 2009) appear to be the most selective inhibitors of cardiac late INa reported to date. Ranolazine has been demonstrated to be safe (Morrow 2007) in patients with non-ST-elevation acute coronary syndromes and angina pectoris. Ranolazine inhibits late INa and IKr with potencies of 6 and 12–14 μmol/L, respectively, (Antzelevitch et al. 2004) and slightly prolongs the QT interval in humans (Chaitman 2006). It also blocks both α- and β-adrenergic receptors with low potency (Zhao et al. 2011).
Most drugs that selectively inhibit cardiac late INa are believed to bind to the local anesthetic site in the Nav1.5 Na+ channel vestibule, and they cause both useand voltage-dependent block of the Na+ current (Zygmunt et al. 2011; Nesterenko et al. 2011). Local anesthetic binding to the Na+ channel is state dependent, and high-affinity binding depends on the availability of a channel conformation in which certain amino acids are arranged in a specific 3D relationship to form a binding domain (Lipkind and Fozzard 2005). In any different conformation (e.g., the closed state), the same amino acids are present but in different relative 3D positions that do not form a local anesthetic binding site of high affinity. The amino acids that form the putative local anesthetic site in open/inactivated Na+ channel include F1759 and Y1766 (F1760 and Y1767 in hH1) in DIVS6, but much data indicate that other amino acids contribute to local anesthetic binding, and more than one binding site may be present [for review, see Mike and Lukacs (2010)]. It should be noted that the binding site for batrachotoxin in the Na+ channel vestibule overlaps that for local anesthetics (however, at a molecular weight of 539, batrachotoxin is larger than most local anesthetics) and displacement of its binding has been used to identify potential use-dependent Na+ channel blockers (Carter et al. 2000; Grauert et al. 2002).
The antiarrhythmic effects of drugs and agents that reduce late INa have been demonstrated by many investigators using many different cardiac preparations (e.g., (Antzelevitch et al. 2004, 2011; Ruan et al. 2009; Undrovinas et al. 1999; Wu et al. 2004; Sicouri et al. 1997a; Scirica et al. 2007; Antzelevitch and Belardinelli 2006; Song et al. 2004; Sheu and Lederer 1985; Fedida et al. 2006; Burashnikov et al. 2007; Pignier et al. 2010)). In these studies, late INa is enhanced as a result of disease (e.g., heart failure), ischemia or hypoxia, gain-of-function mutations in SCN5A, or application of toxins (e.g., ATX-II, veratridine, aconitine), intermediary metabolites (e.g., palmitoyl-l-carnitine), or H2O2. Inhibition of late INa has been shown to improve function and reduce arrhythmogenic activity in ventricular, atrial, pulmonary vein sleeve, and nodal cardiac tissues. Diastolic depolarization, triggered activity (EADs, DADs), AP duration and variability, and cytosolic concentrations of Na+ and Ca2+ are reduced following inhibition of a pathologically enhanced late INa. Reduction of late INa increases repolarization reserve (shortens AP duration) and attenuates the proarrhythmic effects of IKr blockers. Spontaneous and pause-triggered arrhythmic activity (i.e., TdP) induced by amiodarone, quinidine, moxifloxacin, cisapride, and ziprasidone in the female rabbit isolated heart is reduced by the late INa inhibitor ranolazine (Wu et al. 2006, 2008b, c). In the dog heart, inhibition of late INa reduces AP duration more in myocytes with longer AP durations (Purkinje fibers and M cells) than in myocytes with short AP duration (epicardial cells) and thus decreases the transmural dispersion of repolarization (Antzelevitch and Belardinelli 2006; Shimizu and Antzelevitch 1997b). No risks associated with the block of cardiac late INa have been identified to date.
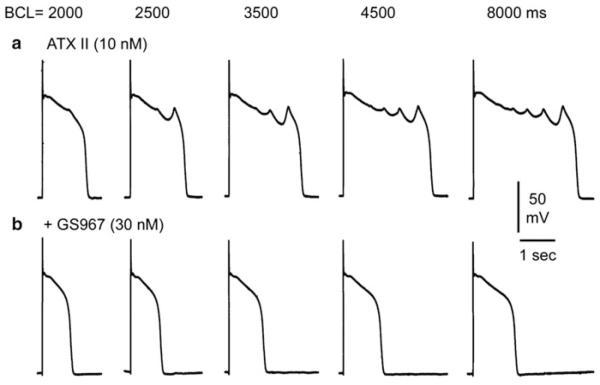
GS-458967, a recently introduced Gilead Sciences compound, is a selective late INa inhibitor with an IC50 of 200 nM. The compound was shown to cause modest abbreviation of APD and to prevent or abolish both ATX-II and E-4031-induced (i.e., models of LQT3 and LQT2, respectively) ventricular tachycardias in rabbit hearts (Belardinelli et al. 2013). Other recent studies have also demonstrated the effect of GS-458967 to abolish EADs and EAD-induced triggered activity elicited by exposure of canine Purkinje fibers to ATX-II (Fig. 5), increased extracellular calcium, and isoproterenol (Sicouri et al. 2013). GS-458967 may be useful to confirm the pathologic roles of late INa and to investigate physiologic and pathologic effects of inhibiting late INa in other excitable tissues.
Fig. 5.
GS-458967 abolishes early afterdepolarizations (EADs) and EAD-induced triggered activity elicited by exposure to ATX-II in a canine Purkinje fiber. (a) ATX-II (10 nM) elicited EADs and EAD-induced triggered activity at basic cycle lengths (BCLs) of 2,000, 2,500, 3,500, 4,500, and 8,000 ms. GS-458967 (30 nM) abolished all EADs and triggered activity. From Sicouri et al. (2013) with permission
Conclusion
Evidence implicating enhancement of late INa in pathophysiological electrical and mechanical instabilities of the heart is steadily increasing (Fig. 6). Selective inhibition of late INa is expected to be an effective strategy for correcting these channelopathies and cardiomyopathies. Unlike most other antiarrhythmic drugs, selective inhibitors of late INa do not appear to be proarrhythmic. Future direction focused on development of highly selective late INa blockers are needed to test this hypothesis in the clinic.
Fig. 6.
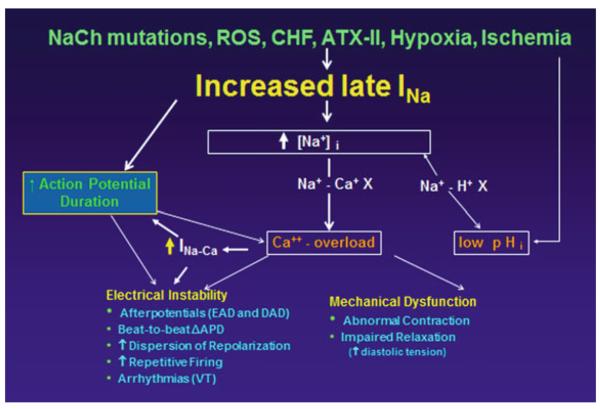
Mechanisms contributing to electrical instability and mechanical dysfunction in acquired and congenital conditions that enhance late INa
Acknowledgments
Support Supported by grants HL47678 from NHLBI, NIH (CA), C026424 from NYSTEM (CA), Gilead Sciences, Inc. and the Masons of New York State, Florida, Massachusetts Connecticut, Maryland, Rhode Island, and Wisconsin.
Footnotes
Conflicts of Interest Dr. Antzelevitch is a consultant to Gilead Sciences and Dr. Belardinelli, Shryock, and Rajamani are employed by Gilead Sciences.
References
- Abrahamsson C, Carlsson L, Duker G. Lidocaine and nisoldipine attenuate almokalant-induced dispersion of repolarization and early afterdepolarizations in vitro. J Cardiovasc Electrophysiol. 1996;7:1074–1081. doi: 10.1111/j.1540-8167.1996.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Abriel H. Cardiac sodium channel Na(v)1.5 and interacting proteins: physiology and pathophysiology. J Mol Cell Cardiol. 2010;48:2–11. doi: 10.1016/j.yjmcc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Ackerman MJ, Mohler PJ. Defining a new paradigm for human arrhythmia syndromes: phenotypic manifestations of gene mutations in ion channel- and transporter-associated proteins. Circ Res. 2010;107:457–465. doi: 10.1161/CIRCRESAHA.110.224592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern GP, Hsu SF, Klyachko VA, Jackson MB. Induction of persistent sodium current by exogenous and endogenous nitric oxide. J Biol Chem. 2000;275:28810–28815. doi: 10.1074/jbc.M003090200. [DOI] [PubMed] [Google Scholar]
- Ahern CA, Zhang JF, Wookalis MJ, Horn R. Modulation of the cardiac sodium channel NaV1.5 by Fyn, a Src family tyrosine kinase. Circ Res. 2005;96:991–998. doi: 10.1161/01.RES.0000166324.00524.dd. [DOI] [PubMed] [Google Scholar]
- Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- Aiba T, Hesketh GG, Liu T, Carlisle R, Villa-Abrille MC, O'Rourke B, Akar FG, Tomaselli GF. Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res. 2010;85:454–463. doi: 10.1093/cvr/cvp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin AS, Tan HL, Wilde AAM. Cardiac ion channels in health and disease. Heart Rhythm. 2010;7:117–135. doi: 10.1016/j.hrthm.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, Sung RJ. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther. 1998;287:996–1006. [PubMed] [Google Scholar]
- Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C. Heterogeneity and cardiac arrhythmias: an overview. Heart Rhythm. 2007;4:964–972. doi: 10.1016/j.hrthm.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C. Drug-induced spatial dispersion of repolarization. Cardiol J. 2008;15:100–121. [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Belardinelli L. The role of sodium channel current in modulating transmural dispersion of repolarization and arrhythmogenesis. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S79–S85. doi: 10.1111/j.1540-8167.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Oliva A. Amplification of spatial dispersion of repolarization underlies sudden cardiac death associated with catecholaminergic polymorphic VT, long QT, short QT and Brugada syndromes. J Intern Med. 2006;259:48–58. doi: 10.1111/j.1365-2796.2005.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Sicouri S. Mechanisms underlying arrhythmogenesis in long QT syndrome. Card Electrophysiol Clin. 2012;4:17–27. [Google Scholar]
- Antzelevitch C, Shimizu W, Yan GX, Sicouri S, Weissenburger J, Nesterenko VV, Burashnikov A, Di Diego JM, Saffitz J, Thomas GP. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, Cordeiro JM, Thomas GP. Electrophysiologic effects of ranolazine: a novel anti-anginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Guerchicoff A, Pollevick GD. The role of spatial dispersion of repolarization in sudden cardiac death. ISHNE World Wide Internet Symposium on Sudden Cardiac Death; 2006. http://hf2010.ishne.org/vs/scd-2006/lectures/ing_antzelevitch_charles.pdf. [Google Scholar]
- Antzelevitch C, Burashnikov A, Sicouri S, Belardinelli L. Electrophysiological basis for the antiarrhythmic actions of ranolazine. Heart Rhythm. 2011;8:1281–1290. doi: 10.1016/j.hrthm.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach DS, Grzda KR, Furspan PB, Sato PY, Mironov S, Jalife J. Structural heterogeneity promotes triggered activity, reflection and arrhythmogenesis in cardiomyocyte monolayers. J Physiol. 2011;589:2363–2381. doi: 10.1113/jphysiol.2010.200576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci U S A. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-Martinez H, Hu D, Goodrow RJ, Jr, Joyce F, Antzelevitch C. Electrophysiologic characteristics and pharmacologic response of human cardiomyocytes isolated from a patient with hypertrophic cardiomyopathy. Pacing Clin Electrophysiol. 2013;36:1512–1515. doi: 10.1111/pace.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett ME, Madgwick DK, Takemoto DJ. Protein kinase C as a stress sensor. Cell Signal. 2007;19:1820–1829. doi: 10.1016/j.cellsig.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruscotti M, DiFrancesco D, Robinson RB. Na(+) current contribution to the diastolic depolarization in newborn rabbit SA node cells. Am J Physiol Heart Circ Physiol. 2000;279:H2303–H2309. doi: 10.1152/ajpheart.2000.279.5.H2303. [DOI] [PubMed] [Google Scholar]
- Bean BP, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L, Antzelevitch C, Fraser H. Inhibition of late (sustained/persistent) sodium current: a potential drug target to reduce intracellular sodium-dependent calcium overload and its detrimental effects on cardiomyocyte function. Eur Heart J. 2004;(Suppl 6):i3–i7. [Google Scholar]
- Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92(Suppl 4):iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L, Liu G, Smith-Maxwell C, Wang WQ, El-Bizri N, Hirakawa R, Karpinski S, Kornyeyev D, Li CH, Hu L, Li XJ, Crumb W, Wu L, Koltun D, Zablocki J, Yao L, Dhalla AK, Rajamani S, Shryock J. A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias. J Pharmacol Exp Ther. 2013;344:23–32. doi: 10.1124/jpet.112.198887. [DOI] [PubMed] [Google Scholar]
- Ben CE, Boutjdir M, Himel HD, El-Sherif N. Role of subendocardial Purkinje network in triggering torsade de pointes arrhythmia in experimental long QT syndrome. Europace. 2008;10:1218–1223. doi: 10.1093/europace/eun248. [DOI] [PubMed] [Google Scholar]
- Benito B, Brugada R, Perich RM, Lizotte E, Cinca J, Mont L, Berruezo A, Tolosana JM, Freixa X, Brugada P, Brugada J. A mutation in the sodium channel is responsible for the association of long QT syndrome and familial atrial fibrillation. Heart Rhythm. 2008;5:1434–1440. doi: 10.1016/j.hrthm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- Benoist D, Stones R, Drinkhill MJ, Benson AP, Yang Z, Cassan C, Gilbert SH, Saint DA, Cazorla O, Steele DS, Bernus O, White E. Cardiac arrhythmia mechanisms in rats with heart failure induced by pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H2381–H2395. doi: 10.1152/ajpheart.01084.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaufox AD, Tristani-Firouzi M, Seslar S, Sanatani S, Trivedi B, Fischbach P, Paul T, Young ML, Tisma-Dupanovic S, Silva J, Cuneo B, Fournier A, Singh H, Tanel RE, Etheridge SP. Congenital long QT 3 in the pediatric population. Am J Cardiol. 2012;109:1459–1465. doi: 10.1016/j.amjcard.2012.01.361. [DOI] [PubMed] [Google Scholar]
- Boutjdir M, El-Sherif N. Pharmacological evaluation of early afterdepolarisations induced by sea anemone toxin (ATXII) in dog heart. Cardiovasc Res. 1991;25:815–819. doi: 10.1093/cvr/25.10.815. [DOI] [PubMed] [Google Scholar]
- Bryant SM, Wan X, Shipsey SJ, Hart G. Regional differences in the delayed rectifier current (IKr and IKs) contribute to the differences in action potential duration in basal left ventricular myocytes in guinea-pig. Cardiovasc Res. 1998;40:322–331. doi: 10.1016/s0008-6363(98)00133-3. [DOI] [PubMed] [Google Scholar]
- Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnes JE, Ghanem RN, Waldo AL, Rudy Y. Imaging dispersion of myocardial repolarization, I: comparison of body- surface and epicardial measures. Circulation. 2001;104:1299–1305. doi: 10.1161/hc3601.094276. [DOI] [PubMed] [Google Scholar]
- Capogrossi MC, Houser SR, Bahinski A, Lakatta EG. Synchronous occurrence of spontaneous localized calcium release from the sarcoplasmic reticulum generates action potentials in rat cardiac ventricular myocytes at normal resting membrane potential. Circ Res. 1987;61:498–503. doi: 10.1161/01.res.61.4.498. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Slow inactivation of the sodium current in rabbit cardiac Purkinje fibers. Pflugers Arch. 1987a;408:18–26. doi: 10.1007/BF00581835. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Voltage-dependent block by tetrodotoxin of the sodium channel in rabbit cardiac Purkinje fibers. Biophys J. 1987b;51:109–114. doi: 10.1016/S0006-3495(87)83315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AJ, Grauert M, Pschorn U, Bechtel WD, Bartmann-Lindholm C, Qu Y, Scheuer T, Catterall WA, Weiser T. Potent blockade of sodium channels and protection of brain tissue from ischemia by BIII 890 CL. Proc Natl Acad Sci U S A. 2000;97:4944–4949. doi: 10.1073/pnas.040577097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation. 2006;113:2462–2472. doi: 10.1161/CIRCULATIONAHA.105.597500. [DOI] [PubMed] [Google Scholar]
- Chandler NJ, Greener ID, Tellez JO, Inada S, Musa H, Molenaar P, DiFrancesco D, Baruscotti M, Longhi R, Anderson RH, Billeter R, Sharma V, Sigg DC, Boyett MR, Dobrzynski H. Molecular architecture of the human sinus node: insights into the function of the cardiac pacemaker. Circulation. 2009;119:1562–1575. doi: 10.1161/CIRCULATIONAHA.108.804369. [DOI] [PubMed] [Google Scholar]
- Chandra R, Starmer CF, Grant AO. Multiple effects of KPQ deletion mutation on gating of human cardiac Na+ channels expressed in mammalian cells. Am J Physiol. 1998;274:H1643–H1654. doi: 10.1152/ajpheart.1998.274.5.H1643. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Chen SA, Chang MS, Lin CI. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: implication for the genesis of atrial fibrillation. Cardiovasc Res. 2000;48:265–273. doi: 10.1016/s0008-6363(00)00179-6. [DOI] [PubMed] [Google Scholar]
- Cheung DW. Electrical activity of the pulmonary vein and its interaction with the right atrium in the guinea-pig. J Physiol. 1981;314:445–456. doi: 10.1113/jphysiol.1981.sp013718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy CE, Rudy Y. Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature. 1999;400:566–569. doi: 10.1038/23034. [DOI] [PubMed] [Google Scholar]
- Clancy CE, Tateyama M, Kass RS. Insights into the molecular mechanisms of bradycardia-triggered arrhythmias in long QT-3 syndrome. J Clin Invest. 2002;110:1251–1262. doi: 10.1172/JCI15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colatsky TJ. Mechanisms of action of lidocaine and quinidine on action potential duration in rabbit cardiac Purkinje fibers: an effect on steady-state sodium current? Circ Res. 1982;50:17–27. doi: 10.1161/01.res.50.1.17. [DOI] [PubMed] [Google Scholar]
- Coppini R, Ferrantini C, Yao L, Fan P, Del LM, Stillitano F, Sartiani L, Tosi B, Suffredini S, Tesi C, Yacoub M, Olivotto I, Belardinelli L, Poggesi C, Cerbai E, Mugelli A. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation. 2013;127:575–584. doi: 10.1161/CIRCULATIONAHA.112.134932. [DOI] [PubMed] [Google Scholar]
- Coraboeuf E, Deroubaix E, Coulombe A. Effect of tetrodotoxin on action potentials of the conducting system in the dog heart. Am J Physiol. 1979;236:H561–H567. doi: 10.1152/ajpheart.1979.236.4.H561. [DOI] [PubMed] [Google Scholar]
- Curran J, Brown KH, Santiago DJ, Pogwizd S, Bers DM, Shannon TR. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca(2+)-calmodulin-dependent protein kinase II. J Mol Cell Cardiol. 2010;49:25–32. doi: 10.1016/j.yjmcc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano BP, Stump GL, Yagel SK. Investigation of electrophysiologic mechanisms for the antiarrhythmic actions of R 56865 in cardiac glycoside toxicity. J Cardiovasc Pharmacol. 1991;18:415–428. doi: 10.1097/00005344-199109000-00015. [DOI] [PubMed] [Google Scholar]
- Darbar D, Kannankeril PJ, Donahue BS, Kucera G, Stubblefield T, Haines JL, George AL, Jr, Roden DM. Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation. 2008;117:1927–1935. doi: 10.1161/CIRCULATIONAHA.107.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denac H, Mevissen M, Scholtysik G. Structure, function and pharmacology of voltage-gated sodium channels. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:453–479. doi: 10.1007/s002100000319. [DOI] [PubMed] [Google Scholar]
- Di Diego JM, Antzelevitch C. Pinacidil-induced electrical heterogeneity and extrasystolic activity in canine ventricular tissues. Does activation of ATP-regulated potassium current promote phase 2 reentry? Circulation. 1993;88:1177–1189. doi: 10.1161/01.cir.88.3.1177. [DOI] [PubMed] [Google Scholar]
- Eddlestone GT, Zygmunt AC, Antzelevitch C, Eddlestone GT, Zygmunt AC, Antzelevitch C. Larger late sodium current contributes to the longer action potential of the M cell in canine ventricular myocardium. Pacing Clin Electrophysiol. 1996;19(Pt 2):569. Abstract. [Google Scholar]
- Edrich T, Wang SY, Wang GK. State-dependent block of human cardiac hNav1.5 sodium channels by propafenone. J Membr Biol. 2005;207:35–43. doi: 10.1007/s00232-005-0801-4. [DOI] [PubMed] [Google Scholar]
- Eigel BN, Gursahani H, Hadley RW. ROS are required for rapid reactivation of Na+/Ca2+ exchanger in hypoxic reoxygenated guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol. 2004;286:H955–H963. doi: 10.1152/ajpheart.00721.2003. [DOI] [PubMed] [Google Scholar]
- Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev. 2011;91:889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escande D, Coraboeuf E, Planche C, Lacour-Gayet F. Effects of potassium conductance inhibitors on spontaneous diastolic depolarization and abnormal automaticity in human atrial fibers. Basic Res Cardiol. 1986;81:244–257. doi: 10.1007/BF01907407. [DOI] [PubMed] [Google Scholar]
- Fedida D. Vernakalant (RSD1235): a novel, atrial-selective antifibrillatory agent. Expert Opin Investig Drugs. 2007;16:519–532. doi: 10.1517/13543784.16.4.519. [DOI] [PubMed] [Google Scholar]
- Fedida D, Noble D, Rankin AC, Spindler AJ. The arrhythmogenic transient inward current Iti and related contraction in isolated guinea-pig ventricular myocytes. J Physiol (London) 1987;392:523–542. doi: 10.1113/jphysiol.1987.sp016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D, Orth PM, Hesketh JC, Ezrin AM. The role of late I and antiarrhythmic drugs in EAD formation and termination in Purkinje fibers. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S71–S78. doi: 10.1111/j.1540-8167.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- Ferrier GR, Saunders JH, Mendez C. A cellular mechanism for the generation of ventricular arrhythmias by acetylstrophanthidin. Circ Res. 1973;32:600–609. doi: 10.1161/01.res.32.5.600. [DOI] [PubMed] [Google Scholar]
- Fish JM, Antzelevitch C. Cellular mechanism and arrhythmogenic potential of T-wave alternans in the Brugada syndrome. J Cardiovasc Electrophysiol. 2008;19:301–308. doi: 10.1111/j.1540-8167.2007.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser H, Belardinelli L, Wang L, Light PE, McVeigh JJ, Clanachan AS. Ranolazine decreases diastolic calcium accumulation caused by ATX-II or ischemia in rat hearts. J Mol Cell Cardiol. 2006;41:1031–1038. doi: 10.1016/j.yjmcc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Tanaka H, Mani H, Nakagami T, Takamatsu T. Burst emergence of intracellular Ca2+ waves evokes arrhythmogenic oscillatory depolarization via the Na+-Ca2+ exchanger: simultaneous confocal recording of membrane potential and intracellular Ca2+ in the heart. Circ Res. 2008;103:509–518. doi: 10.1161/CIRCRESAHA.108.176677. [DOI] [PubMed] [Google Scholar]
- Galinier M, Vialette JC, Fourcade J, Cabrol P, Dongay B, Massabuau P, Boveda S, Doazan JP, Fauvel JM, Bounhoure JP. QT interval dispersion as a predictor of arrhythmic events in congestive heart failure. Importance of aetiology. Eur Heart J. 1998;19:1054–1062. doi: 10.1053/euhj.1997.0865. [DOI] [PubMed] [Google Scholar]
- Gautier M, Zhang H, Fearon IM. Peroxynitrite formation mediates LPC-induced augmentation of cardiac late sodium currents. J Mol Cell Cardiol. 2008;44:241–251. doi: 10.1016/j.yjmcc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Gavillet B, Rougier JS, Domenighetti AA, Behar R, Boixel C, Ruchat P, Lehr HA, Pedrazzini T, Abriel H. Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ Res. 2006;99:407–414. doi: 10.1161/01.RES.0000237466.13252.5e. [DOI] [PubMed] [Google Scholar]
- Gelband H, Bush HL, Rosen MR, Myerburg RJ, Hoffman BF. Electrophysiologic properties of isolated preparations of human atrial myocardium. Circ Res. 1972;30:293–300. doi: 10.1161/01.res.30.3.293. [DOI] [PubMed] [Google Scholar]
- Ghanem RN, Burnes JE, Waldo AL, Rudy Y. Imaging dispersion of myocardial repolarization, II: noninvasive reconstruction of epicardial measures. Circulation. 2001;104:1306–1312. doi: 10.1161/hc3601.094277. [DOI] [PubMed] [Google Scholar]
- Glass A, Sicouri S, Antzelevitch C. Development of a coronary-perfused interventricular septal preparation as a model for studying the role of the septum in arrhythmogenesis. J Electrocardiol. 2007;40:S142–S144. doi: 10.1016/j.jelectrocard.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AO, Trantham JL, Brown KK, Strauss HC. pH-dependent effects of quinidine on the kinetics of dV/dtmax in guinea pig ventricular myocardium. Circ Res. 1982;50:210–217. doi: 10.1161/01.res.50.2.210. [DOI] [PubMed] [Google Scholar]
- Grant AO, Dietz MA, Gilliam FR, III, Starmer CF. Blockade of cardiac sodium channels by lidocaine. Single-channel analysis. Circ Res. 1989;65:1247–1262. doi: 10.1161/01.res.65.5.1247. [DOI] [PubMed] [Google Scholar]
- Grauert M, Bechtel WD, Weiser T, Stransky W, Nar H, Carter AJ. Synthesis and structure-activity relationships of 6,7-benzomorphan derivatives as use-dependent sodium channel blockers for the treatment of stroke. J Med Chem. 2002;45:3755–3764. doi: 10.1021/jm020875j. [DOI] [PubMed] [Google Scholar]
- Guo T, Zhang T, Mestril R, Bers DM. Ca2+/calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- Guo D, Young LH, Wu Y, Belardinelli L, Kowey PR, Yan GX. Increased late sodium current in left atrial myocytes of rabbits with left ventricular hypertrophy: its role in the genesis of atrial arrhythmias. Am J Physiol Heart Circ Physiol. 2010;298:H1375–H1381. doi: 10.1152/ajpheart.01145.2009. [DOI] [PubMed] [Google Scholar]
- Haigney MC, Lakatta EG, Stern MD, Silverman HS. Sodium channel blockade reduces hypoxic sodium loading and sodium-dependent calcium loading. Circulation. 1994;90:391–399. doi: 10.1161/01.cir.90.1.391. [DOI] [PubMed] [Google Scholar]
- Hammarstrom AK, Gage PW. Hypoxia and persistent sodium current. Eur Biophys J. 2002;31:323–330. doi: 10.1007/s00249-002-0218-2. [DOI] [PubMed] [Google Scholar]
- Harris DR, Green WL, Craelius W. Acute thyroid hormone promotes slow inactivation of sodium current in neonatal cardiac myocytes. Biochim Biophys Acta. 1991;1095:175–181. doi: 10.1016/0167-4889(91)90081-8. [DOI] [PubMed] [Google Scholar]
- Hartmann HA, Tiedeman AA, Chen S-F, Brown AM, Kirsch GE. Effects of III-IV linker mutations on human heart Na+ channel inactivation gating. Circ Res. 1994;75:114–122. doi: 10.1161/01.res.75.1.114. [DOI] [PubMed] [Google Scholar]
- Heath BM, Cui Y, Worton S, Lawton B, Ward G, Ballini E, Doe CP, Ellis C, Patel BA, McMahon NC. Translation of flecainide- and mexiletine-induced cardiac sodium channel inhibition and ventricular conduction slowing from nonclinical models to clinical. J Pharmacol Toxicol Methods. 2011;63:258–268. doi: 10.1016/j.vascn.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- Hoeker GS, Katra RP, Wilson LD, Plummer BN, Laurita KR. Spontaneous calcium release in tissue from the failing canine heart. Am J Physiol Heart Circ Physiol. 2009;297:H1235–H1242. doi: 10.1152/ajpheart.01320.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PM, Davis LD. Evidence for specialized fibers in the canine right atrium. Circ Res. 1968;23:387–396. doi: 10.1161/01.res.23.3.387. [DOI] [PubMed] [Google Scholar]
- Honerjager P. Cardioactive substances that prolong the open state of sodium channels. Rev Physiol Biochem Pharmacol. 1982;92:1–74. doi: 10.1007/BFb0030502. [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L, Llach A, Bayes-Genis A, Roura S, Rodriguez FE, Aris A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- Hoyer K, Song Y, Wang D, Phan D, Balser J, Ingwall JS, Belardinelli L, Shryock JC. Reducing the late sodium current improves cardiac fuction during sodium pump inhibition by ouabain. J Pharmacol Exp Ther. 2011;337:513–523. doi: 10.1124/jpet.110.176776. [DOI] [PubMed] [Google Scholar]
- Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, Rasband MN, Anderson ME, Mohler PJ. A beta(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata N, Ishihara T. Mechanism of inhibition by SUN 1165, a new Na channel blocking antiarrhythmic agent, of cardiac glycoside-induced triggered activity. Eur J Pharmacol. 1988;145:313–322. doi: 10.1016/0014-2999(88)90435-9. [DOI] [PubMed] [Google Scholar]
- Isenberg G, Ravens U. The effects of the anemonia sulcata toxin (ATX II) on membrane currents of isolated mammalian myocytes. J Physiol. 1984;357:127–149. doi: 10.1113/jphysiol.1984.sp015493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Li B, Reed TD, Lorenz JN, Kaetzel MA, Dedman JR. Targeted inhibition of Ca2+/calmodulin-dependent protein kinase II in cardiac longitudinal sarcoplasmic reticulum results in decreased phospholamban phosphorylation at threonine 17. J Biol Chem. 2003;278:25063–25071. doi: 10.1074/jbc.M302193200. [DOI] [PubMed] [Google Scholar]
- Jia S, Lian J, Guo D, Xue X, Patel C, Yang L, Yuan Z, Ma A, Yan GX. Modulation of the late sodium current by the toxin, ATX-II, and ranolazine affects the reverse use-dependence and proarrhythmic liability of I(Kr) blockade. Br J Pharmacol. 2011;164:308–316. doi: 10.1111/j.1476-5381.2010.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GW, Letienne R, Le GB, Pignier C, Vacher B, Patoiseau JF, Colpaert FC, Coulombe A. KC 12291: an atypical sodium channel blocker with myocardial antiischemic properties. Cardiovasc Drug Rev. 2004;22:17–26. doi: 10.1111/j.1527-3466.2004.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Josephson IR, Sperelakis N. Tetrodotoxin differentially blocks peak and steady-state sodium channel currents in early embryonic chick ventricular myocytes. Pflugers Arch. 1989;414:354–359. doi: 10.1007/BF00584639. [DOI] [PubMed] [Google Scholar]
- Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol. 1996;497(Pt 2):337–347. doi: 10.1113/jphysiol.1996.sp021772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass RS, Lederer WJ, Tsien RW, et al. Role of calcium ions in transient inward currents and aftercontractions induced by strophantidin in cardiac Purkinje fibers. J Physiol (London) 1978;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung ECH, Aronson RS. Transmembrane action potentials and the electrocardiogram in rats with renal hypertension. Cardiovasc Res. 1981;15:611–614. doi: 10.1093/cvr/15.11.611. [DOI] [PubMed] [Google Scholar]
- Kirchhefer U, Schmitz W, Scholz H, Neumann J. Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res. 1999;42:254–261. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- Kiyosue T, Arita M. Late sodium current and its contribution to action potential configuration in guinea pig ventricular myocytes. Circ Res. 1989;64:389–397. doi: 10.1161/01.res.64.2.389. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M, Fichtner H, Frobe U. Metabolites of the glycolytic pathway modulate the activity of single cardiac Na+ channels. FASEB J. 1989;3:1963–1967. doi: 10.1096/fasebj.3.8.2542113. [DOI] [PubMed] [Google Scholar]
- Kort AA, Lakatta EG, Marban E, Stern MD, Wier WG. Fluctuations in intracellular calcium concentration and their effect on tonic tension in canine cardiac Purkinje fibres. J Physiol (London) 1985;367:291–308. doi: 10.1113/jphysiol.1985.sp015825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand B, Talmant JM, Rieu JP, Patoiseau JF, Colpaert FC, John GW. Investigation of the mechanism by which ketanserin prolongs the duration of the cardiac action potential. J Cardiovasc Pharmacol. 1995;26:803–809. doi: 10.1097/00005344-199511000-00018. [DOI] [PubMed] [Google Scholar]
- Li Z, Ai T, Samani K, Xi Y, Tzeng HP, Xie M, Wu S, Ge S, Taylor MD, Dong JW, Cheng J, Ackerman MJ, Kimura A, Sinagra G, Brunelli L, Faulkner G, Vatta M. A ZASP missense mutation, S196L, leads to cytoskeletal and electrical abnormalities in a mouse model of cardiomyopathy. Circ Arrhythm Electrophysiol. 2010;3:646–656. doi: 10.1161/CIRCEP.109.929240. [DOI] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol Pharmacol. 2005;68:1611–1622. doi: 10.1124/mol.105.014803. [DOI] [PubMed] [Google Scholar]
- Liu Y, DeFelice LJ, Mazzanti M. Na channels that remain open throughout the cardiac action potential plateau. Biophys J. 1992;63:654–662. doi: 10.1016/S0006-3495(92)81635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Atkins J, Kass RS. Common molecular determinants of flecainide and lidocaine block of heart Na + channels: evidence from experiments with neutral and quaternary flecainide analogues. J Gen Physiol. 2003;121:199–214. doi: 10.1085/jgp.20028723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Williams JB, Sumpter BR, Bevensee MO. Inhibition of the Na/bicarbonate cotransporter NBCe1-A by diBAC oxonol dyes relative to niflumic acid and a stilbene. J Membr Biol. 2007;215:195–204. doi: 10.1007/s00232-007-9018-z. [DOI] [PubMed] [Google Scholar]
- Lopez-Santiago LF, Meadows LS, Ernst SJ, Chen C, Malhotra JD, McEwen DP, Speelman A, Noebels JL, Maier SK, Lopatin AN, Isom LL. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol. 2007;43:636–647. doi: 10.1016/j.yjmcc.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HR, De CF. R 56 865, a Na+/Ca(2+)-overload inhibitor, protects against aconitine-induced cardiac arrhythmias in vivo. J Cardiovasc Pharmacol. 1993;22:120–125. doi: 10.1097/00005344-199307000-00019. [DOI] [PubMed] [Google Scholar]
- Ma J, Luo A, Wu L, Wan W, Zhang P, Ren Z, Zhang S, Qian C, Shryock JC, Belardinelli L. Calmodulin kinase II and protein kinase C mediate the effect of increased intracellular calcium to augment late sodium current in rabbit ventricular myocytes. Am J Physiol Cell Physiol. 2012;302:C1141–C1151. doi: 10.1152/ajpcell.00374.2011. [DOI] [PubMed] [Google Scholar]
- Maier LS. A novel mechanism for the treatment of angina, arrhythmias, and diastolic dysfunction: inhibition of late INa using ranolazine. J Cardiovasc Pharmacol. 2009;54:279–286. doi: 10.1097/FJC.0b013e3181a1b9e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. Distinct subcellular localization of different sodium channel a and b subunits in single ventricular myocytes from mouse heart. Circulation. 2004;109:1421–1427. doi: 10.1161/01.CIR.0000121421.61896.24. [DOI] [PubMed] [Google Scholar]
- Makielski JC, Farley AL. Na(+) current in human ventricle: implications for sodium loading and homeostasis. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S15–S20. doi: 10.1111/j.1540-8167.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- Makita N, Horie M, Nakamura T, Ai T, Sasaki K, Yokoi H, Sakurai M, Sakuma I, Otani H, Sawa H, Kitabatake A. Drug-induced long-QT syndrome associated with a subclinical SCN5A mutation. Circulation. 2002;106:1269–1274. doi: 10.1161/01.cir.0000027139.42087.b6. [DOI] [PubMed] [Google Scholar]
- Makita N, Behr E, Shimizu W, Horie M, Sunami A, Crotti L, Schulze-Bahr E, Fukuhara S, Mochizuki N, Makiyama T, Itoh H, Christiansen M, McKeown P, Miyamoto K, Kamakura S, Tsutsui H, Schwartz PJ, George AL, Jr, Roden DM. The E1784K mutation in SCN5A is associated with mixed clinical phenotype of type 3 long QT syndrome. J Clin Invest. 2008;118:2219–2229. doi: 10.1172/JCI34057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev VA, Undrovinas AI. A multi-modal composition of the late Na+ current in human ventricular cardiomyocytes. Cardiovasc Res. 2006;69:116–127. doi: 10.1016/j.cardiores.2005.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev VA, Sabbah HN, Higgins RS, Silverman N, Lesch M, Undrovinas AI. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 1998;98:2545–2552. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Sabbah HN, Undrovinas AI. Late sodium current is a novel target for amiodarone: studies in failing human myocardium. J Mol Cell Cardiol. 2001;33:923–932. doi: 10.1006/jmcc.2001.1355. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail. 2007;9:219–227. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev VA, Reznikov V, Undrovinas NA, Sabbah HN, Undrovinas A. Modulation of late sodium current by Ca2+, calmodulin, and CaMKII in normal and failing dog cardiomyocytes: similarities and differences. Am J Physiol Heart Circ Physiol. 2008;294:H1597–H1608. doi: 10.1152/ajpheart.00484.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev VA, Kyle JW, Undrovinas A. Late Na+ current produced by human cardiac Na+ channel isoform Nav1.5 is modulated by its b1 subunit. J Physiol Sci. 2009;59:217–225. doi: 10.1007/s12576-009-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban E, Robinson SW, Wier WG. Mechanism of arrhythmogenic delayed and early afterdepolarizations in ferret muscle. J Clin Invest. 1986;78:1185–1192. doi: 10.1172/JCI112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary-Rabine L, Hordof AJ, Danilo P, Malm JR, Rosen MR. Mechanisms for impulse initiation in isolated human atrial fibers. Circ Res. 1980;47:267–277. doi: 10.1161/01.res.47.2.267. [DOI] [PubMed] [Google Scholar]
- Mazzone A, Strege PR, Tester DJ, Bernard CE, Faulkner G, Degiorgio R, Makielski JC, Stanghellini V, Gibbons SJ, Ackerman MJ, Farrugia G. A mutation in telethonin alters Nav1.5 function. J Biol Chem. 2008;283:16537–16544. doi: 10.1074/jbc.M801744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair WP, Ku L, Taylor MR, Fain PR, Dao D, Wolfel E, Mestroni L. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia1. Circulation. 2004;110:2163–2167. doi: 10.1161/01.CIR.0000144458.58660.BB. [DOI] [PubMed] [Google Scholar]
- Meadows LS, Isom LL. Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovasc Res. 2005;67:448–458. doi: 10.1016/j.cardiores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, Ye B, Valdivia C, Ueda K, Canizales-Quinteros S, Tusie-Luna MT, Makielski JC, Ackerman MJ. SCN4B-encoded sodium channel b4 subunit in congenital long-QT syndrome. Circulation. 2007;116:134–142. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mike A, Lukacs P. The enigmatic drug binding site for sodium channel inhibitors. Curr Mol Pharmacol. 2010;3:129–144. doi: 10.2174/1874467211003030129. [DOI] [PubMed] [Google Scholar]
- Milberg P, Reinsch N, Wasmer K, Monnig G, Stypmann J, Osada N, Breithardt G, Haverkamp W, Eckardt L. Transmural dispersion of repolarization as a key factor of arrhythmogenicity in a novel intact heart model of LQT3. Cardiovasc Res. 2005;65:397–404. doi: 10.1016/j.cardiores.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Mines GR. On circulating excitations in heart muscles and their possible relation to tachycardia and fibrillation. Trans R Soc Can. 1914;8:43–52. [Google Scholar]
- Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT, Bennett V. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci U S A. 2004;10:9137–9142. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JD, Clancy CE. Pathophysiology of the cardiac late Na current and its potential as a drug target. J Mol Cell Cardiol. 2012;52:608–619. doi: 10.1016/j.yjmcc.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow DA. MERLIN-TIMI-36 (Metabolic Efficiency with Ranolazine for Less Ischemia in NSTE ACS) Clin Cardiol. 2007;30:418–419. [Google Scholar]
- Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol. 2008;19:1289–1293. doi: 10.1111/j.1540-8167.2008.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KT, Hu NN, Daw JR, Shin HG, Watson MT, Mashburn AB, George AL., Jr Functional effects of protein kinase C activation on the human cardiac Na+ channel. Circ Res. 1997;80:370–376. doi: 10.1161/01.res.80.3.370. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Tetrodotoxin: a brief history. Proc Jpn Acad Ser B Phys Biol Sci. 2008;84:147–154. doi: 10.2183/pjab.84.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- Nesterenko VV, Zygmunt AC, Rajamani S, Belardinelli L, Antzelevitch C. Mechanisms of atrial-selective block of Na + channels by ranolazine: II. Insights from a mathematical model. Am J Physiol Heart Circ Physiol. 2011;301:H1615–H1624. doi: 10.1152/ajpheart.00243.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D, Noble PJ. Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium-calcium overload. Heart. 2006;92(Suppl 4):iv1–iv5. doi: 10.1136/hrt.2005.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nof E, Burashnikov A, Antzelevitch C. Cellular basis for atrial fibrillation in an experimental model of short QT1: implications for a pharmacological approach to therapy. Heart Rhythm. 2010;7:251–257. doi: 10.1016/j.hrthm.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth PM, Hesketh JC, Mak CK, Yang Y, Lin S, Beatch GN, Ezrin AM, Fedida D. RSD1235 blocks late I(Na) and suppresses early afterdepolarizations and torsades de pointes induced by class III agents. Cardiovasc Res. 2006;70:486–496. doi: 10.1016/j.cardiores.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Patel C, Antzelevitch C. Pharmacological approach to the treatment of long and short QT syndromes. Pharmacol Ther. 2008a;118:138–151. doi: 10.1016/j.pharmthera.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel C, Antzelevitch C. Cellular basis for arrhythmogenesis in an experimental model of the SQT1 form of the short QT syndrome. Heart Rhythm. 2008b;5:585–590. doi: 10.1016/j.hrthm.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak JB, Ortiz M. Slow currents through single sodium channels of the adult rat heart. J Gen Physiol. 1985;86:89–104. doi: 10.1085/jgp.86.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, Hatem SN, Coulombe A, Abriel H. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ Res. 2011;108:294–304. doi: 10.1161/CIRCRESAHA.110.228312. [DOI] [PubMed] [Google Scholar]
- Pignier C, Rougier JS, Vie B, Culie C, Verscheure Y, Vacher B, Abriel H, Le GB. Selective inhibition of persistent sodium current by F 15845 prevents ischaemia-induced arrhythmias. Br J Pharmacol. 2010;161:79–91. doi: 10.1111/j.1476-5381.2010.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinet C, Algalarrondo V, Sablayrolles S, Le GB, Pignier C, Cussac D, Perez M, Hatem SN, Coulombe A. Protease-activated receptor-1 mediates thrombin-induced persistent sodium current in human cardiomyocytes. Mol Pharmacol. 2008;73:1622–1631. doi: 10.1124/mol.107.043182. [DOI] [PubMed] [Google Scholar]
- Remme CA, Verkerk AO, Nuyens D, Van Ginneken AC, Belterman CN, Wilders R, van Roon MA, Tan HL, Wilde AA, Carmeliet P, de Bakker JM, Veldkamp MW, Bezzina CR. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation. 2006;114:2584–2594. doi: 10.1161/CIRCULATIONAHA.106.653949. [DOI] [PubMed] [Google Scholar]
- Restivo M, Caref EB, Kozhevnikov DO, El-Sherif N. Spatial dispersion of repolarization is a key factor in the arrhythmogenicity of long QT syndrome. J Cardiovasc Electrophysiol. 2004;15:323–331. doi: 10.1046/j.1540-8167.2004.03493.x. [DOI] [PubMed] [Google Scholar]
- Rivolta I, Abriel H, Tateyama M, Liu H, Memmi M, Vardas P, Napolitano C, Priori SG, Kass RS. Inherited Brugada and LQT-3 syndrome mutations of a single residue of the cardiac sodium channel confer distinct channel and clinical phenotypes. J Biol Chem. 2001;276:30623–30630. doi: 10.1074/jbc.M104471200. [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Bhogal MS, Colyer J. Stoichiometric phosphorylation of cardiac ryanodine receptor on serine 2809 by calmodulin-dependent kinase II and protein kinase A. J Biol Chem. 2003;278:38593–38600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- Rook MB, Evers MM, Vos MA, Bierhuizen MF. Biology of cardiac sodium channel Nav1.5 expression. Cardiovasc Res. 2012;93:12–23. doi: 10.1093/cvr/cvr252. [DOI] [PubMed] [Google Scholar]
- Rosen MR, Danilo P., Jr Effects of tetrodotoxin, lidocaine, verapamil and AHR-266 on ouabain induced delayed afterdepolarizations in canine Purkinje fibers. Circ Res. 1980;46:117–124. doi: 10.1161/01.res.46.1.117. [DOI] [PubMed] [Google Scholar]
- Rota M, Vassalle M. Patch-clamp analysis in canine cardiac Purkinje cells of a novel sodium component in the pacemaker range. J Physiol. 2003;548:147–165. doi: 10.1113/jphysiol.2003.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y, Liu N, Priori SG. Sodium channel mutations and arrhythmias. Nat Rev Cardiol. 2009;6:337–348. doi: 10.1038/nrcardio.2009.44. [DOI] [PubMed] [Google Scholar]
- Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, Opiela MK, Backs J, Olson EN, Brown JH, Neef S, Maier SK, Maier LS. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail. 2009;2:664–675. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint DA. The role of the persistent Na(+) current during cardiac ischemia and hypoxia. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S96–S103. doi: 10.1111/j.1540-8167.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- Saint DA. The cardiac persistent sodium current: an appealing therapeutic target? Br J Pharmacol. 2008;153:1133–1142. doi: 10.1038/sj.bjp.0707492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint DA, Ju YK, Gage PW. A persistent sodium current in rat ventricular myocytes. J Physiol (London) 1992;453:219–231. doi: 10.1113/jphysiol.1992.sp019225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B, Spindler AJ, Bryant SM, Linz KW, Noble D. Distribution of a persistent sodium current across the ventricular wall in guinea-pigs. Circ Res. 2000;87:910–914. doi: 10.1161/01.res.87.10.910. [DOI] [PubMed] [Google Scholar]
- Sarhan MF, Van PF, Ahern CA. A double tyrosine motif in the cardiac sodium channel domain III–IV linker couples calcium-dependent calmodulin binding to inactivation gating. J Biol Chem. 2009;284:33265–33274. doi: 10.1074/jbc.M109.052910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawanobori T, Hirano Y, Hiraoka M. Aconitine-induced delayed afterdepolarization in frog atrium and guinea pig papillary muscles in the presence of low concentrations of Ca2+ Jpn J Physiol. 1987;37:59–79. doi: 10.2170/jjphysiol.37.59. [DOI] [PubMed] [Google Scholar]
- Scherer D, von Lowenstern K, Zitron E, Scholz EP, Bloehs R, Kathofer S, Thomas D, Bauer A, Katus HA, Karle CA, Kiesecker C. Inhibition of cardiac hERG potassium channels by tetracyclic antidepressant mianserin. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:73–83. doi: 10.1007/s00210-008-0289-4. [DOI] [PubMed] [Google Scholar]
- Scherf D, Romano FJ, Terranova R. Experimental studies on auricular flutter and auricular fibrillation. Am Heart J. 1948;36:241–251. doi: 10.1016/0002-8703(48)90403-7. [DOI] [PubMed] [Google Scholar]
- Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ Res. 2000;87:774–780. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- Schreibmayer W, Lindner W. Stereoselective interactions of (R)- and (S)-propafenone with the cardiac sodium channel. J Cardiovasc Pharmacol. 1992;20:324–331. doi: 10.1097/00005344-199208000-00020. [DOI] [PubMed] [Google Scholar]
- Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, Molhoek P, Verheugt FW, Gersh BJ, McCabe CH, Braunwald E. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the metabolic efficiency with ranolazine for less ischemia in non ST-elevation ACUTE CORONARY syndrome thrombolysis in myocardial infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- Shao D, Okuse K, Djamgoz MB. Protein-protein interactions involving voltage-gated sodium channels: post-translational regulation, intracellular trafficking and functional expression. Int J Biochem Cell Biol. 2009;41:1471–1481. doi: 10.1016/j.biocel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Shattock MJ, Bers DM. Rat vs. rabbit ventricle: Ca flux and intracellular Na assessed by ion-selective microelectrodes. Am J Physiol. 1989;256:C813–C822. doi: 10.1152/ajpcell.1989.256.4.C813. [DOI] [PubMed] [Google Scholar]
- Sheu SS, Lederer WJ. Lidocaine's negative inotropic and antiarrhythmic actions: dependence on shortening of action potential duration and reduction of intracellular sodium activity. Circ Res. 1985;57:578–590. doi: 10.1161/01.res.57.4.578. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade de pointes in LQT2 as well as LQT3 models of the long QT syndrome. Pacing Clin Electrophysiol. 1997a;20:1234. doi: 10.1161/01.cir.96.6.2038. Abstract. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade de pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation. 1997b;96:2038–2047. doi: 10.1161/01.cir.96.6.2038. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Cellular basis for the ECG features of the LQT1 form of the long QT syndrome: effects of b-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes. Circulation. 1998;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Spontaneous and stimulation-induced Torsade de Pointes in LQT1, LQT2 and LQT3 models of the long QT syndrome. Circulation. 1999a;100(II):359. doi: 10.1161/01.cir.102.6.706. Abstract. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Cellular basis for long QT, transmural dispersion of repolarization, and Torsade de Pointes in the long QT syndrome. J Electrocardiol. 1999b;32(Suppl):177–184. doi: 10.1016/s0022-0736(99)90077-8. [DOI] [PubMed] [Google Scholar]
- Shryock JC, Song Y, Rajamani S, Antzelevitch C, Belardinelli L. The arrhythmogenic consequences of increasing late INa in the cardiomyocyte. Cardiovasc Res. 2013;99:600–611. doi: 10.1093/cvr/cvt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicouri S, Fish J, Antzelevitch C. Distribution of M cells in the canine ventricle. J Cardiovasc Electrophysiol. 1994;5:824–837. doi: 10.1111/j.1540-8167.1994.tb01121.x. [DOI] [PubMed] [Google Scholar]
- Sicouri S, Quist M, Antzelevitch C. Evidence for the presence of M cells in the guinea pig ventricle. J Cardiovasc Electrophysiol. 1996;7:503–511. doi: 10.1111/j.1540-8167.1996.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Sicouri S, Antzelevitch D, Heilmann C, Antzelevitch C. Effects of sodium channel block with mexiletine to reverse action potential prolongation in in vitro models of the long QT syndrome. J Cardiovasc Electrophysiol. 1997a;8:1280–1290. doi: 10.1111/j.1540-8167.1997.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Sicouri S, Moro S, Litovsky SH, Elizari MV, Antzelevitch C. Chronic amiodarone reduces transmural dispersion of repolarization in the canine heart. J Cardiovasc Electrophysiol. 1997b;8:1269–1279. doi: 10.1111/j.1540-8167.1997.tb01018.x. [DOI] [PubMed] [Google Scholar]
- Sicouri S, Glass A, Ferreiro M, Antzelevitch C. Transseptal dispersion of repolarization and its role in the development of torsade de pointes arrhythmias. J Cardiovasc Electrophysiol. 2010;21:441–447. doi: 10.1111/j.1540-8167.2009.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicouri S, Blazek J, Belardinelli L, Antzelevitch C. Electrophysiological characteristics of canine superior vena cava sleeve preparations. Effect of ranolazine. Circ Arrhythm Electrophysiol. 2012a;5:371–379. doi: 10.1161/CIRCEP.111.969493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicouri S, Pourrier M, Gibson JK, Lynch JJ, Antzelevitch C. Comparison of electrophysio-logical and antiarrhythmic effects of vernakalant, ranolazine, and sotalol in canine pulmonary vein sleeve preparations. Heart Rhythm. 2012b;9:422–429. doi: 10.1016/j.hrthm.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicouri S, Blazek J, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of the highly-selective late sodium channel current blocker GS 458967 in canine Purkinje fibers and pulmonary vein sleeve preparations. Heart Rhythm. 2012c;9:S186. doi: 10.1016/j.hrthm.2013.03.023. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicouri S, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of the highly-selective late sodium channel current blocker GS-458967. Heart Rhythm. 2013;10(7):1036–1043. doi: 10.1016/j.hrthm.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorov VY, Uzelac I, Wikswo JP. Regional increase of extracellular potassium leads to electrical instability and reentry occurrence through the spatial heterogeneity of APD restitution. Am J Physiol Heart Circ Physiol. 2011;301:H209–H220. doi: 10.1152/ajpheart.01141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Huang CS, Nagata K, Yeh JZ, Narahashi T. Differential action of riluzole on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J Pharmacol Exp Ther. 1997;282:707–714. [PubMed] [Google Scholar]
- Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004;44:192–199. doi: 10.1097/00005344-200408000-00008. [DOI] [PubMed] [Google Scholar]
- Song Y, Shryock J, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- Song Y, Shryock JC, Belardinelli L. An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H2031–H2039. doi: 10.1152/ajpheart.01357.2007. [DOI] [PubMed] [Google Scholar]
- Song Y, Shryock JC, Belardinelli L. A slowly inactivating sodium current contributes to spontaneous diastolic depolarization of atrial myocytes. Am J Physiol Heart Circ Physiol. 2009;297:H1254–H1262. doi: 10.1152/ajpheart.00444.2009. [DOI] [PubMed] [Google Scholar]
- Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts–role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol. 2008;45:32–43. doi: 10.1016/j.yjmcc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, Schmitto JD, Seipelt R, Schondube FA, Hasenfuss G, Belardinelli L, Maier LS. Altered Na+ currents in atrial fibrillation: effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol. 2010;55:2330–2342. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- Sossalla S, Maurer U, Schotola H, Hartmann N, Didie M, Zimmermann WH, Jacobshagen C, Wagner S, Maier LS. Diastolic dysfunction and arrhythmias caused by overexpression of CaMKIIdelta(C) can be reversed by inhibition of late Na(+) current. Basic Res Cardiol. 2011;106:263–272. doi: 10.1007/s00395-010-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CI, Sham JS. Mechanisms underlying the effects of the pyrethroid, tefluthrin, on action potential duration in isolated rat ventricular myocytes 1. J Pharmacol Exp Ther. 2005;315:16–23. doi: 10.1124/jpet.105.084822. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Tateyama M, Clancy CE, Malhotra A, Beggs AH, Cappuccio FP, Sagnella GA, Kass RS, Keating MT. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science. 2002;297:1333–1336. doi: 10.1126/science.1073569. [DOI] [PubMed] [Google Scholar]
- Starmer CF, Nesterenko VV, Undrovinas AI, Grant AO, Rosenshtraukh LV. Lidocaine blockade of continuously and transiently accessible sites in cardiac sodium channels. J Mol Cell Cardiol. 1991;23(Suppl I):73–83. doi: 10.1016/0022-2828(91)90026-i. [DOI] [PubMed] [Google Scholar]
- Stern MD, Capogrossi MC, Lakatta EG. Spontaneous calcium release from the sarcoplasmic reticulum in myocardial cells: mechanisms and consequences. Cell Calcium. 1988;9:247–256. doi: 10.1016/0143-4160(88)90005-x. [DOI] [PubMed] [Google Scholar]
- Sunami A, Fan Z, Sawanobori T, Hiraoka M. Use-dependent block of Na + currents by mexiletine at the single channel level in guinea-pig ventricular myocytes. Br J Pharmacol. 1993;110:183–192. doi: 10.1111/j.1476-5381.1993.tb13790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamareille S, Le GB, John GW, Feuvray D, Coulombe A. Anti-ischemic compound KC 12291 prevents diastolic contracture in isolated atria by blockade of voltage-gated sodium channels. J Cardiovasc Pharmacol. 2002;40:346–355. doi: 10.1097/00005344-200209000-00003. [DOI] [PubMed] [Google Scholar]
- Tan BH, Pundi KN, Van Norstrand DW, Valdivia CR, Tester DJ, Medeiros-Domingo A, Makielski JC, Ackerman MJ. Sudden infant death syndrome–associated mutations in the sodium channel beta subunits. Heart Rhythm. 2010;7:771–778. doi: 10.1016/j.hrthm.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Joung B, Ogawa M, Chen PS, Lin SF. Intracellular calcium dynamics, shortened action potential duration, and late-phase 3 early after depolarization in langendorff-perfused rabbit ventricles. J Cardiovasc Electrophysiol. 2012;23:1364–1371. doi: 10.1111/j.1540-8167.2012.02400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein W, Kassebaum DG, Nelson RM, HECHTHH Electrophysiological study of human heart muscle. Circ Res. 1962;10:306–312. doi: 10.1161/01.res.10.3.306. [DOI] [PubMed] [Google Scholar]
- Tsuchida K, Otomo S. Electrophysiological effects of Monensin, a sodium ionophore, on cardiac Purkinje fibers. Eur J Pharm. 1990;190:313–320. doi: 10.1016/0014-2999(90)94195-4. [DOI] [PubMed] [Google Scholar]
- Tweedie D, Harding SE, MacLeod KT. Sarcoplasmic reticulum Ca content, sarcolemmal Ca influx and the genesis of arrhythmias in isolated guinea-pig cardiomyocytes. J Mol Cell Cardiol. 2000;32:261–272. doi: 10.1006/jmcc.1999.1070. [DOI] [PubMed] [Google Scholar]
- Ueda N, Zipes DP, Wu J. Prior ischemia enhances arrhythmogenicity in isolated canine ventricular wedge model of long QT 3. Cardiovasc Res. 2004;63:69–76. doi: 10.1016/j.cardiores.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Ueda K, Valdivia C, Medeiros-Domingo A, Tester DJ, Vatta M, Farrugia G, Ackerman MJ, Makielski JC. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc Natl Acad Sci U S A. 2008;105:9355–9360. doi: 10.1073/pnas.0801294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undrovinas A, Maltsev VA. Late sodium current is a new therapeutic target to improve contractility and rhythm in failing heart. Cardiovasc Hematol Agents Med Chem. 2008a;6:348–359. doi: 10.2174/187152508785909447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undrovinas A, Maltsev VA. Late sodium current is a new therapeutic target to improve contractility and rhythm in failing heart. Cardiovasc Hematol Agents Med Chem. 2008b;6:348–359. doi: 10.2174/187152508785909447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undrovinas AI, Fleidervish IA, Makielski JC. Inward sodium current at resting potentials in single cardiac myocytes induced by the ischemic metabolite lysophosphatidylcholine. Circ Res. 1992;71:1231–1241. doi: 10.1161/01.res.71.5.1231. [DOI] [PubMed] [Google Scholar]
- Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: role of sustained inward current. Cell Mol Life Sci. 1999;55:494–505. doi: 10.1007/s000180050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undrovinas AI, Maltsev VA, Kyle JW, Silverman N, Sabbah HN. Gating of the late Na+ channel in normal and failing human myocardium. J Mol Cell Cardiol. 2002;34:1477–1489. doi: 10.1006/jmcc.2002.2100. [DOI] [PubMed] [Google Scholar]
- Undrovinas AI, Undrovinas NA, Belardinelli L, Sabbah HN. Ranolazine inhibits late sodium current in isolated left ventricular myocytes of dogs with heart failure. J Am Coll Cardiol. 2004;43(supplA):178A. Abstract. [Google Scholar]
- Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17:S161–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undrovinas NA, Maltsev VA, Belardinelli L, Sabbah HN, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci. 2010;60:245–257. doi: 10.1007/s12576-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher B, Pignier C, Letienne R, Verscheure Y, Le GB. F 15845 inhibits persistent sodium current in the heart and prevents angina in animal models. Br J Pharmacol. 2009;156:214–225. doi: 10.1111/j.1476-5381.2008.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Vatta M, Faulkner G. Cytoskeletal basis of ion channel function in cardiac muscle. Future Cardiol. 2006;2:467–476. doi: 10.2217/14796678.2.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114:2104–2112. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- Verdonck F, Bielen FV, Ver DL. Preferential block of the veratridine-induced, non-inactivating Na + current by R56865 in single cardiac Purkinje cells. Eur J Pharmacol. 1991;203:371–378. doi: 10.1016/0014-2999(91)90893-u. [DOI] [PubMed] [Google Scholar]
- Vermeulen JT, Tan HL, Rademaker H, Schumacher CA, Loh P, Opthof T, Coronel R, Janse MJ. Electrophysiologic and extracellular ionic changes during acute ischemia in failing and normal rabbit myocardium. J Mol Cell Cardiol. 1996;28:123–131. doi: 10.1006/jmcc.1996.0012. [DOI] [PubMed] [Google Scholar]
- Vollmer B, Meuter C, Janssen PA. R 56865 prevents electrical and mechanical signs of ouabain intoxication in guinea-pig papillary muscle. Eur J Pharmacol. 1987;142:137–140. doi: 10.1016/0014-2999(87)90663-7. [DOI] [PubMed] [Google Scholar]
- Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive oxygen species-activated Ca/calmodulin kinase IIdelta is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Shen J, Li Z, Timothy KW, Vincent GM, Priori SG, Schwartz PJ, Keating MT. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum Mol Genet. 1995;4:1603–1607. doi: 10.1093/hmg/4.9.1603. [DOI] [PubMed] [Google Scholar]
- Wang DW, Yazawa K, George AL, Jr, Bennett PB. Characterization of human cardiac Na+ channel mutations in the congenital long QT syndrome. Proc Natl Acad Sci U S A. 1996;93:13200–13205. doi: 10.1073/pnas.93.23.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DW, Crotti L, Shimizu W, Pedrazzini M, Ikeda T, Schwartz PJ, George AL. Malignant perinatal variant of long-QT syndrome caused by a profoundly dysfunctional cardiac sodium channel. Circ Arrhythm Electrophysiol. 2008;1:370–378. doi: 10.1161/CIRCEP.108.788349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DW, Mistry AM, Kahlig KM, Kearney JA, Xiang J, George AL., Jr Propranolol blocks cardiac and neuronal voltage-gated sodium channels. Front Pharmacol. 2010;1:144. doi: 10.3389/fphar.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997;500(Pt 3):631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CA, Bazzazi H, Clark RB, Nygren A, Giles WR. Actions of emigrated neutrophils on Na(+) and K(+) currents in rat ventricular myocytes. Prog Biophys Mol Biol. 2006;90:249–269. doi: 10.1016/j.pbiomolbio.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Weiss S, Benoist D, White E, Teng W, Saint DA. Riluzole protects against cardiac ischaemia and reperfusion damage via block of the persistent sodium current. Br J Pharmacol. 2010;160:1072–1082. doi: 10.1111/j.1476-5381.2010.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JW, Patton DE, Scheuer T. A cluster of hydrophobic amino acid residues required for fast Na+ channel inactivation. Proc Natl Acad Sci U S A. 1992;89:10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D, Himmel H, Ravens U, Peters T. Characterization of the interaction of R 56865 with cardiac Na- and L-type Ca channels. Br J Pharmacol. 1991;104:483–489. doi: 10.1111/j.1476-5381.1991.tb12455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LD, Jeyaraj D, Wan X, Hoeker GS, Said TH, Gittinger M, Laurita KR, Rosenbaum DS. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm. 2009;6:251–259. doi: 10.1016/j.hrthm.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wit AL, Cranefield PF. Triggered and automatic activity in the canine coronary sinus. Circ Res. 1977;41:435–445. doi: 10.1161/01.res.41.4.434. [DOI] [PubMed] [Google Scholar]
- Wit AL, Rosen MR. Pathophysiologic mechanisms of cardiac arrhythmias. Am Heart J. 1983;106:798–811. doi: 10.1016/0002-8703(83)90003-0. [DOI] [PubMed] [Google Scholar]
- Witchel HJ, Dempsey CE, Sessions RB, Perry M, Milnes JT, Hancox JC, Mitcheson JS. The low-potency, voltage-dependent HERG blocker propafenone–molecular determinants and drug trapping. Mol Pharmacol. 2004;66:1201–1212. doi: 10.1124/mol.104.001743. [DOI] [PubMed] [Google Scholar]
- Wu J, Corr PB. Palmitoylcarnitine increases [Na+]i and initiates transient inward current in adult ventricular myocytes. Am J Physiol. 1995;268:H2405–H2417. doi: 10.1152/ajpheart.1995.268.6.H2405. [DOI] [PubMed] [Google Scholar]
- Wu L, Song Y, Shryock JC, Li Y, Antzelevitch C, Belardinelli L. Ranolazine attenuates the prolongation of ventricular monophasic action potential and suppresses ventricular tachycardia caused by sea anemone toxin, ATX-II, in guinea pig isolated hearts. PACE. 2003;26:1023. Abstract. [Google Scholar]
- Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther. 2004;310:599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- Wu L, Shryock JC, Song Y, Belardinelli L. An increase in late sodium current potentiates the proarrhythmic activities of low-risk QT-prolonging drugs in female rabbit hearts. J Pharmacol Exp Ther. 2006;316:718–726. doi: 10.1124/jpet.105.094862. [DOI] [PubMed] [Google Scholar]
- Wu G, Ai T, Kim JJ, Mohapatra B, Xi Y, Li Z, Abbasi S, Purevjav E, Samani K, Ackerman MJ, Qi M, Moss AJ, Shimizu W, Towbin JA, Cheng J, Vatta M. a-1-syntrophin mutation and the long-QT syndrome: a disease of sodium channel disruption. Circ Arrhythm Electrophysiol. 2008a;1:193–201. doi: 10.1161/CIRCEP.108.769224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Guo D, Li H, Hackett J, Yan GX, Jiao Z, Antzelevitch C, Shryock JC, Belardinelli L. Role of late sodium current in modulating the proarrhythmic and antiarrhythmic effects of quinidine. Heart Rhythm. 2008b;5:1726–1734. doi: 10.1016/j.hrthm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Rajamani S, Shryock JC, Li H, Ruskin J, Antzelevitch C, Belardinelli L. Augmentation of late sodium current unmasks the proarrhythmic effects of amiodarone. Cardiovasc Res. 2008c;77:481–488. doi: 10.1093/cvr/cvm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Song Y, Belardinelli L, Shryock JC. The late Na+ current (INa) inhibitor ranolazine attenuates effects of Palmitoyl-L-Carnitine to increase late INa and cause ventricular diastolic dysfunction. J Pharmacol Exp Ther. 2009a;330:550–557. doi: 10.1124/jpet.109.151936. [DOI] [PubMed] [Google Scholar]
- Wu L, Rajamani S, Li H, January CT, Shryock JC, Belardinelli L. Reduction of repolarization researve unmasks the pro-arrhythmic role of endogenous late sodium current in the heart. Am J Physiol Heart Circ Physiol. 2009b;297:H1048–H1057. doi: 10.1152/ajpheart.00467.2009. [DOI] [PubMed] [Google Scholar]
- Wu L, Ma J, Li H, Wang C, Grandi E, Zhang P, Luo A, Bers DM, Shryock JC, Belardinelli L. Late sodium current contributes to the reverse rate-dependent effect of IKr inhibition on ventricular repolarization. Circulation. 2011;123:1713–1720. doi: 10.1161/CIRCULATIONAHA.110.000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao XH, Allen DG. Role of Na(+)/H(+) exchanger during ischemia and preconditioning in the isolated rat heart. Circ Res. 1999;85:723–730. doi: 10.1161/01.res.85.8.723. [DOI] [PubMed] [Google Scholar]
- Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced after depolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan GX, Wu Y, Liu T, Wang J, Marinchak RA, Kowey PR. Phase 2 early afterdepolarization as a trigger of polymorphic ventricular tachycardia in acquired long-qt syndrome: direct evidence from intracellular recordings in the intact left ventricular wall. Circulation. 2001;103:2851–2856. doi: 10.1161/01.cir.103.23.2851. [DOI] [PubMed] [Google Scholar]
- Yao L, Fan P, Jiang Z, Viatchenko-Karpinski S, Wu Y, Kornyeyev D, Hirakawa R, Budas GR, Rajamani S, Shryock JC, Belardinelli L. Nav1.5-dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice. Am J Physiol Cell Physiol. 2011;301:C577–C586. doi: 10.1152/ajpcell.00125.2011. [DOI] [PubMed] [Google Scholar]
- Yatani A, Akaike N. Blockage of the sodium current in isolated single cells from rat ventricle with mexiletine and disopyramide. J Mol Cell Cardiol. 1985;17:467–476. doi: 10.1016/s0022-2828(85)80051-1. [DOI] [PubMed] [Google Scholar]
- Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac “late sodium current”. Pharmacol Ther. 2008;119:326–339. doi: 10.1016/j.pharmthera.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Zeiler RH, Gough WB, El-Sherif N. Electrophysiologic effects of propafenone on canine ischemic cardiac cells. Am J Cardiol. 1984;54:424–429. doi: 10.1016/0002-9149(84)90210-8. [DOI] [PubMed] [Google Scholar]
- Zellerhoff S, Pistulli R, Monnig G, Hinterseer M, Beckmann BM, Kobe J, Steinbeck G, Kaab S, Haverkamp W, Fabritz L, Gradaus R, Breithardt G, Schulze-Bahr E, Bocker D, Kirchhof P. Atrial arrhythmias in long-QT syndrome under daily life conditions: a nested case control study. J Cardiovasc Electrophysiol. 2009;20:401–407. doi: 10.1111/j.1540-8167.2008.01339.x. [DOI] [PubMed] [Google Scholar]
- Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH. CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem. 2007;282:35078–35087. doi: 10.1074/jbc.M707083200. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Yamada S, Barry WH. Ranolazine inhibits an oxidative stress-induced increase in myocyte sodium and calcium loading during simulated-demand ischemia. J Cardiovasc Pharmacol. 2008;51:443–449. doi: 10.1097/FJC.0b013e318168e711. [DOI] [PubMed] [Google Scholar]
- Zhang T, Yong SL, Drinko JK, Popovic ZB, Shryock JC, Belardinelli L, Wang QK. LQTS mutation N1325S in cardiac sodium channel gene SCN5A causes cardiomyocyte apoptosis, cardiac fibrosis and contractile dysfunction in mice. Int J Cardiol. 2011;147:239–245. doi: 10.1016/j.ijcard.2009.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Walsh E, Shryock JC, Messina E, Wu Y, Zeng D, Xu X, Ochoa M, Baker SP, Hintze TH, Belardinelli L. Antiadrenergic and hemodynamic effects of ranolazine in conscious dogs. J Cardiovasc Pharmacol. 2011;57:639–647. doi: 10.1097/FJC.0b013e31821458e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicha S, Maltsev VA, Nattel S, Sabbah HN, Undrovinas AI. Post-transcriptional alterations in the expression of cardiac Na+ channel subunits in chronic heart failure1. J Mol Cell Cardiol. 2004;37:91–100. doi: 10.1016/j.yjmcc.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer T, Surber R. SCN5A channelopathies - an update on mutations and mechanisms. Prog Biophys Mol Biol. 2008;98:120–136. doi: 10.1016/j.pbiomolbio.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Zygmunt AC, Eddlestone GT, Thomas GP, Nesterenko VV, Antzelevitch C. Larger late sodium conductance in M cells contributes to electrical heterogeneity in canine ventricle. Am J Physiol. 2001;281:H689–H697. doi: 10.1152/ajpheart.2001.281.2.H689. [DOI] [PubMed] [Google Scholar]
- Zygmunt AC, Nesterenko VV, Rajamani S, Hu D, Barajas-Martinez H, Belardinelli L, Antzelevitch C. Mechanisms of atrial-selective block of sodium channel by ranolazine I. Experimental analysis of the use-dependent block. Am J Physiol Heart Circ Physiol. 2011;301:H1606–H1614. doi: 10.1152/ajpheart.00242.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]