ABSTRACT
The germ cell lineages are among the best characterized of all cell lineages in mammals. This characterization includes precise nomenclature that distinguishes among numerous, often subtle, changes in function or morphology as development and differentiation of germ cells proceed to form the gametes. In male rodents, there are at least 41 distinct cell types that occur during progression through the male germ cell lineage that gives rise to spermatozoa. However, there is one period during male germ cell development—that which occurs immediately following the primordial germ cell stage and prior to the spermatogonial stage—for which the system of precise and informative cell type terminology is not adequate. Often, male germ cells during this period are referred to simply as “gonocytes.” However, this term is inadequate for multiple reasons, and it is suggested here that nomenclature originally proposed in the 1970s by Hilscher et al., which employs the terms M-, T1-, and T2-prospermatogonia, is preferable. In this Minireview, the history, proper utilization, and advantages of this terminology relative to that of the term gonocytes are described.
Keywords: gonocytes, male germ cells, prospermatogonia
A case for updating the terminology of male germ cells during the fetal and neonatal stages in male rodents.
INTRODUCTION
In both male and female mammals, the germ cell lineages are typically the first future adult cell type to be specified in the embryo proper [1, 2]. Once specified, these lineages follow dynamic differentiation pathways that are largely sexually dimorphic and include distinguishable stages throughout embryonic, fetal, neonatal, pubertal, and adult development [3–8]. In both sexes, these different stages are typically distinguished by distinct terminology for each different cell type within each developing lineage. Thus, germ cells initially appear as primordial germ cells (PGCs) in the developing embryo at a stage approximately coincident with gastrulation in rodents [1, 4, 9–11]. As in all other vertebrate (and several invertebrate) species studied to date, mammalian PGCs do not arise in the same region as the gonadal anlagen, but are rather derived from the epiblast and subsequently migrate to and colonize the developing genital ridges so as to be properly located to participate in gametogenesis [1, 10]. During the PGC stage, the germ cells appear similar in both males and females. However, following initiation of sexual differentiation of the gonads, which occurs in gonadal somatic cells and does not require the presence of germ cells [5, 12, 13], ovarian germ cells enter an oogenic pathway, whereas testicular germ cells enter a spermatogenic pathway [7]. From this point on, development and differentiation of the germ cells is dimorphic in males and females.
Following gonadal sex differentiation in fetal female rodents, mitotically active germ cells are termed “oogonia.” These cells then enter meiosis as “primary oocytes,” and once they reach the dictyate stage of first meiotic prophase, they enter a meiotic arrest that is maintained until adulthood [7]. A series of terms are used to characterize different stages of developing follicles and the oocytes within these follicles (for review, see Ref. 14). These terms distinguish different stages of maturation of the follicles as well as distinguishing oocytes in different stages of first or second meiosis prior to fertilization.
In fetal male rodents, PGCs give rise to germ cells that are initially mitotically active, but which then enter a mitotic arrest during mid-late fetal development that persists until a few days after birth, when mitotic activity resumes in these cells such that, a few days later, they give rise to spermatogonia, which are premeiotic spermatogenic cells. There is more than one system of terminology used to distinguish different stages of spermatogonial development and/or different types of spermatogonia in rodents, and other nomenclature systems are used to describe spermatogonia in other mammalian species, including primates (see detailed review in Ref. 15). All systems designate at least two types of spermatogonia—type A and type B, with type A being further subdivided to include undifferentiated spermatogonia (As-Apr-Aal) with little or no heterochromatin visible in the nucleus and early differentiating spermatogonia (A1-A4) with visible heterochromatin in the nucleus, and type B referring to later differentiating spermatogonia, also with visible heterochromatin. The most common terminology system used to describe various types of spermatogonia in rodents includes 11 types: As, Apr, Aal4, Aal8, Aal16–32, A1, A2, A3, A4, In, and B spermatogonia [15]. Among these spermatogonial types, the As spermatogonia are believed to represent functional spermatogonial stem cells (SSCs) [15].
Subsequent to the spermatogonial stage in male mammals, spermatogenic cells enter meiosis, which includes several specific stages during first meiotic prophase (preleptotene, leptotene, zygotene, pachytene, and diplotene), followed by metaphase I and anaphase I to give rise to secondary spermatocytes (at least eight types of spermatocytes) that then rapidly divide to yield postmeiotic, haploid spermatids. Spermatids are divided into round spermatids, elongating or condensing spermatids, and elongated or condensed spermatids, and these cells give rise to testicular spermatozoa. Spermatid differentiation is also known as spermiogenesis, and this process has been divided into at least 16 different steps [16]. Testicular spermatozoa are released from the seminiferous epithelium by a process called spermiation [16], and are then collected in the rete testis from where they exit the testis and enter the epididymis. Epididymal spermatozoa can be distinguished on the basis of the portion of the epididymis in which they reside at any one time. This includes at least three different regions (the caput, corpus, and cauda epididymis), but has sometimes been divided into more segments [17, 18]. It is in the epididymis that spermatozoa acquire the traits of motility and fertility [17]. Finally, the spermatozoa move out of the epididymis into the vas deferens from where they are subsequently released upon ejaculation. Thus, it is possible to distinguish at least five types of spermatozoa in the adult male (testicular, caput epididymal, corpus epididymal, cauda epididymal, and vas deferens spermatozoa).
A PERIOD FOR WHICH CURRENT TERMINOLOGY IS DEFICIENT
The nomenclature described above reflects the facts that germ cell development and differentiation are quite well characterized in each sex, and that these are highly dynamic processes for which extensive cell type-specific terminology is used to distinguish the numerous different stages and/or processes involved in spermatogenic or oogenic differentiation. As described above, in the case of male germ cell development and differentiation, at least 41 distinct cell type designations span the PGC (1 designation), spermatogonial (11 designations), spermatocyte (8 designations), spermatid (16 designations), and spermatozoon (5 designations) phases of the male germ line. However, there is one notable exception to the rule that different developmental stages, morphologies, or functions in developing male germ cells are commonly distinguished by the use of distinct cell type-specific terms—the period during fetal and neonatal development of the male germ cell lineage that immediately follows the PGC stage and precedes the spermatogonial stage in rodents. In male mice, this period typically extends from approximately 13.5 days postcoitum (dpc) to approximately 3–6 days postpartum (dpp). Some authors have extended the use of the term PGCs to describe fetal/neonatal male germ cells [19]. Others have used the term “fetal spermatogonia” [20–22]. However, the most commonly used term to describe male germ cells during this period has been “gonocyte(s)” [20] or “I-gonocyte(s)” or “II-gonocyte(s)” [23]. The term gonocyte was first introduced in the literature by Clermont and Perey [24], and has been frequently used since then [e.g. 15, 25–50]. While this term carries significant historical value and has had widespread use, it is otherwise less than optimal as a means of describing the various types of male germ cells that occur during this period.
DEFICIENCIES ASSOCIATED WITH USE OF THE TERM GONOCYTE
Deficiencies presented by use of the term gonocyte can be grouped into at least two categories: 1) failure of this term to implicitly convey the intended cell identity, and 2) the need for more than one term to describe the various types of, and/or activities ongoing in, male germ cells during this period. Taken literally, the term gonocyte means simply “gonadal cell.” As such, this term does not implicitly convey the identity of this cell as a germ cell rather than a somatic cell. Nor does this term provide any indication of the sex of the individual in which this cell type is found, as opposed to all other terms for specific germ cell types following the PGC stage, which uniformly include the root “oo” to indicate female, oogenic germ cells or “spermato” to indicate male, spermatogenic germ cells. Indeed, there are examples in the literature where the term gonocyte(s) has been used to refer to both male and female germ cells [22, 51]. Furthermore, the term gonocyte provides no implicit indication of the developmental stage at which these cells occur relative to other cell types in the male germ lineage. By comparison, the terminology used to describe female germ cells that occur during this same developmental period includes the term oogonia for the cells that are still dividing mitotically, and primary oocytes for the cells that have entered first meiotic prophase. These terms effectively convey the identity of these cells as female germ cells (by use of the root oo) and as either premeiotic (by use of the root “gonia/um”) or meiotic (by use of the root “cyte[s]”) germ cells, and the fact that these oocytes precede later types of oocytes in the female germ cell lineage (by use of the term “primary”). In contrast, the term gonocyte(s) fails to convey any of the equivalent information regarding male germ cells during this same developmental period.
As noted above, development of fetal and neonatal male germ cells prior to the spermatogonial stage includes a period of mitotic activity, followed by mitotic arrest, followed by resumption of mitotic activity. The term gonocyte fails to distinguish among these periods of cellular activity. Thus, different authors have used the single term, gonocyte(s), to describe different populations of male germ cells, in some cases referring to the mitotically active male germ cells that immediately follow the PGC stage (e.g., Ref. 41), or, in other cases, referring to the mitotically quiescent cells that occur in the male germ line during the latter portion of fetal development and/or early neonatal development (e.g., Ref. 25), or, in still other cases, referring to the mitotically active male germ cells that immediately precede the formation of spermatogonia (e.g., Ref. 31).
We now know that a variety of different functions are achieved during each of these phases of fetal/neonatal male germ cell development. Expansion of the male germ cell (and future spermatogonial) pool appears to be a primary product of the first round of mitotic activity in male germ cells immediately following the PGC stage. During the phase in which male germ cells are in mitotic arrest, extensive epigenetic reprogramming occurs [52–57]. Whether or not mitotic quiescence is required to facilitate this epigenetic reprogramming is not known, but the correlation between these two events is intriguing. Finally, during the phase of renewed mitotic activity of male germ cells just prior to the spermatogonial stage, there is further expansion of the male germ cell pool, and there is also evidence that differences in developmental potential among subsequent spermatogonia are first manifest at this time [58–66]. These distinctions in cellular activity among male germ cells at various stages of fetal and neonatal development warrant distinguishing terms to identify each different male germ cell function or activity during this period.
PREFERABLE TERMINOLOGY FOR FETAL AND NEONATAL MALE GERM CELLS IN RODENTS
A nomenclature that avoids all of the deficiencies of the gonocyte terminology described above was introduced in a series of studies published in the 1970s by Hilscher et al. [29, 67–70]. These authors made the case that male germ cell development can be divided into three major phases, which they termed the “pregonadal period” (the period of PGC specification and migration to the genital ridges), “prespermatogenesis” (a phase during fetal and neonatal development in male rodents characterized by proliferation and differentiation of the precursors of the mature germ cells later found in the adult testis), and “spermatogenesis” (the maturation of the male germ cells to form spermatozoa in the pubertal and adult testis). To describe the male germ cells that occur during the prespermatogenesis phase, Hilscher et al. [70] proposed the term “prospermatogonia” (plural) or “prospermatogonium” (singular). Thus, these terms have existed in the literature for nearly 40 yr. This terminology has the advantage that it clearly conveys germ cell identity based on inclusion of the root gonia or gonium. It further clearly conveys the identity of a male germ cell, because it contains the root spermato. In addition, this terminology implicitly conveys the fact that these are mitotic cells, because the root gonia/um is similar to the use of the same root in the terms oogonia/um or spermatogonia/um, both of which are premeiotic germ cells (note that the term gonocyte is further problematic in this regard, because the root cyte used in this term to identify mitotic cells leads to confusion with the terms spermatocytes or oocytes that designate meiotic stages of the male and female germ cell lineages). Finally, the term prospermatogonia/um conveys the timing of occurrence of this cell type in the developing male germ cell lineage on the basis of inclusion of the root “pro,” which implies that these cells occur prior to the spermatogonia/um stage.
Hilscher et al. [70] also proposed terminology to distinguish among the three different periods of replication activity (or lack thereof) that occur in fetal/neonatal male germ cells in rodents. Thus, these authors proposed the terms “M-prospermatogonia” to represent the “multiplying prospermatogonia” that occur immediately following the PGC stage, “T1-prospermatogonia” to represent “primary transitional prospermatogonia” that are in “the first state of transition between the M-prospermatogonia and the A-spermatogonia” and are also in mitotic arrest, and “T2-prospermatogonia” to represent “secondary transitional prospermatogonia” that are in “the second state of transition to the A-spermatogonia” and are mitotically active [70]. After undergoing a few rounds of mitotic replication, T2-prospermatogonia give rise to type A spermatogonia, and this marks the end of the prespermatogenesis phase and the beginning of the spermatogenesis phase. The occurrence of each type of prospermatogonium is largely consecutive (Table 1), although it is possible to have both M- and T1- or T1- and T2-prospermatogonia present simultaneously during the transition period between each cell type. Note—the term “prespermatogonia/um” has also been used in the literature to describe these same cell types [71], but this tends to confuse the terminology for the specific cell types with the terminology for the process of prespermatogenesis, and so is more confusing than informative.
TABLE 1.
The male germ cell line in rodents.*
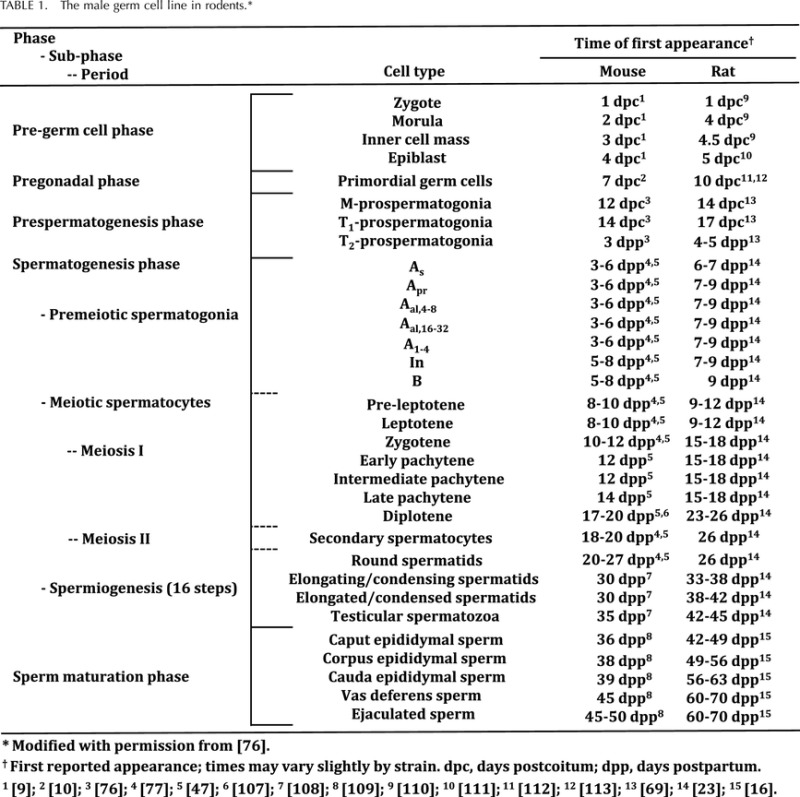
ˆ Modified from McCarrey [76].
First reported appearance; times may vary slightly by strain.
dpc = days postcoitum; dpp = days postpartum.
[9]; 2 [10]; 3 [76]; 4 [77]; 5 [47]; 6 [78]; 7 [79]; 8 [80]; 9 [81]; 10 [82]; 11 [83]; 12 [84]; 13 [69]; 14 [23]; 15 [16].
Interestingly, Hilscher et al. [29, 67–70] made the point that the morphology of M-prospermatogonia is initially similar to that of oogonia at the equivalent developmental period, and that sexual dimorphism between developing male and female germ cells is really first visible on the basis of distinctions between T1-prospermatogonia, which enter mitotic arrest, and primary oocytes, which have entered first meiotic prophase at the equivalent developmental stage. However, in a later publication, Hilscher and Hilscher [72] noted that a more subtle distinction between male and female germ cell development may be seen slightly earlier, at the end the first proliferation wave of M-prospermatogonia and oogonia, respectively, when the cell cycle is seen to be slightly shorter in male germ cells than in female germ cells [73]. In their publication in 1974, Hilscher et al. [70] provided a schematic comparison of development of male and female germ cells that is reproduced here as Figure 1.
FIG. 1.
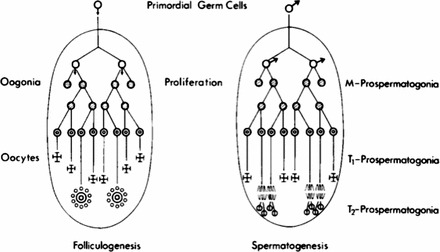
Oogenesis and prespermatogenesis as originally presented by Hilscher and colleagues. The diagram, reproduced with permission from Hilscher et al. [70], shows the parallel differentiation pathways of female (left) and male (right) germ cell development emphasizing the period between the PGC stage and folliculogenesis (in females) or spermatogenesis (in males). This marked the original usage of the terms “M-prospermatogonia,” “T1-prospermatogonia,” and “T2-prospermatogonia” to describe male germ cells during the fetal and neonatal periods in rodents, also known as the period of prespermatogenesis.
This reveals striking parallels between male and female germ cell development, despite the fact that these lineages are distinguished by entry of fetal female germ cells into meiosis while fetal male germ cells remain mitotic [6]. Hischer et al. [70] described this parallel development of female and male germ cells as follows: “The final comparison of the female and male gametogenesis shows that the ‘gonia stage' of the female germ cells is limited to one proliferation wave only (first part of oogenesis), whereas the ‘gonia stage' of the male germ cells consists of a first proliferation wave—comparable to that of oogonia—(first part of prespermatogenesis), a preparation phase to initiate spermatogenesis (second part of prespermatogenesis), and a second proliferation wave with renewal and differentiation of the spermatogonia (third part of prespermatogenesis).” Hilscher and colleagues also provided extensive detailed descriptions of the morphological features that characterize and distinguish M-, T1-, and T2-prospermatogonia in the rat [23, 68–70], although the identification of convenient protein markers for each prospermatogonial cell type is still lacking. They later extended the application of this terminology to the mouse [72], and have also discussed its potential use to describe early male germ cells in humans as well [74, 75].
The terms M-, T1-, and T2-prospermatogonia are congruent with those used to describe male germ cells at all other stages of male gametogenesis. Table 1 (modified from McCarrey [76]) demonstrates how the M-, T1-, and T2-prospermatogonia can be integrated into the terminology system used to describe the complete development and differentiation of the male germ line—from PGCs to spermatozoa.
HISTORICAL PERSPECTIVES
As noted above, there is no doubt that the term gonocyte has a long-term and widespread history in the literature, including descriptions from pioneers in the field of male germ cell biology, such as Clermont and Perey [24], Huckins and Clermont [30], Holstein and Wartenberg [20], Wartenberg et al. [21], and many others (e.g., Refs. 15, 25–50]. Interestingly, however, Wartenberg [32] was subsequently one of the first to adopt the prospermatogonia nomenclature proposed by Hilscher et al. [70].
Thus, gonocyte(s) has been, indisputably, the most commonly used term in the literature to describe fetal and neonatal male germ cells in rodents, and often in primates as well. However, the frequency of use of a term does not necessarily establish it as the preferable choice as the most precise or informative term. Indeed, it is worth considering the context in which this term was initially proposed. In the original paper in which the term gonocyte was first employed, Clermont and Perey used this term to refer to “large cells” contained in the sex cords in rat fetuses at 15–21 dpc and in newborn rats at 1 dpp [24]. They provided the following description of these cells: “The gonocytes, located in the central region of the sex cords, had a large, light, spherical nucleus containing fine chromatin granules and two or more globular nucleoli, as well as a clearly visible cytoplasmic membrane. These cells were numerous in the late fetus and less abundant thereafter.” [24]. These authors also discussed a second cell type within the seminiferous cords, which they referred to as “supporting cells.” These cells were described as “located along the basement membrane, with variable size nuclei containing coarse chromatin granulations which delineated both nuclear membrane and nucleolus as well as by a thin, scarcely visible cytoplasmic membrane” [24]. They concluded that, “although many gonocytes degenerated, some of them produced type A spermatogonia, which, in turn, produced all the other cells of the germ cell lineage.” They also concluded that “the supporting cells eventually became Sertoli cells and played no role in the production of definitive germ cells” [24]. Thus, this latter, supporting somatic cell type is presumably what is commonly referred to as “pre-Sertoli cells” in the current literature [77], and this stands as an example of a more precise and informative term (pre-Sertoli cells) replacing an earlier, less descriptive term (supporting cells).
It seems likely that the rather generic term, gonocyte(s), was considered adequate when it was first used to describe fetal and neonatal male germ cells, because, for the majority of the time during this period, the male germ cells are quiescent with respect to replication activity, and undergo only relatively minor changes in morphology. Clermont and Perey [24] referred to some changes in the size of gonocytes or their nuclei, as well as the appearance of mitotic figures in some of these cells, and they also noted that many gonocytes degenerated, but no discussion of function(s) ongoing in these cells was presented. Indeed it is possible that, to many early investigators, it was not clear that these cells performed any significant function other than spanning the period between initial development of PGCs in male embryos and the subsequent appearance of spermatogonia and initiation of spermatogenesis in prepubertal-adult males. In this regard, the fetal/neonatal period of male germ cell development in rodents has more recently been termed “the forgotten cells of the (male) germ cell lineage” [45].
Thus, it is to the credit of the Hilschers and their colleagues that they recognized the subtle changes ongoing in fetal-neonatal male germ cells and took the initiative to propose terms to distinguish specific male germ cell types within this period. Specifically, they proposed terms that not only distinguished different stages during this period, but also conveyed the identity of these cells as male germ cells that precede spermatogonia. Although use of the term gonocyte(s) has persisted in the literature, several investigators have adopted the prospermatogonia/um terminology. As noted above, one of the earliest of these was Wartenberg [32]. In addition, Dr. Anne McLaren also adopted this terminology early on, and actually initiated direct contact with the Hilschers to learn about the proper use of this (then) new nomenclature, which then led to a Ph.D. student, Ms. Maxine Sutcliffe, from the group of Anne McLaren and Paul Burgoyne, traveling to the Hilschers' laboratory to learn how to recognize the different prospermatogonial stages in mice (P. Burgoyne, personal communication), especially for subsequent use in investigations of the effects of sex chromosomes and sex-linked genes on germ cell development and germ cell sex differentiation [78–81]. Thus, while it was a father of the field of spermatogenesis (Clermont) who coined the term gonocyte(s) [24], it was another father of the field (Wartenberg) [32] as well as the mother of the field of germ cell developmental biology (McLaren) [78–82] who recognized the value and promoted the use of the more precise and informative prospermatogonia/um terminology following its original introduction by Hilscher et al. [70]. This has been followed by use of the prospermatogonia/um terminology by many other authors and laboratories (e.g., Refs. 52, 76, 83–95). Therefore, while gonocyte(s) remains the most frequently used term in the literature to describe fetal/neonatal male germ cells in rodents, the term prospermatogonia/um has also experienced, and continues to gain, widespread use.
FUNCTIONAL PERSPECTIVES
Findings published in recent years have informed our understanding of molecular processes ongoing during fetal and neonatal male germ cell development in rodents. These include studies of gene expression patterns and factors that regulate differential gene expression in developing male germ cells [45]. However, one of the most active molecular processes ongoing during the prospermatogonial stages in male germ cells and the equivalent oogonia/oocyte stages in female germ cells is epigenetic reprogramming. Significant epigenetic reprogramming occurs in the preimplantation embryo [52, 96–99]. Somatic cell lineages largely retain global methylation patterns established in the early embryo and subsequently undergo only locus- and lineage-specific changes in DNA methylation associated with regulation of tissue-specific gene expression characteristic of each individual somatic cell type [96, 98]. On the other hand, the PGCs undergo another, germline-specific wave of global genomic reprogramming that begins with erasure of nearly all DNA methylation, including that at imprinted genes and at many repeated sequences, which begins as the migrating PGCs colonize the developing genital ridges and is complete by the M-prospermatogonia stage in males and the oogonia stage in females [52, 99]. Thus, fetal germ cells in both sexes reach a unique “epigenetic ground state” [100, 101] that is not found in any other cell type in the body at any other developmental stage. This is followed by yet another wave of global remethylation of germ cell genomes, which takes place primarily in T1-prospermatogonia in males and during oocyte development and maturation in females [52, 102].
This second major wave of epigenetic reprogramming, which is unique to the germ line in both sexes, and specific to T1- and T2-prospermatogonia in males, achieves multiple functions. It has been demonstrated that potentiation of expression of germ cell-specific genes that will be subsequently expressed during later stages of gametogenesis begins in fetal germ cells [52, 103]. This is also believed to be a period when the germline genomes become programmed to properly direct development of progeny produced by natural reproduction [99, 101]. In addition, this is the stage at which imprinted genes are biallelically reprogrammed in each sex such that paternally imprinted genes will become biallelically methylated in spermatogenic cells, while maternally imprinted genes will be biallelically methylated in oogenic cells [104]. Finally, this epigenetic reprogramming process that is unique to the developing germ line has recently been shown to correct certain types of epimutations induced by conditions associated with assisted reproductive technologies [105].
Global remethylation of the male germline genome is initiated in T1-prospermatogonia, and is largely completed before the appearance of T2-prospermatogonia [52, 102]. However, some gene-specific reprogramming, including completion of acquisition of DNA methylation associated with certain paternally imprinted genes [106] and gene-specific demethylation that precedes transcriptional activation of certain spermatogenesis-specific genes [52, 103], continues in T2-prospermatogonia and even in early spermatogonia, at least during the initial wave of spermatogenesis. The timing of this major wave of epigenetic reprogramming in prospermatogonia is uniquely advantageous, because it precedes the stage of the adult SSC. Thus, epigenetic reprogramming that is achieved once in prospermatogonia merely needs to be maintained thereafter in SSCs and subsequent spermatogenic cell types. If, on the other hand, de novo epigenetic programming were to normally occur in spermatogenic cells following the SSC stage, it would have to be accomplished anew during each subsequent wave of spermatogenesis, which would seem to involve an undesirable, repeated expenditure of energy throughout the lifetime of the male.
While T1-prospermatogonia represent a stage during which major epigenetic reprogramming occurs in developing male germ cells, T2-prospermatogonia appear to represent a stage at which discrimination among early male germ cells is manifest to determine which prospermatogonia will give rise to adult SSCs and, hence, to all subsequent waves of spermatogenesis in each individual male. Thus, a variety of lines of evidence indicate that the population of T2-prospermatogonia present in the neonatal male is heterogeneous with respect to potential to form SSCs. For instance, the first wave of spermatogenesis in male rodents appears to derive from a population of spermatogonia that is distinct from subsequent SSCs in that the spermatogonial cells that give rise to the first wave do not undergo self-renewal, whereas the SSCs that produce all subsequent waves do undergo self-renewal [58]. In addition, Yoshida et al. [58–61], Shinohara et al. [62, 63], and others [65] have reported results that raise the possibility that distinct subpopulations of early type A spermatogonia can be distinguished on the basis of marker gene expression, and that these different subpopulations may show differences in their potential to become functional SSCs. Finally, a recent analysis of the frequencies of spontaneous mutations detectable at different stages of prespermatogenesis and spermatogenesis also indicated the existence of distinct subpopulations of T1- and T2-prospermatogonia and early spermatogonia that differ with respect to the potential to form functional SSCs [66].
FUTURE PERSPECTIVES
If anything, the emerging evidence regarding distinct functions ongoing in various types of prospermatogonia during the prespermatogenesis phase may soon compel an even more complex nomenclature system to accurately describe male germ cells during the fetal and neonatal periods that further subcategorizes these cells to an extent even greater than that provided by the M-, T1-, and T2-prospermatogonia terminology. Thus, while we should always honor the history of our field and those who forged many of the early studies to begin to describe and understand the development and differentiation of male germ cells in mammals, we must also continue to heed new information as it becomes available and to allow our system of nomenclature to evolve by incorporating this information accordingly. In this way, we can preserve a system of terminology that effectively conveys a maximum amount of information regarding the designated cell types in a manner that distinguishes, as precisely as possible, differences that develop in the male germ line as prespermatogenesis and spermatogenesis progress.
ACKNOWLEDGMENT
The author thanks Drs. Paul Burgoyne, Brian Hermann, and Jon Oatley for reading the manuscript and providing valuable insights and suggestions, and Dr. Paul Burgoyne for providing personal historical accounts. The author is the Robert and Helen Kleberg Distinguished Chair in Cellular & Molecular Biology at the University of Texas at San Antonio.
REFERENCES
- McLaren A. Development of primordial germ cells in the mouse. Andrologia 1992; 24: 243 247. [DOI] [PubMed] [Google Scholar]
- Hayashi K. de Sousa Lopes SM, Surani MA. Germ cell specification in mice. Science 2007; 316: 394 396. [DOI] [PubMed] [Google Scholar]
- O WS, Short RV. Sex determination and differentiation in mammalian germ cells. Birth Defects Orig Artic Ser 1977; 13: 1 12. [PubMed] [Google Scholar]
- McCarrey JR, Abbott UK. Mechanisms of genetic sex determination, gonadal sex differentiation, and germ-cell development in animals. Adv Genet 1979; 20: 217 290. [DOI] [PubMed] [Google Scholar]
- McCarrey JR, Abbott UK. Chick gonad differentiation following excision of primordial germ cells. Dev Biol 1978; 66: 256 265. [DOI] [PubMed] [Google Scholar]
- Handel MA, Hunt PA, Kot MC, Park C, Shannon M. Role of sex chromosomes in the control of male germ-cell differentiation. Ann N Y Acad Sci 1991; 637: 64 73. [DOI] [PubMed] [Google Scholar]
- McLaren A. Germ cells and germ cell sex. Philos Trans R Soc Lond B Biol Sci 1995; 350: 229 233. [DOI] [PubMed] [Google Scholar]
- McLaren A. Germ and somatic cell lineages in the developing gonad. Mol Cell Endocrinol 2000; 163: 3 9. [DOI] [PubMed] [Google Scholar]
- Eddy EM. Germ plasm and the differentiation of the germ cell line. Int Rev Cytol 1975; 43: 229 280. [DOI] [PubMed] [Google Scholar]
- Eddy EM, Hahnel AC. Establishment of the germ line in mammals In: McLaren A, Wylie CC. (eds.), Current Problems in Germ Cell Differentiation, Symposium of British Society for Developmental Biology. Cambridge, UK: Cambridge University Press; 1983: 41 69. [Google Scholar]
- Ginsburg M, Snow MHL, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development 1990; 110: 521 528. [DOI] [PubMed] [Google Scholar]
- Handel MA, Eppig JJ. Sertoli cell differentiation in the testes of mice genetically deficient in germ cells. Biol Reprod 1979; 20: 1031 1038. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Buehr M, Koopman P, Rossant J, McLaren A. Cell-autonomous action of the testis-determining gene: Sertoli cells are exclusively XY in XX-XY chimaeric mouse testes. Development 1988; 102: 443 450. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Growth and development of mammalian oocytes in vitro. Arch Pathol Lab Med 1992; 116: 379 382. [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl 2000; 21: 776 798. [PubMed] [Google Scholar]
- Oakberg EF. Description of spermiogenesis in the mouse and its use in analysis of the cycle of the seminiferous epithelium and germ cell renewal. Am J Anat 1956; 99: 391 413. [DOI] [PubMed] [Google Scholar]
- Robaire B, Hermo L. Efferent ducts, epididymis, and vas deferens: structure, functions, and their regulation. : Knobil E, Neill J, et al. (eds.), The Physiology of Reproduction. Raven Press, New York; 1988: 999 1080. [Google Scholar]
- Ariel M, Cedar H, McCarrey JR. Developmental changes in methylation of spermatogenesis-specific genes include reprogramming in the epidymis. Nat Genet 1994; 7: 59 63. [DOI] [PubMed] [Google Scholar]
- Beaumont HM, Mandl AM. A quantitative study of primordial germ cells in the male rate. J Embryol Exp Morphol 1963; 11: 715 740. [PubMed] [Google Scholar]
- Holstein AF, Wartenberg H. On the cytomorphology of human spermatogenesis In: Schirren C. (ed.), Fortschritte Der Andrologie, vol. 1. Berlin: Grosse; 1970: 8 12. [Google Scholar]
- Wartenberg H, Holstein AF, Vossmeyer J. Cytology of the prenatal development of human gonads. II. Electronmicroscopic studies on the cytogenesis of gonocytes and foetal spermatogonia in the testis. Z Anat Entwicklungsgesch 1971; 134: 165 185. [PubMed] [Google Scholar]
- Wartenberg H. Spermatogenese-oogenese: ein cytomorphologischer vergleich. Versamml Der Anat Ges in Lausanne 1973; 68: 8 12. [PubMed] [Google Scholar]
- Hilscher W. Experimental asbestosis of the Wistar rat [in German]. Zentralbl Allg Pathol 1972; 116: 413 416. [PubMed] [Google Scholar]
- Clermont Y, Perey B. Quantitative study of the cell population of the seminiferous tubules in immature rats. Am J Anat 1957; 100: 241 267. [DOI] [PubMed] [Google Scholar]
- Sappsford CS. Changes in the cells of the sex cords and the seminiferous tubules during development of the testis of the rat and mouse. Austr J Zool 1962; 10: 178 192. [Google Scholar]
- Sappsford CS. Changes in the size of germ cell nuclei during the development of the tesits of the ram and rat. Austr J Zool 1964; 12: 127 149. [Google Scholar]
- Sappsford CS. The synthesis of DNA and nuclear protein by gonocytes in the testes of normal and X-irradiated rats. Austr J Exp Biol Med Sci 1965; 18: 653 664. [DOI] [PubMed] [Google Scholar]
- Novi AM, Saba P. An electron microscopic study of the development of rat testis in the first 10 postnatal days [in German]. Z Zellforsch 1968; 86: 313 326. [DOI] [PubMed] [Google Scholar]
- Hilscher W, Makoski HB. Histologische und autoradiographische untersuchungen zur praspermatogenese der ratte. Z Zellforsch 1968; 86: 327 350. [PubMed] [Google Scholar]
- Huckins C, Clermont Y. Evolution of gonocytes in the rat testis during late embryonic and post-natal life. Arch Anat Histo Embryol 1968; 51: 3434 354. [PubMed] [Google Scholar]
- Roosen-Runge EC, Leik J. Gonocyte degeneration in the postnatal male rat. Am J Anat 1968; 122: 275 300. [DOI] [PubMed] [Google Scholar]
- Wartenberg H. Comparative cytomorphologic aspects of the male germ cells, especially of the “gonia”. Andrologia 1976; 8: 117 130. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Kihara T, Tanimura T. Effect of ethinyl estradiol on the differentiation of mouse fetal testis. Teratology 1985; 32: 113 118. [DOI] [PubMed] [Google Scholar]
- Orth JM, Boehm R. Functional coupling of neonatal rat Sertoli cells and gonocytes in coculture. Endocrinology 1990; 127: 2812 2820. [DOI] [PubMed] [Google Scholar]
- Jorgensen N, Biwercman A, Hansen SW, Skakkebaek NE. Testicular cancer after vasectomy: origin from carcinoma in situ of the testis. Eur J Cancer 1993; 29A: 1062 1064. [DOI] [PubMed] [Google Scholar]
- van Dissel-Emiliani FM, de Boer-Brouwer M, Spek ER, van der Donk JA, de Rooji DG. Survival and proliferation of rat gonocytes in vitro. Cell Tissue Res 1993; 273: 141 147. [DOI] [PubMed] [Google Scholar]
- van Dissel-Emiliani FM, de Boer-Brouwer M, de Rooji DG. Effect of fibroblast growth factor-2 on Sertoli cells and gonocytes in coculture during the perinatal period. Endocrinology 1996; 137: 647 654. [DOI] [PubMed] [Google Scholar]
- Orth JM, Jester WF, Li LH, Laslett AL. Gonocyte-Sertoli cell interactions during development of the neonatal rodent testis. Curr Top Dev Biol 2000; 50: 103 124. [DOI] [PubMed] [Google Scholar]
- Orwig KE, Ryu BY, Avarbock MR, Brinster RL. Male germ-line stem cell potential is predicted by morphology of cells in neonatal rat testis. Proc Natl Acad Sci U S A 2002; 99: 11706 11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, Sharpe RM. Human ‘testicular dysgenesis syndrome': a possible model using in-utero exposure of the rat to dibutlyl phthalate. Hum Reprod 2003; 18: 1383 1394. [DOI] [PubMed] [Google Scholar]
- Li H, Kim KH. Retinoic acid inhibits rat XY gonad development by blocking mesonephric cell migration and decreasing the number of gonocytes. Biol Reprod 2004; 70: 687 693. [DOI] [PubMed] [Google Scholar]
- Lue Y, Jentsch JD, Wang C, Rao PN, Hikim AP, Salameh W, Swerdloff RS. XXY mice exhibit gonadal and behavioral phenotypes similar to Klinefelter syndrome. Endocrinology 2005; 146: 4148 4154. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Behboodi E, Hausler CL, Blash S, Ayres S, Azuma C, Echelard Y, Dobrinski I. Depletion of endogenous germ cells in male pigs and goats in preparation for germ cell transplantation. J Androl 2005; 26: 698 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNapoli L, Batchvarov J, Capel B. FGF9 promotes survival of germ cells in the fetal testis. Development 2006; 133: 1519 1527. [DOI] [PubMed] [Google Scholar]
- Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C Embryo Today 2009; 87: 1 26. [DOI] [PubMed] [Google Scholar]
- Thuillier R, Mazer M, Manku G, Boisvert A, Wang Y, Culty M. Interdependence of platelet-derived growth factor and estrogen-signaling pathways in inducing neonatal rat testicular gonocytes proliferation. Biol Reprod 2010; 82: 825 836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itman C, Wong C, Whiley PA, Fernando D, Loveland KL. TGFβ superfamily signaling regulators are differentially expressed in the developing and adult testis. Spermatogenesis 2011; 1: 63 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumond AL, Meistrich ML, Chiarini-Garcia H. Spermatogonial morphology and kinetics during testis development in mice: a high-resolution light microscopy approach. Reproduction 2011; 142: 145 155. [DOI] [PubMed] [Google Scholar]
- Manku G, Wing SS, Culty M. Expression of the ubiquitin proteasome system in neonatal rat gonocytes and spermatogonia: role in gonocyte differentiation. Biol Reprod 2012; 87: 44. [DOI] [PubMed] [Google Scholar]
- Garcia TX, Defalco T, Capel B, Hofmann MC. Constitutive activation of NOTCH1 signaling in Sertoli cells causes gonocyte exit from quiescence. Dev Biol 2013; 377 (1): 188 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova-Tian-Shanskaia AG, Patkin EL. Change in the gonocytic nuclei at different stages of their differentiation in early human female embryos [in Russian]. Arkh Anat Gistol Embriol 1978; 74: 91 97. [PubMed] [Google Scholar]
- Kafri T, Ariel M, Brandeis M, Shemer R, Urven L, McCarrey J, Cedar H, Razin A. Developmental pattern of gene-specific DNA methylation in the mouse embryo and germ line. Genes Dev 1992; 6: 705 714. [DOI] [PubMed] [Google Scholar]
- Santos F, Dean W. Epigenetic reprogramming during early development in mammals. Reproduction 2004; 127: 643 651. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Zhang C, Okada Y, Okamoto K. Erasure of methylation imprint at the promoter and CTCF-binding site upstream of H19 in human testicular germ cell tumors of adolescents indicate their fetal germ cell origin. Oncogene 2006; 25: 3225 3236. [DOI] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Ogonuki N, Miki H, Inoue K, Morimoto T, Morimoto H, Ogura A, Shinohara T. Heritable imprinting defect caused by epigenetic abnormalities in mouse spermatogonial stem cells. Biol Reprod 2009; 80: 518 527. [DOI] [PubMed] [Google Scholar]
- Lee J, Shinohara T. Epigenetic modifications and self-renewal regulation of mouse germline stem cells. Cell Res 2011; 21: 1164 1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sasaki H. Genomic imprinting in mammals: its life cycle, molecular mechanisms and reprogramming. Cell Res 2011; 21: 466 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 2006; 133: 1495 1505. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Nabeshima Y, Nakagawa T. Stem cell heterogeneity: actual and potential stem cell compartments in mouse spermatogenesis. Ann N Y Acad Sci 2007; 1120: 47 58. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sada A, Yoshida S, Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol 2009; 336: 222 231. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 2010; 328: 62 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto H, Kanatsu-Shinohara M, Takashima S, Chuma S, Nakatsuji N, Takehashi M, Shinohara T. Phenotypic plasticity of mouse spermatogonial stem cells. PLoS One 2009; 4: e7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Ishii K, Kanatsu-Shinohara M. Unstable side population phenotype of mouse spermatogonial stem cells in vitro. J Reprod Dev 2011; 57: 288 295. [DOI] [PubMed] [Google Scholar]
- Grisanti L, Falciatori I, Grasso M, Dovere L, Fera S, Muciaccia B, Fuso A, Berno V, Boitani C, Stefanini M, Vicini E. Identification of spermatogonial stem cell subsets by morphological analysis and prospective isolation. Stem Cells 2009; 27: 3043 3052. [DOI] [PubMed] [Google Scholar]
- Zheng K, Wu X, Kaestner KH, Wang PJ. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev Biol 2009; 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey P, McLean DJ, McMahan A, Walter CA, McCarrey JR. Enhanced genetic integrity in mouse germ cells. Biol Reprod 2013; 88: 1 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilscher W. Kinetik der praspermatogenese und spermatogenese. Verh Anat Ges 1974; 68: 39 62. [PubMed] [Google Scholar]
- Hilscher W, Hilscher B. Comparative studies on oogenesis and prespermatogenesis in the Wistar rat under normal and pathological conditions. Ann Biol Anim Biochim Biophys 1973; 13: 127 136. [Google Scholar]
- Hilscher B, Hilscher W, Delbruck G, Lerouge-Bénard B. Autoradiographische bestimmung der S-phasen-dauer der gonocyten bei der Wistarratte durch einfach- und doppel-markierung. Z Zellforsch 1972; 125: 229 251. [PubMed] [Google Scholar]
- Hilscher B, Hilscher W, Bulthoff-Ohnolz B, Kramer U, Birke A, Pelzer H, Gauss G. Kinetics of gametogenesis: I. Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell Tissue Res 1974; 154: 443 470. [DOI] [PubMed] [Google Scholar]
- Byskov AG. Differentiation of mammalian embryonic gonad. Physiol Rev 1986; 66: 71 117. [DOI] [PubMed] [Google Scholar]
- Hilscher W, Hilscher B. Details of the female and male pathway of the Keimbahn determined by enzyme histochemical and autoradiographic studies. Basic Appl Histochem 1990; 34: 21 34. [PubMed] [Google Scholar]
- Miles DC, van den Bergen JA, Sinclair AH, Western PS. Regulation of the female mouse germ cell cycle during entry into meiosis. Cell Cycle 2010; 9: 408 418. [DOI] [PubMed] [Google Scholar]
- Hilscher B, Engemann A. Hitological and morphometric studies on the kinetics of germ cells and immature Sertoli cells during human prespermatogenesis. Andrologia 1992; 24: 7 10. [DOI] [PubMed] [Google Scholar]
- Hilscher W. The genetic control and germ cell kinetics of the female and male germ line in mammals including man. Hum Reprod 1991; 6: 1416 1425. [DOI] [PubMed] [Google Scholar]
- McCarrey JR. Development of the germ cell. : Desjardins C, Ewing LL. (eds.), Cell and Molecular Biology of the Testis. Oxford: Oxford University Press; 1993: 58 89. [Google Scholar]
- Skinner MK, Griswold MD. Sertoli Cell Biology. Amsterdam: Elsevier Academic Press; 2005. [Google Scholar]
- Burgoyne PS. The role of the mammalian Y chromosome in spermatogenesis. Development 1987; 101 (suppl): 133 141. [DOI] [PubMed] [Google Scholar]
- McLaren A, Monk M. X-chromosome activity in the germ cells of sex-reversed mouse embryos. J Reprod Fertil 1981; 63: 533 537. [DOI] [PubMed] [Google Scholar]
- McLaren A, Burgoyne PS, Daughterless X. Sxr/Y Sxr mice. Genet Res 1983; 42: 345 349. [DOI] [PubMed] [Google Scholar]
- Levy ER, Burgoyne PS. The fate of XO germ cells in the testes of XO/XY and XO/XY/XYY mouse mosaics: evidence for a spermatogenesis gene on the mouse Y chromosome. Cytogenet Cell Genet 1986; 42: 208 213. [DOI] [PubMed] [Google Scholar]
- McLaren A, Buehr M. Development of mouse germ cells in cultures of fetal gonads. Cell Differ Dev 1990; 31: 185 195. [DOI] [PubMed] [Google Scholar]
- Enders GC. Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic day 11 to adult in male and female mice. Dev Biol 1994; 163: 331 340. [DOI] [PubMed] [Google Scholar]
- Reijo R, Seligman J, Dinulos MB, Jaffe T, Brown LG, Disteche CM, Page DC. Mouse autosomal homolog of DAZ, a candidate male sterility gene in humans, is expressed in male germ cells before and after puberty. Genomics 1996; 35: 346 352. [DOI] [PubMed] [Google Scholar]
- Nikolova DB, Martinova YS, Seidensticker M, Bellvé AR. Leukaemia inhibitory factor stimulates proliferation of prospermatogonial stem cells. Reprod Fertil Dev 1997; 9: 717 721. [DOI] [PubMed] [Google Scholar]
- Seligman J, Page DC. The Dazh gene is expressed in male and female embryonic gonads before germ cell sex differentiation. Biochem Biophys Res Commun 1998; 245: 878 882. [DOI] [PubMed] [Google Scholar]
- De Felici M. Regulation of primordial germ cell development in the mouse. Int J Dev Biol 2000; 44: 575 580. [PubMed] [Google Scholar]
- Farini D, Scaldaferri ML, Iona S, La Sala G, De Felici M. Growth factors sustain primordial germ cell survival, proliferation and entering into meiosis in the absence of somatic cells. Dev Biol 2005; 285: 49 56. [DOI] [PubMed] [Google Scholar]
- Lefevre C, Mann JR. RNA expression microarray analysis in mouse prospermatogonia: identification of candidate epigenetic modifiers. Dev Dyn 2008; 237: 1082 1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Ross A, Capel B. Expression and functional analysis of Gm114, a putative mammalian ortholog of Drosophila bam. Dev Biol 2008; 318: 73 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Tanaka T, Hosokawa M, Reuter M, Stark A, Kato Y, Kondoh G, Okawa K, Chujo T, Suzuki T, Hata K, Martin SL, et al. The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev Cell 2009; 17: 775 787. [DOI] [PubMed] [Google Scholar]
- van der Heijden GW, Castaneda J, Bortvin A. Bodies of evidence—compartmentalization of the piRNA pathway in mouse fetal prospermatogonia. Curr Opin Cell Biol 2010; 22: 752 757. [DOI] [PubMed] [Google Scholar]
- Abe M, Tsai SY, Jin SG, Pfeifer GP, Szabo PE. Sex-specific dynamics of global chromatin changes in fetal mouse germ cells. PLoS One 2011; 6: e23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Lee DH, Szabo PE. More than insulator: multiple roles of CTCF at the H19-Igf2 imprinted domain. Front Genet 2012; 3: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo D, Cheng Q, O'Connor AE, Deboer KD, Lo CY, Beaulieu E, De Seram M, Hobbs RM, O'Bryan MK, Morand EF. Glucocorticoid-induced leucine zipper (GILZ) regulates testicular FOXO1 activity and spermatogonial stem cell (SSC) function. PLoS One 2013; 8: e59149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet 2013; 14: 204 220. [DOI] [PubMed] [Google Scholar]
- Smith ZD, Chan MM, Mikkelsen TS, Gu H, Gnirke A, Regev A, Meissner A. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature 2012; 484: 339 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A, Cedar H. DNA methylation and embryogenesis. EXS 1993; 64: 343 357. [DOI] [PubMed] [Google Scholar]
- Seisenberger S, Andrews S, Krueger F, Arand J, Walter J, Santos F, Popp C, Thienpont B, Dean W, Reik W. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol Cell 2012; 48: 849 862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surani MA, Hajkova P. Epigenetic reprogramming of mouse germ cells toward totipotency. Cold Spring Harb Symp Quant Biol 2010; 75: 211 218. [DOI] [PubMed] [Google Scholar]
- Hajkova P. Epigenetic reprogramming in the germline: towards the ground state of the epigenome. Philos Trans R Soc Lond B Biol Sci 2011; 366: 2266 2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles KM, Chan D, La Salle S, Oakes CC, Trasler JM. Critical period of non-promoter DNA methylation acquisition during prenatal male germ cell development. PLoS One 2011; 6: e24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer CB, Kiefer CM, Yang TP, McCarrey JR. Ontogeny of a demethylation domain and its relationship to activation of tissue-specific transcription. Biol Reprod 2004; 71: 837 844. [DOI] [PubMed] [Google Scholar]
- Bartolomei MS, Ferguson-Smith AC. Mammalian genomic imprinting. Cold Spring Harb Perspect Biol 2011; 3: a002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal E, Yamazaki Y, Ingale P, Bartolomei MS, Yanagimachi R, McCarrey JR. Primary epimutations introduced during intracytoplasmic sperm injection (ICSI) are corrected by germline-specific epigenetic reprogramming. Proc Natl Acad Sci U S A 2012; 109: 4163 4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Yang GJ, McCarrey JR, Bartolomei MS. The H19 methylation imprint is erased and re-established differentially on the parental alleles during male germ cell development. Hum Mol Genet 2000; 9: 2885 2894. [DOI] [PubMed] [Google Scholar]
- Bellvé AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse, isolation and morphological characterization. J Cell Biol 1977; 74: 68 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel BR, Amarose AP, Hackert EM. Calendar of gametogenic development in the prepuberal male mouse. Science 1961; 134: 832 833. [DOI] [PubMed] [Google Scholar]
- Kramer JM, Erickson RP. Developmental program of PGK-1 and PGK-2 isozymes in spermatogenic cells of the mouse: specific activities and rates of synthesis. Dev Biol 1981; 87: 37 45. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, Hughes TH, Bruce WR. Alteration of epididymal sperm transport and maturation in mice by oestrogen and testosterone. Nature 1975; 145 147. [DOI] [PubMed]
- Beaudoin AR. Embryology and teratology In: Baker HJ, JR Lindsey, Weisbroth SH. (eds.), The Laboratory Rat, vol. II. New York: Academic Press; 1980: 75 101. [Google Scholar]
- Beddington R. The origin of the foetal tissues during gastrulation in the rodent In: Johnson MH. (ed.), Development in Mammals, vol. 5. New York: Elsevier; 1983: 1 32. [Google Scholar]
- Kemper CH, Peters PWJ. Migration and proliferation of primordial germ cells in the rat. Teratology 1987; 36: 117 124. [DOI] [PubMed] [Google Scholar]
REBUTTAL FROM MARTINE CULTY
John McCarrey's article constitutes a thorough review on male and female rodent germ cell development, describing in detail prespermatogenesis and spermatogenesis. He proposes to call prospermatogonia rather than gonocytes the fetal to neonatal male germ cells between PGCs and spermatogonia. As explained in both reviews, these phases present similarities, such as being periods of intense DNA methylation, but they also present differences that have led to distinguishing three cell types in rodents and humans. We agree that the better understanding of these phases should be reflected in using more precise terminologies to identify the cells, and we both propose adding letters in front of the cell names, including mitotic, transitional, and, in my case, quiescent, to help delineate these cell types in the literature. Therefore, the only difference between our lines of thought seems to be that I recommend using the historical term gonocyte, whereas he proposes shifting to the newer term prospermatogonia.
Without going into detail about the reasoning behind keeping the term gonocyte presented in my review, I will respond to two points, referred to as “deficiencies” by John McCarrey:
Regarding the “failure of this term [gonocyte] to implicitly convey the intended cell,” this is clearly not the case, since the term “gonocyte” chosen by Clermont and Perey in the 1950s [24] contains the prefix “gon(o),” indicating “seed, semen” in ancient Greek, and “cyte,” meaning “cell.” Moreover, Dr. McCarrey extends the meaning of terms beyond their definition, such as when stating that cyte conveys the indication of a “meiotic cell,” while it simply stands for cell. He argues that calling a cell “prespermatogonia/um” would confuse the cell types with the process of prespermatogenesis. Surely, anybody with minimal biology/English knowledge can distinguish these words by recognizing the suffix “-esis” as qualifying a process and not a cell! Moreover, “pre” has a single meaning in both Latin and Greek, whereas “pro” has different meanings. Therefore, pre should be less confusing than pro as a prefix used by people preferring a term related to spermatogonia.
Regarding the need for using a nomenclature more informative about the functional and/or mitotic status of cells, I support this proposal, but I believe it can be done independently of the name chosen for the cells. I agree that using M (mitotic) and T (transitional) would help to specify the first and last periods of gonocyte development. However, I propose Q (quiescent) instead of “T1” for cells in “the first state of transition in mitotic arrest.”
In summary, we agree with Drs. Hilscher, Wartenberg, McLaren, and many authors cited in my review that complementing the name of the cells with adjectives reflecting their status would be helpful. However, in view of the lack of consensus for newer terminologies and the need for a better characterization of the cells, I propose keeping the original name gonocyte, encompassing a specific location, morphological appearance, and period of development, applicable to both humans and rodents. Independent of the term favored, one has to remember that these phases overlap.
Supplementary Material
Footnotes
Results described in this review were generated in part on the basis of National Institutes of Health funding.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


