ABSTRACT
The epithelium that lines the epididymal duct establishes the optimal milieu in which spermatozoa mature, acquire motility, and are stored. This finely tuned environment also protects antigenic sperm against pathogens and autoimmunity, which are potential causes of transient or permanent infertility. The epididymal epithelium is pseudostratified and contains basal cells (BCs) that are located beneath other epithelial cells. Previous studies showed that in the mouse epididymis, BCs possess macrophage-like characteristics. However, we previously identified a dense population of cells belonging to the mononuclear phagocyte (MP) system (comprised of macrophages and dendritic cells) in the basal compartment of the mouse epididymis and showed that a subset of MPs express the macrophage marker F4/80. In the present study, we evaluate the distribution of BCs and MPs in the epididymis of transgenic CD11c-EYFP mice, in which EYFP is expressed exclusively in MPs, using antibodies against the BC marker keratin 5 (KRT5) and the macrophage marker F4/80. Immunofluorescence labeling for laminin, a basement membrane marker, showed that BCs and most MPs are located in the basal region of the epithelium. Confocal microscopy showed that in the initial segment, both BCs and MPs project intraepithelial extensions and establish a very intricate network. Flow cytometry experiments demonstrated that epididymal MPs and BCs are phenotypically distinct. BCs do not express F4/80, and MPs do not express KRT5. Therefore, despite their proximity and some morphological similarities with peritubular macrophages and dendritic cells, BCs do not belong to the MP system.
Keywords: basal cells, dendritic cells, epididymis, immunology, macrophages, male reproductive tract, reproductive immunology
The basal region of the murine epididymal duct is heavily populated by basal cells, macrophages, and dendritic cells, which are morphologically and phenotypically distinct.
INTRODUCTION
Immature spermatozoa released from the testis gain the ability to become motile and to recognize and fertilize the oocyte during a series of events mediated by a tightly regulated exchange of fluids, solutes, proteins, lipids, and extracellular vesicles. This complex and sequential maturation process is controlled by the highly specialized, pseudostratified epithelium that lines the epididymis, a long and convoluted tube located between the testis and the vas deferens [1–7]. The epididymal lumen is continuous, but the peritubular interstitium is segmented and confers functional and morphological specificities to each segment. The number of segments varies among species, but they are generally grouped into four major anatomical regions named, from the most proximal to the most distal, the initial segment (IS), the caput, the corpus, and the cauda. The epididymal epithelium contains a variable number of several cell types described as principal, basal, narrow, clear, and apical cells [4–6, 8–12]. In addition, the epididymal epithelium is the major anatomical component of the blood-epididymis barrier (BEB). The BEB creates a physical, physiological, and immunological separation between the “blood” compartment (the basolateral side of the epithelium) and the luminal content [13–18]. Indeed, due to their postmeiotic origin and their arrival long after the maturation of the immune system, sperm are autoantigenic and must avoid destruction by the immune system of the host [19, 20].
Among epithelial cells, principal cells reside along the entire length of the epididymal tubule and are primarily responsible for fluid and nutrient exchange and protein secretion [1, 2]. They are typically identified by their apical stereocilia, in which the water channel aquaporin 9 (AQP9) is abundantly expressed [21–23]. Narrow cells located in the IS and clear cells located in the caput, corpus, and cauda express high levels of vacuolar H+-ATPase (V-ATPase) and secrete protons to regulate the luminal pH, a process critical for sperm maturation and viability [24–31]. Basal cells (BCs) reside in all regions of the epididymal duct and are generally identified by their round nuclei located at the base of epithelium and beneath adjacent epithelial cells [4, 8, 32, 33]. They exhibit segment-specific morphological characteristics, varying from a dome-shaped cell located beneath epithelial cells [34, 35] to cells that extend a long and narrow body process projecting into the lumen between adjacent epithelial cells [36]. Epididymal BCs specifically display high expression of cyclooxygenase-1 [36–38], claudin-1 [36, 39], and keratin (KRT5) [40].
Contrasting with the generally well-documented description and functional characterization of major subsets of epithelial cells, the nature and function of epididymal immune cells is relatively confusing [19]. Interstitial and peritubular epididymal macrophages, which are thought to be the most prominent leukocytes in the epididymis, have been identified on the basis of ultrastructural observation (electron microscopy) and detection of macrophage markers by immunohistochemistry. However, some confusion has arisen with respect to the exact nature of peritubular macrophages and BCs [12, 20, 41–47]. The expression of macrophage markers in BCs has been reported, suggesting a lineage relationship between macrophages and BCs as well as immunological functions for the latter [41–43]. Furthermore, the description of an abundant population of F4/80-positive cells displaying a dendriform appearance in the proximal epididymis of the mouse highlights the complexity between intraepithelial macrophages and BCs [45]. Our previous work further suggested that the basal compartment of the epithelium might, in fact, be more complex than generally portrayed in the literature. Indeed, using transgenic mouse models expressing fluorescent proteins under the control of the CD11c (integrin alpha X chain) and CX3CR1 (fractalkine receptor), two markers of mononuclear phagocytes (MPs), we described a very abundant and heterogeneous population of immune cells (macrophages and dendritic cells) in the peritubular and interstitial compartments of the mouse epididymis [48]. Owing to the notorious heterogeneity of the MP system and the long-standing controversy that surrounds the respective origin, function, and nomenclature of macrophages and dendritic cells [49–53], we now prefer to use the less restrictive term “epididymal mononuclear phagocytes” (eMPs) to describe these cells. We found that peritubular eMPs establish various degrees of interaction with the epithelium—for instance, by projecting slender dendrites toward the luminal compartment in the proximal epididymis (a property of dendritic cells described in other mucosal systems such as the small intestine [54]) or simply by spreading in the basal compartment in the more distal segments. Facilitating confusion, this morphological characteristic is strikingly similar to the property of epididymal BCs, which occasionally project narrow extensions across apical tight junctions to sample the luminal content [36].
In the present study, we hypothesized that despite their extremely close peritubular colocalization and intriguing morphological similarities, epididymal BCs and MPs constitute two morphologically, phenotypically, and functionally distinct cell types. We identified carefully and unequivocally, using fluorescence microscopy and flow cytometry, the morphological and phenotypical characteristics of BCs and MPs in the mouse epididymis. The close interaction between immune and sensu stricto epithelial cells as well as the possible functional implications of our findings in the unraveling of the mechanisms that control sperm maturation in the epididymis are discussed.
MATERIALS AND METHODS
Mice
Adult male C56BL/6J wild-type, CD11c-EYFP [55], and CX3CR1-GFP [56] mice were purchased from The Jackson Laboratory. CD11c is the integrin alpha X chain, encoded by Itgax. CX3CR1 is the receptor that binds fractalkine (CX3CL1). Both CD11c-EYFP and CX3CR1-GFP can be used to identify eMPs in situ. The genotype of transgenic mice was confirmed by PCR analysis of tail-snip DNA. All mice were maintained free of common rodent pathogens and on a standard lab chow diet. Mouse protocols were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee in accordance with National Institutes of Health, Department of Agriculture, and Accreditation of Laboratory Animal Care requirements.
Tissue Fixation and Preparation
Mice were anesthetized with sodium pentobarbital (50 mg/kg i.p.), and whole-body fixation was performed by perfusion through the left ventricle with periodate-lysine-paraformaldehyde (PLP) or 4% paraformaldehyde (PFA) in PBS as described previously [36, 48]. Epididymides were then harvested and further fixed by immersion in PLP or PFA for 3 h at room temperature. Tissues were washed in PBS and preserved at 4°C in PBS containing 0.02% sodium azide until sectioning. Fixed tissues were cryoprotected in PBS with 30% sucrose overnight, embedded in Tissue-Tek Optimal Cutting Temperature compound (Sakura Finetek), and frozen on a cutting block. Epididymides were sectioned in a CM3050S cryostat (Leica Microsystems) at thicknesses of 5–50 μm, placed onto Superfrost Plus microscope slides (Fisher Scientific), and stored at 4°C or −20°C until use.
Antibodies, Immunofluorescence, and Microscopy Analyses
Epididymal MPs were identified by the expression of CD11c-EYFP, and a monoclonal anti-F4/80 antibody (BM8; eBioscience) was used to label macrophages as previously described [48]. For BC identification, we used a polyclonal rabbit anti-KRT5 antibody (Abcam), as we have previously reported that the protein is specific for BCs [40]. A polyclonal rabbit anti-laminin antibody (Sigma-Aldrich) was used to label the basal lamina, which represents a major component of the basement membrane. Single- and double-immunofluorescence labeling were performed as described previously [36, 48]. Briefly, epididymal cryosections were hydrated in PBS for 5 min, followed by an antigen retrieval step with PBS containing 1% SDS for 4 min. Nonspecific antibody binding was minimized by incubating sections in 1% bovine serum albumin in PBS for 30 min at room temperature. Samples were then incubated with primary antibodies for 90 min at room temperature or overnight at 4°C. Sections were washed and then incubated with the appropriate affinity-purified secondary antibody (Jackson ImmunoResearch Laboratories) for 60 min at room temperature. Washed slides were mounted in Vectashield medium (Vector Labs) with 4′,6-diamidino-2-phenylindole. Primary and secondary antibodies were diluted in Antibody Diluent (Dako). Conventional epifluorescence images, including large-field-of-view images and z stacks, were obtained using a motorized Nikon Eclipse 90i microscope with NIS-Elements (Nikon Instruments) and an Orca-100 CCD camera (Hamamatsu). Confocal images were acquired on a Nikon A1R confocal microscope with NIS-Elements. Digital images were processed with NIS Elements, Volocity 6 (Perkin Elmer), ImageJ (http://imagej.nih.gov/ij/), Adobe Photoshop, and Apple QuickTime. Visualizing MPs in situ requires the use of relatively thick tissue sections (thicknesses of up to 50 μm). The extended depth of field in two-dimensional (2D) images was obtained by performing a maximum-intensity projection (MIP) of epifluorescence and confocal z stacks. Occasionally, MIP of complex three-dimensional (3D) structures generates colocalization artifacts (yellow areas). Careful analysis of all the z stacks used in the present article showed that the red and green colors did not colocalize in individual z planes; consequently, yellow areas resulted from processing artifacts.
Flow Cytometry
Epididymides from five C57BL/6J adult mice were dissected and divided into three regions representing the proximal epididymis (IS and proximal caput; region 1), central epididymis (distal caput and corpus; region 2), and distal epididymis (cauda; region 3), respectively. Individual cell suspensions were prepared as previously described [48]. Briefly, dissected epididymis fragments were minced in RPMI medium containing collagenase type I and type II (1 mg/ml each). Tissues were incubated for 45 min at 37°C with gentle shaking. Cell suspensions were passed through a 70-μm nylon mesh to remove cell aggregates, washed in PBS with 0.5% bovine serum albumin and 2 mM ethylenediaminetetraacetic acid, and stored on ice until processing. For negative selection of unwanted cells, suspensions were incubated with a cocktail of phycoerythrin (PE)-labeled monoclonal antibodies against T cells (CD90-PE, 53-2.1), B cells (B220-PE, RA3-6B2), natural killer cells (CD49b-PE, DX5; NK1.1-PE, PK136), and granulocytes (Ly-6G-PE, 1A8) and were depleted using anti-PE microbeads and a magnetic-activated cell sorting column (Miltenyi Biotec) as previously described [48]. MP-enriched cell suspensions were then labeled with CD11b-APC-Cy7 (M1/70), CD11c-Alexa 700 (HL3), F4/80-PE-Cy7 (BM8), and KRT5 plus APC-conjugated secondary antibody. Antibodies were purchased from BD Biosciences and eBioscience. Because the KRT5 epitope is intracellular, cells were permeabilized and fixed using Cytofix/Cytoperm reagents (BD Biosciences) before the addition of antibodies, following the manufacturer's instructions. Flow cytometry data were acquired in triplicate on an LSR II Flow Cytometer (BD Biosciences) and analyzed with FlowJo 9 (Tree Star).
RESULTS
BCs and MPs Are Present in All Mouse Epididymal Segments
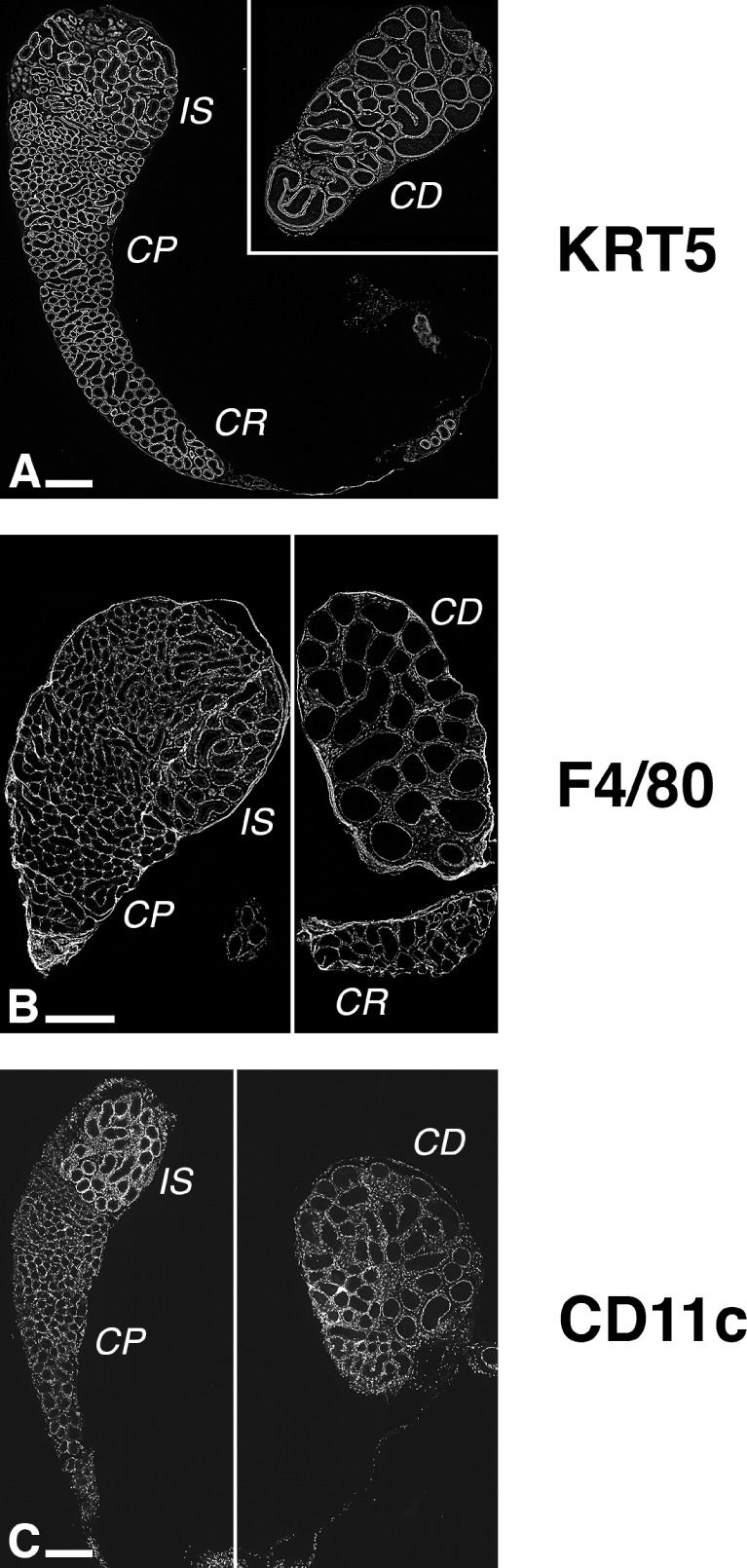
The expression of KRT5, F4/80, and CD11c, which are used traditionally as markers of BCs, macrophages, and dendritic cells, respectively, has been previously reported in the epididymis [40, 42, 43, 45, 48]. Figure 1 shows low-magnification fluorescence micrographs displaying the overall expression patterns of KRT5, F4/80, and CD11c in mouse epididymis sections (for high-resolution images, refer to Supplemental Figs. S1–S3; all Supplemental Data are available online at www.biolreprod.org). BCs (KRT5-positive; Fig. 1A and Supplemental Fig. S1), macrophages (F4/80-positive; Fig. 1B and Supplemental Fig. S2), and dendritic cells (CD11c-postive; Fig. 1C and Supplemental Fig. S3) were located in all epididymal segments (the narrowest region of the corpus is occasionally absent from the sections), from the most proximal region, the IS, to the caput, the corpus, and the most distal region, the cauda. BCs lined the base of the entire epididymal tubule, and the level of expression of KRT5 appeared to be similar in all segments. The F4/80 signal was more heterogeneous, probably reflecting variable levels of expression of the protein in different subsets of peritubular and interstitial macrophages. In contrast, the network of peritubular CD11c-positive cells appeared to be more dense in the IS than in more distal segments, due to the IS-specific presence of abundant intraepithelial dendrites. These data show that BCs are a relatively well-defined and homogeneous population of epithelial cells, whereas MPs, which include macrophages and dendritic cells, represent several subsets of cells with heterogeneous tissue distribution and morphologies.
FIG. 1.
Distribution of BCs, macrophages, and dendritic cells in the adult mouse epididymis. C57BL/6J mouse epididymis sections were labeled with an anti-KRT5 (A) or an anti-F4/80 (B) antibody. CD11c-EYFP mice express EYFP under the control of Itgax (coding for CD11c) promoter, a widely used marker of mouse dendritic cells (C). These low-magnification images reveal that BCs (KRT-positive), macrophages (F4/80-postive), and dendritic cells (CD11c-positive) populate the entire mouse epididymis. BCs line the base of the epididymal duct, but F4/80 and CD11c highlight several subsets of MPs in the peritubular region as well as in the interstitium. See also Supplemental Figures S1–S3. CP, caput; CR, corpus; CD, cauda. Bars = 500 μm.
Most Peritubular MPs Reside on the Epithelial Side of the Basement Membrane
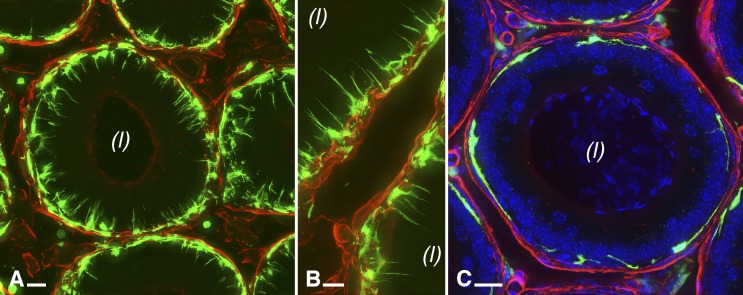
Fluorescence microscopy was used to visualize more precisely the localization of tubule-associated CD11c-positive and CX3CR1-positive MPs (Fig. 2). In the IS, several MPs (green) were closely associated with the epithelium and sent intraepithelial dendrites toward the lumen (Fig. 2, A and B). The cellular bodies of these peritubular MPs were located on the epithelial side of the basement membrane (labeled for laminin in red). Some MPs were also located in the interstitium. In the caput (Fig. 2C) as well as in all distal epididymal segments (not shown), peritubular MPs located between the basement membrane and epithelial cells were also detected, but they did not project intraepithelial dendrites toward the lumen.
FIG. 2.
Visualization of laminin and MPs in the mouse epididymis. CD11c-EYFP (A; green), and CX3CR1-GFP (B and C; green) are expressed in MPs, whereas the basal lamina, which is one component of the basement membrane, was immunolabeled with an anti-laminin antibody (red). Laminin is also abundant in the basement membrane of capillaries. Cross-sections of the epididymal tubule in the IS (A and B) and of the epididymal tubule in the distal caput (C) are shown. Nuclei were labeled with 4′,6-diamidino-2-phenylindole. All images are MIPs of 25-μm z stacks. (l), lumen. Bars = 10 μm.
Epididymal BCs and MPs Are Morphologically Distinct
Double-immunofluorescence labeling of wild-type epididymal cryosections for KRT5 and F4/80 (Fig. 3) and single-immunofluorescence labeling of CD11c-EYFP sections for KRT5 (Fig. 4) were carried out, and high-magnification images were obtained to determine the morphological details and localization relationship among the three cell types.
FIG. 3.
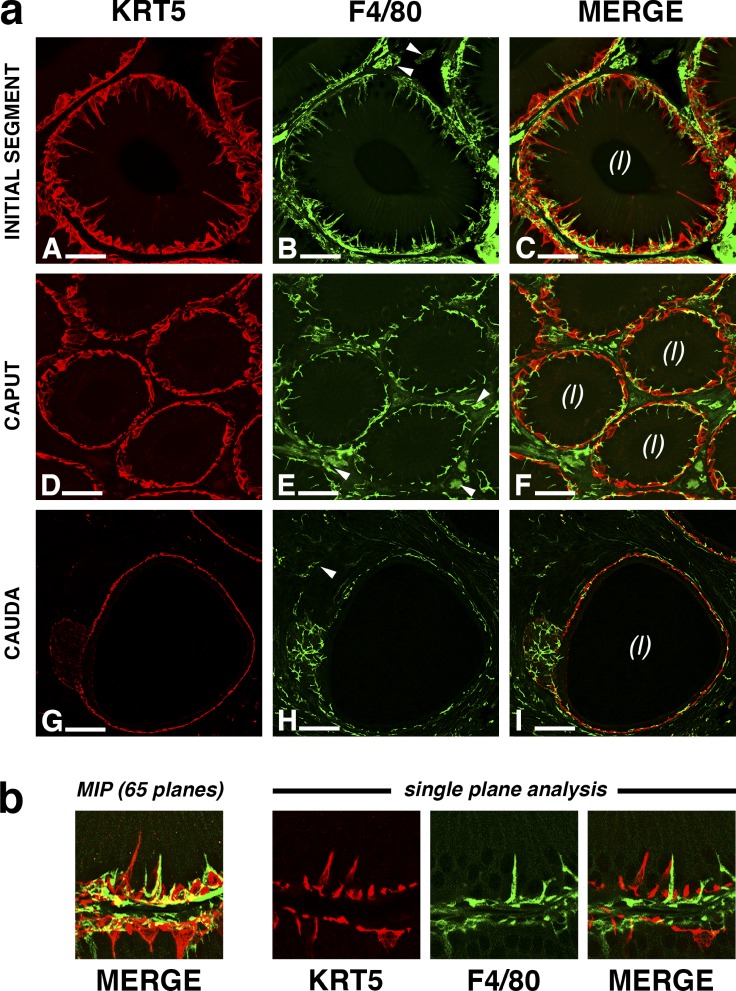
Double-immunolabeling of KRT5 (red) and F4/80 (green) in the mouse epididymis. a) Cross-section of the epididymal tubule in the IS (A–C) shows that BCs (A) sit at the base of the epithelium and occasionally project a single, narrow extension toward the lumen. A strong F4/80 immunoreactivity is also detected at the base of the epithelium (B); however, each F4/80-positive cell projects several slender intraepithelial dendrites. The merged picture (C) shows that although both KRT5- and F4/80-positive cells are present in the basolateral region of the epithelium, they represent two clearly morphologically distinct cell types. Cross-sections of the tubule in more distal regions of the epididymis (D–I) show that BCs do not extend projections toward the lumen but continue to populate the basal region (D and G). F4/80-positive MPs extend short, intraepithelial dendrites in the proximal caput (E) but remain exclusively basal in the cauda (H). Merged images (F and I) show that similar to the pattern observed in the IS, KRT5 and F4/80 are expressed in neighboring but distinct cells. Arrowheads indicate F4/80-positive cells that are located in the interstitium. (l), lumen. Bars = 20 μm (A–C), 30 μm (D–F), and 60 μm (G–I). b) Creating 2D images from a z stack may cause colocalization artifacts. An enlarged area of aC is shown (left) The presence of yellow areas in the 2D image obtained by performing an MIP of the z stack might suggest some degree of colocalization of KRT5 and F4/80. However, when the z stack is analyzed at the single-plane level (right), KRT5 and F4/80 do not colocalize, indicating that the yellow areas visible in MIPs represent processing artifacts.
FIG. 4.
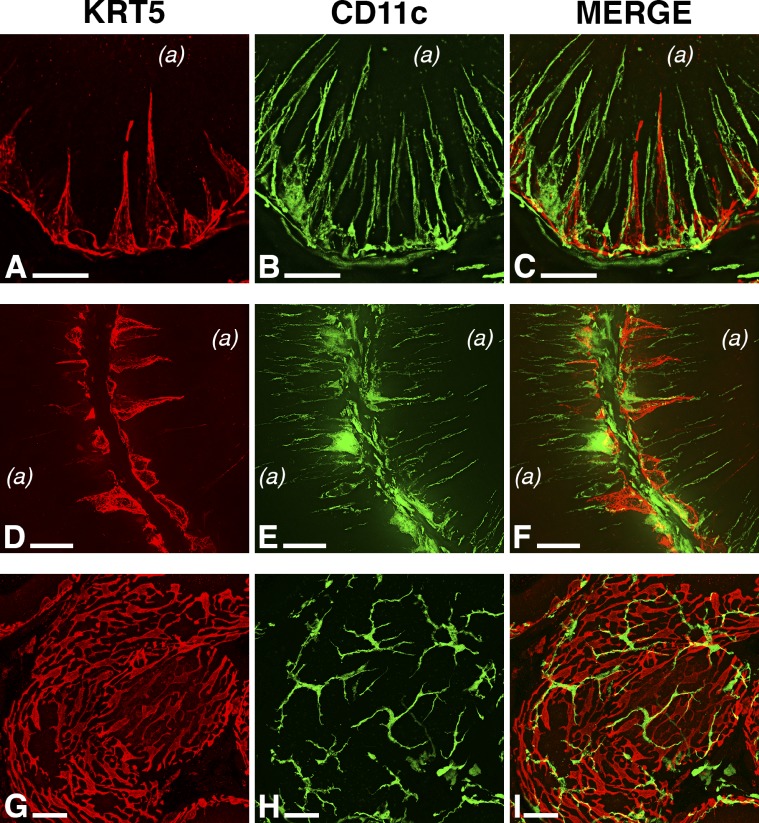
Expression of KRT5 and CD11c in the mouse epididymis. The distribution of KRT5-positive BCs is described in Figure 2. The distribution pattern of CD11c-positive cells (expressing EYFP; green) resembles the immunolabeling pattern of F4/80-positive cells described in Figure 2, although the intraepithelial CD11c-positive cells appear to be more numerous than the F4/80-positive cells. In the IS (A–C), CD11c-positive cells project multiple intraepithelial dendrites, which are occasionally adjacent to BC extensions. High-resolution fluorescence microscopy images demonstrate that KRT5 and CD11c are expressed in morphologically distinct cell types (see also Supplemental Movie S1). In the caput (D–F) and cauda (G–I), MP intraepithelial dendrites and BC projections are absent, whereas cellular bodies of both BCs and CD11c-positive MPs line the base of the epididymal epithelium. (l), lumen; arrowheads, interstitial CD11c-positive cells. Bars = 20 μm (A–C), 25 μm (D–F), and 40 μm (G–I).
In all regions of the mouse epididymis, KRT5 (red) and F4/80 (green) did not colocalize (Fig. 3). KRT5-positive BCs and F4/80-positive macrophages were mostly located, as expected, at the base of the epithelium. The interstitial compartment contained a subset of F4/80-positive cells (arrowheads) that were morphologically distinct from the epithelium-associated MPs. In the IS (Fig. 3a, A–C), both KRT5-positive BCs and F4/80-positive cells were extremely close to each other and projected long and slender intraepithelial extensions toward the luminal compartment. In agreement with our previous study [48], each F4/80-positive cell projected numerous intraepithelial dendrites. In contrast, BCs projected no more than one protrusion per cell, as was previously shown in the rat epididymis [35, 36]. In the caput (Fig. 3a, D–F), the projections from KRT5-positive BCs were no longer present, whereas F4/80-positive macrophages still projected short extensions occasionally infiltrating between epithelial cells. Neither BCs nor F4/80-positive macrophages showed intraepithelial protrusions in the distal epididymis (Fig. 3a, G–I). Most cellular bodies were restricted to the basal region of the tubule, whereas a subset of macrophages expressing lower levels of F4/80 remained strictly interstitial (arrowheads). Imaging large and ramified cells such as MPs requires the use of thick tissue sections (thicknesses of up to 50 μm) and the subsequent generation of z stacks. MIP of z stacks is commonly used to generate focused, 2D images from a z stack. A single-plane analysis (Fig. 3b) showed that the yellow areas visible in the merged MIP (left) were processing artifacts generated by the projection and channel merging rather than colocalization of KRT5 and F4/80. This observation applies to all 2D images in the present study.
Labeling of CD11c-EYFP mouse epididymis sections for KRT5 (Fig. 4) revealed relatively similar results; however, the visualization of CD11c-positive MPs was greatly facilitated by the high level of expression of cytoplasmic EYFP. Similar to the F4/80-positive cells described above, CD11c-positive MPs projected numerous intraepithelial dendrites toward the lumen in the IS, whereas KRT5-positive BCs had, strictly, one or no visible extension (Fig. 4, A–C). In the more distal regions of the epididymis (caput, Fig. 4, D–F; cauda, Fig. 4, G–I), both BCs and MPs remained peritubular and devoid of intraepithelial projections. A subset of strictly interstitial and morphologically distinct CD11c-positive MPs, less abundant than F4/80-positive interstitial macrophages, was observed in all epididymal segments (arrowheads).
Next, we used scanning confocal microscopy to generate higher z-resolution images of BCs and MPs (Fig. 5 and Supplemental Movie S1). In tubule cross-sections, the characteristic “pyramidal” shape of BC bodies was visible at the base of the epithelium (Fig. 5, A and D), and BCs occasionally projected long extensions toward the apical side. In contrast, CD11c-positive MPs were not more abundant but projected numerous and very slender dendrites between epithelial cells (Fig. 5, B and E). The merged images (Fig. 5, C and F) as well as the 3D rendering of a z stack captured in the IS (Supplemental Movie S1) confirmed that basal cellular bodies and intraepithelial processes of both cell types are perfectly distinct, although closely localized. A different view of the basal region of the epithelium in a more distal region (caput) revealed another significant difference between BCs and MPs: Whereas MPs (Fig. 5H) were scattered at the base of the epithelium and did not seem to be interconnected, BCs (Fig. 5G) formed a dense and possibly continuous network of basal cellular bodies. The lack of KRT5 and CD11c colocalization (Fig. 5I, red and green) confirmed, again, that BCs and MPs are two independent cell types.
FIG. 5.
Confocal imaging of BCs and CD11c-positive MPs in the mouse epididymis. High-resolution scanning confocal micrographs reveal the significant morphological differences between BCs (expressing KRT5; red) and MPs (expressing CD11c; green). BC bodies appear as domes at the base of the epithelium and project single extensions toward the luminal compartment (A and D). In contrast, CD11c-positive cells project multiple slender dendritic processes into the epithelium (B and E). Merged images (C and F) show independent expression of KRT5 and CD11c in BCs and MPs, respectively; however, BCs and MPs often appear to be closely related. Contrasting with cross-sections of the epididymal tubule, G–I show the basal region of the epithelium in the caput region. BCs form a continuous mesh of basal cellular bodies (G and I), whereas MPs are scattered, do not form a continuous network, and are particularly ramified (H and I). (a), apical side of the epithelium. Bars = 10 μm (A–F) and 20 μm (G–I).
Epididymal BCs and Macrophages Are Phenotypically Distinct
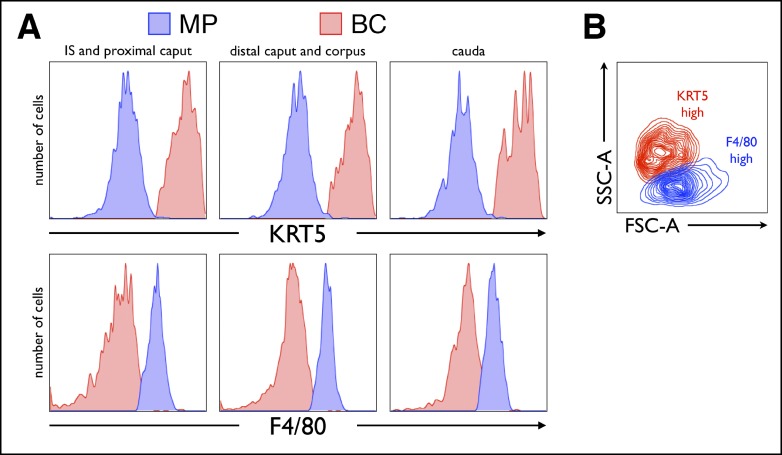
To distinguish between BCs and MPs in a more quantitative manner, we performed a flow cytometry-based analysis using cell suspensions prepared from three major regions, proximal (IS and proximal caput), central (distal caput and corpus), and distal (cauda) of the mouse epididymis, and labeled with a cocktail of antibodies directed against KRT5 and a selection of MP markers. In agreement with our microscopy results described above, this analysis confirmed that F4/80-positive MPs do not express high amounts of KRT5 (Fig. 6A, top), whereas BCs do not express high levels of F4/80 (Fig. 6A, bottom), in all epididymal regions. In addition, the side-scatter/forward-scatter plot (Fig. 6B), which represents cells exclusively on the basis of morphological parameters (side scatter represents the cellular internal complexity, whereas forward scatter is an indicator of cell size), showed that isolated high levels of F4/80 and of KRT5 represent two clearly nonoverlapping cellular populations.
FIG. 6.
Flow cytometry of KRT5 and F4/80 expression in cell suspensions from the mouse epididymis. Cell suspensions represent three regions of the mouse epididymis: proximal (IS and proximal caput), central (distal caput and corpus), and distal (cauda). Histograms representing the number of cells versus the intensity of KRT5 and F4/80 immunolabeling (A) show unequivocally that in all epididymal regions, BCs (red) express abundant KRT5 and low levels of F4/80, whereas MPs (blue) express low levels of KRT5 and high levels of F4/80. In addition, the side-scatter (SSC-A; representing the inner cellular complexity) versus forward-scatter (FSC-A; representing the cell size) plot (B) shows, again, that cells with high levels of KRT5 (BCs) and low levels of F4/80 (MPs) are morphologically very distinct.
DISCUSSION
Our present work, based on the use of specific markers (KRT5, CD11c, and F4/80, identifying BCs, dendritic cells, and macrophages, respectively), demonstrates that epididymal BCs are morphologically and phenotypically distinct from peritubular MPs (dendritic cells and macrophages). BCs form a homogeneous network in the basal region of all mouse epididymal segments and extend single intraepithelial processes exclusively in the IS. This contrasts with rat BCs, which showed luminal-reaching projections in more distal segments [35, 36, 40]. Most MPs are located between epithelial cells and the basement membrane, where they form a basolateral network that is intertwined with the network made by BCs. In the IS, these peritubular MPs establish a greater opportunity for interactions with neighboring epithelial cells via numerous, slender intraepithelial dendritic processes. In addition, some interstitial MPs are separated from the epithelium by the basement membrane.
The epididymis creates and maintains an intricate milieu in which spermatozoa mature in transit and are ultimately stored before ejaculation. Such a complex function requires multiple interactions between several cellular populations, and to date, most studies have been focused on the pseudostratified epithelium that lines the epididymal duct. Epididymal epithelial cells control the exchange of fluids, solutes, ions, proteins, and microvesicles between the basolateral and luminal compartments [1, 2, 11, 57], and recent studies have highlighted the importance of functional crosstalk between distinct subsets of epithelial cells that line the epididymal duct [27, 36–38, 58]. Epithelial cells communicate with each other and exert their respective functions in a concerted and segment-specific manner to continuously adjust the composition of the luminal microenvironment. Surprisingly, a very important physiological aspect related to the immunological regulation of the posttesticular environment has been poorly studied: The excurrent duct contains a unique mucosal immune system, and the nature and functions of the interactions between immune and nonimmune cells in the epididymis remain mostly unknown [19, 20]. Mucosal immune systems are primarily involved in the regulation of the delicate balance between immunity and tolerance as well as maintaining the integrity of epithelial barriers. In the epididymis, the mucosa must orchestrate the peripheral tolerance to antigenic spermatozoa while eliminating ascending pathogens. Numerous cell types, including epithelial and nonepithelial cells, are potential candidates for the mediation of local immunoregulatory mechanisms. Interestingly, Yeung et al. [41] reported ultrastructural and antigenic similarities between BCs and peritubular macrophages in the human epididymis. This article was followed by several others from the same group, who suggested that epididymal BCs from primates and rodents may originate from circulating progenitors and exert immunological functions [42–44, 47]. However, we recently reported that during postnatal development of the rat epididymis, at the onset of epithelial cell differentiation, BCs have morphological features similar to those of adjacent epithelial cells, and we proposed that BCs originate from the nondifferentiated columnar epithelial cells, as was suggested by Sun and Flickinger [33]. Interestingly, despite their hematopoietic origin, we found that peritubular MPs appear to coexist with epithelial cells and must, therefore, have the ability to cross the basement membrane. In agreement with this notion, F4/80-positive cells located between the basement membrane and the epithelium have also been described in the mouse epididymis [45]. Future studies following the appearance of MPs in the epithelium during postnatal development are required to further characterize the origin of MPs.
Mucosal immunity has been extensively studied in organs that are particularly vulnerable to infection by pathogens. Among the most studied mucosal systems, the gut and the lungs are lined by thin and permeable epithelia, which exert their respective specialized physiological functions (food absorption in the gut and gas exchange in the lungs) while preventing invasion of pathogens coming from the external environment. To date, epithelial BCs have not been shown to play a significant immunological role in these two well-characterized mucosal immune systems. BCs (or an equivalent cell type) have not been formally described in the gut epithelium, indicating that the presence of BCs is not necessary for the immune function of this continuously challenged mucosa. Furthermore, in the airways, BCs are primarily progenitor cells involved in the renewal of other epithelial cell populations [59–63]. In contrast, MPs play a critical role in the gut and the lung by constantly sampling the environment and transmitting antigens to local lymph nodes to orchestrate immunogenic or tolerogenic immune responses [64–68]. MPs are also in charge of clearing apoptotic epithelial cells and debris, which is essential for tissue homeostasis [69]. The epididymal mucosa strikingly resembles the small intestine mucosa: Epididymal MPs express makers such as CD11c, CD103, CX3CR1, and F4/80; they are located in the basal region of the epithelium and establish close interactions with neighboring epithelial cells, particularly in the IS, where they project intraepithelial dendrites that are very similar to the luminal-sampling structures described in the small intestine [54]. Although the precise role of MPs in the epididymis remains to be elucidated, they likely have functions similar to those observed in other mucosal systems by sampling the local environment, regulating the balance between immunogenic and tolerogenic responses to luminal antigens (including sperm antigens), and contributing to epithelial homeostasis. Whereas epididymal BCs have the ability to sample the luminal compartment to regulate epithelial functions [36], no data to our knowledge support the idea that BCs have, like MPs, the ability to take up, process, and present antigens or functionally interact with other immunologically active cells. Therefore, the complexity of the basolateral compartment of the epididymal epithelium may have been underestimated, and whereas both BCs and MPs reside in the basal region (basolateral in the IS) and are often extremely close to each other, their cohabitation might be purely coincidental, indicating that two populations of cells with distinct origins and functions might be required to share a limited basolateral space. The structural integrity of the epithelium likely relies not only on the direct attachment of epithelial cells on the basement membrane but also on complex cell-cell interactions that involve all epithelial cell types as well as immune cells. In addition, as conventional microscopy provides snapshots that do not fully reflect the complexity of cellular physiology, the dynamics of epithelium-MP and -BC interactions remain to be elucidated. Finally, as was proposed in the gut [67], the maintenance of epididymal immune homeostasis most likely involves the concerted participation of immune and epithelial cells, and even if BCs do not belong to the MP system, our data do not exclude their possible role in the regulation of this unique mucosal immune system.
Supplementary Material
Footnotes
Supported by National Institutes of Health grants R01HD069623 (N.D.S.), RO1DK085715 (S.B.), and R01AI084880 (M.J.P.). S.B. is a Massachusetts General Hospital (MGH) Charles and Ann Sanders Research Scholar. The Microscopy Core facility of the MGH Program in Membrane Biology receives support from the Boston Area Diabetes and Endocrinology Research Center (DK057521) and the MGH Center for the Study of Inflammatory Bowel Disease (DK043351).
REFERENCES
- Belleannee C, Thimon V, Sullivan R. Region-specific gene expression in the epididymis. Cell Tissue Res 2012; 349: 717–731. [DOI] [PubMed] [Google Scholar]
- Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update 2009; 15: 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgebin-Crist MC, Danzo BJ, Davies J. Endocrine control of the development and maintenance of sperm fertilizing ability in the epididymis. : DW Hamilton, Greep RO. (eds.), Handbook of Physiology, Section 7, Endocrinology, vol. 5. Washington, DC: American Society of Physiologists; 1975: 319–339. [Google Scholar]
- Robaire B, Hermo L. Efferent ducts, epididymis, and vas deferens: structure, functions, and their regulation. : Knobil E, Neil J. (eds.), The Physiology of Reproduction. New York: Raven Press; 1988: 999–1080. [Google Scholar]
- Robaire B, Hinton BT. The Epididymis: From Molecular to Clinical Practice. A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. New York: Kluwer Academic/Plenum; 2002. [Google Scholar]
- Turner TT. On the epididymis and its role in the development of the fertile ejaculate. J Androl 1995; 16: 292–298. [PubMed] [Google Scholar]
- Turner TT. De Graaf's thread: the human epididymis. J Androl 2008; 29: 237–250. [DOI] [PubMed] [Google Scholar]
- Hermo L, Robaire B. Epididymal cell types and their functions. : Robaire B, Hinton BT. (eds.), The Epididymis: From Molecular to Clinical Practice. A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. New York: Kluwer Academic/Plenum; 2002: 81–102. [Google Scholar]
- Serre V, Robaire B. Distribution of immune cells in the epididymis of the aging Brown Norway rat is segment-specific and related to the luminal content. Biol Reprod 1999; 61: 705–714. [DOI] [PubMed] [Google Scholar]
- Flickinger CJ, Howards SS, Baran ML, Pessoa N, Herr JC. Appearance of ‘natural' antisperm autoantibodies after sexual maturation of normal Lewis rats. J Reprod Immunol 1997; 33: 127–145. [DOI] [PubMed] [Google Scholar]
- Robaire B, Hinton BT, Orgebin-Crist MC. The epididymis. : Neill JD. (ed.), Knobil and Neill's Physiology of Reproduction, vol 1, 3rd ed Amsterdam: Elsevier; 2006: 1072–1148. [Google Scholar]
- Arrighi S. Are the basal cells of the mammalian epididymis still an enigma? Reprod Fertil Dev (in press). Published online ahead of print 21 October 2013; DOI 10.1071/RD13301. [DOI] [PubMed]
- Friend DS, Gilula NB. Variations in tight and gap junctions in mammalian tissues. J Cell Biol 1972; 53: 758–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod 2011; 84: 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer AP, Hinton BT. Morphological evidence for a blood-epididymis barrier and the effects of gossypol on its integrity. Biol Reprod 1984; 30: 991–1004. [DOI] [PubMed] [Google Scholar]
- Levy S, Robaire B. Segment-specific changes with age in the expression of junctional proteins and the permeability of the blood-epididymis barrier in rats. Biol Reprod 1999; 60: 1392–1401. [DOI] [PubMed] [Google Scholar]
- Dube E, Cyr DG. The blood-epididymis barrier and human male fertility. Adv Exp Med Biol 2012; 763: 218–236. [DOI] [PubMed] [Google Scholar]
- Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol 2012; 763: 237–259. [PubMed] [Google Scholar]
- Hedger MP. Immunophysiology and pathology of inflammation in the testis and epididymis. J Androl 2011; 32: 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger MP, Hales DB. Immunophysiology of the male reproductive tract. : Neill JD. (ed.), Knobil and Neill's Physiology of Reproduction, vol 1, 3rd ed Amsterdam: Elsevier; 2006: 1195–1286. [Google Scholar]
- Elkjaer M, Vajda Z, Nejsum LN, Kwon T, Jensen UB, Amiry-Moghaddam M, Frokiaer J, Nielsen S. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem Biophys Res Commun 2000; 276: 1118–1128. [DOI] [PubMed] [Google Scholar]
- Pastor-Soler N, Bagnis C, Sabolic I, Tyszkowski R, McKee M, Van Hoek A, Breton S, Brown D. Aquaporin 9 expression along the male reproductive tract. Biol Reprod 2001; 65: 384–393. [DOI] [PubMed] [Google Scholar]
- Badran HH, Hermo LS. Expression and regulation of aquaporins 1, 8, and 9 in the testis, efferent ducts, and epididymis of adult rats and during postnatal development. J Androl 2002; 23: 358–373. [PubMed] [Google Scholar]
- Breton S, Smith PJ, Lui B, Brown D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med 1996; 2: 470–472. [DOI] [PubMed] [Google Scholar]
- Da Silva N, Shum WW, El-Annan J, Paunescu TG, McKee M, Smith PJ, Brown D, Breton S. Relocalization of the V-ATPase B2 subunit to the apical membrane of epididymal clear cells of mice deficient in the B1 subunit. Am J Physiol Cell Physiol 2007; 293: C199–C210. [DOI] [PubMed] [Google Scholar]
- Da Silva N, Shum WW, Breton S. Regulation of vacuolar proton pumping ATPase-dependent luminal acidification in the epididymis. Asian J Androl 2007; 9: 476–482. [DOI] [PubMed] [Google Scholar]
- Shum WW, Ruan YC, Da Silva N, Breton S. Establishment of cell-cell crosstalk in the epididymis: control of luminal acidification. J Androl 2011; 32: 576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Da Silva N, Brown D, Breton S. Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk. J Exp Biol 2009; 212: 1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidarsson H, Westergren R, Heglind M, Blomqvist SR, Breton S, Enerback S. The forkhead transcription factor Foxi1 is a master regulator of vacuolar H-ATPase proton pump subunits in the inner ear, kidney and epididymis. PLoS ONE 2009; 4: e4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist SR, Vidarsson H, Soder O, Enerback S. Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. EMBO J 2006; 25: 4131–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton S, Brown D. Regulation of luminal acidification by the V-ATPase. Physiology (Bethesda) 2013; 28: 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont Y, Flannery J. Mitotic activity in the epithelium of the epididymis in young and old adult rats. Biol Reprod 1970; 3: 283–292. [DOI] [PubMed] [Google Scholar]
- Sun EL, Flickinger CJ. Proliferative activity in the rat epididymis during postnatal development. Anat Rec 1982; 203: 273–284. [DOI] [PubMed] [Google Scholar]
- Veri JP, Hermo L, Robaire B. Immunocytochemical localization of the Yf subunit of glutathione S-transferase P shows regional variation in the staining of epithelial cells of the testis, efferent ducts, and epididymis of the male rat. J Androl 1993; 14: 23–44. [PubMed] [Google Scholar]
- Robaire B, Viger RS. Regulation of epididymal epithelial cell functions. Biol Reprod 1995; 52: 226–236. [DOI] [PubMed] [Google Scholar]
- Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell 2008; 135: 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung GP, Cheung KH, Leung CT, Tsang MW, Wong PY. Regulation of epididymal principal cell functions by basal cells: role of transient receptor potential (Trp) proteins and cyclooxygenase-1 (COX-1). Mol Cell Endocrinol 2004; 216: 5–13. [DOI] [PubMed] [Google Scholar]
- Cheung KH, Leung GP, Leung MC, Shum WW, Zhou WL, Wong PY. Cell-cell interaction underlies formation of fluid in the male reproductive tract of the rat. J Gen Physiol 2005; 125: 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory M, Dufresne J, Hermo L, Cyr D. Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology 2001; 142: 854–863. [DOI] [PubMed] [Google Scholar]
- Shum WW, Hill E, Brown D, Breton S. Plasticity of basal cells during postnatal development in the rat epididymis. Reproduction 2013; 146: 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung CH, Nashan D, Sorg C, Oberpenning F, Schulze H, Nieschlag E, Cooper TG. Basal cells of the human epididymis—antigenic and ultrastructural similarities to tissue-fixed macrophages. Biol Reprod 1994; 50: 917–926. [DOI] [PubMed] [Google Scholar]
- Seiler P, Wenzel I, Wagenfeld A, Yeung CH, Nieschlag E, Cooper TG. The appearance of basal cells in the developing murine epididymis and their temporal expression of macrophage antigens. Int J Androl 1998; 21: 217–226. [DOI] [PubMed] [Google Scholar]
- Seiler P, Cooper TG, Yeung CH, Nieschlag E. Regional variation in macrophage antigen expression by murine epididymal basal cells and their regulation by testicular factors. J Androl 1999; 20: 738–746. [PubMed] [Google Scholar]
- Seiler P, Cooper TG, Nieschlag E. Sperm number and condition affect the number of basal cells and their expression of macrophage antigen in the murine epididymis. Int J Androl 2000; 23: 65–76. [DOI] [PubMed] [Google Scholar]
- Mullen TE, Jr, , Kiessling RL, Kiessling AA. Tissue-specific populations of leukocytes in semen-producing organs of the normal, hemicastrated, and vasectomized mouse. AIDS Res Hum Retroviruses 2003; 19: 235–243. [DOI] [PubMed] [Google Scholar]
- Pollanen P, Cooper TG. Immunology of the testicular excurrent ducts. J Reprod Immunol 1994; 26: 167–216. [DOI] [PubMed] [Google Scholar]
- Holschbach C, Cooper TG. A possible extratubular origin of epididymal basal cells in mice. Reproduction 2002; 123: 517–525. [DOI] [PubMed] [Google Scholar]
- Da Silva N, Cortez-Retamozo V, Reinecker HC, Wildgruber M, Hill E, Brown D, Swirski FK, Pittet MJ, Breton S. A dense network of dendritic cells populates the murine epididymis. Reproduction 2011; 141: 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 2010; 10: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity 2011; 35: 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev 2010; 234: 55–75. [DOI] [PubMed] [Google Scholar]
- Hume DA, Mabbott N, Raza S, Freeman TC. Can DCs be distinguished from macrophages by molecular signatures? Nat Immunol 2013; 14: 187–189. [DOI] [PubMed] [Google Scholar]
- Randolph G, Merad M. Reply to: “Can DCs be distinguished from macrophages by molecular signatures?” Nat Immunol 2013; 14: 189–190. [DOI] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005; 307: 254–258. [DOI] [PubMed] [Google Scholar]
- Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol 2004; 5: 1243–1250. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 2000; 20: 4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PY, Gong XD, Leung GP, Cheuk BL. Formation of the epididymal fluid microenvironment. : Robaire B, Hinton BT. (eds.), The Epididymis: From Molecular to Clinical Practice. A Comprehensive Survey of the Efferent Ducts, the Epididymis and the Vas Deferens. New York: Kluwer Academic/Plenum; 2002: 119–130. [Google Scholar]
- Belleannee C, Da Silva N, Shum WW, Brown D, Breton S. Role of purinergic signaling pathways in V-ATPase recruitment to apical membrane of acidifying epididymal clear cells. Am J Physiol Cell Physiol 2010; 298: C817–C830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A 2009; 106: 12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature 2011; 479: 189–193. [DOI] [PubMed] [Google Scholar]
- Ousset M, Van Keymeulen A, Bouvencourt G, Sharma N, Achouri Y, Simons BD, Blanpain C. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nat Cell Biol 2012; 14: 1131–1138. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 2010; 18: 8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, Medoff BD, Rajagopal J. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 2013; 503: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varol C, Zigmond E, Jung S. Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat Rev Immunol 2010; 10: 415–426. [DOI] [PubMed] [Google Scholar]
- Niess JH, Reinecker HC. Dendritic cells: the commanders-in-chief of mucosal immune defenses. Curr Opin Gastroenterol 2006; 22: 354–360. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol 2008; 8: 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 2008; 8: 411–420. [DOI] [PubMed] [Google Scholar]
- Chang SY, Song JH, Guleng B, Cotoner CA, Arihiro S, Zhao Y, Chiang HS, O'Keeffe M, Liao G, Karp CL, Kweon MN, Sharpe AH. et al. Circulatory antigen processing by mucosal dendritic cells controls CD8+ T cell activation. Immunity 2013; 38: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol 2010; 189: 1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.