ABSTRACT
Progesterone supplementation is recommended to prevent preterm birth in women with a short cervix, but the mechanism is unclear. We hypothesize that progesterone acts by altering the composition of the cervical extracellular matrix (ECM). We tested this hypothesis using human cervical fibroblasts in both two-dimensional (2D) and three-dimensional (3D) cultures. For 2D culture, cells were seeded in 6-well plates and cultured with media supplemented with estradiol (10−8 M), progesterone (10−7 or 10−6 M), and vehicle. For 3D culture, the cells were cultured on a porous silk protein scaffold system. Progesterone and estrogen receptors were documented by immunohistochemistry and Western blot analysis. In both 2D and 3D cultures, decreased collagen synthesis was seen with increased progesterone concentration. Three-dimensional cultures could be maintained significantly longer than 2D cultures, and the morphology of 3D cultures appeared similar to native cervical tissue. Thus, further studies were performed in 3D culture. To determine the effect of progesterone concentration, the 3D scaffolds were cultured with estradiol (10−8 M) and five conditions: vehicle; 10−9, 10−8, or 10−7 M progesterone; or 10−7 M progesterone plus 10−6 M mifepristone. The highest progesterone concentration correlated with the least amount of collagen synthesis. Collagen synthesis progressively increased as progesterone concentration decreased. This effect was partially antagonized by mifepristone, suggesting the mechanism is mediated by the progesterone receptor. This hormonally responsive 3D culture system supports the hypothesis that progesterone has a direct effect on remodeling cervical ECM during pregnancy. The 3D culture system could be useful for studying the mechanism of progesterone effects on the cervix.
Keywords: cervix, human, pregnancy, preterm birth, progesterone
Using a hormonally responsive, three-dimensional culture system and human cervical fibroblasts, estradiol increased formation of cervical-like tissue; this effect was opposed by progesterone.
INTRODUCTION
Preterm birth affects 15 million babies worldwide, leading to one million newborn deaths and lifelong disabilities in survivors [1]. In the United States, preterm birth is responsible for 21 billion dollars in health care costs per year [2]. Although preterm birth has multiple causes [3], cervical dysfunction is an important contributor [4–6]. In current clinical practice, women at high-risk for preterm birth are offered cervical length measurement because a short cervix is strongly associated with subsequent preterm birth [7]. Offering cervical length measurement to all pregnant women is currently being debated [8]. Driving the debate on the cervical length measurement are the clinical studies that demonstrate supplemental vaginal progesterone prevents preterm birth in women with a short cervix [9, 10].
Although multiple pathogenic mechanisms lead to a short cervix, in some women, cervical shortening is caused by dysregulated remodeling of the collagen-rich, extracellular matrix (ECM) of the cervical stroma [11, 12]. The temporal sequence of cervical remodeling prior to labor is divided into two stages: softening and ripening [13–15]. During the softening phase, which occurs slowly throughout pregnancy, there is progressive reorganization of collagen fibers resulting in increased collagen turnover, decreased collagen cross-links, and changes in fiber microstructure [16]. These biochemical changes are correlated to decreased tissue strength [17, 18], which is seen as early as midgestation in the murine pregnancy [19]. During the ripening phase, which is proximal to parturition, further softening is coupled with effacement and dilation, which facilitates successful labor.
Progesterone could regulate softening and ripening by separate and distinct mechanisms [13, 14]. For cervical softening, rising serum progesterone levels could progressively modulate cell function, leading to altered ECM composition and organization. Studies with human cervical fibroblasts show a dose-dependent effect of progesterone on glycosaminoglycan (GAG) synthesis [20], which affects ECM organization. Sex steroids also affect ECM synthesis in cardiac [21], vascular [22], and ligament fibroblasts [23].
In addition to cervical softening, multiple studies show a role for progesterone and cervical ripening. First, cervical ripening occurs close to parturition, a period during which the uterus changes from a quiescent to a contractile state [24, 25]. In the uterus, high serum levels of progesterone maintain quiescence and a functional progesterone withdrawal contributes to the initiation of parturition [26, 27]. In the cervix, there is evidence for a similar functional withdrawal [28], suggesting that falling local progesterone levels contribute to cervical ripening. Second, knockout mice models show that abnormalities of progesterone metabolism lead to high local progesterone levels and impaired cervical ripening [29]. Last, mifepristone reliably causes preterm birth in most animal species and cervical ripening in women [30, 31].
Although progesterone has an important role in cervical softening and ripening, less clear is the biological mechanism. Multiple investigators liken cervical ripening to an inflammatory process [32–34], and progesterone's anti-inflammatory effects could modulate this process [35, 36]. However, elucidating the biological mechanisms has been challenging [37], and studies in rats show that the therapeutic effect depends on the specific progestogen, the route of administration, and properties of the vehicle [38]. In addition, the role of cervical inflammation as an important mediator of cervical ripening is being debated [39, 40]. Adding further complexity is that progesterone is one of several mediators affecting cervical ripening; studies with both humans and animals demonstrate important roles for prostaglandins [41, 42], nitric oxide [43, 44], and relaxin [45]. Although challenging, a better understanding of progesterone's role in cervical softening and ripening is critical to understanding cervical dysfunction and its influence on spontaneous preterm birth.
Motivated by the fact that 1) a short cervix is important for selecting patients for progesterone therapy, 2) a short cervix could be caused by abnormal remodeling of the cervical ECM, and 3) progesterone could influence remodeling of the cervical ECM, our purpose was to study the effect of progesterone on long-term culture of human cervical fibroblasts. We recently described a three-dimensional (3D) culture system using cervical fibroblasts and porous silk protein scaffolds [46, 47]. The culture system produced cervical-like tissue that responded to changes in the nutritional environment of the culture system. A key advantage of the 3D system is the ability to study cell-matrix interactions in a biologically relevant microenvironment without the complexity of an animal model. In the present study, cervical fibroblasts were cultured in two-dimensions (2D) and 3D and characterized by ECM production, histology, and gene expression. We hypothesized that the 3D culture system would facilitate long-term culture and that progesterone would influence the synthesis of cervical-like tissue.
MATERIALS AND METHODS
Subject Demographics
Nonpregnant, premenopausal women having a hysterectomy for benign gynecological indications were approached to participate. Informed consent was obtained from all the subjects, and the study was approved by the Institutional Review Board at Tufts Medical Center. Biopsies were obtained from three premenopausal women. The 2D and first 3D experiments were performed with cells from a 38-yr-old Caucasian, para 5 having a hysterectomy for endometriosis. The second 3D experiment was performed with cells from two women (two replicates): a 42-yr-old Asian, para 5 having a hysterectomy for adenomyosis; a 52-yr-old Caucasian para 0 having a hysterectomy for fibroids. There was no history of preterm birth in the study subjects. Once the surgical specimen was removed from the body, a cervical biopsy was performed under sterile conditions. The biopsy was obtained from the midcanal region taking care to avoid the endocervical epithelium. The biopsy was rinsed in normal saline and stored on ice-cold culture media during transport to the culture hood.
Cell Culture
Cervical fibroblasts were obtained using an explant method as previously described [47]. Briefly, the cervical biopsy was minced in sterile 6-well plates. The minced pieces were cultured in expansion media (see below) in a humidified incubator at 37°C, 5% CO2/95% air, and 95% relative humidity. After 10 days, cells were confluent around the explants. The cells were trypsinized, and the culture expanded and cryopreserved using standard techniques. Passages four and five were used for all the experiments.
Culture Media
Two culture media were used: expansion media and phenol red-free media. Expansion media consisted of Dulbecco modified Eagle medium (11995-065; Life Technologies) with 10% fetal bovine serum (160000-044; Life Technologies), 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (15240-062; Life Technologies). Phenol red-free media consisted of phenol red-free Dulbecco modified Eagle medium (31053-036; Life Technologies) supplemented with 5% charcoal-stripped fetal bovine serum (12676-029; Life Technologies), 1 mM sodium pyruvate (11360-070; Life Technologies), 2 mM l-glutamine (25030-081; Life Technologies), 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B (15240-062; Life Technologies), and freshly prepared 50 μg/ml ascorbic acid 2-phosphate (A8960; Sigma-Aldrich). Phenol red-free media was additionally supplemented with estradiol (E2758; Sigma), progesterone (8783; Sigma); or mifepristone (M8046; Sigma) according to the experimental protocol. Steroids were prepared as 10 mM stock solutions using 200-proof ethanol and serially diluted to the appropriate concentration. The final concentration of ethanol did not exceed 0.01%.
Two-Dimensional Culture
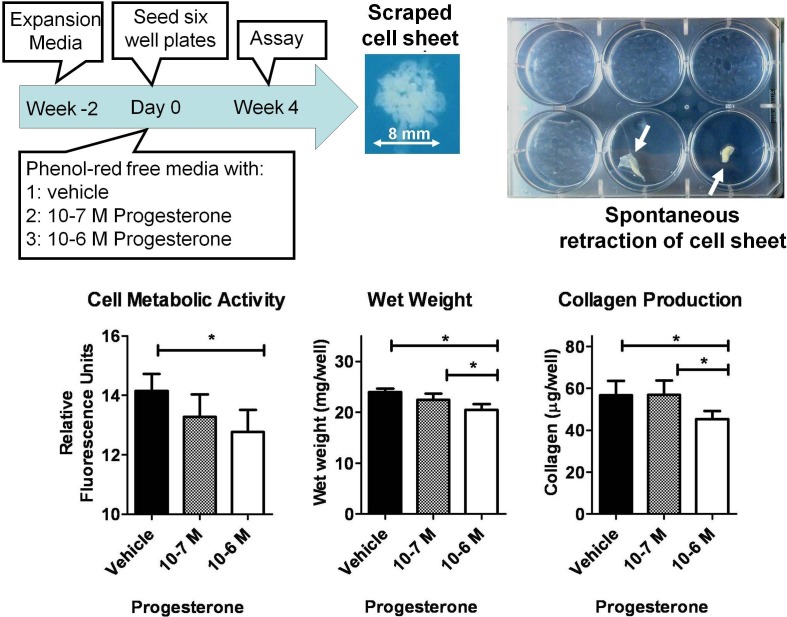
For 2D culture (Fig. 1), cervical fibroblasts were expanded in culture for 2 wk in expansion media. The media was switched to phenol red-free media and supplemented with progesterone (10−7 or 10−6 M) or vehicle. Cells were seeded in 6-well plates at 10 000 cells/cm2 and allowed to grow undisturbed for 4 wk (Fig. 1). Media supplemented with progesterone or vehicle was changed twice a week. After 4 wk, the cell sheet was scraped and assayed for wet weight, cell metabolic activity, and collagen concentration (assays below). Of note, in several wells, the cell sheet spontaneously contracted into a small tissue sample (Fig 1). These wells were discarded.
FIG. 1.
Two-dimensional culture of cervical fibroblasts. After 4 wk of culture, the cell sheet was scraped and assayed for metabolic activity, wet weight, and collagen production. In several wells, the cell sheet spontaneously retracted (top, right), and these wells were discarded. Compared to vehicle, high concentrations of progesterone (10−6 M) correlated with significantly decreased cell metabolic activity (*P < 0.05), tissue wet weight (*P < 0.01), and collagen production (*P < 0.01). Data presented as mean ± SD of four replicates.
Three-Dimensional Culture
For 3D culture, the protocol was adapted from a previously described technique with minor modifications [47]. Briefly, silk fibroin protein was purified from cocoons of Bombyx mori silkworms (Tajima Shoji Co., LTD) [48]. The cocoons were cut into dime-sized pieces and boiled in an aqueous solution of 0.02 M Na2CO3 for 30 min. Fibrous silk protein was rinsed and solubilized in 9.3 M LiBr solution at 60°C for 4 h. The solubilized silk solution was dialyzed in a Slide-A-Lyzer dialysis cassette (66110; Pierce Protein) against six changes of distilled water over 48 h to a final purified silk concentration of 6% (w/w). Silk fibroin solution (2 ml) was poured into the well of a 24-well plate followed by 4 g granular NaCl (particle size 500–600 μm). Gelation of the silk occurred after 24 h at room temperature and 4 h at 60°C. The 24-well plate was immersed in water for 2 days to remove the NaCl. The scaffold was removed from the well and washed for an additional 24 h. A 6 mm cylindrical punch biopsy (no. 33–36 Miltex) was used to create a cylindrical scaffold and a blade was used to cut the scaffold to a 4 mm height. The scaffolds were autoclaved for sterilization and coated with collagen as previously described [46]. The scaffolds were placed in 12-well plates and seeded by applying 100 μl of a concentrated cell solution (20 × 106 cells/ml; 2.0 × 106 cells/scaffold) in a drop-wise fashion to the scaffold surface. The scaffolds were placed in the incubator for 1 h to allow cell attachment. After 1 h, the scaffolds were cultured in 3.0 ml of expansion media for 24 h. After 24 h, the scaffolds were moved to a new 12-well plate and cultured in static conditions until the start of the experiment.
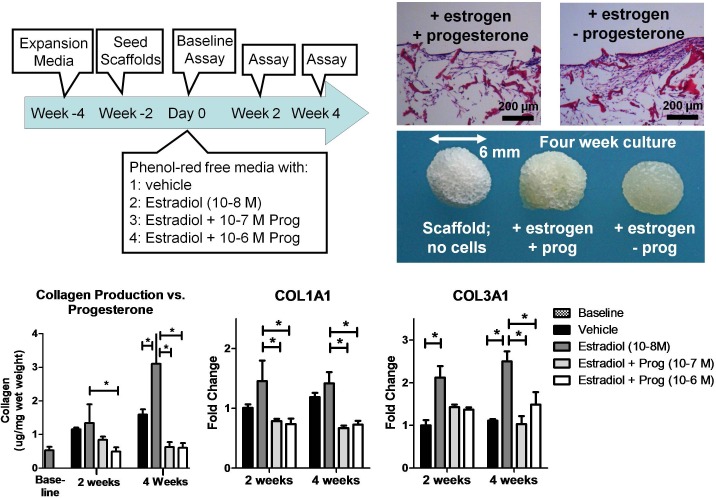
Two 3D experiments were performed. In the first experiment (Fig. 2), seeded scaffolds were cultured in expansion media for 2 wk in static conditions. The experiment commenced when the scaffolds were transferred to spinner flasks as previously described [47]. The media was switched to phenol red-free media supplemented with estradiol (10−8 M), progesterone (10−7 or 10−6 M), or vehicle. Scaffolds were removed from the spinner flask and flash frozen at Day 0 (baseline assay), Wk 2, and Wk 4.
FIG. 2.
Three-dimensional culture of cervical fibroblasts. In the presence of estradiol, the scaffold was covered in cervical-like tissue. However, the addition of progesterone resulted in nonuniform distribution of cervical-like tissue (top, right). Collagen protein and COL1A1 and COL3A1 gene expression correlated with the histology results. Estradiol significantly increased collagen protein and gene expression while progesterone opposed these effects. Control scaffolds with no cervical-like tissue showed no collagen signal. Each data point represented the mean ± SD of four replicates.
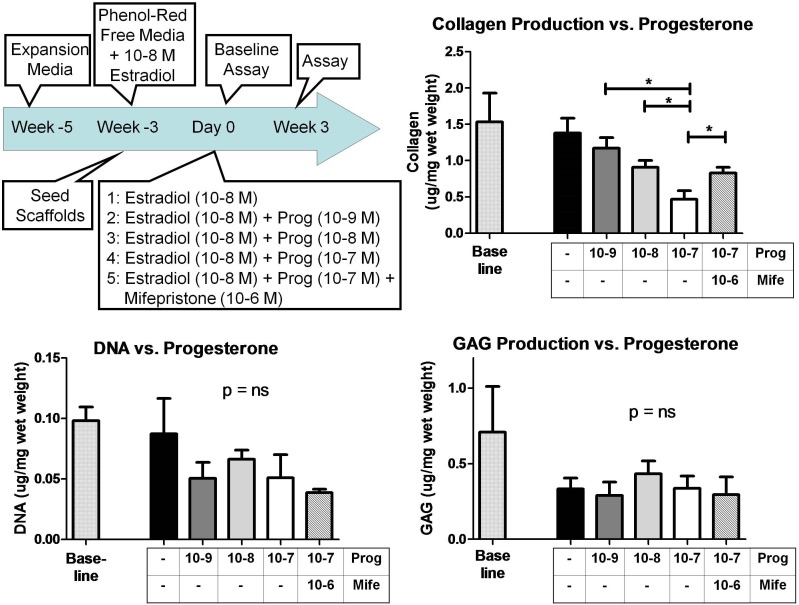
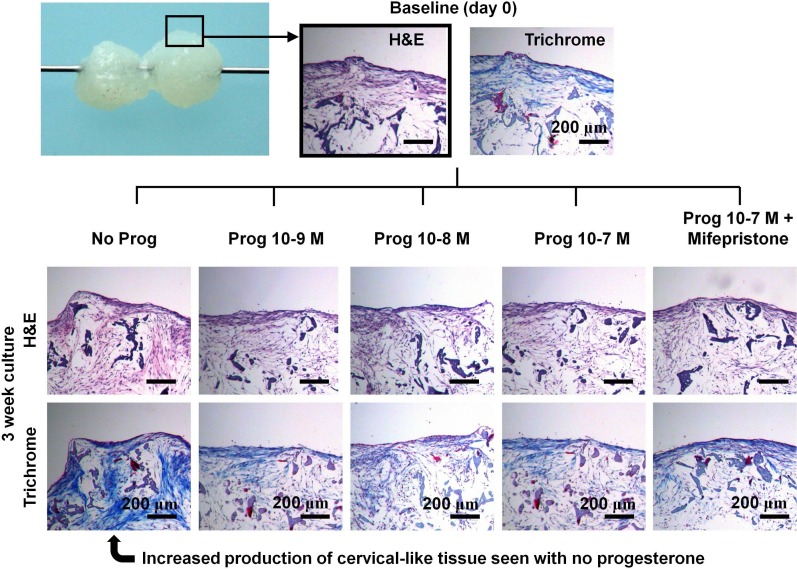
In the second 3D experiment (Fig. 3), scaffolds were seeded and cultured in phenol red-free media supplemented with estradiol (10−8 M) for 3 wk. Next, the scaffolds were transferred to spinner flasks with phenol red-free media plus estradiol (10−8 M) supplemented with progesterone (10−9, 10−8, or 10−7 M), progesterone (10−7 M) plus mifepristone (10× excess or 10−6 M), or vehicle. Scaffolds were removed from the spinner flask and flash frozen at Day 0 (baseline) and Wk 3. The experiment was duplicated with cells from two different women (see Subject Demographics above).
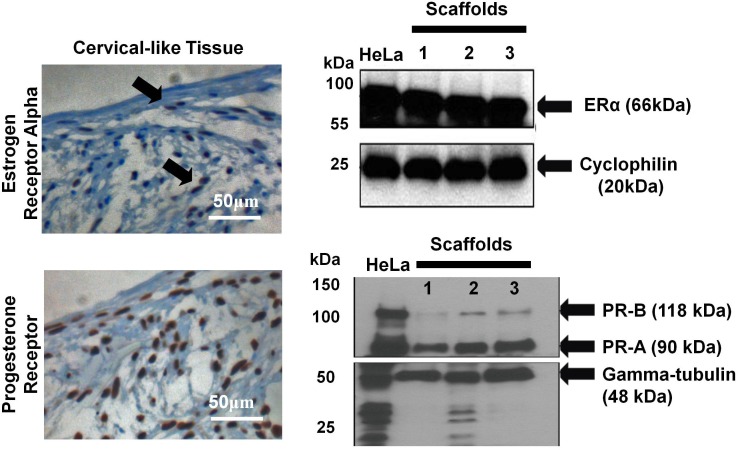
FIG. 3.
Immunohistochemistry and Western blot analysis showing the presence of the estrogen receptor alpha and progesterone receptor in cervical-like tissue. HeLa cells were used as a positive control. Arrows (top, left figure) indicated cells positive for estrogen receptor alpha.
Choices of steroid hormone concentrations were guided by serum levels during pregnancy. In the first 3D experiment, progesterone levels were chosen to approximate serum levels in the first trimester (10−7 M) and third trimester (10−6 M) [49]. Estradiol levels were chosen to correspond to the third trimester (10−8 M). In the second 3D experiment, we desired to study the effect of a wider range of progesterone concentrations (10−9, 10−8, or 10−7 M) as well as 10−7 M progesterone plus 10−6 M mifepristone on synthesis of cervical-like tissue.
Steroid Receptors
To confirm steroid receptors were present in the 3D culture system, immunohistochemical and Western blot analyses were performed. For immunohistochemical analysis, the following antibodies were used: anti-human estrogen receptor (790-4324; Ventana Medical Systems) and anti-progesterone receptor (790-2223; Ventana Medical Systems) using a Benchmark XT (Ventana Medical Systems) and following the manufacturer's protocol (antibody concentrations were approximately 1 μg/ml). Breast tissue was used as a positive control.
For Western blot analysis, scaffolds were flash frozen at the baseline time point (Fig. 2, total time in culture was 4 wk) and stored at −80°C. The scaffolds were broken into pieces on a dry-ice chilled, metallic hard surface. Then 100 μl of chilled lysis buffer (Fermentas) supplemented with 0.2 μg/ml PMSF, 0.1% SDS, and a protease inhibitor cocktail (Roche Applied Science) was added and homogenized. The lysates were centrifuged to separate the scaffold material from the lysate. Subsequently, equal amounts of protein (40 μg) were then loaded on precast 4%–15% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) under reducing conditions and transferred to a nitrocellulose membrane. The rabbit anti-human estrogen-receptor alpha (ER-α) (Santa Cruz Biotechnology, Inc.) and rabbit anti-human progesterone receptor (PR-A/B) (Cell Signaling Technologies) were used as primary antibody. Membranes were incubated overnight with primary antibody in 5% milk in phosphate-buffered saline with Tween-20 at 4°C. Detection was performed using biotinylated goat anti-rabbit secondary antibody (1:2000; Jackson Immunoresearch) followed by streptavidin-linked horseradish peroxidase (1:8000; Amersham Biosystems), chemiluminescence (ECL-Plus; Pierce), and a timed 5-min exposure to film (Kodak Biomax). Mouse anti-human alpha-tubulin (1:4000; Abcam) and rabbit anti-human cyclophilin (1:4000; Abcam) served as internal controls.
Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction
Gene expression was determined after 2 and 4 wk of 3D culture (Fig. 2). Total RNA was extracted using the RNeasy Fibrous Tissue Kit (Qiagen) as previously described [47]. The A260/A280 ratio was above 2.0 for all the samples tested (Nanodrop 2000; Thermo Scientific). The RNA concentration was 30–100 ng/μg (Nanodrop 2000). High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) was used for reverse transcription reactions using 50 μl RNA and 50 μl reverse transcription master mix. The cDNA was stored at −20°C. Quantitative gene expression was determined with the Stratagene Mx 3000P QPCR System (Stratagene) as previously described [47]. A 50 μl reaction consisted of 25 μl TaqMan Gene Expression Master Mix, 5 μl cDNA template, 17.5 μl RNase-free water, and 2.5 μl TaqMan Gene Expression Assay. Expression of collagen type I, alpha 1 (COL1A1, assay ID Hs00164004_m1) and collagen type III, alpha 1 (COL3A1, assay ID Hs00164103_m1) were studied. Gene expression was normalized by the geometric mean of two housekeeping genes: glyceraldehyde-3-phosphate dehydrogenase (GAPDH, assay ID Hs99999905_m1) and actin beta (ACTB, assay ID Hs99999903_m1). Cycle threshold was calculated from baseline-corrected, normalized fluorescence data using instrument software. The data was expressed as fold-change relative to the gene expression of vehicle controls using the 2ˆ(delta delta Ct) method.
Biochemical Characterization
Collagen concentration.
Collagen was measured using the QuickZyme collagen assay (QuickZyme Biosciences) following the manufacturer's protocol. Briefly, sections of frozen scaffold with cervical-like tissue (approximately 50 mg) were homogenized using a Bessman tissue pulverizer precooled with liquid nitrogen. The frozen, crushed powder was transferred to screw-capped tubes and weighed. Then 6 M HCl was added at a concentration of 1.0 ml/50 mg scaffold. The tubes were incubated at 95°C for 20 h to hydrolyze the sample. Hydrolyzed samples were centrifuged, and the supernatant was diluted twofold with 4 M HCl and assayed. Collagen concentrations were normalized by sample wet weight. Collagen concentrations were not normalized by sample dry weight because previous reports with this model system showed hydration of cervical-like tissue was 90% and was not affected by experimental conditions [46].
Cell metabolic activity.
The alamarBlue reagent (Life Technologies) was used to measure cell metabolic activity in 2D culture. The 10× reagent was diluted with expansion media, and 2.5 ml was added to each well of a 6-well plate. The wells were incubated for 1 h at 37°C, and 100 μl of media was transferred to an opaque 96-well plate. The fluorescence was read at 560 nm excitation and 590 nm emission.
Sulfated glycosaminoglycan.
Sections of scaffold (approximately 50 mg) were homogenized under liquid nitrogen and incubated in 1.0 ml of papain extraction reagent at 65°C for 3 h. The extraction reagent was 0.2 M sodium phosphate buffer (pH 6.4) with 0.1 M sodium acetate, 0.01 M disodium ethylenediaminetetraacetic acid, 0.005 M cysteine HCl, and 15–20 mg papain/100 ml extraction buffer (P3125; Sigma). The digestion was centrifuged at 10 000 × g for 10 min, and the supernatant was used to determine sulfated GAGs with a 1,9-dimethylmethylene blue dye label (Blyscan assay kit; Biocolor). Sulfated GAG concentrations were normalized by sample wet weight.
DNA concentration.
Sections of scaffold (approximately 35 mg) were homogenized under liquid nitrogen and digested in 400 μl of 1.0 mg/ml proteinase K (19131; Qiagen) in digestion buffer (50 mM Tris HCl, 1 mM ethylenediaminetetraacetic acid, pH 8.0) at 50°C overnight. The digestions were clarified with centrifugation at 10 000 × g for 10 min. The supernatants were assayed for DNA using a fluorescent nucleic acid stain (Quant-iT PicoGreen ds DNA kit; Life Technologies). DNA concentrations were normalized by sample wet weight.
Collagen extractability.
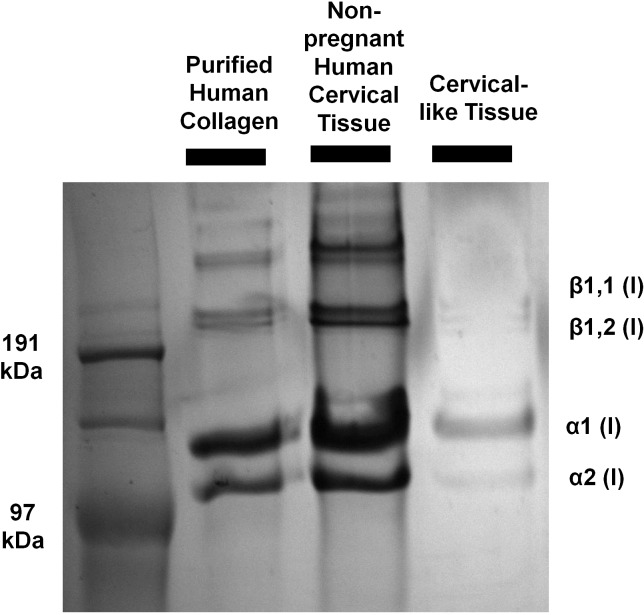
The electrophoretic mobility of collagen extracted from cervical-like tissue was compared to collagen extracted from native cervical tissue. Cervical-like tissue was grown for 3 wk (Fig. 4). The scaffold (50 mg) was pulverized under liquid nitrogen and placed in extraction reagent (1.0 ml of 0.5 M acetic acid and 1 mg/ml pepsin [P7012; Sigma]) on ice. Collagen was extracted for 48 h with gentle agitation at 4°C. The extract was clarified at 10 000 × g for 30 min at 4°C. Native cervical tissue (20 mg) was studied using the same protocol. The extracts were run on SDS-PAGE under reducing conditions and stained with Coomassie Blue. Purified human collagen (VitroCol; Advanced BioMatrix) was used as a positive control.
FIG. 4.
Increasing progesterone concentration results in a decrease in collagen concentration in cervical-like tissue after 3 wk of culture. The negative effect of progesterone was partially abrogated by mifepristone. Progesterone had no effect on DNA synthesis and GAGs production in this culture system. This experiment was performed with cervical fibroblasts from two different women with similar results. Data presented as mean ± SD of four replicates; *P < 0.05.
Statistical Analysis
Data was expressed as mean ± standard deviation. Means were calculated from four to five replicates as indicated. Statistical tests were one-way or two-way ANOVA with Bonferroni posttests as appropriate (GraphPad Prism version 5.04 for Windows; GraphPad Software). A P < 0.05 was considered significant.
RESULTS
Two-Dimensional Culture
Compared with vehicle control, increasing concentration of progesterone (10−7 or 10−6 M) was associated with decreased metabolic activity, decreased tissue wet weight, and decreased collagen production after 6 wk of culture. However, in some wells, the cell sheet spontaneously contracted prior toward the end of the experiment, which is a well-known feature of 2D culture [46, 50]. This observation motivated us to pursue 3D cultures in subsequent experiments.
Three-Dimensional Culture
In the first 3D experiment (Fig. 2), collagen production was significantly increased in the presence of estradiol (10−8 M), and this effect was opposed by progesterone (10−7 or 10−6 M). The effect of progesterone could be seen by gross inspection of the scaffolds. Scaffolds cultured in the presence of estradiol were covered in a smooth surface of cervical-like tissue. However, in the presence of progesterone, cervical-like tissue did not cover the scaffold uniformly. On histology, the gross impressions were confirmed: increased cervical-like tissue was seen in the presence of estradiol but progesterone opposed this effect. Measurement of collagen production showed estradiol significantly increased collagen production at 2 wk of culture, and the effect was more pronounced at 4 wk of culture. The presence of progesterone reduced collagen production to levels seen in vehicle controls. The effect of estradiol was also seen at the transcript level with increased gene expression of COL1A1 and COL3A1 at 2 and 4 wk of culture. In addition, the presence of the estrogen receptor and progesterone receptor was confirmed on histology and Western blot analysis after 4 wk of scaffold culture (Fig. 3).
The objective of the second 3D experiment was to 1) study a wider range of progesterone concentrations and 2) study the effect of mifepristone. We did not include the highest level of progesterone (10−6 M) in the second 3D experiment because the first 3D experiment showed no difference between 10−7 M and 10−6 M. In the second 3D experiment, after 3 wk of culture, progesterone inhibited synthesis of cervical-like tissue (Figs. 4 and 5) and the highest level of progesterone (10−7 M) corresponded to the least amount of collagen production. The presence of mifepristone (10−6 M) partially abrogated the effect of progesterone. There was no significant effect of progesterone on DNA synthesis or GAG production. On histology, the most amount of collagen synthesis was seen with no progesterone present (Fig. 5).
FIG. 5.
Histology from the experiment in Figure 4. All the scaffolds were cultured in the presence of estradiol (10−8 M). The figure at the top shows how cervical-like tissue can bridge a small gap between adjacent scaffolds. At Wk 3, the highest production of cervical-like tissue was seen in the absence of progesterone.
The electrophoretic mobility of collagen chains extracted from 3D culture was similar to both purified collagen and collagen extracted from human cervical tissue. Both α-chains and β-chains (cross-linked dimers) were seen, suggesting that collagen processing is preserved in 3D culture (Fig. 6). As expected, there was quantitatively less collagen extracted from cervical-like tissue because collagen content in native tissue is over 10 times greater than engineered tissue [46].
FIG. 6.
The electrophoretic mobility of collagen chains extracted from cervical-like tissue was similar to purified human collagen and collagen extracted from cervical tissue.
DISCUSSION
Progesterone inhibited collagen synthesis in 3D cultures from human cervical fibroblasts. The progesterone effect was strongest at the higher progesterone concentrations. This effect was abrogated by mifepristone, suggesting that the mechanism is mediated in part by the progesterone receptor. Progesterone did not affect tissue DNA content or GAG synthesis. Compared with 2D culture, longer culture times were possible with 3D culture while maintaining hormone responsiveness.
An improved understanding of collagen remodeling is critical to understanding the contribution of cervical dysfunction to preterm birth. In clinical obstetrics, there is intense interest in cervical assessment because a short cervix predicts subsequent preterm birth [7, 51]. The likelihood of cervical shortening depends in part on mechanical properties of the stroma, which arise from its collagen-rich connective tissue [11]. In human pregnancy, collagen concentration decreases during pregnancy [11], a period during which serum progesterone levels rise by over two orders of magnitude [49]. Here, we also show an inverse correlation with progesterone and collagen concentration in cervical-like tissue. We speculate that collagen remodeling seen in the present study corresponds to ECM changes seen with cervical softening.
It is important to emphasize that cervical softening also depends on factors not measured in this study, that is, collagen solubility [11], collagen cross-links [16], collagen morphology [52], and tissue permeability [53] also influence cervical properties. In addition, the mechanical properties of cervical-like tissue were not measured directly, and future studies are needed to determine how collagen concentration in cervical-like tissue affects mechanical properties. Nevertheless, this study is important for correlating collagen remodeling in 3D tissue with the hormonal environment of pregnancy.
It is not clear how to reconcile the results of this study with the therapeutic effects of progesterone supplementation given for a short cervix. This study does not support the concept that supplemental progesterone strengthens the cervix, a concept that is proposed in the clinical literature [54]. Rather, the study suggests that rising serum progesterone levels during pregnancy act to soften the cervix by a direct effect on cervical fibroblast function. Whether progesterone-mediated cervical softening is important for cervical shortening and preterm birth is not known. The study highlights the need for a more complete understanding of the cellular and biochemical aspects of progesterone effects on cervical remodeling for an improved understanding of progesterone's therapeutic efficacy. In addition, progesterone's therapeutic efficacy may be related to effects on tissues independent of the cervix such as the myometrium, decidua, or fetal membranes.
The 3D culture system presented here addresses some of the limitations of current model systems for studying cervical remodeling. Current model systems can be divided into two general categories: human models and animal models. In prior work, we used human cervical tissue from pregnant and nonpregnant hysterectomy specimens to measure mechanical properties and biochemical constituents. We showed pregnancy was associated with marked changes in tissue mechanical properties, and these changes correlated with changes in ECM composition [17, 18]. However, experiments with human cervical tissue are challenging because it is difficult to obtain sufficient cervical tissue during pregnancy [55]. Animal models, including the mouse, rat, primate, and guinea pig [29, 56–60] have been important for our current understanding of cervical remodeling. However, key differences exist between animal and human pregnancy. In addition, the complex environment of an animal model makes it difficult to distinguish cause and effect from multiple factors present in vivo. The present 3D model allows long-term culture of human cells in a microenvironment similar to native tissue, which makes it a valuable alternative to current model systems. Future 3D models could incorporate cocultures of fibroblasts and epithelial cells, which would permit studies of the interaction between the stroma and endocervical epithelium.
Although many studies highlight the importance of cervical ripening as an inflammatory response [32, 33], here we show inflammatory cells are not required for cervical remodeling. Progesterone, acting directly on cervical fibroblasts, affected ECM formation. This is not to discount the potential role for an inflammatory response and cervical remodeling. Indeed, cross-talk between cervical fibroblasts and the immune system has been demonstrated [36, 61–65]. Rather, this study supports the hypothesis that multiple pathways are important for preparing the cervix for parturition [66]. In addition, the cervical effects of progesterone are likely part of a larger mechanism of premature delivery because progesterone is known to act on the myometrium, decidua, and fetal membranes [26].
Although we demonstrated an important role for progesterone in remodeling cervical-like tissue, the specific biological mechanism requires further study. Both PR-B and PR-A were coexpressed in cervical-like tissue. Thus, it is likely that the functional response to progesterone depends on the genomic activity of both progesterone receptors [26]. For example, uterine quiescence is thought to be a PR-B effect. We hypothesize that cervical softening is also mediated by PR-B because softening (but not ripening) occurs slowly over the course of gestation. In future experiments, it will be interesting to correlate changes in the PR-A:PR-B ratio with changes in collagen synthesis in cervical-like tissue. Also, it is known that mifepristone has both antiprogesterone and antiglucocorticoid activity. To what extent glucocorticoid signaling is important in this model system is unknown. Estradiol increased synthesis of cervical-like tissue and progesterone opposed this effect, which may relate to progesterone inhibition of the ERα expression [67]. Last, we previously demonstrated that synthesis of cervical-like tissue was strongly dependent on cell nutritional environment, a mechanism that could relate to steroid hormonal environment [46, 47].
In summary, we have shown that progesterone has an inhibitory effect on cervical tissue formation in 3D culture. This novel model system allows long-term culture of human cervical fibroblasts in a 3D microenvironment similar to native tissue. We expect future studies targeting the mechanism of steroid effects on cervical-like tissue will have an important impact on cervical biology in pregnancy.
ACKNOWLEDGMENT
We gratefully acknowledge the Department of Gynecology at Tufts Medical Center for assistance with obtaining cervical biopsy specimens.
Footnotes
Supported by the Reproductive Scientist Development Program (NIH grant #2K12HD000849-21) and the March of Dimes Birth Defects Foundation. Additional funding from the Tissue Engineering Resource Center (TERC) from the National Institute of Biomedical Imaging and Bioengineering (EB002520) is gratefully acknowledged. Presented in part at the 59th Annual Meeting of the Society for Gynecologic Investigation, March 21–24, 2012, San Diego, California.
REFERENCES
- March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon: The Global Action Report on Preterm Birth. CP Howson, MV Kinney, JE Lawn (eds.). Geneva: World Health Organization; 2012.
- National Research Council. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: The National Academies Press, 2007. [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008; 371: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iams JD, Berghella V. Care for women with prior preterm birth. Am J Obstet Gynecol 2010; 203: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med 1996; 334: 567–572. [DOI] [PubMed] [Google Scholar]
- Owen J, Yost N, Berghella V, Thom E, Swain M, Dildy GA, III, , Miodovnik M, Langer O, Sibai B, McNellis D. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA 2001; 286: 1340–1348. [DOI] [PubMed] [Google Scholar]
- Society for Maternal-Fetal Medicine Publications Committee. Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol 2012; 206: 376–386. [DOI] [PubMed] [Google Scholar]
- Parry S, Simhan H, Elovitz M, Iams J. Universal maternal cervical length screening during the second trimester: pros and cons of a strategy to identify women at risk of spontaneous preterm delivery. Am J Obstet Gynecol 2012; 207: 101–106. [DOI] [PubMed] [Google Scholar]
- Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med 2007; 357: 462–469. [DOI] [PubMed] [Google Scholar]
- Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, Vijayaraghavan J, Trivedi Y, Soma-Pillay P, Sambarey P, Dayal A, Potapov V, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 2011; 38: 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House M, Kaplan DL, Socrate S. Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy. Semin Perinatol 2009; 33: 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol 1995; 38: 267–279. [DOI] [PubMed] [Google Scholar]
- Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction 2012; 143: 429–438. [DOI] [PubMed] [Google Scholar]
- Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med 2007; 25: 69–79. [DOI] [PubMed] [Google Scholar]
- Shi L, Shi SQ, Saade GR, Chwalisz K, Garfield RE. Studies of cervical ripening in pregnant rats: effects of various treatments. Mol Hum Repro 2000; 6: 382–389. [DOI] [PubMed] [Google Scholar]
- Akins ML, Luby-Phelps K, Bank RA, Mahendroo M. Cervical softening during pregnancy-regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse. Biol Reprod 2011; 84: 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Paskaleva AP, House M, Socrate S. Mechanical and biochemical properties of human cervical tissue. Acta Biomater 2008; 4: 104–116. [DOI] [PubMed] [Google Scholar]
- Myers KM, Socrate S, Paskaleva AP, House MA. Study of the anisotropy and tension/compression behavior of human cervical tissue. J Biomech Eng 2010; 132: 021003–021015. [DOI] [PubMed] [Google Scholar]
- Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction 2007; 134: 327–340. [DOI] [PubMed] [Google Scholar]
- Carbonne B, Dallot E, Haddad B, Ferre F, Cabrol D. Effects of progesterone on prostaglandin E(2)-induced changes in glycosaminoglycan synthesis by human cervical fibroblasts in culture. Mol Hum Reprod 2000; 6: 661–664. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Jackson EK, Keller PJ. 17Beta-estradiol, its metabolites, and progesterone inhibit cardiac fibroblast growth. Hypertension 1998; 31: 522–528. [DOI] [PubMed] [Google Scholar]
- Natoli AK, Medley TL, Ahimastos AA, Drew BG, Thearle DJ, Dilley RJ, Kingwell BA. Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension 2005; 46: 1129–1134. [DOI] [PubMed] [Google Scholar]
- Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthop Relat Res 2001; 268–281. [DOI] [PubMed]
- Norwitz ER, Robinson JN, Challis JR. The control of labor. N Engl J Med 1999; 341: 660–666. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev 2000; 21: 514–550. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci 2011; 18: 6–19. [DOI] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci U S A 2003; 100: 9518–9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernholm-Vladic Y, Wang H, Stygar D, Ekman G, Sahlin L. Differential regulation of the progesterone receptor A and B in the human uterine cervix at parturition. Gynecol Endocrinol 2004; 18: 41–46. [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol 1999; 13: 981–992. [DOI] [PubMed] [Google Scholar]
- Radestad A, Christensen NJ, Stromberg L. Induced cervical ripening with mifepristone in first trimester abortion. A double-blind randomized biomechanical study. Contraception 1988; 38: 301–312. [DOI] [PubMed] [Google Scholar]
- Wing DA, Fassett MJ, Mishell DR. Mifepristone for preinduction cervical ripening beyond 41 weeks' gestation: a randomized controlled trial. Obstet Gynecol 2000; 96: 543–548. [DOI] [PubMed] [Google Scholar]
- Sennstrom MB, Ekman G, Westergren-Thorsson G, Malmstrom A, Bystrom B, Endresen U, Mlambo N, Norman M, Stabi B, Brauner A. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod 2000; 6: 375–381. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Benson M, Scholz P, Daum J, Beier HM, Hegele-Hartung C. Cervical ripening with the cytokines interleukin 8, interleukin 1 beta and tumour necrosis factor alpha in guinea-pigs. Hum Reprod 1994; 9: 2173–2181. [DOI] [PubMed] [Google Scholar]
- Bokstrom H, Brannstrom M, Alexandersson M, Norstrom A. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Hum Reprod 1997; 12: 586–590. [DOI] [PubMed] [Google Scholar]
- Elovitz M, Wang Z. Medroxyprogesterone acetate, but not progesterone, protects against inflammation-induced parturition and intrauterine fetal demise. Am J Obstet Gynecol 2004; 190: 693–701. [DOI] [PubMed] [Google Scholar]
- Loudon JA, Elliott CL, Hills F, Bennett PR. Progesterone represses interleukin-8 and cyclo-oxygenase-2 in human lower segment fibroblast cells and amnion epithelial cells. Biol Reprod 2003; 69: 331–337. [DOI] [PubMed] [Google Scholar]
- Nold C, Maubert M, Anton L, Yellon S, Elovitz MA. Prevention of preterm birth by progestational agents: what are the molecular mechanisms? Am J Obstet Gynecol 2013; 208: 223.e1–223.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuon RJ, Shi SQ, Maul H, Sohn C, Balducci J, Maner WL, Garfield RE. Pharmacologic actions of progestins to inhibit cervical ripening and prevent delivery depend on their properties, the route of administration, and the vehicle. Am J Obstet Gynecol 2010; 202: 455.e1–455.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod 2006; 74: 236–245. [DOI] [PubMed] [Google Scholar]
- Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab 2010; 21: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing DA, Jones MM, Rahall A, Goodwin TM, Paul RH. A comparison of misoprostol and prostaglandin E2 gel for preinduction cervical ripening and labor induction. Am J Obstet Gynecol 1995; 172: 1804–1810. [DOI] [PubMed] [Google Scholar]
- Rath W, Osmers R, Adelmann-Grill BC, Stuhlsatz HW, Szevereny M, Kuhn W. Biochemical changes in human cervical connective tissue after intracervical application of prostaglandin E2. Prostaglandins 1993; 45: 375–384. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Garfield RE. Nitric oxide as the final metabolic mediator of cervical ripening. Hum Reprod 1998; 13: 245–248. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Lunan CB, Ledingham M, Howat RC, Cameron IT, Greer IA, Norman JE. Randomised trial of nitric oxide donor versus prostaglandin for cervical ripening before first-trimester termination of pregnancy. Lancet 1998; 352: 1093–1096. [DOI] [PubMed] [Google Scholar]
- Luque EH, Munoz de Toro MM, Ramos JG, Rodriguez HA, Sherwood OD. Role of relaxin and estrogen in the control of eosinophilic invasion and collagen remodeling in rat cervical tissue at term. Biol Reprod 1998; 59: 795–800. [DOI] [PubMed] [Google Scholar]
- House M, Sanchez CC, Rice WL, Socrate S, Kaplan DL. Cervical tissue engineering using silk scaffolds and human cervical cells. Tissue Eng Part A 2010; 16: 2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House M, Daniel J, Elstad K, Socrate S, Kaplan DL. Oxygen tension and formation of cervical-like tissue in two-dimensional and three-dimensional culture. Tissue Eng Part A 2012; 18: 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials 2005; 26: 2775–2785. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Hancock KW, Oakey RE. Non-protein-bound oestradiol and progesterone in human peripheral plasma before labour and delivery. J Endocrinol 1985; 104: 7–15. [DOI] [PubMed] [Google Scholar]
- Dennis RG, Kosnik PE., II. Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim 2000; 36: 327–335. [DOI] [PubMed] [Google Scholar]
- Feltovich H, Hall TJ, Berghella V. Beyond cervical length: emerging technologies for assessing the pregnant cervix. Am J Obstet Gynecol 2012; 207: 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akins ML, Luby-Phelps K, Mahendroo M. Second harmonic generation imaging as a potential tool for staging pregnancy and predicting preterm birth. J Biomed Opt 2010; 15: 026020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M, Vink J, Yoshida K, Wapner R, Myers KM. Direct measurement of the permeability of human cervical tissue. J Biomech Eng 2013; 135: 021024. [DOI] [PubMed] [Google Scholar]
- Campbell S. Universal cervical-length screening and vaginal progesterone prevents early preterm births, reduces neonatal morbidity and is cost saving: doing nothing is no longer an option. Ultrasound Obstet Gynecol 2011; 38: 1–9. [DOI] [PubMed] [Google Scholar]
- Keeler SM, Rust OA, Kiefer DG, Prutsman WJ, Proudfit CL, Naftolin F. Controlled fine needle biopsy of the uterine cervix during pregnancy. Reprod Sci 2011; 18: 737–742. [DOI] [PubMed] [Google Scholar]
- Ji H, Dailey TL, Long V, Chien EK. Androgen-regulated cervical ripening: a structural, biomechanical, and molecular analysis. Am J Obstet Gynecol 2008; 198 (543): e541–e549. [DOI] [PubMed] [Google Scholar]
- Simon C, Einspanier A. The hormonal induction of cervical remodeling in the common marmoset monkey (Callithrix jacchus). Reproduction 2009; 137: 517–525. [DOI] [PubMed] [Google Scholar]
- El Maradny E, Kanayama N, Kobayashi H, Hossain B, Khatun S, Liping S, Kobayashi T, Terao T. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Hum Reprod 1997; 12: 1080–1088. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Shao-Qing S, Garfield RE, Beier HM. Cervical ripening in guinea-pigs after a local application of nitric oxide. Hum Reprod 1997; 12: 2093–2101. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Ebner CA, Elovitz MA. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reprod Sci 2009; 16: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmstrom E, Sennstrom M, Holmberg A, Frielingsdorf H, Eklund E, Malmstrom L, Tufvesson E, Gomez MF, Westergren-Thorsson G, Ekman-Ordeberg G, Malmstrom A. The importance of fibroblasts in remodelling of the human uterine cervix during pregnancy and parturition. Mol Hum Reprod 2007; 13: 333–341. [DOI] [PubMed] [Google Scholar]
- Dubicke A, Akerud A, Sennstrom M, Hamad RR, Bystrom B, Malmstrom A, Ekman-Ordeberg G. Different secretion patterns of matrix metalloproteinases and IL-8 and effect of corticotropin-releasing hormone in preterm and term cervical fibroblasts. Mol Hum Reprod 2008; 14: 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson AE, Hyatt K, Kenkel C, Hanson K, Myers DA. Interleukin 1beta regulates progesterone metabolism in human cervical fibroblasts. Reprod Sci 2012; 19: 271–281. [DOI] [PubMed] [Google Scholar]
- Kim MG, Shim JY, Pak JH, Jung BK, Won HS, Lee PR, Kim A. Progesterone modulates the expression of interleukin-6 in cultured term human uterine cervical fibroblasts. Am J Reprod Immunol 2012; 67: 369–375. [DOI] [PubMed] [Google Scholar]
- Fukuyama A, Tanaka K, Kakizaki I, Kasai K, Chiba M, Nakamura T, Mizunuma H. Anti-inflammatory effect of proteoglycan and progesterone on human uterine cervical fibroblasts. Life Sci 2012; 90: 484–488. [DOI] [PubMed] [Google Scholar]
- Holt R, Timmons BC, Akgul Y, Akins ML, Mahendroo M. The molecular mechanisms of cervical ripening differ between term and preterm birth. Endocrinology 2011; 152: 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluska GJ, West NB, Novy MJ, Brenner RM. Uterine estrogen receptors are increased by RU486 in late pregnant rhesus macaques but not after spontaneous labor. J Clin Endocrinol Metab 1990; 70: 181–186. [DOI] [PubMed] [Google Scholar]