ABSTRACT
Female ESR2-null mice (betaERKO) display defects in ovarian function and are subfertile. Follicular maturation is impaired and explains smaller litters, but betaERKO also produce fewer litters, which may be partially due to inadequate ovulatory signals. To test this, the amplitude and timing of the naturally occurring luteinizing hormone (LH) surge was measured in individual intact betaERKO and wild-type (WT) mice. Vaginal cytology was evaluated daily, and blood samples were taken from mice in proestrus. The amplitude of the LH surge was severely blunted in betaERKO mice compared to WT, but pituitary LH levels revealed no differences. The betaERKO mice did not produce a preovulatory estradiol surge. To determine if the smaller LH surges and the reduced number of litters in betaERKO were due to the lack of ESR2 in the hypothalamic-pituitary axis or due to the absence of ESR2 in the ovary, ovaries were transplanted from WT into betaERKO mice and vice versa. The size of the LH surge was reduced only in mice lacking ESR2 within the ovary, and these mice had fewer litters. Fertility and size of the LH surge were rescued in betaERKO mice receiving a WT ovary. These data provide the first experimental evidence that the LH surge is impaired in betaERKO females and may contribute to their reduced fertility. ESR2 is not necessary within the pituitary and hypothalamus for the generation of a normal LH surge and for normal fertility, but ESR2 is essential within the ovary to provide proper signals.
Keywords: ESR2, estrogen receptor beta, fertility, HPO axis, naturally occurring luteinizing hormone surge, ovarian transplants, positive feedback
Lack of ESR2 expression in the ovary, but not in the hypothalamus, leads to reduced fertility and diminished LH surges.
INTRODUCTION
Estrogen's role in the classical hypothalamic-pituitary-ovarian (HPO) axis feedback loop is well described, most prominently the negative feedback on the hypothalamus and pituitary resulting in suppression of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) release [1]. Furthermore, the positive feedback mechanism is just as important for successful reproduction. A preovulatory estrogen surge is produced by the granulosa cells of maturing follicles, and this large increase in estrogen triggers the generation of a GnRH and subsequent LH surge. The increase in estradiol is absolutely required for the hypothalamus to initiate an LH surge, and the LH surge is required to trigger successful ovulation, a key factor for fertility [1–3].
Estrogen function is mediated by two nuclear receptors ESR1 (estrogen receptor alpha [ERα]) and ESR2 (estrogen receptor beta [ERβ]). These two receptors are transcribed from two different genes but have a degree of homology and bind estradiol with similar affinity [4]. However, there are differences in the ligand binding domain of these receptors, differences in transactivation of target genes, and differences in tissue-specific expression and distribution [5].
The role of ESR1 in maintaining normal reproductive function is undisputed. ESR1-null mice (αERKO) are infertile. ESR1 is found in the theca cell layer of growing follicles, in the hypothalamus and the pituitary, but not in GnRH neurons [6, 7]. The αERKO mice have hemorrhagic follicles in the ovary and systemically high levels of estrogen and testosterone [6, 7]. The lack of ESR1 expression in the hypothalamus disrupts the negative feedback, resulting in high levels of circulating LH. This chronic high level of LH is the underlying reason for the ovarian dysfunction and subsequent infertility in female αERKO mice [6, 7].
In contrast to ESR1, the role of ESR2 in the maintenance of normal reproduction and fertility has not been fully elucidated. Female ESR2-null mice (βERKO) are subfertile compared to wild type (WT), as characterized by fewer pregnancies, fewer litters, and smaller litter sizes [8, 9]. The role of ESR2 in mediating feedback within the HPO axis is poorly understood. ESR2 is expressed in GnRH neurons and in granulosa cells within the ovarian follicles [10, 11]. The role of ESR2 in negative feedback regulation seems to be minor [12]. While some experimental models find no role of ESR2 on positive feedback in GnRH neurons [13, 14], other studies clearly show a role of ESR2 on activation of these neurons resulting in the LH surge [15–18].
Ovaries of adult βERKO mice contain a reduced number of corpora lutea indicating fewer ovulations, and ovulation rates cannot be rescued by exogenous gonadotropins [8, 11, 19]. Follicular maturation is impaired in βERKO mice and results in a reduced number of follicles responsive to LH, which could explain why βERKO mice have smaller litters.
The fact that βERKO mice have fewer pregnancies and produce fewer litters may also be due to fewer adequate ovulatory signals (i.e., LH surges). Cultured follicles from βERKO follicles produce less estrogen than WT follicles [19, 20] and may provide an inappropriate stimulus to trigger physiologically relevant LH surges. To date, no differences in peripheral concentrations of LH or estradiol in βERKO mice compared to WT mice are documented. However, estradiol or LH has not been measured during the preovulatory period or during the time of the LH surge.
We hypothesized that the naturally occurring LH surge in βERKO mice is inadequate to support consistent ovulation. To test this hypothesis, we measured the amplitude and timing of the naturally occurring LH surge in individual intact βERKO mice and compared the levels to naturally occurring LH surges in WT mice. This novel approach was chosen because traditional models, based on ovariectomized, hormone-supplemented mice, are unable to mimic the complexity of communication within the intact HPO axis. Further, we performed ovarian transplants between WT and βERKO mice in order to investigate if our observations were due to a hypothalamic defect in βERKO mice or due to an inappropriate stimulus from the ovary.
Our serial bleeding technique and use of a high-sensitivity LH assay allowed, for the first time, documentation of the naturally occurring LH surge in individual mice. We describe that the LH surge and number of litters in βERKO mice is significantly diminished compared to WT mice. Use of ovarian transplantation uncovered that for a normally occurring LH surge and subsequent fertility, expression of ESR2 in the ovary is critical.
MATERIALS AND METHODS
Animals
All animals were handled according to National Institutes of Health (NIH) guidelines and in compliance with the National Institute of Environmental Health Sciences (NIEHS) Institutional Animal Care and Use Committee. All animals were maintained in standard plastic mouse cages, unless otherwise described, in temperature controlled rooms and fed NIH-31 mouse chow and water ad libitum. Numbers of animals used are described with the respective experiments.
The generation of ESR2-null mice (B6.129P2-Esr2tm1Unc/J), referred to hereafter as βERKO, has been described previously [8]. The generation of ESR1-null mice (B6.129P2-Esr1tm1Ksk/J), referred to hereafter as αERKO, has been described previously [6]. WT, βERKO, and αERKO mice all on a C57BL/6 background were obtained from Taconic Farms or from our breeding colony at NIEHS. WT litter mates of the knockout mice were used. Mice were weaned at 21 days of age and genotyped as described previously [21].
Fertility of female mice in our breeding colony was tested in a continuous mating scheme under controlled lighting (12L:12D). Breeding pairs of adult females (2–3 mo of age) and proven WT males (C57BL/6 males at 5–7 mo of age (Charles River) were housed together (one pair per cage), and males were removed after 16 wk. Cages were checked twice a day during this time and for an additional 23 days after removal of males, and number and size of litters were recorded.
Optimized Housing Conditions for the Measurement of the Naturally Occurring LH Surge
Housing conditions were optimized to provide a stress-free environment conducive to regular cyclicity. Female mice were housed in large plastic rat cages (five mice per cage) to avoid crowding-induced stress. Mature males were housed in separate cages next to the female cages, and all cages were covered only with felt tops to allow exposure to male pheromones. Lights were on for 14 h (14L:10D) as described by Bronson et al. and Goldman [22–27].
Vaginal Cytology
Vaginal washes (smears) were taken from each animal every morning throughout the experimental period in order to monitor the estrous cycles. Samples were immediately fixed on glass slides (Safetex; Andwin Scientific) dried, and hematoxylin and eosin stained using standard protocols. All slides were scored by the same individual (F.L.J.) and classified into one of four phases of the estrous cycle [28]. Briefly, a smear containing primarily nucleated epithelial cells with no or few cornified cells indicated proestrus (PRO), a smear containing predominantly cornified cells indicated estrus (E), a smear containing epithelial cells clumped in masses and leucocytes indicated metestrus (MET), and a thin smear containing predominantly leucocytes with some rounded or cornified epithelial cells indicated diestrus (DI).
Blood and Tissue Samples
Blood samples for the determination of the LH surge were obtained using the mandibular puncture technique (MEDIpoint Inc.) [29], which allows for the collection of small blood samples so that repeated sampling from the same animal is possible. Preliminary experiments under the guidance of NIEHS veterinarians determined that our repeat sampling scheme did not significantly affect the hematocrit and well-being of the animals.
Samples for the determination of LH surges (<100 μl whole blood) were taken on the evening of proestrus, the time when LH surges are predicted to occur [26, 27]. Animals were sampled at 2 h before dark (D − 2 h), at lights-out (D), and at 2 h after lights-out (D + 2 h). Samples were additionally obtained in the evening of the following day in order to detect if the LH surge may have been delayed in βERKO mice. Serum was stored at −80 C° until assayed. Each female was sampled during one to four different proestrous periods within the 50-day experimental period.
At the termination of the experiments, animals were euthanized by CO2 asphyxiation according to NIH guidelines. Blood samples were collected from the inferior vena cava. Pituitaries were collected, snap frozen, and stored at −80°C until assayed for LH content.
LH Assay
Serum and pituitary LH levels were measured in ng/ml using a sensitive low-volume assay based on the Dissociation-Enhanced Lanthanide Fluorescent Immunoassay (DELFIA; Wallac/PerkinElmer; Haavisto et al. [30]). We validated this for the measurement of mouse LH using the NIDDK reference preparation mLH-RP AFP5306A obtained from the National Hormone and Peptide Program. The lowest standard used in our assays was 0.03 ng/ml, and samples below 0.03 were assigned a value of 0.02 ng/ml before data were analyzed. Serum samples (20 μl) from sequential bleeds were assayed in singlet.
Pituitary LH content was determined after sonication of whole pituitaries in 500 μl saline for 60 sec followed by 1/100 dilution in assay buffer. Samples were assayed in duplicate with a maximum coefficient of variation of 7.6% between duplicates.
Estradiol Assay
Estradiol concentrations in serum were measured in pg/ml using a time resolved DELFIA (Wallac/PerkinElmer) following the manufacturer's instructions. The kit was validated for the use of mouse serum (25 μl), and all samples were assayed in duplicate in the same assay run. The lowest standard used was 6.8 pg/ml.
Ovarian Transplants
In the ovarian transplant experiments, the WT mice used as donors or recipients were Tyrosinase albino C57BL/6J-Tyrc-2J/J obtained from the Jackson Laboratory. All recipient mice were bred with Tyrosinase albino males C57BL/6J-Tyrc-2J/J males. Pups born could immediately and easily be identified as albino or black, indicating whether pups originated from residual host oocytes or from the transplanted ovary.
Bilateral ovarian transplants were performed. Both ovaries from the recipient were removed, and one-half of a donor ovary was placed within the empty bursa. The donor mouse was euthanized, and the ovaries were removed, halved, and placed in sterile 0.9% saline. The recipient mouse was anesthetized using isoflurane, oxygen, and buprenorphine (0.1 mg/kg). The recipient mouse was placed in ventral recumbency, and ovaries were exposed through a skin incision 1 cm lateral and perpendicular to the spine over the left cranial lumbar muscles. The surgeon cut with microdissecting scissors through both the pericapsular fat and the ovarian bursa. The bursa was cut only to the extent that allowed the retraction of the dorsal portion of the bursa until the ovary extruded through the incision. An assistant then placed Dumont forceps under the ovary, elevating it above the bursa and away from the infundibulum so that the surgeon could cut out the ovary. It was critical that the entire ovary was removed and that the infundibulum was not damaged. Homeostasis was accomplished by applying pressure with a swab pretreated with 1:10 000 epinephrine. Donor ovary was then transferred from the sterile saline and placed into the void created by the recipient's ovariectomy. The surgeon pulled the dorsal portion of the bursa over the donated ovary, ensuring its complete encapsulation. The abdominal musculature was sutured with 6-0 Vicryl (Ethicon Inc., Johnson & Johnson Family of Companies) in a simple interrupted pattern. The skin was closed with autoclips. The contralateral ovary was approached in the same manner and was replaced with the other half of the donor ovary. Ovarian transplant recipients were allowed to recover from surgery for 1 wk. Thereafter, vaginal smears were taken every day for 21 days to monitor the estrous cycle followed by a continuous mating study with proven Tyrosinase albino males for 8–16 wk. Blood samples for the determination of the LH surge were taken from a group of control and transplanted animals 6 wk after surgery.
Calculations and Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 5.00 or 6.00 for Windows (GraphPad Software) and SAS version 9.2 (SAS Institute, Inc.).
Vaginal cytology.
Estrus cycle phases are known to sometimes be shorter than 1 day. As long as the cycle progressed in the expected biological order (MET → DI → PRO → E), the cycle was counted in the analysis even if one of the phases (i.e., MET, DI, or PRO) was not detected [31]. Statistical approach was based on the total number of cycles, total number of study days, average cycle length (study days/number of cycles), and total number of days in DI, PRO, E, and MET. Percentages were transformed using arcsine transformations to improve normality. Genotypes were compared using unpaired two-sided t-test; P < 0.001.
LH surges, maximum LH, pituitary LH, and systemic estradiol.
For all 29 animals used in the first LH surge experiment, we identified the highest LH value during each 2-day sampling period. All three samples from the day containing the highest value were included in LH surge calculations, whereas all three samples from the other day were used for baseline LH calculations. Baseline LH did not differ between genotypes and was calculated to be 0.08 ± 0.12 ng/ml (mean ± SD). All samples with a value of three standard deviations above the baseline (>0.43 ng/ml) were counted as part of an LH surge. This cutoff value was also used in all subsequent experiments.
LH surges were only included in the analysis if the sampling period yielded LH data for all three time points (D − 2 h, D, and D + 2 h). Maximum LH was defined as the highest LH concentration of those three points. Maximum LH and area under the curve for each LH surge were highly correlated; therefore, only data on the maximum LH are presented. Quartile distributions were analyzed by chi-square analysis with maximum LH compared using the Mann-Whitney test; P-values are two sided. Pituitary LH content was analyzed by two-way analysis of variance (ANOVA) with the Bonferroni multiple comparison posttest, and preplanned comparison of the time point closest to the dark period was also done by t-tests; P-values are two sided. Systemic estradiol was compared using t-tests; P-values are two sided. Unless otherwise indicated, all results are reported as mean ± SEM.
LH surge after estradiol supplementation.
LH surges were compared using two-way ANOVA with the Bonferroni posttest. No statistical difference were noted, P > 0.05.
Ovarian transplant.
Maximum LH surges were compared using one-way ANOVA with the Dunnett multiple comparison posttest, P < 0.05. Fertility in animals with ovarian transplants were compared using one-way ANOVA with the Tukey multiple comparison posttest, P < 0.05.
RESULTS
βERKO Mice Are Subfertile
All genetic models of βERKO mice are subfertile [32]; however, colony phenotypes may ultimately depend on husbandry conditions and breeding protocols and may change over time [33]. Therefore, we reevaluated the severity of subfertility in the mice from our βERKO colony. Our results are consistent with the previously described βERKO fertility phenotypes [8], and we used a larger group of mice (n = 37) and a longer breeding period (16 wk). βERKO dams produced fewer litters than WT dams (litter mates). βERKO females produced 90% fewer pups than WT mice due to both 67% smaller litters and 69% fewer litters than WT mice (Supplemental Table S1 and Supplemental Figure S1; all Supplemental Data are available online at www.biolreprod.org). For our studies, we also had to confirm that our mice had at least some fertile cycles indicating that effective LH surges had occurred. We observed that 65% of our βERKO dams had one or more litters.
Regular Cyclicity Is Observed in WT and βERKO Mice
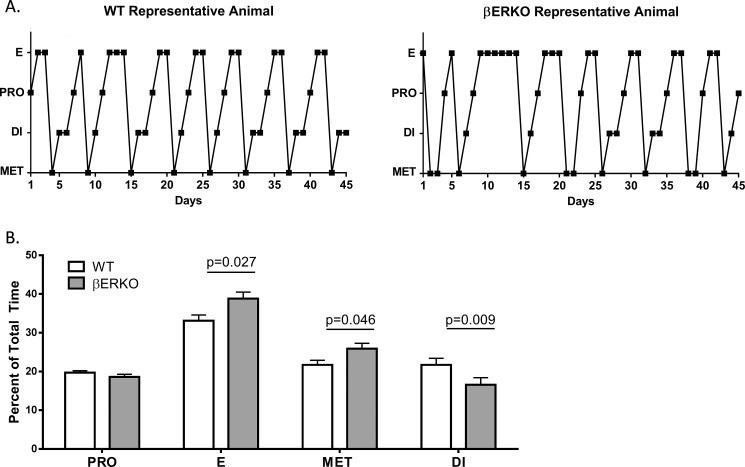
The reproductive cycle of adult WT (n = 20) and βERKO (n = 9) mice was monitored by vaginal cytology (smears) daily for 45–50 consecutive days. Smears were categorized as proestus (PRO), estrus (E), metestrus (MET), and diestrus (DI) as detailed in Materials and Methods. Occasionally, smears were difficult to categorize, especially in the βERKO animals, because they contained cells characteristic of several phases (nucleated cells, cornified cells, and leukocytes). Prolonged cornification was detected, meaning that a considerable number of cornified epithelial cells persisted over several days, often with leukocytes and nucleated cells present at the same time in βERKO animals. However, PRO was clearly detectable by the predominance of nucleated cells following DI or following a predominantly cornified smear.
WT and βERKO animals cycled regularly (Fig. 1A). The average cycle length did not differ (5.6 ± 0.2 days), and we detected an average of 7.1 ± 0.2 cycles per animal during the experimental period. βERKO animals overall spent more days in E and MET and fewer days in DI than WT mice (Fig. 1B). No differences were noted between WT and βERKO animals for time spent in PRO. Based on these cycle data, an equal chance of finding βERKO and WT animals in PRO for blood sampling was attainable. The observation that animals continued to cycle regularly after repeated blood sampling provides additional confirmation that the sampling procedure did not interfere with the well-being of the mice. These data demonstrate that this cohort of βERKO mice spend more days in E and MET with fewer days in DI but have equal cycle lengths when compared to WT mice.
FIG. 1.
βERKO mice spend more time in E and MET but less time in DI compared to WT mice. Cyclicity in WT and βERKO mice as determined by daily vaginal smears. A) Vaginal smears were taken for 45–50 consecutive days and scored to be either PRO, E, MET, or DI. Representative profiles of one WT and one βERKO animal are shown. B) Percentage of time spent in each stage of the cycle (mean ± SEM). Significant P-values between WT and βERKO mice in each stage are shown. Genotypes were compared using two-sample t-tests; P-values are two sided. PRO, proestrus; MET, metestrus; DI, diestrus; E, estrus.
The LH Surge of βERKO Mice Is Severely Blunted Compared to WT Mice
Blood samples used for the determination of the LH surge were taken only when the vaginal cytology clearly indicated PRO. Three blood samples were taken from each animal in PRO at 2 h before dark (D − 2 h), at lights-out (D) and at 2 h after lights-out (D + 2 h) as described in Materials and Methods. Samples were additionally obtained on the evening of the following day in order to detect if the LH surge may have been delayed in βERKO mice. In WT animals, 94.1% of all surges occurred on the first day, and in similar fashion, in the βERKO animals, 95.8% of all surges also occurred on the first day, indicating that there was no difference in the timing of the detected LH surges.
Within the 50-day experimental period, blood samples were obtained from up to four different PRO periods per animal. There was no difference between the WT and the βERKO mice in the number of surges detected per animal throughout the experimental period (surge = highest LH >0.43 ng/ml).
Overall, an LH surge was detected 71 ± 5% in WT mice and 77 ± 9% in βERKO mice of the times an LH surge was predicted to occur. A total of 51 LH surges were detected in WT animals, and a total of 24 LH surges were detected in βERKO animals throughout the experimental period.
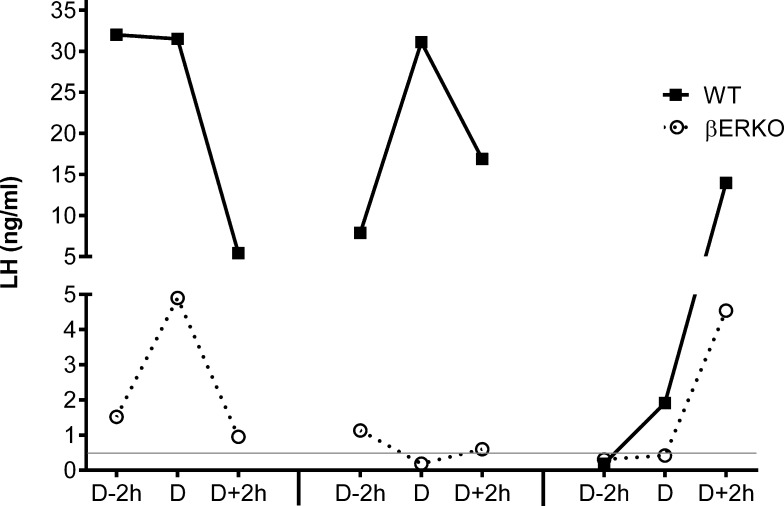
First, we evaluated the shape of the LH surges. Figure 2 shows the LH surge profiles of representative mice from both genotypes (surge = highest LH >0.43 ng/ml). Individual animals displayed surges with a maximum LH value not only at D (37.2% of WT; 16.7% of βERKO) but also at D − 2 h (19.6% of WT; 25.0% of βERKO). Most LH surges were measured with a maximum at D + 2 h (43.1% of WT; 58.3% of βERKO). There was no significant difference in time of maximum LH between genotypes, and individual animals displayed a full range of LH amplitudes (small and large). No correlation was detected between the size of the LH surge and individual mice, and each mouse displayed some smaller and some larger LH surges during the 50-day experimental period. These data demonstrate that the shape of the LH surge in WT and βERKO is comparable.
FIG. 2.
Timing and shape of the LH surge are similar between WT and βERKO mice. Consecutive LH surge profiles are shown from one representative WT and one βERKO mouse. Each animal was sampled during proestrus of three different cycles during the 50-day experimental period. Each LH surge profile is defined by three LH values measured in blood samples taken at D − 2 h, D, and D + 2 h. An LH surge threshold was defined as >0.43 ng/ml (represented by the gray line).
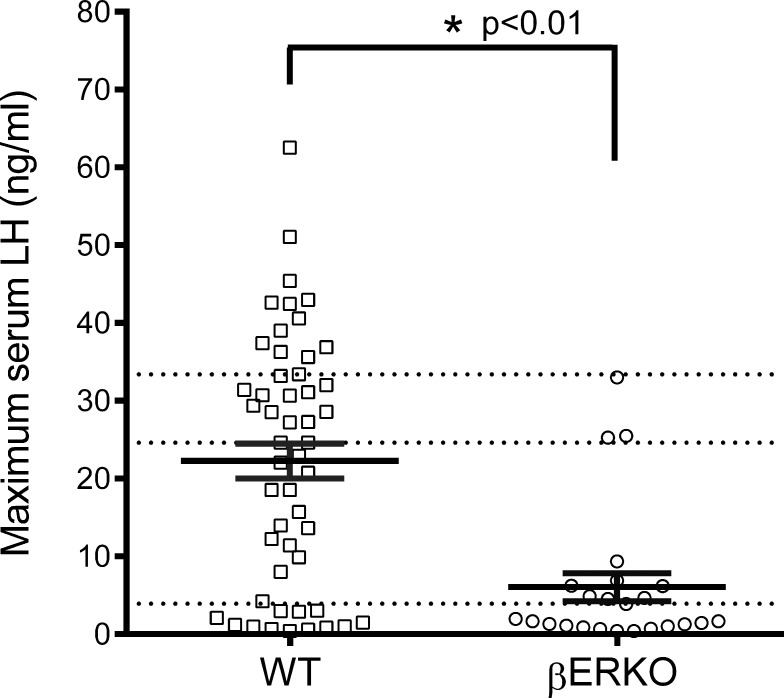
Next, we evaluated the size of the LH surges. The maximum LH value obtained from each LH surge was examined. There was no difference between WT and βERKO mice whether the largest surge occurred early or late in the 50-day experimental period. The average maximum LH in the WT was 22.3 ± 2.2 ng/ml (median concentration of 24.6 ng/ml) with the highest maximum LH value of 62.5 ng/ml (Fig. 3). In the βERKO animals, the average maximum LH was 6.1 ± 1.8 ng/ml (median of 1.8 ng/ml) with the highest maximum LH value of 33 ng/ml (Fig. 3). The distribution of maximum LH values in WT were divided into quartiles (Fig. 3, dotted lines) to analyze the distribution of βERKO maximum LH values within the same ranges. The distribution of maximum LH values differed between WT and βERKO (P < 0.001): 58.3% of βERKO maximum LH values were in the lowest WT quartile, 29.2% of βERKO maximum LH values were in the second quartile, 12.5% of βERKO maximum LH values were in the third quartile, and no βERKO maximum LH values fell into the highest WT quartile. Additionally, the three βERKO maximum LH values that fell into the third quartile originated from three different mice. These data demonstrate that the LH surge in βERKO mice is impaired (3.6-fold lower) relative to the WT surge levels.
FIG. 3.
Maximum LH measured in βERKO mice is significantly decreased relative to WT mice. Maximum LH values of LH surges detected in blood samples from WT and βERKO mice in the evening of proestrus. The solid bars represents the mean ± SEM, while the individual markers represent the distribution of all values (WT, n = 51; βERKO, n = 24). The distribution of WT maximum LH into quartiles is indicated by the horizontal dotted lines, and the percentile distributions of maximum LH values for βERKO within those quartiles are lowest quartile (58.3%), second quartile (29.2%), third quartile (12.5%), and fourth quartile (0%). Quartile distributions were analyzed by chi-square analysis. *P < 0.01. Maximum LH was compared using Mann-Whitney test; P-values are two sided.
Pituitary LH Stores in WT and βERKO Mice Are Comparable
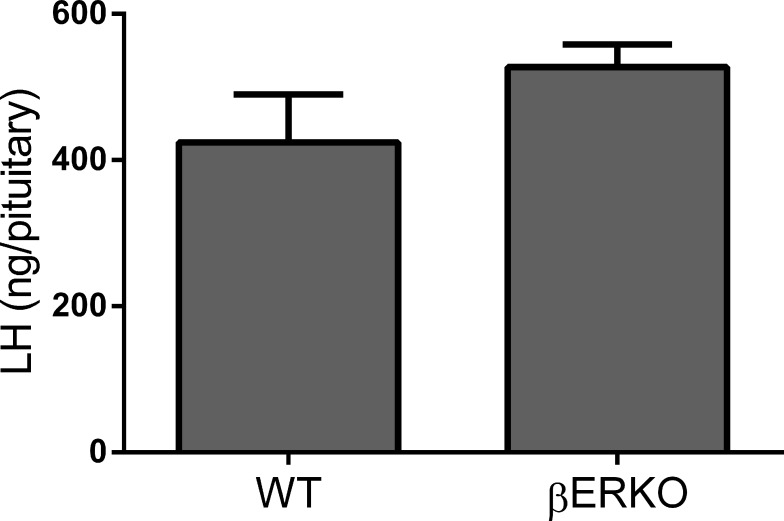
To determine if pituitary LH stores in βERKO mice are adequate to support a full-size LH surge, we measured the LH content in pituitaries collected from WT (n = 24) and βERKO (n = 22) female mice housed under the same optimized conditions as in the previous experiment. Cycles were monitored daily by vaginal cytology, and pituitaries were collected on the day of PRO at 12, 9, and 5 h before dark (D − 12 h, n = 13; D − 9 h, n = 17; D − 5 h, n = 16). Pituitary LH content did not differ between time points (P > 0.5). Within time points, the LH content in βERKO pituitaries was never smaller than in WT. Figure 4 shows that on the evening of proestrus, at D − 5 h (the time point closest to the expected LH surge), pituitary LH stores in βERKO were not different from pituitary LH stores in WT (P > 0.1), demonstrating that sufficient LH stores were present in βERKO animals.
FIG. 4.
LH pituitary content is not different between WT and βERKO mice. LH content in pituitaries collected from intact cycling mice (mean ± SEM) 5 h before dark on the day of proestrus. Pituitaries from βERKO mice contained similar amounts of LH as WT. P > 0.1 by t-tests; P-values are two sided. n = 8 per group.
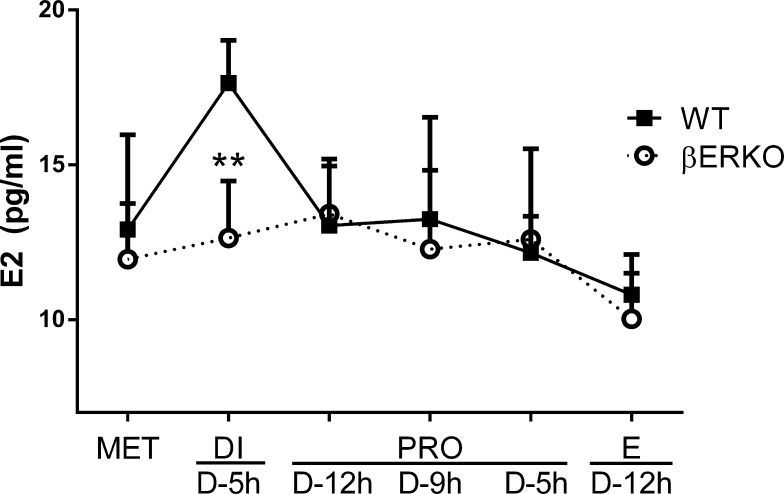
βERKO Mice Fail to Mount a Preovulatory Estradiol Surge
To examine if the abnormal LH surges in the βERKO animals could be due to suboptimal estradiol (E2) concentrations reaching the hypothalamus and pituitary, we collected trunk blood from WT (n = 48) and βERKO (n = 39) mice at every stage of the cycle for the determination of estradiol concentrations in peripheral serum. Mice were housed under optimized conditions as in the LH surge experiments, and vaginal cytology was monitored daily. Of particular interest were the estradiol levels during the periovulatory period, as a preovulatory E2 maximum is described in the rat and in other species [34–36]. Therefore, samples were collected at MET, DI (D − 5 h), PRO (D − 12, D − 9, and D − 5 h), and E (D − 12 h; Fig. 5). A preovulatory E2 surge was detectable in WT mice (Fig. 5) during the afternoon of DI, but βERKO mice failed to show a significant preovulatory increase of E2. These data show that βERKO mice fail to mount a preovulatory E2 surge, which may consequently affect the ensuing LH surge.
FIG. 5.
Systemic estradiol (E2) does not increase in βERKO mice during DI. E2 concentrations in peripheral blood samples collected during all stages of the estrous cycle at different times before dark (D) as indicated on the x-axis. ** P < 0.01 compared to WT by t-tests; P-values are two sided. Sample sizes: MET (WT, n = 9; βERKO, n = 6), DI D − 5 h (WT, n = 8; βERKO, n = 8), PRO D − 12 h (WT, n = 7; βERKO, n = 6), PRO D − 9 h (WT, n = 9; βERKO, n = 7), PRO D − 5 h (WT, n = 8; βERKO, n = 8), E D− 12 h (WT, n = 7; βERKO, n = 4).
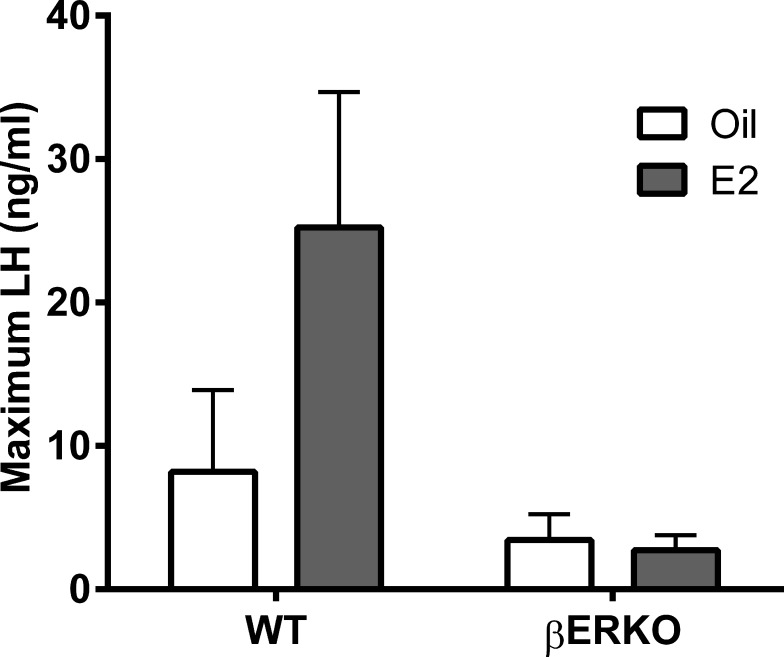
Estradiol Supplementation Does Not Rescue the LH Surge in βERKO Mice
To determine if supplementation of E2 during the afternoon of DI would allow for a normal-size LH surge in βERKO mice, WT (n = 16) and βERKO (n = 14), female mice were housed under optimized conditions, and vaginal cytology was monitored daily. On the afternoon of DI (D − 5 h), mice were injected with E2 (10 μg/kg body weight [BW]; n = 15) or vehicle (sesame oil; n = 15). This dose of E2 has been shown to be effective in mice [37]. On the following evening (PRO), three sequential blood samples were taken from every mouse for the determination of LH levels. Consistent with our earlier experiments, we detected 78% of all predicted LH surges. The LH amplitudes (Fig. 6) in WT animals (E2: 23.9 ± 8.8; oil: 12.6 ± 5.7) were greater than in βERKO mice (E2: 2.7 ± 0.9; oil: 4.1 ± 1.6); P < 0.03). There was no effect of treatment with E2 in either genotype. The injection of estradiol (10 μg/kg BW) into βERKO mice on the afternoon of DI did not rescue the amplitude of the LH surge.
FIG. 6.
Estradiol (E2) supplementation does not rescue the LH surge in βERKO mice. Maximum serum LH concentrations (mean ± SEM) during the LH surge in animals injected with oil vehicle or estradiol (10 μg/kg BW) on the afternoon of DI. Sample sizes: WT, n = 6 per group; βERKO oil, n = 5; and βERKO E2, n = 4. P > 0.1, two-way ANOVA with Bonferroni posttest.
The Expression of ESR2 in the Ovary Is Required for an Ovulatory LH Surge
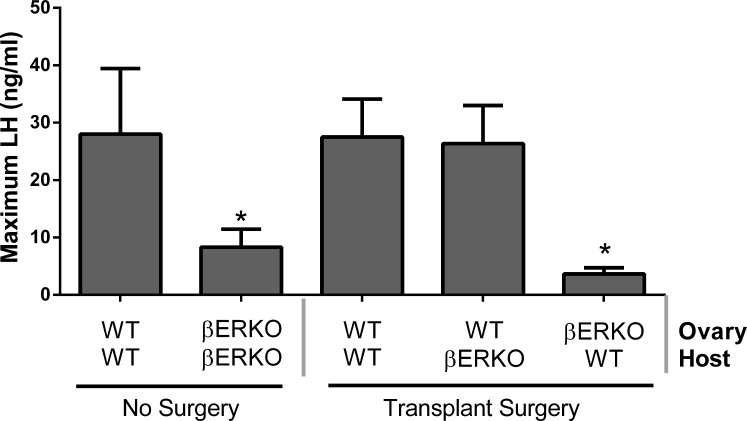
Female βERKO mice are known to display defects in ovarian function, and in addition to estrogen production, other ovarian factors may be affected. In order to address the question if the small/dampened LH surges and if the reduced number of litters in βERKO are due to the lack of ESR2 in the hypothalamic-pituitary axis or due to the absence of ESR2 in the ovary, we transplanted ovaries from WT mice into βERKO mice and vice versa. Groups of mice included WT ovary to WT host (WT in WT; n = 20), WT ovary to βERKO host (WT in βERKO; n = 18), and βERKO ovary to WT host (βERKO in WT; n = 21). Additionally, αERKO (ESR1 knockout animals) transplants were used as controls because the primary defect in αERKO is the hypothalamus/pituitary, as conditional knockout of ESR1 in the gonadotrophs results in female infertility [38] and agonists for ESR1 but not ESR2 are capable of inducing LH secretion in E2-primed pituitaries in vitro [39, 40]. Groups included ovary to WT host (αERKO in WT; n = 13), and WT ovary to αERKO host (WT in αERKO; n = 10).
Blood samples for the determination of the LH surge were taken from control and transplanted animals 6 wk after surgery (intact WT [n = 6], intact βERKO [n = 13], WT in WT [n = 4], WT in βERKO [n = 5], and βERKO in WT [n = 5]). Estrus cycles were monitored daily for 21 days by vaginal cytology, and three sequential blood samples were taken from each animal in the evening of PRO at 2 h before dark (D − 2 h), at lights-out (D), and at 2 h after lights-out (D + 2 h) as described in Materials and Methods. Every animal was sampled during one to two different proestrus periods within the 21-day experimental period. The average LH surge amplitude (maximum LH) in nonsurgery controls was greater in WT mice (mean: 28.0 ± 11.4; median 41.9 ng/ml) than in βERKO mice (mean 8.3 ± 3.1; median 2.1 ng/ml; Fig. 7), similar to the values observed in the initial LH surge experiment (Fig. 3). Maximum LH was not affected in mice with transplants between WTs serving as surgery controls and averaged 27.5 ± 6.6 ng/ml (median 31.1 ng/ml). Transplantation of a WT ovary into a βERKO mouse rescued the size of the LH surge (mean 26.4 ± 6.6; median 27.1 ng/ml), and transplantation of a βERKO ovary into a WT mouse reduced the size of the LH surge to 3.6 ± 1.1 (median 3.7 ng/ml), a value not different from the maximum LH in intact βERKO mice. These data strongly suggest that the LH surge defect in the βERKO mice is due to the absence of ESR2 expression and function in the ovary.
FIG. 7.
The serum LH level increases to WT levels when a WT ovary is transplanted into a βERKO host. Maximum serum LH concentrations (mean ± SEM) during the LH surge in animals with ovarian transplants. *P < 0.03; one-way ANOVA with the Dunnett multiple comparison posttest. Sample sizes: WT no surgery, n = 6; βERKO no surgery, n = 13; WT in WT, n = 4; WT in βERKO, n = 5; and βERKO in WT, n = 5.
Cyclicity (Supplemental Figure S2) and fertility (Supplemental Table S2) of these mice and ovarian histology from transplanted ovaries (Supplemental Figure S3) were examined. Genotypes of pups were determined, and only animals that produced litters originating exclusively from the transplanted ovary were included in the results. Our results reconfirm that expression of ESR1 in the hypothalamus is required for normal fertility (Supplemental Table S2). In transplants involving βERKO and WT animals, the number of litters did not differ between animals containing a WT ovary, but litter numbers were smaller in animals with a βERKO ovary (Supplemental Table S2). Of the control animals (WT ovary into a WT host), 90% became pregnant. In the βERKO ovary into a WT host group, only 19% of the animals became pregnant, but of the WT ovary into a βERKO host group, 83% of the animals became pregnant. These data correlate with the LH surge data, indicating that ESR2 expression in the ovary is essential for fertility and that ESR2 expression in the hypothalamic/pituitary axis is not required for normal reproductive function.
DISCUSSION
This is the first experimental evidence that the naturally occurring LH surge is severely impaired in the absence of ESR2 and the first time that the naturally occurring LH surge has been documented in individual intact mice. Our approach allowed for the assessment of communication within the intact HPO axis and the role of ESR1 and ESR2. Ovarian transplants of WT ovaries that express normal levels of ESR2 within βERKO mice rescued the size of the LH surge, and confirmed the critical role of ESR2 in the ovary. The signals sent from the ovary to induce an LH surge were often inadequate in the absence of ESR2. The resulting LH surges were significantly smaller, and we hypothesized that most were ineffective to induce ovulation. The occurrence of smaller LH surges was directly associated with reduced numbers of litters. The dampening of the LH surge was not based on limited stores of pituitary LH. The role of the ovary was critical in the induction of an appropriately sized LH surge, and we determined that systemic estradiol concentrations during the preovulatory period were indeed lower in βERKO mice than in WT mice. Ovarian transplants confirmed the defect in βERKO mice is mediated by ESR2 in the ovary and not ESR2 in the hypothalamic pituitary axis. Taken together, these observations are especially interesting and informative, as they implicate that ovarian factor(s) trigger the LH surge.
The physiological functions of ESR2 are still poorly understood and sometimes controversial. We consistently found βERKO mice to be subfertile, and these findings are in agreement with previous studies [7, 8, 32]. We confirmed the phenotype of smaller litters and fewer litters in our βERKO mice. It has generally been accepted that impairment of follicular maturation is the primary reproductive defect in βERKO mice [8], with fewer mature, preovulatory follicles explaining smaller litter sizes. However, we hypothesized that the reduced number of litters in mice lacking ESR2 also could be due to inadequate LH surges, resulting in anovulatory cycles. Previous reports have described systemic estradiol and LH concentrations only from randomly cycling animals, and no differences were found between WT and βERKO animals [8, 11, 19].
Herein, detailed data of systemic estradiol and LH concentrations during the periovulatory period are provided and report distinct differences between WT and βERKO mice. In independent experiments, the maximum LH concentrations in the evening of PRO were significantly smaller in mice lacking ESR2 in the ovary than in WT mice. An objective and purely statistical measure was applied to identify LH surges since no data are available on what constitutes an effective, ovulation-inducing LH surge in mice. An LH surge threshold has been proposed as necessary in order for ovulation to occur; however, the absolute value is not known in mice. Ryan et al. [41] observed LH values characteristic of the LH surge (>10 ng/ml) in mice, but their study used a different LH-standard preparation. In the rat, it is postulated that the LH surge needed is 14% of the maximum measured [42]. Analyzing our data with a subjective cutoff of 9 ng/ml (based on the 14% cutoff postulated in the rat data [42]) for a presumably effective LH surge showed that the success rate in βERKO mice was only 24% of WT mice. This is virtually the same proportion of βERKO mice (25%) that produced two or more litters when compared to WT mice that all had two or more litters during the breeding study. It is likely that many of the LH surges counted in our study for βERKO animals did not qualify as physiologically effective surges and were ineffective in triggering ovulation. Ultimately, if ∼9 ng/ml LH is not the absolute level of the cutoff value in mice, we can still definitely conclude that the lack of ESR2 results in smaller or fewer effective LH surges, leading to fewer ovulatory cycles and fewer litters. Consistently, the fewer litters were associated with smaller LH surges. This correlation between βERKO litter numbers and amplitude of the LH surge has not previously been described.
The smaller LH surges in βERKO mice could have been due to inadequate pituitary LH content or improper pituitary responsiveness to GnRH. Changes in responsiveness of the pituitary were not explored because no clear evidence for the presence of ESR2 in the mouse pituitary is described [7, 43]. Pituitary LH content and storage is documented to be stimulated by low-frequency GnRH pulses from the hypothalamus, and GnRH neurons contain ESR2 [15, 44]. Negative feedback of rising estradiol concentrations is known to induce LH production and storage within the pituitary as well as prepare the hypothalamus for the coordinated release of GnRH, both needed for the large amounts of LH released during the LH surge. On investigation, the LH stores in βERKO mice during the preovulatory period showed that they were adequate, implying that a lack of stored LH was not the underlying reason for the smaller LH surges. In addition, the ovarian transplant experiments would have uncovered changes in pituitary responsiveness due to the lack of ESR2 in the pituitary, yet none were detected.
Very little data have been published on the naturally occurring LH surge in the intact mouse. Most research on the generation and regulation of the LH surge has been done in the rat, as they allow for larger blood samples and cycle more regularly than mice. However, Bronson [24–27] used more than 2000 CF-1 mice for his research on the components affecting the timing and size of the LH surge. It has since been accepted that the maximum of the LH surge is expected around the time of lights-out. Nevertheless, strain differences are to be expected, and Ryan [41] reports maximum LH in White Swiss mice 4 h after lights-out (dark). Our unique experimental design allowed for the documentation of several LH surges from individual mice and, therefore, a better evaluation of the timing and the size of the surges. In our experiments using C57BL/6 mice, the highest LH concentrations were detected at 2 h after lights-out (D + 2 h) about 50% of the time. Both genotypes also generated LH surges with a maximum at 2 h before dark as well as at the time of lights-out. Certainly, additional time points would be helpful to substantiate our observations, but our close examination of the preovulatory period, especially around the time of lights-out, allowed us to establish that in C57BL/6 mice lacking ESR2, the timing and shape but not the amplitude of the LH surge is similar to WT mice.
Our data revealed that the abnormal LH surges in the βERKO animals are due to a suboptimal signal from the ovary. Estradiol was the most obvious candidate. A preovulatory estrogen surge is absolutely necessary in most species to trigger positive feedback, release of GnRH, and subsequent LH surge. ESR2 is expressed in the granulosa cells, the estrogen-producing cells, within the ovarian follicle. We had previously established that follicular maturation is impaired in βERKO mice [19] and that follicles acquire fewer LH receptors [20]. Occasionally, a few mature follicles in βERKO animals can respond to an ovulatory stimulus, but treatment with exogenous gonadotropins (superovulation) did not improve ovulation rates [8, 11]. This is in line with our current observations and the observation that βERKO mice produce fewer pups per litter. Cultured follicles from βERKO follicles produce less estrogen than WT follicles [19, 20], and inappropriate amounts of estrogen may be secreted by the βERKO follicles in vivo during the critical time period. This may provide an inappropriate stimulus to trigger physiologically relevant LH surges, though, until now, this assumption had not been confirmed, and morning samples from randomly cycling WT and βERKO mice had shown no difference in serum estradiol concentration [8, 11, 19]. Here we report E2 concentrations during the preovulatory period and show that an increase in E2 was observed in WT mice on the afternoon of diestrus, but this increase was absent in the βERKO mice. An increase in E2 usually occurs during proestrus in the rat [35, 45], but reports in mice vary and are affected by housing conditions and strain of mice [41, 46–48]. Nevertheless, our data suggest that the hypothalamus/pituitary of the βERKO mouse is exposed to less estradiol during the preovulatory period, a probable reason for ineffective positive feedback resulting in small LH surges.
Interestingly, when estrogen was supplemented during the expected time of the estrogen surge, the size of the LH surge was not rescued in mice lacking ESR2. It is possible that the timing and dose of estradiol supplementation was not ideal; however, in WT mice, the same dose of estradiol supplementation is commonly used and effective in other experiments [37]. Our observations from the ovarian transplant studies are especially interesting and informative because they clearly implicate that ovarian factors not only trigger an LH surge but also affect the size of the LH surge. It was thought that there needed to be only a threshold amount of estrogen during the preovulatory period in order to trigger a GnRH and a subsequent LH surge [27]. In contrast, our experiments suggest that a much more complicated and coordinated set of signals from the ovary is required for a proper LH surge. Beyond the scope of our current study, future investigation will now need to address the potential combination of ovarian factors needed to rescue the LH surge in βERKO mice. Important to ovulation and found in granulosa cells are inhibin and activin, which modulate the secretion of FSH by increasing GnRH receptor formation and collectively creating a feedback loop needed for ovulation [44]. Additionally, future studies could explore the concentration and replacement of progesterone, as it is recognized that adult βERKO ovaries contain a reduced number of corpora lutea, indicating fewer ovulations than WT mice [11]. Most likely, the fine-tuning of the size of the LH surge is a combination of the aforementioned hormones, secreted peptides, dose, and timing.
By utilizing WT ovarian transplants, we demonstrate the rescue of the βERKO subfertility supporting the critical nature of ESR2 in the ovary and establish that the size of the LH surge was reduced only in mice lacking ESR2 within the ovary, that the same mice experienced lower fertility (i.e., fewer litters per animal), and that the ESR2 status within the hypothalamus and pituitary did not affect fertility or the size of the LH surge. Therefore, we conclude that ESR2 is not necessary within the pituitary and hypothalamus for the generation of a normal-size LH surge and for normal fertility but that ESR2 is essential within the ovary for the generation of a normal-size LH surge and for normal fertility. Our results also establish that the signals sent from the βERKO ovary are triggering smaller-than-normal LH surges and that for a normal LH surge to occur, the presence of ESR2 is required within the ovary.
Supplementary Material
ACKNOWLEDGMENT
We thank Sylvia Hewitt and Dr. Heather Franco for critical review of this article. From the NIEHS Comparative Medicine branch, we thank James Clark and Page Myers for the ovarian transplantation surgery and Clay Rouse for help with the mandibular bleeding technique. We thank Debbie Best at the U.S. Environmental Protection Agency for assistance and reagents for the DELFIA LH assay, Mariana Yates and Katherine Hamilton for staining of vaginal smears, and John Couse and Judith Emmen for helpful discussion.
Footnotes
Supported by the Division of Intramural Research of the National Institute of Environmental Health Sciences through Z01 ES70065 to K.S.K. Presented in part at the 39th Annual Meeting of the Society for the Study of Reproduction, 20 July–1 August 2006, Omaha, NE; the 41st Annual Meeting of the Society for the Study of Reproduction, 17–30 May 2008, Kailua-Kona, Hawaii; and the Reproductive Tract Biology Gordon Conference, 3–8 August 2008, Andover, NH.
These authors contributed equally to this work.
REFERENCES
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 1998; 19: 302–330. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J Neuroendocrinol 2009; 21: 305–311. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology 2008; 149: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci 2004; 80: 14–25. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 1997; 277: 1508–1510. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Reproductive phenotypes in the estrogen receptor-alpha knockout mouse. Ann Endocrinol (Paris) 1999; 60: 143–148. [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 1999; 20: 358–417. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A 1998; 95: 15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 2000; 127: 4277–4291. [DOI] [PubMed] [Google Scholar]
- Ng Y, Wolfe A, Novaira HJ, Radovick S. Estrogen regulation of gene expression in GnRH neurons. Mol Cell Endocrinol 2009; 303: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 2005; 146: 3247–3262. [DOI] [PubMed] [Google Scholar]
- Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology 2003; 78: 204–209. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Elzer J, Herbison AE, Fritzemeier KH, Schutz G. Genetic dissection of estrogen receptor signaling in vivo. Ernst Schering Found Symp Proc 2006; 1: 25–44. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 2006; 52: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci 2009; 29: 5616–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci 2003; 23: 5771–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moenter SM, Chu Z, Christian CA. Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones. J Neuroendocrinol 2009; 21: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham IM, Herbison AE. Major sex differences in non-genomic estrogen actions on intracellular signaling in mouse brain in vivo. Neuroscience 2005; 131: 945–951. [DOI] [PubMed] [Google Scholar]
- Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER)α and ERβ null mice indicate a role for ERβ in follicular maturation. Endocrinology 2005; 146: 2817–2826. [DOI] [PubMed] [Google Scholar]
- Rodriguez KF, Couse JF, Jayes FL, Hamilton KJ, Burns KA, Taniguchi F, Korach KS. Insufficient luteinizing hormone-induced intracellular signaling disrupts ovulation in preovulatory follicles lacking estrogen receptor-β. Endocrinology 2010; 151: 2826–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol 2003; 17: 1039–1053. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms 2001; 16: 283–301. [DOI] [PubMed] [Google Scholar]
- Coquelin A, Bronson FH. Release of luteinizing hormone in male mice during exposure to females: habituation of the response. Science 1979; 206: 1099–1101. [DOI] [PubMed] [Google Scholar]
- Bronson FH. The reproductive ecology of the house mouse. Q Rev Biol 1979; 54: 265–299. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Light intensity and reproduction in wild and domestic house mice. Biol Reprod 1979; 21: 235–239. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Vom Saal FS. The preovulatory surge of luteinizing hormone secretion in mice: variation in magnitude due to ambient light intensity. Biol Reprod 1979; 20: 1005–1008. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology 1979; 104: 1247–1255. [DOI] [PubMed] [Google Scholar]
- Allen E. The oestrous cycle in the mouse. Am J Anat 1922; 30: 297–353. [Google Scholar]
- Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 2005; 34: 39–43. [DOI] [PubMed] [Google Scholar]
- Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology 1993; 132: 1687–1691. [DOI] [PubMed] [Google Scholar]
- Zhai J, Morris RWA. Markov chain model for animal estrous cycling data. Biometrics 2005; 61: 141–150. [DOI] [PubMed] [Google Scholar]
- Harris HA. Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol 2007; 21: 1–13. [DOI] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Sterility Mark M. and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci U S A 2008; 105: 2433–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahesh VB, Muldoon TG. Integration of the effects of estradiol and progesterone in the modulation of gonadotropin secretion. J Steroid Biochem 1987; 27: 665–675. [DOI] [PubMed] [Google Scholar]
- Chapman SC, Woodruff TK. Betaglycan localization in the female rat pituitary: implications for the regulation of follicle-stimulating hormone by inhibin. Endocrinology 2003; 144: 5640–5649. [DOI] [PubMed] [Google Scholar]
- Pau KY, Berria M, Hess DL, Spies HG. Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology 1993; 133: 1650–1656. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Li L, Grimm SA, Chen Y, Liu L, Li Y, Bushel PR, Fargo D, Korach KS. Research resource: whole-genome estrogen receptor alpha binding in mouse uterine tissue revealed by ChIP-seq. Mol Endocrinol 2012; 26: 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieske MC, Kim HJ, Legan SJ, Koo Y, Krust A, Chambon P, Ko C. Pituitary gonadotroph estrogen receptor-alpha is necessary for fertility in females. Endocrinology 2008; 149: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Criado JE. Martin De Las Mulas J, Bellido C, Tena-Sempere M, Aguilar R, Blanco A. Biological role of pituitary estrogen receptors ERalpha and ERbeta on progesterone receptor expression and action and on gonadotropin and prolactin secretion in the rat. Neuroendocrinology 2004; 79: 247–258. [DOI] [PubMed] [Google Scholar]
- Sanchez-Criado JE, de Las Mulas JM, Bellido C, Aguilar R, Garrido-Gracia JC. Gonadotrope oestrogen receptor-alpha and -beta and progesterone receptor immunoreactivity after ovariectomy and exposure to oestradiol benzoate, tamoxifen or raloxifene in the rat: correlation with LH secretion. J Endocrinol 2005; 184: 59–68. [DOI] [PubMed] [Google Scholar]
- Ryan KD, Schwartz NB. Changes in serum hormone levels associated with male-induced ovulation in group-housed adult female mice. Endocrinology 1980; 106: 959–966. [DOI] [PubMed] [Google Scholar]
- Greig F, Weisz J. Preovulatory levels of luteinizing hormone, the critical period and ovulation in rats. J Endocrinol 1973; 57: 235–245. [DOI] [PubMed] [Google Scholar]
- Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 1997; 138: 4613–4621. [DOI] [PubMed] [Google Scholar]
- Falcone T, Hurd WT. (eds) Clinical Reproductive Medicine and Surgery: A Practical Guide. New York: Springer Sciences; 2013: 31–42. [Google Scholar]
- Mahesh VB, Brann DW. Regulation of the preovulatory gonadotropin surge by endogenous steroids. Steroids 1998; 63: 616–629. [DOI] [PubMed] [Google Scholar]
- Fata JE, Chaudhary V, Khokha R. Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17beta-estradiol during the estrous cycle. Biol Reprod 2001; 65: 680–688. [DOI] [PubMed] [Google Scholar]
- Walmer DK, Wrona MA, Hughes CL, Nelson KG. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology 1992; 131: 1458–1466. [DOI] [PubMed] [Google Scholar]
- Hedrich H. The Laboratory Mouse, 2nd ed. New York: Academic Press; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.