Rapid eosinophil accumulation around nematode species occurs via leukotriene induction, which is necessary for nematode-induced eosinophil accumulation in the lung.
Abstract
Eosinophil accumulation is a defining feature of the immune response to parasitic worm infection. Tissue-resident cells, such as epithelial cells, are thought to initiate eosinophil recruitment. However, direct recognition of worms by eosinophils has not been explored as a mechanism for amplifying eosinophil accumulation. Here, we report that eosinophils rapidly migrate toward diverse nematode species in three-dimensional culture. These include the mammalian parasite Nippostrongylus brasiliensis and the free-living nematode Caenorhabditis elegans. Surprisingly, collective migration toward worms requires paracrine leukotriene B4 signaling between eosinophils. In contrast, neutrophils show a minimal response to nematodes, yet are able to undergo robust leukotriene-dependent migration toward IgG-coated beads. We further demonstrate that eosinophils accumulate around C. elegans in the lungs of mice. This response is not dependent on bacterial products, CCR3, or complement activation. However, mice deficient in leukotriene signaling show markedly attenuated eosinophil accumulation after injection of C. elegans or N. brasiliensis. Our findings establish that nematode-derived signals can directly induce leukotriene production by eosinophils and that leukotriene signaling is a major contributor to nematode-induced eosinophil accumulation in the lung. The similarity of the eosinophil responses to diverse nematode species suggests that conserved features of nematodes are recognized during parasite infection.
Leukotrienes are eicosanoid lipid mediators generated from arachidonic acid by the enzyme ALOX5 (5-lipoxygenase; Haeggström and Wetterholm, 2002). Leukotriene B4 (LTB4) contributes to leukocyte accumulation in many inflammatory diseases, and its production by neutrophils is triggered by multiple stimuli (Sadik et al., 2012). Dual expression of ALOX5 and the LTB4 receptor LTB4R1 in neutrophils is required for their recruitment in a model of arthritis, revealing a paracrine amplification loop in this setting (Chen et al., 2006; Sadik et al., 2012). LTB4R1 is also highly expressed on human and mouse eosinophils (Tager et al., 2000), and human eosinophils produce LTB4 (Henderson et al., 1984), raising the possibility of paracrine leukotriene signaling between eosinophils analogous to that established for neutrophils.
Eosinophil accumulation is highly associated with infection by multicellular endoparasites, and this association is conserved from zebrafish to humans (Klion and Nutman, 2004; Balla et al., 2010). Sentinel cells in tissues are thought to instigate eosinophil accumulation by releasing the cytokines TSLP, IL-25, and IL-33 in response to worm-induced injury (Licona-Limón et al., 2013). These cytokines stimulate innate lymphoid cells, which enable eosinophil survival through IL-5 production. However, the signals that attract eosinophils into tissues during infection, and whether any are helminth derived, remain unclear. Mice deficient in CCL11 (eotaxin-1) exhibit reductions in eosinophil accumulation in some models of helminth infection (Klion and Nutman, 2004). However, the eotaxin receptor CCR3 is also important in regulating basal numbers of eosinophils in tissues, confounding these studies (Matthews et al., 1998). Furthermore, eosinophil recruitment is intact in Ccr3−/− mice during Nippostrongylus brasiliensis infection, indicating that other unknown recruitment factors are involved (Knott et al., 2009).
RESULTS AND DISCUSSION
Eosinophils migrate toward diverse nematode species
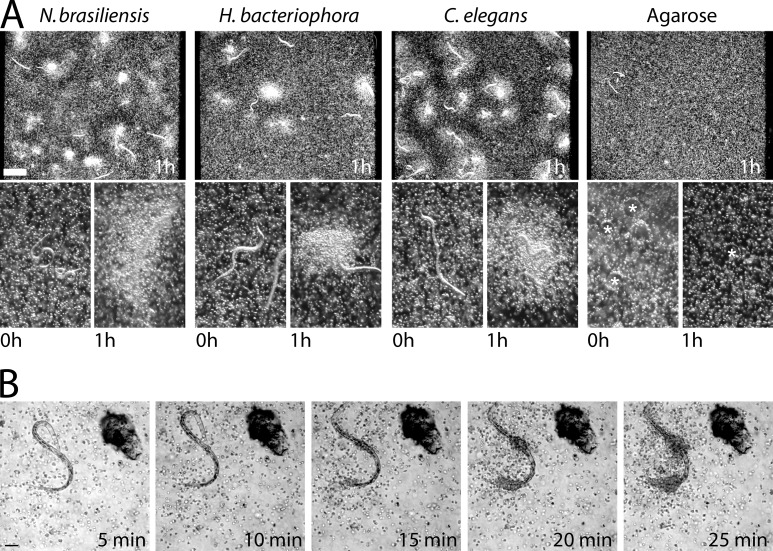
To determine whether live nematodes induce eosinophil migration directly, we suspended Caenorhabditis elegans, Heterorhabditis bacteriophora, or N. brasiliensis larvae in a three-dimensional (3D) Matrigel matrix along with mouse eosinophils (cultured from bone marrow progenitors). After 1 h, thousands of eosinophils had accumulated around worms of all three species (Fig. 1 A). Eosinophils did not accumulate around agarose (Fig. 1 A), chitin, or Teflon beads (not depicted), ruling out a general response to foreign surfaces. Accumulation also occurred in collagen gels, with heat- or formaldehyde-killed worms, and with eosinophils isolated from the blood or spleen of IL-5Tg mice (not depicted).
Figure 1.
Eosinophil migration in response to nematodes. (A) Darkfield dissection scope images of bone marrow–derived eosinophils cultured with nematodes. Low-power images (top) show eosinophil accumulation after 1 h. High-power images (bottom) show nematodes before and 1 h after eosinophil accumulation. Agarose beads are marked with asterisks. (B) Images taken from a differential interference contrast time-lapse video (Video 1) of eosinophils migrating toward a C. elegans dauer larva. (A and B) Results represent three independent experiments. Bars: (A) 500 µm; (B) 50 µm.
To determine whether directed migration of eosinophils was involved, we performed time-lapse imaging of eosinophils mixed with C. elegans dauers (Fig. 1 B and Video 1). Eosinophils migrated en mass over distances of up to 300 µm toward C. elegans. The cells congregated at specific points on each nematode (Fig. 1 B and Video 1), but these points differed from worm to worm. This indicated that no single anatomical structure was responsible for triggering migration. Eosinophil cell spreading was evident on the worm cuticle and was accompanied by migration along its length (Videos 1 and 2). Eosinophils from human peripheral blood also exhibited robust migration toward nematodes with similar cell-spreading interactions (Video 3).
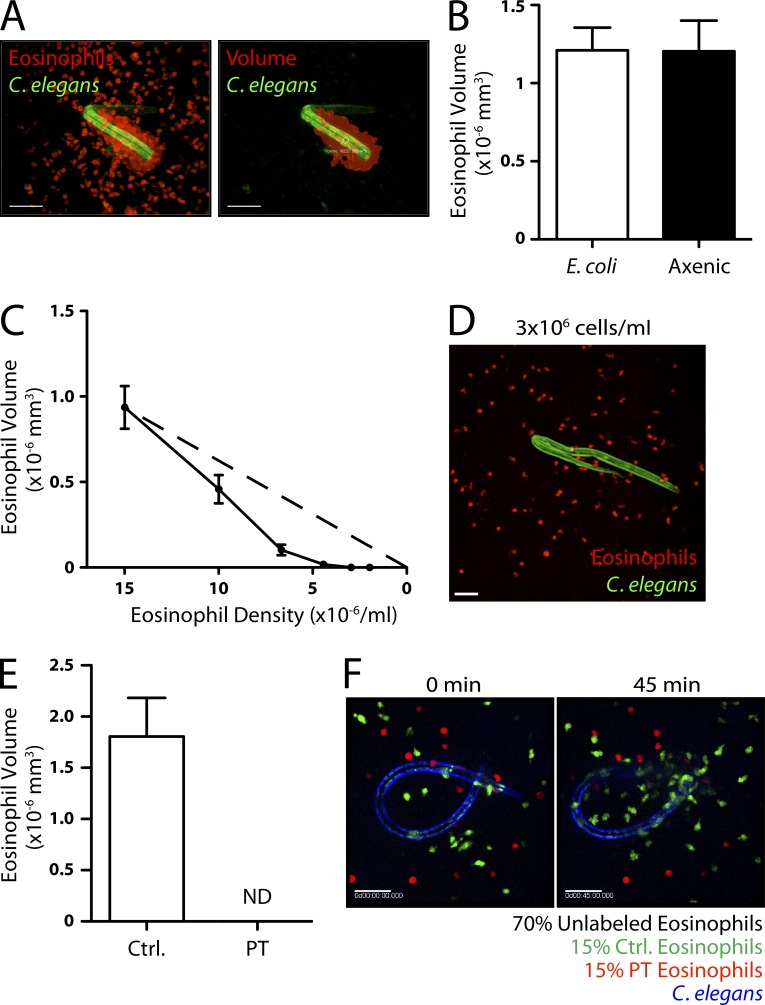
To quantify eosinophil migration, we used fluorescence confocal microscopy and image analysis to calculate the volume of eosinophils associated with each nematode after 1 h (Fig. 2 A). Many nematodes, including the species we used, feed on bacteria. Thus, we used only extensively washed, nonfeeding larval stages for which LPS was undetectable in worm supernatants (Limulus test). We also grew C. elegans in axenic liquid culture for more than three generations to eliminate bacterial products (Rao et al., 2005). There was no difference in eosinophil aggregation whether C. elegans was grown on Escherichia coli or in axenic medium (Fig. 2 B).
Figure 2.
Evidence for paracrine signaling in collective eosinophil migration. (A) An example of a 3D eosinophil volume calculation using Imaris software. The raw image (left) and artificial volume (right) are shown. (B) Eosinophil accumulation around monoxenic C. elegans dauers grown on E. coli and dauers grown in axenic culture (n = 10). (C) Eosinophil accumulation as a function of eosinophil density (n = 10). The dashed line represents linear proportionality with an x intercept at the origin. (D) Eosinophils after 2 h of culture with dauers at a density of 3 × 106 cells/ml. (E) Control and PT-treated eosinophil accumulation around C. elegans dauers (n = 6). (F) Images taken from a fluorescence microscopy time-lapse video (Video 4) of control and PT-treated eosinophils migrating toward a C. elegans dauer. The larva is shown at 0 min (left) and after 45 min (right) of culture. (A–F) Results represent three (A and B) or two (C–F) independent experiments. Error bars indicate standard error of the mean. Bars, 50 µm.
The preferential migration of eosinophils toward areas of growing eosinophil accumulation suggested that eosinophils themselves could be the source of chemoattractants. To investigate this possibility, we incubated C. elegans with varying densities of eosinophils and measured accumulation on the worms. As expected, at high densities, (>5 × 106 cells/ml) the volume of eosinophils that accumulated decreased with decreasing cell density (Fig. 2 C). However, at <3 × 106 cells/ml, there was no detectable migration toward worms (Fig. 2 D). A nematode-derived chemoattractant would be expected to attract eosinophils within the vicinity of the worm, independent of cell density. Thus, the observed threshold density requirement suggested that signaling between eosinophils was involved.
Collective migration is dependent on eosinophil-derived LTB4
We therefore suspected that eosinophil migration toward nematodes was mediated by a soluble, eosinophil-derived factor. Incubation of eosinophils with pertussis toxin (PT), to block Gαi/0-coupled chemoattractant receptors, completely abolished eosinophil accumulation around worms (Fig. 2 E). Next, we mixed equal numbers of differentially labeled PT-treated and control eosinophils and observed their migration. Although control cells migrated robustly toward C. elegans, PT-treated eosinophils failed to migrate toward the same worms, demonstrating that the effect of PT was intrinsic to the treated eosinophils (Fig. 2 F and Video 4).
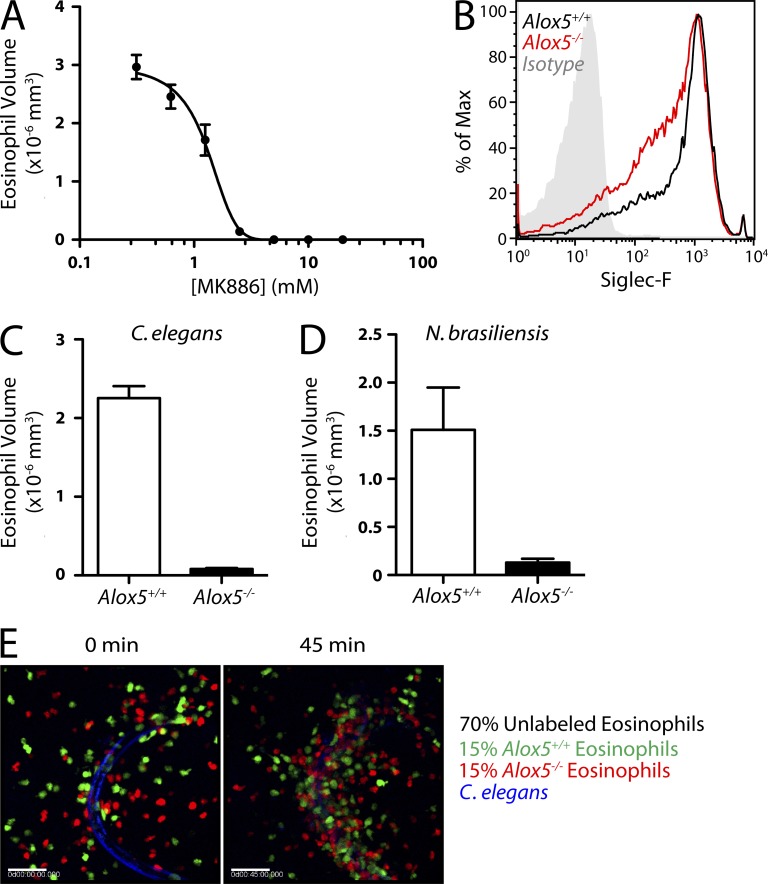
Neutrophils attract other neutrophils via LTB4 in vitro (Afonso et al., 2012), as well as in several models of inflammation (Sadik et al., 2011; Lämmermann et al., 2013). We found that MK886, a leukotriene synthesis inhibitor (Rouzer et al., 1990), prevented the accumulation of eosinophils around C. elegans in a dose-dependent manner (IC50 of ∼0.5 µM; Fig. 3 A). Furthermore, eosinophils derived from Alox5−/− bone marrow (Fig. 3 B) were >90% impaired in accumulating around C. elegans and N. brasiliensis (Fig. 3, C and D). To determine whether paracrine leukotriene signaling accounted for eosinophil migration toward nematodes, we attempted to rescue the migration of Alox5−/− cells by mixing in wild-type cells. We combined fluorescently labeled Alox5−/− cells in equal proportion with fluorescently labeled wild-type cells in the presence of abundant (70%) unlabeled wild-type cells. The migration of Alox5−/− eosinophils was indistinguishable from wild-type cells (Fig. 3 E and Video 5).
Figure 3.
Involvement of leukotrienes in collective eosinophil migration. (A) Eosinophil accumulation in response to C. elegans dauers in the presence of MK886 at the indicated concentrations in equal volumes of DMSO (n = 5). (B) Siglec-F staining on bone marrow–derived eosinophils from wild-type and Alox5−/− mice. (C and D) Accumulation of wild-type and Alox5−/− eosinophils in response to C. elegans dauers (n = 9; C) or N. brasiliensis larvae (n = 9; D). (E) Images taken from a fluorescence microscopy time-lapse video (Video 5) of wild-type and Alox5−/− eosinophils migrating toward a C. elegans dauer. The larva is shown at 0 min (left) and after 45 min (right) of culture. Bars, 50 µm. (A–E) Results represent two independent experiments. Error bars indicate standard error of the mean.
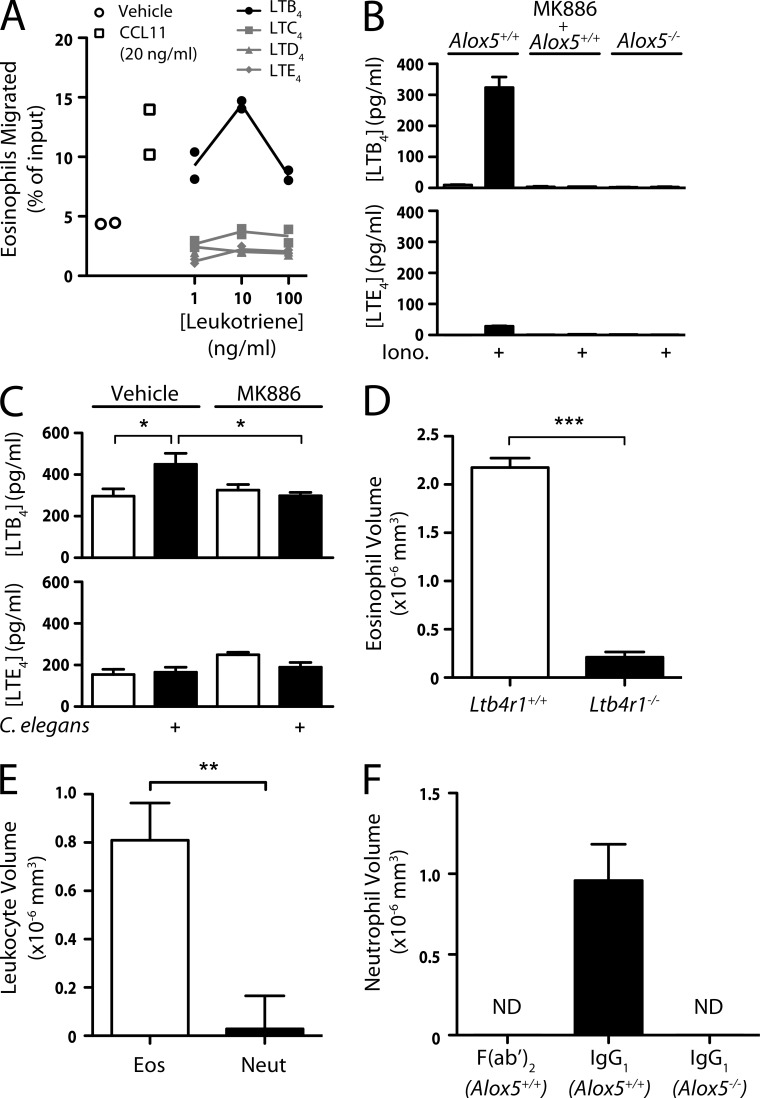
Because ALOX5 is required for the production of both LTB4 and cysteinyl leukotrienes (cysLTs), we next asked which leukotrienes were involved in the response to nematodes. Although eosinophils produce cysLTs upon activation (Kanaoka and Boyce, 2004), the CSYLTR1 antagonist montelukast (10 µM) failed to alter eosinophil migration to nematodes (not depicted). Because LTB4 is a chemoattractant for eosinophils in vitro (Spada et al., 1994), we tested whether our eosinophil preparations migrated toward LTB4 or the cysLTs in Transwell assays. We confirmed robust migration toward LTB4, as well toward CCL11, but there was no detectable response to LTC4, LTD4, or LTE4 (Fig. 4 A). When we activated eosinophils with ionomycin, there was ∼10-fold more LTB4 than LTE4 in the supernatant (Fig. 4 B). Furthermore, mixing of eosinophils with C. elegans stimulated eosinophil LTB4 production, which was completely inhibited by MK886 (Fig. 4 C). In contrast, there was no detectable production of LTE4 by eosinophils in response to worms. Finally, Ltb4r1−/− eosinophils exhibited a >90% reduction in accumulation around worms (Fig. 4 D), establishing that LTB4 signaling is required for Alox5-dependent migration and accumulation.
Figure 4.
Involvement of LTB4 in collective eosinophil migration. (A) Migration of eosinophils across Transwell membranes in response to CCL11 or leukotrienes. (B) LTB4 and LTE4 production in wild-type or Alox5−/− eosinophils treated with 1 µM ionomycin. Where indicated, cells were treated with 10 µM MK886. (C) LTB4 and LTE4 production in wild-type eosinophils incubated with C. elegans dauers. Where indicated, cells were treated with 10 µM MK886. (D) Accumulation of wild-type and Ltb4r1−/− eosinophils in response to C. elegans dauers (n ≥ 12 per experiment). (E) Eosinophil (Eos) and neutrophil (Neut) accumulation around C. elegans dauers (n ≥ 18). (F) Accumulation of wild-type and Alox5−/− neutrophils around IgG1- or F(ab′)2-coated agarose beads (n ≥ 8). (A–F) Results represent two (A–C, E, and F) or are pooled from four (D) independent experiments. Error bars indicate standard error of the mean. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Neutrophils are also recruited into tissues during parasite infection, although they are often far outnumbered by eosinophils (Makepeace et al., 2012). The known involvement of LTB4 in neutrophil migration prompted us to test whether neutrophils would respond to nematodes. Neutrophils isolated from mouse bone marrow exhibited >30-fold reduced migration toward C. elegans compared with eosinophils (Fig. 4 E). Sephadex beads, which are recognized by SIGN-R1 (Kang et al., 2003), also induced migration of eosinophils in an Alox5-dependent fashion, whereas neutrophils did not respond at all (not depicted). Neutrophils are known to produce LTB4 in response to Fc receptor engagement (Sadik et al., 2012). We therefore tested whether IgG1-coated beads would induce collective migration of neutrophils. Neutrophils migrated robustly toward beads coated with intact IgG1 (Fig. 4 F) but not to F(ab′)2-coated beads, confirming a requirement for Fc receptor engagement. There was no accumulation of Alox5−/− neutrophils around IgG1-coated beads. Collectively, these results show that leukotriene-dependent collective migration of granulocytes can be triggered by various primary stimuli and that nematodes selectively initiate this process in eosinophils.
Our findings suggest a novel mechanism of signal relay in which contact with a stimulatory particle induces leukotriene production by granulocytes, drawing increasing numbers of cells into association with the particle. Our data also highlight the ability of both eosinophils and neutrophils to produce and respond to LTB4. During the early stages of some fungal infections and many helminth infections, both eosinophils and neutrophils accumulate (Makepeace et al., 2012). Thus, these granulocytes may have complementary functions during acute inflammation that make common recruitment factors, like LTB4, advantageous.
Leukotrienes amplify eosinophil accumulation in the lung in response to nematodes
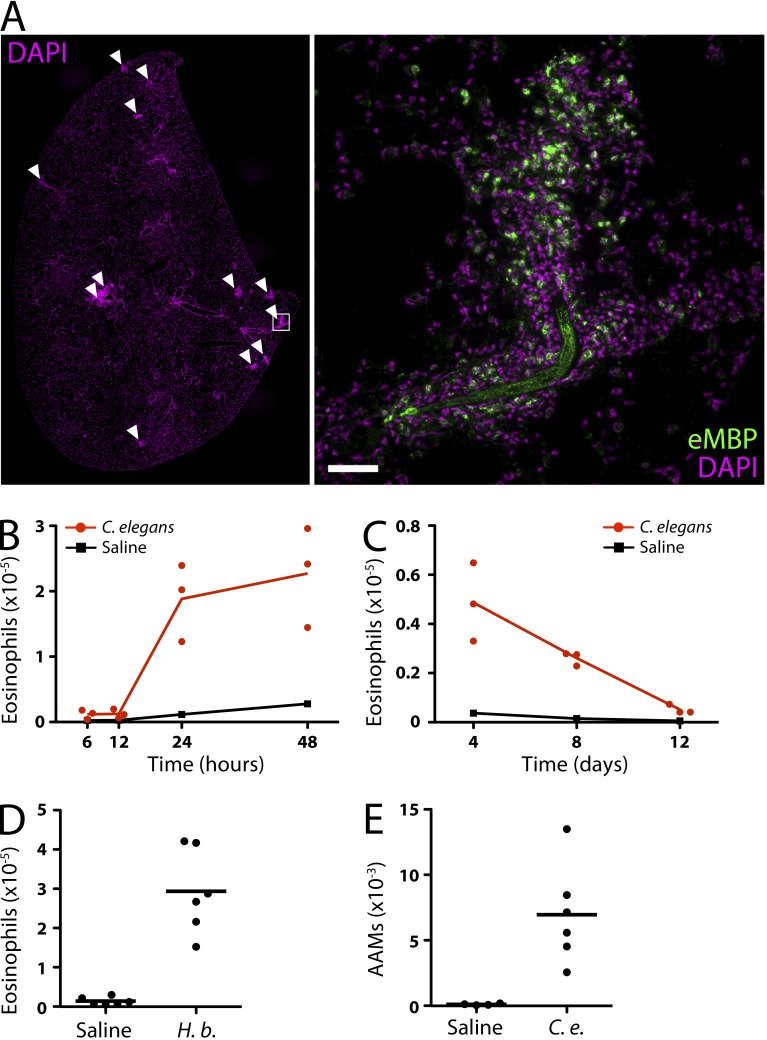
We next investigated the contribution of leukotrienes to eosinophil accumulation in mice in response to nematodes. Within 5 min after i.v. injection, we observed live C. elegans dauers immobilized in the capillaries of the lung (not depicted). After 24 h, abundant eosinophils (identified by major basic protein) had accumulated around the worms (Fig. 5 A). To quantify eosinophils, we digested lungs with collagenase and analyzed leukocytes by flow cytometry. Eosinophils (identified by Siglec-F and low autofluorescence) were rare (<20 × 103 cells) before and even 12 h after nematode injection (Fig. 5 B). By 24 h, eosinophils in the lung increased ∼10-fold, after which they gradually returned to baseline over the course of 2 wk (Fig. 5 C). Correspondingly, there was an increase in the percentage of eosinophils among total lung leukocytes after injection of C. elegans (11.2 ± 1.3%; n = 11) compared with saline (2.9 ± 0.6%; n = 9). The response was similar in both C57BL/6 (Fig. 5 B) and BALB/c mice (Fig. 6 C) and after injection of the insect parasite, H. bacteriophora (Fig. 5 D). Helminth infections typically induce alternative macrophage activation, which is characterized by the expression of arginase-1 (Arg1; Reese et al., 2007). Using Arg1-YFP reporter mice, we found a significant increase in alternative activation of macrophages 24 h after C. elegans injection (Fig. 5 E), highlighting another similarity in the innate immune responses to parasitic and nonparasitic nematodes.
Figure 5.
Eosinophil accumulation in the lung in response to C. elegans dauers. (A) Tiled composite image of a DAPI (magenta) stained tissue section of the entire left lung lobe (left) 24 h after i.v. injection of 500 C. elegans dauers. Multiple inflammatory foci are visible (arrowheads). High-power inset (right) shows a larva surrounded by inflammatory cells, including eMBP+ eosinophils. The dauer exhibits nonspecific fluorescence. Bar, 50 µm. (B–D) Flow cytometry analysis of eosinophil accumulation in the lung at various time points after injection of C. elegans dauers (B and C) or H. bacteriophora larvae (D). (E) Flow cytometry analysis of alternatively activated macrophages (AAMs) in the lung 24 h after injection of C. elegans dauers or saline. (A–E) Results represent two (A–C) or are pooled from two (D and E) independent experiments. Horizontal bars indicate the mean.
Figure 6.
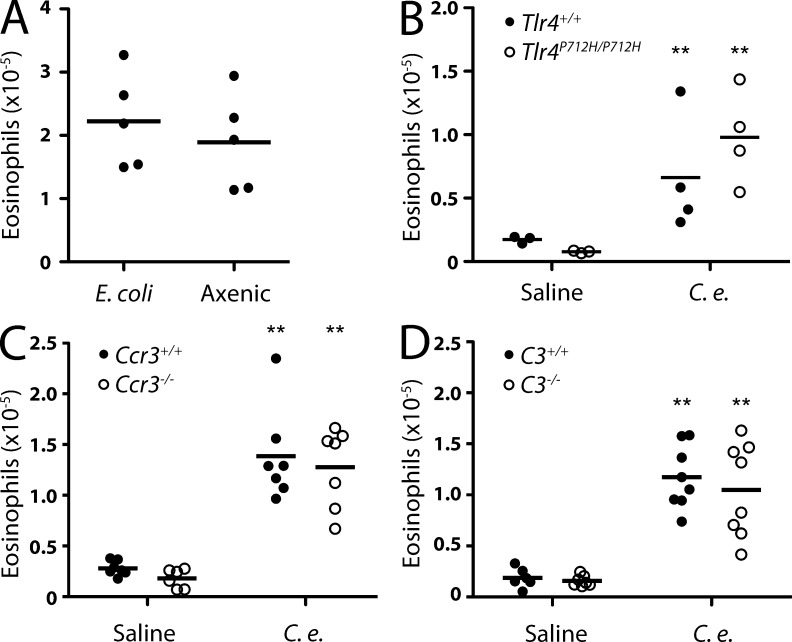
Eosinophil accumulation in the absence of bacterial products, TLR4, CCR3, and complement. (A) Flow cytometry analysis of eosinophils in the lung 24 h after injection of C. elegans grown on E. coli or in axenic medium. (B–D) Flow cytometry analysis of eosinophil accumulation in the lungs of Tlr4P712H/P712H (B), Ccr3−/− (C), and C3−/− (D) mice after injection of C. elegans dauers or saline. (A–D) Results represent two (A and B) or are pooled from two (C and D) independent experiments. Horizontal bars indicate the mean. **, P < 0.01.
C. elegans dauers grown in axenic culture induced similar levels of eosinophil accumulation as conventionally grown dauers (Fig. 6 A), indicating that the eosinophil response was independent of bacterial products. Additionally, mice deficient in LPS recognition (Tlr4P712H mice) were not impaired in their ability to respond to C. elegans (Fig. 6 B). CCR3 and its ligands, CCL11 and CCL24, are known to be involved in the recruitment of eosinophils to tissues in several settings (Humbles et al., 2002). In addition, C3-dependent complement deposition can mediate interactions between eosinophils and helminths (Klion and Nutman, 2004). However, we found that eosinophil accumulation in Ccr3−/− and C3−/− mice did not differ from that in wild-type mice (Fig. 6, C and D).
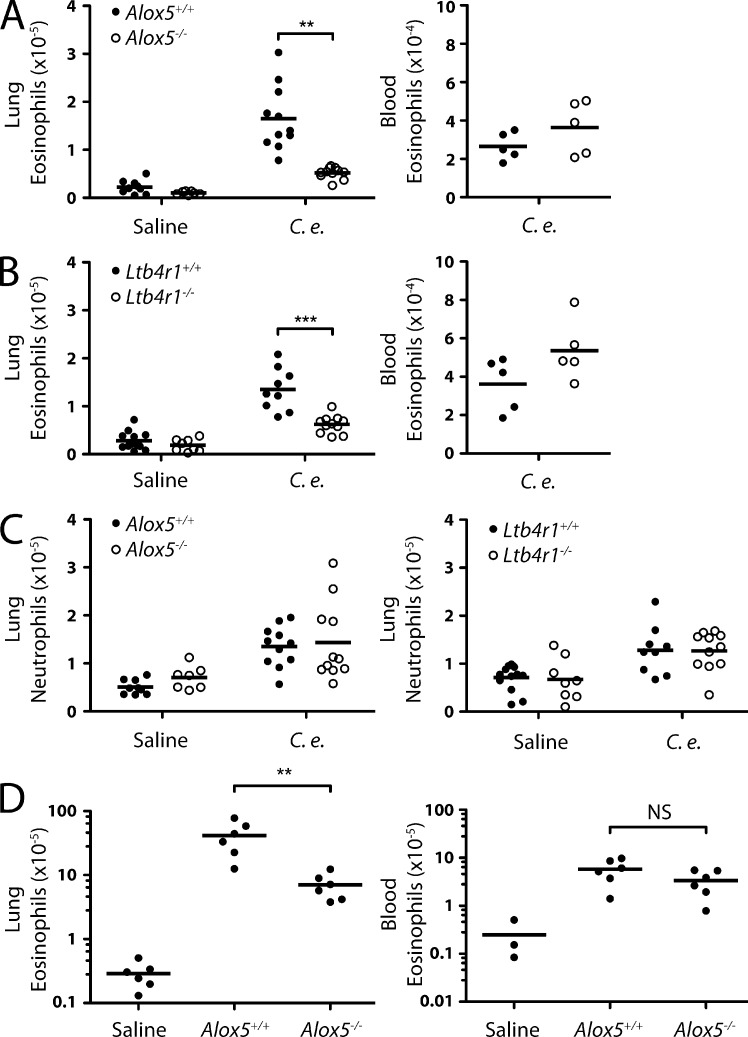
In light of our 3D culture findings, we next investigated the contribution of leukotriene signaling. The number of eosinophils that accumulated in the lung 24 h after C. elegans injection was decreased by 70% in Alox5−/− mice (Fig. 7 A). This decrease was not likely caused by systemic defects in eosinophil production or survival because Alox5−/− mice had normal numbers of eosinophils in peripheral blood. In Ltb4r1−/− mice, eosinophils were 55% reduced in the lung (Fig. 7 B), whereas there was no reduction in the blood. Neutrophil accumulation did not exhibit this dependency on leukotriene signaling (Fig. 7 C). To determine whether leukotrienes were also important for the response to a bonafide rodent parasite, we infected Alox5−/− mice with N. brasiliensis. Again, there was no difference in the number of peripheral blood eosinophils between wild-type and Alox5−/− mice during infection (Fig. 7 D). However, leukotriene deficiency resulted in a >80% reduction in eosinophils in the lung 9 d after infection.
Figure 7.
Involvement of leukotrienes in eosinophil accumulation in the lung. (A and B) Flow cytometry analysis of eosinophil accumulation in the lungs (left; three experiments pooled) and blood (right) of Alox5−/− (A) and Ltb4r1−/− (B) mice 24 h after injection of C. elegans dauers or saline. (C) Flow cytometry analysis of neutrophil accumulation in the lungs of Alox5−/−, Ltb4r1−/−, and wild-type mice 24 h after injection of C. elegans dauers or saline. (D) Flow cytometry analysis of eosinophil accumulation in the lungs (left) and blood (right) of Alox5−/− and wild-type mice 9 d after infection with N. brasiliensis larvae. Numbers of eosinophils in uninfected mouse lung (from A) are shown for comparison. (A–D) Results are pooled from three (A–C) or represent two (D) independent experiments. Horizontal bars indicate the mean. **, P < 0.005; ***, P < 0.0005.
It is possible that the residual eosinophil accumulation in Ltb4r1−/− mice is caused by cysLTs because human eosinophils can migrate toward cysLTs in Transwell assays (Spada et al., 1994). However, we do not favor an involvement of cysLTs in collective migration because mouse eosinophils were not attracted to LTC4, LTD4, or LTE4 in Transwells and exhibited unperturbed migration to C. elegans in the presence of a CYSLTR1 antagonist. Moreover, mice deficient in cysLTs do not have defects in eosinophil recruitment to the skin in a model of atopic dermatitis (Oyoshi et al., 2012). It is conceivable that cysLTs exert effects on eosinophils by promoting their survival in the lung (e.g., by modulating IL-5).
This study establishes that eosinophils exhibit collective migration toward both parasitic and nonparasitic nematode species in the absence of adaptive immunity and stromal cell injury. This strongly points to recognition of conserved features of nematodes during eosinophil accumulation and raises intriguing questions regarding the nature of these features. The in vitro assay that we describe is amenable to genetic screens in C. elegans. Used in conjunction with the ability of C. elegans to elicit eosinophil accumulation in mouse lung, this model provides a methodology for the discovery of nematode factors that initiate immune responses during helminth infection.
MATERIALS AND METHODS
Mice.
YARG(Arg1YFP) (Reese et al., 2007), Tlr4P712H (Dumont and Barrois, 1976), Ccr3−/− (Humbles et al., 2002), C3−/− (Klion and Nutman, 2004), Alox5−/− (Chen et al., 1994), Ltb4r1−/− (Tager et al., 2000), and Il5Tg (Lee et al., 1997) mice have been described previously. All strains were maintained on the C57BL/6J background except Ccr3−/−, which was maintained on the BALB/c background. Wild-type C57BL/6J and BALB/cJ mice were obtained from the Jackson Laboratory. All animal procedures were approved by the University of California, San Francisco (UCSF), Institutional Animal Care and Use Committee and performed in accordance with the guidelines established by the National Institutes of Health.
Nematodes.
C. elegans strains carrying the daf-2(e1370), rmIs126[unc-54p::Q0::YFP] alleles were obtained from the Caenorhabditis Genetics Center and crossed with the aid of him-5 RNAi provided by C. Kenyon (UCSF, San Francisco, CA) to generate a daf-2(e1370); rmIs126[unc-54p::Q0::YFP] strain. Worms carrying a [myo3p::CFP] extra-chromosomal array on the daf-2(31368) background were obtained from A. Bethke (UCSF). C. elegans dauers were generated by culturing synchronized L1 daf-2 mutants at 27°C for 4 d in sealed Petri dishes and then collecting larvae from the sides and lids of the dishes. Dauers were >99% pure based on body morphology and an absence of pharyngeal pumping. For axenic culture, mCeHR medium was prepared as described previously (Rao et al., 2005). Axenic cultures were routinely tested for sterility by culture on brain heart infusion agar plates (BD) at 30°C and 37°C. Additionally, LPS was not detectable by Limulus amebocyte lysate testing (Pyrotell) in axenic cultures or in supernatants of E. coli fed C. elegans dauers after washing (as described below). H. bacteriophora–infective juveniles were provided by T.A. Ciche (Michigan State University, East Lansing, MI) and maintained using RET16 Photorhabdus luminescens cultures, as described previously (Hallem et al., 2007). N. brasiliensis infection was performed as described previously (Patnode et al., 2013). In brief, mice were anesthetized using isoflurane and injected subcutaneously at the base of the tail with 500 N. brasiliensis L3 larvae. Mice were maintained on water containing 2 g/liter neomycin sulfate and 100 mg/liter polymyxin B for 5 d and sacrificed after 9 d. For administration of C. elegans, 500 live dauers were injected into the caudal tail vein in 150 µl of 0.01% BSA in saline.
3D and Transwell migration assays.
Nematodes were washed by six rounds of suspension in 1.5 ml of wash buffer (0.01% BSA in 0.9% NaCl), 5-s centrifugation at ∼1,000 g, and aspiration of supernatant. Nematodes were suspended at a concentration of 6,000 larvae/ml in Matrigel without phenol red (BD) or 1 mg/ml collagen (Invitrogen) and kept on ice. For bead-based assays, streptavidin-agarose beads (Sigma-Aldrich) were incubated with 10 µg/ml mouse IgG1-biotin or mouse F(ab′)2-biotin for 1 h. Eosinophils were obtained from bone marrow cultures, as described previously (Patnode et al., 2013). In brief, bone marrow from femurs and tibiae from two mice per group were flushed with RPMI-1640 (RPMI) and treated with water to lyse erythrocytes. Cells were then cultured in the presence of recombinant SCF and Flt3L (R&D Systems) for 4 d and subsequently cultured in the presence of recombinant IL-5 and collected after 10 additional days. Neutrophils were isolated from mouse bone marrow as described previously (Boxio et al., 2004). Human eosinophils were obtained from peripheral blood using a Human Eosinophil Isolation kit (Miltenyi Biotec) according to the manufacturer’s instructions. Purified human eosinophils were >98% Siglec-8+ and <2% CD16high, whereas human neutrophils were <1% Siglec-8+ and >99% CD16high. Eosinophils from two separate donors exhibited similar cell surface staining and migration responses. Mouse eosinophils or neutrophils were incubated in 1 µM chloromethyl tetramethylrhodamine or CFSE in RPMI containing 2% FBS for 25 min at 37°C. Cells were washed, and some samples were treated with 250 ng/ml Bordetella pertussis toxin for 1 h and then washed with RPMI. Other samples were treated with various concentrations of MK886 in equal volumes of DMSO for 10 min before being mixed with larvae. Cells were washed and resuspended at 40 × 106/ml, unless otherwise noted, in 0.1% BSA in RPMI without phenol red. Leukocyte and nematode suspensions were mixed in a 1:1 ratio, loaded into 6-lane or 18-well chamber imaging slides (Ibidi), and cultured at 37°C for 2 h. Eosinophil density titration was performed after 1-h incubation. Transwell migration assays were performed exactly as described previously (Allen et al., 2004), using leukotrienes (Cayman Chemical) or CCL11 (R&D Systems).
Microscopy.
Images were acquired using a microscope setup that has been described previously (Gilden et al., 2012). In brief, three lasers (argon 488 nm, krypton 568 nm, and indium gallium nitride 406 nm) were connected to a spinning disk confocal scan-head (CSU-10b; Yokogawa Corporation of America; modified by Solamere Technology Group), which was connected to a motorized, inverted microscope (Axiovert 200M inverted fluorescence microscope; Carl Zeiss). Emission light was passed through an automated filter wheel (FW-1000; Applied Scientific Instrumentation) and detected by an electron-multiplying charge-coupled device (EMCCD) camera (iXon3; Andor Technology). Images were collected through 10× Fluor 0.5 NA and 20× Fluor air objectives. For time-lapse imaging, cultures were maintained at 37°C on a heated stage and imaged starting roughly 15 min after the start of the culture. For endpoint leukocyte accumulation assays, image volumes were acquired for all nematodes within 100 µm from the bottom of the imaging slide (three to seven larvae per well) from at least two wells per condition. Images were analyzed using Imaris (BitPlane) software. Volumes representing the accumulated leukocytes were generated in parallel by applying single, manually set fluorescence intensity and volume thresholds to all larvae from all wells. For bead-induced accumulation, volumes were calculated for beads in several random fields. Accumulations of cells that were not in contact with the surface of larvae or beads were rare and omitted from the volume measurement. Immunofluorescence staining of lung tissue sections was performed as described previously (Patnode et al., 2013).
Leukotriene ELISAs.
Detection of leukotrienes was performed using LTB4 and LTE4 Enzyme Immunoassay kits (Cayman Chemical) according to the manufacturer’s instructions. Bone marrow–derived eosinophils (106/well) were incubated with 1 µM ionomycin or DMSO in 0.1% BSA in RPMI for 2 h at 37°C. Incubation with C. elegans dauers (1,000/well) was performed in parallel on wells coated with Matrigel. For all experiments, separate groups of cells were treated with 10 µM MK886 or DMSO before the start of the culture.
Flow cytometry.
Mice were sacrificed and all lobes of lungs, except the left, were collected. Lungs were injected with digestion buffer consisting of 0.2 U/ml Liberase DL (Roche) and 10 µg/ml DNase (Sigma-Aldrich) in HBSS (without Ca2+/Mg2+). Lungs were minced using razor blades and incubated with digestion buffer for 25 min at 37°C. Digestion was stopped by adding EDTA to 10 mM and 2% FBS in RPMI, and tissue was crushed onto 70-µm cell strainers (BD). Cells were treated with ammonium chloride buffer (150 mM NH4Cl, 10 mM KHCO3, and 100 µM EDTA) to lyse erythrocytes. Cells were incubated with 10 µg/ml anti–mouse CD16/32 (clone 93; eBioscience) to block Fc receptors before staining with PE anti–Siglec-F (E50; BD) and APC anti–Ly-6G (1A8; BioLegend). Viability was determined by adding 50 µl/ml 7-aminoactinomycin D solution (BD) or 0.5 µg/ml DAPI. Cells were analyzed using a FACSort cytometer (BD) equipped with CellQuest software (BD). Further analysis was performed using FlowJo software (Tree Star).
Statistical analysis.
Bar graphs are plotted as means plus the standard error of the mean using Prism 5 software (GraphPad Software). Where individual mice are plotted, horizontal bars represent the mean. Student’s t test was used to evaluate the statistical significance of differences between groups.
Online supplemental material.
Videos 1 and 2 show eosinophil migration toward C. elegans. Video 3 shows human eosinophil migration toward C. elegans. Video 4 shows control and PT-treated eosinophil migration toward C. elegans. Video 5 shows wild-type and Alox5−/− eosinophil migration toward C. elegans. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20132336/DC1.
Supplementary Material
Acknowledgments
We thank Jakob von Moltke and Sebastian Peck for technical assistance, Creg Darby for helpful discussions, James Lee, Cynthia Kenyon, Todd A. Ciche, Axel Bethke, and Iqbal Hamza for advice and reagents. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by National Institutes of Health (NIH) Office of Research Infrastructure Programs (P40 OD010440). Access to imaging equipment and analysis software was provided by the Biological Imaging Development Center at the University of California, San Francisco, which is funded in part by the Sandler Asthma Basic Research Center.
This work was supported by NIH grants GM-23547 and GM-57411 (to S.D. Rosen), P01 HL024136 (to M.F. Krummel), and AI-26918 and AI-30663 (to R.M. Locksley).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- cysLT
- cysteinyl leukotriene
- PT
- pertussis toxin
References
- Afonso P.V., Janka-Junttila M., Lee Y.J., McCann C.P., Oliver C.M., Aamer K.A., Losert W., Cicerone M.T., Parent C.A. 2012. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell. 22:1079–1091 10.1016/j.devcel.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C.D., Ansel K.M., Low C., Lesley R., Tamamura H., Fujii N., Cyster J.G. 2004. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 5:943–952 10.1038/ni1100 [DOI] [PubMed] [Google Scholar]
- Balla K.M., Lugo-Villarino G., Spitsbergen J.M., Stachura D.L., Hu Y., Bañuelos K., Romo-Fewell O., Aroian R.V., Traver D. 2010. Eosinophils in the zebrafish: prospective isolation, characterization, and eosinophilia induction by helminth determinants. Blood. 116:3944–3954 10.1182/blood-2010-03-267419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxio R., Bossenmeyer-Pourié C., Steinckwich N., Dournon C., Nüsse O. 2004. Mouse bone marrow contains large numbers of functionally competent neutrophils. J. Leukoc. Biol. 75:604–611 10.1189/jlb.0703340 [DOI] [PubMed] [Google Scholar]
- Chen M., Lam B.K., Kanaoka Y., Nigrovic P.A., Audoly L.P., Austen K.F., Lee D.M. 2006. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med. 203:837–842 10.1084/jem.20052371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.S., Sheller J.R., Johnson E.N., Funk C.D. 1994. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 372:179–182 10.1038/372179a0 [DOI] [PubMed] [Google Scholar]
- Dumont F., Barrois R. 1976. Electrokinetic properties of splenic lymphocytes from the low-lipopolysaccharide responder C3H/Hej mice. Folia Biol. (Praha). 12:145–150 [PubMed] [Google Scholar]
- Gilden J.K., Peck S., Chen Y.C., Krummel M.F. 2012. The septin cytoskeleton facilitates membrane retraction during motility and blebbing. J. Cell Biol. 196:103–114 10.1083/jcb.201105127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeggström J.Z., Wetterholm A. 2002. Enzymes and receptors in the leukotriene cascade. Cell. Mol. Life Sci. 59:742–753 10.1007/s00018-002-8463-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem E.A., Rengarajan M., Ciche T.A., Sternberg P.W. 2007. Nematodes, bacteria, and flies: a tripartite model for nematode parasitism. Curr. Biol. 17:898–904 10.1016/j.cub.2007.04.027 [DOI] [PubMed] [Google Scholar]
- Henderson W.R., Harley J.B., Fauci A.S. 1984. Arachidonic acid metabolism in normal and hypereosinophilic syndrome human eosinophils: generation of leukotrienes B4, C4, D4 and 15-lipoxygenase products. Immunology. 51:679–686 [PMC free article] [PubMed] [Google Scholar]
- Humbles A.A., Lu B., Friend D.S., Okinaga S., Lora J., Al-Garawi A., Martin T.R., Gerard N.P., Gerard C. 2002. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc. Natl. Acad. Sci. USA. 99:1479–1484 10.1073/pnas.261462598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaoka Y., Boyce J.A. 2004. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J. Immunol. 173:1503–1510 10.4049/jimmunol.173.3.1503 [DOI] [PubMed] [Google Scholar]
- Kang Y.S., Yamazaki S., Iyoda T., Pack M., Bruening S.A., Kim J.Y., Takahara K., Inaba K., Steinman R.M., Park C.G. 2003. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int. Immunol. 15:177–186 10.1093/intimm/dxg019 [DOI] [PubMed] [Google Scholar]
- Klion A.D., Nutman T.B. 2004. The role of eosinophils in host defense against helminth parasites. J. Allergy Clin. Immunol. 113:30–37 10.1016/j.jaci.2003.10.050 [DOI] [PubMed] [Google Scholar]
- Knott M.L., Matthaei K.I., Foster P.S., Dent L.A. 2009. The roles of eotaxin and the STAT6 signalling pathway in eosinophil recruitment and host resistance to the nematodes Nippostrongylus brasiliensis and Heligmosomoides bakeri. Mol. Immunol. 46:2714–2722 10.1016/j.molimm.2009.05.016 [DOI] [PubMed] [Google Scholar]
- Lämmermann T., Afonso P.V., Angermann B.R., Wang J.M., Kastenmüller W., Parent C.A., Germain R.N. 2013. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 498:371–375 10.1038/nature12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N.A., McGarry M.P., Larson K.A., Horton M.A., Kristensen A.B., Lee J.J. 1997. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J. Immunol. 158:1332–1344 [PubMed] [Google Scholar]
- Licona-Limón P., Kim L.K., Palm N.W., Flavell R.A. 2013. TH2, allergy and group 2 innate lymphoid cells. Nat. Immunol. 14:536–542 10.1038/ni.2617 [DOI] [PubMed] [Google Scholar]
- Makepeace B.L., Martin C., Turner J.D., Specht S. 2012. Granulocytes in helminth infection — who is calling the shots? Curr. Med. Chem. 19:1567–1586 10.2174/092986712799828337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews A.N., Friend D.S., Zimmermann N., Sarafi M.N., Luster A.D., Pearlman E., Wert S.E., Rothenberg M.E. 1998. Eotaxin is required for the baseline level of tissue eosinophils. Proc. Natl. Acad. Sci. USA. 95:6273–6278 10.1073/pnas.95.11.6273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyoshi M.K., He R., Kanaoka Y., ElKhal A., Kawamoto S., Lewis C.N., Austen K.F., Geha R.S. 2012. Eosinophil-derived leukotriene C4 signals via type 2 cysteinyl leukotriene receptor to promote skin fibrosis in a mouse model of atopic dermatitis. Proc. Natl. Acad. Sci. USA. 109:4992–4997 10.1073/pnas.1203127109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnode M.L., Cheng C.W., Chou C.C., Singer M.S., Elin M.S., Uchimura K., Crocker P.R., Khoo K.H., Rosen S.D. 2013. Galactose 6-O-sulfotransferases are not required for the generation of Siglec-F ligands in leukocytes or lung tissue. J. Biol. Chem. 288:26533–26545 10.1074/jbc.M113.485409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A.U., Carta L.K., Lesuisse E., Hamza I. 2005. Lack of heme synthesis in a free-living eukaryote. Proc. Natl. Acad. Sci. USA. 102:4270–4275 10.1073/pnas.0500877102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese T.A., Liang H.E., Tager A.M., Luster A.D., Van Rooijen N., Voehringer D., Locksley R.M. 2007. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 447:92–96 10.1038/nature05746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C.A., Ford-Hutchinson A.W., Morton H.E., Gillard J.W. 1990. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J. Biol. Chem. 265:1436–1442 [PubMed] [Google Scholar]
- Sadik C.D., Kim N.D., Luster A.D. 2011. Neutrophils cascading their way to inflammation. Trends Immunol. 32:452–460 10.1016/j.it.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadik C.D., Kim N.D., Iwakura Y., Luster A.D. 2012. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcγR signaling. Proc. Natl. Acad. Sci. USA. 109:E3177–E3185 10.1073/pnas.1213797109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada C.S., Nieves A.L., Krauss A.H., Woodward D.F. 1994. Comparison of leukotriene B4 and D4 effects on human eosinophil and neutrophil motility in vitro. J. Leukoc. Biol. 55:183–191 [DOI] [PubMed] [Google Scholar]
- Tager A.M., Dufour J.H., Goodarzi K., Bercury S.D., von Andrian U.H., Luster A.D. 2000. Bltr mediates leukotriene B4–induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J. Exp. Med. 192:439–446 10.1084/jem.192.3.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.