Abstract
Soon after the 9–11 attacks, politicians and scientists began to question our ability to cope with a large-scale radiological terrorism incident. The outline of what was needed was fairly obvious: the ability to prevent such an attack; methods to cope with the medical consequences; the ability to clean up afterwards; and the tools to figure out who perpetrated the attack and bring them to justice. The medical response needed three components: the technology to rapidly determine the radiation doses received by a large number of people, methods for alleviating acute hematological radiation injuries, and therapies for mitigation and treatment of chronic radiation injuries. Research done to date has shown that a realistic medical response plan is scientifically possible, but the regulatory and financial barriers to achieving this may currently be insurmountable.
Keywords: radiological terrorism, nuclear accident, medical countermeasures, biodosimetry, acute radiation syndrome
INTRODUCTION
Soon after the 11 September 2001 attacks on the World Trade Center and the Pentagon, scientists and politicians in the United States began to question the country’s ability to cope with a radiological terrorism incident (Moulder 2002). The framework of what was needed was fairly obvious: the ability to prevent such an attack; methods to cope with the medical consequences; the ability to clean up afterwards; and the tools to figure out who did it (Fig. 1). This review will focus of the current status of the medical components needed to manage a mass-casualty radiological or nuclear incident: determining dose (biodosimetry), methods for alleviating acute radiation injuries, and therapies for chronic radiation injuries. (Pellmar and Rockwell 2005, Rojas-Palma et al. 2009).
Figure 1.
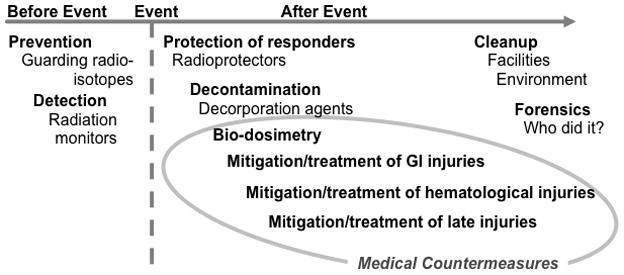
The components of a complete radiological terrorism countermeasures program. Adapted with permission (Moulder and Medhora 2011).
All three components are needed. Biodosimetry will be of little practical use unless there are effective therapies. Therapies for acute injuries will have little long-term benefit unless there are therapies for the late effects that will occur in people who receive high doses and for whom hematological lethality is prevented. Conversely, therapy for chronic radiation injuries will be of little use without development of better biodosimetry tools, and better methods for decreasing acute hematological toxicity.
WHY BE CONCERNED ABOUT ORGAN SYSTEMS OTHER THAN BONE MARROW?
At the Chernobyl accident the acute 50% lethal dose (LD50) was about 6 Gy (Fig 2). If a similar-size incident were to occur now, advances in treatment of hematological injuries are such that there would probably be survivors with exposures as high as 8–10 Gy (Fig. 2). Upper-body doses this high would cause radiation pneumonitis (Fig. 2), and might also cause cognitive impairment (Raber et al. 2004, Acevedo et al. 2008).(Baker et al. 2011) Lower-body doses this high would result in severe prodromal emesis and diarrhea (Anno et al. 1995, Otterson et al. 1996), but would probably not cause acute gastrointestinal lethality (Fig 2). However, lower-body doses this high would exceed renal tolerance (Fig. 2) and probably also cause indirect cardiac injury (Lenarczyk et al. 2013). Thus an effective medical counter measures program needs to deal with both acute hematological injury and delayed injury to organ systems as diverse as kidney, lung, heart and brain (Fig 3).
Figure 2.
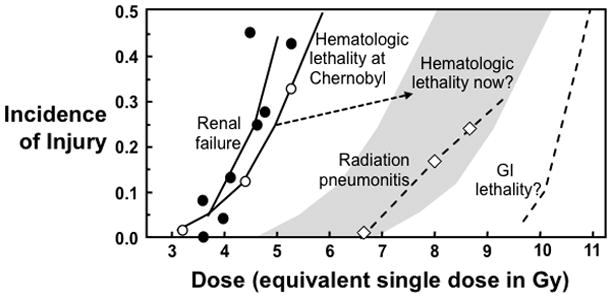
The relationship between total body irradiation (TBI) dose and morbidity. Data is shown for hematopoietic lethality after the Chernobyl accident (Mould 1988) and for what that lethality dose-response curve might look if a similar incident happened now (gray area). Data are also shown for radiation-induced chronic renal failure (Moulder and Cohen 2005), and pneumonitis (van Dyk et al. 1981) in humans. The dose-response curve for human GI lethality is speculative, based on data for nonhuman primates (MacVittie et al. 2012) and the limited data that is available for humans (Anno et al. 2003). Adapted with permission (Moulder and Medhora 2011).
Figure 3.
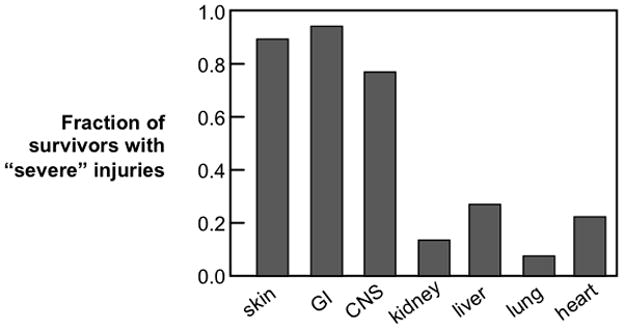
Non-hematological injuries in radiation accident survivors who had severe hematological injuries and who survived at least 90 days (Fliedner et al. 2005).
CONTAMINATION VS. EXPOSURE VS. DOSE
The distinction between assessing contamination and measuring dose is not well-understood outside the radiation community. While there are sophisticated and widely-available instruments for assessing contamination of people and property, the available tools for retroactive assessment of radiation dose are either primitive or not widely available (Rojas-Palma et al. 2009, Swartz et al. 2010, Fenech 2011). But more importantly, for medical intervention organ-specific doses are needed (Bertho et al. 2008, Rojas-Palma et al. 2009, Prasanna et al. 2010), and our methods for doing this are poorly-developed. This lack of methods for assessing dose will severely impede triage and medical intervention.
If a mass-casualty incident occurred now, the only method for rapid (less than 12 hours) radiation dose assessment would be “time to emesis” (Goans and Waselenko 2005). This is not actually very practical, since not all irradiated people (even those with large abdominal doses) vomit, and in many mass casualty scenarios there will be other events that will cause people to vomit (Demidenko et al. 2009, Swartz et al. 2010).
If more time were available, dose estimates could be made based on lymphocyte depletion kinetics, but that takes at least a day (Swartz et al. 2010, Rothkamm et al. 2013). In theory, doses could also be based on chromosome aberrations in blood lymphocytes, but this assay takes days and requires that samples be sent to central laboratories whose capacity is limited (Rojas-Palma et al. 2009, Swartz et al. 2010, Rothkamm et al. 2013).
Recent research has shown that faster and more deployable systems are theoretically possible. One approach has been the development of portable electron paramagnetic resonance (EPR) dosimeters that can measure low-LET radiation doses from teeth and finger nails (Wilcox et al. 2010, Williams et al. 2010). The EPR signal is durable, and the technique may be able to detect doses as low as 2 Gy (Swartz et al. 2010, Wilcox et al. 2010, Williams et al. 2010). A very different approach is development of an automated device for measuring radiation-induced micronuclei, g-H2AX fluorescence and/or dicentrics in blood lymphocytes (Garty et al. 2010, Vaurijoux et al. 2012). However, machines such as these will not be deployed anytime soon, as currently there is no market to support the cost of manufacturing the units and/or the cost of getting them approved by the required authorities (e.g., in the U.S.A., by the Food and Drug Administration).
A third approach is to use genomic (Megid et al. 2007, Lam et al. 2012, Riecke et al. 2012), proteomic (Ménard et al. 2006, Sharma and Moulder 2013) and/or metabolomic (Johnson et al. 2012, Lalkakis et al. 2012) signals from blood (Ménard et al. 2006, Riecke et al. 2012), urine (Johnson et al. 2012, Lalkakis et al. 2012, Sharma and Moulder 2013), feces (Lam et al. 2012) and/or buccal cells (Megid et al. 2007). All of these “omic” approaches show some promise, and most have the advantage that portable (or at least potentially-portable) tools for rapid assessment have already been developed for other reasons. The main scientific barrier to deployment is establishing a signal (or package of signals) unique to radiation; the method must work in a diverse population (e.g., a range of health, age, ethnicity, social class), and in the presence of multiple stressors and combined injuries. In addition, the signal(s) needs to be stable over at least 72 hours, as in many scenarios the time(s) of exposure may not be known with any accuracy.
All of these approaches also face an uncertain regulatory climate, as the legal framework for getting the approval has not been clearly established. Will medically-irradiated humans (or animals) be accepted as surrogates for accident/terrorism victims? Will ex-vivo irradiations be accepted as surrogates for human exposure? In the US, there is a legal framework for approval of therapies that cannot be ethically tested in humans (Aebersold 2012), but it is not clear that this policy applies to biodosimetry tools.
PROTECTION VS. MITIGATION VS. TREATMENT
The term “radioprotector” has long been used in radiobiology to refer to prophylactic agents that must be given before radiation exposure; mitigators are agents that are given after exposure, but before the appearance of overt evidence of injury; and treatment refers to agents that are given after overt symptoms develop (Fig. 4).
Figure 4.

Recommended terminology for therapeutic approaches to radiation-induced normal tissue injuries (Stone et al. 2004).
All three approaches have been assessed in clinical or preclinical studies. Most, but not all, prophylactic agents developed to date are free radical scavengers or cytokines (Ryan et al. 2011, Bourgier et al. 2012, Koukourakis 2012, Singh et al. 2012). Mitigators include suppressors of the renin-angiotensin system (Kohl et al. 2007, Jenrow et al. 2011, Moulder et al. 2011, Cohen et al. 2012, Lee et al. 2012, Medhora et al. 2012) and suppressors of chronic oxidative stress (Rosenthal et al. 2011, Kim et al. 2012, Mahmood et al. 2012). Treatment agents include some of the same drugs that are effective as mitigators (Moulder and Cohen 2007, Bourgier et al. 2012), but also include agents such as pentoxifylline to treat radiation fibrosis (Boerma et al. 2008, Hamama et al. 2012) and growth factors to facilitate recovery from hematological injury (Dainiak 2010).
PROGRESS ON TREATMENT OF ACUTE RADIATION SYNDROME
A number of groups have devoted considerable effort to improving treatment of the acute radiation syndromes (Brown et al. 2010, DiCarlo et al. 2011, Farese et al. 2013, Moroni et al. 2013). Interestingly, high-quality supportive care may be far more important than any of the new biologicals or pharmaceuticals. For example, Dr. George Georges’s group at the Fred Hutchinson Cancer Research Center (Seattle, Washington, U.S.A.) has shown that the canine LD50 can be increased from less than 4 Gy to about 8 Gy by providing human-standard supportive care (e.g., hydration, antibiotics, transfusions). Adding state-of-the-art cytokine therapy to this supportive care did not further increase the LD50, although it did accelerate the time to neutrophil and platelet recovery (Georges et al. 2009).
After a radiation terrorism incident with doses above 4 Gy, providing supportive care to a large number of victims will be the immediate challenge. This challenge is made more severe by the fact that it is not clear which aspects of supportive care are most important. Dissecting supportive care to find out what really matters will be difficult, as the studies needed are considered problematic by many of the authorities that regulate animal studies (e.g., in the U.S.A., by the Institutional Animal Care and Use Committees).
PROGRESS ON MITIGATION OF LATE TISSUE INJURY
Agents for mitigation, rather than for treatment and protection are urgently needed for medical management of a radiological terrorism event. Protectors will be of little use, as there is very unlikely to be any warning. Protectors might be of use for first responders; but they would have to work quickly, have a significant duration of efficacy, and have no acute toxicity that would interfere with responder performance. So far such agents have not been identified. Treatment agents would be of use, but mitigation would be better than treatment. First, victims will want something done immediately, and the ability to offer this may be crucial to avoiding wide-spread panic. Second, all currently-known post-irradiation interventions work best if started before injury is clinically evident.
The ideal mitigator for late effects (Table 1) would work if started many days after exposure. Efficacy when started after 72 hours is critical, as it will take at least that long for preliminary triage, and because there will probably be other medical priorities (e.g., burns, trauma). An additional issue is that the victims in need of therapy will not be able to take oral meds over the first 24–72 hours because of prodromal nausea and vomiting. Efficacy when started even later is preferred, as unless the drug has essentially no toxicity, it should not be given until biodosimetry (or symptoms) determine who is at risk.
Table 1.
Requirements for a practical mitigator of chronic radiation-induced normal tissue injury.
|
The ideal mitigator (Table 1) would either be orally available or a skin patch (although thermal or radiation skin injury could be a problem for skin patches). Intravenous administration is not suitable for a mass-casualty event, although intramuscular or subcutaneous routes might be practical if the drug schedule was short (e.g., days rather than weeks). The drug should also be reasonably stable, so that it can be stock-piled. Ideally the drug should also have efficacy in multiple organ systems so that multiple drugs are not required for injuries to different organ systems. Finally, it would be advantageous if the drug were already approved for use for other indications, so that toxicity and pharmacokinetics would already be known.
Although these requirements (Table 1) are severe, there is at least one class of agents that appears to meet these requirements, the angiotensin converting enzyme inhibitors (ACEIs). The ACEIs (e.g., captopril, enalapril, ramipril, lisinopril) were developed as hypertensive agents, but they have found efficacy against a range of renal and cardiac diseases. In the laboratory a wide range of late radiation-induced normal tissue injuries can be mitigated using ACEI therapy that is not started until days to weeks after irradiation. Such mitigation was first demonstrated in 1988 using a rat model of lung injury (Ward et al. 1988); (Moulder et al. 1993, Cohen et al. 1997, Moulder et al. 1997) a more recent study of mitigation of radiation-induced lung injury is shown in Fig 5. Subsequently, the ACEIs were also shown to mitigate experimental radiation-induced renal (Fig 5), brain (Fig 6), and cardiac (Baker et al. 2011) injuries. The ACEI’s have now made the transition from bench-to-bedside (Fig 7), and have shown promise for mitigation of radiation-induced renal (Cohen et al. 2012) and lung (Jenkins and Watts 2011, Cohen et al. 2012, Kharofa et al. 2012) injury in humans.
Figure 5.
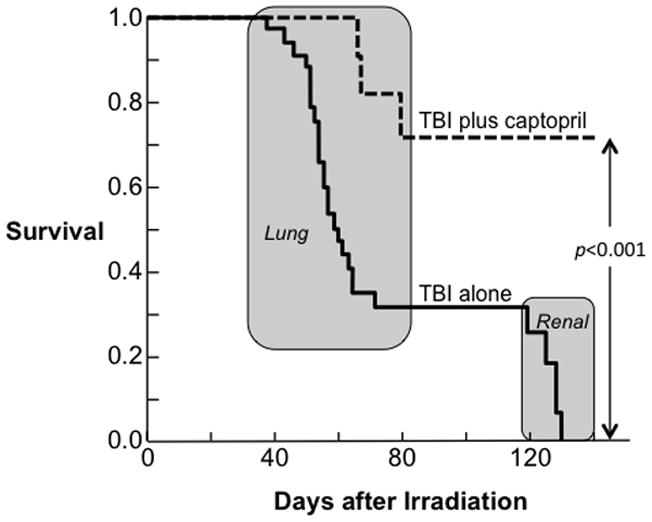
Mitigation of multiple organ system failure by the ACE inhibitor, captopril, after 11.5 Gy TBI plus a bone marrow transplant in a rat model. Kaplan-Meier plots for morbidity show the effects of captopril (176 mg/m2/day) started 7 days after irradiation and continued. Captopril significantly reduced morbidity during both the pneumonitis and the nephropathy phases as determined by Peto-Peto Wilcoxon tests. Adapted with permission (Medhora et al. 2013).
Figure 6.
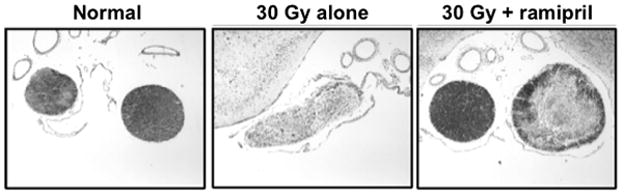
Effect of the ACE inhibitor ramipril on radiation injury to the optic nerve (Kim et al. 2004). Shown are histological sections (stained with Luxol Fast Blue for myelin) from representative rats 180 days after receiving 30 Gy to the optic chiasm: an untreated age-matched control rat, a rat that received irradiation alone, and a rat that received irradiation and daily administrations of ramipril (1.5mg/kg/day) in the drinking water starting 2 weeks after irradiation. Images are “blow-ups” of the optic nerve regions obtained using 4x magnification with a 10x eye lens objective. The corresponding semi-quantitative histology analysis (Luxol Fast Blue Optical Density, scale 1–256) yielded: 209±18 (mean±sd) for the control (n=3), 66±10 for irradiation alone (n=4) and 160±44 for irradiation plus ramipril (n=4).
Figure 7.
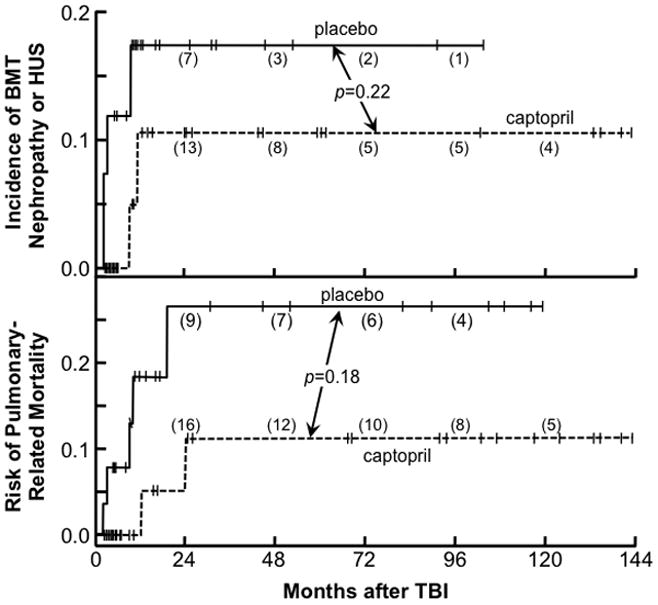
The cumulative incidence of bone marrow transplant (BMT) nephropathy (top) and pulmonary-related mortality (bottom) after total body irradiation (TBI) as conditioning for BMT according to use of captopril or placebo (Cohen et al. 2012). Cases censored are shown as vertical bars and the number at risk at 24 month intervals is shown in parentheses; not shown are placebo cases censored for pulmonary-related mortality at 1.8, 2.2, and 2.3 months and for nephropathy at 1.8, 1.9, 2.2, 2.3 and 2.7 months.
A second class of agents that has shown promise for mitigation of late radiation injuries are the superoxide and catalase mimetics (Jourdan et al. 2009, Gauter-Fleckenstein et al. 2010, Vorotnikova et al. 2010, Rosenthal et al. 2011, Mahmood et al. 2012). Unfortunately, the superoxide/catalase mimetics that show promise as radiation mitigators in laboratory studies are not yet approved for human use.
GETTING MITIGATORS APPROVED FOR HUMAN USE
To make radiation mitigators available for use after a radiological terrorism incident will not be easy, at least not in the U.S. Current regulations in the U.S. require that for agents to be available for use after a radiological terrorism incident, they must not only be approved for human use, but they must be specifically labeled for use as radiation counter measures. Even use of a drug to mitigate radiation injuries in cancer patients (e.g., captopril (Cohen et al. 2012)) is not considered by regulators in the U.S. to be proof that it is suitable for use as a radiological terrorism countermeasure. Since people cannot be irradiated to test whether candidate mitigators are effective, their efficacy will need to be proven using animal studies (Aebersold 2012).
In the U.S. efficacy for mitigators of radiation injury will have to be established under the “Animal Rule” (Table 2). According to FDA, this is the “least approved route of approval”, but there appears to be no other option for mitigators. The burden-of-proof required by this rule is huge, and it may be insurmountable for mitigators of non-hematologic radiation injuries. The biological stumbling block is the need to understand the mechanisms of action of both radiation and mitigation, while the reality is that the mechanistic basis of late radiation-induced tissue injury to not completely understood. It is also not completely clear how the best-known mitigators (i.e., ACEIs and superoxide/catalase mimetics) are working as mitigators. A practical stumbling block is the FDA’s desire for endpoints based on “survival or prevention of major morbidity”, as this requires endpoints that by many of the authorities that regulate animal studies (e.g., in the U.S.A., by the Institutional Animal Care and Use Committees) are reluctant to approve. The requirement for multiple animal species (almost certainly including large animals) will be quite expensive to meet, both because of housing costs and because large animal radiation models are poorly developed. Finally, the need for the work to be done under “Good Laboratory Practices (GLP)” is a roadblock because the number of animal radiobiology facilities that are GLP-certified is very small (perhaps as few as three in the entire U.S.).
Table 2.
Basic requirements of the FDA “Animal Rule” (Aebersold 2012)
|
Repurposing (i.e., relabeling) approved, but inexpensive and off-patent drugs, as mitigators of radiation injuries (e.g., the ACEIs) is a particularly severe problem, as the lack of intellectual property protections means that there is no way that a company could adequately recover costs. To date, no drug appears to have been approved in the U.S. for a mitigator of radiation injuries. As discussed above, it is not clear that the required studies are even feasible; but at a minimum satisfying the regulations will require a time- and money-intensive effort. How other countries will handle this issue is not yet clear.
WHAT IF AN INCIDENT HAPPENED NOW?
The good news from the lab is that we now have “proof of principle” that the critical elements of an effective medical countermeasures program are possible: methods for rapid large-volume biodosimetry could be developed and deployed; the acute effects of radiation can be alleviated; the chronic effects of radiation on normal tissues can be mitigated. The bad news is that moving from laboratory studies to a deployed program will not be easy. The work that still needs to be done is expensive and time-consuming, and the move from the lab to the field faces severe regulatory and financial barriers.
The best current role for the health physics community is to use their resources and expertise to make sure that radiological terrorism and nuclear accidents do not occur. But all potential first responders should also have an emergency response database such as REMM (Radiation Emergency Medical Management <http://www.remm.nlm.gov>) loaded on their mobile devices.
Acknowledgments
The work reported here was supported by a contract (AI067734) from the U.S. National Institutes of Health
I thank my colleagues John Baker, Eric Cohen, Brian Fish and Meetha Medhora for their insightful reviews of the Dade Moeller lecture and of this resulting manuscript.
References
- Acevedo SF, McGinnis G, Raber J. Effects of 137Cs γ irradiation on cognitive performance and measures of anxiety in Apoe−/− and wild-type female mice. Radiat Res. 2008;170:422–428. doi: 10.1667/rr1494.1. [DOI] [PubMed] [Google Scholar]
- Aebersold P. FDA experience with medical countermeasures under the Animal Rule. Adv Prev Med. 2012;2012:507571. doi: 10.1155/2012/507571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anno GH, McClellan GE, Dore MA. Protracted radiation-induced performance decrement. Volume 1: Model development. Santa Monica, United States: Pacific-Sierra Research Corporation; 1995. [Google Scholar]
- Anno GH, Young RW, Bloom RM, Mercier JR. Dose response relationships for acute ionizing-radiation lethality. Health Phys. 2003;84:565–75. doi: 10.1097/00004032-200305000-00001. [DOI] [PubMed] [Google Scholar]
- Baker JE, Moulder JE, Hopewell JW. Radiation as a risk factor for cardiovascular disease. Antiox Redox Signal. 2011;15:1945–1956. doi: 10.1089/ars.2010.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertho JM, Roy L, Souidi M, Benderitter M, Gueguen Y, Lataillade JJ, Prat M, Fagot T, De Revel T, Gourmelon P. New biological indicators to evaluate and monitor radiation-induced damage: an accident case report. Radiat Res. 2008;169:543–550. doi: 10.1667/RR1259.1. [DOI] [PubMed] [Google Scholar]
- Boerma M, Roberto KA, Hauer-Jensen M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Rad Oncol Biol Phys. 2008;71:170–177. doi: 10.1016/j.ijrobp.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgier C, Levy A, Vozenin MC, Deutsch E. Pharmacological strategies to spare normal tissues from radiation damage: useless or overlooked therapeutics? Cancer Metas Rev. 2012;31:699–712. doi: 10.1007/s10555-012-9381-9. [DOI] [PubMed] [Google Scholar]
- Brown SL, Kolozsvary A, Liu J, Jenrow KA, Ryu S, Kim JH. Antioxidant diet supplementation starting 24 hours after exposure reduces radiation lethality. Radiat Res. 2010;173:462–468. doi: 10.1667/RR1716.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EP, Bedi M, Irving AA, Jacobs ER, Tomic R, Klein JP, Lawton CA, Moulder JE. Mitigation of late renal and pulmonary injury after hematopoietic stem cell transplantation. Int J Rad Oncol Biol Phys. 2012;83:292–296. doi: 10.1016/j.ijrobp.2011.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EP, Fish BL, Moulder JE. Successful brief captopril treatment in radiation nephropathy. J Lab Clin Med. 1997;129:536–547. doi: 10.1016/s0022-2143(97)90008-1. [DOI] [PubMed] [Google Scholar]
- Dainiak N. Rationale and recommendations for treatment of radiation injury with cytokines. Health Phys. 2010;98:838–842. doi: 10.1097/HP.0b013e3181b3fce5. [DOI] [PubMed] [Google Scholar]
- Demidenko E, Williams BB, Swartz HM. Radiation dose prediction using data on time to emesis in the case of nuclear terrorism. Radiat Res. 2009;171:310–319. doi: 10.1667/RR1552.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo AL, Poncz M, Cassatt DR, Shah JR, Czarniecki CW, Maidment BW. Development and licensure of medical countermeasures for platelet regeneration after radiation exposure. Radiat Res. 2011;176:134–137. doi: 10.1667/rr2610.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, Macvittie TJ. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 2013;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M. Current status, new frontiers and challenges in radiation biodosimetry using cytogenetic, transcriptomic and proteomic technologies. Radiat Measure. 2011;46:737–741. [Google Scholar]
- Fliedner TM, Dörr HD, Meineke V. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. Brit J Radiol Suppl. 2005;27:1–8. [Google Scholar]
- Garty G, Chen Y, Salerno A, Turner H, Zhang J, Lyulko O, Bertucci A, Xu Y, Wang H, Simaan N, Randers-Pehrson G, Yao YL, Amundson SA, Brenner DJ. The RABIT: a rapid automated biodosimetry tool for radiological triage. Health Phys. 2010;98:209–217. doi: 10.1097/HP.0b013e3181ab3cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Rebouças JS, Batinic-Haberle I, Vujaskovic Z. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Rad Biol Med. 2010;48:1034–1043. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges G, Lesnikov V, Jordan R, Lesnikova M, Yang JYL, Aragon A. Hematopoietic stem cells (HSC) survive and reconstitute hematopoiesis after 8 Gy total body irradiation (TBI) in dogs given intensive supportive care and cytokine treatment. Annual Meeting of the Radiation Research Society; Savannah, Georgia, U.S.A. 2009. pp. 30–31. [Google Scholar]
- Goans RE, Waselenko JK. Medical management of radiological casualties. Health Phys. 2005;89:505–512. doi: 10.1097/01.hp.0000172144.94491.84. [DOI] [PubMed] [Google Scholar]
- Hamama S, Gilbert-Sirieix M, Vozenin MC, Delanian S. Radiation-induced enteropathy: Molecular basis of pentoxifylline-vitamin E anti-fibrotic effect involved TGF-beta(1) cascade inhibition. Radiother Oncol. 2012;105:305–312. doi: 10.1016/j.radonc.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Jenkins P, Watts J. An improved model for predicting radiation pneumonitis incorporating clinical and dosimetric variables. Int J Rad Oncol Biol Phys. 2011;80:1023–1029. doi: 10.1016/j.ijrobp.2010.03.058. [DOI] [PubMed] [Google Scholar]
- Jenrow KA, Liu JG, Brown SL, Kolozsvary A, Lapanowski K, Kim JH. Combined atorvastatin and ramipril mitigate radiation-induced impairment of dentate gyrus neurogenesis. J Neuro-Oncol. 2011;101:449–456. doi: 10.1007/s11060-010-0282-x. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Patterson AD, Krausz KW, Kalinich JF, Tyburski JB, Kang DW, Luecke H, Gonzalez FJ, Blakely WF, Idle JR. Radiation metabolomics. 5. Identification of urinary biomarkers of ionizing radiation exposure in nonhuman primates by mass spectrometry-based metabolomics. Radiat Res. 2012;178:328–340. doi: 10.1667/rr2950.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan MM, Olasz EB, Moulder JE, Fish BL, Mäder M, Schock A, Morrow N, Semenenko VA, Doctrow SR, Lazarova Z. Mitigation of combined radiation and skin wound injury by SOD/catalase mimetic EUK-207. Annual Meeting of the Radiation Research Society; Savannah, Georgia, U.S.A. 2009. pp. 64–65. [Google Scholar]
- Kharofa JR, Cohen EP, Tomic R, Xiang Q, Gore E. Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin-converting enzyme inhibitors and thoracic radiation therapy. Int J Rad Oncol Biol Phys. 2012;84:238–243. doi: 10.1016/j.ijrobp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Kim JH, Brown SL, Kolozsvary A, Jenrow KA, Ryu S, Rosenblum ML, Carretero OA. Modification of radiation injury by Ramipril, inhibitor of angiotensin converting enzyme, on optic neuropathy in the rat. Radiat Res. 2004;161:137–142. doi: 10.1667/rr3124. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kolozsvary A, Jenrow KA, Brown SL. Plerixafor, a CXCR4 antagonist, mitigates skin radiation-induced injury in mice. Radiat Res. 2012;178:202–206. doi: 10.1667/rr2886.1. [DOI] [PubMed] [Google Scholar]
- Kohl RR, Kolozsvary A, Brown SL, Zhu G, Kim JH. Differential radiation effect in tumor and normal tissue after treatment with ramipril, an angiotensin-converting enzyme inhibitor. Radiat Res. 2007;168:440–445. doi: 10.1667/RR0707.1. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI. Radiation damage and radioprotectants: new concepts in the era of molecular medicine. Brit J Radiol. 2012;85:313–330. doi: 10.1259/bjr/16386034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalkakis EC, Hyduke DR, Fornace AJ. Comparison of mouse urinary metabolic profiles after exposure to the inflammatory stressors γ radiation and lipopolysaccharide. Radiat Res. 2012;177:187–199. doi: 10.1667/rr2771.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam V, Moulder JE, Salzman NH, Dubinsky EA, Anderson GL, Baker JE. Intestinal microbiota as novel biomarkers of prior radiation exposure. Radiat Res. 2012;177:573–583. doi: 10.1667/rr2691.1. [DOI] [PubMed] [Google Scholar]
- Lee TC, Greene-Schloesser D, Payne V, Diz DI, Hsu FC, Kooshki M, Mustafa R, Riddle DR, Zhao W, Chan MD, Robbins ME. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat Res. 2012;178:46–56. doi: 10.1667/rr2731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarczyk M, Lam V, Jensen E, Fish BL, Su J, Kaprowski S, Komoriwski RA, Harmann L, Migrino RQ, Li XA, Hopewell JW, Moulder JE, Baker JE. Cardiac injury following 10 Gy total body irradiation: indirect role of effects on abdominal organs. Radiat Res. 2013;180:247–258. doi: 10.1667/RR3292.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Bennett A, Gelfond D, Shea-Donohue T, Tudor G, Booth C, McFarland E, Jackson W., 3rd The acute gastrointestinal subsyndrome of the acute radiation syndrome: a rhesus macaque model. Health Phys. 2012;103:411–426. doi: 10.1097/HP.0b013e31826525f0. [DOI] [PubMed] [Google Scholar]
- Mahmood J, Jelveh S, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury with EUK-207 and genistein: effects in adolescent rats. Radiat Res. 2012;179:125–134. doi: 10.1667/RR2954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Gao F, Fish BL, Jacobs ER, Moulder JE, Szabo A. Dose-modifying factor for captopril for mitigation of radiation injury to normal lung. J Rad Res. 2012;53:633–640. doi: 10.1093/jrr/rrs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhora M, Gao F, Jacobs E, Moulder JE, Fish BL. Model development and use of ACE inhibitors for preclinical mitigation of radiation-induced injury to multiple organs. Radiat Res. 2013 doi: 10.1667/RR13425.1. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megid WA, Ensenberger MG, Halberg RB, Stanhope SA, Kent-First MG, Prolla TA, Bacher JW. A novel method for biodosimetry. Radiat Environ Biophys. 2007;46:147–154. doi: 10.1007/s00411-006-0072-1. [DOI] [PubMed] [Google Scholar]
- Ménard C, Johann D, Lowenthal M, Muanza T, Sproull M, Ross S, Gulley J, Petricoin E, Coleman CN, Whiteley G, Liotta L, Camphausen K. Discovering clinical biomarkers of ionizing radiation exposure with serum proteomic analysis. Cancer Res. 2006;66:1844–1850. doi: 10.1158/0008-5472.CAN-05-3466. [DOI] [PubMed] [Google Scholar]
- Moroni M, Ngudiankama BF, Christensen C, Olsen CH, Owens R, Lombardini ED, Holt RK, Whitnall MH. The Gottingen minipig Is a model of the hematopoietic acute radiation syndrome: G-colony stimulating factor stimulates hematopoiesis and enhances survival from lethal total-body γ-irradiation. Int J Rad Oncol Biol Phys. 2013;86:986–992. doi: 10.1016/j.ijrobp.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould RF. Chernobyl: The real story. Oxford, U.K: Pergamon Press; 1988. [Google Scholar]
- Moulder JE. Report on an interagency workshop on the radiobiology of nuclear terrorism. Radiat Res. 2002;158:118–124. doi: 10.1667/0033-7587(2002)158[0118:roaiwo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Moulder JE, Cohen EP. Radiation-induced multi-organ involvement and failure: the contribution of radiation effects on the renal system. Brit J Radiol Suppl. 2005;27:82–88. [Google Scholar]
- Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Sem Rad Oncol. 2007;17:141–148. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total body irradiation. Radiat Res. 2011;175:29–36. doi: 10.1667/RR2400.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JE, Cohen EP, Fish BL, Hill P. Prophylaxis of bone marrow transplant nephropathy with captopril, an inhibitor of angiotensin-converting enzyme. Radiat Res. 1993;136:404–407. [PubMed] [Google Scholar]
- Moulder JE, Fish BL, Cohen EP. Noncontinuous use of angiotensin converting enzyme inhibitors in the treatment of experimental bone marrow transplant nephropathy. Bone Marrow Transplant. 1997;19:729–736. doi: 10.1038/sj.bmt.1700732. [DOI] [PubMed] [Google Scholar]
- Moulder JE, Medhora M. Advances in mitigation of injuries from radiological terrorism or nuclear accidents. Def Sci J. 2011;61:99–104. [Google Scholar]
- Otterson MF, Sarna SK, Moulder JE. The effects of radiation on gastrointestinal motility and potential therapies. In: MacVittie TJ, Weiss JF, Brownes D, editors. Advances in the Treatment of Radiation Injuries. New York, U.S.A: Elsevier; 1996. pp. 207–216. [Google Scholar]
- Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–123. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- Prasanna PGS, Blakely WF, Bertho JM, Chute JP, Cohen EP, Goans RE, Grace MB, Lillis-Hearne PK, Lloyd DC, Lutgens LC, Meineke V, Ossetrova NI, Romanyukha A, Saba JD, Weisdorf DJ, Wojcik A, Yukihara EG, Pellmar TC. Synopsis of partial-body radiation diagnostic biomarkers and medical management of radiation injury workshop. Radiat Res. 2010;173:245–253. doi: 10.1667/RR1993.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Riecke A, Rufa CG, Cordes M, Hartmann J, Meineke V, Abend M. Gene expression comparisons performed for biodosimetry purposes on in vitro peripheral blood cellular subsets and irradiated individuals. Radiat Res. 2012;178:234–43. doi: 10.1667/rr2738.1. [DOI] [PubMed] [Google Scholar]
- Rojas-Palma C, Liland A, Jerstad AN, Etherington G, del Rosario Pérez M, Rahola T, Smith K. TMT HANDBOOK - Triage, monitoring and treatment - handbook for management of the public in the event of malevolent use of radiation. Østerås, Norway: Norwegian Radiation Protection Agency; 2009. [Google Scholar]
- Rosenthal RA, Fish B, Hill RP, Huffman KD, Lazarova Z, Mahmoud J, Medhora M, Molthen R, Moulder JE, Sonis ST, Tofilon PJ, Doctrow SR. Salen Mn complexes mitigate radiation injury in normal tissues. Anti-Cancer Agents Med Chem. 2011;11:359–372. doi: 10.2174/187152011795677490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkamm K, Beinke C, Romm H, Badie C, Balagurunathan Y, Barnard S, Bernard N, Boulay-Greene H, Brengues M, De Amicis A, De Sanctis S, Greither R, Herodin F, Jones A, Kabacik S, Knie T, Kulka U, Lista F, Martigne P, Missel A, Moquet J, Oestreicher U, Peinnequin A, Poyot T, Roessler U, Scherthan H, Terbrueggen B, Thierens H, Valente M, Vral A, Zenhausern F, Meineke V, Braselmann H, Abend M. Comparison of established and emerging biodosimetry assays. Radiat Res. 2013;180:111–119. doi: 10.1667/RR3231.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JL, Krishnan S, Movsas B, Coleman CN, Vikram B, Yoo SS. Decreasing the adverse effects of cancer therapy: An NCI workshop on the preclinical development of radiation injury mitigators/protectors. Radiat Res. 2011;176:688–691. doi: 10.1667/rr2704.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Moulder JE. The urine proteome as a radiation biodosimeter. Adv Exper Med Biol. 2013;990:87–100. doi: 10.1007/978-94-007-5896-4_5. [DOI] [PubMed] [Google Scholar]
- Singh VK, Ducey EJ, Brown DS, Whitnall MH. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int J Radiat Biol. 2012;88:296–310. doi: 10.3109/09553002.2012.652726. [DOI] [PubMed] [Google Scholar]
- Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Greenberger JS, Hauer-Jensen M, Hill RP, Kolesnick RN, Macvittie TJ, Marks C, McBride WH, Metting N, Pellmar T, Purucker M, Robbins ME, Schiestl RH, Seed TM, Tomaszewski JE, Travis EL, Wallner PE, Wolpert M, Zaharevitz D. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI workshop, December 3–4, 2003. Radiat Res. 2004;162:711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Flood AB, Gougelet RM, Rea ME, Nicolalde RJ, Williams BB. A critical assessment of biodosimetry methods for large-scale incidents. Health Phys. 2010;98:95–108. doi: 10.1097/HP.0b013e3181b8cffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyk J, Keane TJ, Kan S, Rider WD, Fryer CJH. Radiation pneumonitis following large single dose irradiation: a re-evaluation based on absolute dose to the lung. Int J Rad Oncol Biol Phys. 1981;7:461–467. doi: 10.1016/0360-3016(81)90131-0. [DOI] [PubMed] [Google Scholar]
- Vaurijoux A, Gregoire E, Roch-Lefevre S, Voisin P, Martin C, Voisin P, Roy L, Gruel G. Detection of partial-body exposure to ionizing radiation by the automatic detection of dicentrics. Radiat Res. 2012;178:357–364. doi: 10.1667/rr2728.1. [DOI] [PubMed] [Google Scholar]
- Vorotnikova E, Rosenthal RA, Tries M, Doctrow SR, Braunhut S. Novel synthetic SOD/catalase mimetics can mitigate capillary endothelial cell apoptosis caused by ionizing radiation. Radiat Res. 2010;173:748–759. doi: 10.1667/RR1948.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WF, Kim YT, Molteni A, Solliday NH. Radiation-induced pulmonary endothelial dysfunction in rats: Modification by an inhibitor of angiotensin converting enzyme. Int J Rad Oncol Biol Phys. 1988;15:135–140. doi: 10.1016/0360-3016(88)90357-4. [DOI] [PubMed] [Google Scholar]
- Wilcox DE, He X, Gui J, Ruuge AE, Li H, Williams BB, Swartz HM. Dosimetry based on EPR spectral analysis of fingernail clippings. Health Phys. 2010;98:309–317. doi: 10.1097/HP.0b013e3181b27502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Dong R, Kmiec M, Burke G, Demidenko E, Gladstone D, Nicolalde RJ, Sucheta A, Lesniewski P, Swartz HM. Development of in vivo tooth EPR for individual radiation dose estimation and screening. Health Phys. 2010;98:327–338. doi: 10.1097/HP.0b013e3181a6de5d. [DOI] [PMC free article] [PubMed] [Google Scholar]


