Abstract
Glutamate carboxypeptidase II (GCPII) in the central nervous system is referred to as the prostate-specific membrane antigen (PSMA) in the periphery. PSMA serves as a target for imaging and treatment of prostate cancer and because of its expression in solid tumor neovasculature has the potential to be used in this regard for other malignancies as well. An overview of GCPII/PSMA in cancer, as well as a discussion of imaging and therapy of prostate cancer using a wide variety of PSMA-targeting agents is provided.
Keywords: Prostate-specific membrane antigen, PSMA, prostate cancer, molecular imaging, PET, SPECT
PSMA IN CANCER
As has been discussed in previous chapters, enzymatic activity of glutamate carboxypeptidase II (GCPII) is observed in a number of normal and diseased tissues inside and outside of the central nervous system (CNS). GCPII expression within the brush border of the jejunum is one of two locations for which the function of GCPII activity is definitely known. The other is GCPII expression on astrocytes, which cleaves N-acetylaspartylglutamate (NAAG) into NAA and glutamate, as discussed in Chapter 6. On the luminal side of brush border cells in the jejunum GCPII is known as folate hydrolase I, which cleaves all γ-linked glutamates from food-derived folate species, enabling folate transport across the intestine and loading onto folate carrier proteins for distribution throughout the body [1–3]. Once these γ-linked monoglutamyl folates are imported into cells through folate receptors, they are then reduced and re-poly-γ-glutamated by folylpoly-γ-glutamate synthetase [4] for storage. GCPII is also expressed in prostate adenocarcinomas [5–9], where it is referred to as the prostate-specific membrane antigen (PSMA), as well as within a few subtypes of bladder carcinoma [10], schwannoma [11] and on the tumor neovasculature of many solid tumors [12–15]. It is also present in the neoendothelium of benign tissues such as endometrium and keloid scar vasculature [16], for which the endogenous substrate(s) and function(s) remain unknown, although new hypotheses have been introduced and will be outlined below.
PSMA Expression in Prostate Cancer
PSMA expression and localization in the normal human prostate is associated with the cytoplasm and apical side of the epithelium surrounding prostatic ducts but not basal epithelium, neuroendocrine or stromal cells [17]. Cytoplasmic PSMA is an N-terminally truncated form of PSMA called PSM′, which has no folate hydrolase activity or capacity to hydrolyze NAAG [18–20]. It appears to be the product of post-translational modification rather than mRNA splicing. The function of PSM′ is unknown. Dysplastic and neoplastic transformation of prostate tissue results in the targeting of PSMA from the apical membrane to the luminal membrane of the ducts [21,22]. Further transformation eventually leads to expression on the plasma membrane of less differentiated epithelial cells, which is associated with the transition into and achievement of androgen growth independence [23–25]. As tumor cells advance in Gleason grade, the ratio of PSMA/PSM′ reliably increases [18] and PSMA expression is thought to circumvent decreased androgen signaling by contributing to an unknown ligand-induced alternative signal transduction pathway [17]. Indeed, androgen receptor (AR) signaling down-regulates the expression of full-length PSMA [5], which may contribute to increasing expression of PSMA by tumor cells following androgen ablation therapy [26,27] and to heterogeneous expression within tumor cells of increasing malignancy in intact males.
Interestingly, dogs are the only other mammalian species known to express PSMA in the prostate (by RT-PCR) and also are the only other species known to develop prostate cancer spontaneously, which is also frequently PSMA-positive [28,29]. Canine prostate cancer, like human prostate cancer, also becomes more aggressive following castration and exhibits greater PSMA expression following castration. The biological function of PSMA expression on prostate cancer cell membranes remains unknown. Speculations range from alternative folate transporter mechanisms [30,31], a receptor for an as yet unknown endogenous ligand or possibly as a source of glutamate. Because nearly 100% of distant metastases express PSMA, PSMA activity is very likely contributing to successful travel to and coloniziation of distant sites such as bone [32,33].
PSMA has both sequence homology and biological behavior similar to that of the transferrin receptor [34,35]. Both receptors are membrane bound and both bind their ligands as a dimer before internalization through clathrin-coated pits [36,37]. PSMA also exists on the membrane surface as a monomer but is enzymatically active only as a dimer [5]. Following substrate, small-molecule antagonist or specific antibody binding, PSMA-bound ligands are then internalized within the cell and are either retained in lysosomal compartments along with the degrading PSMA receptor [5], or bound ligands may be released to distribute within the cell or diffuse out of the cell as labile metabolites [38,39]). Some internalized PSMA proteins are also recycled intact back to the membrane surface through the recycling endosomal compartment (REC) [5, 40]. The fate of bound ligands through that pathway remains unknown. It is possible that bound ligands might be delivered intact to the cytosol, as is the case for endogenous folates [31]. The cytoplasmic tail of PSMA contains consensus sequences for protein kinase C (PKC) recognition and phosphorylation. Phosphorylation of various Thr and Tyr residues by PKC may direct PSMA interaction with selected adaptor proteins, which control receptor sorting into either lysosomes or the REC for recycling [5].
PSMA contains ten putative glycosylation sites and is highly glycosylated [41]. Glycosylation is necessary for enzymatic activity as well as for protein folding and membrane targeting [42] within prostate cancer cells. Interestingly, different human prostate cancer lines and tumors within patients have been found to express PSMA with different glycosylation patterns and correspondingly different enzymatic activity profiles [43,42]. Control of glycosylation for membrane targeting and enzymatic activity is likely involved in metastasis. Indeed, various human prostate cancer cell lines reflecting androgen sensitive and insensitive growth differed in their PSMA enzymatic activity (using N-acetylaspartylglutamate as the substrate) where the androgen insensitive C4-2B cell line, having the highest metastatic potential in mouse models, had 109 times the carboxypeptidase activity of LAPC-4, an androgen sensitive non-metastatic cell line [44], and three to five times the activity of two common androgen insensitive and one sensitive, but less aggressive cell lines. Differential glycosylation of PSMA may be controlled by residual androgen signaling since three of four cell lines described above that displayed growth-related androgen insensitivity also possess mutant ARs with disparate residual signaling capacity. Growth sensitivity to androgens is not always tied to AR transcriptional control of other prostate cancer markers, such as for prostate-specific antigen (PSA) or kallikrein 2 expression, and differs by cell line [44]. Notably, the only two isolated prostate cancer cell lines devoid of AR expression (PC-3 and DU-145) are also similarly devoid of PSMA expression [45].
PSMA in the Neovasculature
PSMA is also expressed on the apical and luminal surface of new blood endothelial cells associated with most solid tumors as well as the neovasculature of some normal proliferative tissues such as endometrium and wounds within normal tissues such as heart valve injuries, pleural lesions and some keloid scars [16]. PSMA expression in tumor neovasculature has so far only been reported in humans although the search for PSMA-expressing neovasculature in tumors from murine hosts continues. The function of PSMA expression on solid tumor neovasculature remains unknown although two biochemical pathway interactions augmented by PSMA folate hydrolase activity have been reported as important to vasculogenesis. One report describes a feedback loop connection between PSMA, γ1-integrin, and p21-associated kinase (PAK) signaling in which PSMA activity activates γ1-integrin (coordinates cell adhesion to and movement across laminin, a component of the extracellular matrix) and γ1-integrin activates PAK, which phosphorylates and deactivates filamin A. Deactivation of filamin A promotes outreach of invasive filopodia by the cytoskeleton, which is important for endothelial cell invasion [46]. Gordon et al. [16] speculate that the folate hydrolase activity of PSMA on endothelial plasma membranes scavenges released extracellular poly-γ-glutamyl folates and internalizes these folates, which are stored and converted into 5-methyl-tetrahydrofolate (THF). THF reduces and recycles the tetrahydrobiopterin (BH4) cofactor of the endothelial isoform of nitric oxide synthase (eNOS), allowing eNOS catalyzed production of NO, a potent stimulator of angiogenesis [47,48].
Various folate receptors that enable import of all species of extracellular folate are ubiquitous on all cell types [49–51] and intracellular pools of stored folates are frequently adequate due to intake from fortified dietary sources [52,53]. When considering the effect of glutamate carboxypeptidase/folate hydrolase activity, it is important to remember that PSMA enzymatic activity upon endogenous folates results in more than just folic acid. Cleavage also results in the generation of multiple glutamates, which activate glutamate receptors.
PSMA and Glutamate Signaling
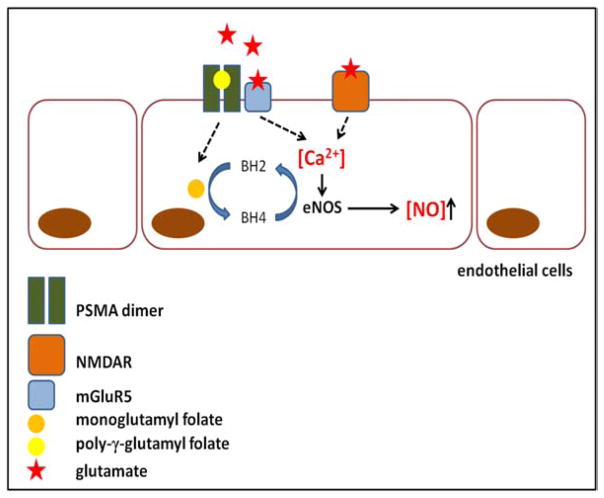
It has recently been recognized that functional glutamate signaling occurs in several cancer types outside of the CNS: melanoma [54], head and neck cancer [55], osteosarcoma [56], colorectal cancer [57], squamous cell carcinoma [58] and in prostate cancer [59]. Signaling can involve ionotropic (N-methyl-D-aspartate receptor, NMDAR) or metabotropic glutamate receptors (mGluR) as well as glutamate transporters [60]. The synthesis of NO by eNOS is particularly sensitive to intracellular calcium concentration, where increasing calcium elevates production of NO [61,62]. Intracellular calcium concentration is increased by excitatory NMDAR and group I mGluR signaling, both of which are activated by glutamate binding. PSMA is co-expressed with mGluR5, a group I mGluR, in prostate cancer cells [59] (C.A. Foss unpublished results) and also mGluR1 [59] and the NMDAR is expressed in prostate cancer of increasing grade [63]. The functional significance and therapeutic implications of possible PSMA-augmented excitatory glutamate signaling is the subject of current work by the authors. Currently, NMDAR vascular presence has been reported only in brain microvasculature [64,65] while excitatory group I mGluRs have so far only been reported in brain and dermal microvasculature [66–68]. It is possible that excitatory glutamate signaling in the neovasculature of any origin has not been investigated due to the continuing focus of glutamate transmission on CNS and PNS neuronal and glial cell functioning. However, PSMA-gated glutamate-evoked calcium-dependant NO generation in pre-vascular endothelial cells to stimulate angiogenesis is an intriguing mechanistic possibility. PSMA enzymatic cleavage would simultaneously supply a folate moiety to facilitate eNOS cofactor BH4 turnover while raising intracellular calcium concentration through NMDAR and/or mGluR1/5 glutamate signaling, leading to increased NO generation and angiogenesis (Fig. 1).
Fig. (1).
Diagram of PSMA-augmented generation of NO in nascent endothelial cells. PSMA folate hydrolase activity may boost NO production in two complementary ways including scavenging extra folate (orange circle) to increase BH4 eNOS cofactor turnover and generation of extra glutamate (stars), which raises intracellular calcium levels to increase expression of eNOS. NO is important for angiogenesis and expression of PSMA on neovasculature and may increase the efficiency of vessel formation.
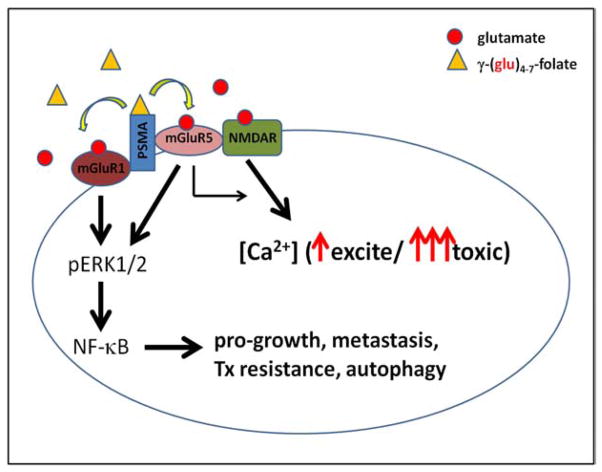
PSMA expression alone in prostate cancers has been shown to be ineffective in predicting the course of the disease, although metastatic tumors almost universally express large amounts of PSMA [26,69]. It is possible that the combination of expression of membrane-bound PSMA with excitatory glutamate receptors, which may express differentially according to environment and accumulated genetic changes, shape malignancy and/or metastatic potential in prostate tumors. Serum and tissue glutamate levels are typically 30–40 times higher in the periphery [70] than in resting synapses (40–60 μM vs. 1–2 μM), where potentially toxic signaling is prevented by control of synaptic glutamate [71]. Peripheral NMDARs are not voltage dependant and can be activated in the presence of both glycine and glutamate [72]. Peripheral group I mGluRs are constitutively active in the presence of glutamate [73] and are regulated in part by frequent internalization via clathrin coated pits [74]. PSMA is also internalized via clathrin coated pits and has been postulated to interact with mGluR5 through filamin A binding [59.] It is possible that poly-γ-glutamyl folate binding and/or cleavage by PSMA may cause a conformational change that activates adjacent mGluR5 and/or mGluR1, enabling folate-released or tissue glutamate binding and subsequent specific downstream signaling events such as NF-γB activation, which is known to be constitutively active in prostate cancers, possibly through this mechanism (Fig. 2). Similarly, glutamate signaling through group I mGluRs in astrocytes has been shown to activate NF-γB signaling [75]. More work in this area is needed to illuminate these interactions and functional consequences of PSMA and excitatory glutamate receptor co-expression.
Fig. (2).
Diagram of the possible consequences of PSMA folate hydrolase activity in prostate cancer cells. Scavenged poly-γ-glutamyl folate substrates could feed nearby or interacting mGluR1/5 or NMDARs with released glutamates, maintaining both the downstream activation of NF-γB and raising intracellular calcium levels indirectly through mGluR5 signaling or directly through NMDAR channel opening. Small amounts of glutamate might be beneficial while large amounts of glutamate could cause excitotoxicity.
Because the expression of membrane-bound PSMA is very restricted in normal tissues and is abundantly expressed in prostate cancers and the neovasculature of most solid tumors, it is an attractive target for both diagnostic imaging of metastatic tumors and targeted therapies for these tumors, which will be described further below. Increased knowledge concerning the connection between PSMA folate hydrolase activity and glutamate signaling in both prostate tumors and in neoangiogenesis would also be helpful in terms of drug development, diagnostic and prognostic significance. Radiolabeled small molecule probes for the NMDAR [76], mGluR5 [77–82] and mGluR1 [83–85] have been developed to allow patient selection for targeted therapies to augment or disrupt interactions between these proteins. Radiolabeled small molecule probes targeting PSMA for positron emission tomography (PET) and single photon emission computed tomography (SPECT) imaging have also been developed and will be described in the following sections.
MOLECULAR IMAGING OF CANCER
Molecular imaging broadly defined is the non-invasive detection and measurement of cellular and molecular processes in whole living beings using a variety of existing modalities including PET, SPECT, magnetic resonance (MR), computed tomography (CT), ultrasound, fluorescence, or bioluminescence [86–88]. Imaging has become an indispensable tool in cancer research, clinical trials and medical practice. In the era of molecular oncology and personalized medicine, development of molecular imaging methodologies can detect processes related to metabolism, angiogenesis, and hypoxia as well as image other cellular processes such as gene expression, receptor expression, and signaling pathways. Molecular imaging in cancer promises to address the following issues in cancer management: (1) detection of the presence of malignancy or to direct biopsy; (2) staging; (3) therapeutic monitoring – particularly early after initiation of therapy; (4) provision of a prognostic biomarker differentiating aggressive from indolent disease; (5) improvement and acceleration of development of novel therapeutics. PET/CT imaging has emerged over the last decade as an important molecular imaging modality in oncology as evidenced by the rapid rise in the total number of [18F]fluorodeoxyglucose (FDG) PET/CT scans performed for clinical use, with more than 1.5 million FDG PET/CT or PET scans in the United States in 2006 [89].
MOLECULAR IMAGING OF PROSTATE CANCER
Prostate cancer is the mostly commonly diagnosed cancer and the second leading cause of cancer death among men in the United States and second most common cancer in men worldwide [90,91]. Conventional imaging modalities, including bone scintigraphy (bone scan), CT, ultrasound, and MR imaging, are currently used to detect primary prostate cancer and metastatic disease for staging and risk stratification. However, there is a need for imaging beyond current capabilities to improve management and selection of appropriate therapy in the following clinical scenarios: (1) initial diagnosis (accurate diagnosis and anatomic localization directly within the prostate to guide biopsy and determine the likely effectiveness of focal therapy; risk stratification to determine whether the lesion represents indolent versus aggressive disease; accurate staging); (2) biochemical recurrence after initial primary therapy (detection of local and metastatic disease); and, (3) progressive metastatic disease (detection and localization of metastases; assessment of overall metastatic burden; assessment of castrate-sensitive and -resistant disease; assessment of early treatment response). By targeting the biological mechanisms unique to prostate cancer, molecular imaging can help address many of the above mentioned unmet clinical needs and hasten the development of new therapeutics. PET is the current premier translational molecular imaging technique and will provide the basis for the following discussion. It is against the existing and more conventional molecular imaging agents discussed in this section that the newer agents that target PSMA must be judged.
FDG PET
Although PET as a functional imaging modality, in particular FDG PET, has played an increasingly important role in tumor detection, staging, and therapeutic monitoring in a variety of other cancers, the role of FDG PET has been limited in prostate cancer for detection of primary disease and soft tissue and osseous metastases [92–94]. FDG PET detection of metastatic prostate cancer can however be improved with optimal detection of metastatic disease based on receiver operating characteristic (ROC) analysis of PSA ≥ 2.4 and PSA velocity of 1.3 ng/mL/year [95]. When there is detectable FDG uptake in metastatic prostate cancer, increasing FDG uptake (> 33% increase in maximum standardized uptake value [SUVmax]) on chemotherapy proved to be a prognostic marker and outcome measure comparable to a combination of imaging and biochemical markers (serum PSA, bone scan, CT and/or MRI) in patients with advanced hormone-refractory metastatic disease [96].
[18F]Fluoride PET
[18F]Fluoride-labeled NaF ([18F]NaF )has long been recognized as an excellent radiopharmaceutical for bone imaging, but fell out of use when more widely available, kit-based [99mTc]diphos-phonates emerged for skeletal scintigraphy [97]. [18F]NaF uptake in osteoblastic activity is 2-fold greater than [99mTc]methylene diphosphonate (MDP). Through use of PET, which is of inherently higher sensitivity and resolution than SPECT, with its semi-quantitative capacity and the recent availability of hybrid (PET/CT imaging systems), [18F]NaF has been revisited as a viable radiopharmaceutical for imaging bone metastasis [98]. A prospective study of [18F]NaF PET/CT, [18F]NaF PET, MDP SPECT, and MDP planar imaging for detection of bone metastases in patients with prostate cancer demonstrated the high sensitivity and specificity of [18F]NaF PET/CT, with higher specificity than PET alone and higher sensitivity and specificity than standard MDP SPECT or planar bone scintigraphy [99]. However, the added clinical value of improved and earlier detection of bone metastasis by [18F]NaF PET/CT must be balanced against providing increased radiation exposure and cost. The National Oncology PET Registry (NOPR) sought and recently obtained approval from the Center for Medicare and Medicaid Services (CMS) to begin a registry to perform the necessary collection of data to allow for coverage of [18F]NaF PET scans for detection of bony metastases in cancer [100].
Radiolabeled Choline Analogs
Choline uptake in prostate cancer cells is associated with cell membrane phospholipid metabolism, with extracellular uptake dependent on choline uptake through a choline transporter and phosphorylation through a choline kinase [101–103]. [11C]Choline and [18F]fluorocholine uptake mechanisms are similar with rapid tumor uptake. However, in addition to differences in physical half-life (20 min for 11C versus 109 minutes for 18F), there is substantial urinary excretion of [18F]fluorocholine but only minimal urinary excretion of [11C]choline [104]. [11C]Choline and [18F]fluorocholine are the most studied PET radiopharmaceuticals for prostate cancer, but due to issues inherent in what are usually small studies and the lack of histopathologic verification of imaging findings, the role of these agents in prostate cancer remains unclear and awaits further assessment in large, prospective, multi-center trials.
For detection of primary prostate cancer, [11C]choline PET is limited due to significant overlap with uptake seen in prostatitis or benign prostatic hypertrophy. There has been no correlation between radiolabeled choline uptake and tumor histologic grade, volume of disease or PSA level [105–107]. However, other studies claim to be able to differentiate benign from malignant processes by visual evaluation or to differentiate aggressive prostate cancer (Gleason 4+3 or higher from 3+4 or lower) through use of tumor-to-background radiotracer uptake ratios [108,109]. When compared to pelvic MR imaging with 3D MR spectroscopy, [11C]choline demonstrated lower sensitivity for localization of primary prostate cancer [110]. [18F]Fluorocholine performed better for detection of primary prostate cancer, i.e., it was able to localize dominant areas of malignancy, but was unable to detect small lesions. Addition of dual time point imaging enabled differentiation between benign and malignant primary prostate lesions [111, 112]. However, another study did not recommend [18F]fluorocholine for localizing prostate cancer, although in highly selected patients [18F]fluorocholine PET/CT allowed the identification of neoplastic regions within the prostate [113].
For staging and detection of metastatic disease, an early study demonstrated relatively high sensitivity and specificity (80% and 96%, respectively) for detection of nodal metastases for [11C]choline, although this technique possessed lower sensitivity and comparable specificity compared to MRI or CT for detection of LN metastases [114]. For detection of lymph node metastases at PSA relapse, [11C]choline showed a high positive predictive value for detection of metastases in patients with PSA ≥ 2.0 with the detection rate directly related to serum PSA level. However, the negative predictive value and sensitivity were low, attributable to the inability to detect microscopic disease in subcentimeter lymph nodes due to PET detectability limits [115,116]. In patients with advanced metastatic disease, [11C]choline PET/CT affected management in 24% of patients [117]. Notably, [11C]choline uptake declined after anti-androgen therapy in primary and metastatic prostate cancer in one study but reportedly did not significantly affect the rate of detection of metastases in two other studies [105–117]. [18F]Fluorocholine could reliably detect metastases in PSA recurrence but only when PSA levels were> 4 ng/mL [118]. A systematic review and critical analysis of published results with [18F]fluorocholine reported recommendation for its use for initial staging among intermediate- to high-risk populations (PSA > 10 ng/mL or Gleason score ≥ 7) and restaging for recurrent disease, optimally if PSA > 2 ng/mL or there are short PSA doubling times [119].
Acetate
[11C]Acetate uptake in prostate cancer is associated with fatty acid metabolism and related to levels of expression of fatty acid synthase (FAS) [120]. Although less extensively studied than choline PET radiotracers, [11C]acetate PET imaging is thought to have similar accuracy for detection of recurrent disease [121]. Other studies of [11C]acetate at PSA relapse demonstrated detection of metastatic disease with findings influencing patient management in 28% of patients, but also demonstrated a false positive uptake in 15% of patients [122–124]. A review of several studies for detection of local, nodal and osseous metastases in recurrent prostate cancer was unable to recommend definitively between choline or acetate PET radiotracers for these indications [125]. [18F]Fluoroacetate has also been synthesized and reported to have potential application in prostate cancer [126].
[18F]FACBC
[18F]FACBC (anti-1-amino-3-[18F]fluorocyclobutane-1-carboxylic acid) is a synthetic, non-metabolized amino acid analog of L-leucine that demonstrated detection of primary prostate tumor and local pelvic nodal metastatic disease as well as suspected sites of recurrence in an initial pilot study of 15 patients [127]. A larger study of 50 patients comparing [18F]FACBC with that of [111In]capromab pendetide (ProstaScint™) in the detection of recurrent prostate cancer found [18F]FACBC to be more sensitive for detection of intraprostatic and extraprostatic sites of disease [128]. The mechanism of uptake in prostate cancer is thought to be via the amino acid transporter system ASC transporter 2 (ASCT2), which sodium-dependent. [18F]FACBC is not incorporated into proteins [129].
[18F]FDHT
[18F]FDHT (16β-fluoro-5α-dihydrotestosterone) is an [18F]-labeled analog of dihydrotestosterone, the endogenous ligand for AR. Initial studies evaluating [18F]FDHT PET imaging for AR imaging in patients with castrate-resistant prostate cancer confirmed receptor mediated uptake with diminished uptake after competition with testosterone treatment. [18F]FDHT was present within 78% of lesions seen by conventional imaging in one study, demonstrating a sensitivity of 63% on a patient-by-patient basis and 86% sensitivity on a lesion-by-lesion basis [130, 131]. As a radiotracer designed against a well characterized receptor, a pharmacokinetic model of [18F]FDHT behavior was able to provide a surrogate measure of free AR concentration [132].
Other
Other PET radiopharmaceuticals have also been studies in prostate cancer in a limited number of studies, including [11C]methionine, [18F]FMAU (1-(2′-deoxy-2′-fluoro-β-D-arabinofuranosyl) thymine), [18F]FLT (3′-deoxy-3′-[18F]fluorothymidine) [133–135].
IMAGING PSMA: ANTIBODIES
Monoclonal antibodies are widely used in basic research and are often used for targeted therapy in oncology. Aside from therapeutic use, monoclonal antibodies have also been applied to molecular imaging, enabling specific targeting in vivo. Radiolabeled antibodies are detected with PET and SPECT in analogy to the low molecular weight agents discussed above [136]. Several recent reviews have appeared that outline the use of monoclonal antibodies and antibody fragments for imaging, and the strategies used to obviate problems of non-specific binding and long circulation times that often accompany the use of antibodies for this indication [137].
As discussed above, PSMA is expressed in virtually all prostate cancers and that expression progressively increases with cancer grade [138]. Unlike prostate cancer-specific secretory molecules such as PSA and prostatic acid phosphatase (PAP), PSMA is an integral membrane protein [139]. Prostate cancer cells overexpress this protein by several orders of magnitude over normal prostate cells, making PSMA an ideal target for detection and treatment of prostate cancer. Currently, the only monoclonal antibody approved by the U.S. Food and Drug Administration (FDA) available for imaging prostate cancer is that based on 7E11/CYT-356.
7E11/CYT-356
7E11/CYT-356 is a monoclonal antibody against PSMA that binds to a six amino acid sequence on the intracellular surface of the protein. The 111In-labelled version of 7E11/CYT-356, is what is commonly known as ProstaScint™, introduced above. ProstaScint™ was approved by the FDA in 1997 for human imaging [140]. As stated by the FDA, ProstaScint™ was approved for usage “...in newly diagnosed patients with biopsy-proven prostate cancer thought to be clinically localized... who are at high risk for pelvic lymph node metastasis” and “...in post-prostatectomy patients with a rising PSA and a negative or equivocal standard metastatic evaluation in whom there is a high clinical suspicion of occult metastatic disease.” ProstaScint™ is reported to stage primary prostate cancer with 68% accuracy, 62% sensitivity and 72% specificity [141], which suggests that this agent may be useful but suboptimal. ProstaScint™ is also sequestered to a large extent in liver, kidney, and other non-prostate sites [142]. Non-specific tissue uptake, especially that which occurs in the lower abdomen and pelvis, creates background signal that often masks the prostate. Long circulating times must also be addressed, usually through subtraction of a blood pool image of the pelvic vasculature.
One reason that images from ProstaScint™ are difficult to interpret may be due to the fact that the antibody binds to an intracellular epitope on prostate cancer cells, and has access to target only through destruction of the cell membrane as occurs in states of necrosis and apoptosis [143]. In tissues such as bone, where access to target is even more limited than in soft tissue, ProstaScint™ has proved unable to detect metastases reliably [144]. ProstaScint™ is injected approximately one week prior to imaging to allow time for maximal delivery to target, and for clearance of non-specifically bound signal. Over the course of the week, the 111In radiolabel signal decays significantly, due to its 2.8 day physical half-life. Decay correction of the signal generated one week post-injection has proved challenging [145]. That difficulty in image interpretation is magnified further by continued nonspecifically-bound signal even after allowing for one week of clearance [146]. Nevertheless, new techniques specifically designed to interpret images due to ProstaScint™ can overcome some of those limitations. Localization of the SPECT signal is aided by superimposing anatomic CT data on the SPECT image. Difficulties still obtain, however, with superimposing these two different data sets due to changes in the shape and volume of the rectum and/or bladder and due to patient movement between their acquisition [147]. While such efforts have greatly reduced the incidence of false positives in the diagnosis of primary prostate cancer, ProstaScint™ has proved less reliable than biopsies of nearby organs such as the seminal vesicles [148].
J591
J591 is a deimmunized monoclonal antibody that is specific for the extracellular domain of PSMA [149]. Unlike 7E11, which binds to an intracellular epitope, J591 binds viable cells expressing PSMA on the outside of the cell membrane. The antibody is prepared for imaging or therapy by conjugating it with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) a chelator for a variety of radiometals [150]. The J591 antibody has been radiolabeled with 111In and 99mTc for imaging and with 90Y and 177Lu for therapy [149, 151, 152]. Also, although beyond the scope of this review, near-infrared (NIR) fluorophores have also been attached to J591 for use as imaging agents [153].
As in the case of ProstaScint™, [177Lu]J591requires time to reach its target, also requiring about a week to reach metastatic lesions. Similar to ProstaScint™, this radiotracer also appears in other non-target organs, most noticeably in the liver, kidneys, and spleen, as is common to antibody based imaging agents. However, in comparison to ProstaScint™, [177Lu]J591 demonstrates higher target-to-background ratios [149]. Most recently the J591 antibody, labeled with 64Cu, has proved capable of reporting on AR signaling, opening up an entirely new use for PSMA-based imaging agents [25].
3/A12, 3/F11, 3/E7
Three monoclonal antibodies specific for PSMA, 3/A12, 3/F11, 3/E7,are currently under development as molecular imaging agents for prostate cancer. As for J591, 3/A12, 3/F11 and 3/E7 are all conjugated to DOTA [152]. The conjugated DOTA is then radiolabeled with 64Cu, a radionuclide that decays through positron emission, γ-decay and electron capture, and is imaged using PET [154]. These antibodies all demonstrate specific uptake in PSMA-positive tumors, with 3/A12 displaying significantly more uptake in the kidneys than the others. In flow cytometry assays for competitive binding alongside J591, the antibodies showed comparable half-maximal saturation concentrations with 14 nM for 3/A12, 17nM for 3/E7, and 9nM for 3/F11, while J591 had a half-maximal saturation concentration of 16 nM [155]. In animal studies, combined PET/MR imaging allows for the easy identification of tumors with low antibody uptake [156]. The high specificity of these antibodies seems to warrant further studies and cautionary optimism for use in prostate cancer.
IMAGING PSMA: LOW MOLECULAR WEIGHT AGENTS
As stated above, one of the drawbacks of using monoclonal antibodies for imaging is the long time required for the antibody to clear from non-target tissues, often a span of several days between injection of the agent and imaging. Strategies for addressing the poor pharmacokinetics of antibodies for imaging include the use of aptamers or small molecules as the PSMA targeting agent. Aptamers are either 8–15KDa oligonucleotides or peptides, isolated from combinatorial libraries, which can be selected for specific binding to target molecules through affinity maturation [157]. Their specificity and affinity for targets are similar to antibodies. 99mTc-Labeled aptamer TTA1 demonstrated uptake in breast, colon, glioblastoma, and lung tumor xenografts [158] and demonstrates the promise of this approach. Most work so far with PSMA-binding aptamers has concentrated on the delivery of therapeutics including short hairpin RNA (shRNA) [159]. Aptamer A10 [160] as well as aptamer-doxorubicin [161] showed specific binding to PSMA-positive cells in vitro. A10-Apatamer-siRNA and A10-Aptamer-shRNA chimera have demonstrated tumor regression in PSMA-positive tumor xenografts [162] and increased the sensitization of PSMA-positive tumor xenografts to external radiation [163]. Co-delivery of doxorubicin and shRNA against Bcl-xl using a PSMA aptamer-conjugated polyplex was more toxic to PSMA positive cells than a simple mixture of the drug and shRNA, which demonstrates the potential of targeted co-delivery of therapeutic agents [164]. Bi-specific apatamers consisting of a PSMA targeting aptamer and an agonistic 4-1BB aptamer inhibited PSMA-positive tumor xenograft growth via 4-1BB co-stimulation of tumor-specific CD8+ T cells [165] and could be useful in developing a targeted immune response to cancer.
The A10 aptamer has also been utilized as the PSMA targeting moiety (20 nM affinity, [166,167]) for aptamer-nanoparticle [164, 168–172] and aptamer-quantum dot [173] conjugates. Several of these preparations, when loaded with either doxorubicin or cisplatin, demonstrated either PSMA-specific cell growth inhibition in vitro [164, 170, 172, 173] or tumor regression from a single intratumoral injection [168]. Only recently has an aptamer to PSMA been radiolabeled. In this report, the positron emitter 64Cu complexed to DOTA was conjugated to the aptamer. However, no in vivo images have yet been produced [174].
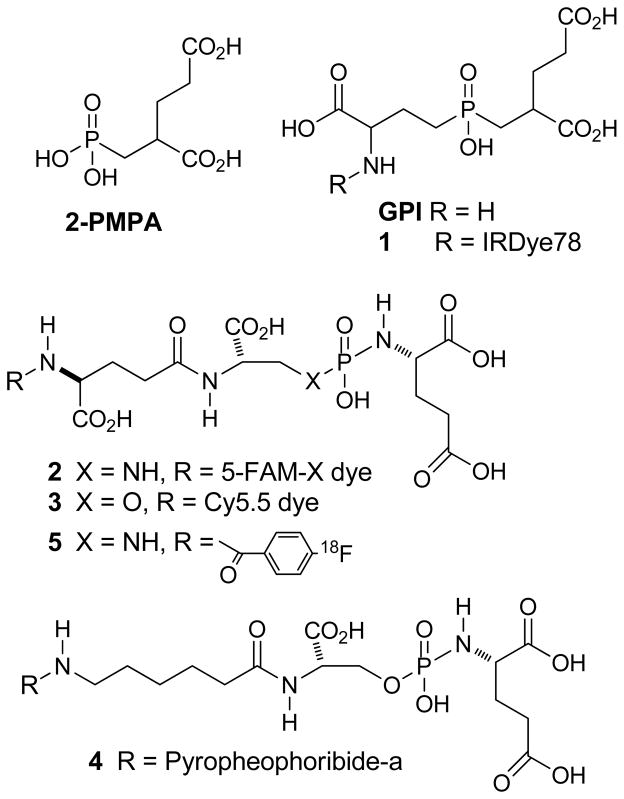
As discussed above, in addition to providing a target for monoclonal antibodies such as ProstaScint™ and J591, PSMA possesses an enzymatic site in its extracellular domain that cleaves endogenous substrates such as NAAG and poly-γ-glutamyl folic acid. The crystal structure of PSMA with and without inhibitors in the enzymatic site has been described [175–180]. The enzymatic site contains two zinc ions, and is composed of two pockets, the glutamate-sensing pocket (S1′ pocket) and the non-pharmacophore pocket (S1 pocket). A tunnel of an approximate length of 20 Ǻ connects the enzymatic site to the surface. Many small molecule substrates and inhibitors for this enzyme have been prepared and tested, many prior to the reporting of the crystal structure of PSMA. This topic has been reviewed recently [181, 182]. Small molecule PSMA inhibitors are generally zinc binding compounds attached to a glutamate or glutamate isostere [183] and fall into three families of compounds: (1) phosphonate-, phosphate-, and phosphoramidate compounds; (2) thiols; and (3) ureas. The initial work on the phosphonate and phosphate inhibitors, which included the potent GCPII inhibitor 2-(phosphonomethyl) pentanedioic acid, 2-PMPA (IC50 ~ 0.9 nM, [184]), as well as the thiol based GCPII inhibitors, came from research conducted at ZENECA and then Guilford Pharmaceuticals [185, 186]. Later, extensive studies with the phosphoramidate inhibitors came from Berkman’s group (IC50s ~ 0.5–20 nM), [187–189]. The initial preparation and testing of urea-based inhibitors was reported by Kozikowski et al. [190]. Research on new imaging agents for prostate cancer based on small molecule PSMA inhibition have concentrated on the use of phosphoramidates and ureas. Of the agents derived from 2-PMPA the first optical imaging agent was prepared by conjugating IRdye78 to the known GCPII inhibitor GPI to form 1 (Fig. 4). That agent possessed a high binding affinity (low nanomolar) for PSMA positive cells and clearly stained PSMA positive cells in vitro. However, its rapid clearance from the blood limits the time for uptake in tumors [191]. Berkman’s group reported compound 2, which was also a potent PSMA inhibitor and demonstrated internalization of the fluorescent molecule [188]. Changing to the near-IR dye Cy5.5 to produce 3 also resulted in a potent inhibitor and may be useful for in vivo imaging although no in vivo images were reported [192]. Conjugation of a photodynamic therapeutic agent, pyropheophorbide-a to a phosphoramidate resulted in agent 4,which was phototoxic to PSMA-positive cells in vitro and could eventually lead to a treatment for primary prostate cancer [192]. A [18F]fluorobenzoyl phosphoramidate, compound 5, demonstrated specific uptake in PSMA positive xenografts [193].
Fig. (4).
Phosphorus-containing GCPII imaging and therapeutic agents.
The first urea based small molecule PSMA inhibitor was [11C]methyl-cysteine-glutamate urea 6 [194], followed by radioiodinated tyrosine-glutamate urea 7 [195] (Fig. 5). Other radiolabeled cysteine-glutamate ureas include 4-[18F]fluorobenzyl-, and 4-[125I]iodobenzyl-cysteine-glutamate ureas, compounds 8 [196] and 9 [197]. The lysine glutamate urea 10 and its tri-esters are important platforms on which to add radiolabeled prosthetic groups. We have used it to prepare compounds 11-13 while Molecular Insight Pharmaceuticals, Inc. (Cambridge, MA) has used it to prepare 14 and 15 [198–201]. All of those radiolabeled PSMA inhibitors (6-15) showed high affinity (very low nanomolar), selective binding to PSMA-positive cells and specific uptake in PSMA-positive xenografts. All compounds exhibited urinary excretion and high uptake in the kidneys due to the presence of PSMA in mouse kidneys. Clinical SPECT imaging with 123I-labeled 14 and 15 detected metastatic [202] lesions while PET/CT imaging with [18F]8 (N-[N-[(S)-1,3-dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine, DCFBC) detected both bone and lymph node metastases [203]. In the latter study several lesions in areas considered suspicious for metastases but not seen on either CT or bone scan were clearly visualized. Additional clinical studies using these new agents and possibly others will be required in order to determine which agent performs best in detecting metastatic disease and determining its usefulness in imaging primary disease and for staging.
Fig. (5).
Urea-based GCPII imaging agents.
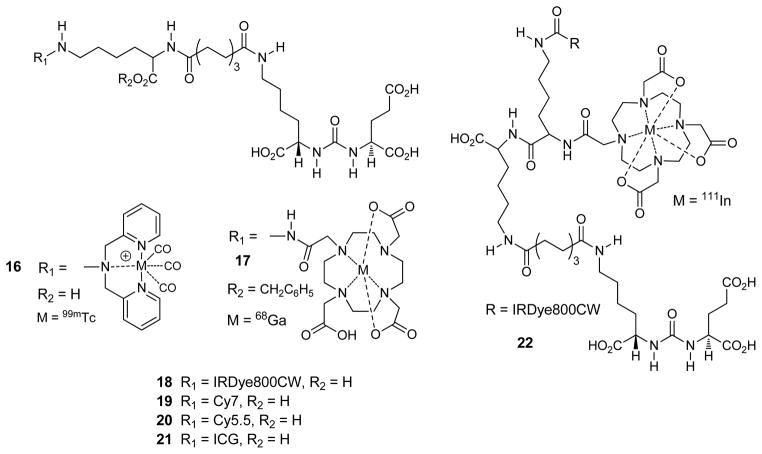
We have also used compound 10 and its esters to add a linking group to tether a bulky radiometal chelate to the urea, allowing the urea to reach the enzymatic site while keeping the metal chelate on the exterior. This has produced inhibitors containing [99mTc]tricarbonyl, and [68Ga]DOTA, compounds 16, and 17 respectively (Fig. 6) as well as a series of 99mTcVO+3 complexes” [204–206], all which showed high specific uptake in PSMA positive xenografts. The use of the positron emitter 68Ga is especially exciting because it is produced within a generator, which would eliminate the need for a local cyclotron. We have also used this tethered urea to form optical agents 18-21, which all demonstrated high specific uptake in PSMA positive xenografts [201,207]. Recently we reported the dual modality agent 22, in which a single injection of 1mCi (37 MBq) of [111In]22 permitted the visualization of tumor xenografts by both SPECT and near-IR optical imaging [208]. Finally, this tethered urea has been used to prepare PSMA-targeted antibody recruiting molecules [209] and may provide new platforms for radiolabeling and imaging of prostate cancer.
Fig. (6).
Optical and radiometallated GCPII imaging agents.
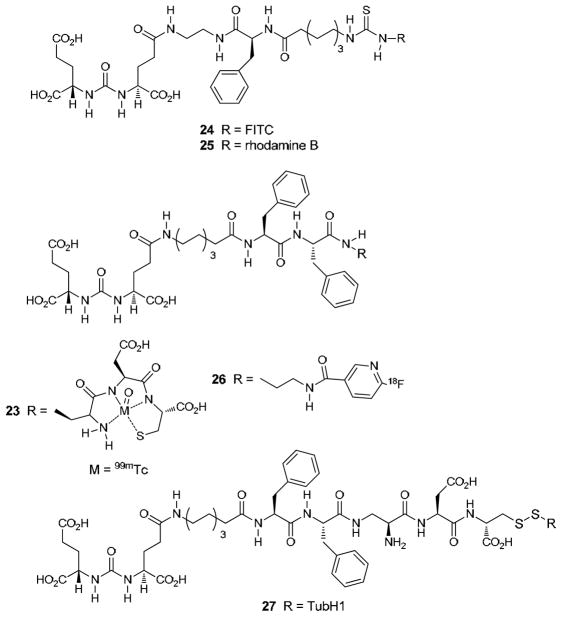
Bis-glutamate ureas are also important platforms on which to construct useful PSMA binding compounds. Low’s group prepared a series of 99mTc-labeled agents, one of which is 23 (Fig. 7), and optical agents 24 and 25 [210, 211]. All of those compounds showed specific uptake in PSMA-positive xenografts. An 18F-labeled version, 26, has also been prepared, but no imaging or biodistribution data has yet been reported [212].
Fig. (7).
Additional urea-based imaging agents for GCPII.
Like aptamers, conjugates of small molecule PSMA inhibitors are also being investigated for targeted delivery of therapeutics and radiotherapeutics. Tethered 10 has been used to deliver drug loaded nanoparticles to PSMA positive cells [213]. Kozikowski has reported a urea-doxorubicin conjugate that retained its high binding affinity but had poor anti-tumor activity most likely due to the fact that the agent lacked a mechanism to release the active drug [214]. To address the drug release problem, Low’s group has prepared a series of urea–pro-drug conjugates, such as 27, containing a cleavable disulfide bond. Several of their conjugates demonstrated high affinity binding to PSMA and low nanomolar cytotoxicity to PSMA positive cells [215]. MIP-1375, a 131I-labeled version of 14 inhibited the growth of PSMA positive xenografts [216], which suggests that targeted radiotherapy using small molecule PSMA inhibitors may be possible either with [131I] or linker-tethered radiometal complexes.
PERSPECTIVE
While PSMA itself may not be a critical therapeutic target for prostate cancer, as the biology and significance of PSMA in this disease is still being elucidated, PSMA represents a validated target for delivering important vehicles – such as imaging and therapeutic agents. It can also report on other, arguably more important therapeutic targets, such as AR signaling [25]. It has been used to enhance vaccines against prostate cancer [217]. PSMA is often used as a target for the proof-of-principle for nano-agents [218]. It has been the target for enzymatically activated prodrugs, with one product in clinical trials [219]. Its expression in tumor neovasculature has not been extensively explored in non-prostate tumor imaging and therapy. Accordingly, in addition to its well characterized presence as GCPII in the CNS, PSMA in the periphery will likely continue to be leveraged for imaging and treating prostate as well as a variety of PSMA-expressing cancers and other tissues.
Fig. (3).
FDG PET showing FDG uptake at sites of widely metastatic disease in a patient with advanced castrate-resistant prostate cancer. (A) liver metastases (white arrows); (B) lumbar and right pelvic bone metastases (white arrows). (Johns Hopkins PET center).
Acknowledgments
We thank CA92871 and CA134675 for financial support.
References
- 1.Halsted CH. The intestinal absorption of folates. The American journal of clinical nutrition. 1979;32(4):846–55. doi: 10.1093/ajcn/32.4.846. [DOI] [PubMed] [Google Scholar]
- 2.Sirotnak FM, Tolner B. Carrier-mediated membrane transport of folates in mammalian cells. Annual review of nutrition. 1999;19:91–122. doi: 10.1146/annurev.nutr.19.1.91. [DOI] [PubMed] [Google Scholar]
- 3.Fowler B. The folate cycle and disease in humans. Kidney International Supplement. 2001;78:S221–9. doi: 10.1046/j.1523-1755.2001.59780221.x. [DOI] [PubMed] [Google Scholar]
- 4.Moran RG. Roles of folylpoly-gamma-glutamate synthetase in therapeutics with tetrahydrofolate antimetabolites: an overview. Seminars in Oncology. 1999;26(2 Suppl 6):24–32. [PubMed] [Google Scholar]
- 5.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91(3):528–39. doi: 10.1002/jcb.10661. [DOI] [PubMed] [Google Scholar]
- 6.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3(1):81–5. [PubMed] [Google Scholar]
- 7.Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, Penetrante R. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology. 2007;50(4):472–83. doi: 10.1111/j.1365-2559.2007.02635.x. [DOI] [PubMed] [Google Scholar]
- 8.Rovenska M, Hlouchova K, Sacha P, Mlcochova P, Horak V, Zamecnik J, Barinka C, Konvalinka J. Tissue expression and enzymologic characterization of human prostate specific membrane antigen and its rat and pig orthologs. The Prostate. 2008;68(2):171–82. doi: 10.1002/pros.20676. [DOI] [PubMed] [Google Scholar]
- 9.Antunes AA, Leite KR, Sousa-Canavez JM, Camara-Lopes LH, Srougi M. The role of prostate specific membrane antigen and pepsinogen C tissue expression as an adjunctive method to prostate cancer diagnosis. The Journal of Urology. 2009;181(2):594–600. doi: 10.1016/j.juro.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Samplaski MK, Heston W, Elson P, Magi-Galluzzi C, Hansel DE. Folate hydrolase (prostate-specific antigen) 1 expression in bladder cancer subtypes and associated tumor neovasculature. Modern Pathology. 2011;24(11):1521–1529. doi: 10.1038/modpathol.2011.112. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Tavora F, Sharma R, Eisenberger M, Netto GJ. PSMA expression in Schwannoma: a potential clinical mimicker of metastatic prostate carcinoma. Urologic Oncology. 2009;27(5):525–8. doi: 10.1016/j.urolonc.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Chang SS, O’Keefe DS, Bacich DJ, Reuter VE, Heston WD, Gaudin PB. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res. 1999;5(10):2674–81. [PubMed] [Google Scholar]
- 13.Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59(13):3192–8. [PubMed] [Google Scholar]
- 14.Chang SS, Reuter VE, Heston WD, Gaudin PB. Metastatic renal cell carcinoma neovasculature expresses prostate-specific membrane antigen. Urology. 2001;57(4):801–5. doi: 10.1016/s0090-4295(00)01094-3. [DOI] [PubMed] [Google Scholar]
- 15.Haffner MC, Kronberger IE, Ross JS, Sheehan CE, Zitt M, Muhlmann G, Ofner D, Zelger B, Ensinger C, Yang XJ, Geley S, Margreiter R, Bander NH. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Human Pathology. 2009;40(12):1754–61. doi: 10.1016/j.humpath.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Ilyssa O, Gordon MST. Amy E Noffsinger, John Hart, Victor E Reuter and Hikmat A Al-Ahmadie, Prostate-specific membrane antigen expression in regeneration and repair. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21(12):1421–1427. doi: 10.1038/modpathol.2008.143. [DOI] [PubMed] [Google Scholar]
- 17.DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361(9361):955–64. doi: 10.1016/S0140-6736(03)12779-1. [DOI] [PubMed] [Google Scholar]
- 18.Su SL, Huang IP, Fair WR, Powell CT, Heston WD. Alternatively spliced variants of prostate-specific membrane antigen RNA: ratio of expression as a potential measurement of progression. Cancer Research. 1995;55(7):1441–3. [PubMed] [Google Scholar]
- 19.Heston WD. Characterization and glutamyl preferring carboxypeptidase function of prostate specific membrane antigen: a novel folate hydrolase. Urology. 1997;49(3A Suppl):104–12. doi: 10.1016/s0090-4295(97)00177-5. [DOI] [PubMed] [Google Scholar]
- 20.Grauer LS, Lawler KD, Marignac JL, Kumar A, Goel AS, Wolfert RL. Identification, purification, and subcellular localization of prostate-specific membrane antigen PSM′ protein in the LNCaP prostatic carcinoma cell line. Cancer research. 1998;58(21):4787–9. [PubMed] [Google Scholar]
- 21.Yao V, Parwani A, Maier C, Heston WD, Bacich DJ. Moderate expression of prostate-specific membrane antigen, a tissue differentiation antigen and folate hydrolase, facilitates prostate carcinogenesis. Cancer Res. 2008;68(21):9070–7. doi: 10.1158/0008-5472.CAN-08-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright GL, Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urologic Oncology. 1995;1(1):18–28. doi: 10.1016/1078-1439(95)00002-y. [DOI] [PubMed] [Google Scholar]
- 23.Denmeade SR, Sokoll LJ, Dalrymple S, Rosen DM, Gady AM, Bruzek D, Ricklis RM, Isaacs JT. Dissociation between androgen responsiveness for malignant growth vs. expression of prostate specific differentiation markers PSA, hK2, and PSMA in human prostate cancer models. The Prostate. 2003;54(4):249–57. doi: 10.1002/pros.10199. [DOI] [PubMed] [Google Scholar]
- 24.Birtle AJ, Freeman A, Masters JR, Payne HA, Harland SJ. Tumour markers for managing men who present with metastatic prostate cancer and serum prostate-specific antigen levels of <10 ng/mL. BJU International. 2005;96(3):303–7. doi: 10.1111/j.1464-410X.2005.05619.x. [DOI] [PubMed] [Google Scholar]
- 25.Evans MJ, Smith-Jones PM, Wongvipat J, Navarro V, Kim S, Bander NH, Larson SM, Sawyers CL. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci U S A. 2011;108(23):9578–82. doi: 10.1073/pnas.1106383108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathology Oncology Research : POR. 2009;15(2):167–72. doi: 10.1007/s12253-008-9104-2. [DOI] [PubMed] [Google Scholar]
- 27.Wright GL, Jr, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, Troyer J, Konchuba A, Schellhammer PF, Moriarty R. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48(2):326–34. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 28.Lai CL, van den Ham R, van Leenders G, van der Lugt J, Mol JA, Teske E. Histopathological and immunohistochemical characterization of canine prostate cancer. The Prostate. 2008;68(5):477–88. doi: 10.1002/pros.20720. [DOI] [PubMed] [Google Scholar]
- 29.Lai CL, van den Ham R, van Leenders G, van der Lugt J, Teske E. Comparative characterization of the canine normal prostate in intact and castrated animals. The Prostate. 2008;68(5):498–507. doi: 10.1002/pros.20721. [DOI] [PubMed] [Google Scholar]
- 30.Yao V, Bacich DJ. Prostate specific membrane antigen (PSMA) expression gives prostate cancer cells a growth advantage in a physiologically relevant folate environment in vitro. The Prostate. 2006;66(8):867–75. doi: 10.1002/pros.20361. [DOI] [PubMed] [Google Scholar]
- 31.Yao V, Berkman CE, Choi JK, O’Keefe DS, Bacich DJ. Expression of prostate-specific membrane antigen (PSMA), increases cell folate uptake and proliferation and suggests a novel role for PSMA in the uptake of the non-polyglutamated folate, folic acid. The Prostate. 2010;70(3):305–16. doi: 10.1002/pros.21065. [DOI] [PubMed] [Google Scholar]
- 32.Barwe SP, Maul RS, Christiansen JJ, Anilkumar G, Cooper CR, Kohn DB, Rajasekaran AK. Preferential association of prostate cancer cells expressing prostate specific membrane antigen to bone marrow matrix. Int J Oncol. 2007;30(4):899–904. [PubMed] [Google Scholar]
- 33.Ananias HJ, van den Heuvel MC, Helfrich W, de Jong IJ. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. The Prostate. 2009;69(10):1101–8. doi: 10.1002/pros.20957. [DOI] [PubMed] [Google Scholar]
- 34.Mahadevan D, Saldanha JW. The extracellular regions of PSMA and the transferrin receptor contain an aminopeptidase domain: implications for drug design. Protein science : a publication of the Protein Society. 1999;8(11):2546–9. doi: 10.1110/ps.8.11.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert LA, Mitchell SL. Molecular Evolution of the Transferrin Receptor/Glutamate Carboxypeptidase II Family. J Mol Evol. 2007;64(1):113–28. doi: 10.1007/s00239-006-0137-4. [DOI] [PubMed] [Google Scholar]
- 36.Schulke N, Varlamova OA, Donovan GP, Ma D, Gardner JP, Morrissey DM, Arrigale RR, Zhan C, Chodera AJ, Surowitz KG, Maddon PJ, Heston WD, Olson WC. The homodimer of prostate-specific membrane antigen is a functional target for cancer therapy. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(22):12590–5. doi: 10.1073/pnas.1735443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dautry-Varsat A. Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor. Biochimie. 1986;68(3):375–81. doi: 10.1016/s0300-9084(86)80004-9. [DOI] [PubMed] [Google Scholar]
- 38.Commandeur LC, Parsons JR. Degradation of halogenated aromatic compounds. Biodegradation. 1990;1(2–3):207–20. doi: 10.1007/BF00058837. [DOI] [PubMed] [Google Scholar]
- 39.Carrasquillo J. Imaging and dosimetry determinations using radiolabeled antibodies. Cancer Treatment and Research. 1993;68:65–97. doi: 10.1007/978-1-4615-3076-3_4. [DOI] [PubMed] [Google Scholar]
- 40.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nature reviews Molecular cell biology. 2009;10(9):597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barinka C, Sacha P, Sklenar J, Man P, Bezouska K, Slusher BS, Konvalinka J. Identification of the N-glycosylation sites on glutamate carboxypeptidase II necessary for proteolytic activity. Protein science : a publication of the Protein Society. 2004;13(6):1627–35. doi: 10.1110/ps.04622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosh A, Heston WD. Effect of carbohydrate moieties on the folate hydrolysis activity of the prostate specific membrane antigen. The Prostate. 2003;57(2):140–51. doi: 10.1002/pros.10289. [DOI] [PubMed] [Google Scholar]
- 43.Holmes EH, Greene TG, Tino WT, Boynton AL, Aldape HC, Misrock SL, Murphy GP. Analysis of glycosylation of prostate-specific membrane antigen derived from LNCaP cells, prostatic carcinoma tumors, and serum from prostate cancer patients. The Prostate Supplement. 1996;7:25–9. [PubMed] [Google Scholar]
- 44.Denmeade SR, Litvinov I, Sokoll LJ, Lilja H, Isaacs JT. Prostate-specific antigen (PSA) protein does not affect growth of prostate cancer cells in vitro or prostate cancer xenografts in vivo. Prostate. 2003;56(1):45–53. doi: 10.1002/pros.10213. [DOI] [PubMed] [Google Scholar]
- 45.Laidler P, Dulinska J, Lekka M, Lekki J. Expression of prostate specific membrane antigen in androgen-independent prostate cancer cell line PC-3. Archives of Biochemistry and Biophysics. 2005;435(1):1–14. doi: 10.1016/j.abb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Conway RE, Petrovic N, Li Z, Heston W, Wu D, Shapiro LH. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Molecular and Cellular Biology. 2006;26(14):5310–24. doi: 10.1128/MCB.00084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duda DG, Fukumura D, Jain RK. Role of eNOS in neovascularization: NO for endothelial progenitor cells. Trends in Molecular Medicine. 2004;10(4):143–5. doi: 10.1016/j.molmed.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Bivalacqua TJ, Musicki B, Usta MF, Champion HC, Kadowitz PJ, Burnett AL, Hellstrom WJ. Endothelial nitric oxide synthase gene therapy for erectile dysfunction. Current Pharmaceutical Design. 2005;11(31):4059–67. doi: 10.2174/138161205774913345. [DOI] [PubMed] [Google Scholar]
- 49.Antony AC. Folate receptors. Annual Review of Nutrition. 1996;16:501–21. doi: 10.1146/annurev.nu.16.070196.002441. [DOI] [PubMed] [Google Scholar]
- 50.Matherly LH, Goldman DI. Membrane transport of folates. Vitamins and Hormones. 2003;66:403–56. doi: 10.1016/s0083-6729(03)01012-4. [DOI] [PubMed] [Google Scholar]
- 51.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Advanced Drug Delivery Reviews. 2004;56(8):1067–84. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Bailey LB. Folate status assessment. The Journal of Nutrition. 1990;120(Suppl 11):1508–11. doi: 10.1093/jn/120.suppl_11.1508. [DOI] [PubMed] [Google Scholar]
- 53.Rader JI. Folic acid fortification, folate status and plasma homocysteine. The Journal of Nutrition. 2002;132(8 Suppl):2466S–2470S. doi: 10.1093/jn/132.8.2466S. [DOI] [PubMed] [Google Scholar]
- 54.Marin YE, Chen S. Involvement of metabotropic glutamate receptor 1, a G protein coupled receptor, in melanoma development. Journal of Molecular Medicine. 2004;82(11):735–49. doi: 10.1007/s00109-004-0566-8. [DOI] [PubMed] [Google Scholar]
- 55.Haas HS, Linecker A, Pfragner R, Sadjak A. Peripheral glutamate signaling in head and neck areas. Head & Neck. 2010;32(11):1554–72. doi: 10.1002/hed.21438. [DOI] [PubMed] [Google Scholar]
- 56.Kalariti N, Lembessis P, Koutsilieris M. Characterization of the glutametergic system in MG-63 osteoblast-like osteosarcoma cells. Anticancer Research. 2004;24(6):3923–9. [PubMed] [Google Scholar]
- 57.Chang HJ, Yoo BC, Lim SB, Jeong SY, Kim WH, Park JG. Metabotropic glutamate receptor 4 expression in colorectal carcinoma and its prognostic significance. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2005;11(9):3288–95. doi: 10.1158/1078-0432.CCR-04-1912. [DOI] [PubMed] [Google Scholar]
- 58.Park SY, Lee SA, Han IH, Yoo BC, Lee SH, Park JY, Cha IH, Kim J, Choi SW. Clinical significance of metabotropic glutamate receptor 5 expression in oral squamous cell carcinoma. Oncology Reports. 2007;17(1):81–7. [PubMed] [Google Scholar]
- 59.Pissimissis N, Papageorgiou E, Lembessis P, Armakolas A, Koutsilieris M. The glutamatergic system expression in human PC-3 and LNCaP prostate cancer cells. Anticancer Res. 2009;29(1):371–7. [PubMed] [Google Scholar]
- 60.Hinoi E, Takarada T, Ueshima T, Tsuchihashi Y, Yoneda Y. Glutamate signaling in peripheral tissues. European Journal of Biochemistry/FEBS. 2004;271(1):1–13. doi: 10.1046/j.1432-1033.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 61.Wu KK. Regulation of endothelial nitric oxide synthase activity and gene expression. Annals of the New York Academy of Sciences. 2002;962:122–30. doi: 10.1111/j.1749-6632.2002.tb04062.x. [DOI] [PubMed] [Google Scholar]
- 62.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2003;284(1):R1–12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 63.Abdul M, Hoosein N. N-methyl-D-aspartate receptor in human prostate cancer. J Membr Biol. 2005;205(3):125–8. doi: 10.1007/s00232-005-0777-0. [DOI] [PubMed] [Google Scholar]
- 64.Legros H, Launay S, Roussel BD, Marcou-Labarre A, Calbo S, Catteau J, Leroux P, Boyer O, Ali C, Marret S, Vivien D, Laudenbach V. Newborn- and adult-derived brain microvascular endothelial cells show age-related differences in phenotype and glutamate-evoked protease release. Journal of Cerebral Blood Flow and Metabolism. 2009;29(6):1146–58. doi: 10.1038/jcbfm.2009.39. [DOI] [PubMed] [Google Scholar]
- 65.Neuhaus W, Freidl M, Szkokan P, Berger M, Wirth M, Winkler J, Gabor F, Pifl C, Noe CR. Effects of NMDA receptor modulators on a blood-brain barrier in vitro model. Brain Research. 2011;1394:49–61. doi: 10.1016/j.brainres.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Filosa JA, Nelson MT, Gonzalez Bosc LV. Activity-dependent NFATc3 nuclear accumulation in pericytes from cortical parenchymal microvessels. American Journal of Physiology Cell Physiology. 2007;293(6):C1797–805. doi: 10.1152/ajpcell.00554.2006. [DOI] [PubMed] [Google Scholar]
- 67.Collard CD, Park KA, Montalto MC, Alapati S, Buras JA, Stahl GL, Colgan SP. Neutrophil-derived glutamate regulates vascular endothelial barrier function. The Journal of Biological Chemistry. 2002;277(17):14801–11. doi: 10.1074/jbc.M110557200. [DOI] [PubMed] [Google Scholar]
- 68.Krizbai IA, Deli MA, Pestenacz A, Siklos L, Szabo CA, Andras I, Joo F. Expression of glutamate receptors on cultured cerebral endothelial cells. Journal of Neuroscience Research. 1998;54(6):814–9. doi: 10.1002/(SICI)1097-4547(19981215)54:6<814::AID-JNR9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 69.Ross JS, Sheehan CE, Fisher HA, Kaufman RP, Jr, Kaur P, Gray K, Webb I, Gray GS, Mosher R, Kallakury BV. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9(17):6357–62. [PubMed] [Google Scholar]
- 70.Ashina M, Jorgensen M, Stallknecht B, Mork H, Bendtsen L, Pedersen JF, Olesen J, Jensen R. No release of interstitial glutamate in experimental human model of muscle pain. European Journal of Pain. 2005;9(3):337–43. doi: 10.1016/j.ejpain.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nature Reviews Neuroscience. 2002;3(9):748–55. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- 72.Gill SS, Mueller RW, McGuire PF, Pulido OM. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicologic Pathology. 2000;28(2):277–84. doi: 10.1177/019262330002800207. [DOI] [PubMed] [Google Scholar]
- 73.Dale LB, Babwah AV, Ferguson SS. Mechanisms of metabotropic glutamate receptor desensitization: role in the patterning of effector enzyme activation. Neurochemistry International. 2002;41(5):319–26. doi: 10.1016/s0197-0186(02)00073-6. [DOI] [PubMed] [Google Scholar]
- 74.Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. The Journal of Neuroscience. 2009;29(11):3590–602. doi: 10.1523/JNEUROSCI.5824-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sitcheran R, Comb WC, Cogswell PC, Baldwin AS. Essential role for epidermal growth factor receptor in glutamate receptor signaling to NF-kappaB. Molecular and Cellular Biology. 2008;28(16):5061–70. doi: 10.1128/MCB.00578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sobrio F, Gilbert G, Perrio C, Barre L, Debruyne D. PET and SPECT imaging of the NMDA receptor system: an overview of radiotracer development. Mini Reviews in Medicinal Chemistry. 2010;10(9):870–86. doi: 10.2174/138955710791608299. [DOI] [PubMed] [Google Scholar]
- 77.Wang JQ, Tueckmantel W, Zhu A, Pellegrino D, Brownell AL. Synthesis and preliminary biological evaluation of 3-[(18)F]fluoro-5-(2-pyridinylethynyl)benzonitrile as a PET radiotracer for imaging metabotropic glutamate receptor subtype 5. Synapse. 2007;61(12):951–61. doi: 10.1002/syn.20445. [DOI] [PubMed] [Google Scholar]
- 78.Treyer V, Streffer J, Wyss MT, Bettio A, Ametamey SM, Fischer U, Schmidt M, Gasparini F, Hock C, Buck A. Evaluation of the metabotropic glutamate receptor subtype 5 using PET and 11C-ABP688: assessment of methods. Journal of Nuclear Medicine. 2007;48(7):1207–15. doi: 10.2967/jnumed.107.039578. [DOI] [PubMed] [Google Scholar]
- 79.Wyss MT, Ametamey SM, Treyer V, Bettio A, Blagoev M, Kessler LJ, Burger C, Weber B, Schmidt M, Gasparini F, Buck A. Quantitative evaluation of 11C-ABP688 as PET ligand for the measurement of the metabotropic glutamate receptor subtype 5 using autoradiographic studies and a beta-scintillator. NeuroImage. 2007;35(3):1086–92. doi: 10.1016/j.neuroimage.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 80.Ametamey SM, Kessler LJ, Honer M, Wyss MT, Buck A, Hintermann S, Auberson YP, Gasparini F, Schubiger PA. Radiosynthesis and preclinical evaluation of 11C-ABP688 as a probe for imaging the metabotropic glutamate receptor subtype 5. Journal of Nuclear Medicine. 2006;47(4):698–705. [PubMed] [Google Scholar]
- 81.Patel S, Ndubizu O, Hamill T, Chaudhary A, Burns HD, Hargreaves R, Gibson RE. Screening Cascade and Development of Potential Positron Emission Tomography Radiotracers for mGluR5: In vitro and In vivo Characterization. Mol Imaging Biol. 2005:1–10. doi: 10.1007/s11307-005-0005-4. [DOI] [PubMed] [Google Scholar]
- 82.Hamill TG, Krause S, Ryan C, Bonnefous C, Govek S, Seiders TJ, Cosford ND, Roppe J, Kamenecka T, Patel S, Gibson RE, Sanabria S, Riffel K, Eng W, King C, Yang X, Green MD, O’Malley SS, Hargreaves R, Burns HD. Synthesis, characterization, and first successful monkey imaging studies of metabotropic glutamate receptor subtype 5 (mGluR5) PET radiotracers. Synapse. 2005;56(4):205–16. doi: 10.1002/syn.20147. [DOI] [PubMed] [Google Scholar]
- 83.Hostetler ED, Eng W, Joshi AD, Sanabria-Bohorquez S, Kawamoto H, Ito S, O’Malley S, Krause S, Ryan C, Patel S, Williams M, Riffel K, Suzuki G, Ozaki S, Ohta H, Cook J, Burns HD, Hargreaves R. Synthesis, characterization, and monkey PET studies of [(1)F]MK-1312, a PET tracer for quantification of mGluR1 receptor occupancy by MK-5435. Synapse. 2011;65(2):125–35. doi: 10.1002/syn.20826. [DOI] [PubMed] [Google Scholar]
- 84.Satoh A, Nagatomi Y, Hirata Y, Ito S, Suzuki G, Kimura T, Maehara S, Hikichi H, Satow A, Hata M, Ohta H, Kawamoto H. Discovery and in vitro and in vivo profiles of 4-fluoro-N-[4-[6-(isopropylamino)pyrimidin-4-yl]-1, 3-thiazol-2-yl]-N-methy lbenzamide as novel class of an orally active metabotropic glutamate receptor 1 (mGluR1) antagonist. Bioorganic & Medicinal Chemistry Letters. 2009;19(18):5464–8. doi: 10.1016/j.bmcl.2009.07.097. [DOI] [PubMed] [Google Scholar]
- 85.Huang Y, Narendran R, Bischoff F, Guo N, Zhu Z, Bae SA, Lesage AS, Laruelle M. A positron emission tomography radioligand for the in vivo labeling of metabotropic glutamate 1 receptor: (3-ethyl-2-[11C]methyl-6-quinolinyl)(cis-4-methoxycyclohexyl)methanone. Journal of Medicinal Chemistry. 2005;48(16):5096–9. doi: 10.1021/jm050263+. [DOI] [PubMed] [Google Scholar]
- 86.Glunde K, Pathak AP, Bhujwalla ZM. Molecular-functional imaging of cancer: to image and imagine. Trends Mol Med. 2007;13(7):287–97. doi: 10.1016/j.molmed.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 87.Higgins LJ, Pomper MG. The evolution of imaging in cancer: current state and future challenges. Semin Oncol. 2011;38(1):3–15. doi: 10.1053/j.seminoncol.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452(7187):580–9. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weber WA, Grosu AL, Czernin J. Technology Insight: advances in molecular imaging and an appraisal of PET/CT scanning. Nat Clin Pract Oncol. 2008;5(3):160–70. doi: 10.1038/ncponc1041. [DOI] [PubMed] [Google Scholar]
- 90.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 91.Worldwide Prostate Cancer Statistics. http://globocan.iarc.fr/factsheets/cancers/prostate.asp.
- 92.Hofer C, Laubenbacher C, Block T, Breul J, Hartung R, Schwaiger M. Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur Urol. 1999;36(1):31–5. doi: 10.1159/000019923. [DOI] [PubMed] [Google Scholar]
- 93.Yeh SD, Imbriaco M, Larson SM, Garza D, Zhang JJ, Kalaigian H, Finn RD, Reddy D, Horowitz SM, Goldsmith SJ, Scher HI. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol. 1996;23(6):693–7. doi: 10.1016/0969-8051(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 94.Morris MJ, Akhurst T, Osman I, Nunez R, Macapinlac H, Siedlecki K, Verbel D, Schwartz L, Larson SM, Scher HI. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology. 2002;59(6):913–8. doi: 10.1016/s0090-4295(02)01509-1. [DOI] [PubMed] [Google Scholar]
- 95.Schoder H, Herrmann K, Gonen M, Hricak H, Eberhard S, Scardino P, Scher HI, Larson SM. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res. 2005;11(13):4761–9. doi: 10.1158/1078-0432.CCR-05-0249. [DOI] [PubMed] [Google Scholar]
- 96.Morris MJ, Akhurst T, Larson SM, Ditullio M, Chu E, Siedlecki K, Verbel D, Heller G, Kelly WK, Slovin S, Schwartz L, Scher HI. Fluorodeoxyglucose positron emission tomography as an outcome measure for castrate metastatic prostate cancer treated with antimicrotubule chemotherapy. Clin Cancer Res. 2005;11(9):3210–6. doi: 10.1158/1078-0432.CCR-04-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49(1):68–78. doi: 10.2967/jnumed.106.037200. [DOI] [PubMed] [Google Scholar]
- 98.Even-Sapir E, Mishani E, Flusser G, Metser U. 18F-Fluoride positron emission tomography and positron emission tomography/computed tomography. Semin Nucl Med. 2007;37(6):462–9. doi: 10.1053/j.semnuclmed.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 99.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47(2):287–97. [PubMed] [Google Scholar]
- 100.CMS National Coverage Analysis (NCA) for Positron Emission Tomography (NaF-18) to Identify Bone Metastasis of Cancer. http://www.cms.gov/medicare-coverage-database/details/nca-details.aspx?NCAId=233&NcaName=Positron+Emission+Tomography+(NaF-18)+to+Identify+Bone+Metastasis+of+Cancer&NCDId=331&ncdver=3&IsPopup=y&.
- 101.Hara T, Bansal A, DeGrado TR. Choline transporter as a novel target for molecular imaging of cancer. Mol Imaging. 2006;5(4):498–509. [PubMed] [Google Scholar]
- 102.Ramirez de Molina A, Rodriguez-Gonzalez A, Gutierrez R, Martinez-Pineiro L, Sanchez J, Bonilla F, Rosell R, Lacal J. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun. 2002;296(3):580–3. doi: 10.1016/s0006-291x(02)00920-8. [DOI] [PubMed] [Google Scholar]
- 103.Roivainen A, Forsback S, Gronroos T, Lehikoinen P, Kahkonen M, Sutinen E, Minn H. Blood metabolism of [methyl-11C]choline; implications for in vivo imaging with positron emission tomography. Eur J Nucl Med. 2000;27(1):25–32. doi: 10.1007/pl00006658. [DOI] [PubMed] [Google Scholar]
- 104.Beer AJ, Eiber M, Souvatzoglou M, Schwaiger M, Krause BJ. Radionuclide and hybrid imaging of recurrent prostate cancer. Lancet Oncol. 2011;12(2):181–91. doi: 10.1016/S1470-2045(10)70103-0. [DOI] [PubMed] [Google Scholar]
- 105.Giovacchini G, Picchio M, Coradeschi E, Scattoni V, Bettinardi V, Cozzarini C, Freschi M, Fazio F, Messa C. [(11)C]choline uptake with PET/CT for the initial diagnosis of prostate cancer: relation to PSA levels, tumour stage and anti-androgenic therapy. Eur J Nucl Med Mol Imaging. 2008;35(6):1065–73. doi: 10.1007/s00259-008-0716-2. [DOI] [PubMed] [Google Scholar]
- 106.Sutinen E, Nurmi M, Roivainen A, Varpula M, Tolvanen T, Lehikoinen P, Minn H. Kinetics of [(11)C]choline uptake in prostate cancer: a PET study. Eur J Nucl Med Mol Imaging. 2004;31(3):317–24. doi: 10.1007/s00259-003-1377-9. [DOI] [PubMed] [Google Scholar]
- 107.Souvatzoglou M, Weirich G, Schwarzenboeck S, Maurer T, Schuster T, Bundschuh RA, Eiber M, Herrmann K, Kuebler H, Wester HJ, Hoefler H, Gschwend J, Schwaiger M, Treiber U, Krause BJ. The sensitivity of [11C]choline PET/CT to localize prostate cancer depends on the tumor configuration. Clin Cancer Res. 2011;17(11):3751–9. doi: 10.1158/1078-0432.CCR-10-2093. [DOI] [PubMed] [Google Scholar]
- 108.Piert M, Park H, Khan A, Siddiqui J, Hussain H, Chenevert T, Wood D, Johnson T, Shah RB, Meyer C. Detection of aggressive primary prostate cancer with 11C-choline PET/CT using multimodality fusion techniques. J Nucl Med. 2009;50(10):1585–93. doi: 10.2967/jnumed.109.063396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scher B, Seitz M, Albinger W, Tiling R, Scherr M, Becker HC, Souvatzogluou M, Gildehaus FJ, Wester HJ, Dresel S. Value of 11C-choline PET and PET/CT in patients with suspected prostate cancer. Eur J Nucl Med Mol Imaging. 2007;34(1):45–53. doi: 10.1007/s00259-006-0190-7. [DOI] [PubMed] [Google Scholar]
- 110.Testa C, Schiavina R, Lodi R, Salizzoni E, Corti B, Farsad M, Kurhanewicz J, Manferrari F, Brunocilla E, Tonon C, Monetti N, Castellucci P, Fanti S, Coe M, Grigioni WF, Martorana G, Canini R, Barbiroli B. Prostate cancer: sextant localization with MR imaging, MR spectroscopy, and 11C-choline PET/CT. Radiology. 2007;244(3):797–806. doi: 10.1148/radiol.2443061063. [DOI] [PubMed] [Google Scholar]
- 111.Kwee SA, Thibault GP, Stack RS, Coel MN, Furusato B, Sesterhenn IA. Use of step-section histopathology to evaluate 18F-fluorocholine PET sextant localization of prostate cancer. Mol Imaging. 2008;7(1):12–20. [PubMed] [Google Scholar]
- 112.Kwee SA, Wei H, Sesterhenn I, Yun D, Coel MN. Localization of primary prostate cancer with dual-phase 18F-fluorocholine PET. J Nucl Med. 2006;47(2):262–9. [PubMed] [Google Scholar]
- 113.Igerc I, Kohlfurst S, Gallowitsch HJ, Matschnig S, Kresnik E, Gomez-Segovia I, Lind P. The value of 18F-choline PET/CT in patients with elevated PSA-level and negative prostate needle biopsy for localisation of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35(5):976–83. doi: 10.1007/s00259-007-0686-9. [DOI] [PubMed] [Google Scholar]
- 114.de Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJ. 11C-choline positron emission tomography for the evaluation after treatment of localized prostate cancer. Eur Urol. 2003;44(1):32–8. doi: 10.1016/s0302-2838(03)00207-0. discussion 38–9. [DOI] [PubMed] [Google Scholar]
- 115.Scattoni V, Picchio M, Suardi N, Messa C, Freschi M, Roscigno M, Da Pozzo L, Bocciardi A, Rigatti P, Fazio F. Detection of lymph-node metastases with integrated [11C]choline PET/CT in patients with PSA failure after radical retropubic prostatectomy: results confirmed by open pelvic-retroperitoneal lymphadenectomy. Eur Urol. 2007;52(2):423–9. doi: 10.1016/j.eururo.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 116.Krause BJ, Souvatzoglou M, Tuncel M, Herrmann K, Buck AK, Praus C, Schuster T, Geinitz H, Treiber U, Schwaiger M. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35(1):18–23. doi: 10.1007/s00259-007-0581-4. [DOI] [PubMed] [Google Scholar]
- 117.Tuncel M, Souvatzoglou M, Herrmann K, Stollfuss J, Schuster T, Weirich G, Wester HJ, Schwaiger M, Krause BJ. [(11)C]Choline positron emission tomography/computed tomography for staging and restaging of patients with advanced prostate cancer. Nucl Med Biol. 2008;35(6):689–95. doi: 10.1016/j.nucmedbio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 118.Cimitan M, Bortolus R, Morassut S, Canzonieri V, Garbeglio A, Baresic T, Borsatti E, Drigo A, Trovo MG. [18F]fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur J Nucl Med Mol Imaging. 2006;33(12):1387–98. doi: 10.1007/s00259-006-0150-2. [DOI] [PubMed] [Google Scholar]
- 119.Bauman G, Belhocine T, Kovacs M, Ward A, Beheshti M, Rachinsky I. (18)F-fluorocholine for prostate cancer imaging: a systematic review of the literature. Prostate Cancer Prostatic Dis. 2011 doi: 10.1038/pcan.2011.35. [DOI] [PubMed] [Google Scholar]
- 120.Vavere AL, Kridel SJ, Wheeler FB, Lewis JS. 1–11C-acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. J Nucl Med. 2008;49(2):327–34. doi: 10.2967/jnumed.107.046672. [DOI] [PubMed] [Google Scholar]
- 121.Nanni C, Castellucci P, Farsad M, Rubello D, Fanti S. 11C/18F-choline PET or 11C/18F-acetate PET in prostate cancer: may a choice be recommended? Eur J Nucl Med Mol Imaging. 2007;34(10):1704–5. doi: 10.1007/s00259-007-0491-5. [DOI] [PubMed] [Google Scholar]
- 122.Albrecht S, Buchegger F, Soloviev D, Zaidi H, Vees H, Khan HG, Keller A, Bischof Delaloye A, Ratib O, Miralbell R. (11)C-acetate PET in the early evaluation of prostate cancer recurrence. Eur J Nucl Med Mol Imaging. 2007;34(2):185–96. doi: 10.1007/s00259-006-0163-x. [DOI] [PubMed] [Google Scholar]
- 123.Sandblom G, Sorensen J, Lundin N, Haggman M, Malmstrom PU. Positron emission tomography with C11-acetate for tumor detection and localization in patients with prostate-specific antigen relapse after radical prostatectomy. Urology. 2006;67(5):996–1000. doi: 10.1016/j.urology.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 124.Wachter S, Tomek S, Kurtaran A, Wachter-Gerstner N, Djavan B, Becherer A, Mitterhauser M, Dobrozemsky G, Li S, Potter R, Dudczak R, Kletter K. 11C-acetate positron emission tomography imaging and image fusion with computed tomography and magnetic resonance imaging in patients with recurrent prostate cancer. J Clin Oncol. 2006;24(16):2513–9. doi: 10.1200/JCO.2005.03.5279. [DOI] [PubMed] [Google Scholar]
- 125.Morris MJ, Scher HI. (11)C-acetate PET imaging in prostate cancer. Eur J Nucl Med Mol Imaging. 2007;34(2):181–4. doi: 10.1007/s00259-006-0281-5. [DOI] [PubMed] [Google Scholar]
- 126.Matthies A, Ezziddin S, Ulrich EM, Palmedo H, Biersack HJ, Bender H, Guhlke S. Imaging of prostate cancer metastases with 18F-fluoroacetate using PET/CT. Eur J Nucl Med Mol Imaging. 2004;31(5):797. doi: 10.1007/s00259-003-1437-1. [DOI] [PubMed] [Google Scholar]
- 127.Schuster DM, Votaw JR, Nieh PT, Yu W, Nye JA, Master V, Bowman FD, Issa MM, Goodman MM. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48(1):56–63. [PubMed] [Google Scholar]
- 128.Schuster DM, Savir-Baruch B, Nieh PT, Master VA, Halkar RK, Rossi PJ, Lewis MM, Nye JA, Yu W, Bowman FD, Goodman MM. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology. 2011;259(3):852–61. doi: 10.1148/radiol.11102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Okudaira H, Shikano N, Nishii R, Miyagi T, Yoshimoto M, Kobayashi M, Ohe K, Nakanishi T, Tamai I, Namiki M, Kawai K. Putative transport mechanism and intracellular fate of trans-1-amino-3-18F-fluorocyclobutanecarboxylic acid in human prostate cancer. J Nucl Med. 2011;52(5):822–9. doi: 10.2967/jnumed.110.086074. [DOI] [PubMed] [Google Scholar]
- 130.Dehdashti F, Picus J, Michalski JM, Dence CS, Siegel BA, Katzenellenbogen JA, Welch MJ. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur J Nucl Med Mol Imaging. 2005;32(3):344–50. doi: 10.1007/s00259-005-1764-5. [DOI] [PubMed] [Google Scholar]
- 131.Larson SM, Morris M, Gunther I, Beattie B, Humm JL, Akhurst TA, Finn RD, Erdi Y, Pentlow K, Dyke J, Squire O, Bornmann W, McCarthy T, Welch M, Scher H. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45(3):366–73. [PubMed] [Google Scholar]
- 132.Beattie BJ, Smith-Jones PM, Jhanwar YS, Schoder H, Schmidtlein CR, Morris MJ, Zanzonico P, Squire O, Meirelles GS, Finn R, Namavari M, Cai S, Scher HI, Larson SM, Humm JL. Pharmacokinetic assessment of the uptake of 16beta-18F-fluoro-5alpha-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. J Nucl Med. 2010;51(2):183–92. doi: 10.2967/jnumed.109.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Toth G, Lengyel Z, Balkay L, Salah MA, Tron L, Toth C. Detection of prostate cancer with 11C-methionine positron emission tomography. J Urol. 2005;173(1):66–9. doi: 10.1097/01.ju.0000148326.71981.44. discussion 69. [DOI] [PubMed] [Google Scholar]
- 134.Sun H, Sloan A, Mangner TJ, Vaishampayan U, Muzik O, Collins JM, Douglas K, Shields AF. Imaging DNA synthesis with [18F]FMAU and positron emission tomography in patients with cancer. Eur J Nucl Med Mol Imaging. 2005;32(1):15–22. doi: 10.1007/s00259-004-1713-8. [DOI] [PubMed] [Google Scholar]
- 135.Oyama N, Ponde DE, Dence C, Kim J, Tai YC, Welch MJ. Monitoring of therapy in androgen-dependent prostate tumor model by measuring tumor proliferation. J Nucl Med. 2004;45(3):519–25. [PubMed] [Google Scholar]
- 136.Wu AM, Olafsen T. Antibodies for molecular imaging of cancer. Cancer J. 2008;14(3):191–7. doi: 10.1097/PPO.0b013e31817b07ae. [DOI] [PubMed] [Google Scholar]
- 137.Goldenberg DM, Sharkey RM. Advances in cancer therapy with radiolabeled monoclonal antibodies. Q J Nucl Med Mol Imaging. 2006;50(4):248–64. [PubMed] [Google Scholar]
- 138.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82(11):2256–61. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 139.Akhtar NH, Pail O, Saran A, Tyrell L, Tagawa ST. Prostate-specific membrane antigen-based therapeutics. Adv Urol. 2012;2012:973820. doi: 10.1155/2012/973820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ellis RJ, Kaminsky DA, Zhou EH, Fu P, Chen WD, Brelin A, Faulhaber PF, Bodner D. Ten-year outcomes: the clinical utility of single photon emission computed tomography/computed tomography capromab pendetide (Prostascint) in a cohort diagnosed with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):29–34. doi: 10.1016/j.ijrobp.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 141.Zuckier LS, DeNardo GL. Trials and tribulations: oncological antibody imaging comes to the fore. Semin Nucl Med. 1997;27(1):10–29. doi: 10.1016/s0001-2998(97)80033-5. [DOI] [PubMed] [Google Scholar]
- 142.Mease RC. Radionuclide based imaging of prostate cancer. Curr Top Med Chem. 2010;10(16):1600–16. doi: 10.2174/156802610793176774. [DOI] [PubMed] [Google Scholar]
- 143.Troyer JK, Beckett ML, Wright GL., Jr Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate. 1997;30(4):232–42. doi: 10.1002/(sici)1097-0045(19970301)30:4<232::aid-pros2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 144.Bander NH, Nanus DM, Milowsky MI, Kostakoglu L, Vallabahajosula S, Goldsmith SJ. Targeted systemic therapy of prostate cancer with a monoclonal antibody to prostate-specific membrane antigen. Semin Oncol. 2003;30(5):667–76. doi: 10.1016/s0093-7754(03)00358-0. [DOI] [PubMed] [Google Scholar]
- 145.Haseman MK, Rosenthal SA, Polascik TJ. Capromab Pendetide imaging of prostate cancer. Cancer Biother Radiopharm. 2000;15(2):131–40. doi: 10.1089/cbr.2000.15.131. [DOI] [PubMed] [Google Scholar]
- 146.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–63. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 147.Sodee DB, Sodee AE, Bakale G. Synergistic value of single-photon emission computed tomography/computed tomography fusion to radioimmunoscintigraphic imaging of prostate cancer. Semin Nucl Med. 2007;37(1):17–28. doi: 10.1053/j.semnuclmed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 148.Manyak MJ. Indium-111 capromab pendetide in the management of recurrent prostate cancer. Expert Rev Anticancer Ther. 2008;8(2):175–81. doi: 10.1586/14737140.8.2.175. [DOI] [PubMed] [Google Scholar]
- 149.Vallabhajosula S, Kuji I, Hamacher KA, Konishi S, Kostakoglu L, Kothari PA, Milowski MI, Nanus DM, Bander NH, Goldsmith SJ. Pharmacokinetics and biodistribution of 111In- and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu? J Nucl Med. 2005;46(4):634–41. [PubMed] [Google Scholar]
- 150.Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, Navarro V, Hunter CJ, Bastidas D, Bander NH. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res. 2000;60(18):5237–43. [PubMed] [Google Scholar]
- 151.Bander NH, Trabulsi EJ, Kostakoglu L, Yao D, Vallabhajosula S, Smith-Jones P, Joyce MA, Milowsky M, Nanus DM, Goldsmith SJ. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urol. 2003;170(5):1717–21. doi: 10.1097/01.ju.0000091655.77601.0c. [DOI] [PubMed] [Google Scholar]
- 152.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23(21):4591–601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 153.Nakajima T, Mitsunaga M, Bander NH, Heston WD, Choyke PL, Kobayashi H. Targeted, activatable, in vivo fluorescence imaging of prostate-specific membrane antigen (PSMA) positive tumors using the quenched humanized J591 antibody-indocyanine green (ICG) conjugate. Bioconjug Chem. 2011;22(8):1700–5. doi: 10.1021/bc2002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alt K, Wiehr S, Ehrlichmann W, Reischl G, Wolf P, Pichler BJ, Elsasser-Beile U, Buhler P. High-resolution animal PET imaging of prostate cancer xenografts with three different 64Cu-labeled antibodies against native cell-adherent PSMA. Prostate. 2010;70(13):1413–21. doi: 10.1002/pros.21176. [DOI] [PubMed] [Google Scholar]
- 155.Wolf P, Freudenberg N, Buhler P, Alt K, Schultze-Seemann W, Wetterauer U, Elsasser-Beile U. Three conformational antibodies specific for different PSMA epitopes are promising diagnostic and therapeutic tools for prostate cancer. Prostate. 2010;70(5):562–9. doi: 10.1002/pros.21090. [DOI] [PubMed] [Google Scholar]
- 156.Elsasser-Beile U, Reischl G, Wiehr S, Buhler P, Wolf P, Alt K, Shively J, Judenhofer MS, Machulla HJ, Pichler BJ. PET imaging of prostate cancer xenografts with a highly specific antibody against the prostate-specific membrane antigen. J Nucl Med. 2009;50(4):606–11. doi: 10.2967/jnumed.108.058487. [DOI] [PubMed] [Google Scholar]
- 157.Cerchia L, Giangrande PH, McNamara JO, de Franciscis V. Cell-specific aptamers for targeted therapies. Methods Mol Biol. 2009;535:59–78. doi: 10.1007/978-1-59745-557-2_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hicke BJ, Stephens AW, Gould T, Chang YF, Lynott CK, Heil J, Borkowski S, Hilger CS, Cook G, Warren S, Schmidt PG. Tumor targeting by an aptamer. J Nucl Med. 2006;47(4):668–78. [PubMed] [Google Scholar]
- 159.Vorhies JS, Nemunaitis JJ. Nucleic acid aptamers for targeting of shRNA-based cancer therapeutics. Biologics. 2007;1(4):367–76. [PMC free article] [PubMed] [Google Scholar]
- 160.Lupold SE, Hicke BJ, Lin Y, Coffey DS. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 2002;62(14):4029–33. [PubMed] [Google Scholar]
- 161.Bagalkot V, Farokhzad OC, Langer R, Jon S. An aptamer-doxorubicin physical conjugate as a novel targeted drug-delivery platform. Angew Chem Int Ed Engl. 2006;45(48):8149–52. doi: 10.1002/anie.200602251. [DOI] [PubMed] [Google Scholar]
- 162.Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR, Meyerholz DK, McCaffrey AP, McNamara JO, 2nd, Giangrande PH. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat Biotechnol. 2009;27(9):839–49. doi: 10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ni X, Zhang Y, Ribas J, Chowdhury WH, Castanares M, Zhang Z, Laiho M, DeWeese TL, Lupold SE. Prostate-targeted radiosensitization via aptamer-shRNA chimeras in human tumor xenografts. J Clin Invest. 2011;121(6):2383–90. doi: 10.1172/JCI45109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim D, Jeong YY, Jon S. A drug-loaded aptamer-gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer. ACS Nano. 2010;4(7):3689–96. doi: 10.1021/nn901877h. [DOI] [PubMed] [Google Scholar]
- 165.Pastor F, Kolonias D, McNamara JO, II, Gilboa E. Targeting 4-1BB Costimulation to Disseminated Tumor Lesions With Bi-specific Oligonucleotide Aptamers. Mol Ther. 2011 doi: 10.1038/mt.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chu TC, Marks JW, 3rd, Lavery LA, Faulkner S, Rosenblum MG, Ellington AD, Levy M. Aptamer:toxin conjugates that specifically target prostate tumor cells. Cancer Res. 2006;66(12):5989–92. doi: 10.1158/0008-5472.CAN-05-4583. [DOI] [PubMed] [Google Scholar]
- 167.Lupold SE, Hicke BJ, Lin Y, Coffey DS. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 2002;62(14):4029–33. [PubMed] [Google Scholar]
- 168.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103(16):6315–20. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Farokhzad OC, Jon S, Khademhosseini A, Tran TN, Lavan DA, Langer R. Nanoparticle-aptamer bioconjugates: a new approach for targeting prostate cancer cells. Cancer Res. 2004;64(21):7668–72. doi: 10.1158/0008-5472.CAN-04-2550. [DOI] [PubMed] [Google Scholar]
- 170.Wang AZ, Bagalkot V, Vasilliou CC, Gu F, Alexis F, Zhang L, Shaikh M, Yuet K, Cima MJ, Langer R, Kantoff PW, Bander NH, Jon S, Farokhzad OC. Superparamagnetic iron oxide nanoparticle-aptamer bioconjugates for combined prostate cancer imaging and therapy. ChemMedChem. 2008;3(9):1311–5. doi: 10.1002/cmdc.200800091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang L, Radovic-Moreno AF, Alexis F, Gu FX, Basto PA, Bagalkot V, Jon S, Langer RS, Farokhzad OC. Co-delivery of hydrophobic and hydrophilic drugs from nanoparticle-aptamer bioconjugates. ChemMedChem. 2007;2(9):1268–71. doi: 10.1002/cmdc.200700121. [DOI] [PubMed] [Google Scholar]
- 172.Dhar S, Gu FX, Langer R, Farokhzad OC, Lippard SJ. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc Natl Acad Sci U S A. 2008;105(45):17356–61. doi: 10.1073/pnas.0809154105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R, Farokhzad OC. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett. 2007;7(10):3065–70. doi: 10.1021/nl071546n. [DOI] [PubMed] [Google Scholar]
- 174.Rockey WM, Huang L, Kloepping KC, Baumhover NJ, Giangrande PH, Schultz MK. Synthesis and radiolabeling of chelator- RNA aptamer bioconjugates with copper-64 for targeted molecular imaging. Bioorg Med Chem. 2011 doi: 10.1016/j.bmc.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Barinka C, Byun Y, Dusich CL, Banerjee SR, Chen Y, Castanares M, Kozikowski AP, Mease RC, Pomper MG, Lubkowski J. Interactions between human glutamate carboxypeptidase II and urea-based inhibitors: structural characterization. J Med Chem. 2008;51(24):7737–43. doi: 10.1021/jm800765e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Barinka C, Rovenska M, Mlcochova P, Hlouchova K, Plechanovova A, Majer P, Tsukamoto T, Slusher BS, Konvalinka J, Lubkowski J. Structural Insight into the Pharmacophore Pocket of Human Glutamate Carboxypeptidase II. Journal of Medicinal Chemistry. 2007;50(14):3267–3273. doi: 10.1021/jm070133w. [DOI] [PubMed] [Google Scholar]
- 177.Barinka C, Starkova J, Konvalinka J, Lubkowski J. A high-resolution structure of ligand-free human glutamate carboxypeptidase II. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007;63(Pt 3):150–3. doi: 10.1107/S174430910700379X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Davis MI, Bennett MJ, Thomas LM, Bjorkman PJ. Crystal structure of prostate-specific membrane antigen, a tumor marker and peptidase. Proc Natl Acad Sci U S A. 2005;102(17):5981–6. doi: 10.1073/pnas.0502101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mesters JR, Barinka C, Li W, Tsukamoto T, Majer P, Slusher BS, Konvalinka J, Hilgenfeld R. Structure of glutamate carboxypeptidase II, a drug target in neuronal damage and prostate cancer. Embo J. 2006;25(6):1375–84. doi: 10.1038/sj.emboj.7600969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mesters JR, Henning K, Hilgenfeld R. Human glutamate carboxypeptidase II inhibition: structures of GCPII in complex with two potent inhibitors, quisqualate and 2-PMPA. Acta Crystallogr D Biol Crystallogr. 2007;63(Pt 4):508–13. doi: 10.1107/S090744490700902X. [DOI] [PubMed] [Google Scholar]
- 181.Supuran C, Winum J-Y, Byun Y, Mease RC, Lupold SE, Pomper MG. Chapter 36 Recent Deveopment of Diagnostic and Therapeutic Agents Targeting Glutamate Carboxypeptidase II (GCPII) John Wiley & Sons; Hoboken, New Jersey: 2009. Drug Design of Zinc-Enzyme inhibitors. [Google Scholar]
- 182.Zhou J, Neale JH, Pomper MG, Kozikowski AP. NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat Rev Drug Discov. 2005;4(12):1015–26. doi: 10.1038/nrd1903. [DOI] [PubMed] [Google Scholar]
- 183.Wang H, Byun Y, Barinka C, Pullambhatla M, Bhang HE, Fox JJ, Lubkowski J, Mease RC, Pomper MG. Bioisosterism of urea-based GCPII inhibitors: Synthesis and structure-activity relationship studies. Bioorg Med Chem Lett. 2010;20(1):392–7. doi: 10.1016/j.bmcl.2009.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Slusher BS, Vornov JJ, Thomas AG, Hurn PD, Harukuni I, Bhardwaj A, Traystman RJ, Robinson MB, Britton P, Lu XC, Tortella FC, Wozniak KM, Yudkoff M, Potter BM, Jackson PF. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat Med. 1999;5(12):1396–402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- 185.Jackson PF, Cole DC, Slusher BS, Stetz SL, Ross LE, Donzanti BA, Trainor DA. Design, synthesis, and biological activity of a potent inhibitor of the neuropeptidase N-acetylated alpha-linked acidic dipeptidase. J Med Chem. 1996;39(2):619–22. doi: 10.1021/jm950801q. [DOI] [PubMed] [Google Scholar]
- 186.Majer P, Jackson PF, Delahanty G, Grella BS, Ko YS, Li W, Liu Q, Maclin KM, Polakova J, Shaffer KA, Stoermer D, Vitharana D, Wang EY, Zakrzewski A, Rojas C, Slusher BS, Wozniak KM, Burak E, Limsakun T, Tsukamoto T. Synthesis and biological evaluation of thiol-based inhibitors of glutamate carboxypeptidase II: discovery of an orally active GCP II inhibitor. J Med Chem. 2003;46(10):1989–96. doi: 10.1021/jm020515w. [DOI] [PubMed] [Google Scholar]
- 187.Anderson MO, Wu LY, Santiago NM, Moser JM, Rowley JA, Bolstad ES, Berkman CE. Substrate specificity of prostate-specific membrane antigen. Bioorg Med Chem. 2007;15(21):6678–86. doi: 10.1016/j.bmc.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu T, Wu LY, Kazak M, Berkman CE. Cell-Surface labeling and internalization by a fluorescent inhibitor of prostate-specific membrane antigen. Prostate. 2008;68(9):955–64. doi: 10.1002/pros.20753. [DOI] [PubMed] [Google Scholar]
- 189.Maung J, Mallari JP, Girtsman TA, Wu LY, Rowley JA, Santiago NM, Brunelle AN, Berkman CE. Probing for a hydrophobic a binding register in prostate-specific membrane antigen with phenylalkylphosphonamidates. Bioorg Med Chem. 2004;12(18):4969–79. doi: 10.1016/j.bmc.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 190.Kozikowski AP, Nan F, Conti P, Zhang J, Ramadan E, Bzdega T, Wroblewska B, Neale JH, Pshenichkin S, Wroblewski JT. Design of remarkably simple, yet potent urea-based inhibitors of glutamate carboxypeptidase II (NAALADase) J Med Chem. 2001;44(3):298–301. doi: 10.1021/jm000406m. [DOI] [PubMed] [Google Scholar]
- 191.Humblet V, Lapidus R, Williams LR, Tsukamoto T, Rojas C, Majer P, Hin B, Ohnishi S, De Grand AM, Zaheer A, Renze JT, Nakayama A, Slusher BS, Frangioni JV. High-affinity near-infrared fluorescent small-molecule contrast agents for in vivo imaging of prostate-specific membrane antigen. Mol Imaging. 2005;4(4):448–62. doi: 10.2310/7290.2005.05163. [DOI] [PubMed] [Google Scholar]
- 192.Liu T, Wu LY, Hopkins MR, Choi JK, Berkman CE. A targeted low molecular weight near-infrared fluorescent probe for prostate cancer. Bioorg Med Chem Lett. 2010;20(23):7124–6. doi: 10.1016/j.bmcl.2010.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lapi SE, Wahnishe H, Pham D, Wu LY, Nedrow-Byers JR, Liu T, Vejdani K, VanBrocklin HF, Berkman CE, Jones EF. Assessment of an 18F-labeled phosphoramidate peptidomimetic as a new prostate-specific membrane antigen-targeted imaging agent for prostate cancer. J Nucl Med. 2009;50(12):2042–8. doi: 10.2967/jnumed.109.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pomper MG, Musachio JL, Zhang J, Scheffel U, Zhou Y, Hilton J, Maini A, Dannals RF, Wong DF, Kozikowski AP. 11C-MCG: synthesis, uptake selectivity, and primate PET of a probe for glutamate carboxypeptidase II (NAALADase) Mol Imaging. 2002;1(2):96–101. doi: 10.1162/15353500200202109. [DOI] [PubMed] [Google Scholar]
- 195.Foss CA, Mease RC, Fan H, Wang Y, Ravert HT, Dannals RF, Olszewski RT, Heston WD, Kozikowski AP, Pomper MG. Radiolabeled small-molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer. Clin Cancer Res. 2005;11(11):4022–8. doi: 10.1158/1078-0432.CCR-04-2690. [DOI] [PubMed] [Google Scholar]
- 196.Mease RC, Dusich CL, Foss CA, Ravert HT, Dannals RF, Seidel J, Prideaux A, Fox JJ, Sgouros G, Kozikowski AP, Pomper MG. N-[N-[(S)-1, 3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-L-cysteine, [18F]DCFBC: a new imaging probe for prostate cancer. Clin Cancer Res. 2008;14(10):3036–43. doi: 10.1158/1078-0432.CCR-07-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dusich CL, Chen Y, Pullambhatla M, Nimmagadda S, Foss CA, Fox JJ, Castanares M, Mease RC, Pomper MG. Synthesis and in-vivo evaluation of 2-{-[1-carboxy-2-(4-[125I]iodo-benzylsulfanyl)-ethyl]-ureido}-pentanedioic acid) [125I]DCFBC) Journal of Labelled Compounds and Radiopharmaceuticals. 2009;52(Supplement 1):S18. (abstract) [Google Scholar]
- 198.Chen Y, Foss CA, Byun Y, Nimmagadda S, Pullambhatla M, Fox JJ, Castanares M, Lupold SE, Babich JW, Mease RC, Pomper MG. Radiohalogenated prostate-specific membrane antigen (PSMA)-based ureas as imaging agents for prostate cancer. J Med Chem. 2008;51(24):7933–43. doi: 10.1021/jm801055h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hillier SM, Maresca KP, Femia FJ, Marquis JC, Foss CA, Nguyen N, Zimmerman CN, Barrett JA, Eckelman WC, Pomper MG, Joyal JL, Babich JW. Preclinical Evaluation of Novel Glutamate-Urea-Lysine Analogues That Target Prostate-Specific Membrane Antigen as Molecular Imaging Pharmaceuticals for Prostate Cancer. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Maresca KP, Hillier SM, Femia FJ, Keith D, Barone C, Joyal JL, Zimmerman CN, Kozikowski AP, Barrett JA, Eckelman WC, Babich JW. A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. J Med Chem. 2009;52(2):347–57. doi: 10.1021/jm800994j. [DOI] [PubMed] [Google Scholar]
- 201.Chen YPM, Foss C, Nimmagadda S, Byun Y, Fox JJ, Mease RC, Pomper MG. Synthesis and biological evaluation of [18F]DCFPyL for PSMA-targeted imaging of prostate cancer. J Nucl Med (Abstract) 2011;52 (Supplement 1):88P. [Google Scholar]
- 202.Barrett JA, LaFrance N, Coleman RE, Goldsmith SJ, Stubbs JB, Petry NA, Vallabhajosula S, Maresca KP, Femia FJ, Babich JW. Targeting metastatic prostate cancer [PCa] in patients with 123I-MIP1072 and 123I-MIP1095. J Nucl Med. 2009;50S:136P. [Google Scholar]
- 203.Cho SY, Mease R, Holt D, Dannals RF, Eisenberger M, Rodriguez R, Carducci M, Rojas C, Slusher BS, Pomper MG. Initial clinical assessment of DCFBC-PET for metastatic prostate cancer (PCa). (Abstract) J Nuc Med. 2011;52(Supplement 1):12P. [Google Scholar]
- 204.Banerjee SR, Pullambhatla M, Byun Y, Green G, Fox JJ, Horti AG, Mease R, Pomper MG. Ga-68- and In-111-labeled small molecule inhibitors of PSMA for prostate cancer imaging. Journal of Labelled Compounds and Radiopharmaceuticals. 2009;52(Supplement 1):S19. (abstract) [Google Scholar]
- 205.Banerjee SR, Foss CA, Castanares M, Mease RC, Byun Y, Fox JJ, Hilton J, Lupold SE, Kozikowski AP, Pomper MG. Synthesis and evaluation of technetium-99m- and rhenium-labeled inhibitors of the prostate-specific membrane antigen (PSMA) J Med Chem. 2008;51(15):4504–17. doi: 10.1021/jm800111u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ray SPM, Falk AS, Byun Y, Foss C, Nimmagadda S, Fox JJ, Mease RC, Pomper MG. 99mTc-Labeled inhibitors for prostate-specific membrane antigen (PSMA) for imaging prostate cancer. J Nucl Med (Abstract) 2011;52(Supplement 1):88P. [Google Scholar]
- 207.Chen Y, Dhara S, Banerjee SR, Byun Y, Pullambhatla M, Mease RC, Pomper MG. A low molecular weight PSMA-based fluorescent imaging agent for cancer. Biochem Biophys Res Commun. 2009;390(3):624–9. doi: 10.1016/j.bbrc.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Banerjee SR, Pullambhatla M, Byun Y, Nimmagadda S, Foss CA, Green G, Fox JJ, Lupold SE, Mease RC, Pomper MG. Sequential SPECT and optical imaging of experimental models of prostate cancer with a dual modality inhibitor of the prostate-specific membrane antigen. Angew Chem Int Ed Engl. 2011;50 doi: 10.1002/anie.201102872. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Murelli RP, Zhang AX, Michel J, Jorgensen WL, Spiegel DA. Chemical Control over Immune Recognition: A Class of Antibody- Recruiting Small Molecules That Target Prostate Cancer. J Am Chem Soc. 2009;131(47):17090–17092. doi: 10.1021/ja906844e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kularatne SA, Wang K, Santhapuram HK, Low PS. Prostate-specific membrane antigen targeted imaging and therapy of prostate cancer using a PSMA inhibitor as a homing ligand. Mol Pharm. 2009;6(3):780–9. doi: 10.1021/mp900069d. [DOI] [PubMed] [Google Scholar]
- 211.Kularatne SA, Zhou Z, Yang J, Post CB, Low PS. Design, synthesis, and preclinical evaluation of prostate-specific membrane antigen targeted (99m)Tc-radioimaging agents. Mol Pharm. 2009;6(3):790–800. doi: 10.1021/mp9000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Malik N, Machulla HJ, Solbach C, Winter G, Reske SN, Zlatopolskiy B. Radiosynthesis of a new PSMA targeting ligand ([18F]FPy-DUPA-Pep) Appl Radiat Isot. 2011;69(7):1014–8. doi: 10.1016/j.apradiso.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 213.Chandran SS, Banerjee SR, Mease RC, Pomper MG, Denmeade SR. Characterization of a targeted nanoparticle functionalized with a urea-based inhibitor of prostate-specific membrane antigen (PSMA) Cancer Biol Ther. 2008;7(6):974–82. doi: 10.4161/cbt.7.6.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jayaprakash S, Wang X, Heston WD, Kozikowski AP. Design and synthesis of a PSMA inhibitor-doxorubicin conjugate for targeted prostate cancer therapy. ChemMedChem. 2006;1(3):299–302. doi: 10.1002/cmdc.200500044. [DOI] [PubMed] [Google Scholar]
- 215.Kularatne SA, Venkatesh C, Santhapuram HK, Wang K, Vaitilingam B, Henne WA, Low PS. Synthesis and biological analysis of prostate-specific membrane antigen-targeted anticancer prodrugs. J Med Chem. 2010;53(21):7767–77. doi: 10.1021/jm100729b. [DOI] [PubMed] [Google Scholar]
- 216.Hillier SMMR, Maresca K, Zimmerman CN, Barrett JA, Tesson M, Eckelman WC, Mairs RJ, Joyal JL, Babich JW. [131I]MIP-1375, a small molecule prostate-specific membrane antigen (PSMA) inhibitor for targeting therapy of prostate cancer (PCa) J Nucl Med (Abstract) 2011;52(Supplement):107P. [Google Scholar]
- 217.Slovin SF. Emerging role of immunotherapy in the management of prostate cancer. Oncology (Williston Park) 2007;21(3):326–33. discussion 334, 338, 346–8. [PubMed] [Google Scholar]
- 218.Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22(8):969–76. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 219.Denmeade SR, Jakobsen CM, Janssen S, Khan SR, Garrett ES, Lilja H, Christensen SB, Isaacs JT. Prostate-specific antigen-activated thapsigargin prodrug as targeted therapy for prostate cancer. J Natl Cancer Inst. 2003;95(13):990–1000. doi: 10.1093/jnci/95.13.990. [DOI] [PubMed] [Google Scholar]