Abstract
Objective
Evaluate speech, hearing, and neuropsychological correlates to reading among children, adolescents and young adults with non-syndromic cleft of the lip and/or palate (NSCL/P).
Method
All testing was completed in one visit at a Midwestern university hospital. Subjects in both the NSCL/P (n = 80) and control group (n = 62) ranged in age from 7 to 26 years (average age = 17.60 and 17.66, respectively). Subjects completed a battery of standardized tests evaluating intelligence, neuropsychological skills, and word reading. Subjects with NSCL/P also underwent speech assessment and past audiology records were evaluated.
Results
After controlling for age and SES, subjects with cleft performed significantly worse on a test of word reading. For subjects with cleft, word reading deficits were not associated with measures of speech or hearing, but were correlated with impairments in auditory memory.
Conclusions
These findings show poorer reading among subjects with NCL/P compared to those without. Further work needs to focus on correlates of reading among subjects with cleft to allow early identification and appropriate intervention/accommodation for those at risk.
Keywords: Cleft, Reading, Learning, Speech, Hearing
Research extending back 50 years has documented a strong occurrence of language disorders (LD) among children with non-syndromic cleft of the lip and/or palate (NSCL/P; Conrad, Richman, Nopoulos, & Dailey, 2009; Goldstein et al., 2007; Hentges et al., 2011; Lamb, Wilson, & Leeper, 1972; Nopoulos, Berg, VanDemark, et al., 2002; Roberts, Mathias, & Wheaton, 2012). This is most often reflected in a high occurrence of Dyslexia in children with NSCL/P (Broder, Richman, & Matheson, 1998; Chapman, 2011; Collett, Stott-Miller, Kapp-Simon, Cunningham, & Speltz, 2010; Richman, Eliason, & Lindgren, 1988; Richman & Ryan, 2003; Richman, Wilgenbusch, & Hall, 2005). For some subgroups this appears to be a developmental lag where reading skills improve in adolescence, though others continue to demonstrate a deficit into adulthood (Richman, et al., 1988).
Early on, it was hypothesized that the high rates of Dyslexia and LD were due either to poor articulation resulting from the abnormal oral cavity or hearing loss secondary to frequent episodes of Otitis Media (Amaral, Martins, & Santos, 2010; Chapman, 2011; Collett, Stott-Miller, et al., 2010). These disruptions to sensory input at critical developmental times were hypothesized to impair phonological awareness, a key skill required for reading (Ramus, 2003). Research supporting this theory has found correlations between early speech issues (Chapman, 2011), poor hearing (Boscariol, Andre, & Feniman, 2009; Collett, Stott-Miller, et al., 2010) and reading outcomes.
However, there have been some criticisms of this theory. Research within the general population has shown normal phonological development in persons with severe speech disabilities (Ramus, Pidgeon, & Frith, 2003), which suggests that phonological representations are not solely a product of speech articulation (Ramus et al., 2003). Second, there are studies among people with NSCL/P that demonstrate no relationship between articulation, hearing, and reading or language outcome (Ceponiene, Haapanen, Ranta, Naatanen, & Hukki, 2002; Hentges, et al., 2011; Lamb, et al., 1972; Shriver, Canady, Richman, Andreasen, & Nopoulos, 2006). These findings suggest that there may be something more than disrupted speech or hearing influencing language and reading skills among people with NSCL/P.
In order to better understand the neurological underpinnings of these outcomes, some research has evaluated neuropsychological skills associated with reading (i.e., phonological awareness, rapid labeling, and auditory/visual memory). Studies have shown related deficiencies in rapid labeling and auditory/visual memory compared to controls (Brennan & Cullinan, 1974; Ceponiene, et al., 2002; Ceponiene et al., 1999; Nopoulos, Berg, VanDemark, et al., 2002; Richman & Ryan, 2003; Richman, et al., 2005; Smith & McWilliams, 1968). Although, there has been some research that has found no differences in reading or these related skills (Chapman, 2011; Collett, Leroux, & Speltz, 2010; Smith & McWilliams, 1968).
In support of a theory of neuropsychological deficits, our laboratory has documented abnormal brain structure in both children (Nopoulos, Langbehn, Canady, Magnotta, & Richman, 2007) and adults with NSCL/P (Nopoulos, Berg, Canady, et al., 2002; Nopoulos et al., 2005). These changes in brain structure are hypothesized to be due to abnormal brain development that occurs in parallel with the abnormality in facial development. Establishing a relationship between reading performance and specific language-based neuropsychological skills would support the notion that the reading disabilities may be rooted in abnormal brain structure and function, rather than a secondary effect from deprivation of sensory input.
One weakness in this body of literature is that the majority of recent research evaluating language and reading skills are focused on younger subjects (maximum age around 8 years old), in whom reading skills are just starting to develop. A close evaluation of articulation, hearing, neuropsychological skill, and reading among an older sample, where reading skill is stronger or more established, is lacking.
The purpose of this study was to obtain measures of word reading, neuropsychological skill, speech production, and history of hearing in children, adolescents, and young adults with NSCL/P. Key questions for evaluation included: 1) What is the effect of sex, age and group membership on reading outcome for subjects with and without cleft? 2) What is the relationship of reading skill to measures of neuropsychological skill, speech, and hearing? It was hypothesized that reading skill would increase with age for all subjects; however, there may be a group-by-age interaction where reading for subjects with NSCL/P is discrepant at younger ages and “catches-up” in young adulthood. It was also expected that neuropsychological skill would be associated to reading outcome, but results for speech and hearing were not predicted.
Methods
Subjects were tested as part of a longitudinal study on cognitive and behavioral outcomes and brain development in children, adolescents, and young adults with NSCL/P. Previous work from this study has reported lower verbal expression and memory in comparison to controls (Conrad, et al., 2009). The current sample comprises 77 subjects (n = 34 NSCL/P and 43 Controls) who returned for follow-up assessment, as well as 65 new subjects (n = 46 NSCL/P and 19 Controls) who were not enrolled in the initial evaluation.
Subjects with an oral cleft were recruited from clinic lists; reports from clinical evaluations by geneticists were reviewed and only those without a potential genetic syndrome were contacted. Subjects without cleft were recruited through local advertisements. Control subjects were screened (interview with parent) and excluded for potential learning disabilities as well as exceptional academic performance (defined as participation in a talented/gifted program). This screening methodology was implemented in order to obtain a sample of subjects with average reading skill. Both case and control subjects with a history of head trauma or other major medical disorder (aside from the cleft) were excluded.
Socioeconomic status (SES) was obtained via parental report and rated on a modified 5-point Hollingshead scale where 1 = highest SES and 5 = lowest SES (Hollingshead, 1975; Hollingshead & Redlich, 1958). However, this information was not returned for 26 subjects (primarily older participants). In order to include this measure in analyses, parental SES was estimated based on parental education for 10 of these subjects. Imputation of group means was used for subjects without a measure of SES (NSCL/P n = 9 and Control n = 7).
Subjects, ages 7 to 26 years, were recruited and tested between March of 2009 and June 2012. The case group consisted of 80 subjects with NSCL/P (55% male); 18 had a cleft lip only (CLO; 14 unilateral / 4 bilateral), 19 had a cleft palate only (CPO; 11 soft and hard palate / 8 soft palate) and 43 had cleft lip and palate (CLP; 23 unilateral / 17 bilateral / 3 severity unknown). These cleft type distributions are similar to reported prevalence rates (American Academy of Otolaryngology - Head and Neck Surgery, 2011). Based on Analyses of Variance, there were no differences between cleft type on age (F (2, 77) = 1.939, p = 151), SES (F (2, 77) = 2.137, p = .125), nor Full Scale IQ (F (2, 76) = 0.470, p = .627) or neuropsychological outcome measures (F (12,138) = 1.049, p = .408) after controlling for SES. Therefore, the three groups were combined for analyses in comparison to controls.
There were 62 subjects in the control group (44% male). Both groups spoke English as their primary language and were predominantly Caucasian (case = 84% [next highest racial group = 8% Asian] and control = 97%), consistent with the demographics of the region. Case and control subjects were of the same average age (case = 17.60 [4.57] and control = 17.66 [3.28]; Mean Difference = 0.059; 95% CI [-1.299, 1.417]). Subjects with NSCL/P had significantly lower reported parental SES (case = 2.691 [0.062] and control = 2.381 [0.070]; Mean Difference = -0.311; 95% CI [0.126, 0.495]). See Table 1.
Table 1.
Group Means and Standard Deviations for Demographics and Measures of Interest
| Control |
NSCL/P |
Mean Difference | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | 95% CI | ||
| Age | 17.66 | 3.28 | 17.60 | 4.57 | −1.299, 1.417 | |
| SES | 2.38 | 0.51 | 2.69 | 0.58 | −0.498, −0.126*** | |
| FSIQa | 111.41 | 1.63 | 107.16 | 1.43 | −0.111, 8.604 | |
| Neuropsychological | ||||||
| Verbal Fluencya | −0.33 | 0.13 | −0.42 | 0.12 | −0.274, 0.446 | |
| Rapid Labelinga | 0.30 | 0.11 | −0.06 | 0.10 | 0.070, 0.655* | |
| Sustained Attentiona | 0.27 | 0.14 | −0.24 | 0.12 | 0.124, 0.880** | |
| Auditory Memorya | −0.18 | 0.12 | −0.26 | 0.11 | −0.235, 0.403 | |
| Visual Memorya | 0.19 | 0.12 | 0.13 | 0.10 | −0.254, 0.375 | |
| Speech | ||||||
| Nasal Passage | 58.75 | 9.31 | ||||
| Low Pressure Passage | 20.32 | 13.85 | ||||
| Zoo Passage | 20.52 | 16.39 | ||||
| Hearing | ||||||
| Best | 5.36 | 9.26 | ||||
| Worst | 24.64 | 16.11 | ||||
| Average | 13.17 | 10.20 | ||||
Note. NSCL/P = Nonsyndromic Cleft Lip and/or Palate; SES = Socioeconomic Status; FSIQ = Full Scale IQ. FSIQ and Word Reading are age-based index scores. All neuropsychological measures are age-based z-scores, where higher values indicate better performance. For Nasal Passage, scores of 50 or higher are considered normal, and for Low Pressure Passage and Zoo Passage, scores lower than 30 are considered normal. For audiology measures, lower values indicate better hearing.
Means are adjusted for SES and Standard Error is reported in place of Standard Deviation.
p < .05
p < .01
p < .001
The protocol was approved by the Institutional Review Board and all minor subjects gave assent and parents gave written consent. Subjects who were 18 or older provided their own written consent. Subjects underwent neuropsychological testing by a trained research assistant and speech was evaluated by a certified speech-language pathologist. Hospital records for subjects with NSCL/P were reviewed and audiology assessment results were recorded.
Measures
Intelligence
FSIQ was pro-rated from select subtests of the Wechsler Intelligence Scale for Children, 3rd Edition (WISC-III; Wechsler, 1991) and Wechsler Adult Intelligence Scale, 3rd Edition (WAIS-III; Wechsler, 1997a). At the start of this study, the 3rd edition was the most current version of the Wechsler scales available, and testing on this version continued throughout the length of the study. Subjects ages 7 to 16 were administered the WISC-III and those 17 and older were administered the WAIS-III. Vocabulary and Similarities were administered to calculate a pro-rated Verbal IQ (sum of scaled scores = (Vocabulary + Similarities) * 2.5), Block Design and Picture Completion subtests were administered to calculate a pro-rated Perceptual IQ (sum of scaled scores = (Block Design + Picture Completion) * 2.5). Verbal and Perceptual IQ’s were combined to calculate Full Scale IQ.
Verbal Fluency
Subjects under the age of 13 were administered the phonemic trial of Verbal Fluency from the NEPSY (Korkman, Kirk, & Kemp, 1998) while those 13 and older were administered the Verbal Fluency subtest from the Multi-lingual Aphasia Exam (MAE; Benton, Hamsher, & Sivan, 1994). This division of tests by age was established with the original study and continued in the current methodology for consistency. Both tests evaluated the subject’s ability to rapidly generate words with a specific beginning letter. To combine these tests and evaluate as one “Verbal Fluency” measure, raw scores from the MAE were used (based on three letters; CLF), and raw phonemic score from the NEPSY (based on two letters; SF) was pro-rated to be comparable to the MAE raw score.
Auditory Memory
The Digit Span subtest from the WISC-III (Wechsler, 1991) and WAIS-III (Wechsler, 1997a) was included as a measure of auditory memory.
Visual Memory
For visual memory, Spatial Span from the WISC-III-PI (Kaplan, Fein, Kramer, Delis, & Morris, 1999) and Wechsler Memory Scale, 3rd Edition (WMS-III; Wechsler, 1997b) was administered.
Rapid Labeling
The Color/Word Interference subtest of the Delis-Kaplin Executive Function System (DKEFS; Delis, Kaplan, & Kramer, 2001) was administered to assess rapid labeling. Time to complete trial 1 (Color Naming) was transposed so higher scores were indicative of faster (better) performance.
Sustained Attention
The Connor’s Continuous Performance Test, 2nd edition (CPT-II; Conners, 2000) was administered to assess sustained attention. The score for sustained attention (number of omission errors) was transposed so higher scores were indicative of better performance.
Reading Achievement
The Word Reading subtest from the Wide Range Achievement Test, 3rd Edition (WRAT-III; Wilkinson, 1993) was administered to all subjects to evaluate reading achievement.
Speech
Speech and nasal resonance assessments for 67 (84%) of the subjects with NSCL/P were provided by a certified speech pathologist with extensive experience evaluating children with speech disorders associated with velopharyngeal inadequacy (VPI). Assessment included standardized articulation testing via the Goldman-Fristoe Test of Articulation, Second Edition (Goldman & Fristoe, 2000; GFTA-2). Percent consonants correct was calculated for the single word production subtest. Objective acoustic measurements of nasalance were obtained with a Nasometer II (model 6400) developed by Kay Elemetrics (now KayPENTAX; Adams, 1988). Nasalance measures included hyponasality (Nasal Passage; demonstrating a lack of nasal emission of air; scores above 50 are normative) and hypernasality (Low Pressure Passage and Zoo Passage; demonstrating excessive nasal emission of air; scores below 30 are normative). See Table 1.
Hearing
Audiology records were reviewed for all NSCL/P subjects for whom these data were available (n = 70; 88%). This subsample was 50% male and an average of 17.74 years of age (SD = 4.47; range = 7.5 – 26.33) at cognitive assessment. Values for all Speech Detection Threshold and Speech Reception Threshold were recorded with lower threshold scores indicating better hearing. Number of assessments per subject ranged from 1 to 24 (Average = 8.17). Since hearing tests were not conducted at the same age/time intervals for all participants, three measures were utilized to summarize hearing levels. From all available data, the best score, worst score, and average score were all recorded. Values ranged from 0 to 100 dB, where 0 was best and values above 15 - 20 dB can be indicative of a hearing issue depending upon the age of subject at testing (First Years, 2004). See Table 1.
Analyses
An a-priori analysis of group differences in FSIQ and neuropsychological skills was conducted with a Multivariate Analysis of Variance, controlling for SES (MANCOVA). Also, for informational purposes, a distribution graph of Word Reading normative scores between subjects with and without NSCL/P was produced. See Figure 1.
Figure 1.
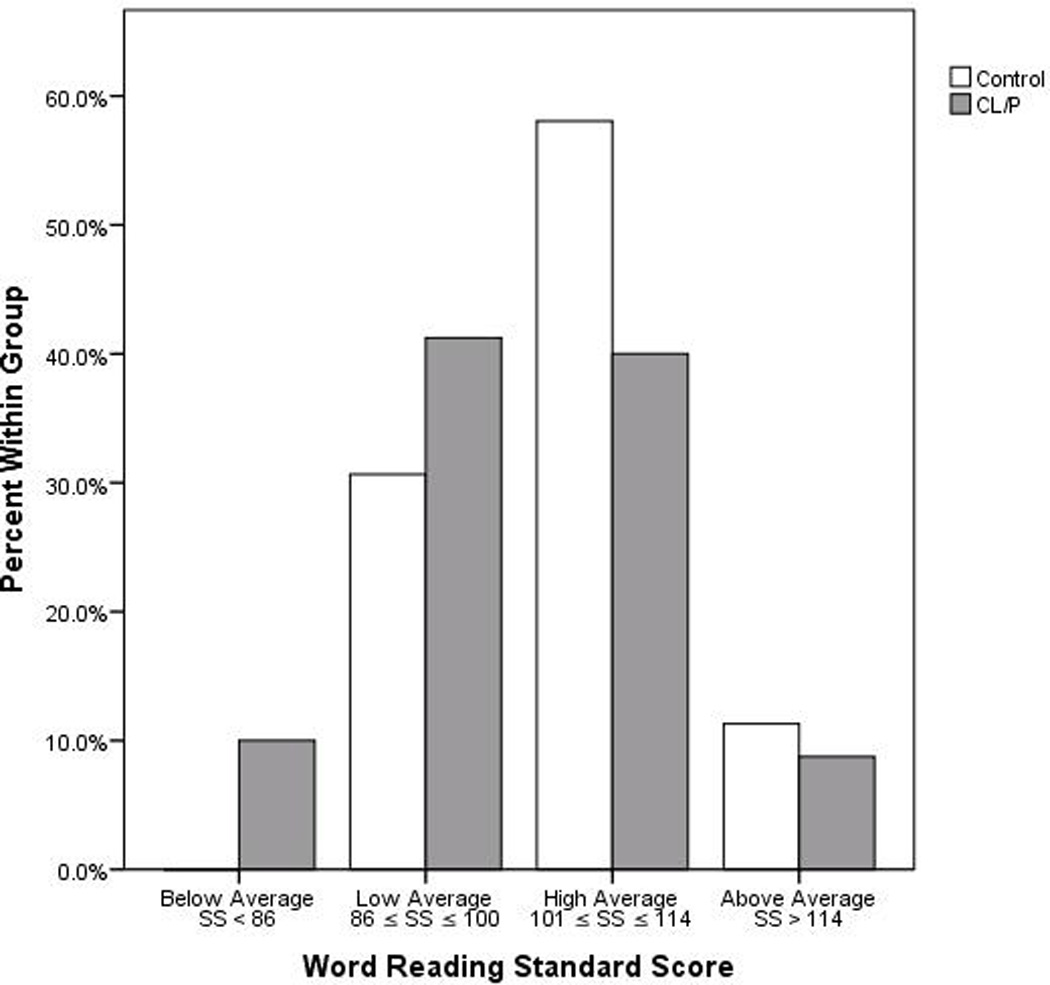
Distribution of Word Reading Standard Scores by Group.
Next, to evaluate the influence of sex, age and group membership on word reading outcome, a hierarchical regression was run; with Word Reading raw score as the dependent variable, SES was entered in step one. Next, sex was entered in step two, and then age and group were entered in step three, evaluating their potential main effects after controlling for SES. Finally, the interaction of age and group were entered in step four.
To assess the contribution of neuropsychological skill, current speech quality, and history of hearing performance to reading achievement, a partial Pearson correlation was run between Word Reading (raw score), neuropsychological measures (raw scores; verbal fluency, rapid labeling, auditory memory, visual memory, and sustained attention), speech measures and hearing performance (best, worst, and average score). Age and SES were entered as covariates. This analysis was run separately for controls and subjects with NSCL/P.
Results
The a-priori MANCOVA was significant for group differences after controlling for SES (F (6, 129) = 2.806, p = .013). Subjects with NSCL/P had slower rapid naming (Mean Difference = 0.362; 95% CI [0.070, 0.655]) and worse sustained attention (Mean Difference = 0.502; 95% CI [0.124, 0.880]. Of note, although within the average range, there was a strong trend for FSIQ in subjects with NSCL/P (Adjusted Mean = 107.16) to be lower than controls (Adjusted Mean = 111.41; Mean Difference = 4.247; 95% CI [-0.111, 8.604]). The average estimated IQ for the state of Iowa is 103.2 (McDaniel, 2006), with the average being higher for the University town where this study took place. See Table 1.
The regression predicting reading outcome demonstrated a non-significant trend for SES in step 1 (F (1, 140) = 3.585, p = .060). The addition of sex did not contribute significantly to the prediction of Word Reading (F Change (1, 139) = 3.060, p = .082). However, the addition of age and group did contributed significantly after controlling for SES and sex (F Change (2, 137) = 46.272, p < .001), and SES became significantly predictive. Subjects who performed better on Word Reading had higher SES, were older and more likely to be in the control group. Finally, the potential interaction of age and group on reading outcome was non-significant (F change (1, 136) = 0.200, p = .656). See Table 2.
Table 2.
Hierarchical Linear Regression Predicting Word Reading Raw Score
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| β | β | β | β | |
| SES | −.158 | −.160 | −.150* | −.146* |
| Sex | −.145 | −.074 | −.075 | |
| Age | .598*** | .552*** | ||
| Group | −.174* | −.175* | ||
| Age*Group | .055 |
Note. SES = Socioeconomic Status. β = Standardized Beta. Dependent Variable = Word Reading raw score. Model 1 = SES entered. Model 2 = SES and Sex entered. Model 3 = SES, Sex, Age, and Group entered. Model 4 = SES, Sex Age, Group, and Age*Group entered.
p < .05
p < .001
Among the five neuropsychological measures evaluated, all were significantly correlated to word reading after controlling for age and SES for control subjects. After Bonferroni correction, the correlations to Verbal fluency (r = .452, p = .001), Auditory Memory (r = .478, p < .001), and Visual Memory (r = .331, p = .050) remained significant. Subjects with NSCL/P demonstrated a similar pattern, but after Bonferroni correction, only the correlation to Auditory Memory (r = .524, p < .001) remained significant. See Table 3.
Table 3.
Relationship of Measures to Word Reading.
| Control |
NSCL/P |
|||||
|---|---|---|---|---|---|---|
| Correlation | Sig. | df | Correlation | Sig. | df | |
| Neuropsychological | ||||||
| Verbal Fluency | .452 | .001 | 58 | .284 | .060 | 76 |
| Rapid Labeling | .278 | .155 | 58 | .245 | .155 | 76 |
| Sustained Attention | .323 | .065 | 57 | .265 | .110 | 73 |
| Auditory Memory | .478 | < .001 | 58 | .524 | < .001 | 76 |
| Visual Memory | .331 | .050 | 58 | .220 | .265 | 76 |
| Speech | ||||||
| Nasal Passage | −.025 | > .999 | 61 | |||
| Low Pressure Passage | .164 | .970 | 62 | |||
| Zoo Passage | .168 | .920 | 62 | |||
| Hearing | ||||||
| Best | .016 | > .999 | 66 | |||
| Worst | .030 | > .999 | 66 | |||
| Average | .028 | > .999 | 66 | |||
Note. Correlations control for Age and FSIQ. NSCL/P = Non-Syndromic Cleft Lip and/or Palate. All measures are raw values. A linear transformation was applied to Rapid Labeling and Sustained Attention so all neuropsychological variables had high scores reflecting better performance. p-values are adjusted for Bonferroni Correction.
There was a ceiling effect for articulation, where only 11 subjects scored under 100%, and all but one were above 90% correct. Therefore, only nasalance measures were included in the analyses. Correlations of word reading to speech measures found no relationship to hyponasality (Nasal Passage (r = -.025, p = .845)) or hypernasality (Low Pressure Passage (r = .164, p = .194) and Zoo Passage (r = .168, p = .184)). Additionally, there were no significant correlations between measures of hearing and word reading skill (Best (r = .016, p = .898), Worst (r = .030, p = .807), and Average (r = .028, p = .822)). Correlations for speech and audiology measures were deemed non-significant prior to Bonferroni correction. See Table 3.
Discussion
This study evaluated the relationship of word reading in children, adolescents, and young adults with NSCL/P to speech, hearing, and neuropsychological skill. Similar to previous work, FSIQ was within the average range, though somewhat lower in the NSCL/P group (Conrad et al., 2009). Despite average FISQ, subjects with NSCL/P had significantly slower rapid labeling and worse sustained attention compared to those without. The finding of slower labeling is consistent with past work (Conrad, et al., 2009; Richman & Ryan, 2003). It is interesting that this was found among an older population that is post speech therapy and had overall high articulation scores, so it is less likely that altered speech was influencing speed. Little research among subjects with NSCL/P has evaluated computerized measures of sustained attention. In the previous publication with this sample (Conrad, et al., 2009) the measure of executive functioning was a non-verbal measure of organizational planning (i.e., Tower) was used, and it was not significantly different. Here, the measure was the CPT, which is based on letter recognition and evaluates more sustained attention. Multiple modes of measurement will need to be incorporated into future work to further evaluate strengths and weaknesses within executive functioning skills for people with NSCL/P.
After controlling for SES, those with NSCL/P did show deficiencies in reading achievement compared to those without. Similar to previous findings within this population, the word reading skills were within the average range, though significantly discrepant from same-aged geographical peers. This suggests that some students with cleft may perform average nationally, but struggle when competing with their peers in the classroom. It is important for those evaluating children with potential reading problems to obtain information on how the child is doing within the classroom rather than relying soly on the results of tests norms.
Age was a significant predictor of word reading (as expected), but the interaction of group by age was non-significant. In previous work, it has been suggested that subjects with cleft experience a developmental delay where they perform lower than peers at younger ages and ‘catch up’ in adolescence (Richman, et al., 1988). The results of the current study seem to support greater consistency in the discrepancy between the two groups across ages. However, cleft type differences have also been referenced where boys with CPO continue to show issues in adolescence, but boys with CLP ‘catch up’ (Richman, et al., 1988). Unfortunately, the number of subjects by cleft type was too low to evaluate this potential cleft type by age interaction. Further work with larger subgroups is needed to better understand the interplay of these variables on outcome.
In evaluating correlates of reading, control subjects had a variety of cognitive skills that were significantly related to word reading outcome. In contrast, only auditory memory was significant for subjects with NSCL/P. Additionally, no measures of current speech quality or past hearing ability were correlated to reading. This suggests that unlike controls, children with NSCL/P seem to rely primarily on auditory memory for reading, whereas the control children had several different cognitive skills that they relied on. Auditory memory skills may be compensating for other cognitive tools that aren’t being utilized in the process of reading. This also may explain why their overall score in auditory memory was not different than controls. Interestingly, auditory memory was not significantly discrepant for this sample as it was in the previous study (Conrad, et al., 2009). Previously, Sentence Repetition was used to assess auditory memory, while Digit Span (which assesses more rote auditory memory) was the measure for the current sample. When reading, rote (or short-term) auditory memory is essential for phonological processing: letters on the page are transferred into sounds, the sounds are briefly held within working memory, and then blended into words (Shaywitz, 2003). It is unclear if auditory memory is impacting reading through a deficit in phonological awareness, or if it is primarily a memory issue. However, previous work has found deficits in auditory memory without lower phonological awareness among subjects with NSCL/P (Richman & Ryan, 2003), supporting the notion that it may be auditory memory and not phonologic awareness that is the primary issue related to poor reading.
Finally, post-hoc analysis demonstrated no association between audiology scores and auditory memory (Average Audiology Score r = -.096, p = .438). This would suggest against the increased Otitis-Media theory where disrupted hearing results in abnormal phonological development. These findings may lend support, instead, to a more neurodevelopmental theory. It is possible that whatever unknown factor that impacts abnormal facial development, is also causing abnormal brain development and resulting deficits in function. Our laboratory has reported on abnormal brain structure in children and adults with NSCL/P. More importantly, we have found specific relationships between abnormal brain structure of regions known to govern language-related skills and scores on cognitive tests of language-specific skills. In a study of adult males with NSCL/P (Shriver, et al., 2006), the superior temporal plane (a region commonly associated with language and reading function; Frank & Pavlakis, 2001) was found to be abnormally enlarged – a phenomenon known as ‘pathologic enlargement’. This means that not only was this brain region enlarged, but it was directly related to language skills – the larger this region, the lower the Verbal IQ. In addition, the abnormality of the brain region had no relationship to hearing scores. In children, we found a very similar finding in which the temporal pole, a region known to govern verbal labeling, was pathologically enlarged in male NSCL/P subjects, in particular on the left side. Again, the enlargement was directly related to the cognitive skill: The larger the brain volume, the lower the verbal labeling skill (DeVolder, Conrad, Richman, Magnotta, & Nopoulos, 2013). Finally, a recent study on infants with NSCL/P (Yang, McPherson, Shu, Xie, & Xiang, 2012) found abnormal brain structure in the left temporal lobe, supporting the notion that this region may be abnormal early in development.
Lack of specific assessments for predictor and outcome measures as well as records of intervention limit the interpretations of the current study. Phonological awareness (which is the hypothesized language skill related to hearing loss/speech problems) was not assessed as part of the neuropsychological battery. Given the hypothesized impact hearing may have on phonological development as well as its importance to word reading, this is a key limitation to the study. A measure of phonological awareness is needed in future work to help clarify the relationships of hearing, auditory memory, reading, and brain development. Further, the measures of hearing taken from record reviews were administered at different ages by different examiners and were not conducted at the time of cognitive testing. Results from these comparisons should be considered with caution and further work with planned audiology assessments at specified age points should be conducted to better understand the potential influence hearing may have on reading outcome. Additionally, the outcome measure was limited to word reading. As subjects age, other measures of reading achievement (such as comprehension and fluency) are more distinctive of poor versus good readers (Shaywitz & Shaywitz, 2005). Measures of reading comprehension and fluency may yield different results and should be evaluated. Finally, data was not obtained on which subjects had received any intervention. It is possible that intervention may have improved reading skills for some and masked relevant associations.
Regardless of why auditory memory is so strongly associated to word reading skill in people with NSCL/P, this connection does provide key information in how to approach remediation and accommodation. Because of its strong association to phonological awareness, the most evidence-based treatment would be multi-sensory work with phonetics (e.g. Orton-Gillingham (Vickery, Reynolds, & Cochran, 1987)) and training in memory tools (e.g., pneumonics or imagery; Riccio, Sullivan, & Cohen, 2010). Helpful classroom accommodations may include being provided outlines for lectures in advance, additional time, and multi-sensory instruction (use of visual aids or hands-on projects).
Future work should include assessment of a larger sample, with evaluations of history of hearing/speech, phonological skills as well as other language skills, and a variety of reading achievement assessments. Also, work with structural and functional neuroimaging will assist in determining the contribution brain development may have on these skills. There have been initial findings related to abnormal temporal lobe structure; it will be interesting to see if functional findings are similar. Findings from this line of work are important in early identification of subjects at the highest risk for learning problems. This will lead to early intervention, which can be more specifically designed to work with the skill sets most strongly influencing reading outcome.
Acknowledgments
Funding/Support: This study was supported by a grant from the National Institutes of Health, R01 DEO14399-05 (Dr Nopoulos, PI).
References
- Adams LE. Nasometer 6200-2 Instruction Manual. Pine Brooks, NJ: Kay Elemetrics Corp.; 1988. [Google Scholar]
- Amaral MI, Martins JE, Santos MF. A study on the hearing of children with non-syndromic cleft palate/lip. Brazilian journal of otorhinolaryngology. 2010;76(2):164–171. doi: 10.1590/S1808-86942010000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Otolaryngology - Head and Neck Surgery. Fact Sheet: Cleft Lip and Cleft Palate. 2011 Retrieved May 31, 2013, 2013, from http://www.entnet.org/HealthInformation/cleftLipPalate.cfm.
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3rd Edition. Iowa City, IA: AJA Associates; 1994. [Google Scholar]
- Boscariol M, Andre KD, Feniman MR. Cleft palate children: performance in auditory processing tests. Brazilian journal of otorhinolaryngology. 2009;75(2):213–220. doi: 10.1016/S1808-8694(15)30780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan DG, Cullinan WL. Object identification and naming in cleft palate children. Cleft Palate Journal. 1974;11:188–195. [PubMed] [Google Scholar]
- Broder HL, Richman LC, Matheson PB. Learning disability, school achievement, and grade retention among children with cleft: a two-center study. Cleft Palate Craniofac J. 1998;35(2):127–131. doi: 10.1597/1545-1569_1998_035_0127_ldsaag_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Haapanen ML, Ranta R, Naatanen R, Hukki J. Auditory sensory impairment in children with oral clefts as indexed by auditory event-related potentials. The Journal of craniofacial surgery. 2002;13(4):554–566. doi: 10.1097/00001665-200207000-00016. discussion 567. [DOI] [PubMed] [Google Scholar]
- Ceponiene R, Hukki J, Cheour M, Haapanen ML, Ranta R, Naatanen R. Cortical auditory dysfunction in children with oral clefts: relation with cleft type. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 1999;110(11):1921–1926. doi: 10.1016/s1388-2457(99)00152-2. [DOI] [PubMed] [Google Scholar]
- Chapman KL. The relationship between early reading skills and speech and language performance in young children with cleft lip and palate. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2011;48(3):301–311. doi: 10.1597/08-213. [DOI] [PubMed] [Google Scholar]
- Collett BR, Leroux B, Speltz ML. Language and early reading among children with orofacial clefts. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2010;47(3):284–292. doi: 10.1597/08-172.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett BR, Stott-Miller M, Kapp-Simon KA, Cunningham ML, Speltz ML. Reading in children with orofacial clefts versus controls. Journal of pediatric psychology. 2010;35(2):199–208. doi: 10.1093/jpepsy/jsp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. Connors' Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual. North Tonawanda, NY: Multi-Health Systems; 2000. [Google Scholar]
- Conrad AL, Richman L, Nopoulos P, Dailey S. Neuropsychological functioning in children with non-syndromic cleft of the lip and/or palate. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2009;15(5):471–484. doi: 10.1080/09297040802691120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- DeVolder I, Conrad AL, Richman L, Magnotta V, Nopoulos P. Naming deficits in children with isolated cleft lip and/or palate: Relationship to left inferior temporal lobe and temporal pole. 2013 Manuscript submitted for publication. [Google Scholar]
- First Years. (2004, September 2011). How to Read and Audiogram: Auditory Thresholds. Retrieved March 27, 2013, 2013, from http://www.firstyears.org/lib/howtoread.htm.
- Frank Y, Pavlakis SG. Brain imaging in neurobehavioral disorders. Pediatric neurology. 2001;25(4):278–287. doi: 10.1016/s0887-8994(01)00282-x. [DOI] [PubMed] [Google Scholar]
- Goldman R, Fristoe M. Goldman-Fristoe Test of Articulation. 2nd ed. Bloomington, MN: Pearson Assessments; 2000. [Google Scholar]
- Goldstein M, Brendel G, Tuescher O, Pan H, Epstein J, Beutel M, Silbersweig D. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. NeuroImage. 2007;36(3):1026–1040. doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Hentges F, Hill J, Bishop DV, Goodacre T, Moss T, Murray L. The effect of cleft lip on cognitive development in school-aged children: a paradigm for examining sensitive period effects. Journal of child psychology and psychiatry, and allied disciplines. 2011;52(6):704–712. doi: 10.1111/j.1469-7610.2011.02375.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. New Haven: Department of Sociology, Yale University; 1975. [Google Scholar]
- Hollingshead AB, Redlich FC. Social Class and Mental Illness. New York: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Fein D, Kramer JH, Delis D, Morris R. Weschler Intelligence Scale for Children, 3rd Edition - Process Instrument. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY: A Developmental Neuropsychological Assessment. Washington, DC: The Psychological Corp.; 1998. [Google Scholar]
- Lamb MM, Wilson FB, Leeper HA., Jr A comparison of selected cleft palate children and their siblings on the variables of intelligence, hearing loss, and visual-perceptual-motor abilities. Cleft Palate Journal. 1972;9:218–228. [PubMed] [Google Scholar]
- McDaniel MA. Estimating state IQ: Measurement challenges and preliminary correlates. Intelligence. 2006;34:607–619. [Google Scholar]
- Nopoulos P, Berg S, Canady J, Richman L, Van Demark D, Andreasen NC. Structural brain abnormalities in adult males with clefts of the lip and/or palate. Genetics in Medicine. 2002;4(1):1–9. doi: 10.1097/00125817-200201000-00001. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Berg S, VanDemark D, Richman L, Canady J, Andreasen NC. Cognitive dysfunction in adult males with non-syndromic clefts of the lip and/or palate. Neuropsychologia. 2002;40(12):2178–2184. doi: 10.1016/s0028-3932(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Choe I, Berg S, Van Demark D, Canady J, Richman L. Ventral frontal cortex morphology in adult males with isolated orofacial clefts: relationship to abnormalities in social function. Cleft Palate Craniofacial Journal. 2005;42(2):138–144. doi: 10.1597/03-112.1. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Langbehn DR, Canady J, Magnotta V, Richman L. Abnormal brain structure in children with isolated clefts of the lip or palate. Arch Pediatr Adolesc Med. 2007;161(8):753–758. doi: 10.1001/archpedi.161.8.753. [DOI] [PubMed] [Google Scholar]
- Ramus F. Developmental dyslexia: specific phonological deficit or general sensorimotor dysfunction? Current opinion in neurobiology. 2003;13(2):212–218. doi: 10.1016/s0959-4388(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ramus F, Pidgeon E, Frith U. The relationship between motor control and phonology in dyslexic children. Journal of child psychology and psychiatry, and allied disciplines. 2003;44(5):712–722. doi: 10.1111/1469-7610.00157. [DOI] [PubMed] [Google Scholar]
- Ramus F, Rosen S, Dakin SC, Day BL, Castellote JM, White S, Frith U. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain : a journal of neurology. 2003;126(Pt 4):841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Sullivan JR, Cohen MJ, editors. Neuropsychological Assessment and Intervention for Childhood and Adolescent Disorders. Hoboken, New Jersey: John Wiley & Sons, Inc.; 2010. [Google Scholar]
- Richman LC, Eliason MJ, Lindgren SD. Reading disability in children with clefts. Cleft Palate J. 1988;25(1):21–25. [PubMed] [Google Scholar]
- Richman LC, Ryan SM. Do the reading disabilities of children with cleft fit into current models of developmental dyslexia? Cleft Palate Craniofac J. 2003;40(2):154–157. doi: 10.1597/1545-1569_2003_040_0154_dtrdoc_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Richman LC, Wilgenbusch T, Hall T. Spontaneous verbal labeling: visual memory and reading ability in children with cleft. Cleft Palate Craniofac J. 2005;42(5):565–569. doi: 10.1597/04-128r.1. [DOI] [PubMed] [Google Scholar]
- Roberts RM, Mathias JL, Wheaton P. Cognitive functioning in children and adults with nonsyndromal cleft lip and/or palate: a meta-analysis. Journal of pediatric psychology. 2012;37(7):786–797. doi: 10.1093/jpepsy/jss052. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE. Overcoming Dyslexia: A new and complete science-based program for reading problems at any level. New York, NY: Alfred A. Knopf; 2003. [Google Scholar]
- Shaywitz SE, Shaywitz BA. Dyslexia (specific reading disability) Biological psychiatry. 2005;57(11):1301–1309. doi: 10.1016/j.biopsych.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Shriver AS, Canady J, Richman L, Andreasen NC, Nopoulos P. Structure and function of the superior temporal plane in adult males with cleft lip and palate: pathologic enlargement with no relationship to childhood hearing deficits. Journal of Child Psychology and Psychiatry. 2006;47(10):994–1002. doi: 10.1111/j.1469-7610.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Smith RM, McWilliams BJ. Psycholinguistic abilities of children with clefts. Cleft Palate Journal. 1968;5:238–249. [PubMed] [Google Scholar]
- Vickery KS, Reynolds VA, Cochran SW. Multisensory teaching appraoch for reading, spelling, and handwriting, Orton-Gillingham based curriculum, in a public school setting. Annals of Dyslexia. 1987;37:189–200. doi: 10.1007/BF02648066. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children, 3rd Edition, manual. Washington, DC: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale, 3rd Edition, manual. Washington, DC: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Weschler Memory Scale, 3rd Edition. San Antonio: The Psychological Corporation; 1997b. [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test, Administration Manual. Wilmington, Delaware: Wide Range, Inc.; 1993. [Google Scholar]
- Yang FF, McPherson B, Shu H, Xie N, Xiang K. Structural abnormalities of the central auditory pathway in infants with nonsyndromic cleft lip and/or palate. The Cleft palate-craniofacial journal : official publication of the American Cleft Palate-Craniofacial Association. 2012;49(2):137–145. doi: 10.1597/11-014. [DOI] [PubMed] [Google Scholar]


