The main side-effect of transfusion is alloimunization against red blood cell (RBC) antigens. Thirteen percent of the general population were shown to be responders and 30% of responders make antibodies indicating a rate of alloimmunization of 3.9%.1 However, alloimmunization is more frequent in sickle cell disease (SCD) patients2,3 and is associated with life-threatening complications.4–6 This is a major concern in transfusion medicine since transfusion is widely used to treat the complications of SCD and to prevent their occurrence.
To identify which SCD patients are at risk for RBC immunization, and which mismatch antigens are the most immunogenic, we investigated immunization in a cohort of 403 D-positive SCD transfused in the same center. The only inclusion criterion was a known history of transfusion in the center. Patient records were accessed in accordance with protocols approved by the local ethics board (Medical Ethics Committee, agreement n. 10-011). Patients were phenotyped as fully as possible prior to transfusion and were genotyped only when phenotype was not feasible. Transfusion-protocol was based on phenotypically-matched leuko-reduced RBC units for ABO, Rh and Kell. Once a patient developed a clinically significant antibody, matching is extended to the antigen against which the antibody was produced. Patients received 1–940 RBC units (total 41,349) and 170 of 403 patients (42.1%) were immunized with 1–10 antibodies (total 460). Immunization was defined as the presence of reactivity detected by the screening test either in the patient’s history or on the day of inclusion, including antibodies of undetermined specificity because such antibodies: i) are frequently related to antibodies against low-prevalence antigens; and ii) may be important allo-antibodies in development.7 Since the antibody count was weakly dependent on the number of transfusion (Spearman r=0.2856), we assessed whether SCD patients had a responder phenotype by applying the procedure developed by Higgins and Sloan.1 The distribution of the numbers of patients producing different numbers of antibodies was geometric; the frequency of producing an additional antibody was 61.0%, and 69% of the SCD patients were responders (Figure 1). This model was strengthened using an independent validation set of SCD patients (n=198) undergoing transfusion under the same protocol. Among the 460 antibodies detected, 33.5% were directed against Rh antigens (154 antibodies in 93 patients). Seventy of 154 anti-Rh antibodies were developed in patients negative for the corresponding antigen; this was unexpected in view of the routine practice of Rh/Kell-matched RBCs for transfusion in France. Similar observations were also reported by Chou et al.8 with a similar rate for unexplained anti-Rh. Other anti-Rh antibodies (n=84) were found in patients with a positive phenotype for the corresponding antigen and could be linked to expression of partial-Rh phenotype.9,10 Thus, to determine the contribution of Rh variants to alloimmunization, we characterized the RHD and RHCE variant alleles in patients (Online Supplementary Appendix). Based on the presence of homozygosity or compound heterozygosity of partial allele(s), we deduced that 34 (8.4%) of the 403 patients of our population carried a partial-D phenotype; 21 (20.8%) of 101 C-positive patients were predicted to express a partial-C phenotype; 14 (3.5%) of 400 could be considered partial-e (Table 1). The occurrence of anti-D was more prevalent in partial-D (17.6%) than in wild-type (3.4%) individuals. Similarly, anti-C was more common in partial than non-partial-C individuals. These differences were not statistically significant, but this was probably due to the small numbers of patients with the various partial phenotypes. Unexpectedly, anti-e was the most frequent anti-Rh antibodies observed, although only 3.5% of patients had a predicted partial-e phenotype. Moreover, anti-e was equally prevalent in partial individuals and non-partial individuals with or without altered RHCE alleles suggesting that they cannot always be explained by RHCE diversity and therefore these antibodies may be auto-antibodies. In view of the high rate of anti-e (approx. 10% of all antibodies), and also the rarity of anti-e alone (only one case among 42), it is unclear whether the development of anti-e could be a marker of high-responder status. A prospective study addressing this point would be useful. Overall, among 84 anti-Rh antibodies developed by patients positive for the corresponding antigen, only 10 could be considered to be allo-antibodies, based on the deduced partial phenotype. No correlation between RH alleles or haplotypes and immunization could be established in our study because of the small numbers of individuals with identical genotypes; large multi-center studies are required to provide more rigorous data concerning this issue.
Figure 1.
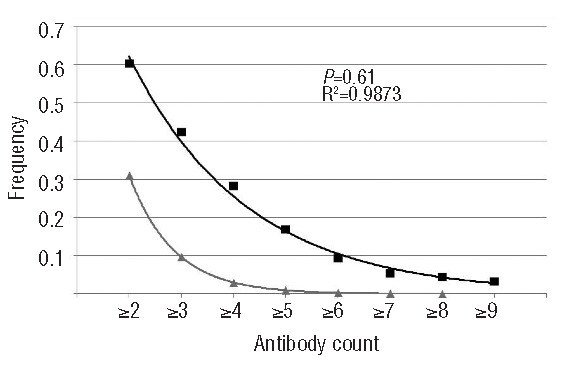
Stochastic model of RBC immunization in SCD patients. Frequencies of patients with different numbers of antibodies. The frequency in our SCD patient population is shown in black. Expected frequency according to the Sloan and Higgins model is shown in gray.1
Table 1.
Anti-RH immunization in SCD patients, consequences of RH alleles for anti-Rh immunization and comparison with anti-Jkb and anti-S immunization.
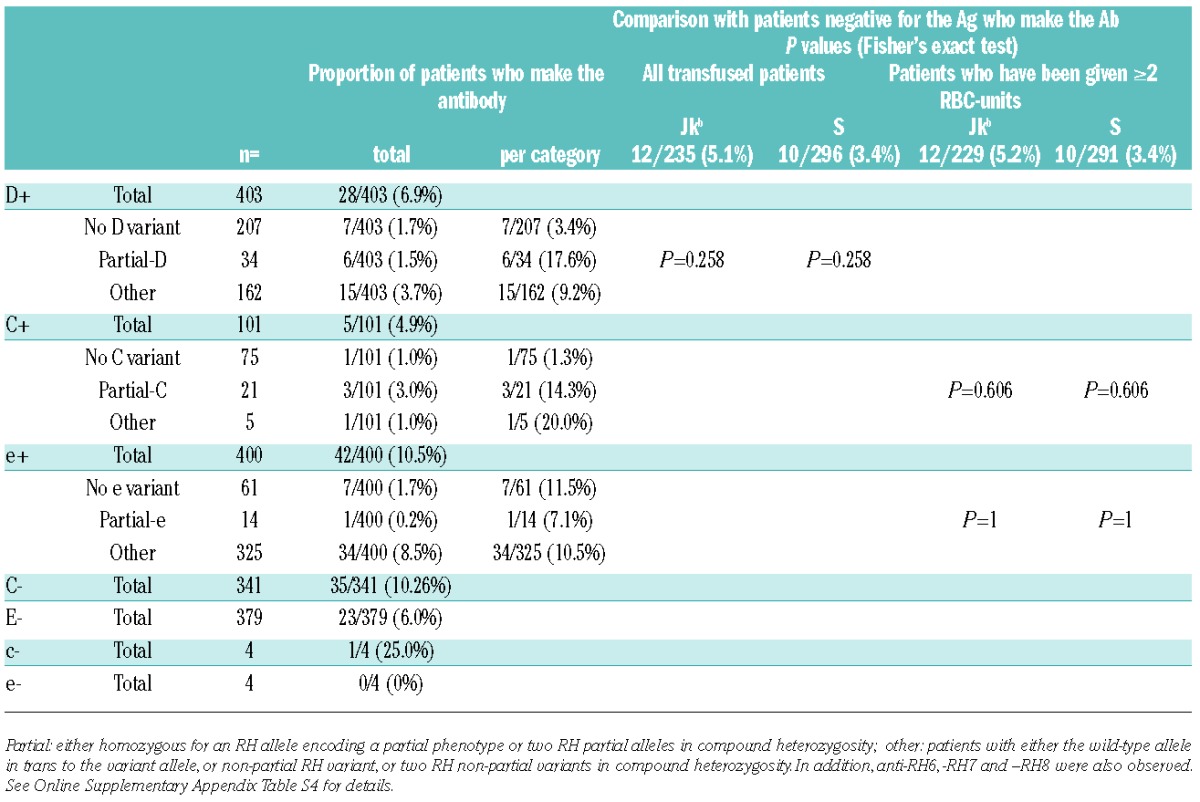
Considering that partial-D, Jkb-negative and S-negative patients are exposed at similar frequencies to the corresponding immunogenic antigens, whereas partial-C and -e patients were exposed twice as frequently as Jkb-negative and S-negative patients (data not shown). We compared the risk of a partial-Rh patient producing allo-anti-Rh antibodies when exposed to the complete antigen with those of Jkb negative and S-negative patients receiving Jkb-positive and S-positive RBC units. The risk of producing the antibody is higher in partial-D and partial-C situations than the risk of producing antibody against a common antigen (Jkb and S) suggesting that primary prevention targeting Rh variants would be beneficial. However, various other issues have to be taken into account: i) all antibodies related to partial-Rh antigens represent only 2.2% of the total number of antibodies produced (10 of 460) and primary prevention targeting Rh variants would only slightly reduce the immunization rate according to our findings; ii) the clinical significance of these antibodies has not been demonstrated; and iii) systematic prevention of anti-D in partial-D would require the use of already scarce resources and would also increase exposure to Fya, Jkb and S, because D-negative RBC are more frequent in the Caucasian population.11 Thus, real efforts are needed to promote donation in Afro-Caribbean donors, and to keep fully phenotyped units available for immunized patients.
This study shows that responder SCD patients are at a 61% increased risk of producing additional antibodies. Interestingly, anti-e was the most prevalent antibody independent of the e variant status of the patients. The partial-D and -C phenotypes seem to be more immunogenic than Jkb and S mismatches but account for only 2% of alloimmunization. This suggests that it may be beneficial to extend matching to the MNS, JK and FY blood groups and the variant profile as soon as the first antibody appears, including antibodies of undetermined specificity. A prospective international trial would be of great value in order to determine whether deeper Rh typing could reduce allo- and auto-antibody formation in SCD patients.
Acknowledgments
We acknowledge the contributions of Thomas Granier, Beley Sophie, and Kevin Gaillard for their expert technical assistance, Isabelle Dettori for providing information about the SCD cohort from EFS-Alpes Méditerranée, and the biologists of EFS IdF.
Footnotes
Funding: this study was funded by Établissement Français du Sang, France, (all co-authors, APR-2010-12) and partly by the ANR (SCD-TRANSFU-2011–2013, EFS Ile de France, UPEC, INSERMU955).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders. Blood. 2008;112(6):2546–53 [DOI] [PubMed] [Google Scholar]
- 2.Bashmri LA. Red cell alloimmunization in sickle-cell anaemia patients. East Mediterr Health J. 2007;13(5):1181–9 [DOI] [PubMed] [Google Scholar]
- 3.Winkler AM, Josephson CD. Transfusion practices for patients with sickle cell disease at major academic medical centers participating in the Atlanta Sickle Cell Consortium. Immunohematology. 2012;28(1):24–6 [PubMed] [Google Scholar]
- 4.Petz LD, Calhoun L, Shulman IA, Johnson C, Herron RM. The sickle cell hemolytic transfusion reaction syndrome. Transfusion. 1997;37(4):382–92 [DOI] [PubMed] [Google Scholar]
- 5.Scheunemann LP, Ataga KI. Delayed hemolytic transfusion reaction in sickle cell disease. Am J Med Sci. 2010;339(3):266–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120(3):528–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Grossman BJ. Antibody of undetermined specificity: frequency, laboratory features, and natural history. Transfusion. 2013;53(5):931–8 [DOI] [PubMed] [Google Scholar]
- 8.Chou ST, Jackson T, Vege S, Smith-Whitley K, Friedman DF, Westhoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122(6):1062–71 [DOI] [PubMed] [Google Scholar]
- 9.Noizat-Pirenne F, Lee K, Pennec PY, Simon P, Kazup P, Bachir D, et al. Rare RHCE phenotypes in black individuals of Afro-Caribbean origin: identification and transfusion safety. Blood. 2002;100(12): 4223–31 [DOI] [PubMed] [Google Scholar]
- 10.Silvy M, Di Cristofaro J, Beley S, Papa K, Rits M, Chiaroni J, et al. Identification of RHCE and KEL alleles in large cohorts of Afro-Caribbean and Comorian donors by multiplex SNaPshot and fragment assays: a transfusion support for sickle cell disease patients. Br J Haematol. 2011;154(2):260–70 [DOI] [PubMed] [Google Scholar]
- 11.Badjie KS, Tauscher CD, van Buskirk CM, Wong C, Jenkins SM, Smith CY, et al. Red blood cell phenotype matching for various ethnic groups. Immunohematology. 2011;27(1):12–9 [PubMed] [Google Scholar]


