Abstract
Heptaprenyl diphosphate (C35-PP) is an isoprenoid intermediate in the synthesis of both menaquinone and the sesquarterpenoids. We demonstrate that inactivation of ytpB, encoding a C35-PP utilizing enzyme required for sesquarterpenoid synthesis, leads to an increased sensitivity to bacitracin, an antibiotic that binds undecaprenyl pyrophosphate (C55-PP), a key intermediate in cell wall synthesis. Genetic studies indicate that bacitracin sensitivity is due to accumulation of C35-PP, rather than the absence of sesquarterpenoids. Sensitivity is accentuated in a ytpB menA double mutant, lacking both known C35-PP consuming enzymes, and in a ytpB strain overexpressing the HepST enzyme that synthesizes C35-PP. Conversely, sensitivity in the ytpB background is suppressed by mutation of hepST or by supplementation with 1,4-dihydroxy-2-naphthoate, a co-substrate with C35-PP for MenA. Bacitracin sensitivity results from impairment of the BceAB and BcrC resistance mechanisms by C35-PP: in a bceAB bcrC double mutant disruption of ytpB no longer increases bacitracin sensitivity. These results suggest that C35-PP inhibits both BcrC (a C55-PP phosphatase) and BceAB (an ABC transporter that confers bacitracin resistance). These findings lead to a model in which BceAB protects against bacitracin by transfer of the target, C55-PP, rather than the antibiotic across the membrane.
Keywords: Bacillus subtilis, bacitracin, undecaprenyl pyrophosphate, antibiotic resistance, peptidoglycan
Introduction
Bacillus subtilis serves as a model system for investigating antibiotic resistance mechanisms in Gram-positive bacteria. This organism inhabits the soil, a highly competitive environment where it encounters producers of all types of antibiotics. As a consequence, B. subtilis has evolved numerous systems to respond to, and defend against, antibiotics (Jordan et al., 2008). The characterization of these systems is pertinent because many of them are homologous to clinically relevant resistance mechanisms in human pathogens (Wright, 2007).
Bacitracin is a cyclic lipopeptide produced by Bacillus licheniformis spp. that inhibits cell wall synthesis by binding to undecaprenyl pyrophosphate (UPP, C55-PP) (Stone & Strominger, 1971). C55-PP is an essential precursor for the lipid I and lipid II species needed for bacterial cell wall biosynthesis, both of which contain C55-P lipid anchors (Valvano, 2008). C55-P also serves as a lipid anchor for the synthesis of wall teichoic acids and, in Gram-negative bacteria, lipopolysaccharide (Tatar et al., 2007). The transglycosylase step of peptidoglycan synthesis releases C55-PP as a product which must be efficiently recycled to sustain continued cell wall synthesis (Sanyal & Menon, 2009). C55-PP is flipped to the cytosolic face of the membrane and dephosphorylated by a cytosolic UPP phosphatase (UppP) to generate undecaprenyl phosphate (UP, C55-P). Dephosphorylation may also occur on the outer face of the membrane, prior to translocation, by the action of the BcrC phosphatase (Bernard et al., 2005). The identity of the translocase responsible for transfer of the C55-P(P) across the membrane is not yet clear.
Bacitracin inhibits cell wall synthesis by binding tightly, together with a divalent metal ion (usually zinc), to C55-PP on the outer leaflet of the plasma membrane (Ming & Epperson, 2002). Structural analysis reveals that bacitracin completely envelops the pyrophosphate group which prevents phosphatases from accessing the compound and converting it to C55-P (Economou et al., 2013) and is also presumed to prevent membrane translocation. The diminished recycling of C55-P restricts synthesis of peptidoglycan and other cell envelope components that require C55-P as a membrane anchor.
Bacitracin is one of the most commonly used antibiotics worldwide as a frequent component in both livestock feed (Sarmah et al., 2006) and topical ointments (Drucker, 2012). The bacitracin scaffold is also an appealing candidate for the structure-based design of new therapeutic agents that overcome bacitracin’s existing limitations (Economou et al., 2013) (Wagner et al., 2006). Thus, a complete understanding of how bacteria resist this antimicrobial compound is of vital importance.
In B. subtilis, the majority of intrinsic bacitracin resistance is conferred by the BceRS two component system which regulates the ABC transporter BceAB (Bernard et al., 2003, Mascher et al., 2003, Ohki et al., 2003). BceRS is strongly induced by even sub-inhibitory levels of bacitracin (Rietkötter et al., 2008), and, remarkably, absolutely requires BceAB for bacitracin sensing (Bernard et al., 2007). Disrupting either BceR or BceAB increases bacitracin sensitivity 50–100 fold (Mascher et al., 2003, Ohki et al., 2003). In B. subtilis, three operons encode ABC transporters closely linked to two-component systems, and in each case the transporter complex is required for sensing of peptide antibiotics (Staron et al., 2011). A similar relationship has been shown in Staphylococcus aureus for systems that sense cationic antimicrobial peptides including bacitracin and nisin (Hiron et al., 2011, Falord et al., 2012). Indeed, genomic analyses reveal that this functional coupling of transporter and regulatory proteins is widespread in the Firmicutes (Revilla-Guarinos et al., 2014), with overall 250 examples noted (Dintner et al., 2011). Recent results suggest that the sensing and transport functions of BceAB can be separated by mutations affecting BceB (Kallenberg et al., 2013).
Despite its effectiveness, the exact function of BceAB has long been puzzling (Rietkötter et al., 2008). Since most ABC transporters involved in antibiotic resistance pump toxic compounds out of the cell, it was initially suggested that BceAB acts as a bacitracin efflux pump (Podlesek et al., 1995). However, the target of bacitracin (C55-PP) is on the external face of the membrane, so exporting bacitracin would not be expected to provide resistance. Although the BceAB-BceRS bacitracin resistance module has been well studied, the mechanism by which BceAB-BceRS senses bacitracin remains unclear (Revilla-Guarinos et al., 2014).
The only other known bacitracin resistance determinant in B. subtilis is BcrC, a phosphatase that degrades C55-PP to C55-P on the extracellular face of the membrane so it is no longer recognized by bacitracin (Bernard et al., 2003, Cao & Helmann, 2002, Bernard et al., 2005). The σM-dependent bcrC gene exhibits a moderate level of basal activity and can be upregulated 2–4 fold by bacitracin levels high enough to impair peptidoglycan synthesis and induce a σECF response (Eiamphungporn & Helmann, 2008). Cells without a functional copy of bcrC or σM exhibit a 4–8 fold increase in bacitracin sensitivity (Cao & Helmann, 2002). It is generally presumed that C55-PP, released during transglycosylation, is flipped to the internal face of the membrane where it can be dephosphorylated to C55-P to serve as substrate for lipid I synthesis. BcrC provides an alternative pathway in which C55-PP can be dephosphorylated on the outer leaflet of the membrane, with the resultant C55-P flipped to the inner leaflet. These two pathways are likely to be functionally redundant, as previously reported in Escherichia coli where the cytosolic activity is due to BacA/UppP and there are multiple proteins that act on the external face of the membrane (Bickford & Nick, 2013). In B. subtilis, the corresponding phosphatases appear to be BcrC (external leaflet) and YubB (a BacA ortholog) in the inner leaflet (Bickford & Nick, 2013, Inaoka & Ochi, 2012). The flippase(s) involved in C55-P or C55-PP translocation across the membrane have yet to be identified, and only recently have the corresponding lipid II flippases (FtsW and RodA) involved in export of C55-P linked cell wall precursors been characterized (Szwedziak & Lowe, 2013).
Here, we report that a B. subtilis 168 (trpC2) strain lacking the tetraprenyl-β-curcumene synthase YtpB exhibits a bacitracin sensitive phenotype. We show that this phenotype arises because the substrate heptaprenyl diphosphate (C35-PP) accumulates in the absence of YtpB. Our analyses lead us to propose that C35-PP acts as a molecular mimic of C55-PP and antagonizes both BceAB and BcrC. By extension, we postulate that BceAB may function by transfer of the bacitracin target C55-PP from the external to the internal leaflet of the inner membrane, where it is no longer susceptible to binding by bacitracin. Thus, antibiotic resistance in this system may arise from transmembrane relocation (flipping) of the target of the antibiotic, rather than transfer of the antibiotic itself.
Results and Discussion
Antibiotic susceptibility of null mutants of the σM-regulated ytpA and ytpB genes
The ytpAB operon is activated as part of the σM stress response (Eiamphungporn & Helmann, 2008). Upregulation of σM is elicited by compounds that inhibit peptidoglycan biosynthesis, and many σM regulated genes are involved in cell wall homeostasis (Eiamphungporn & Helmann, 2008). A common feature of the many conditions that induce the σM regulon is interference with lipid II cycling (the bactoprenol cycle). These conditions include antibiotics that bind lipid II or C55-PP (Cao et al., 2002, Eiamphungporn & Helmann, 2008, Mascher et al., 2003), a reduction in the level of UppS (Lee & Helmann, 2013), and depletion of bactoprenol by the action of the CsbB glycosyltransferase (Inoue et al., 2013).
Here, we initiated a genetic and physiological analysis to determine if the antibiotic-mediated induction of YtpA and YtpB provided protection against one or more antibiotics. YtpA is a phospholipase annotated as a bacilysocin synthase. YtpA cleaves fatty acids from the 2 position of phosphatidylglycerol (PhG) (Tamehiro et al., 2002). The resulting product is similar to lysophosphatidic acid, an intermediate in de novo synthesis, except that it carries a glycerol headgroup. The function of PhG de-acylation is unknown, but it may be involved in membrane remodeling. Deacylated lipids may be re-acylated using newly synthesized fatty acids, possibly by YtpA itself in a transesterification reaction. Alternatively, PlsC (1-acylglycerol-phosphate acyltransferase), which normally uses acyl-ACPs and lysophosphatidic acid (Yao & Rock, 2013), might re-acylate lysoPhG, the product of the YtpA reaction. We therefore speculated that YtpA might be involved in membrane remodeling, possibly as a mechanism to protect against membrane-disrupting compounds.
YtpB catalyzes the first committed step in C35 terpenoid biosynthesis (Fig. 1) (Sato, 2013, Sato et al., 2011). The first C35 terpenes identified in the Bacilli were isolated from spore preparations and designated as sporulenes (Kontnik et al., 2008, Bosak et al., 2008). However, subsequent studies indicate that sporulenes are non-enzymatic products resulting from oxidation and thermal dehydration of a precursor compound during spore preparation (Takigawa et al., 2010). This precursor (Fig. 1) has recently been designated as baciterpenol A (Sato, 2013). The roles of C35 terpenoids (designated as sesquarterpenes; Sato et al., 2011) are largely unexplored, although a minor role in H2O2 resistance was reported (Bosak et al., 2008). Recent evidence indicates that C35terpenes are present in growing cells, and only trace amounts were detected in the spores of B. subtilis KSM 6–10 (Takigawa et al., 2010). This observation, together with the up-regulation of ytpB in response to antibiotic stress (Eiamphungporn & Helmann, 2008), suggests that C35 terpenes may function in a cell stress responsive pathway to modulate the properties of the membrane.
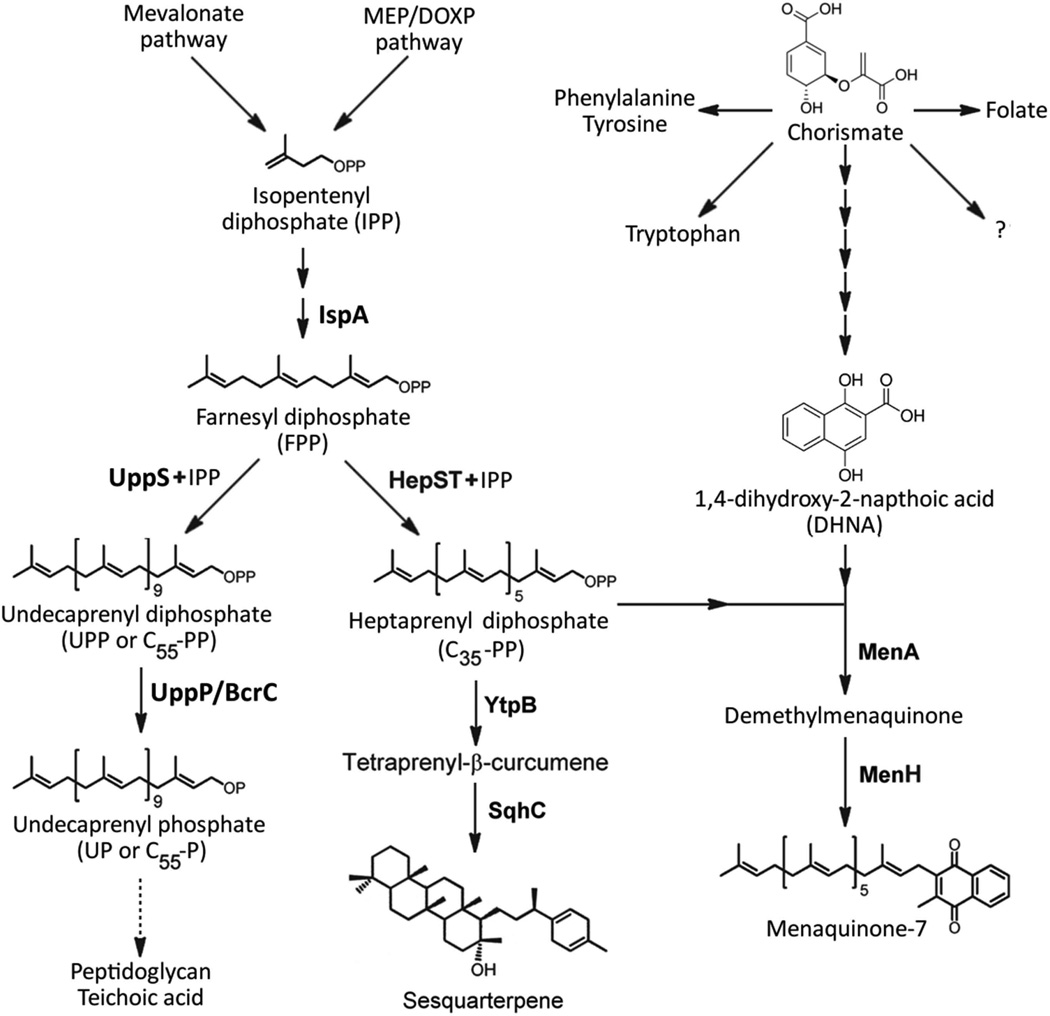
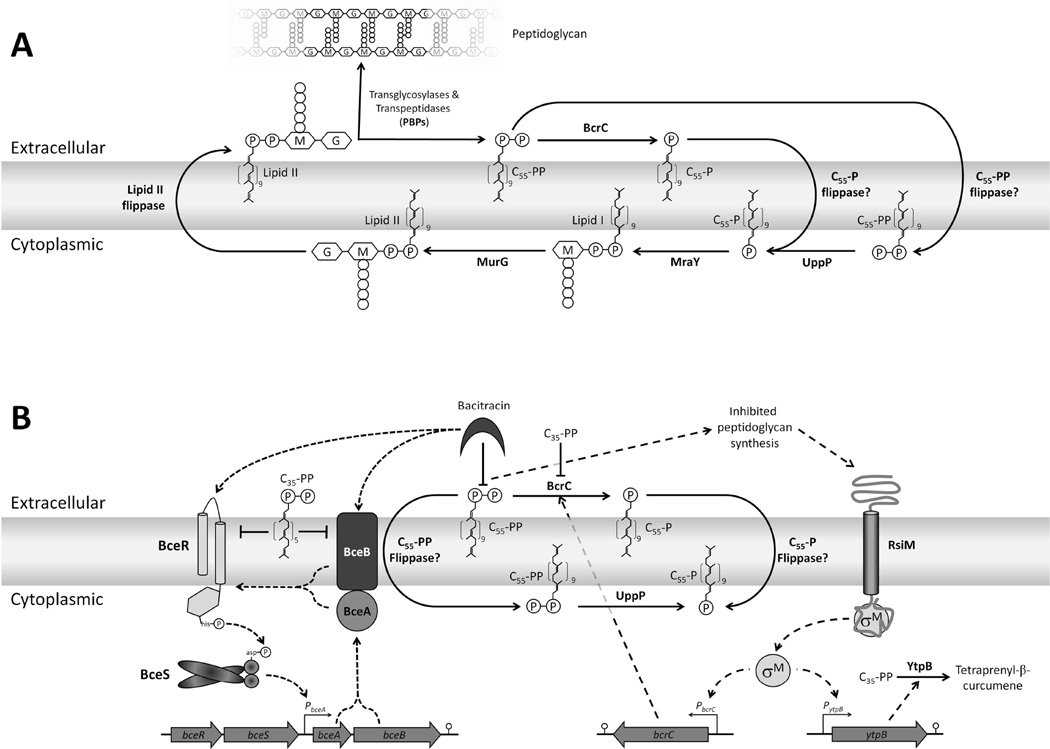
Figure 1.
Pathways of isoprenoid biosynthesis and utilization in Bacillus subtilis. Isopentenyl diphosphate is synthesized via the methylerythritol phosphate (MEP) pathway (Wagner et al., 2000). UppS adds eight consecutive isopentenyl diphosphate groups to the C15 farnesyl diphosphate (FPP) to generate undecaprenyl pyrophosphate (UPP=C55-PP), a key intermediate in the synthesis of cell wall components (Teng & Liang, 2012). The heterodimeric enzyme HepST also functions as a prenyltransferase and adds four isoprenyl units in head-to-tail condensation with FPP to generate the C35 intermediate, heptaprenyl diphosphate (C35-PP) (Zhang et al., 1998). The C35-PP intermediate is a substrate for YtpB, catalyzing the committed step in the synthesis of sesquarterpenes (Sato et al., 2011). The sesquarterpene shown has recently been named baciterpenol A (Sato, 2013). C35-PP is also a substrate for MenA, a central enzyme of the menaquinone-7 biosynthesis pathway (Suvarna et al., 1998). Depending on the growth conditions, MenA activity can be limited by the availability of the co-substrate 1,4-dihydroxy-2-naphthoic acid (DHNA) (see text for details), a metabolite derived from the central branch point intermediate chorismate.
To investigate the possible roles of the σM-activated ytpA and ytpB genes in membrane remodeling and antibiotic resistance, we generated null mutants in both genes and screened the disruptants for changes in antibiotic sensitivity. The ΔytpA null strain did not exhibit any major sensitivity phenotypes (data not shown), but we observed a striking bacitracin sensitivity phenotype in the ytpB null mutant. As described below, this phenotype results in part from the serendipitous presence of the trpC2 mutation in the B. subtilis 168 strain. In this study we focus on this property of the ytpB null mutant and report genetic and physiological studies that address the mechanistic basis of this effect.
Bacitracin is bacteriolytic for a ΔytpB strain
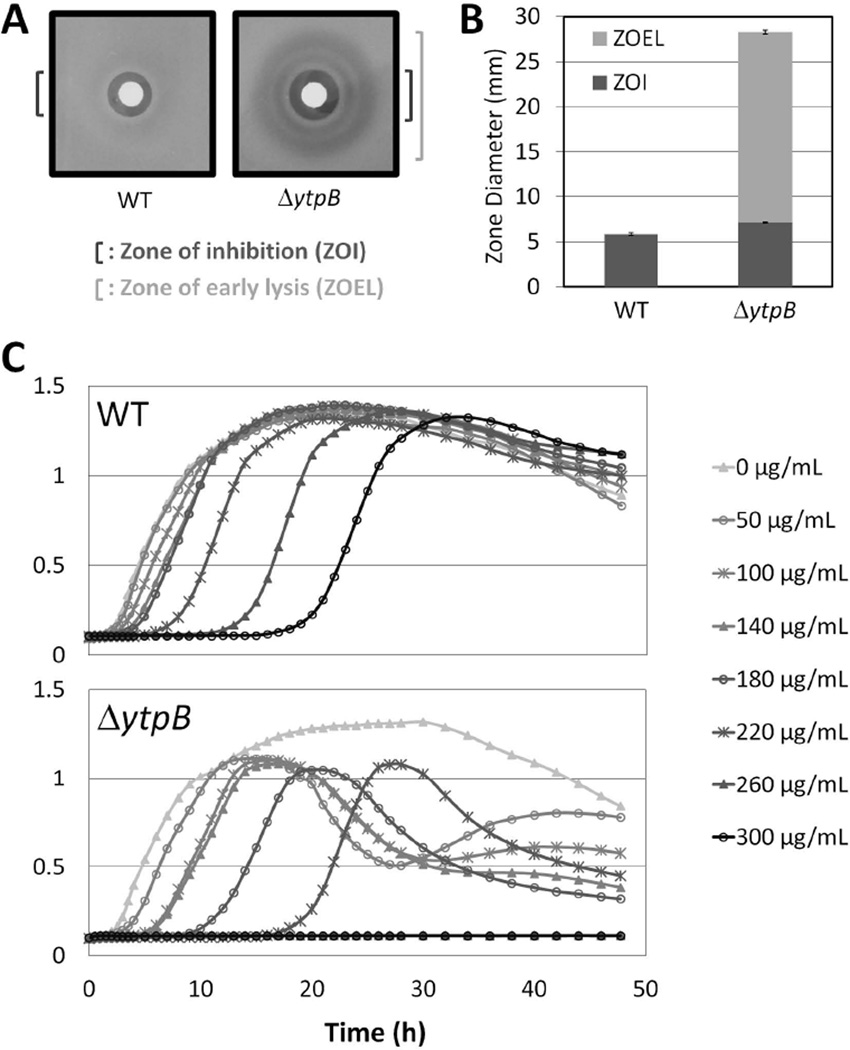
In a disk diffusion assay, wild-type (WT) cells of B. subtilis 168 (trpC2) exposed to bacitracin exhibit a clear zone of growth inhibition (Fig. 2A & B). In the ΔytpB strain, this clear zone is only slightly larger than that of WT cells, but a large halo of diminished cell density can be observed around the disk. To further investigate this sensitivity phenotype, we analyzed the effects of bacitracin on cell growth in liquid culture. We used bacitracin concentrations ranging from 50 µg ml−1, which had little effect on WT cell growth, up to 300 µg ml−1, a concentration able to increase the lag phase of WT cells to over 20 hours. This assay revealed several notable differences between WT and ΔytpB cells (Fig. 2C). The ΔytpB strain exhibited a moderately lower MIC than the WT strain (190±10 µg ml−1 vs. 250±13 µg ml−1), and its lag phase was longer than that of the corresponding WT culture for each bacitracin concentration tested. Intriguingly, even relatively low levels of bacitracin caused ΔytpB cultures to lyse shortly after reaching stationary phase (a reduction in OD600 of ~50% over ~10 hours). We speculated that this stationary phase bacteriolytic effect seen in liquid cultures might account for the large halo of reduced cell density observed in disk diffusion assays. Indeed, visual inspection of disk diffusion assays as a function of time confirmed that the large clear zone seen with the ΔytpB strain resulted from cells that initially grew to moderate density and then lysed on plates. Thus, we labeled this halo as a zone of early lysis.
Figure 2.
Bacitracin sensitivity of the ΔytpB strain. (A) Sample image of a bacitracin disk diffusion assay with WT (168) and ΔytpB (HB13321) cells on MH medium. The zone of inhibition (ZOI) and the larger zone of early lysis (ZOEL) are designated by dark and light brackets, respectively. (B) Graphical depiction of the disk diffusion assay from panel A. For this and subsequent graphs, the y-axis shows the ZOI, expressed as total diameter (in millimeters) minus the diameter of the filter paper disk (5.5 mm) with the ZOEL depicted in lighter shade above the ZOI when it was present. Each bar represents the average from at least three assays performed with three independent clones of each strain, with error bars representing standard error. Statistically significant differences in zone of inhibition were determined with a Student's t-test (P < 0.05). (C) Representative growth curve assay of WT (168) and ΔytpB (HB13321) cells in the presence of bacitracin at the concentrations indicated.
The bacitracin sensitivity phenotype of the ΔytpB strain can be fully complemented by the ectopic expression of the ytpB gene. Introduction of an IPTG-inducible copy of ytpB (Pspac(hy)- ytpB) into a ΔytpB background restored bacitracin resistance to the wild-type level (Fig. S1A). This complementation was observed in the absence of IPTG, indicating that even the low levels of YtpB produced by the uninduced Pspac(hy) promoter are sufficient to restore bacitracin resistance.
The effect of the ytpB disruptant was specific for bacitracin since there was little if any change in sensitivity to detergents (Triton X-100, bile salts, SDS, amitriptyline, dodecyltrimethylammonium bromide, N-lauryl-sarcosine, EDTA, and poly-L-lysine), other cell wall synthesis inhibitors (moenomycin, vancomycin, D-cycloserine, and cefuroxime), membrane active agents (daptomycin, polymyxin B, colistin, and clofazimine), a fatty acid synthesis inhibitor (cerulenin), or a DNA gyrase inhibitor (novobiocin) (Fig. S1B).
The effect of YtpB on bacitracin sensitivity is due to the accumulation of C35-PP rather than the lack of sesquarterpenoids
Since ytpB is essential for the synthesis of sesquarterpenes, we initially hypothesized that the lack of sesquarterpenes resulted in bacitracin sensitivity. However, blocking the subsequent step of the sesquarterpenoid biosynthetic pathway by deleting sqhC (Fig. 1) did not sensitize cells to bacitracin (Fig. 3A). We therefore concluded that the step catalyzed by YtpB is critical to bacitracin resistance, rather than the final product of this pathway. Thus, bacitracin resistance is linked either to the failure of the ΔytpB cells to synthesize tetraprenyl-β-curcumene or to an accumulation of the substrate, C35-PP. Since C35-PP is similar in structure to the target of bacitracin, C55-PP, we hypothesized that it was accumulation of C35-PP that was responsible for the effects on bacitracin resistance.
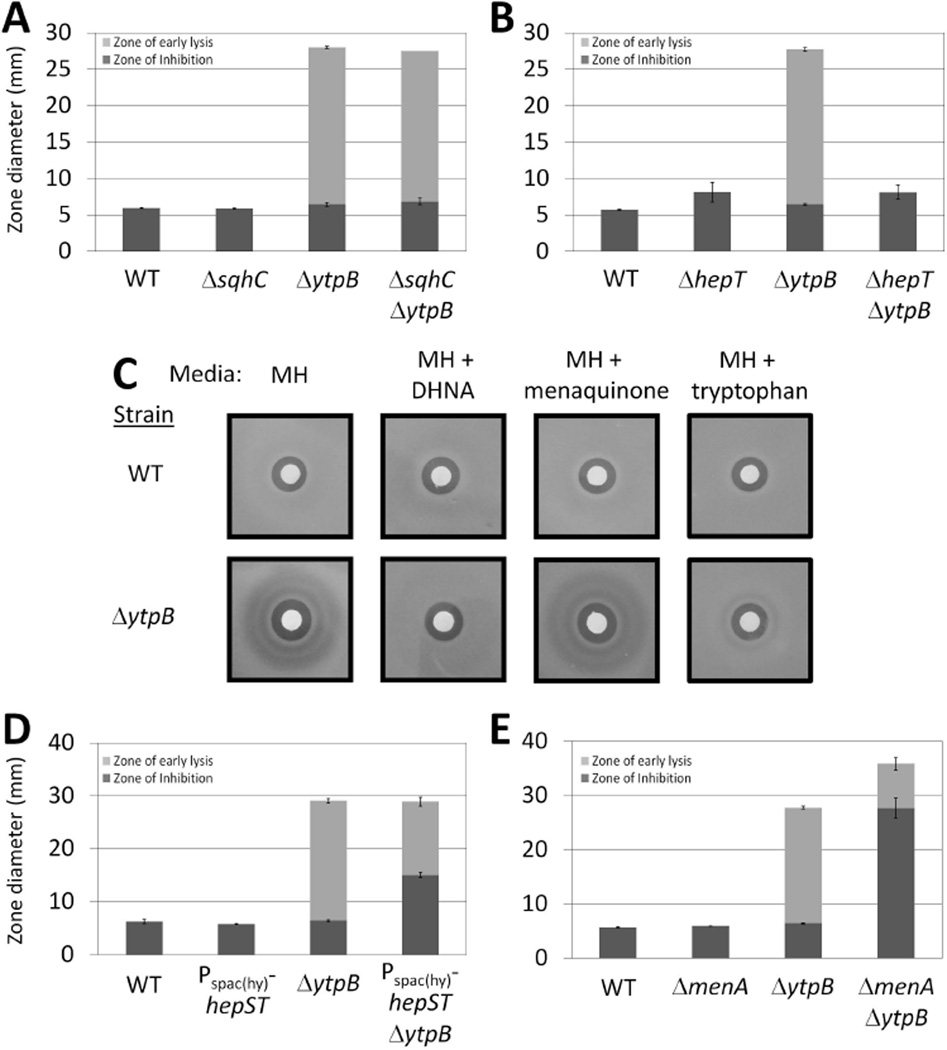
Figure 3.
C35-PP accumulation increases bacitracin sensitivity. Bacitracin disk diffusion assays of (A) WT (168), ΔsqhC (HB13358), ΔytpB (HB13321), and ΔsqhC ΔytpB (HB13360) strains on MH medium; (B) WT (168), ΔhepT (HB13447), ΔytpB (HB13321), and ΔhepT ΔytpB (HB13448) strains on MH media containing 10 µg ml−1 menaquinone; (C) WT (168) and ΔytpB (HB13321) strains on MH containing either no supplement, 100 µg ml−1 tryptophan, 10 µg ml−1 1,4-dihydroxy-2-naphthoic acid (DHNA), or 10 µg ml−1 menaquinone; (D) WT (168), Pspac(hy)-hepSmenHhepT (HB13398), ΔytpB (HB13321), and Pspac(hy)-hepSmenHhepT ΔytpB (HB13402) strains on MH medium containing 1 mM IPTG; and (E) WT (168), ΔmenA (HB13438), ΔytpB (HB13321), and ΔmenA ΔytpB (HB13439) strains on MH media containing 10 µg ml−1 menaquinone.
To test this hypothesis, we first showed that mutations and growth conditions expected to reduce C35-PP levels were able to alleviate the bacitracin sensitivity phenotype in ΔytpB cells. Our attempts to influence available C35-PP levels in the cell were informed by the metabolic pathways described in Fig. 1. First, we tested the effect of deleting hepT, one of the genes required for the synthesis of the C35-PP intermediate, on bacitracin sensitivity. Although the ΔhepT mutation caused a slight increase in the bacitracin zone of inhibition, the ΔhepT ΔytpB double mutant did not exhibit a zone of early lysis (Fig. 3B). This confirms that C35-PP is needed for the sensitivity phenotype to arise.
Next, we sought to increase the conversion of C35-PP into menaquinone with an IPTG inducible copy of menA. However, overexpression of menA did not affect bacitracin sensitivity in the ytpB null background (Fig. S2). However, it was possible that menA overexpression did not influence the rate of this reaction because of limited availability of 1,4-dihydroxy-2-naphthoic acid (DHNA) (Fig. 1). Indeed, supplementation of the growth medium with DHNA completely suppressed the bacitracin sensitivity phenotype of the ΔytpB strain even in the absence of MenA overexpression (Fig. 3C). Moreover, supplementing with menaquinone (MK-4) did not alleviate the bacitracin sensitivity phenotype, consistent with the hypothesis that the effect of DHNA addition on bacitracin sensitivity was due to consumption of C35-PP rather than increased synthesis of menaquinone. These studies confirm that conditions or mutations that decrease C35-PP accumulation prevent the bacteriolytic effects of bacitracin.
The bacteriolytic effect of bacitracin is associated with genetic or physiological conditions that block C35-PP utilization
In a second set of studies, we investigated the consequences of changes that increase C35-PP accumulation. Overexpressing the C35-PP synthesis genes hepST had no effect on bacitracin sensitivity in a WT background, but significantly increased the bacitracin zone of inhibition in a ΔytpB background (Fig. 3D). Similarly, a ΔmenA mutation did not influence bacitracin sensitivity by itself, but caused an increased bacitracin sensitive phenotype when combined with ΔytpB (Fig. 3E). Together, these results indicate that the bacitracin sensitivity phenotype of the ΔytpB strain can be exacerbated by mutations that further increase the amount of C35-PP in the cell.
In the course of these studies we noted that the ability to observe the zone of early lysis seemed to depend on the growth medium: it is reproducibly observed on Mueller-Hinton (MH) medium, but was never seen on LB medium. Further investigation revealed that the higher Trp content of LB medium was responsible for this difference. Since DHNA was able to suppress early lysis, we sought a link between the Trp content of the growth medium and the ability of cells to synthesize DHNA. DHNA derives from the central intermediate chorismate (Fig. 1), which is also used to synthesize a number of essential compounds including Trp. When Trp is depleted from the growth medium there is derepression of Trp biosynthesis enzymes, as well as cotranscribed enzymes for folate biosynthesis. The expression of Trp biosynthesis enzymes is normally feedback regulated at the translational level by the TRAP protein, which senses available Trp, and by transcriptional attenuation, in response to charged tRNATrp (Gollnick et al., 2005). In addition, the flux of chorismate into the Trp biosynthesis pathway is normally feedback inhibited by the binding of Trp to the first enzyme, anthranilate synthase (Nester & Jensen, 1966). Because our B. subtilis 168 lab strains are Trp auxotrophs (trpC2) blocked in the penultimate step of the biosynthetic pathway, we speculate that depletion of Trp during growth on MH medium leads to derepression of Trp biosynthesis enzymes and a failure of feedback inhibition which, in combination, leads to a high demand for chorismate. As a result, there is a limiting amount of chorismate available for synthesizing DHNA, the co-substrate (with C35-PP) for MenA.
In agreement with this model, we found that supplementing the growth medium with Trp eliminated the zone of early lysis due to bacitracin in the ΔytpB trpC2 strain (Fig. 3C), while supplementing the media with Phe or other amino acids failed to suppress this phenotype (data not shown). Similarly, converting our trpC2 strains to trp prototrophy also suppressed the zone of early lysis noted in strains carrying the ΔytpB mutation (Fig. 4B vs. 4A). These results are consistent with the hypothesis that the early lysis phenotype of bacitracin-treated cells is due to accumulation of C35-PP, which in turn results from (i) a lack of the C35-PP utilizing enzyme YtpB and (ii) low activity of the C35-PP utilizing enzyme MenA because of limitation for the DHNA cosubstrate, likely triggered by depletion of the DHNA precursor chorismate due to derepression of Trp biosynthesis enzymes in a strain unable to regulate the flux of chorismate into this pathway by feeback inhibition of anthranilate synthase (due to an inability to complete the synthesis of Trp).
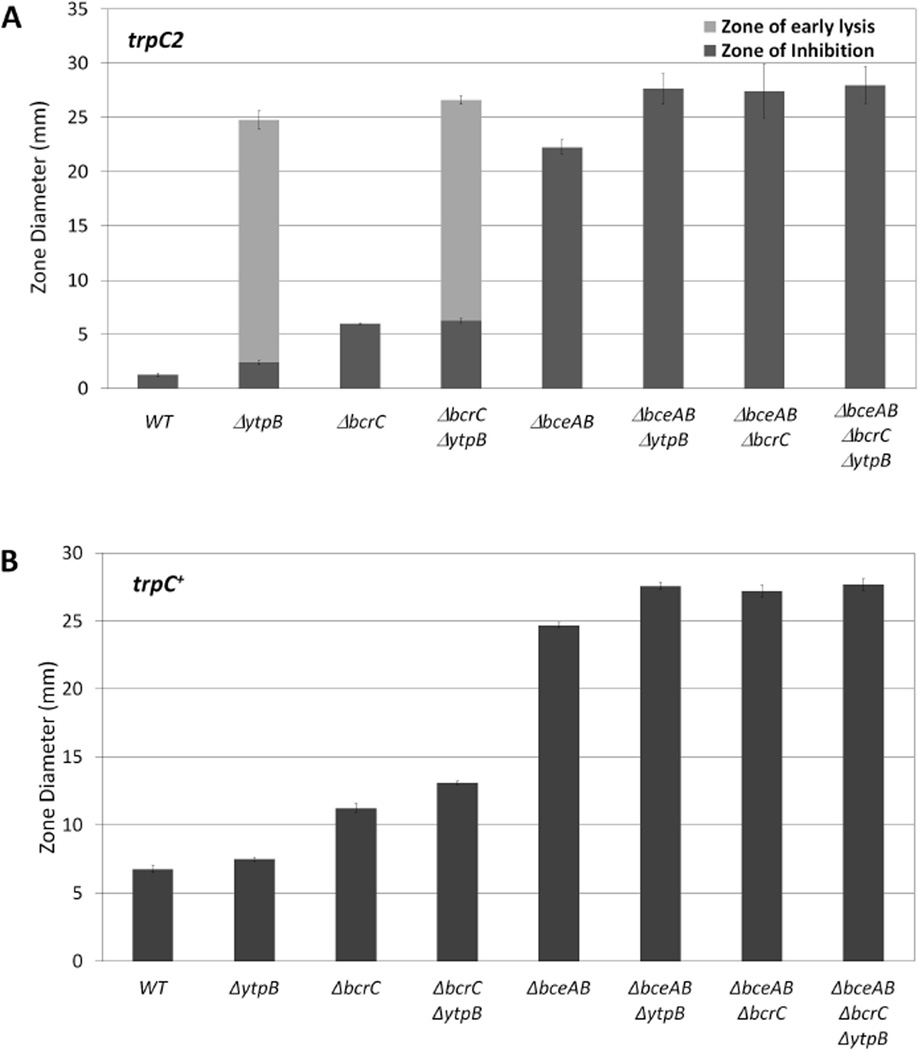
Figure 4.
The effect of deleting ytpB on bacitracin sensitivity is dependent upon BceAB and BcrC in both trpC2 and trp+ backgrounds. (A) Bacitracin disk diffusion assays for WT (168 trpC2) and isogenic strains: ΔytpB (HB13350), ΔbcrC (HB13348), ΔbcrC ΔytpB (HB13351), ΔbceAB (HB0928), ΔbceAB ΔytpB (HB13354), ΔbceAB ΔbcrC (HB13355), and ΔbceAB ΔbcrC ΔytpB (HB13356); (B) Bacitracin disk diffusion assays (using zinc bacitracin) in trp+ background for WT (HB13387) and isogenic strains: ΔytpB (HB13389), ΔbcrC (HB17031), ΔbcrC ΔytpB (HB17033), ΔbceAB (HB13388), ΔbceAB ΔytpB (HB13390), ΔbceAB ΔbcrC (HB17032), and ΔbceAB ΔbcrC ΔytpB (HB17034).
The effect of C35-PP on bacitracin sensitivity is largely mediated by decreased activity of BceAB and BcrC
Next, we sought to define the link between C35-PP accumulation and increased sensitivity to bacitracin. Bacitracin inhibits cell wall synthesis by binding to undecaprenyl pyrophosphate (C55-PP) on the outer leaflet of the cell membrane which prevents the recycling of C55-PP to undecaprenyl phosphate (C55-P). One might expect that accumulated C35-PP could bind and sequester bacitracin, thereby contributing to resistance against bacitracin, but this is clearly not the case since C35-PP sensitizes cells to bacitracin action. We instead hypothesized that C35-PP might serve to competitively inhibit one or more pathways of bacitracin resistance.
We have previously characterized the transcriptional response of B. subtilis to bacitracin and identified the major bacitracin resistance determinants (Mascher et al., 2003). At very low levels of bacitracin, the BceRS-BceAB two-component system transporter module is induced and confers the majority of bacitracin resistance (Rietkötter et al., 2008, Bernard et al., 2007). At higher levels, bacitracin induces the σM stress response which confers resistance by activation of transcription of the gene encoding the undecaprenyl pyrophosphate phosphatase BcrC (Bernard et al., 2005, Cao & Helmann, 2002). These two pathways have additive effects on bacitracin resistance (Mascher et al., 2003).
Comparison of bacitracin sensitivity using disk diffusion assays reveals that the zone of early lysis in the ΔytpB mutant corresponds in diameter to the zone of growth inhibition for the ΔbceAB ΔbcrC strain. Thus, we hypothesized that C35-PP accumulation in cells lacking ytpB results in bacitracin sensitivity because it interferes with either or both of the BceAB and BcrC resistance pathways. Construction of double mutants indicates that introduction of a ytpB null mutation can still sensitize either the ΔbcrC or ΔbceAB single mutant to bacitracin. However, there was no difference in bacitracin sensitivity between the ΔbceAB ΔbcrC double mutant and the ΔbceAB ΔbcrC ΔytpB triple mutant (Fig. 4). This lack of additivity was also observed in experiments with a four-fold lower amount of bacitracin, despite a significantly smaller diameter of the ZOI (data not shown). Thus, the loss of YtpB has no effect on cells lacking both resistance pathways (Fig. 4A). Despite the absence of a zone of early lysis in strains prototrophic for Trp, this same general pattern of epistasis is observed (Fig. 4B), although there were differences in the absolute values between the experiments shown in Fig.4A and 4B due to the use of different preparations of bacitracin. In strains compared in parallel, the sensitivity of both the WT and ΔytpB cells was unaffected by conversion of the trpC2 parent strain to Trp prototrophy. These observations suggest that C35-PP accumulation in the absence of ytpB (and under conditions where MenA activity is limited due to the trpC2 mutation) interferes with the ability of both the primary resistance determinant (the BceAB transporter) and the secondary determinant (the BcrC phosphatase) to confer bacitracin resistance.
The BcrC phosphatase confers resistance to bacitracin by dephosphorylating C55-PP to C55-P, which no longer serves as a target for bacitracin (Bernard et al., 2005). The resultant C55-P is presumably flipped to the internal face of the membrane, perhaps by the same (unidentified) translocase that normally functions with C55-PP. C55-P then serves as a substrate for MraY, the enzyme responsible for lipid I synthesis. Because of their obvious structural similarity, it is easy to imagine that C35-PP could competitively inhibit dephosphorylation of C55-PP. In contrast, the molecular basis for the effects of C35-PP on BceAB function are less obvious, in part due to our poor understanding of how this resistance determinant funtions. As described below, we speculate that C55-PP may also be a substrate for BceAB, and that C35-PP serves as a competitive inhibitor of this resistance pathway.
The level of UppS (C55-PP synthase) modulates the effect of C35-PP on bacitracin sensitivity
If our model is correct, and C55-PP and C35-PP compete as substrates for the BcrC and BceAB resistance proteins, then the observed bacitracin sensitivity should be affected by changes in ratio of C35-PP to C55-PP. Above, we tested this idea by genetic and physiological changes expected to alter the level of C35-PP (Fig. 1). Next, we sought to further explore this model by altering expression of UppS, the C55-PP synthase.
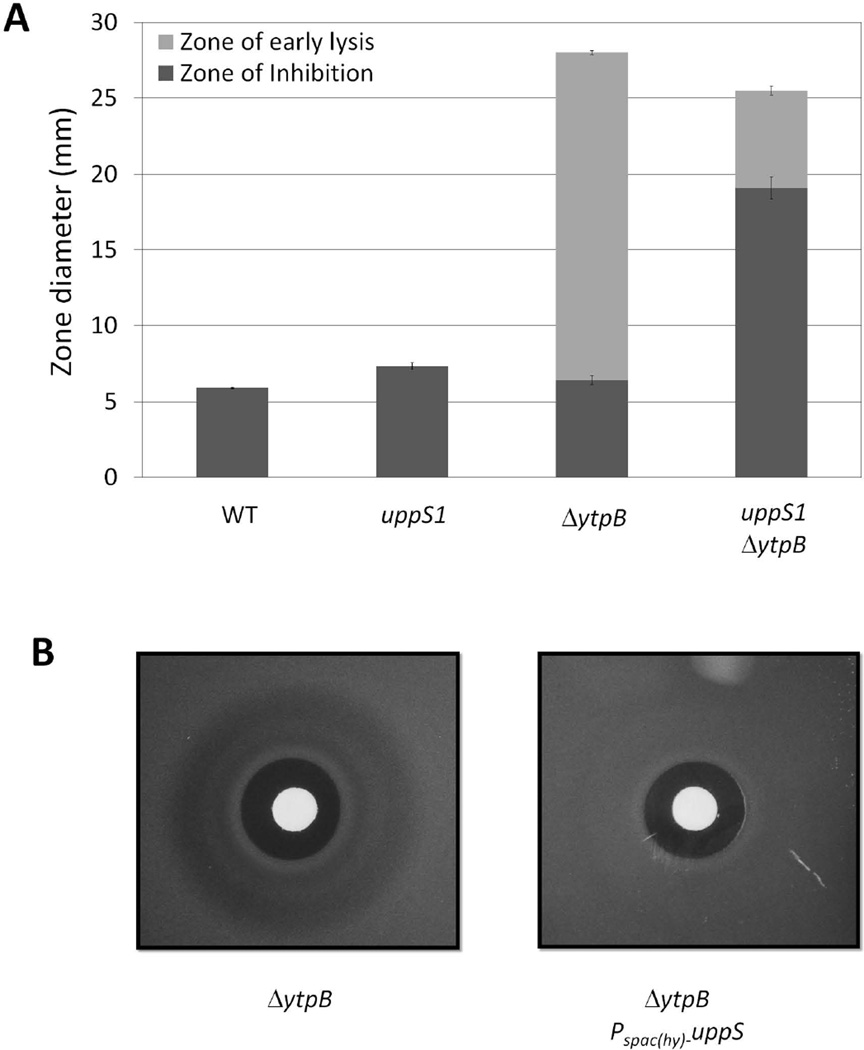
First, we took advantage of a recently identified mutation (uppS1) in the ribosome binding site of the undecaprenyl pyrophosphate synthetase gene that has been shown to reduce the expression of UppS by ~2-fold (Lee & Helmann, 2013). This mutation by itself slightly increases bacitracin sensitivity, consistent with our previously reported results (Lee & Helmann, 2013). However, when the uppS1 allele was introduced into a strain lacking ytpB, the bacitracin zone of inhibition increased dramatically (Fig. 5A). This synergistic effect suggests that the effect of C35-PP on bacitracin sensitivity is enhanced in cells with reduced C55-PP.
Figure 5.
The impact of C35-PP on bacitracin sensitivity is affected by the expression level of UppS. (A) Bacitracin disk diffusion assays for WT (168 trpC2), uppS1 (HB13648), ΔytpB (HB13321), and uppS1 ΔytpB (HB13443) strains on MH medium; (B) Bacitracin disk diffusion assays illustrating the loss of the ZOEL in a strain overexpressing UppS (ΔytpB Pspac(hy)-uppS (HB17057; right) as compared to the isogenic ΔytpB (HB13350) strain (left) when grown on MH medium containing 1 mM IPTG.
Second, we monitored the effect of UppS overproduction using an IPTG-inducible expression system as previously described (Lee & Helmann, 2013). Under conditions of elevated UppS expression, the ΔytpB strain no longer displayed the characteristic zone of early lysis (Fig. 5B). We further note that the zone of growth inhibition was only modestly reduced by UppS induction, despite the fact that C55-PP is the direct target of bacitracin binding. This is consistent with the generally low levels of C55-PP present in cells and the large excess of bacitracin present in the growth medium. Together, these results support a model in which C35-PP interferes with the BcrC and BceAB resistance pathways by acting as a molecular mimic of the normal substrate, C55-PP.
C35-PP accumulation does not prevent bacitracin-sensing by the BceAB-BceRS regulatory system
An intriguing feature of the BceAB transporter is that it is required for the transcriptional response to bacitracin mediated by the BceRS two-component system (Revilla-Guarinos et al., 2014). Thus, in the presence of bacitracin, the BceAB transporter senses the presence of the antibiotic, by an unknown mechanism, and signals to the BceRS system which, in turn, transcriptionally activates expression of the bceRS-bceAB operon. One possible explanation for the effects of C35-PP on bacitracin sensitivity would be interference with the bacitracin-sensing function of BceAB which is necessary for upregulation of the bceRS-bceAB complex operon by BceR (Bernard et al., 2007).
To test this hypothesis, we monitored the concentration dependence of bacitracin induction using a PbceA-lacZ fusion integrated into the amyE locus. Consistent with prior studies (Rietkötter et al., 2008, Mascher et al., 2003), expression of PbceA-lacZ was strongly induced by bacitracin, starting at concentrations of 0.1–1 µg ml−1 and with a peak at approximately 100 µg ml−1 (Fig. S3). In the ytpB null mutant, bacitracin was still able to mediate activation of the bceAB promoter (PbceAB), although with slightly lower overall expression at the highest tested concentrations compared to WT cells. It seems unlikely that such a modest change in BceAB expression could be causing the bacitracin sensitivity seen in the ΔytpB strain.
To further investigate the relationship between BceAB expression level and bacitracin sensitivity, we inserted an IPTG inducible copy of bceAB into WT and ΔytpB cells and tested the sensitivity of these strains to bacitracin with disk diffusion assays. Induction of bceAB slightly decreased the bacitracin zone of inhibition in WT cells, but was unable to eliminate the zone of early lysis in ΔytpB cells (Fig. S2). Thus, the amount of BceAB in the cell does not seem to be a limiting factor, leading us to conclude that the mild reduction in PbceAB induction in response to bacitracin, as seen in the ytpB deletion strain, is unlikely to affect bacitracin sensitivity.
The BceAB resistance determinant may serve as a C55-PP translocase
Since C35-PP accumulation does not substantially affect the induction of bceAB, we speculated that it might interfere with BceAB function. The BceAB transporter has been proposed to mediate translocation (export) of bacitracin (Ohki et al., 2003, Podlesek et al., 1995), although there is no direct evidence to support this notion. Moreover, it is unclear how export could confer resistance to an antibiotic that acts on a target on the external face of the cytoplasmic membrane. An alternative model suggests that perhaps BceAB serves to import bacitracin, thereby allowing degradation in the cytosol (Rietkötter et al., 2008). Both of these models presume that it is bacitracin that is the substrate for BceAB (Revilla-Guarinos et al., 2014).
Our investigation of the effects of C35-PP accumulaton on bacitracin sensitivity suggests an alternative model for BceAB function. We propose that BceAB confers bacitracin resistance not by transporting bacitracin, but by removing the target for bacitracin by the flipping of C55-PP across the membrane (Fig. 6). According to this model, C55-PP (and possibly C55-P) is the substrate for BceAB. In the presence of bacitracin, this transporter may bind the bacitracin:C55-PP complex and this complex (perhaps functioning as a transport inhibitor) may trigger the activation of the BceS sensor kinase.
Figure 6.
Proposed model of (A) C55-P(P) recycling and (B) bacitracin resistance mechanisms in Bacillus subtilis. (A) C55-PP is initially synthesized in the cytoplasm by undecaprenyl pyrophosphate synthase (UppS) and then converted to C55-P by undecaprenyl pyrophosphate phosphatase (UppP). C55-P is a substrate for MraY, which generates lipid I, and then MurG completes the synthesis of the lipid II precursor. The lipid II molecule is then flipped across the membrane for utilization in peptidoglycan synthesis by the transglycosylase activity of penicillin binding proteins (PBPs). C55-PP released on the extracellular face of the membrane may either be dephosphorylated by BcrC outside of the cell and subsequently flipped to the cytoplasmic face by a C55-P flippase, or flipped by a C55-PP flippase, and then dephosphorylated by UppP. The C55-P(P) flippase(s) have yet to be identified, but our results suggest that BceAB may have such an activity. (B) Model for the effects of bacitracin on the lipid II cycle and induction of bacitracin resistance genes. Bacitracin binds to C55-PP to prevent its recycling to C55-P. We suggest that the C55-PP:bacitracin complex inhibits the BceAB transporter and this leads to signal transduction, presumably mediated by protein-protein interactions (Dintner et al., 2011), to the BceRS two component system. BceR then activates expression of both BceRS and the BceAB ABC transporter (short dashed lines). If bacitracin levels are sufficient to inhibit peptidoglycan synthesis, σM will be activated leading to increased expression of BcrC which catalyzes C55-PP dephosphorylation (long dashed lines). Under conditions where C35-PP accumulates, this isoprenoid inhibits BceAB and BcrC leading to increased bacteriolysis. Accumulation of C35-PP in this study results from (i) inactivation of the σM regulated tetraprenyl-β-curcumene synthase YtpB and (ii) the limited activity of MenA resulting from high expression and activity of enzymes that convert chorismate into precursors for Trp biosynthesis upon depletion of Trp from the growth medium.
This model is consistent with the observations that BceRS requires the BceAB transporter to sense bacitracin (Bernard et al., 2007), and that BceAB-dependent bacitracin sensing works even in L-form cells lacking peptidoglycan (Wolf et al., 2012) suggesting that bacitracin is sensed directly, rather than through its effects on peptidoglycan biosynthesis. In the context of this model, the striking effects of the ytpB mutation on bacitracin sensitivity can be explained. We postulate that C35-PP competes with C55-PP for the BceAB translocase, thereby interfering with removal of C55-PP from the outer leaflet where it can be complexed by bacitracin. Since the primary determinants for ligand recognition by bacitracin are only the pyrophosphate group and its closest prenyl group (Economou et al., 2013), bacitracin is expected to bind to C35-PP as readily as it binds to C55-PP. By analogy with the corresponding C55-PP:bacitracin complex, we speculate that the C35-PP:bacitracin complex may also be recognized by BceAB. As suggested above, this may also lead to inhibition of the translocase and signaling to BceRS. Ultimately, reconsitution of BceAB-BceRS signaling in vitro will be required to test these and related hypotheses.
These observations may also have implications for approaches to develop synergistic antibiotic combinations and antibacterial adjuvants. There is growing interest in identifying novel antibiotic adjuvants, but many strategies focus on costly bulk screening methods (Farha & Brown, 2013). Here, we have shown that C35-PP accumulation can enhance the activity of bacitracin and it is likely that further modifications could optimize this activity. Synergy between another isoprenoid, the C15 compound farnesol, and cell wall antibiotics has also been documented (Kuroda et al., 2007). Our genetic results further suggest that compounds that inhibit UppS, analogous in effect to strains containing a uppS1 mutation (Lee & Helmann, 2013), will synergize with bacitracin and other inhibitors of lipid II cycling. In addition, we note that menaquinone biosynthesis, and the MenA enzyme in particular, has recently emerged as an attractive target for antibacterials directed at Mycobacterium tuberculosis (Dhiman et al., 2009, Kurosu & Crick, 2009, Debnath et al., 2012).
Concluding remarks
Our analysis of the bacitracin sensitivity phenotype of a B. subtilis ΔytpB strain had led to unanticipated insights into the molecular mechanisms of bacitracin resistance (Fig. 6B). We found that deleting ytpB interferes with BcrC and BceAB, the two major bacitracin resistance determinants in B. subtilis. We suggest that accumulation of C35-PP, in the absence of YtpB and under conditions limiting the activity of MenA, increases bacitracin sensitivity by acting as a competitive inhibitor for both BceAB and BcrC. This is most simply explained by proposing that C55-PP is the substrate for both of these enzymes: BcrC dephosphorylates C55-PP to C55-P whereas BceAB may flip C55-PP to the inner face of the membrane where it is now protected from bacitracin and can be dephosphorylated by a cytosolic acting UppP. It is likely that there is at least one cytosolic UppP (as in E. coli; Touzé et al., 2008) since C55-PP is normally synthesized cytosolically (by UppS) and must be desphosphorylated to serve as the acceptor for MraY (phospho-N-acetylmuramoyl-pentapeptide transferase). It has previously been proposed that C55-PP:bacitracin is the physiologically relevant inducer for both the BceRS-BceAB module (Bernard et al., 2007) and for the unrelated transmembrane sensor, BcrR (Gebhard et al., 2009, Gauntlett et al., 2008). As an extension of this model, we suggest that BceAB senses the C55-PP:bacitracin complex and this complex may competitively inhibit C55-PP import. This inhibition leads to transmission of a signal to activate BceS leading to phosphorylation of BceR and upregulation of bceAB transcription.
Since the identity of the transporter(s) responsible for the recycling of C55-P (and C55-PP) is unknown, an ability of BceAB to catalyze such a reaction has general significance for our understanding of cell wall synthesis. It is expected that transmembrane flipping of bactoprenol-derived lipids released during the transglycosylation step of cell wall synthesis is essential for rapid cell growth. However, the relevant transporters have yet to be identified, possibly due to functional redundancy. The results presented here suggest that the use of a strain lacking BceAB (and perhaps paralogous transporters) may provide a suitable genetic platform for efforts to identify this elusive activity.
Experimental Procedures
Strains, plasmids, and growth conditions
All B. subtilis strains, plasmids, and oligonucleotides (oligos) used in this study are listed Table S1. Bacteria were grown in liquid lysogeny broth (LB) or Mueller-Hinton (MH) medium at 37°C with vigorous shaking or on solid LB or MH medium containing 1.5% Bacto agar (Difco) with appropriate selection. Plasmids were amplified in Escherichia coli DH5α before transformation of B. subtilis strains. Ampicillin (amp; 100 µg ml−1) was used to select E. coli transformants. For B. subtilis, antibiotics used for selection were: spectinomycin (spec; 100 µg ml−1), kanamycin (kan; 15 µg ml−1), chloramphenicol (cat; 10 µg ml−1), tetracycline (20 µg ml−1), neomycin (neo; 10 µg ml−1), and macrolide-lincosoamide-streptogramin B (MLS; contains 1 µg ml−1 erythromycin and 25 µg ml−1 lincomycin). Mutants unable to synthesize menaquinone-7 were supplemented with 10 µg ml−1 menaquinone-4 (Sigma-Aldrich Co. St. Louis, MO USA) which permitted growth.
Genetic techniques
Chromosomal and plasmid DNA transformations were performed as described previously (Harwood & Cutting, 1990). Unless otherwise stated, all PCR products were generated using 168 chromosomal DNA as a template and all strains were verified by sequence analysis (Cornell University Life Sciences Core Laboratories Center). Gene deletions were generated using long-flanking homology PCR (LFH-PCR) as described previously (Mascher et al., 2003).
Disk diffusion assays
Disk diffusion assays were performed as described (Mascher et al., 2007). Briefly, strains were grown to an OD600 of 0.4. A 100 µl aliquot of these cultures was mixed with 4 ml of 0.7% MH soft agar (kept at 50°C) and directly poured onto MH plates (containing 15 ml of 1.5% MH agar). The plates were dried for 20 min in a laminar airflow hood. Filter paper disks containing the chemicals to be tested were placed on the top of the agar and the plates were incubated at 37°C overnight. The overall diameter of the inhibition zones were measured along two orthogonal lines. Zones of inhibition are reported as the average diameter minus the 5.5 mm dia. of the filter paper disk. For IPTG or menaquinone treated cells, the indicated compound was added to both the soft agar and the plates to a concentration of 1 mM IPTG or 10 µg ml−1 menaquinone. Unless otherwise noted, the following chemicals and quantities were used in the disk diffusion assays: Triton X-100 10 µl of a 10% solution, N-lauryl sarcosine 5 µl of a 10% solution, sodium dodecyl sulfate 500 µg, bile salts 1 mg, colistin 100 µg, clofazimine 1 mg, poly-L-lysine 5 µg, ethylenediaminetetraacetic acid 365 µg, cerulenin 5 µg, polymyxin B 50 µg, novobiocin 50 µg, vancomycin 50 µg, daptomycin 100 µg, D-cycloserine 500 µg, moenomycin 5 µg, cefuroxime 5 µg, dodecyltrimethylammonium bromide (DTAB) 50 µg, Amitriptyline 100 µg, nisin 20 µg, and bacitracin 400 µg. For nisin assays, a 2.5 mg ml−1 nisin stock solution was prepared by dissolving a 2.5% nisin mixture balanced with sodium chloride and denatured milk solids (Sigma-Aldrich Co. St. Louis, MO USA) in 0.02 M HCl. For daptomycin assays, the media was supplemented with 1.25 mM CaCl2. The majority of bacitracin sensitivity assays were performed with bacitracin zinc salt (Sigma-Aldrich Co. St. Louis, MO USA) dissolved in 0.3 M HCl, but some (e.g. the results shown in Fig. 4A) utilized bacitracin (Sigma-Aldrich Co. St. Louis, MO USA) dissolved in water to confirm that pattern of bacitracin sensitivity was not affected by zinc.
Broth Dilution Assays
We determined the minimal inhibitory concentration (MIC) and growth phenotype of B. subtilis strains to bacitrain using a variation of the broth dilution assay described previously (Luo & Helmann, 2012) that follows CLSI standards (Reller et al., 2009). Briefly, strains were grown to an OD600 of 0.4 in MH media. Strains were diluted 1:200 in MH broth (to an approximate cell density of 2 × 105 cells ml−1 (Arrieta et al., 2006), and 200 µl aliquots of the diluted cultures were dispensed in a Bioscreen 100-well microtitre plate. Each strain was grown with bacitracin concentrations ranging from 50 to 300 µg ml−1 with concentrations increasing in increments of 40 µg ml−1. Growth was measured spectrophotometrically (OD600) every 15 min for 24 h using a Bioscreen C incubator (Growth Curves USA, Piscataway, NJ) at 37 °C with continuous shaking. MIC was defined as the lowest concentration that prevented growth (OD600 < 0.15) at the 10 h time point.
β-galactosidase assays
Strains carrying promoter-lacZ fusions were grown to an OD600 of 0.4 in LB. Cultures were then treated with the indicated concentrations of bacitracin or control (H2O) and samples were taken 60 min after treatment. β-galactosidase assays were performed as described by Miller except that cells were lysed with 0.1 mg ml−1 lysozyme for 30 minutes instead of chloroform (Miller, 1972).
Statistical analysis
All experiments were performed with a minimum of three biological replicates. Unless otherwise noted, data is presented as mean ± standard error. Statistical evaluation of the data was performed by the use of unpaired Student's t tests. A value of P ≤ 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
This work was funded by a grant from the National Institutes of Health to J.D.H. (GM-047446).
References
- Arrieta MC, Leskiw BK, Kaufman WR. Antimicrobial activity in the egg wax of the African cattle tick Amblyomma hebraeum (Acari: Ixodidae) Exp Appl Acarol. 2006;39:297–313. doi: 10.1007/s10493-006-9014-5. [DOI] [PubMed] [Google Scholar]
- Bernard R, Ghachi ME, Mengin-Lecreulx D, Chippaux M, Denizot F. BcrC from Bacillus subtilis acts as an undecaprenyl pyrophosphate phosphatase in bacitracin resistance. J Biol Chem. 2005;280:28852–28857. doi: 10.1074/jbc.M413750200. [DOI] [PubMed] [Google Scholar]
- Bernard R, Guiseppi A, Chippaux M, Foglino M, Denizot F. Resistance to bacitracin in Bacillus subtilis: unexpected requirement of the BceAB ABC transporter in the control of expression of its own structural genes. J Bacteriol. 2007;189:8636–8642. doi: 10.1128/JB.01132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard R, Joseph P, Guiseppi A, Chippaux M, Denizot F. YtsCD and YwoA, two independent systems that confer bacitracin resistance to Bacillus subtilis. FEMS Microbiol Lett. 2003;228:93–97. doi: 10.1016/S0378-1097(03)00738-9. [DOI] [PubMed] [Google Scholar]
- Bickford JS, Nick HS. Conservation of the PTEN catalytic motif in the bacterial undecaprenyl pyrophosphate phosphatase, BacA/UppP. Microbiology. 2013;159:2444–2455. doi: 10.1099/mic.0.070474-0. [DOI] [PubMed] [Google Scholar]
- Bosak T, Losick RM, Pearson A. A polycyclic terpenoid that alleviates oxidative stress. PNAS. 2008;105:6725–6729. doi: 10.1073/pnas.0800199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Helmann JD. Regulation of the Bacillus subtilis bcrC bacitracin resistance gene by two extracytoplasmic function σ factors. J Bacteriol. 2002;184:6123–6129. doi: 10.1128/JB.184.22.6123-6129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Wang T, Ye R, Helmann JD. Antibiotics that inhibit cell wall biosynthesis induce expression of the Bacillus subtilisσW and σM regulons. Mol Microbiol. 2002;45:1267–1276. doi: 10.1046/j.1365-2958.2002.03050.x. [DOI] [PubMed] [Google Scholar]
- Debnath J, Siricilla S, Wan B, Crick DC, Lenaerts AJ, Franzblau SG, Kurosu M. Discovery of selective menaquinone biosynthesis inhibitors against Mycobacterium tuberculosis. Journal of medicinal chemistry. 2012;55:3739–3755. doi: 10.1021/jm201608g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman RK, Mahapatra S, Slayden RA, Boyne ME, Lenaerts A, Hinshaw JC, Angala SK, Chatterjee D, Biswas K, Narayanasamy P, Kurosu M, Crick DC. Menaquinone synthesis is critical for maintaining mycobacterial viability during exponential growth and recovery from non-replicating persistence. Mol Microbiol. 2009;72:85–97. doi: 10.1111/j.1365-2958.2009.06625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintner S, Staron A, Berchtold E, Petri T, Mascher T, Gebhard S. Coevolution of ABC transporters and two-component regulatory systems as resistance modules against antimicrobial peptides in Firmicutes Bacteria. J Bacteriol. 2011;193:3851–3862. doi: 10.1128/JB.05175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker CR. Update on topical antibiotics in dermatology. Dermatol Ther. 2012;25:6–11. doi: 10.1111/j.1529-8019.2012.01493.x. [DOI] [PubMed] [Google Scholar]
- Economou NJ, Cocklin S, Loll PJ. High-resolution crystal structure reveals molecular details of target recognition by bacitracin. PNAS. 2013;110:14207–14212. doi: 10.1073/pnas.1308268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiamphungporn W, Helmann JD. The Bacillus subtilis σM regulon and its contribution to cell envelope stress responses. Mol Microbiol. 2008;67:830–848. doi: 10.1111/j.1365-2958.2007.06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falord M, Karimova G, Hiron A, Msadek T. GraXSR proteins interact with the VraFG ABC transporter to form a five-component system required for cationic antimicrobial peptide sensing and resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56:1047–1058. doi: 10.1128/AAC.05054-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farha MA, Brown ED. Discovery of antibiotic adjuvants. Nat Biotechnol. 2013;31:120–122. doi: 10.1038/nbt.2500. [DOI] [PubMed] [Google Scholar]
- Gauntlett JC, Gebhard S, Keis S, Manson JM, Pos KM, Cook GM. Molecular analysis of BcrR, a membrane-bound bacitracin sensor and DNA-binding protein from Enterococcus faecalis. J Biol Chem. 2008;283:8591–8600. doi: 10.1074/jbc.M709503200. [DOI] [PubMed] [Google Scholar]
- Gebhard S, Gaballa A, Helmann JD, Cook GM. Direct stimulus perception and transcription activation by a membrane-bound DNA binding protein. Mol Micro. 2009;73:482–491. doi: 10.1111/j.1365-2958.2009.06787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick P, Babitzke P, Antson A, Yanofsky C. Complexity in Regulation of Tryptophan Biosynthesis in Bacillus subtilis. Annu Rev Genet. 2005;29:47–68. doi: 10.1146/annurev.genet.39.073003.093745. [DOI] [PubMed] [Google Scholar]
- Harwood CR, Cutting SM. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley & Sons Ltd; 1990. [Google Scholar]
- Hiron A, Falord M, Valle J, Debarbouille M, Msadek T. Bacitracin and nisin resistance in Staphylococcus aureus: a novel pathway involving the BraS/BraR two-component system (SA2417/SA2418) and both the BraD/BraE and VraD/VraE ABC transporters. Mol Microbiol. 2011;81:602–622. doi: 10.1111/j.1365-2958.2011.07735.x. [DOI] [PubMed] [Google Scholar]
- Inaoka T, Ochi K. Undecaprenyl pyrophosphate involvement in susceptibility of Bacillus subtilis to rare earth elements. J Bacteriol. 2012;194:5632–5637. doi: 10.1128/JB.01147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Suzuki D, Asai K. A putative bactoprenol glycosyltransferase, CsbB, in Bacillus subtilis activates SigM in the absence of co-transcribed YfhO. Biochemical and biophysical research communications. 2013;436:6–11. doi: 10.1016/j.bbrc.2013.04.064. [DOI] [PubMed] [Google Scholar]
- Jordan S, Hutchings MI, Mascher T. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol Rev. 2008;32:107–146. doi: 10.1111/j.1574-6976.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- Kallenberg F, Dintner S, Schmitz R, Gebhard S. Identification of regions important for resistance and signalling within the antimicrobial peptide transporter BceAB of Bacillus subtilis. J Bacteriol. 2013;195:3287–3297. doi: 10.1128/JB.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontnik R, Bosak T, Butcher RA, Brocks JJ, Losick R, Clardy J, Pearson A. Sporulenes, heptaprenyl metabolites from Bacillus subtilis spores. Org Lett. 2008;10:3551–3554. doi: 10.1021/ol801314k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M, Nagasaki S, Ohta T. Sesquiterpene farnesol inhibits recycling of the C55 lipid carrier of the murein monomer precursor contributing to increased susceptibility to beta-lactams in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2007;59:425–432. doi: 10.1093/jac/dkl519. [DOI] [PubMed] [Google Scholar]
- Kurosu M, Crick DC. MenA is a promising drug target for developing novel lead molecules to combat Mycobacterium tuberculosis. Med Chem. 2009;5:197–207. doi: 10.2174/157340609787582882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Helmann JD. Reducing the level of undecaprenyl pyrophosphate synthase has complex effects on susceptibility to cell wall antibiotics. Antimicrob Agents Ch. 2013;57:4267–4275. doi: 10.1128/AAC.00794-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Helmann JD. Analysis of the role of Bacillus subtilis σM in β-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol Micro. 2012;83:623–639. doi: 10.1111/j.1365-2958.2011.07953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher T, Hachmann AB, Helmann JD. Regulatory overlap and functional redundancy among Bacillus subtilis extracytoplasmic function sigma factors. J Bacteriol. 2007;189:6919–6927. doi: 10.1128/JB.00904-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher T, Margulis NG, Wang T, Ye RW, Helmann JD. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol Microbiol. 2003;50:1591–1604. doi: 10.1046/j.1365-2958.2003.03786.x. [DOI] [PubMed] [Google Scholar]
- Miller JH. Experiments in molecular genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- Ming L-J, Epperson JD. Metal binding and structure–activity relationship of the metalloantibiotic peptide bacitracin. J Inorg Biochem. 2002;40:530–536. doi: 10.1016/s0162-0134(02)00464-6. [DOI] [PubMed] [Google Scholar]
- Nester EW, Jensen RA. Control of aromatic acid biosynthesis in Bacillus subtilis: sequential feedback inhibition. Journal of bacteriology. 1966;91:1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki R, Giyanto, Tateno K, Masuyama W, Moriya S, Kobayashi K, Ogasawara N. The BceRS two-component regulatory system induces expression of the bacitracin transporter, BceAB, in Bacillus subtilis. Mol Micro. 2003;49:1135–1144. doi: 10.1046/j.1365-2958.2003.03653.x. [DOI] [PubMed] [Google Scholar]
- Podlesek Z, Comino A, Herzog-Velikonja B, Zgur-Bertok D, Komel R, Grabnar M. Bacillus licheniformis bacitracin-resistance ABC transporter: relationship to mammalian multidrug resistance. Mol Microbiol. 1995;16:969–976. doi: 10.1111/j.1365-2958.1995.tb02322.x. [DOI] [PubMed] [Google Scholar]
- Reller LB, Weinstein M, Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin Infect Dis. 2009;49:1749–1755. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- Revilla-Guarinos A, Gebhard S, Mascher T, Zuniga M. Defence against antimicrobial peptides: different strategies in Firmicutes. Environmental Microbiology. 2014 doi: 10.1111/1462-2920.12400. (in press). [DOI] [PubMed] [Google Scholar]
- Rietkötter E, Hoyer D, Mascher T. Bacitracin sensing in Bacillus subtilis. Mol Micro. 2008;68:768–785. doi: 10.1111/j.1365-2958.2008.06194.x. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Menon AK. Flipping lipids: why an' what's the reason for? ACS Chem Biol. 2009;4:895–909. doi: 10.1021/cb900163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah AK, Meyer MT, Boxall ABA. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. 2006;65:725–759. doi: 10.1016/j.chemosphere.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Sato T. Unique biosynthesis of sesquarterpenes (C35 terpenes) Bioscience, biotechnology, and biochemistry. 2013;77:1155–1159. doi: 10.1271/bbb.130180. [DOI] [PubMed] [Google Scholar]
- Sato T, Yoshida S, Hoshino H, Tanno M, Nakajima M, Hoshino T. Sesquarterpenes (C35 terpenes) biosynthesized via the cyclization of a linear C35 isoprenoid by a tetraprenyl-beta-curcumene synthase and a tetraprenyl-beta-curcumene cyclase: identification of a new terpene cyclase. J Am Chem Soc. 2011;133:9734–9737. doi: 10.1021/ja203779h. [DOI] [PubMed] [Google Scholar]
- Staron A, Finkeisen DE, Mascher T. Peptide antibiotic sensing and detoxification modules of Bacillus subtilis. Antimicrob Agents Chemother. 2011;55:515–525. doi: 10.1128/AAC.00352-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KJ, Strominger JL. Mechanism of action of bacitracin: Complexation with metal ion and C55-isoprenyl pyrophosphate. PNAS. 1971;68:3223–3227. doi: 10.1073/pnas.68.12.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna K, Stevenson D, Meganathan R, Hudspeth ME. Menaquinone (vitamin K2) biosynthesis: localization and characterization of the menA gene from Escherichia coli. J Bacteriol. 1998;180:2782–2787. doi: 10.1128/jb.180.10.2782-2787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwedziak P, Lowe J. Do the divisome and elongasome share a common evolutionary past? Curr Opin Microbiol. 2013;16:745–751. doi: 10.1016/j.mib.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Takigawa H, Sugiyama M, Shibuya Y. C(35)-terpenes from Bacillus subtilis KSM 6–10. Journal of natural products. 2010;73:204–207. doi: 10.1021/np900705q. [DOI] [PubMed] [Google Scholar]
- Tamehiro N, Okamoto-Hosoya Y, Okamoto S, Ubukata M, Hamada M, Naganawa H, Ochi K. Bacilysocin, a novel phospholipid antibiotic produced by Bacillus subtilis 168. Antimicrob Agents Ch. 2002;46:315–320. doi: 10.1128/AAC.46.2.315-320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar LD, Marolda CL, Polischuk AN, Leeuwen Dv, Valvano MA. An Escherichia coli undecaprenyl-pyrophosphate phosphatase implicated in undecaprenyl phosphate recycling. Microbiol. 2007;153:2518–2529. doi: 10.1099/mic.0.2007/006312-0. [DOI] [PubMed] [Google Scholar]
- Teng KH, Liang PH. Undecaprenyl diphosphate synthase, a cis-prenyltransferase synthesizing lipid carrier for bacterial cell wall biosynthesis. Molecular membrane biology. 2012;29:267–273. doi: 10.3109/09687688.2012.674162. [DOI] [PubMed] [Google Scholar]
- Touzé T, Blanot D, Mengin-Lecreulx D. Substrate specificity and membrane topology of Escherichia coli PgpB, an undecaprenyl pyrophosphate phosphatase. J Biol Chem. 2008;283:16573–16583. doi: 10.1074/jbc.M800394200. [DOI] [PubMed] [Google Scholar]
- Valvano MA. Undecaprenyl phosphate recycling comes out of age. Mol Micro. 2008;67:232–235. doi: 10.1111/j.1365-2958.2007.06052.x. [DOI] [PubMed] [Google Scholar]
- Wagner B, Schumann D, Linne U, Koert U, Marahiel MA. Rational design of bacitracin A derivatives by incorporating natural product derived heterocycles. J Am Chem Soc. 2006;128:10513–10520. doi: 10.1021/ja062906w. [DOI] [PubMed] [Google Scholar]
- Wagner WP, Helmig D, Fall R. Isoprene biosynthesis in Bacillus subtilis via the methylerythritol phosphate pathway. Journal of natural products. 2000;63:37–40. doi: 10.1021/np990286p. [DOI] [PubMed] [Google Scholar]
- Wolf D, Domínguez-Cuevas P, Daniel RA, Mascher T. Cell envelope stress response in cell wall-deficient L-forms of Bacillus subtilis. Antimicrob Agents Chemother. 2012;56:5907–5915. doi: 10.1128/AAC.00770-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GD. The antibiotic resistome: the nexus of chemical and genetic diversity. Nat Rev Microbiol. 2007;5:175–186. doi: 10.1038/nrmicro1614. [DOI] [PubMed] [Google Scholar]
- Yao J, Rock CO. Phosphatidic acid synthesis in bacteria. Biochim Biophys Acta. 2013;1831:495–502. doi: 10.1016/j.bbalip.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Koyama T, Marecak DM, Prestwich GD, Maki Y, Ogura K. Two subunits of heptaprenyl diphosphate synthase of Bacillus subtilis form a catalytically active complex. Biochemistry. 1998;37:13411–13420. doi: 10.1021/bi972926y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.