Abstract
Polyomaviruses encode a Large T antigen (LT), a multifunctional protein essential for the regulation of both viral and host cell gene expression and productive viral infection. Previously, we have shown that stable expression of LT protein results in upregulation of genes involved in the interferon induction and signaling pathway. In this study, we focus on the cellular signaling mechanism that leads to the induction of interferon (IFN) responses by LT. Our results show that ectopic expression of Simian virus 40 (SV40) LT results in the induction of interferon-stimulated genes (ISGs) in human fibroblasts and confers an antiviral state. We describe a LT-initiated DNA-damage response (DDR) that activates IRF1 causing IFNβ production and consequent ISG expression in human cells. This IFNβ and ISG induction is dependent on ATM and Rad3 related (ATR) kinase, but independent of ataxia-telangiectasia mutated (ATM). ATR kinase inhibition using a selective kinase inhibitor (ETP-46464) caused a decrease in IRF1 stabilization and ISG expression. Furthermore, expression of a mutant LT that does not induce DDR, does not induce IFNβ and ISGs. These results show that in the absence of viral infection, LT-initiated activation of ATR-dependent DDR is sufficient for the induction of an IFNβ-mediated innate immune response in human cells. Thus, we have uncovered a novel and critical role for ATR as a mediator of antiviral responses utilizing LT.
Keywords: Large T antigen, interferon, ISG, IRF1, ATR
INTRODUCTION
The polyomavirus (PyV) family consists of small double–stranded DNA viruses that infect a wide variety of hosts. Infection with polyomaviruses is ubiquitous amongst the human population and generally results in subclinical infections in healthy hosts. However, upon the onset of AIDS-related, age related, and iatrogenic immune suppression, manifestations of clinical disease associated with viral infection become apparent (1–3). To date, there are few successful therapies for the treatment of human PyV-related diseases. Thus, understanding the interactions between the virus and the host response to viral infection is crucial for the development of new effective therapies.
The wealth of knowledge of PyV biology has stemmed from studies utilizing the prototypic virus, Simian Virus 40 (SV40). SV40 encodes early proteins, large T antigen (LT), small t antigen and 17k T (early region), and late proteins with structural and auxiliary functions. The SV40 LT is a well-studied multi-functional protein that controls viral DNA replication, host cell proliferation and gene expression, thereby promoting cellular transformation. These functions are largely mediated by interactions between LT and numerous cellular proteins (4). Identifying the cellular processes targeted by LT is crucial for understanding the pathogenesis of polyomaviruses and their transformative phenotype.
Genotoxic stress triggers a signaling cascade, called DNA damage response (DDR), mediated by the kinases ataxia-telangiectasia mutated (ATM) and ATM and Rad3-related (ATR) (5). Activation of these kinases by double-strand DNA breaks and single strand DNA gaps, respectively, results in the induction of the checkpoint proteins and modifications of many other proteins implicated in DNA repair to maintain genome integrity and cell survival (6). Multiple studies have provided evidence that infection with mouse PyV virus (MPyV) (7), JC PyV (JCV) (8), BK PyV (BKV) (9), and SV40 (10–14) causes the activation of DDR to augment viral genome replication. It has also been shown that expression of SV40 LT protein alone is sufficient to activate both ATM and ATR in cells (12). However, the cellular consequences of this DDR activation are not completely understood.
Detection of viral infection triggers cytoplasmic and nuclear sensors that initiate a signaling cascade that results in the induction of type I interferon (IFN) and the expression of IFN-stimulated genes (ISGs). The ISGs have antiviral effector functions that limit the replication of viral genomes, inhibit protein synthesis, and promote cell death (15). Previously, using mouse genome-wide arrays, we reported that SV40 LT expression results in a tissue specific induction of ISGs (16). Although this phenomenon was dependent on various domains of LT (17), it was surprising to find ISG induction in the absence of an active virus infection. Furthermore, the host factors responsible for this phenomenon remained elusive.
In this study, we have examined the cellular signaling pathways that are responsible for the SV40 LT-mediated induction of ISGs in human fibroblasts. LT expression induced upregulation of IRF1 protein causing transcription and secretion of IFNβ and its downstream genes through IFN receptor signaling. Furthermore, we showed that the induction of DDR by LT is needed to induce the expression of IRF1, in an ATR kinase activity dependent manner. Therefore, our data provides a clear mechanistic link between the DDR with the regulation of IFN responses and the induction of innate immune gene expression in the absence of viral infection.
MATERIALS AND METHODS
Cells and reagents
HEK293T (Invitrogen, Carlsbad, CA, Cat no. R700-07) cell lines were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Lonza, Basel, Switzerland) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Flowery Branch, GA) and 100 I.U./ml penicillin and 100 mg/ml streptomycin (Pen/Strep) (Lonza). BJ/TERT cells (12) and derived cell lines were cultured in DMEM with 20% Medium 199 (Invitrogen, Carlsbad, CA, USA), 10% FBS, and Pen/Strep. Primary human foreskin fibroblasts were purchased from Lonza and cultured as per manufacturer’s instructions.
Hexydimethrine bromide (polybrene), cycloheximide (CHX), and Chk1 kinase inhibitor, UCN-01, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Blasticidin and Puromycin were obtained from Invivogen (San Diego, CA, USA). ATM kinase inhibitor, KU60019, was obtained from Astra Zeneca (London, UK). ATR kinase inhibitor ETP-46464 was previously described (18). DMSO (Fisher Scientific, Pittsburgh, PA, USA) was used as vehicle control.
The following antibodies were used in this study for immunoblot analysis (19): SV LT (pAb 416 and 419) (12), ISG56 and ISG60 (20), Sendai virus C-protein (21), OASL (Abgent, San Diego, CA, USA), IRF1, IRF7, IRF9, GFP, phospho-p53 (Serine 15), p53 (DO1), Actin, and Tubulin (Santa Cruz, Dallas, TX, USA), total STAT1, phospho-STAT1 Y701, and phospho-STAT1 S727 (Cell Signaling, Danvers, MA, USA).
Plasmids and viruses
Retroviral plasmids pLBNCX and pLBNCX-LT and plasmid pCMV-LT encoding SV40 LT cDNA have been previously described (12, 22). Plasmids pcDNA3.1/HisA/huIRF1, pCMV/FLAG-IRF7, and pCMV/p48 have been previously described (23, 24). shRNA lentiviral vectors targeting IRF1 (TRCN0000014669), IFNAR1 (TRCN0000059017), and scrambled control were purchased from Sigma-Aldrich. Vesicular stomatitis virus (VSV-GFP) (25) was grown in BHK21 cells. Encephalomyocarditis virus (EMCV), obtained from ATCC (VR-1479) (Manassas, VA, USA), and HSV-1 (K26) (26) were grown in Vero cells. Sendai virus (Cantell strain) was purchased from Charles River Laboratories (Wilmington, MA, USA). Multiplicity of infection (m.o.i) and virus titers were determined in their respective producer cell lines.
Retroviral and lentiviral vectors
Retroviral vectors pLBNCX and pLBNCX-LT were transfected into (1×107) packaging cell line (293-Ampho) using Fugene 6 (Roche, Mannheim, Germany) following manufacturer’s guidelines and processed as previously described (10). pLKO.1 shRNA delivery lentiviral vectors were packaged as previously described (19). BJ/TERT-derived pLKO.1 cells were generated by overnight lentiviral infection with virus packaged from pLKO.1 vectors in the presence of 8µg/ml polybrene. 48 hrs post-infection, cells were selected with 1µg/ml Puromycin for 7 days.
Luciferase reporter assays
HEK293T cells were used for luciferase assays as previously described (19). BJ/TERT LT cells were transfected with 1µg pGL3 Basic or pIFNβ125-luc plasmid using Lipofectamine 2000 (Invitrogen) and luciferase activity was assessed by Dual luciferase assay (Promega, Madison, WI, USA).
Quantitative PCR analysis of gene expression
Total RNA was extracted, cDNA was prepared and subjected to real-time PCR as previously described (19). Primers used for target gene amplification can be provided upon request.
Viruses and viral infections
For microscopy experiments, BJ/TERT vector or LT cells were seeded in a lab-tek® II chamber slide ™ (part #154534) and infected with VSV-GFP at 1 m.o.i. Cells were washed with PBS after 24 h infection and fixed in 4% paraformaldehyde followed by washing mounting in Vectashield (Vector Laboratories, Burlingame, CA) containing DAPI. To determine viral protein expression and viral growth, BJ/TERT vector or LT cells were infected with VSV-GFP (m.o.i 1), HSV-1 (K26) at an m.o.i 5, or EMCV (m.o.i 5). Infected cell supernatants were harvested at the indicated timepoints and kept at −80°C until further use. Infectious virus production was measured by plaque assay and reported as plaque forming units (PFU)/ml. Alternatively, BJ/TERT LT cells were pretreated with CHX (50ng/ml), washed twice with PBS, and incubated with 4 µm ETP-46464 or DMSO for 3 hrs prior to infection with EMCV (m.o.i. 50). Virus growth was quantified by plaque assay on Vero cells and expressed as plaque forming units (PFU)/ml. Cellular viability was determined by crystal violet staining.
IRF3 dimerization assays
BJ/TERT, BJ/TERT VECTOR, and BJ/TERT LT cells (1 × 106) were plated in 10 cm plates. BJ/TERT cells were infected with 300 HAU/ml of SeV for 8hrs. Cells were washed with PBS and lysed in 70 µl of lysis buffer (50mM Tris-HCl, pH7.5; 150 mM NaCl; 1mM EDTA; 1% NP-40; 2 mM Na3VO4; 10 mM NaF; 12 mM β-glycerophosphate). Lysates were then diluted in equal volumes of PAGE loading buffer (2×), resolved by Native PAGE, and blotted using anti-IRF3 antibody as previously described (20).
RESULTS
SV40 Large T antigen induces ISG expression
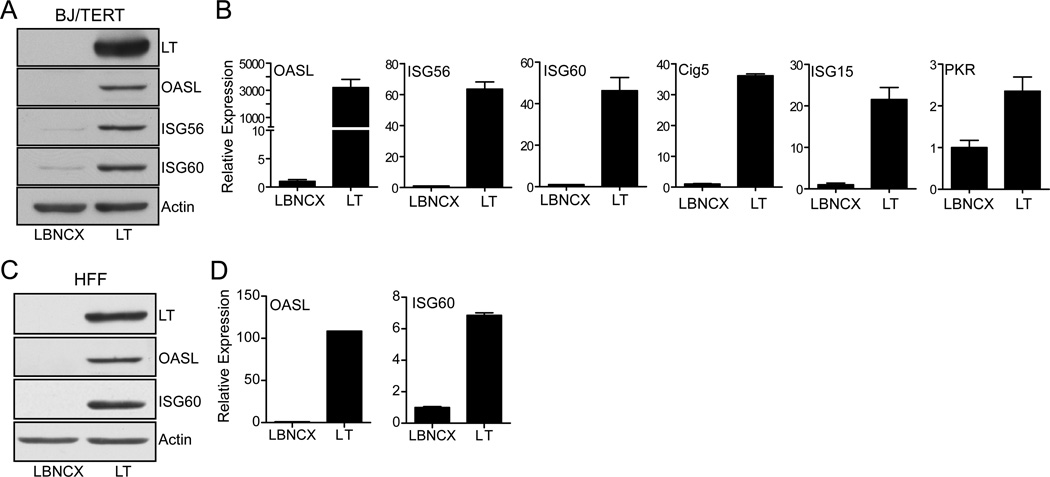
Previous studies that examined global changes in gene expression induced by the SV40 early region identified a significant number of interferon stimulated genes (ISGs) upregulated in mouse embryonic fibroblasts (17). To confirm whether LT alone can induce ISG without small t in human cells, we used retroviral transduction to generate immortalized human fibroblasts (BJ/TERT) stably expressing LT cDNA or empty vector (LBNCX). Expression of LT resulted in an increase in the protein levels of ISG60 (IFIT3) and the related protein ISG56 (IFIT1). Furthermore, another ISG, OASL was upregulated in LT expressing cells (Fig. 1A). The increase in protein synthesis was accompanied by an increase in ISG56, ISG60, and OASL mRNA transcription, suggesting that LT stimulates the transcription of these genes (Fig. 1B). Moreover, ISGs identified in the mouse genome arrays, Cig5, ISG15, and PKR, were also induced in human cells (Fig. 1B). To determine whether the ISG induction by LT is independent of telomerase (TERT) expression and immortalization, we expressed LT in primary human fibroblasts. Similar to BJ/TERT cells, ISG60 and OASL protein expression (Fig. 1C) and concomitant transcriptional upregulation (Fig. 1D) was observed with LT expression in primary human fibroblasts.
Fig. 1. SV40 Large T antigen induces ISGs.
(A) Induction of ISG protein expression in human fibroblasts. Lysates were prepared from BJ/TERT cells transduced by retrovirus infection with SV40 Large T antigen (LT) cDNA or empty vector (LBNCX) and probed with antibodies against LT, OASL, ISG56, IS60, and Actin.
(B) Transcriptional induction of ISG in human fibroblasts. Total RNA was extracted from BJ/TERT cells stably expressing SV40 LT cDNA or empty vector (described above). OASL, ISG56, and IS60, Cig5, ISG15, and PKR mRNA were assessed by quantitative RT-PCR. Expression of mRNA was normalized to the housekeeping gene RPL32 and expressed as fold change compared to empty vector expressing cells (value 1).
(C) Induction of ISG protein expression in primary human fibroblasts (HFF). Lysates were prepared from primary HFF cells transduced by retrovirus infection with SV40 Large T antigen (LT) cDNA or empty vector and probed with antibodies against LT, OASL, IS60, and Actin.
(D) Transcriptional induction of ISG60 and OASL in primary human fibroblasts. Total RNA was extracted from primary HFF cells stably expressing SV40 LT cDNA or empty vector. ISG60 and OASL mRNA was measured by qRT-PCR. Expression of mRNA was normalized to RPL32 and expressed as fold change compared to empty vector expressing cells (value 1).
ISGs expression generates an antiviral state
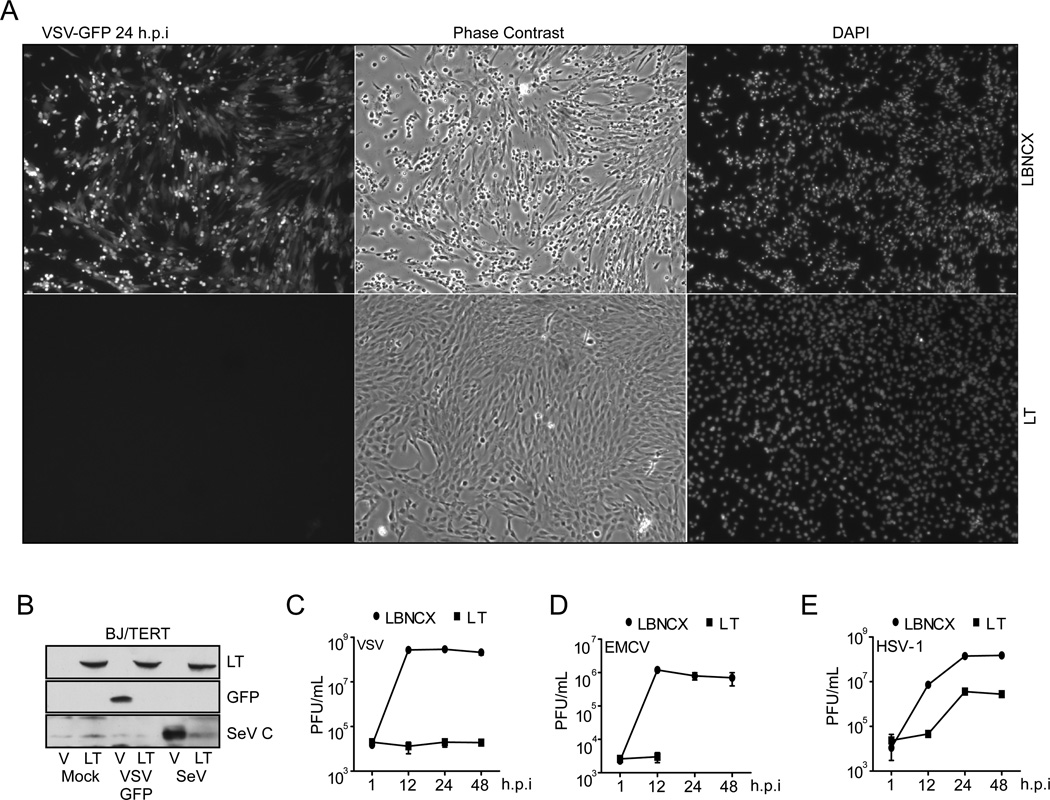
Given the strong upregulation of ISGs, we investigated whether LT-expression protected fibroblasts from viral infection. BJ/TERT cells were infected at an m.o.i of 1 with vesicular stomatitis virus (VSV-GFP), a (−) single-stranded RNA (ssRNA) virus., and incubated for 24hrs following infection. Fluorescence microscopic analysis at 24hrs postinfection showed readily detectable GFP expression in empty vector expressing cells and VSV-induced cytopathic effects, but no detectable GFP expression or cytotoxicity in cells stably expressing LT (Fig. 2A). Similarly, immunoblot analysis of GFP expression in VSV-GFP infected cells showed a marked decrease in GFP protein in cells expressing LT (Fig. 2B). The decrease in viral protein synthesis was also observed after infection with Sendai virus (SeV), another (−) ssRNA virus. BJ/TERT LT cells infected with SeV for 24hrs, failed to synthesize the major capsid protein (C-protein) confirming that LT expression restricts viral protein synthesis upon RNA virus infection (Fig. 2B). To examine the overall effect on virus replication, BJ /TERT expressing LT and vector control cells were infected with VSV and EMCV, a (+) ss-RNA virus, and viral growth was assessed as indicated. The growth of VSV (Fig. 2C) and EMCV (Fig. 2D) was severely impaired in cells expressing LT. We also tested LT-mediated inhibition of a dsDNA virus, HSV-1, and saw a 2-log reduction in virus production relative to control cells (Fig. 2E). Taken together, these results suggest that the LT-mediated increase in ISG expression protects cells from viral infection.
Fig. 2. Expression of LT induces an antiviral state.
(A) GFP expression in VSV-GFP infected BJ/TERT cells. BJ/TERT cells expressing SV40 LT cDNA or vector control were infected with VSV-GFP (m.o.i 1) for 24hrs. GFP expression was detected by fluorescence microscopy (left), cell morphology was examined by phase contrast microscopy (middle). Nuclei were stained with DAPI (right).
(B) Analysis of viral protein expression in infected BJ/TERT cells. BJ/TERT cells expressing SV40 LT cDNA or vector control were infected with VSV-GFP (m.o.i. 5) or SeV (200 HAU/ml) for 24 hrs. Lysates were prepared and probed with antibodies against LT, GFP, and SeV C-protein.
(C) Analysis of VSV growth in BJ/TERT cells. BJ/TERT cells expressing SV40 LT cDNA or vector control were infected with VSV-GFP (m.o.i. 5). Supernatants were harvested at the indicated time-points and virus replication was measured by plaque assay on BHK21 cells. Virus growth is expressed as plaque forming units (PFU)/ml.
(D) Analysis of EMCV growth in BJ/TERT cells. BJ/TERT cells expressing SV40 LT cDNA or vector control were infected with EMCV (m.o.i. 5). Supernatants were harvested at the indicated time-points and virus replication was measured by plaque assay on Vero cells. Virus growth is expressed as PFU/ml.
(E) Analysis of HSV-1 growth in BJ/TERT cells. BJ/TERT cells expressing SV40 LT or vector control were infected with HSV-1 (K26-GFP) (m.o.i. 5). Supernatants were harvested at the indicated time-points and virus replication was measured by plaque assay on Vero cells. Virus growth is expressed as PFU/ml.
IFN receptor amplifies the induction of ISGs
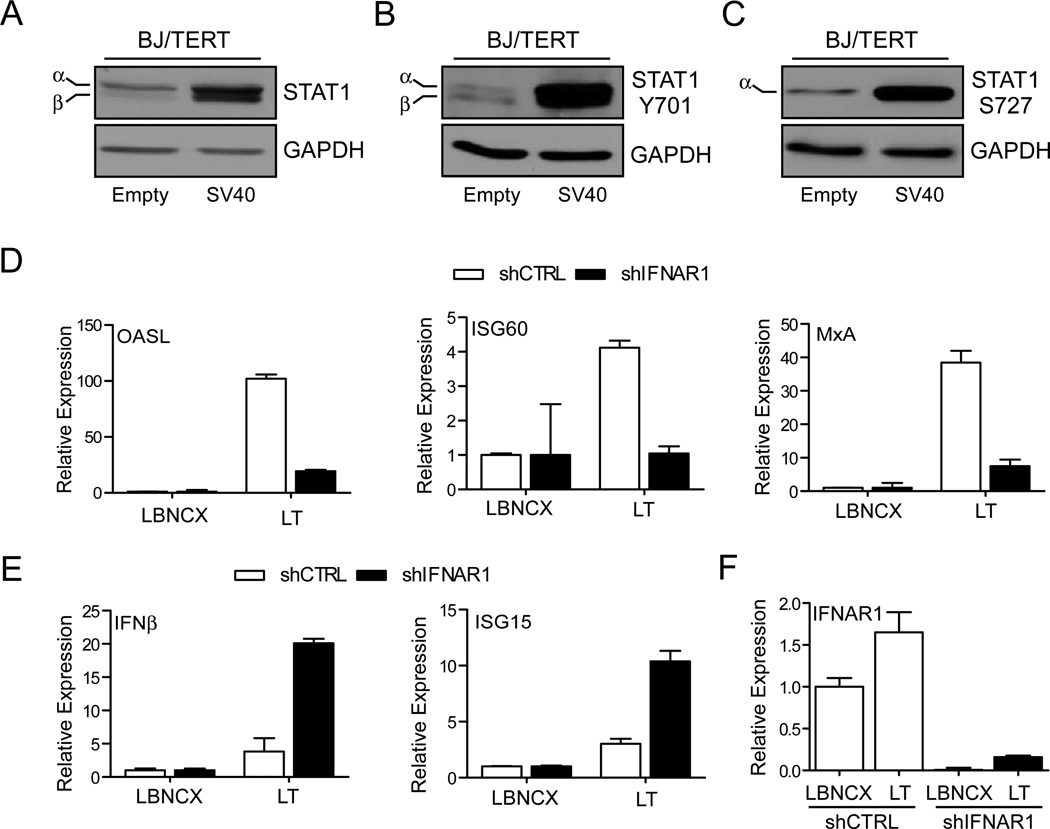
In the context of a viral infection, the induction of ISGs generally follows nucleic acid sensing by cytoplasmic and nuclear receptors, which activate IRF3 and induce IFNβ and ISGs. IFNs then bind to the IFN receptor, IFNAR1, activating the JAK/STAT signaling pathway. This results in the phosphorylation of STAT1 at Tyr701 and Ser727 and the formation of the STAT1/STAT2/IRF9 (ISGF3) signaling complex that further regulates the expression of ISGs (27–29). Analysis of STAT1 protein expression and activation showed that LT cDNA is not only sufficient to induce STAT1 protein expression (Fig. 3A), but also enhances its phosphorylation at Tyr701 (Fig. 3B) and Ser727 (Fig. 3C). These observations suggest that the expression of LT induces IFN signaling. To confirm that the induction of ISGs in human fibroblasts is mediated by IFN signaling, we examined the effect of IFNAR1 knockdown in BJ/TERT cells, which fail to induce ISG expression after stimulation with type I IFN (Supplementary Fig. 1). As expected, expression of LT resulted in increased ISG mRNA expression in cells expressing a non-targeting shRNA. The downregulation of IFNAR1 resulted in a decrease in ISG60, OASL, and the interferon responsive gene, MxA mRNA (Fig. 3D), indicating that a type I IFN protein is involved in the induction of ISGs in human cells.
Fig. 3. IFNAR-dependent and -independent induction of ISGs.
(A–C) STAT1 activation in human fibroblasts by LT expression. BJ/TERT cells were transfected with SV40 early region or vector and placed under selection with Puromycin (1µg/ml) for 7 days. Whole cell extracts were prepared from antibiotic resistant cells. Lysates were probed with antibodies against total STAT1 (A), STAT1 Y701 (B), STAT1 S727 (C), and GAPDH.
(D) Analysis of ISG induction in IFNAR1 knockdown cells. BJ/TERT cells were infected with lentivirus encoding a short hairpin targeting IFNAR1 or scrambled control. Cells were then selected with puromycin (1 µg/ml). Puromycin resistant cells were then infected with a retrovirus encoding the SV40 LT cDNA or empty control (Vector) and selected for resistance to blasticidin (5 µg/ml). Total RNA was harvested and expression of OASL, ISG60, and MxA mRNA was analyzed by qRT-PCR. Samples were normalized to RPL32 and expressed as fold change with respect to vector control cells (value 1).
(E) Analysis of IFNβ and ISG15 induction in IFNAR1 knockdown cells. RNA was harvested from BJ/TERT cells expressing either empty vector or SV40 LT cDNA as well as a short hairpin targeting IFNAR1 (described above). Expression of IFNβ and ISG15 mRNA was analyzed by qRT-PCR. Samples were normalized to RPL32 and expressed as fold change with respect to vector control cells (value 1).
(F) Validation of IFNAR1 knockdown in BJ/TERT cells. RNA was harvested from BJ/TERT cells expressing either empty vector or SV40 LT cDNA as well as a short hairpin targeting IFNAR1. Expression of IFNAR1 mRNA was analyzed by qRT-PCR. Samples were normalized to RPL32 and expressed as fold change with respect to vector control cells expressing the non-targeting shRNA (value 1).
The ectopic expression of LT resulted in the stimulation of IFNβ transcription in cells expressing the scrambled shRNA control (shCTRL). Downregulation of IFNAR1 resulted in enhanced IFNβ expression, likely due to the lack of a negative feedback loop required to dampen its induction (Fig. 3E, left). The transcription of ISG15 followed the same pattern of expression as IFNβ (Fig. 3E, right), suggesting that transcriptional activation of both genes is controlled by the same factor involved in the early phase of the LT-mediated interferon synthesis pathway in contrast the upregulation of ISG60, OASL, and MxA is largely dependent on IFNAR1-mediated signaling. Finally, we verified the downregulation of IFNAR1 expression in shIFNAR1 cells expressing either the empty vector or LT. While the expression of LT led to a slight increase in IFNAR1 mRNA expression, targeting of IFNAR1 with shRNA led to nearly 90% decrease in mRNA expression in both LBNCX and LT expressing cells lines (Fig. 3F). Together, the data suggests that in human fibroblasts, LT activates cellular factors involved in the transcriptional upregulation of IFNβ and consequently inducing ISGs through IFNAR1.
IRF protein expression is upregulated by LT
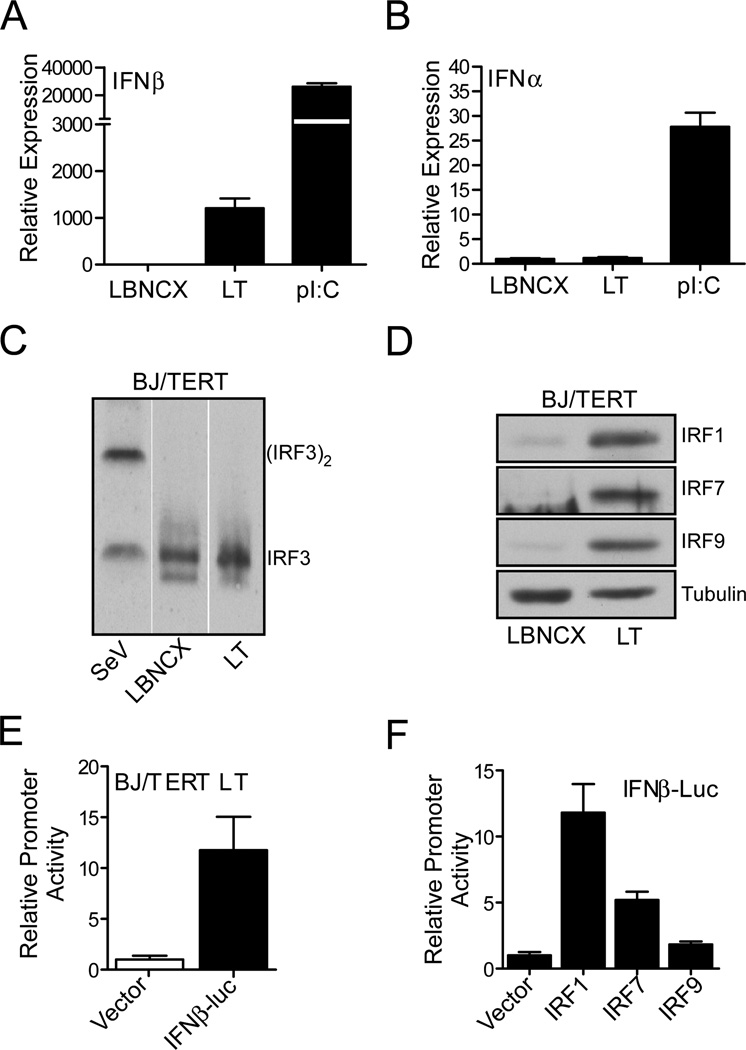
Given that IFNβ gene expression was detected in LT expressing cells, we postulated that the mechanism of ISG induction in human fibroblast relied on factors involved in the regulation of IFN transcription. As observed in shCTRL and shIFNAR1 expressing cells, IFNβ mRNA was readily detectable in BJ/TERT cells stably expressing LT or BJ/TERT cells treated with p(I):(C) overnight. However, the induction of IFNβ mRNA following p(I):(C) treatment was 20 times greater than that observed by LT expression (Fig. 4A). To our surprise, the induction of type I IFN by LT was restricted to the stimulation of IFNβ transcription as no transcription of IFNα genes was detected in BJ/TERT LT cells by qRT-PCR analysis using Pan-IFNα primers. We examined the response of BJ/TERT cells to p(I):(C) and were able to measure a readily detectable induction of IFNα transcription (Fig. 4B). These results indicate that LT activates factors that lead to induction of IFNβ in the absence of IFNα induction.
Fig. 4. SV40 Large T antigen induces IFNβ expression.
(A and B) SV40LT induction of type I IFN mRNA. BJ/TERT cells were stimulated with 100 mg/ml poly(I):(C) for 24hrs or stably transduced with empty vector or SV40 LT cDNA by retroviral infection and blasticidin selection. Total RNA was extracted from 1×107 cells and IFNβ (A) and Pan-IFNα (B) mRNA levels were analysed by qRT-PCR. mRNA expression was normalized to RPL32 and expressed as fold change relative to vector control expressing cells (value 1).
(C) IRF3 dimerization assay. Lysates were prepared from BJ/TERT cells stably expressing vector control, SV40 LT cDNA, or cells infected with 300 HAU/ml for 8hrs. Protein was resolved by Native PAGE and probed with anti-IRF3 antibodies.
(D) Modulation of IRF gene expression by SV40 LT. Lysates were prepared from BJ/TERT cells stably expressing vector control or SV40 LT cDNA and probed with antibodies against the antiviral mediators IRF1, IRF7, and IRF9 as well as Tubulin.
(E) SV40 LT expression induces the IFNβ promoter activity. BJ/TERT LT cells (4×105) were transiently transfected with 1 µg of IFNβ luciferase reporter construct or pGL3 basic vector control using Lipofectamine 2000 at a 1:2 DNA to transfection reagent ratio. Six hours post transfection, fresh culture medium was added to cells and cells were incubated for 48hrs prior to measurement of luciferase activity. Firefly luciferase was measured by Dual-luciferase assay (Promega) and normalized to empty vector expressing cells (value 1).
(F) Induction of IFNβ promoter activity by IRF proteins. HEK293T (1.5×105) cells were co-trasnfected with pcDNA, IRF1, IRF7, or IRF9 cDNA (250ng), IFNβ125-luc (400ng) and pRL-null (50ng). 24hrs post transfection cells were collected by trypsin-EDTA transfection and seeded in white-walled 96-well plates. Twenty four hours later, firefly and renilla luciferase activity was measure by Dual-luciferase assay (Promega). Firefly luciferase activity was normalized to renilla luciferase activity and promoter activity was expressed as fold change relative to vector control transfected cells (value 1).
The major regulators of transcription in the IFN response pathways are the IRF family of proteins. Five of these proteins, IRF1, -3, -5, -7, and -9, are ubiquitously expressed and positively regulate the transcription of IFN and ISGs. The activation of IRF3 leads to its dimerization and translocation into the nucleus, leading to the induction of IFNβ transcription. To determine whether the expression of LT induces the activation of IRF3, we examined the presence of IRF3 dimers in LT and SeV infected cells. While IRF3 dimers were readily detected in BJ/TERT cells 8hrs after SeV infection, the expression of LT or vector control failed to promote IRF3 dimerization (Fig 4C). With the exception of IRF3, the induction of IRF protein synthesis acts as a regulatory step of their transcriptional activities. Analysis of IRF protein expression showed a significant increase in IRF1, IRF7, and IRF9 in cells expressing LT (Fig. 4D). However, the induction of IFNβ is likely mediated by either IRF1 or IRF7, as IRF9 acts downstream of IFNAR1. We verified the transcriptional induction of IFNβ promoter activity by using transient transfection of the IFNβ125-luc in BJ/TERT cells and recorded a 10-fold increase in luciferase activity relative to the luciferase activity observed in pGL3-basic transfected cells (Fig. 4E). To examine the effect of these proteins on IFNβ promoter activation, we co-transfected HEK293T cells with IRF1, IRF7, or IRF9 cDNA and IFNβ125-luc and pRL-null as transfection controls. As expected, expression of IRF1 resulted in over 10-fold increase in promoter activity while IRF7 transduction resulted in a 5-fold induction in promoter activity relative to vector control transfected cells (Fig. 4F). Further, the expression of IRF9 did not significantly activate the IFNβ promoter activity excluding the possibility that IRF9 is involved in the direct activation of IFNβ transcription in BJ/TERT LT cells.
IRF1 is required for the induction of IFNβ by LT in human fibroblasts
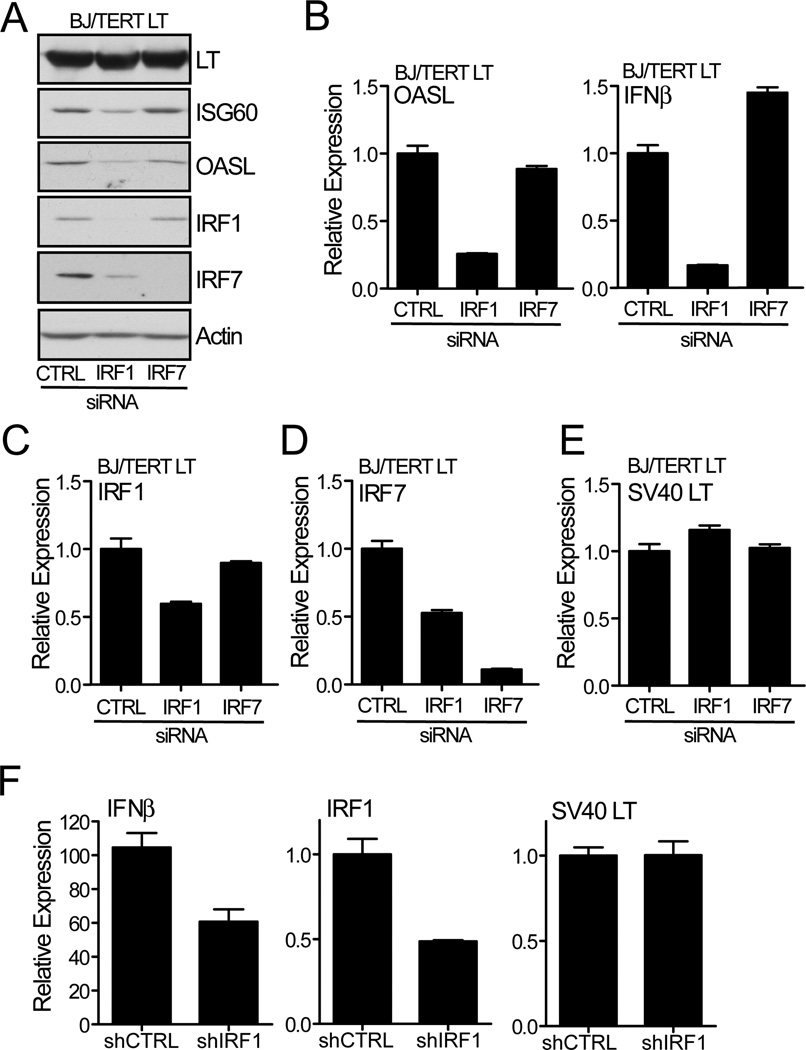
To determine whether either IRF1 or IRF7 is required for the induction of IFNβ by LT, we knocked-down the expression of these genes by transfecting siRNA targeting IRF1 or IRF7 into BJ/TERT LT cells. The downregulation of IRF1, specifically, was accompanied by a decrease in the expression of ISGs, as determined by the loss of OASL and ISG60 protein expression. Further, the loss of IRF1 expression led to a decrease of IRF7, which not only regulates ISG expression but is also an IFNβ-responsive ISG (30). Knockdown of IRF7 did not affect the expression of either ISGs or IRF1 (Fig. 5A). The loss of ISG expression was due to a decrease in transcriptional activation of these genes as determined by the decrease in mRNA levels following the knockdown of IRF1. Downregulation of IRF1 in BJ/TERT LT cells also led to a reduction in IFNβ mRNA, explaining the concomitant loss of ISG expression. Again, IRF7 knockdown had no effect on the transcription of ISGs and resulted in a slight increase in IFNβ transcription (Fig. 5B). Knockdown of either IRF1 or IRF7 had a similar effect on the transcription of OASL, likely due to the loss of IFNβ synthesis. We then examined the effect of knocking down IRF1 and IRF7 on the expression of these genes. Transient targeting of IRF1 resulted in a 50% loss in IRF1 mRNA expression, while targeting IRF7 had no effect on the transcription of IRF1 (Fig. 5C). On the other hand, silencing of IRF1 led to a 50% decrease in IRF7 transcription indicating that the expression of IRF7 is induced downstream of IRF1. Specific targeting of IRF7 led to ~ 90% reduction in mRNA expression (Fig. 5D). The modulation of gene expression that followed the downregulation of IRF1 was not due to changes in LT, as equivalent protein and mRNA expression was detected after downregulation of either gene (Fig. 5 E and Fig. 5A).
Fig. 5. Induction of IRF1 transactivates IFNβ.
(A) Requirement for IRF1 in the induction of ISG protein synthesis. Briefly, BJ/TERT LT cells were plated in 60 mm dishes and transfected with 160 pmoles of siRNAs pools targeting IRF1 (target sequences; 5’-CGTGTGGATCTTGCCACATTT -3’ and 5’-CCTCTGTCTATGGAGACTTTA -3’), IRF7 (M-011810-02-0005, Thermo Fisher, Pittsburgh, PA) or control (D-001220-01-05, Thermo Fisher) using 6 µl of Lipofectamine RNAiMAX (Invitrogen). After 48 hrs, lysates were prepared and probed with antibodies against OASL, ISG60, SV40 LT, IRF1, IRF7, and Actin.
(B–E) IRF1 and IRF7 requirement for the transcriptional induction of IFNβ and OASL. IFNβ (B), IRF1 (C), IRF7 (D), and SV40 LT (E) mRNA levels were assessed by qRT-PCR. BJ/TERT LT cells were transfected with siRNA against IRF1 and IRF7 as described above. Total RNA was harvested and cDNA was synthezised. Expression of target genes was determined by qRT-PCR analysis and normalized to RPL32 and control siRNA transfected cells (value 1).
(F) Analysis of IFNβ mRNA expression in IRF1 knockdown cells. BJ/TERT cells were stably transduced with an shRNA targeting IRF1 or a non-targeting shRNA. Cells were then stably transduced with vector control or LT encoding retrovirus. Total RNA was harvested and mRNA expression was determined by qRT-PCR. Expression of target genes was normalized to RPL32 and reported relative to the enhancement observed over empty vector expressing cells (value 1).
We further supported our siRNA studies by measuring the induction of IFNβ mRNA in cells stably expressing an IRF1-targeting shRNA. IRF1 knockdown in BJ/TERT cells resulted in a 40% loss of IFNβ transcript and a 50% decrease in IRF1 without any effect on the expression of SV40 LT (Fig. 5F). These results suggest IRF1 controls IFNβ transcription and that the response to IFNβ secretion leads to the IFNAR1 dependent activation of IRF7 and IRF9, which then participate in the enhanced expression of ISGs like OASL, ISG60, and MxA.
ATR Kinase activity is necessary for the induction of IRF1 and IFNβ
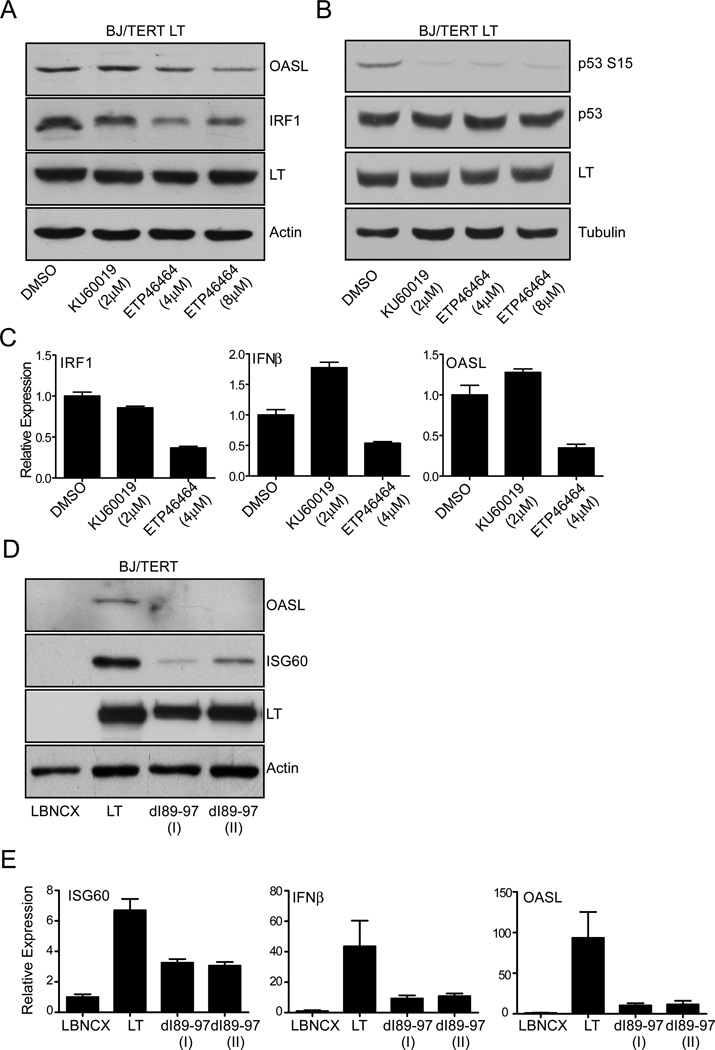
Expression of LT has been shown to induce DNA damage, and ATM and ATR kinase mediated DDR (8, 9, 12). Since the induction of a DDR has previously been associated with the accumulation of IRF1 protein (31), we examined whether the inhibition of either ATM or ATR kinase activity in cells stably expressing LT would cause a decrease in IRF1 and ISG expression. Treatment of BJ/TERT LT cells with a specific ATM kinase inhibitor, KU60019 (32, 33), did not affect the expression of either IRF1 or OASL as compared to controls. Treatment with ETP-46464, a specific ATR kinase inhibitor (33, 34) led to a dose dependent decrease in OASL and IRF1 protein expression (Fig. 6A). While LT is known to be phosphorylated by ATM (11), ATR kinase inhibition did not affect the LT protein stability (Fig. 6A; Supplementary Fig. 2). We validated ATM and ATR kinase inhibition by examining the phosphorylation p53 at Ser15. Both KU60019 and ETP-46464 treatment led to a decrease in Ser15 phosphorylation without affecting the stability of p53 in BJ/TERT LT cells (Fig. 6B). In order to determine whether ATR kinase activity is involved in the transcriptional upregulation of IRF1, we quantified the levels of IRF1 mRNA after treatment of BJ/TERT LT cells with KU60019 and ETP-46464. ATR kinase inhibition with ETP-46464 caused a decrease in the transcription of IRF1 mRNA, while ATM kinase inhibition with KU60019 had no effect on IRF1 mRNA expression. Likewise, ATR kinase inhibition led to a decrease in IFNβ mRNA levels, accounting for both the decrease in OASL mRNA and protein expression (Fig. 6C).
Fig. 6. ATR kinase activity is necessary for the induction of IFNβ and subsequent ISG expression.
(A) Analysis of ISG induction in ATM/ATR kinase inhibitor treated BJ/TERT LT cells. Lysates were prepared from BJ/TERT LT cells stimulated for 24hrs with the indicated doses of KU60019 or ETP46464. OASL, IRF1, LT, and Actin proteins were detected by immunoblot analysis.
(B) Analysis of p53 phosphorylation in ATM/ATR kinase inhibitor treated BJ/TERT LT cells. Cells were treated as described above. Lysates were prepared, and probed with antibodies against phosphorylated p53 (Serine 15), total p53, and Actin.
(C) Analysis of IFNβ mRNA in ATM/ATR kinase inhibitor treated BJ/TERT LT cells. Total RNA was prepared from BJ/TERT cells stimulated for 24hrs with 2 µM KU60019 or 4 µM ETP-46464. Expression of IFNβ, IRF1, and OASL mRNA expression was determined by qRT-PCR. Expression of target genes was normalized to the housekeeping gene RPL32 and reported relative to the enhancement observed over empty vector expressing cells (value 1).
(D) Analysis of induction of ISGs by LT mutants defective in DDR induiction. Lysates were prepared from BJ/TERT cells stably expressing either the wt LT or LT dI89-97. Expression of OASL, ISG60, LT, and Actin was detected by immunoblot analysis.
(E) Analysis of type I IFN and ISG mRNA induction by LT mutant. Total RNA was harvested from BJ/TERT cells expressing LT, dI89-97, or empty vector. ISG60, IFNβ, and OASL mRNA expression was determined by qRT-PCR. Expression of target genes was normalized to the housekeeping gene RPL32 and reported relative to the enhancement observed over empty vector expressing cells (value 1).
It has previously been reported that deletion of amino acids 89–97 in LT (dl89–97) results in the ablation of its binding to Bub1 and a reduction in the activation of DDR by LT (12). To provide genetic evidence to support the involvement of LT-mediated DDR in inducing IFNβ, we analyzed OASL and ISG60 protein expression in cells stably transduced with either full length LT, dl89–97 or vector control (Fig. 6D). For dI89–97 mutant expressing cells we used two different batches of BJ/TERT cells, which were generated independently at two different times (dI89–97(I) and dI89–97(II)). As previously observed, LT expression resulted in a robust induction of both ISG60 and OASL expression. However, dl89–97 expression resulted in attenuated ISG induction in both cell lines expressing dI89–97 (Fig. 6D and Supplementary Fig. 2). The reduction in ISG expression was accompanied by diminished IFNβ, ISG60 and OASL mRNA induction in dl89–97 expressing cells (Fig. 6E).
We then confirmed that the induction of DNA damage, in the absence of LT expression could upregulate the expression of IRF1. BJ/TERT cells were UV irradiated and treated with ATM and ATR kinase inhibitors. UV irradiation resulted in the stabilization of p53 in all instances. The induction of IRF1 was comparable in BJ/TERT cells treated with KU60019 (ATM inhibitor) to the induction observed in DMSO treated cells. ATR kinase inhibition with KU60019 resulted in a marked decrease in the induction of IRF1 protein synthesis (Supplementary Fig. 3A). Thus, activating ATR kinase signaling pathway is sufficient for the induction of IRF1. To further dissect the mechanism of IRF1 induction, we examined the involvement of the ATR substrate Chk1. We treated BJ/TERT LT cells with the Chk1 inhibitor, UCN-01 and observed a decrease in IRF1 and OASL protein expression, relative to vehicle treated cells. Similarly, the levels of phosphorylated p53 were reduced upon Chk1 kinase inhibition (Supplementary Fig. 3B). Together, these results confirm that the activation of the ATR-Chk1 axis in response to DNA damage promotes the expression of IRF1.
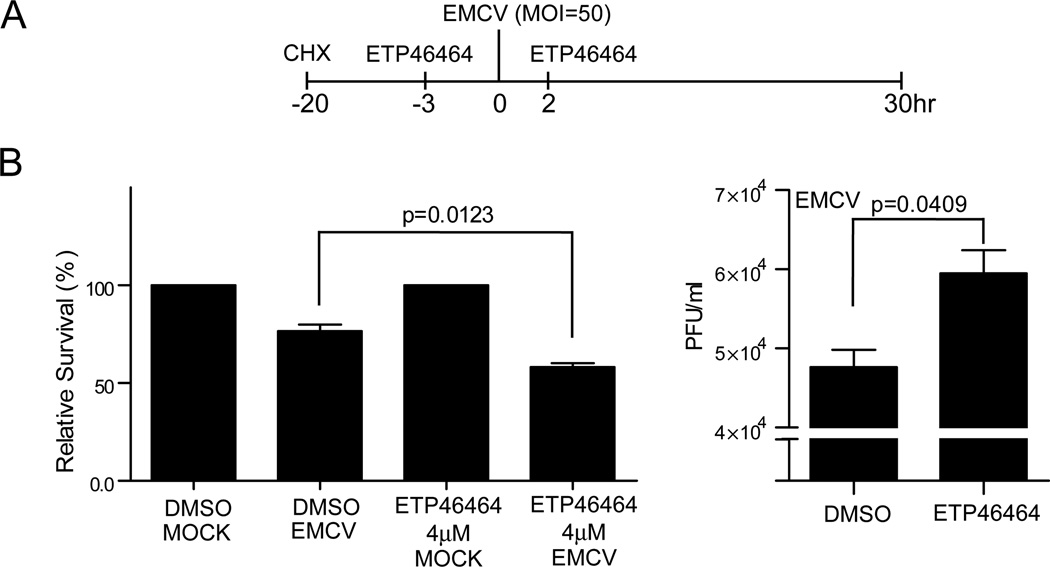
Finally, we asked whether the inhibition of IFNβ expression, through the inhibition of ATR kinase activity, reversed the protection of fibroblast from viral infection afforded by LT expression. First, we treated BJ/TERT LT cells with CHX to inhibit IFNβ protein synthesis. Then, we pre-incubated cells with ATR kinase inhibitor followed by adsorption of EMCV onto the cells and further incubation with either ETP-46464 or DMSO (Fig. 7A). ATR kinase inhibition decreased the LT protection of cells from virus mediated cell death as we observed a significant decrease (~25%) in cell survival relative to the induction of cell death in DMSO treated cells (Fig. 7B, left). The loss in viability was accompanied by a 25% increase in viral growth in BJ/TERT LT cells with inhibited ATR kinase activity (Fig. 7B, right). Taken together, our results suggest that ATR acts as a mediator of antiviral responses through the induction of IFNβ and ISG expression.
Fig. 7. ATR kinase activity is necessary to generate and antiviral state.
(A and B) EMCV growth in BJ/TERT LT cells treated with ETP-46464. In brief, BJ/TERT LT cells were plated in 12-well plates (80% confluency). Cells were treated with CHX (50 ng/ml) for 17hrs prior to viral infection. Three hours prior to EMCV infection, cells were treated with 5 µM of ETP-46464. Cells were then infected with EMCV (m.o.i 50) (A). Infected cells were stained with crystal violet to determine cell survival relative to mock-infected cells. Briefly, Infected cells were stained with 0.1% crystal violet (10% ethanol) overnight. Plates were washed to remove excess stain with distilled water and left to air-dry overnight. The retained crystal violet was solubilized in 600 µl 2% SDS solution in PBS for 30 minutes at room temperature with constant shaking. Absorbance at 550 nm was then used to determine crystal violet retention (B, left). Supernatants were harvested 30hrs post infection and viral growth in DMSO or ATR kinase inhibitor treated infected cells was determined by plaque assay on Vero cells (B, right).
DISCUSSION
We have defined a signaling mechanism through which stable expression of SV40 LT induces the expression of IFN-stimulated genes. Our results indicate that depending on the particular gene, the induction of ISGs occurred in both an IFNAR1-dependent and IFNAR1-independent manner. This suggests that LT expression results in the induction of factors that control the expression of IFNs. Indeed, we observed a strong upregulation of IFNβ mRNA by LT accompanied by increased expression of regulators of IFN responses: IRF1, IRF7, and IRF9. Furthermore, we show that both LT expression and UV irradiation induced DDR results in ATR kinase-dependent increase in IRF1 expression causing upregulation of IFNβ expression. Thus, our study uncovers a unique interface between the DDR and the IFNβ–mediated antiviral response that is largely mediated by ATR kinase.
Classic IFN induction has been viewed as being almost exclusively caused by virus infection and sensing of viral nucleic acid. However, recent studies point to several other situations when infection-independent IFN-ISG induction has been reported (35, 36). Here, we show a unique mechanism for LT mediated induction of IFN that is distinct from previous mechanisms and provide a mechanistic connection between DDR and IFN induction. The DDR is a complex cellular network involved in the detection of genotoxic stress, which triggers cell-cycle arrest and DNA repair or triggers apoptosis if the insult fails to be repaired. Early studies have connected DDR to IRF1 in a different context (31, 37–39). Discovered as an important mediator of IFN responses (40–42), the role of IRF1 in the regulation of other stress responses is still not clearly understood. Studies have implicated IRF1, along with p53, in the induction of genes involved in the regulation of cell growth, susceptibility to transformation by oncogenes and the induction of apoptosis (37). Mechanistic studies examining the regulation of IRF1 expression have linked ATM as well as NF-κB with the induction of IRF-1 in various cells (31). Other studies have also identified NF-κB as a crucial factor involved in the induction of IRF1 and IRF7 expression in human epithelial cells (36, 43). Interestingly, the activation of ATR in Arabidopsis serves to potentiate plant immunity (44). Thus, it is likely that the triggering of DDR is an evolutionarily conserved mechanism to protect genome integrity and to some extent induce an antiviral response against pathogen infection.
In the context of virus infection much attention has been devoted towards understanding the complex molecular interactions between DNA viruses and the DDR (Reviewed in (45, 46)). Detection of viral genomes as damaged DNA, the expression of viral oncogenes that deregulate cell-cycle checkpoints and promote replicative stress, and the induction of reactive oxygen species are all triggers for a response that can have potentially deleterious consequences for both the virus and the infected cell. On the other hand, other studies have elucidated ways in which invading pathogens hijack these pathways during the course of infection to promote efficient replication of their genomes (46). HSV-1 relies on the induction of both ATM and ATR activity early in infection to stimulate the replication of viral genomes, while effectively degrading ATR at later time points to successfully complete the infectious cycle (47, 48). Similarly, the polyomavirus (7–10, 12, 49) and human papillomaviruses (50) also induce the DDR machinery for viral replication while countering these responses during the late phases of viral replication for the completion of the infectious cycle. Here we have shown that while the induction of ATM and ATR-triggered signaling cascades lead to the activation of proteins that benefit viral replication and immune evasion, the ATR-signaling arm specifically acts as a sensor of virus induced replicative stress and potentially promotes viral elimination through the induction of IFN responses in human fibroblasts. Using a virally encoded oncogene and investigating the apical kinases of the DDR, ATM and ATR, we were able to distinguish the signaling pathways and shed light on the connection between DDR and antiviral IFN responses.
Interestingly, RNA viruses, like HIV-1 (51), avian infectious bronchitis virus (52), Hepatitis C virus (53), and Rift Valley Fever (54), have also been shown to trigger DNA-damage responses. Thus, it is likely that these viruses have developed strategies to overcome the blockade imposed by the induction of DDR-mediated IFN induction. Indeed, studies provide evidence that Rift Valley Fever Virus non-structural (NS) proteins induce replicative stress that triggers the ATM signaling pathway and enhances viral replication. On the other hand, NSs target ATR-signaling, specifically, to further promote viral growth (54). While we and others have provided evidence that the ATR signaling pathway is involved in the regulation of immune responses, the overall role of ATR in mediating antiviral responses still remains to be further explored.
DNA tumor viruses not only benefit from the induction of effector genes that can promote genome replication, but also have developed strategies to prevent the arrest in cellular replication, cellular death, and other potential antiviral effects mediated by DDR (45). In particular, SV40 LT can efficiently abrogate the transcriptional functions of p53, preventing the induction of apoptosis (4). This in turn can result in the abrogation of a negative regulation of DDR and contribute to the constitutive secretion of low levels of IFNβ in human fibroblasts transduced with LT. Elevated levels of IFNβ induce the expression of genes involved in the IFN responsive pathway, which have been previously shown to be upregulated in certain types of cancer and promote poor prognosis and response to therapy (55–62). Furthermore, some of the effector genes of the IFN response are epigenetic modifiers that promote nuclear programming and modify cellular gene expression (63). Thus, we propose that the induction of DNA damage and IFN responses by LT, in the absence of viral infection, contributes to the global changes in gene expression observed in LT transformed cells and adds to the transformative capacity of LT.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. Armin Gamper and Tatiana Moiseeva for technical assistance.
This work was supported in part by NIAID AI082673 (SNS), NIH RO1 98956 (JMP) and NIH RO1CA148644 (CJB). This project used the UPCI core facilities and was supported in part by award P30CA047904.
ABBREVIATIONS
- IFN
Interferon
- IRF
Interferon Regulatory Factor
- ISG
Interferon Stimulated Gene
- SV40
Simian Virus 40
- LT
Large T antigen
- ATM
Ataxia-telangiectasia mutated
- ATR
ATM and Rad3 related
- DDR
DNA damage response.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTION
AF and NG performed experiments; AF, OVG, CJB, JMP, and SNS designed experiments; AF and SNS wrote the manuscript.
REFERENCES
- 1.Zurhein G, Chou SM. Particles Resembling Papova Viruses in Human Cerebral Demyelinating Disease. Science. 1965;148:1477–1479. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]
- 2.Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1:1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 3.Bennett SM, Broekema NM, Imperiale MJ. BK polyomavirus: emerging pathogen. Microbes Infect. 2012;14:672–683. doi: 10.1016/j.micinf.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pipas JM. SV40: Cell transformation and tumorigenesis. Virology. 2009;384:294–303. doi: 10.1016/j.virol.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Marechal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. doi: 10.1016/j.cell.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Dahl J, You J, Benjamin TL. Induction and utilization of an ATM signaling pathway by polyomavirus. J Virol. 2005;79:13007–13017. doi: 10.1128/JVI.79.20.13007-13017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orba Y, Suzuki T, Makino Y, Kubota K, Tanaka S, Kimura T, Sawa H. Large T antigen promotes JC virus replication in G2-arrested cells by inducing ATM- and ATR-mediated G2 checkpoint signaling. J Biol Chem. 2010;285:1544–1554. doi: 10.1074/jbc.M109.064311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang M, Zhao L, Gamez M, Imperiale MJ. Roles of ATM and ATR-mediated DNA damage responses during lytic BK polyomavirus infection. PLoS Pathog. 2012;8:e1002898. doi: 10.1371/journal.ppat.1002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boichuk S, Hu L, Hein J, Gjoerup OV. Multiple DNA damage signaling and repair pathways deregulated by simian virus 40 large T antigen. J Virol. 2010;84:8007–8020. doi: 10.1128/JVI.00334-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Dodson GE, Shaikh S, Rundell K, Tibbetts RS. Ataxia-telangiectasia-mutated (ATM) is a T-antigen kinase that controls SV40 viral replication in vivo. J Biol Chem. 2005;280:40195–40200. doi: 10.1074/jbc.C500400200. [DOI] [PubMed] [Google Scholar]
- 12.Hein J, Boichuk S, Wu J, Cheng Y, Freire R, Jat PS, Roberts TM, Gjoerup OV. Simian virus 40 large T antigen disrupts genome integrity and activates a DNA damage response via Bub1 binding. J Virol. 2009;83:117–127. doi: 10.1128/JVI.01515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao F, Hou NB, Yang XL, He X, Liu Y, Zhang YH, Wei CW, Song T, Li L, Ma QJ, Zhong H. Ataxia telangiectasia-mutated-Rad3-related DNA damage checkpoint signaling pathway triggered by hepatitis B virus infection. World journal of gastroenterology : WJG. 2008;14:6163–6170. doi: 10.3748/wjg.14.6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowd GA, Li NY, Fanning E. ATM and ATR activities maintain replication fork integrity during SV40 chromatin replication. PLoS Pathog. 2013;9:e1003283. doi: 10.1371/journal.ppat.1003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikushima H, Negishi H, Taniguchi T. The IRF Family Transcription Factors at the Interface of Innate and Adaptive Immune Responses. Cold Spring Harb Symp Quant Biol. 2013 doi: 10.1101/sqb.2013.78.020321. [DOI] [PubMed] [Google Scholar]
- 16.Cantalupo PG, Saenz-Robles MT, Rathi AV, Beerman RW, Patterson WH, Whitehead RH, Pipas JM. Cell-type specific regulation of gene expression by simian virus 40 T antigens. Virology. 2009;386:183–191. doi: 10.1016/j.virol.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathi AV, Cantalupo PG, Sarkar SN, Pipas JM. Induction of interferon-stimulated genes by Simian virus 40 T antigens. Virology. 2010;406:202–211. doi: 10.1016/j.virol.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamper AM, Rofougaran R, Watkins SC, Greenberger JS, Beumer JH, Bakkenist CJ. ATR kinase activation in G1 phase facilitates the repair of ionizing radiation-induced DNA damage. Nucleic acids research. 2013 doi: 10.1093/nar/gkt833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forero A, Moore PS, Sarkar SN. Role of IRF4 in IFN-Stimulated Gene Induction and Maintenance of Kaposi Sarcoma-Associated Herpesvirus Latency in Primary Effusion Lymphoma Cells. J Immunol. 2013;191:1476–1485. doi: 10.4049/jimmunol.1202514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nature structural & molecular biology. 2004;11:1060–1067. doi: 10.1038/nsmb847. [DOI] [PubMed] [Google Scholar]
- 21.Kato A, Cortese-Grogan C, Moyer SA, Sugahara F, Sakaguchi T, Kubota T, Otsuki N, Kohase M, Tashiro M, Nagai Y. Characterization of the amino acid residues of sendai virus C protein that are critically involved in its interferon antagonism and RNA synthesis down-regulation. J Virol. 2004;78:7443–7454. doi: 10.1128/JVI.78.14.7443-7454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell KS, Mullane KP, Aksoy IA, Stubdal H, Zalvide J, Pipas JM, Silver PA, Roberts TM, Schaffhausen BS, DeCaprio JA. DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Genes & development. 1997;11:1098–1110. doi: 10.1101/gad.11.9.1098. [DOI] [PubMed] [Google Scholar]
- 23.Lin R, Genin P, Mamane Y, Sgarbanti M, Battistini A, Harrington WJ, Jr, Barber GN, Hiscott J. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene. 2001;20:800–811. doi: 10.1038/sj.onc.1204163. [DOI] [PubMed] [Google Scholar]
- 24.Bluyssen HA, Levy DE. Stat2 is a transcriptional activator that requires sequence-specific contacts provided by stat1 and p48 for stable interaction with DNA. J Biol Chem. 1997;272:4600–4605. doi: 10.1074/jbc.272.7.4600. [DOI] [PubMed] [Google Scholar]
- 25.Das SC, Nayak D, Zhou Y, Pattnaik AK. Visualization of intracellular transport of vesicular stomatitis virus nucleocapsids in living cells. J Virol. 2006;80:6368–6377. doi: 10.1128/JVI.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai P, Person S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol. 1998;72:7563–7568. doi: 10.1128/jvi.72.9.7563-7568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 28.Stark GR, Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity. 2012;36:503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 30.Sato M, Hata N, Asagiri M, Nakaya T, Taniguchi T, Tanaka N. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 1998;441:106–110. doi: 10.1016/s0014-5793(98)01514-2. [DOI] [PubMed] [Google Scholar]
- 31.Pamment J, Ramsay E, Kelleher M, Dornan D, Ball KL. Regulation of the IRF-1 tumour modifier during the response to genotoxic stress involves an ATM-dependent signalling pathway. Oncogene. 2002;21:7776–7785. doi: 10.1038/sj.onc.1205981. [DOI] [PubMed] [Google Scholar]
- 32.Golding SE, Rosenberg E, Valerie N, Hussaini I, Frigerio M, Cockcroft XF, Chong WY, Hummersone M, Rigoreau L, Menear KA, O'Connor MJ, Povirk LF, van Meter T, Valerie K. Improved ATM kinase inhibitor KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion. Molecular cancer therapeutics. 2009;8:2894–2902. doi: 10.1158/1535-7163.MCT-09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gamper AM, Rofougaran R, Watkins SC, Greenberger JS, Beumer JH, Bakkenist CJ. ATR kinase activation in G1 phase facilitates the repair of ionizing radiation-induced DNA damage. Nucleic acids research. 2013;41:10334–10344. doi: 10.1093/nar/gkt833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toledo LI, Murga M, Zur R, Soria R, Rodriguez A, Martinez S, Oyarzabal J, Pastor J, Bischoff JR, Fernandez-Capetillo O. A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nature structural & molecular biology. 2011;18:721–727. doi: 10.1038/nsmb.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonova KI, Brodsky L, Lipchick B, Pal M, Novototskaya L, Chenchik AA, Sen GC, Komarova EA, Gudkov AV. p53 cooperates with DNA methylation and a suicidal interferon response to maintain epigenetic silencing of repeats and noncoding RNAs. Proc Natl Acad Sci U S A. 2013;110:E89–E98. doi: 10.1073/pnas.1216922110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter S, Aiello D, Atianand MK, Ricci EP, Gandhi P, Hall LL, Byron M, Monks B, Henry-Bezy M, Lawrence JB, O'Neill LA, Moore MJ, Caffrey DR, Fitzgerald KA. A long noncoding RNA mediates both activation and repression of immune response genes. Science. 2013;341:789–792. doi: 10.1126/science.1240925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka N, Ishihara M, Lamphier MS, Nozawa H, Matsuyama T, Mak TW, Aizawa S, Tokino T, Oren M, Taniguchi T. Cooperation of the tumour suppressors IRF-1 and p53 in response to DNA damage. Nature. 1996;382:816–818. doi: 10.1038/382816a0. [DOI] [PubMed] [Google Scholar]
- 38.Tamura T, Ishihara M, Lamphier MS, Tanaka N, Oishi I, Aizawa S, Matsuyama T, Mak TW, Taki S, Taniguchi T. DNA damage-induced apoptosis and Ice gene induction in mitogenically activated T lymphocytes require IRF-1. Leukemia. 1997;11(Suppl 3):439–440. [PubMed] [Google Scholar]
- 39.Tamura T, Ishihara M, Lamphier MS, Tanaka N, Oishi I, Aizawa S, Matsuyama T, Mak TW, Taki S, Taniguchi T. An IRF-1-dependent pathway of DNA damage-induced apoptosis in mitogen-activated T lymphocytes. Nature. 1995;376:596–599. doi: 10.1038/376596a0. [DOI] [PubMed] [Google Scholar]
- 40.Reis LF, Harada H, Wolchok JD, Taniguchi T, Vilcek J. Critical role of a common transcription factor, IRF-1, in the regulation of IFN-beta and IFN-inducible genes. The EMBO journal. 1992;11:185–193. doi: 10.1002/j.1460-2075.1992.tb05041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujita T, Kimura Y, Miyamoto M, Barsoumian EL, Taniguchi T. Induction of endogenous IFN-alpha and IFN-beta genes by a regulatory transcription factor, IRF-1. Nature. 1989;337:270–272. doi: 10.1038/337270a0. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto M, Fujita T, Kimura Y, Maruyama M, Harada H, Sudo Y, Miyata T, Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988;54:903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- 43.Brzostek-Racine S, Gordon C, Van Scoy S, Reich NC. The DNA damage response induces IFN. J Immunol. 2011;187:5336–5345. doi: 10.4049/jimmunol.1100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan S, Wang W, MarquÈs J, Mohan R, Saleh A, Durrant WE, Song J, Dong X. Salicylic Acid Activates DNA Damage Responses to Potentiate Plant Immunity. Molecular cell. 2013;52:602–610. doi: 10.1016/j.molcel.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikitin PA, Luftig MA. The DNA damage response in viral-induced cellular transformation. British journal of cancer. 2012;106:429–435. doi: 10.1038/bjc.2011.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McFadden K, Luftig MA. Interplay between DNA tumor viruses and the host DNA damage response. Current topics in microbiology and immunology. 2013;371:229–257. doi: 10.1007/978-3-642-37765-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohni KN, Smith S, Dee AR, Schumacher AJ, Weller SK. Herpes Simplex Virus Type 1 Single Strand DNA Binding Protein and Helicase/Primase Complex Disable Cellular ATR Signaling. PLoS Pathog. 2013;9:e1003652. doi: 10.1371/journal.ppat.1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkinson DE, Weller SK. Herpes simplex virus type I disrupts the ATR-dependent DNA-damage response during lytic infection. Journal of cell science. 2006;119:2695–2703. doi: 10.1242/jcs.02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Wang X, Diaz J, Tsang SH, Buck CB, You J. Merkel cell polyomavirus large T antigen disrupts host genomic integrity and inhibits cellular proliferation. J Virol. 2013;87:9173–9188. doi: 10.1128/JVI.01216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moody CA, Laimins LA. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog. 2009;5:e1000605. doi: 10.1371/journal.ppat.1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinclair A, Yarranton S, Schelcher C. DNA-damage response pathways triggered by viral replication. Expert reviews in molecular medicine. 2006;8:1–11. doi: 10.1017/S1462399406010544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu LH, Huang M, Fang SG, Liu DX. Coronavirus infection induces DNA replication stress partly through interaction of its nonstructural protein 13 with the p125 subunit of DNA polymerase delta. J Biol Chem. 2011;286:39546–39559. doi: 10.1074/jbc.M111.242206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen C, He X, Ma H, Hou N, Wei C, Song T, Zhang Y, Sun L, Ma Q, Zhong H. Hepatitis C virus infection downregulates the ligands of the activating receptor NKG2D. Cellular & molecular immunology. 2008;5:475–478. doi: 10.1038/cmi.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baer A, Austin D, Narayanan A, Popova T, Kainulainen M, Bailey C, Kashanchi F, Weber F, Kehn-Hall K. Induction of DNA damage signaling upon Rift Valley fever virus infection results in cell cycle arrest and increased viral replication. J Biol Chem. 2012;287:7399–7410. doi: 10.1074/jbc.M111.296608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khodarev N, Ahmad R, Rajabi H, Pitroda S, Kufe T, McClary C, Joshi MD, MacDermed D, Weichselbaum R, Kufe D. Cooperativity of the MUC1 oncoprotein and STAT1 pathway in poor prognosis human breast cancer. Oncogene. 2010;29:920–929. doi: 10.1038/onc.2009.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duarte CW, Willey CD, Zhi D, Cui X, Harris JJ, Vaughan LK, Mehta T, McCubrey RO, Khodarev NN, Weichselbaum RR, Gillespie GY. Expression signature of IFN/STAT1 signaling genes predicts poor survival outcome in glioblastoma multiforme in a subtype-specific manner. PLoS One. 2012;7:e29653. doi: 10.1371/journal.pone.0029653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khodarev NN, Roizman B, Weichselbaum RR. Molecular pathways: interferon/stat1 pathway: role in the tumor resistance to genotoxic stress and aggressive growth. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:3015–3021. doi: 10.1158/1078-0432.CCR-11-3225. [DOI] [PubMed] [Google Scholar]
- 58.Efimova EV, Liang H, Pitroda SP, Labay E, Darga TE, Levina V, Lokshin A, Roizman B, Weichselbaum RR, Khodarev NN. Radioresistance of Stat1 over-expressing tumour cells is associated with suppressed apoptotic response to cytotoxic agents and increased IL6-IL8 signalling. International journal of radiation biology. 2009;85:421–431. doi: 10.1080/09553000902838566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khodarev NN, Beckett M, Labay E, Darga T, Roizman B, Weichselbaum RR. STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc Natl Acad Sci U S A. 2004;101:1714–1719. doi: 10.1073/pnas.0308102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khodarev NN, Roach P, Pitroda SP, Golden DW, Bhayani M, Shao MY, Darga TE, Beveridge MG, Sood RF, Sutton HG, Beckett MA, Mauceri HJ, Posner MC, Weichselbaum RR. STAT1 pathway mediates amplification of metastatic potential and resistance to therapy. PLoS One. 2009;4:e5821. doi: 10.1371/journal.pone.0005821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pitroda SP, Wakim BT, Sood RF, Beveridge MG, Beckett MA, MacDermed DM, Weichselbaum RR, Khodarev NN. STAT1-dependent expression of energy metabolic pathways links tumour growth and radioresistance to the Warburg effect. BMC medicine. 2009;7:68. doi: 10.1186/1741-7015-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheon H, Holvey-Bates EG, Schoggins JW, Forster S, Hertzog P, Imanaka N, Rice CM, Jackson MW, Junk DJ, Stark GR. IFNbeta-dependent increases in STAT1, STAT2, and IRF9 mediate resistance to viruses and DNA damage. The EMBO journal. 2013 doi: 10.1038/emboj.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–558. doi: 10.1016/j.cell.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.