Abstract
Study Design Case report.
Objective We present the first reported case of spontaneous spinal epidural hematoma secondary to calcium pyrophosphate crystal deposition disease (pseudogout) in a 75-year-old woman.
Methods A retrospective review of the patient's case notes was undertaken and the limited literature on this subject reviewed.
Results This patient presented with sudden-onset lower limb paresis, sensory loss, urinary retention, and back pain. Magnetic resonance imaging showed an epidural hematoma, which was evacuated. Histologic specimens of the clot showed calcium pyrophosphate dihydrate crystal deposits (pseudogout).
Conclusion The importance of histopathologic review of surgical specimens is highlighted when considering the differential diagnosis of apparently spontaneous spinal epidural hematoma.
Keywords: calcium pyrophosphate, spinal, epidural, hematoma
Introduction
Spinal epidural hematoma is a rare cause of acute spinal cord compression. It has a high morbidity even with rapid decompressive surgery. Apparently spontaneously cases of spinal epidural haematoma should be investigated fully to prevent recurrence if a secondary cause is found.
Case Report
History
A 75-year-old woman presented to the emergency department following sudden onset of severe lower back pain, rapidly progressive flaccid diplegia, sensory loss, and painless urinary retention. There was no antecedent trauma and no symptoms or risk factors suggesting malignancy, infection, or degenerative pathology. Symptoms reached maximal intensity over a few minutes. She had no medical history except for controlled hypertension.
Examination
The patient presented to the emergency department after a 2-hour delay. On examination, she had a complete (Medical Research Council grade 0/5) flaccid paralysis of both legs, a complete sensory level to L2 on the left and L1 on the right, and complete areflexia in both legs. She had no periurethral or perianal sensation and no anal tone; her urinary residual volume was 1250 mL.
Investigations
Hematology and blood chemistry were unremarkable and a plain lateral X-ray of the lumbar spine did not show any fracture. Magnetic resonance imaging (Fig. 1) revealed an extra axial lesion compressing the cord at the level of T12 that was low signal on T1 and high signal on T2. It appeared to be occupying epidural space posterior and to the left of the theca. The appearances suggested an acute epidural hematoma.
Fig. 1.
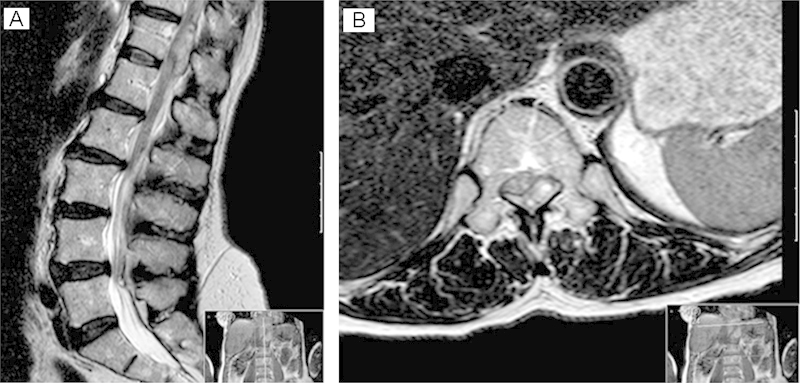
(A, B) Sagittal and axial magnetic resonance image of the thoracolumbar spine showing an extra axial space-occupying lesion compressing the cord at the level of T12. This appears to be occupying epidural space and is posterior and to the left of the theca. There is high signal within the adjacent cord consistent with a compressive myelopathy.
Operation
A T11–L1 laminectomy was performed and a solid hematoma evacuated 9 hours after the onset of symptoms. Systemic investigation did not reveal any malignant disease.
Pathologic Findings
Histopathologic examination of the operative specimen showed fragments of cortical bone with ligament insertion focally covered by synovium and scattered deposits of purple acellular material within the ligamentum flavum, which contained rhomboid-shaped crystals (Fig. 2), features in keeping with calcium pyrophosphate dihydrate deposition disease, or pseudogout.
Fig. 2.
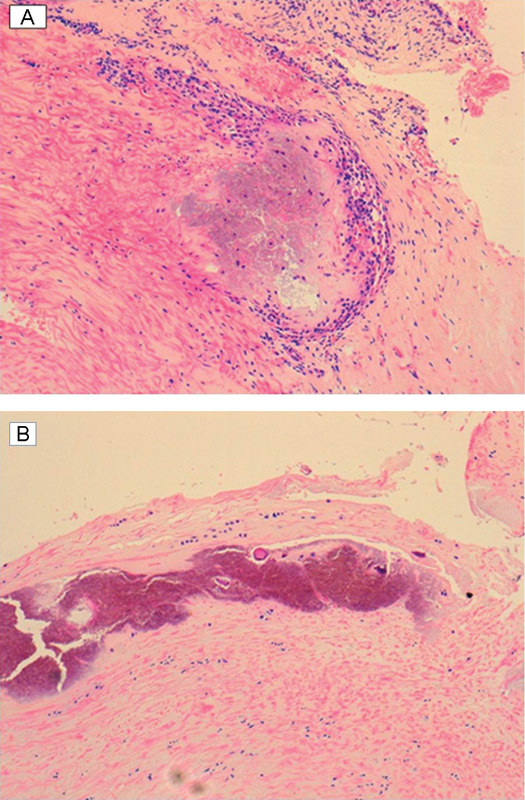
(A) Hematoxylin and eosin–stained sections showing crystalline deposits in the ligament adjacent to the synovium. (B) Hematoxylin and eosin–stained sections showing crystalline deposits in the ligament flavum.
Postoperative Course
The case was reviewed by a rheumatologist but no signs or symptoms of systemic rheumatologic disease were found. The patient was found to have primary hyperparathyroidism, which has a recognized association with pseudogout. The patient was started on colchicine for pseudogout but unfortunately made no neurologic recovery and remains unchanged from her preoperative neurologic status at 1-year follow-up. She is wheelchair-bound and requires assistance for most activities of daily living.
Discussion
This is the first report of spontaneous spinal epidural hematoma secondary to pseudogout. There are reports of pseudogout causing myelopathy, mainly in the cervical cord,1 2 3 and invasion of pyrophosphate crystals into the ligamentum flavum has also been reported in the cervical spine in the setting of pseudogout,4 5 but there was no vascular involvement in these cases. Kawano et al performed electron microscopy of operative specimens and suggested that pyrophosphate crystals are preferentially deposited in the ligamentum flavum and areas of degenerate disk, as these are relatively mobile structures within the vertebral column.4 Extensive bony erosion leading to intradural extension and encasement of the vertebral artery has also been described in the setting of pseudogout.6 A histologic study of 985 theater specimens of cases operated for degenerative spinal disease showed that 2.8% had pyrophosphate crystal deposition and 8.1% had disk neovascularization.7 It is possible that in the case presented, crystal deposition in inflamed, weakened, or neovascularized tissue led to vessel erosion and development of epidural hematoma.
Another histologic study of six cases of lumbar spinal stenosis and facet joint synovial cysts showed that crystal arthropathy was present in all cases.8 Although the usual presentation of pseudogout is described as oligoarthropathy in the distal joints of the upper limb, the literature suggests that crystal arthropathy may be more common than previously thought in clinical practice, especially in an aging population. The diagnosis of pseudogout is usually made by demonstration of chondrocalcinosis on plain X-ray of affected joints and by synovial aspirate containing calcium pyrophosphate dihydrate crystals. A possible association between chondrocalcinosis and spontaneous hemarthrosis, mainly of the knee and shoulder, has been described in the literature.9 10 11 No mechanism for the development of hemarthrosis has been identified, although it may be that calcium pyrophosphate crystals induce an inflammatory reaction in vessel walls and weakens them. Spinal involvement can have serious consequences if not considered in the differential diagnosis of subacute and acute neurologic presentations.
Spontaneous spinal epidural hematoma was first reported as “a case of spinal apoplexy” in the 1800s.12 There is debate in the literature regarding the classification of spinal epidural hematoma. Gopalkrishnan et al reported that hematomas that are not of traumatic or iatrogenic origin are sometimes classified as spontaneous or idiopathic, which is a misnomer if the hematoma is from a vascular malformation, anticoagulant, or other lesion.13 Sarubbo et al discussed the classification in terms of secondary, idiopathic, and spontaneous with co–risk factors. Secondary hematomas are those with a clear definable cause such as an anticoagulant, coagulopathy, vascular malformation, or tumor. Spontaneous hematomas are those without a clear cause but the presence of a risk factor such as inflammatory or metabolic spinal disease and minor trauma. Idiopathic hematomas are those with no identifiable cause or risk factor.14 Table 1 summarizes possible causes for epidural hematoma using the current classifications. It also summarizes the demographics and treatment options. It may be possible to simplify the current classification of spinal epidural hematoma, which would facilitate treatment algorithms, risk management, prognostication, audit, and research. Using the current classification, the presented case would be spontaneous as there is a minor co–risk factor of metabolic bone disease with spinal involvement. The management of acute spinal epidural hematoma is urgent decompressive surgery via laminectomy. In cases where a clear secondary cause exists, correction of coagulopathy or specialist management of underlying vascular malformations/tumors is also required. As demonstrated in the presented case, a co–risk factor may be present but not obvious preoperatively. Therefore, we emphasize the importance of histologic evaluation of operative specimens in cases of spontaneous spinal epidural hematoma. Whether the presence of a co–risk factor in spontaneous hematomas is truly causative or simply strongly associated is unknown. Inflammatory and metabolic bone disease (ankylosing spondylitis, rheumatoid arthritis, Paget disease) with spinal involvement has been reported in association with hematoma frequently enough for it to be discussed in the literature as a co–risk factor. It is possible, therefore, that pseudogout, being a metabolic bone disease, can be a co–risk factor for spontaneous epidural hematoma via the pathophysiologic mechanisms discussed previously.
Table 1. Characteristics of spinal epidural hematoma.
| Incidence | Rare: 0.1–0.3/100,000/y |
| Gender | Equal male and female incidence |
| Spinal levels | Most commonly thoracic and then cervical |
| Age range | Peaks in 20s and then again in 70s |
| Classifications | Spontaneous: existence of a possible co–risk factor such as minor trauma, metabolic or inflammatory bone disease involving the spine Idiopathic: no cause or risk factors identifiable Traumatic Iatrogenic: post–open surgery, lumbar puncture, or epidural injections Secondary: clearly identifiable cause such as coagulopathy, anticoagulation, vascular malformation, or tumor |
| Time course | Acute: sudden neurologic deficit Acute on chronic: stepwise deterioration of neurology Chronic: progressive decline, which can mimic other pathologies such as disk disease |
| Pathophysiology | No single mechanism identified for epidural hematoma without a clear secondary cause; typically venous bleeding in spontaneous cases Evidence of inflammatory/metabolic processes affecting the spinal ligaments as well as bone, leading to vessel inflammation and erosion |
| Management pathway | Clinical assessment Urgent magnetic resonance image scan Reversal of any anticoagulation Treatment of coagulopathy with hematology specialist input Decompressive surgery: emergently for acute cases with neurologic deficit Simultaneous or staged specialist management of secondary causes such as vascular malformations or tumors Send histologic specimens Evaluation of histology postoperatively Treatment of any retrospectively identified co–risk factors Rehabilitation |
In conclusion, spontaneous spinal epidural hematoma, classified variably in the literature, may have a co–risk factor that is not obvious preoperatively. Surgeons obtaining histologic specimens may be valuable in identifying these factors for the individual patient and on a larger scale in reevaluating the classification of spinal epidural hematoma.
Footnotes
Disclosures None of the authors have any conflict of interests to declare. No funding was required for this work.
References
- 1.Caird J, Roberts G, Young S, Brett F. Calcium pyrophosphate dihydrate crystal deposition disease: a case of cervical myelopathy in an elderly woman. J Neurol Neurosurg Psychiatry. 1999;67(4):547–548. doi: 10.1136/jnnp.67.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fye K H, Weinstein P R, Donald F. Compressive cervical myelopathy due to calcium pyrophosphate dihydrate deposition disease: report of a case and review of the literature. Arch Intern Med. 1999;159(2):189–193. doi: 10.1001/archinte.159.2.189. [DOI] [PubMed] [Google Scholar]
- 3.Griesdale D E Jr, Boyd M, Sahjpaul R L. Pseudogout of the transverse atlantal ligament: an unusual cause of cervical myelopathy. Can J Neurol Sci. 2004;31(2):273–275. doi: 10.1017/s0317167100053968. [DOI] [PubMed] [Google Scholar]
- 4.Kawano N, Matsuno T, Miyazawa S. et al. Calcium pyrophosphate dihydrate crystal deposition disease in the cervical ligamentum flavum. J Neurosurg. 1988;68(4):613–620. doi: 10.3171/jns.1988.68.4.0613. [DOI] [PubMed] [Google Scholar]
- 5.Lin S H, Hsieh E T, Wu T Y, Chang C W. Cervical myelopathy induced by pseudogout in ligamentum flavum and retro-odontoid mass: a case report. Spinal Cord. 2006;44(11):692–694. doi: 10.1038/sj.sc.3101890. [DOI] [PubMed] [Google Scholar]
- 6.Sethi K S, Garg A, Sharma M C, Ahmad F U, Sharma B S. Cervicomedullary compression secondary to massive calcium pyrophosphate crystal deposition in the atlantoaxial joint with intradural extension and vertebral artery encasement. Surg Neurol. 2007;67(2):200–203. doi: 10.1016/j.surneu.2006.05.068. [DOI] [PubMed] [Google Scholar]
- 7.Pytel P, Wollmann R L, Fessler R G, Krausz T N, Montag A G. Degenerative spine disease: pathologic findings in 985 surgical specimens. Am J Clin Pathol. 2006;125(2):193–202. doi: 10.1309/89FV-RT04-EGBV-EUD9. [DOI] [PubMed] [Google Scholar]
- 8.Mahmud T, Basu D, Dyson P H. Crystal arthropathy of the lumbar spine: a series of six cases and a review of the literature. J Bone Joint Surg Br. 2005;87(4):513–517. doi: 10.1302/0301-620X.87B4.15555. [DOI] [PubMed] [Google Scholar]
- 9.Cayla J, Huchet B, Rondier J, Menkes C J. [Hemarthrosis of articular chondrocalcinosis. Apropos of 28 cases. Importance of treatment by isotopic synoviorthesis] Rev Rhum Mal Osteoartic. 1982;49(4):281–285. [PubMed] [Google Scholar]
- 10.Menkés C J, Rondier J. Idiopathic haemarthrosis with chondrocalcinosis. Ann Rheum Dis. 1987;46(1):85. doi: 10.1136/ard.46.1.85-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens L W, Spiera H. Hemarthrosis in chondrocalcinosis (pseudogout) Arthritis Rheum. 1972;15(6):651–653. [PubMed] [Google Scholar]
- 12.Jackson R. A case of spinal apoplexy. Lancet. 1869;94(2392):5–6. [Google Scholar]
- 13.Gopalkrishnan C V, Dhakoji A, Nair S. Spontaneous cervical epidural hematoma of idiopathic etiology: case report and review of literature. J Spinal Cord Med. 2012;35(2):113–117. doi: 10.1179/2045772312Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarubbo S, Garofano F, Maida G, Fainardi E, Granieri E, Cavallo M A. Spontaneous and idiopathic chronic spinal epidural hematoma: two case reports and review of the literature. Eur Spine J. 2009;18(11):1055–1061. doi: 10.1007/s00586-009-1175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


