Abstract
Purpose
To examine the impact of a personal health record (PHR) on medication-use safety among older adults.
Background
Online PHRs have potential as tools to manage health information. We know little about how to make PHRs accessible for older adults and what effects this will have.
Methods
A PHR was designed and pretested with older adults and tested in a 6-month randomized controlled trial. After completing mailed baseline questionnaires, eligible computer users aged 65 and over were randomized 3:1 to be given access to a PHR (n=802) or serve as a standard care control group (n=273). Follow-up questionnaires measured change from baseline medication use, medication reconciliation behaviors, and medication management problems.
Results
Older adults were interested in keeping track of their health and medication information. A majority (55.2%) logged into the PHR and used it, but only 16.1% used it frequently. At follow-up, those randomized to the PHR group were significantly less likely to use multiple non-steroidal anti-inflammatory drugs—the most common warning generated by the system (viewed by 23% of participants). Compared with low/non-users, high users reported significantly more changes in medication use and improved medication reconciliation behaviors, and recognized significantly more side effects, but there was no difference in use of inappropriate medications or adherence measures.
Conclusions
PHRs can engage older adults for better medication self-management; however, features that motivate continued use will be needed. Longer-term studies of continued users will be required to evaluate the impact of these changes in behavior on patient health outcomes.
Keywords: Personal Health Record, Medication Management, Patient Safety
Introduction
Personal health records (PHRs) are electronic, secure and private, patient-controlled tools used for management of health information.1–4 This information includes health conditions, medications, health behaviors, test results, healthcare appointments, and other personal information. The data in PHRs can be populated by provider-based electronic records, health system administrative data, and by patients.5 PHRs are an opportunity to increase patient involvement in managing their own health.6–9
Patient safety and self-management have been linked conceptually in a recent effort to join the patient-centeredness of the latter with the systems approach to the former.10 Online PHRs have potential as tools to achieve safety goals by supporting chronic disease self-management. Yet we know little about how to make PHRs accessible for older adults and what effects this will have. The purpose of this study was to examine the impact of a PHR system on medication-use safety among older adults by focusing on supporting self-management. This study was conducted under the Agency for Healthcare Research and Quality funding opportunity, ‘Ambulatory Safety and Quality: Enabling Patient-Centered Care through Health IT.’11
By 2011, 7–10% of the US population reported using a PHR.12 13 However, the designs of PHRs are widely variable, including provider-tethered, payer-tethered, and standalone designs. Furthermore, the evidence on the effects of PHRs is inconclusive.14–19 We designed20 a standalone PHR in partnership with older adults—a priority population group that has a high potential to benefit from increased patient activation but for whom barriers to computer use exist. Because medication management is a complex health behavior requiring daily decision-making to take or not take a medication, and because most older adults take many medications with attendant side effect risk,21 our focus was on the PHR as a means to activate older adults for a more engaged role in medication management. A standalone PHR was chosen because of the limited integrated health informatics capacity in a rural state, the numerous varied electronic medical records being used in rural areas, and to enable easy modification of the user interface. The purpose of this study was to compare patient-reported medication self-management behaviors and safety indicators among older adult participants invited to use the PHR vs a usual care comparison group.
Methods
Study population and study recruitment
This was a single-center open-label parallel-group study with unequal randomization (3:1 ratio) conducted in the USA. To identify participants for the PHR trial, we mailed a brief computer-use screening questionnaire to a simple random sample of adults age 65+from a 2009 list of all registered voters in Iowa. We invited respondents to that questionnaire who reported using a computer in the past month to visit websites or to send or receive email to participate in the trial. The computer could have been at their home or in some other place.
The screening questionnaires were sent to 15 000 registered voters from a list of all registered voters in Iowa age 65 and older. Eligible respondents to the screening questionnaire were mailed a baseline questionnaire. Participants were reimbursed US$10 (via a check) for completing each of the baseline and follow-up questionnaires (for a total of US$20). Of 3963 respondents to the screening questionnaire (26%), 2263 (57%) were eligible to participate in the trial. A baseline questionnaire, accompanied by a cover letter and prepaid business reply envelope was mailed to all 2263 people, and 1163 (51% of eligible) submitted completed baseline questionnaires and enrolled in the trial. Reminder emails were sent to baseline and follow-up questionnaire non-responders who had provided email address information. For the follow-up questionnaire, we mailed a second copy of the questionnaire followed by telephone contact when necessary to non-respondents.
Participants were compared with screening questionnaire respondents who were eligible to participate but did not do so. There were no significant differences in mean (SD) age (72.4 (6.1) vs 72.8 (6.3) years; p=0.1228), gender (56.7% vs 53.9% female; p=0.1646), or the percent with disabilities (20.7% vs 21.8% were limited in activities or used special equipment because of health; p=0.4818). Participants were more likely to have graduated from college (39.8% vs 34.6%; p=0.0047), were more likely to report being comfortable with electronic medical records (77.6% vs 67.8%; p<0.0001), and had a slightly higher health information technology use score (mean (SD) 1.8 (1.2) vs 1.7 (1.2); p=.0586), where the score was the sum of ‘yes’ responses to the following questions: ever searched online for medical information, searched online for information about a doctor, typed in information on a website about eating/exercise/weight, typed in information on a website about chronic illness, sent/received emails from their doctor.
After completing the baseline mailed questionnaire, participants were randomly assigned following simple randomization procedures (computerized random numbers) in a 3:1 ratio to treatment groups: invitation to use the PHR or usual care (control group). We randomized more patients to the intervention than control group because we wanted to be able to have an adequate number of high users for our ‘as-treated’ analysis (see Statistical analysis below). Notification of study group assignment was sent by mail to all trial participants by an investigator with no clinical involvement in the trial. Follow-up questionnaires were mailed to trial participants 6 months after assignment.
Study PHR
Iowa PHR was developed specifically for use by older adults for this study. This was necessary because, after an environmental scan of 58 commercially designed PHRs (see online supplementary appendix 1 for list of PHRs reviewed) and testing the simplest of these in a laboratory setting with 25 participants, we determined that none were likely to work well for older adults. Iowa PHR is a web-based application that features a tabbed interface design. Users can enter, view, and print their current and past medicines, allergies, health conditions, and health event tracking over time. An embedded tutorial video provides assistance with the system. The PHR was developed and refined using participatory design and focus group sessions as well as evaluation in a usability laboratory.20 The resulting design emphasizes the reduction of physical and cognitive demands on users, focusing on simplicity, readability, and quick navigation (see online supplementary appendix 2 for screen shots).
Prior research suggested that individualized user feedback was a key facilitator to health IT adoption by older adults.22 In keeping with this finding and the focus on medication management behaviors and safety, we developed a set of user-friendly medication safety messages based on the Assessing Care of Vulnerable Elders project (ACOVE- 3) medication-use quality indicators.23 24 Iowa PHR displayed a message when a user entered a medication with an associated ACOVE-3 safety concern. This included 16 safety issues for 12 drugs or drug classes with safety concerns ranging from drug–drug interactions (anticholinergics, warfarin) to dosage concerns (acetaminophen, iron), important laboratory monitoring (warfarin, loop diuretics, ACE inhibitors), risk awareness (non-steroidal anti-inflammatory drugs (NSAIDs) and bleeding risk), and drugs that should be avoided by older adults (barbiturates, meperidine, skeletal muscle relaxants). The messages were displayed in three levels of increasing detail and complexity to facilitate tiered information take-up: a brief alert containing the basic reason for concern, a summary level that included recommended actions, and a detailed explanation of the alert. The three levels of text can be viewed in online supplementary appendix 3. In addition to the drug-specific messages, we adapted four general medication-use patient safety indicators from the ACOVE project23 and displayed them to all users on a rotating basis upon login: (1) keeping an up-to-date medication list; (2) receiving an annual medication review; (3) knowing the indication for all current medications; and (4) receiving patient education on the indication, administration, and possible side effects of each medication.
Accompanying the notice of study group assignment, PHR group participants were sent an invitation to use the study PHR for a period of 1 year, a quick-start guide, and their login credentials. Upon initial login to Iowa PHR, users agreed to the terms of an online informed consent document, followed by two user-selected security questions from a predefined list. Participants who did not log in to Iowa PHR were sent a reminder letter 3–4 weeks after the initial invitation.
Measures
Measures were constructed from the baseline and 6-month follow-up questionnaires and from log-tracking of system use. To evaluate changes in medications, a complete medication inventory was collected in each questionnaire. Participants were asked to consult the labels for prescription medications they currently take, and to list the name, strength, date the last prescription was filled, dose, length of time taken, purpose, and side effects they watch for. When more than 10 medications were being taken, only name and strength were queried for medications 11 through a maximum of 20 prescription medications. Name and reason were queried for non-prescription medications taken in the past 2 weeks. To determine rates of potentially inappropriate medications, we compared baseline and follow-up medications with drug lists compiled from the ACOVE project.23 A modified version of the four-item Morisky adherence measure,25 with response options of ‘never’, ‘rarely’, ‘sometimes’, ‘often’, ‘always’ instead of the original yes/no responses, was used to measure self-reported medication adherence. Additional medication management behavior items were developed for the study and included how patients used and maintained medication lists, and how medication lists may facilitate medication reconciliation in the context of healthcare visits. On each questionnaire, participants were asked to indicate whether (yes/no) in the past 3 months they had: started a prescription medication; stopped a prescription medication; changed the strength or dose of a prescription medication; started an over-the-counter medication; stopped an over-the-counter medication; or changed the strength or dose of an over-the-counter medication. The mean number of medication management problems was calculated from endorsed items based on a list of eight problems.26 This list included questions on use of multiple prescribers, multiple pharmacies, use of mail order for prescriptions, confusion about whether medication was taken, taking medication without knowing the indication for use, problems affording medications, feeling that medications are not working, and feeling that medications are not doing what they were intended to do. For descriptive purposes, health status was measured using the 12-item short form health survey (SF-12) and its physical and mental health summary scores,27 and 19 common health conditions were queried. Participant gender, age, ethnicity, race, education, marital status, and living situation were collected at baseline. PHR system-use data for PHR group trial participants were linked to survey data. Event timestamps were logged when users: logged in; visited any major interface tab or sub tab; added, edited, or deleted any information; printed a report; or clicked on the Iowa PHR tutorial.
Statistical analysis
For intention-to-treat analyses, subjects randomized for PHR use and controls were compared before and after the intervention. Independent sample t tests were used to compare group means for continuous variables. Group proportions for categorical variables were compared using χ2 tests. To understand the characteristics of users, we then performed subgroup analyses comparing users and non-users within the intervention group. PHR (intervention) group participants were classified by level of system engagement. High use was defined as multiple user logins over the duration of the trial, with health information entered or edited during the session. Low use was defined as one or more logins, but where health information was entered or edited during only one session. Non-users were examined in two groups: those who logged into the system but did not enter any health-related information and those who never logged in. After observing consistently comparable findings for low users and non-users, we proceeded to dichotomize high use vs all others. In as-treated analyses comparing high users with low/non-users, unadjusted comparisons were performed using independent sample t tests for continuous variables and χ2 tests for categorical variables. Logistic regression and linear regression models were applied to compare post-intervention characteristics for high and non-users, with adjustment for corresponding pre-intervention values and total number of medications.
Analyses were conducted using SAS/STAT software V.9.2.
The protocol was approved by the University of Iowa Institutional Review Board for the Protection of Human Subjects.
Results
All eligible participants were recruited from July 15, 2010 to February 15, 2011. Of 1163 randomized individuals, 23 did not receive their mailed study group assignment because invitation letters were returned by the post office as undeliverable, 62 did not complete the follow-up questionnaire, and survey discrepancies for three suggested that someone other than the subject had completed the survey. The final analytical population of 1075 (7.2% of 15 000) included 802 (91.9%) of those randomized to the PHR intervention and 273 (93.1%) to the control group. Mean age was 72.3 years (SD 6.1), and 56.8% of participants were women.
Intention-to-treat analysis
The study groups were well balanced (tables 1 and 2). At baseline, control group subjects were more likely to have changed the strength or dose of a prescription medication in the past 3 months (p=0.023) (table 2). At follow-up, intervention group participants were less likely to have started an over-the-counter medication (8.9% vs 13.2%, p=0.039) and to be taking two or more NSAIDs (14.1% vs 19.4%, p=0.036) (table 2). All other follow-up comparisons between study groups after the intervention were not significant (p>0.05).
Table 1.
Description of subjects
| Baseline characteristic | Randomized to PHR use (N=802) | Control (N=273) | PHR vs control p value* |
|---|---|---|---|
| Gender | 0.4649 | ||
| Male, n (%) | 341 (42.5) | 123 (45.1) | |
| Female, n (%) | 461 (57.5) | 150 (54.9) | |
| Age, mean (SD) | 72.5 (6.0) | 72.0 (6.3) | 0.2662 |
| Non-Hispanic white†, n (%) | 782 (99.0) | 267 (98.2) | 0.2855 |
| Highest education completed, n (%) | 0.1862 | ||
| Some high school or less | 14 (1.8) | 1 (0.4) | |
| High school diploma or GED | 183 (23.2) | 77 (28.3) | |
| Technical or trade school/some college | 273 (34.6) | 88 (32.4) | |
| Bachelor's degree | 181 (23.0) | 55 (20.2) | |
| Master's degree or higher | 137 (17.4) | 51 (18.8) | |
| Days of computer use in past 7 days, mean (SD) | 6.1 (1.7) | 6.0 (1.6) | 0.5879 |
| Mental health T-score (SF-12), mean (SD) | 55.5 (7.4) | 54.9 (7.9) | 0.3334 |
| Physical health T-score (SF-12), mean (SD) | 45.9 (10.6) | 46.1 (10.3) | 0.7427 |
| Medical conditions (from list of 19), mean (SD) | 3.6 (2.3) | 3.6 (2.2) | 0.9758 |
| Prevalence of individual medical conditions, n (%) | |||
| Acid reflux, GERD, ulcer, or other stomach problems | 275 (34.3) | 94 (34.4) | 0.9657 |
| Anemia | 36 (4.5) | 9 (3.3) | 0.3956 |
| Anxiety | 95 (11.8) | 33 (12.1) | 0.9149 |
| Arthritis | 437 (54.5) | 131 (48.0) | 0.0630 |
| Asthma, emphysema, chronic bronchitis, or COPD | 99 (12.3) | 37 (13.6) | 0.6037 |
| Cancer | 107 (13.3) | 34 (12.5) | 0.7075 |
| Depression | 83 (10.3) | 22 (8.1) | 0.2709 |
| Diabetes | 122 (15.2) | 42 (15.4) | 0.9454 |
| Myocardial infarction, CAD, angina, CHF, or other heart problems | 187 (23.3) | 68 (24.9) | 0.5933 |
| High BP | 432 (53.9) | 142 (52.0) | 0.5965 |
| High cholesterol | 406 (50.6) | 153 (56.0) | 0.1215 |
| Kidney failure | 28 (3.5) | 10 (3.7) | 0.8944 |
| Liver disease | 3 (0.4) | 4 (1.5) | 0.0529 |
| Memory problems | 80 (10.0) | 31 (11.4) | 0.5174 |
| Migraines | 42 (5.2) | 12 (4.4) | 0.5825 |
| Osteoporosis | 165 (20.6) | 51 (18.7) | 0.5003 |
| Prostate problems | 115 (14.3) | 35 (12.8) | 0.5317 |
| Stroke | 27 (3.4) | 7 (2.6) | 0.5129 |
| Thyroid problems | 132 (16.5) | 61 (22.3) | 0.0286* |
*p Values from χ2 tests for categorical variables and t tests for continuous variables comparing group of subjects randomized to use PHR with control group; significance is indicated by an asterisk.
†In all trial analyses, we operationalized race and ethnicity as non-Hispanic white vs other racial/ethnic categories.
BP, blood pressure; CAD, coronary artery disease; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; GED, general educational development; GERD, gastroesophageal reflux disease; PHR, personal health record; SF-12, 12-item short form health survey.
Table 2.
Pre- and post-intervention characteristics of trial participants (N=1075)
| Characteristic | Baseline | Follow-up | ||||
|---|---|---|---|---|---|---|
| Randomized to PHR use (N=802) |
Control (N=273) | PHR vs control p value* | Randomized to PHR use (N=802) |
Control (N=273) |
PHR vs control p value* | |
| Changes in medication use | ||||||
| Number of prescription drugs, mean (SD) | 4.1 (3.2) | 4.2 (3.2) | 0.8444 | 4.0 (3.1) | 4.1 (3.2) | 0.6757 |
| Number of OTC drugs, mean (SD) | 4.1 (2.8) | 4.3 (3.1) | 0.4084 | 3.6 (2.5) | 3.9 (2.7) | 0.0530 |
| Any change in medication use in past 3 months, n (%) | 286 (35.7) | 94 (34.4) | 0.7138 | 349 (43.5) | 124 (45.4) | 0.5839 |
| Started prescription drug, n (%) | 155 (19.3) | 48 (17.6) | 0.5248 | 190 (23.7) | 56 (20.5) | 0.2803 |
| Stopped prescription drug, n (%) | 93 (11.6) | 30 (11.0) | 0.7855 | 123 (15.3) | 39 (14.3) | 0.6750 |
| Changed strength/dose of prescription drug, n (%) | 80 (10.0) | 41 (15.0) | 0.0228* | 110 (13.7) | 38 (13.9) | 0.9328 |
| Started OTC drug, n (%) | 49 (6.1) | 16 (5.9) | 0.8815 | 71 (8.9) | 36 (13.2) | 0.0388* |
| Stopped OTC drug, n (%) | 12 (1.5) | 9 (3.3) | 0.0634 | 37 (4.6) | 12 (4.4) | 0.8815 |
| Changed strength/dose of OTC drug, n (%) | 18 (2.2) | 6 (2.2) | 0.9641 | 19 (2.4) | 12 (4.4) | 0.0840 |
| Medication reconciliation | ||||||
| Keep list of current medications, n (%) | 508 (63.9) | 175 (64.6) | 0.8412 | 559 (70.6) | 196 (72.1) | 0.6432 |
| Reason for medications on list, n (%) | 133 (26.5) | 33 (19.8) | 0.0788 | 210 (37.8) | 59 (30.4) | 0.0635 |
| Usually shows medication list to doctor, n (%) | 404 (80.8) | 131 (78.4) | 0.5080 | 435 (78.2) | 154 (78.6) | 0.9223 |
| Put OTC drugs on list, n (%) | 391 (77.7) | 128 (75.7) | 0.5928 | 435 (78.1) | 155 (79.1) | 0.7734 |
| Updated list in past 3 months, n (%) | 264 (53.1) | 81 (48.5) | 0.3017 | 293 (52.9) | 105 (54.4) | 0.7162 |
| At last doctor visit: | ||||||
| Asked whether keep a medication list, n (%) | 313 (40.1) | 89 (34.4) | 0.1017 | 342 (44.7) | 112 (42.6) | 0.5503 |
| Had medication list, n (%) | 453 (59.3) | 152 (59.4) | 0.9816 | 504 (66.4) | 173 (66.3) | 0.9718 |
| Showed medication list, n (%) | 333 (74.3) | 111 (73.5) | 0.8422 | 378 (75.4) | 127 (73.8) | 0.6734 |
| Someone asked about medication strength at last doctor visit, n (%) | 0.6899 | 0.6687 | ||||
| Yes, for all medications | 251 (32.3) | 91 (35.0) | 301 (39.6) | 112 (42.4) | ||
| Yes, for some medications | 75 (9.7) | 26 (10.0) | 110 (14.5) | 34 (12.9) | ||
| Doctor compared records with what patient said they were taking, n (%) | 514 (66.7) | 180 (70.0) | 0.3175 | 523 (69.0) | 176 (66.9) | 0.5322 |
| Differences found between doctor and patient medication records, n (%) | 63 (8.2) | 21 (8.1) | 0.9445 | 77 (10.1) | 21 (8.0) | 0.3003 |
| Medication problems | ||||||
| Use of potentially inappropriate medications (ACOVE), n (%) | 207 (25.8) | 66 (24.2) | 0.5920 | 164 (20.4) | 53 (19.4) | 0.7129 |
| Taking 2 or more NSAIDS (including aspirin), n (%) | 155 (19.3) | 63 (23.1) | 0.1832 | 113 (14.1) | 53 (19.4) | 0.0355* |
| Number of medication management problems, mean (SD) | 1.4 (1.4) | 1.5 (1.5) | 0.1823 | 1.4 (1.4) | 1.6 (1.5) | 0.1514 |
| Knows how to recognize side effects, n (%) | 566 (73.7) | 201 (75.3) | 0.6110 | |||
| Medication side effects in past 3 months, n (%) | 86 (11.0) | 22 (8.2) | 0.1944 | 100 (12.9) | 33 (12.2) | 0.7883 |
| Modified Morisky adherence score, mean (SD) | 14.2 (1.8) | 14.1 (1.9) | 0.4762 | 13.8 (1.9) | 13.9 (1.9) | 0.9821 |
*p Values from χ2 tests for categorical variables and t tests for continuous variables comparing group of subjects randomized to use PHR with control group; significance is indicated by an asterisk.
ACOVE,Assessing Care of Vulnerable Elders project; NSAID, non-steroidal anti-inflammatory drug;OTC, over-the-counter; PHR, personal health record.
Description of system engagement
Among the PHR group, by the end of the study period, 491 (61.2%) had attempted to log on to the system and 443 (55.2%) performed some type of activity with the PHR; 341 of these (77% of those using the system, 42.5% of PHR group subjects) entered health information. More than 40% of all PHR users entered at least one medication, and the mean (SD) number of medications they entered was 7.1 (4.4). The system displayed at least one medication warning message for 77% of those who used the medications feature (table 3). The most common Iowa PHR-generated medication warnings were about risk factors for stomach bleeding for NSAID users (23% of patients), a reminder about getting regular blood tests for those using ACE inhibitors (11%), and a warning about the maximum daily dosage for acetaminophen (6%).
Table 3.
Description of PHR system engagement
| Action | N | Percentage of PHR participants to whom action applied (N=802) | Percentage of PHR users who entered≥1 medication (N=331) |
|---|---|---|---|
| Login | 491 | 61.2 | |
| Visited at least one feature past login process | 443 | 55.2 | |
| Viewed tutorial video | 374 | 46.6 | |
| Edited allergy | 159 | 19.8 | |
| Entered health condition | 170 | 21.2 | |
| Entered tracking information | 113 | 14.1 | |
| Entered demographic or emergency contact information | 274 | 34.2 | |
| Printed report | |||
| Current medication or wallet card | 284 | 35.4 | |
| Medication warnings | 26 | 3.2 | |
| Other report | 71 | 8.9 | |
| Entered medication | 331 | 41.3 | 100.0 |
| Any warning generated | 255 | 31.8 | 77.0 |
| Specific warning generated | |||
| NSAIDs | 186 | 23.2 | 56.2 |
| ACE inhibitors | 91 | 11.3 | 27.5 |
| Acetaminophen | 50 | 6.2 | 15.1 |
| Anticholinergics | 39 | 4.9 | 11.8 |
| Warfarin | 19 | 2.4 | 5.7 |
| Loop diuretics | 22 | 2.7 | 6.6 |
| Benzodiazepines | 16 | 2.0 | 4.8 |
| Iron | 10 | 1.2 | 3.0 |
| Skeletal muscle relaxants | 6 | 0.7 | 1.8 |
| Barbiturates | 1 | 0.1 | 0.3 |
| Ketorolac | 1 | 0.1 | 0.3 |
NSAID, non-steroidal anti-inflammatory drug;PHR, personal health record.
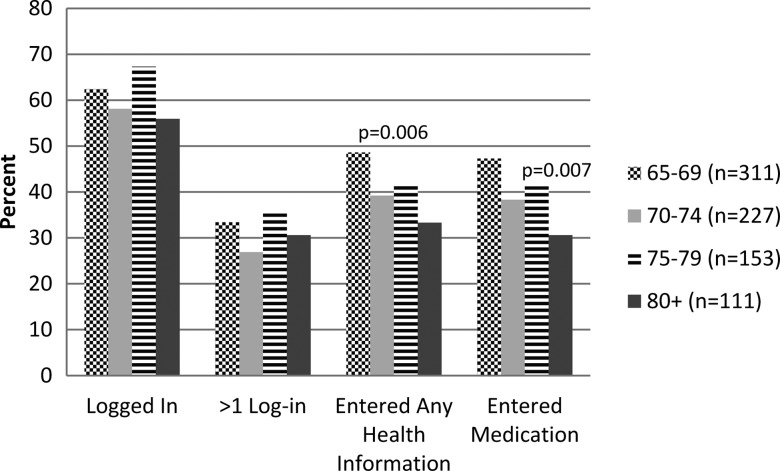
The proportion who logged in, number of log-ins, and mean days between log-ins did not differ by gender or age group. Frequency of medication, health information entries, medication warning messages and user warning clicks did not vary by gender. However, there were significant age group differences in entry of health information (figure 1).
Figure 1.
Usage characteristics by age group.
In subanalyses comparing high users (129 people, 16.1% of PHR group) with all others in the PHR group, high users were more likely than low/non-users to be men (51.2% vs 40.9%, p=0.03), were slightly younger (mean age 71.5 vs 72.7, p=0.025), and were heavier computer users at the time of screening (6.5 days per week vs 6.0 days per week, p=0.0002). There were no significant differences between high and low/non-users in education, marital status, or whether living alone (p>0.27). There were no baseline differences between high users and low/non-users in mean physical or mental health scores, but high users were more likely to report high cholesterol (59.7% vs 48.9%), hypertension (62.0% vs 52.3%), and a larger number of chronic conditions (mean (SD) 4.0 (2.3) vs 3.5 (2.2)). High users reported significantly more medications (mean (SD) 9.3 (4.6)) than low/non-users (8.0 (4.6)) on the baseline questionnaires. They reported significantly more medication problems (mean (SD) 1.6 (1.6) vs 1.3 (1.3); table 4) and were significantly more likely to be already keeping a medication list before the study (76.0% vs 61.6%; table 4).
Table 4.
Comparison of high vs low/non-users on pre-intervention (baseline) and post-intervention (follow-up) characteristics (N=802)
| Characteristic | Baseline | Follow up | |||
|---|---|---|---|---|---|
| High users (N=129) | Low/non-users (N=683) | High users (N=129) | Low/non-users (N=683) | Adjusted† mean difference (SE) or OR (95% CI) for high vs low/non-users | |
| Changes in medication use | |||||
| Number of prescription drugs, mean (SD) | 4.7 (3.0)* | 4.0 (3.2) | 4.6 (3.1)* | 3.9 (3.1) | 0.04 (0.15) |
| Number of OTC drugs, mean (SD) | 4.7 (2.9)* | 4.0 (2.8) | 4.3 (2.9)** | 3.4 (2.4) | 0.48 (0.17)** |
| Any change in medication use in the past 3 months, n (%) | 48 (37.2) | 238 (35.4) | 72 (55.8)** | 277 (41.2) | 1.62 (1.09 to 2.40)* |
| Started prescription drug, n (%) | 27 (20.9) | 128 (19.0) | 45 (34.9)** | 145 (21.5) | 1.79 (1.18 to 2.72)** |
| Stopped prescription drug, n (%) | 17 (13.2) | 76 (11.3) | 35 (27.1)**** | 88 (13.1) | 2.23 (1.40 to 3.56)*** |
| Changed strength/dose of prescription drug, n (%) | 13 (10.1) | 67 (10.0) | 21 (16.3) | 89 (13.2) | 1.13 (0.66 to 1.93) |
| Started OTC drug, n (%) | 9 (7.0) | 40 (5.9) | 17 (13.2) | 54 (8.0) | 1.62 (0.90 to 2.91) |
| Stopped OTC drug, n (%) | 3 (2.3) | 9 (1.3) | 10 (7.8) | 27 (4.0) | 1.81 (0.84 to 3.87) |
| Changed strength/dose of OTC drug, n (%) | 4 (3.1) | 14 (2.1) | 7 (5.4)* | 12 (1.8) | 2.96 (1.13 to 7.75)* |
| Medication reconciliation | |||||
| Keep list of current medications, n (%) | 98 (76.0)** | 410 (61.6) | 113 (88.3)**** | 446 (67.2) | 3.68 (1.83 to 7.37)*** |
| Reason for medications on list, n (%) | 28 (28.6) | 105 (26.1) | 57 (50.4)** | 153 (34.6) | 2.14 (1.26 to 3.64)** |
| Usually shows medication list to doctor, n (%) | 71 (72.4)* | 333 (82.8) | 87 (77.0) | 348 (78.6) | 1.20 (0.62 to 2.34) |
| Put OTC drugs on list, n (%) | 77 (78.6) | 314 (77.5) | 89 (78.8) | 346 (77.9) | 1.09 (0.58 to 2.05) |
| Updated list in past 3 months, n (%) | 59 (60.8) | 205 (51.3) | 62 (55.4) | 231 (52.3) | 1.15 (0.70 to 1.89) |
| At last doctor visit: | |||||
| Had medication list, n (%) | 83 (65.4) | 370 (58.1) | 100 (80.0)*** | 404 (63.7) | 2.48 (1.36 to 4.54)** |
| Showed medication list, n (%) | 61 (74.4) | 272 (74.3) | 75 (75.0) | 303 (75.6) | 0.90 (0.47 to 1.71) |
| Someone asked about medication strength, n (%) | * | 1.61 (1.05 to 2.45)* | |||
| Yes, for all medications | 51 (40.2) | 200 (30.8) | 58 (46.4) | 243 (38.3) | |
| Yes, for some medications | 13 (10.2) | 62 (9.6) | 24 (19.2) | 86 (13.5) | |
| Doctor compared records with what patient said they were taking, n (%) | 89 (70.1) | 425 (66.0) | 95 (76.0) | 428 (67.6) | 1.50 (0.93 to 2.42) |
| Differences found between doctor and patient medication records, n (%) | 15 (11.8) | 48 (7.5) | 24 (19.0)*** | 53 (8.4) | 2.21 (1.27 to 3.85)** |
| Medication problems | |||||
| Use of potentially inappropriate medications (ACOVE), n (%) | 43 (33.3)* | 164 (24.4) | 35 (27.1)* | 129 (19.2) | 1.24 (0.69 to 2.24) |
| Taking 2 or more NSAIDs (including aspirin), n (%) | 30 (23.3) | 125 (18.6) | 25 (19.4) | 88 (13.1) | 1.52 (0.85 to 2.71) |
| Number of medication management problems, mean (SD) | 1.6 (1.6)* | 1.3 (1.3) | 1.8 (1.5)*** | 1.4 (1.4) | 0.15 (0.09) |
| Knows how to recognize side effects, n (%)§ | 104 (81.9)* | 462 (72.1) | 1.76 (1.08 to 2.86)* | ||
| Medication side effects in past 3 months, n (%) | 17 (13.4) | 69 (10.6) | 29 (22.8)*** | 71 (10.9) | 2.24 (1.35 to 3.70)** |
| Modified Morisky adherence score, mean (SD) | 14.2 (1.5) | 14.2 (1.8) | 14.0 (1.6) | 13.8 (2.0) | 0.22 (0.16) |
*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, significantly different from low/non-users. p Values from χ2 tests for categorical variables and t tests for continuous variables comparing group of subjects with high PHR use with low users/non-users within data collection point (baseline or follow-up).
†Linear regression models were used for continuous characteristics and logistic regression models for categorical characteristics (PROC GENMOD procedure was used for both models), models included corresponding characteristic and total number of drugs at baseline.
§Assessed post-intervention only (no baseline); comparisons between high users and low/non-users are adjusted for total number of drugs at baseline.
ACOVE,Assessing Care of Vulnerable Elders project; NSAID, non-steroidal anti-inflammatory drug;OTC, over-the-counter; PHR, personal health record.
As-treated analyses within the PHR group
High use of the PHR was associated with numerous changes in medication use and management at follow-up (table 4). All comparisons between high users and low/non-users at follow-up were adjusted for baseline differences in the characteristic (if baseline was available) as well as for total number of baseline medications (table 4, column 6).
Medication use
After adjustment for baseline differences, on follow-up questionnaires, high users reported significantly higher use of over-the-counter medication compared with low/non-users. They were also significantly more likely to report starting a new prescription medication, stopping a prescription medication, and to have changed the strength or dose of an over-the-counter medication in the past 3 months. Self-reported adherence to medications did not differ between high and low/non-users, at either baseline or follow-up.
Medication lists and medication reconciliation
A greater proportion of high users reported keeping a current medication list compared with low/non-users, adjusted for baseline values. Higher users were also significantly more likely to report including the reasons for taking each medication on their list. When reporting on medication discussions during their last doctor's visit, high users were significantly more likely to report that they had their medication list with them, that someone asked them about the strength of their medications, and that differences were detected between their list and the doctor's records.
Medication problems
High users were significantly more likely to report having a side effect in the past 3 months compared with low/non-users, but they also were more likely to report that they know how to recognize side effects. The crude difference between high and low/non-users in number of medication management problems at follow-up was explained by adjusting for pre-existing differences in medication problems and number of medications. Similarly, the crude difference between high and low/non-users in number of potentially inappropriate medications and number using multiple NSAIDS at follow-up was explained by adjusting for pre-existing differences in these measures.
Discussion
As a result of extensive focus group-based participatory design and usability testing, we had previously learned20 that older adults were interested in keeping track of their health and medication information. Working intensively with a small group of older adults resulted in a web-based PHR system that follows a minimalist approach, tracking the minimal amount of information needed in order to increase adoption. In this randomized controlled trial of the PHR among older adults, the majority (55.2%) logged in and used it. In intention-to-treat comparisons of 802 participants randomized to PHR access vs 273 usual care controls, PHR access alone had minimal effect on medication behaviors: there was improvement in one measure (use of multiple NSAIDs decreased in the PHR group, potentially because this was the most frequent warning, viewed by 23.2% of participants). One possible explanation for the mainly no-difference finding is that only a minority (16.1%) of patients in the PHR intervention group engaged repeatedly with the system. Among the 16.1% of the PHR intervention group who used the system repeatedly, there were several improvements in medication behaviors compared with low/non-users. These improvements included several markers of greater attentiveness to medication safety monitoring: recognizing side effects; keeping a medication list; including reasons for each medication on the list; having their list with them at a doctor visit; having providers query them more extensively about their medications (ie, their strength); and reporting that differences were detected between their record and their doctor's record. Taken together with the greater likelihood of changes in medication therapy among frequent PHR users, this suggests that the PHR engaged and supported them in monitoring more closely, which presumably helped them communicate with their prescribers to adjust therapy. These effects persisted after adjustment for baseline differences between high users and low/non-users, suggesting that they were attributable to the PHR. However, we cannot rule out the possibility that high users were already becoming more attentive to medication management and safety without the PHR.
Improvements in medication behaviors among high users did not translate into other improvements in medication-use safety as measured by the number of potentially inappropriate medications or taking multiple NSAIDs compared with low/non-users. If the high-use patients were more engaged and monitoring more closely, they and their prescribers may have been comfortable in using the riskier drugs—with the belief that the patient would detect adverse effects early enough to prevent serious problems. Self-reported adherence was also no different between high users and low/non-users; however, adherence rates were high at baseline and there was little room for improvement. The duration of follow-up may also have been too short to observe an impact on these variables. The PHR incorporated some individualized feedback, which has been shown to be a key requirement for improving health outcomes.22 28 Research is needed to improve the performance of PHRs and their long-term benefits for the people who are most likely to use them.29
Older adults who engaged with the PHR system tended to have indicators of higher computer self-efficacy and greater health needs: more engaged users were slightly younger, male, used computers more frequently, took more medications, had more medication problems, and were already more likely to be keeping a medication list before being randomized. These findings are consistent with theories of self-management behaviors and reviews of motivators of PHR use, which find that chronic illness and self-efficacy are facilitators for self-management behavior adoption.29–33
There was a doubling in self-reported adverse drug effects from baseline to follow-up for the high users but no change for low/non-users. Because medication safety warnings were triggered for 77% of users who entered medications in the PHR, one possible explanation is that the study intervention increased participant awareness about side effects. Consistent with this interpretation was that they also were significantly more likely to report that they knew how to recognize side effects. Currently, most patient safety problems are detected if patients happen to report them at provider visits or when events lead to hospitalization. One study used between-visit telephone surveillance of diabetes patients and found frequent adverse events, most of which were unknown to primary care physicians.34 Recognition of an adverse effect is a necessary first step toward resolving it. PHRs should develop features that encourage and support interaction with healthcare providers about resolving medication side effects.
The rate of internet use is known to be lower among older people (56% of people aged 65 and over use the internet compared with 83% among people aged 50–65).35 To participate in the study, people had to report using the internet. Because of the cognitive, physical, perceptual, visual, and motor changes that older adults experience, we took great care to reduce interface barriers in designing the PHR. Nevertheless, we found increasing age to be associated with less engagement with the study PHR among internet users enrolled in the study.
Even though over half of older adults logged in, the rate of continued use was low, suggesting that some may not see the value or need for a PHR. High drop-out rates in internet trials of self-help applications are beginning to be recognized as a ‘natural and typical feature’, and intention-to-treat analyses underestimate the impact on a population that continues to use it.36 In a study similar to ours, Krist et al37 also found a low rate of continued use. In their study, of people who registered a PHR account, 49% made a return visit within 3 months and only 10% returned after 3 months. Whether those who chose not to use a PHR would also have benefited remains unanswered. Assuming that such individuals could attain benefits from a PHR if they used one, system design features that reinforce repeated interaction with the system are needed. These may include customizing PHRs to the specific needs of users, providing them with fresh, relevant content, community interaction features, and more options for entering and viewing information, including the use of mobile devices38 39
Although the sampling frame for the trial was population-based, study participants were likely to be more motivated than average,40 limiting the sample representativeness. For example, participants in both study groups had a high rate of keeping a medication list at baseline. In order to participate in the trial, older adults had to first respond to a brief mailed screening questionnaire about computer use, indicate eligibility by using computers to view websites or send or receive email in the past month, and complete a baseline mailed questionnaire.
Standalone PHRs have many advantages, except for the data entry burden. An appeal of standalone PHRs is that they may be particularly of interest for patients who do not have health insurance and for patients with a portal or tethered PHR that does not fully meet their needs. Patients desire a PHR that allows them to enter information while also providing interoperability.41 Standalone PHRs support patient-entered data and also have the potential to integrate patient portal information from multiple systems of care. However, interoperability is not currently typical of standalone PHRs. The study PHR did not have interoperability with medical record or insurance systems. This reflects the reality of health information systems in a rural state at present, but likely underestimates the features and opportunities available elsewhere and in the future for rural communities. In particular, emerging standards such as the meaningful use view, download, and transmit requirements42 should allow patients in the future to transmit their clinical data from healthcare providers to a PHR. To create a sharable medication list and receive medication-related alerts in this study, patients had to enter their medication information. Whether a PHR that can import prescription information from other sources would be used by more patients is an unanswered question. The imported prescription data would have to be demonstrated to be usable to patients, and patients would still need to enter non-prescription medications, which play an important role in medication safety.
A web-based PHR was able to engage patients in medication management and potentially stimulate more complete medication reconciliation discussions with providers and increase patient awareness of medication safety issues. Longer-term studies of continued users will be required to evaluate the impact of these changes in behavior on patient health outcomes. Features that motivate continued use and integration of patient-reported information with systems-derived clinical records should be developed to increase the effects of PHRs on patient self-management and safety.
Supplementary Material
Footnotes
Contributors: EAC made substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data, drafted the article, and provided final approval of the version to be published. JPH, WD, DE, BG, RL, KW, and MM made substantial contributions to conception and design, acquisition of data, and interpretation of data, revised the article critically for important intellectual content, and provided final approval of the version to be published. KF and BL made substantial contributions to conception and design and interpretation of data, revised the article critically for important intellectual content, and provided final approval of the version to be published. EL analyzed the data and made substantial contributions to interpretation of data, revised the article critically for important intellectual content, and provided final approval of the version to be published.
Funding: This work was supported by grant R18HS017034 from the Agency for Healthcare Research and Quality (AHRQ) and grant 2 UL1 TR000442-06 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ or the NIH.
Competing interests: None.
Ethics approval: University of Iowa IRB01.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.National Alliance for Health Information Technology (NAHIT). Report to the Office of the National Coordinator for Health Information Technology on Defining Key Health Information Technology Terms: Department of Health and Human Services (DHHS); 2008. http://healthit.hhs.gov/portal/server.pt/gateway/PTARGS_0_10741_848133_0_0_18/10_2_hit_terms.pdf (accessed 10 Feb 2012).
- 2.Markle Foundation. The personal health working group final report. 2003. http://www.providersedge.com/ehdocs/ehr_articles/The_Personal_Health_Working_Group_Final_Report.pdf (accessed 8 May 2013).
- 3.American Health Information Management Association and the American Medical Informatics Association. The value of personal health records: a joint position statement for consumers of health care. J AHIMA 2007;78:22–4 [PubMed] [Google Scholar]
- 4.Healthcare Information Management and Systems Society. HIMSS personal health records definition and position statement. 2007. http://himss.files.cms-plus.com/HIMSSorg/policy/d/HIMSS_PHR.pdf (accessed 8 May 2013).
- 5.American Health Information Management Association. Personal Health Records. 2012; http://www.ahima.org/resources/phr.aspx (accessed 8 May 2013).
- 6.Aspden P. Institute of Medicine (U.S.). Committee on Identifying and Preventing Medication Errors. Preventing medication errors. National Academies Press, 2007 [Google Scholar]
- 7.Bates DW, Bitton A. The future of health information technology in the patient-centered medical home. Health Aff 2010;29:614–21 [DOI] [PubMed] [Google Scholar]
- 8.Wilson C, Peterson A. Managing personal health information: an action agenda. 2010; (Prepared by Insight Policy Research under Contract No. HHSA290200710072 T). Agency for Healthcare Research and Quality March 2010 Contract No.: AHRQ Publication No. 10-0048-EF
- 9.Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res 2007;42:1443–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar U, Wachter RM, Schroeder SA, et al. Refocusing the Lens: Patient Safety in Ambulatory Chronic Disease Care. Jt Comm J Qual Patient Saf 2009;35:377–83 [DOI] [PubMed] [Google Scholar]
- 11.Ambulatory Safety and Quality: enabling Patient-Centered Care through Health IT (R18) Request for Applications RFA-HS-07–007. http://grants.nih.gov/grants/guide/rfa-files/RFA-HS-07-007.html (accessed 8 May 2013).
- 12.Markle Foundation. PHR adoption on the rise. 2012. http://www.markle.org/publications/1440-phr-adoption-rise (accessed 21 Jan 2012).
- 13.Undeem T. Consumers and health information technology: a national survey. California Health Foundation, 2010. http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/C/PDF%20ConsumersHealthInfoTechnologyNationalSurvey.pdf (accessed 21 Jan 2012). [Google Scholar]
- 14.Palen TE, Ross C, Powers JD, et al. Association of online patient access to clinicians and medical records with use of clinical services. JAMA. 2012;308:2012–19 [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Garrido T, Chock D, et al. The Kaiser Permanente electronic health record: transforming and streamlining modalities of care. Health Aff (Millwood) 2009;28:323–33 [DOI] [PubMed] [Google Scholar]
- 16.Zhou YY, Garrido T, Chin HL, et al. Patient access to an electronic health record with secure messaging: impact on primary care utilization. Am J Manag Care 2007;13:418–24 [PubMed] [Google Scholar]
- 17.Schnipper JL, Gandhi TK, Wald JS, et al. Effects of an online personal healrh record on medication accuracy and safety: a cluster-randomized trial. J Am Med Inform Assoc 2012;19:728–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner PJ, Dias J, Howard S, et al. Personal health records and hypertension control: a randomized trial. J Am Med Inform Assoc 2012;19:626–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krist AH, Woolf SH, Rothemich SF, et al. Interactive preventive health record to enhance delivery of recommended care: A randomized trial. Ann Fam Med 2012;10:312–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hourcade JP, Chrischilles EA, Gryzlak BM, et al. Design Lessons for Older Adult Personal Health Records Software from Older Adults. Proceedings of 6th International Conference on Universal Access in Human-Computer Interaction, held as part of HCI International Lecture Notes in Computer Science, 6766, 2011:176–85 [Google Scholar]
- 21.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003;289:1107–16 [DOI] [PubMed] [Google Scholar]
- 22.Jimison H, Gorman P, Woods S, et al. Barriers and drivers of health information technology use for the elderly, chronically ill, and underserved. Evid Rep Technol Assess (Full Rep) 2008;(175):1–1422 [PMC free article] [PubMed] [Google Scholar]
- 23.Shrank WH, Polinski JM, Avorn J. Quality Indicators for Medication Use in Vulnerable Elders. J Am Geriatr Soc 2007;55:S373–82 [DOI] [PubMed] [Google Scholar]
- 24.MacLean CH, Pencharz JN, Saag KG. Quality indicators for the care of osteoarthritis in vulnerable elders. J Am Geriatr Soc 2007;55:S383–91 [DOI] [PubMed] [Google Scholar]
- 25.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74 [DOI] [PubMed] [Google Scholar]
- 26.Chang E, Doucette W, Pendergast J, et al. Development and initial validation of the Medication Use Self-Evaluation (MUSE) tool. J Am Pharm Assoc 2011;51:258 [Google Scholar]
- 27. Ware JE, Kosinski M, Turner-Bowker DM, et al. How to Score Version 2 of the SF-12v2® Health Survey (With a Supplement Documenting SF-12® Health Survey) Lincoln, RI: QualityMetric Incorporated, 2002. [Google Scholar]
- 28.Gibbons MC, Wilson RF, Samal L, et al. Impact of Consumer Health Informatics Applications. Rockville, MD: Agency for Healthcare Research and Quality, 2009 [Google Scholar]
- 29.Archer N, Fevrier-Thomas U, Lokker C, et al. Personal health records: a scoping review. J Am Med Inform Assoc 2011;515–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unni E, Farris K. Determinants of different types of medication non-adherence in cholesterol lowering and asthma maintenance medications: A theoretical approach. Patient Educ Couns 2011;83:382–90 [DOI] [PubMed] [Google Scholar]
- 31.Lee SK, Kang B, Kim H, et al. Predictions of medication adherence in elderly patients with chronic disease using support vector machine models. Healthc Inform Res 2013;19:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cameron KA, Ross EL, Clayman ML, et al. Patient Educ Couns 2010;80:372–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patient Self-Management Support Programs: An Evaluation: Final Contract Report. November 2007. Rockville, MD: Agency for Healthcare Research and Quality. http://www.ahrq.gov/research/findings/final-reports/ptmgmt/index.html (accessed 16 May 2013).
- 34.Sarkar U, Handley MA, Gupta R, et al. Use of an interactive, telephone-based self-management support program to identify adverse events among ambulatory diabetes patients. J Gen Intern Med 2008;23:459–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pew Research Center, Pew Internet and American Life Project. Demographics of internet users. http://pewinternet.org/Trend-Data-(Adults)/Whos-Online.aspx (accessed 5 Nov 2013).
- 36.Eysenbach G. The Law of Attrition. J Med Internet Res. 2005;7:e11 http://www.jmir.org/2005/1/e11/ (accessed 16 May 2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krist AH, Peele E, Woolf SH, et al. Designing a patient-centered personal health record to promote preventive care. BMC Med Inform Decis Mak 2011;11:73 http://www.biomedcentral.com/content/pdf/1472-6947-11-73.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett GG, Glasgow RE. The delivery of public health interventions via the internet: actualizing their potential. Ann Rev Public Health 2009;30:273–92 [DOI] [PubMed] [Google Scholar]
- 39.Holzinger A, Dorner S, Fodinger M, et al. Chances of increasing youth health awareness through mobile wellness applications. LNC 2010;6389:71–81 [Google Scholar]
- 40.Wanner M, Martin-Diener E, Bauer G, et al. Comparison of Trial Participants and Open Access Users of a Web-Based Physical Activity Intervention Regarding Adherence, Attrition, and Repeated Participation. http://www.jmir.org/2010/1/e3/ (accessed 16 May 2013).
- 41.Fuji KT, Abbott AA, Galt KA, et al. Standalone personal health records in the United States: meeting patient desires. Health Technol 2012;2:197–205 [Google Scholar]
- 42.Medicare and Medicaid EHR Incentive Programs. Stage 2. http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/downloads/Stage2_EPCore_7_PatientElectronicAccess.pdf (accessed 29 May 2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.