Abstract
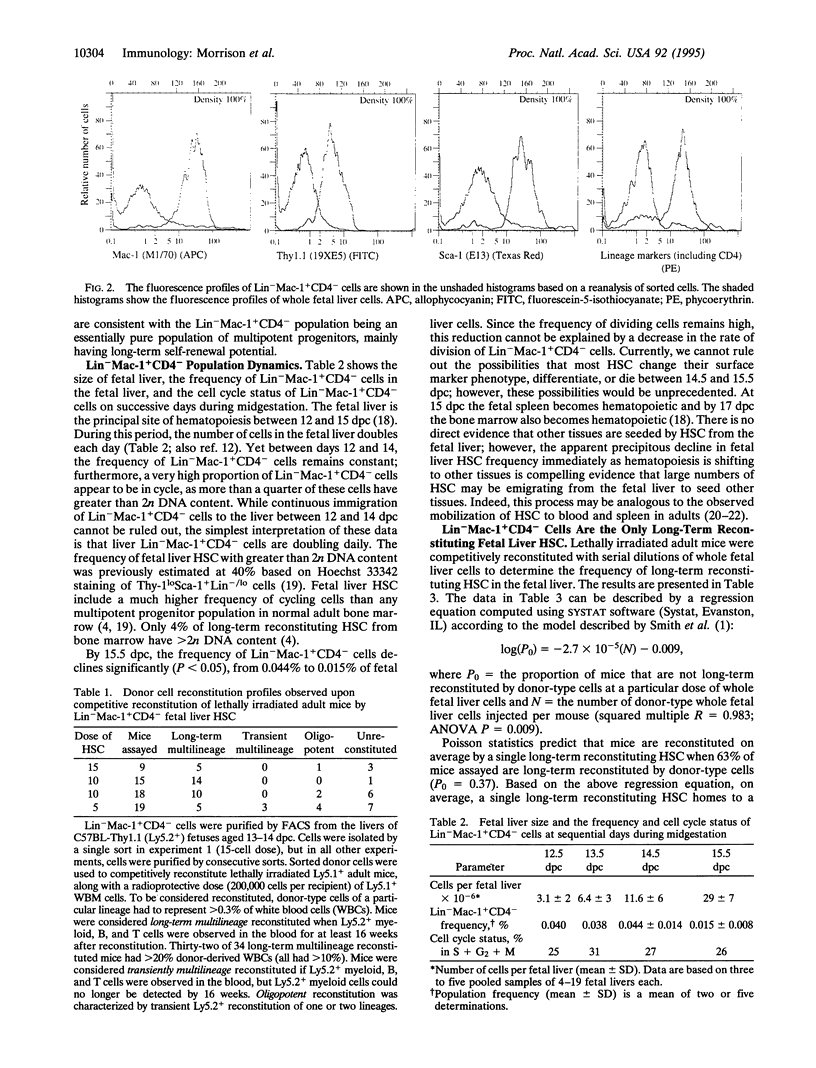
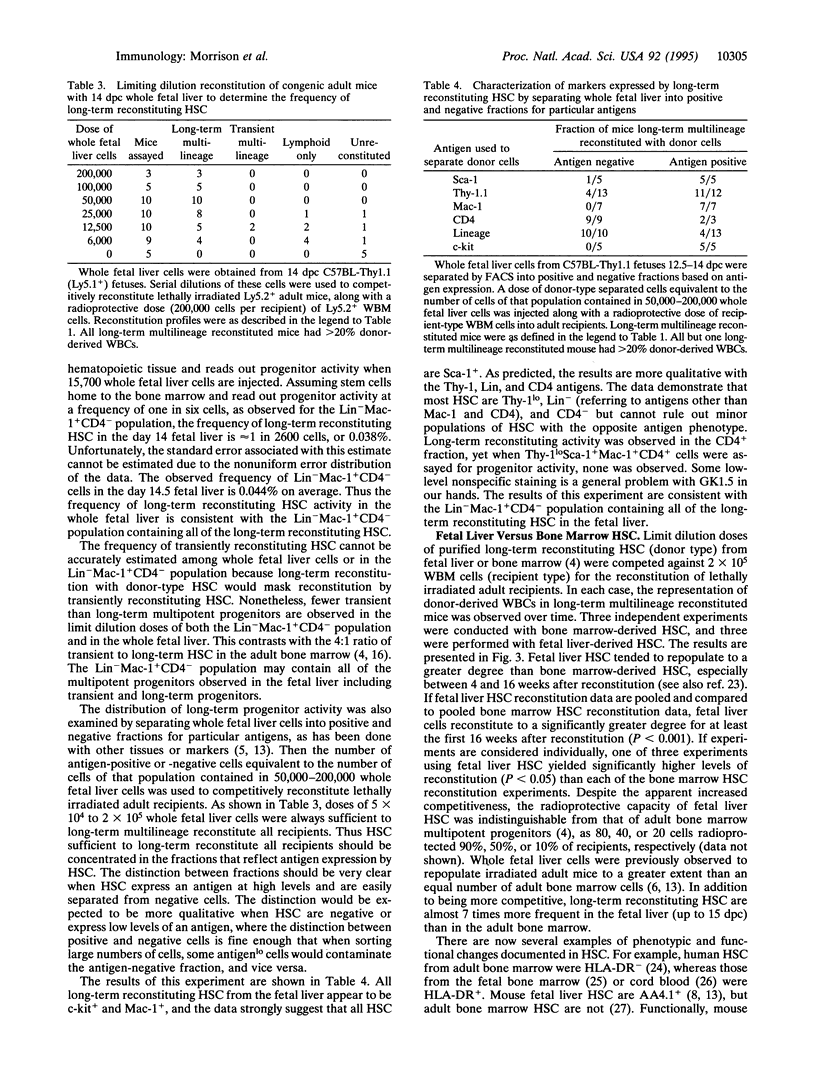
Thy-1loSca-1+Lin-Mac-1+CD4- cells have been isolated from the livers of C57BL-Thy-1.1 fetuses. This population appears to be an essentially pure population of hematopoietic stem cells (HSC), in that injection of only six cells into lethally irradiated adult recipients yields a limit dilution frequency of donor cell-reconstituted mice. Sixty-seven to 77% of clones in this population exhibit long-term multilineage progenitor activity. This population appears to include all long-term multilineage reconstituting progenitors in the fetal liver. A high proportion of cells are in cycle, and the absolute number of cells in this population doubles daily in the fetal liver until 14.5 days postcoitum. At 15.5 days postcoitum, the frequency of this population falls dramatically. Long-term reconstituting HSC clones from the fetal liver give rise to higher levels of reconstitution in lethally irradiated mice than long-term reconstituting HSC from the bone marrow. The precise phenotypic and functional characteristics of HSC vary according to tissue and time during ontogeny.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capel B., Hawley R. G., Mintz B. Long- and short-lived murine hematopoietic stem cell clones individually identified with retroviral integration markers. Blood. 1990 Jun 15;75(12):2267–2270. [PubMed] [Google Scholar]
- Capel B., Hawley R., Covarrubias L., Hawley T., Mintz B. Clonal contributions of small numbers of retrovirally marked hematopoietic stem cells engrafted in unirradiated neonatal W/Wv mice. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4564–4568. doi: 10.1073/pnas.86.12.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbi A. L., Kishimoto T. K., Miller L. J., Springer T. A. The human leukocyte adhesion glycoprotein Mac-1 (complement receptor type 3, CD11b) alpha subunit. Cloning, primary structure, and relation to the integrins, von Willebrand factor and factor B. J Biol Chem. 1988 Sep 5;263(25):12403–12411. [PubMed] [Google Scholar]
- Dührsen U., Villeval J. L., Boyd J., Kannourakis G., Morstyn G., Metcalf D. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood. 1988 Dec;72(6):2074–2081. [PubMed] [Google Scholar]
- Fleischman R. A., Custer R. P., Mintz B. Totipotent hematopoietic stem cells: normal self-renewal and differentiation after transplantation between mouse fetuses. Cell. 1982 Sep;30(2):351–359. doi: 10.1016/0092-8674(82)90233-1. [DOI] [PubMed] [Google Scholar]
- Fleming W. H., Alpern E. J., Uchida N., Ikuta K., Spangrude G. J., Weissman I. L. Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J Cell Biol. 1993 Aug;122(4):897–902. doi: 10.1083/jcb.122.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming W. H., Alpern E. J., Uchida N., Ikuta K., Weissman I. L. Steel factor influences the distribution and activity of murine hematopoietic stem cells in vivo. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3760–3764. doi: 10.1073/pnas.90.8.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunji Y., Nakamura M., Hagiwara T., Hayakawa K., Matsushita H., Osawa H., Nagayoshi K., Nakauchi H., Yanagisawa M., Miura Y. Expression and function of adhesion molecules on human hematopoietic stem cells: CD34+ LFA-1- cells are more primitive than CD34+ LFA-1+ cells. Blood. 1992 Jul 15;80(2):429–436. [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K. A developmental switch in B lymphopoiesis. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11550–11554. doi: 10.1073/pnas.88.24.11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Terstappen L. W. Lymphoid and myeloid differentiation of single human CD34+, HLA-DR+, CD38- hematopoietic stem cells. Blood. 1994 Mar 15;83(6):1515–1526. [PubMed] [Google Scholar]
- Ikuta K., Kina T., MacNeil I., Uchida N., Peault B., Chien Y. H., Weissman I. L. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990 Sep 7;62(5):863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Weissman I. L. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C. T., Astle C. M., Zawadzki J., Mackarehtschian K., Lemischka I. R., Harrison D. E. Long-term repopulating abilities of enriched fetal liver stem cells measured by competitive repopulation. Exp Hematol. 1995 Aug;23(9):1011–1015. [PubMed] [Google Scholar]
- Jordan C. T., McKearn J. P., Lemischka I. R. Cellular and developmental properties of fetal hematopoietic stem cells. Cell. 1990 Jun 15;61(6):953–963. doi: 10.1016/0092-8674(90)90061-i. [DOI] [PubMed] [Google Scholar]
- Kantor A. B., Stall A. M., Adams S., Herzenberg L. A., Herzenberg L. A. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdorp P. M., Dragowska W., Mayani H. Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med. 1993 Sep 1;178(3):787–791. doi: 10.1084/jem.178.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micklem H. S., Ford C. E., Evans E. P., Ogden D. A., Papworth D. S. Competitive in vivo proliferation of foetal and adult haematopoietic cells in lethally irradiated mice. J Cell Physiol. 1972 Apr;79(2):293–298. doi: 10.1002/jcp.1040790214. [DOI] [PubMed] [Google Scholar]
- Molineux G., Pojda Z., Hampson I. N., Lord B. I., Dexter T. M. Transplantation potential of peripheral blood stem cells induced by granulocyte colony-stimulating factor. Blood. 1990 Nov 15;76(10):2153–2158. [PubMed] [Google Scholar]
- Morrison S. J., Uchida N., Weissman I. L. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- Morrison S. J., Weissman I. L. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994 Nov;1(8):661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Parks D. R., Herzenberg L. A. Fluorescence-activated cell sorting: theory, experimental optimization, and applications in lymphoid cell biology. Methods Enzymol. 1984;108:197–241. doi: 10.1016/s0076-6879(84)08086-1. [DOI] [PubMed] [Google Scholar]
- Pytela R. Amino acid sequence of the murine Mac-1 alpha chain reveals homology with the integrin family and an additional domain related to von Willebrand factor. EMBO J. 1988 May;7(5):1371–1378. doi: 10.1002/j.1460-2075.1988.tb02953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. G., Weissman I. L., Heimfeld S. Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude G. J., Brooks D. M. Mouse strain variability in the expression of the hematopoietic stem cell antigen Ly-6A/E by bone marrow cells. Blood. 1993 Dec 1;82(11):3327–3332. [PubMed] [Google Scholar]
- Spangrude G. J., Brooks D. M., Tumas D. B. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: in vivo expansion of stem cell phenotype but not function. Blood. 1995 Feb 15;85(4):1006–1016. [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Srour E. F., Brandt J. E., Briddell R. A., Leemhuis T., van Besien K., Hoffman R. Human CD34+ HLA-DR- bone marrow cells contain progenitor cells capable of self-renewal, multilineage differentiation, and long-term in vitro hematopoiesis. Blood Cells. 1991;17(2):287–295. [PubMed] [Google Scholar]
- Traoré Y., Hirn J. Certain anti-CD34 monoclonal antibodies induce homotypic adhesion of leukemic cell lines in a CD18-dependent and a CD18-independent way. Eur J Immunol. 1994 Oct;24(10):2304–2311. doi: 10.1002/eji.1830241007. [DOI] [PubMed] [Google Scholar]
- Traycoff C. M., Abboud M. R., Laver J., Brandt J. E., Hoffman R., Law P., Ishizawa L., Srour E. F. Evaluation of the in vitro behavior of phenotypically defined populations of umbilical cord blood hematopoietic progenitor cells. Exp Hematol. 1994 Feb;22(2):215–222. [PubMed] [Google Scholar]
- Trevisan M., Iscove N. N. Phenotypic analysis of murine long-term hemopoietic reconstituting cells quantitated competitively in vivo and comparison with more advanced colony-forming progeny. J Exp Med. 1995 Jan 1;181(1):93–103. doi: 10.1084/jem.181.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N., Fleming W. H., Alpern E. J., Weissman I. L. Heterogeneity of hematopoietic stem cells. Curr Opin Immunol. 1993 Apr;5(2):177–184. doi: 10.1016/0952-7915(93)90002-a. [DOI] [PubMed] [Google Scholar]
- Uchida N., Weissman I. L. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin- Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J Exp Med. 1992 Jan 1;175(1):175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J. W., Bauman J. G., Mulder A. H., Eliason J. F., de Leeuw A. M. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984 Jun 1;159(6):1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger D. L., Osman N., Hennings M., McKenzie I. F., Sears D. W., Hogarth P. M. Mouse macrophage beta subunit (CD11b) cDNA for the CR3 complement receptor/Mac-1 antigen. Immunogenetics. 1990;31(3):191–197. doi: 10.1007/BF00211555. [DOI] [PubMed] [Google Scholar]