Abstract
Inflammation contributes to many of the characteristics of plaques implicated in the pathogenesis of acute coronary syndromes (ACS). Moreover, inflammatory pathways not only regulate properties of plaques that precipitate ACS but also modulate the clinical consequences of the thrombotic complications of atherosclerosis. This synthesis will provide an update on the fundamental mechanisms of inflammatory responses that govern ACS, and also highlight the ongoing balance between pro-inflammatory mechanisms and endogenous pathways that can promote the resolution of inflammation. An appreciation of the countervailing mechanisms that modulate inflammation in relation to ACS enriches our fundamental understanding of the pathophysiology of this important manifestation of atherosclerosis. In addition, these insights furnish glimpses into potential novel therapeutic interventions to forestall this ultimate complication of the disease.
Introduction
Inflammation contributes to many of the characteristics of plaques implicated in the pathogenesis of acute coronary syndromes (ACS) (Table 1).1 Two mechanisms precipitate most ACS: a rupture of the plaque's fibrous cap and a superficial erosion of the intima. Fibrous cap fracture causes most fatal acute myocardial infarctions (MIs). The pathways by which inflammation promote fibrous cap rupture indisputably involve inflammation. Superficial erosion has a less clear relationship with inflammation. Therefore this review will focus on plaque rupture. Falk and Virmani have reviewed characteristics of plaques that cause ACS in their contribution to this series. 2 These features (Table 1) include a thin fibrous cap, a multiplicity of macrophages, a large lipid (necrotic) core, spotty calcification, and expansive remodeling. Substantial evidence supports the participation of inflammatory pathways in each of these characteristics associated with plaques that have caused ACS.
Table 1.
Characteristics of Atherosclerotic Plaques Associated with Rupture and Thrombosis.
Inflammation also influences the consequences of a given plaque disruption. While the “solid state” of the atheroma itself determines the propensity to rupture, the “fluid phase” of blood can determine whether a plaque disruption results in a limited mural thrombus that would evade clinical detection or give rise to a sustained occlusive thrombus that could lead to an ST segment elevation MI (Figure 1)
Figure 1. Inflammation in plaque rupture and thrombosis.
This diagram shows a cross section the intima of part of an artery affected by atherosclerosis. Altered hydrodynamics, illustrated in the upper left, cause loss atheroprotective functions of endothelial cells — including vasodilator, anti-inflammatory, pro-fibrinolytic, and anti-coagulant properties. Antigens presented on antigen-presenting cells such as dendritic cells (DCs) can activate Th1 lymphocytes to produce interferon gamma (IFN-γ), which activates macrophages (MΦ, yellow). Other subtypes of lymphocytes (shown in blue) include Th2 lymphocytes, which can elaborate the anti-inflammatory cytokine interleukin 10 (IL-10) and regulatory T cells that secrete the anti-inflammatory cytokine transforming growth factor beta (TFG-β). On its surface, the macrophage contains Toll-like receptors (TLRs) 2 and 4, which can bind PAMPs and DAMPs (see text). The intracellular TLRs 3, 7, and 9 may also contribute to lipid accumulation and other pro-atherogenic functions of the macrophage. Macrophages can undergo stress of the endoplasmic reticulum (ER) under atherogenic conditions. Cholesterol crystals found in plaques can activate the NLRP3 inflammasome (see text) that can generate mature IL-1β from its inactive precursor. The activated macrophage secretes collagenases that can degrade the triple helical interstitial collagen that lends strength to the plaque's fibrous cap. Activated macrophages also express tissue factor, a potent pro-coagulant, and elaborate pro-inflammatory cytokines that amplify and sustain the inflammatory process in the plaque. When the plaque ruptures due to a collagen-poor, weakened fibrous cap, blood in the lumen can contact tissue factor in the lipid core, triggering thrombus formation (red). When the thrombus forms, polymorphonuclear leukocytes (PMNs) can accumulate and elaborate myeloperoxidase (MPO), which in turn elaborates the potent pro-oxidant hypochlorous acid. Dying PMNs extrude DNA that can form neutrophil extracellular traps (NETs), which can entrap leukocytes and promote thrombosis. Other inflammatory cells modulate atherosclerosis. B2 lymphocytes secrete natural antibody that can inhibit plaque inflammation. On the other hand, B1 lymphocytes, in part via B-cell activating factor (BAFF), can promote inflammation and plaque complication. Mast cells can augment atherogenesis by releasing histamine and the cytokines IFN-γ and IL-6. The consequences of a given plaque rupture depend not only on the solid state of the intimal plaque, but also on the fluid phase of blood, as depicted in the upper right. Systemic inflammation can give rise to cytokines, culminating in the overproduction of IL-6, the trigger of the hepatic acute phase response. The acute phase reactant fibrinogen participates directly in thrombus formation. Another acute phase reactant, plasminogen activator inhibitor-1 (PAI-1), can impair fibrinolysis by inhibiting the endogenous fibrinolytic mediators, urokinase and tissue-type plasminogen activators (uPA and tPA).
Maintenance of homeostasis in health and limiting excessive inflammation responses in disease requires countervailing mechanisms. This review will illustrate how inflammation drives the properties of plaques and blood that predispose to ACS, how endogenous anti-inflammatory and resolution measures mitigate over exuberant inflammatory responses, and various therapeutic opportunities afforded by the inflammatory aspects of ACS.
Inflammation Drives ACS
Thin fibrous cap
Plaques that have ruptured and caused fatal acute MI generally have thin fibrous caps, measured in many studies at around 60–70 microns.3 Recent studies with optical coherence tomography (OCT), which provides splendid near field high-resolution views of the intima, have corroborated in humans in vivo the observations of pathologists on autopsy specimens implicating thin fibrous caps in plaque disruption.4 Inflammation decisively governs the metabolism of collagen, a major constituent of the fibrous cap that confers upon it much of its tensile strength. As recently reviewed, inflammatory signals such as the TH1 cytokine gamma interferon impair the ability of the smooth muscle cell (SMC), the source of most arterial interstitial collagen, to synthesize new collagen required to repair and maintain the extracellular matrix of the fibrous cap.1 Considerable biochemical and experimental data support the role of matrix-degrading proteinases, tightly regulated by inflammatory mediators, as contributors to dissolution of interstitial collagen that thins and weakens the fibrous cap and hence renders a plaque susceptible to rupture.5 Death of SMCs in the inflamed atheroma can deplete the plaque of the very cells that synthesize collagen required to form and maintain the fibrous cap.6, 7
Inflammatory cell recruitment
Observations on human atheroma specimens, in vitro studies, and animal experiments have elucidated pathways by which leukocytes from blood adhere to the endothelial cells (ECs) at sites predilected to atheroma formation, enter the intima, and undergo activation to sustain and amplify inflammation locally (Figure 2). Many recent reviews have highlighted the role of pro-inflammatory cytokines as inducers of endothelial-leukocyte adhesion molecules that capture blood leukocytes, most prominently the blood monocyte.8 A series of chemokines that interact with cognate receptors on various classes of leukocytes cause their directed migration to penetrate into the intima. 8, 9 Recent refinements of our understanding of leukocyte accumulation in plaques include an expanded understanding of monocyte/macrophage heterogeneity.10 In particular, in hypercholesterolemic mice, a pro-inflammatory subset of monocytes marked by high levels of expression of the surface marker Ly6c enter plaques early in development of experimental atherosclerotic lesions.11, 12 A preformed pool of pro-inflammatory monocytes in the spleen provides many of these pro-inflammatory monocytes that populate early plaques.13 The Ly6c high monocyte subpopulation uses CCL2/CCR2 or CXCL1/CXCR2 as a major chemokine-receptor dyad related to their recruitment. The less inflammatory subpopulation of monocytes containing low levels of Ly6c may favor fractalkine (CX3CL1) and its receptor (CX3CR1) and CCR5 as trafficking mechanisms.14 The Ly6c low population may arise from Ly6c high monocytes, a transition mediated by the transcription factor Nr4a1 (Nur77.) 15 Mononuclear phagocytes can proliferate in plaques.16 The macrophage scavenger receptor A, known to bind modified lipoproteins, may contribute to mediating this process.17 Macrophage colony stimulating factor (M-CSF/CSF-1) participates in the maturation of monocytes to macrophages, induces the expression of scavenger receptors that permit accumulation of modified lipoproteins within the phagocyte, fostering the formation of foam cells, the hallmark of the atherosclerotic lesion.18 While less apparent morphologically, but functionally pivotal, other leukocyte populations reside in plaques including various classes of T lymphocytes, B lymphocytes, mast cells, and dendritic cells (DCs).9, 19, 20 A recently recognized population of innate response activator B cells can produce granulocyte-macrophage colony stimulating factor (GM-CSF/CSF-2) in the spleen of hypercholesterolemic mice where it can activate dendritic cells which in turn promote TH1 cells capable of producing gamma-interferon that can migrate to atheroma and activate plaque macrophages.21
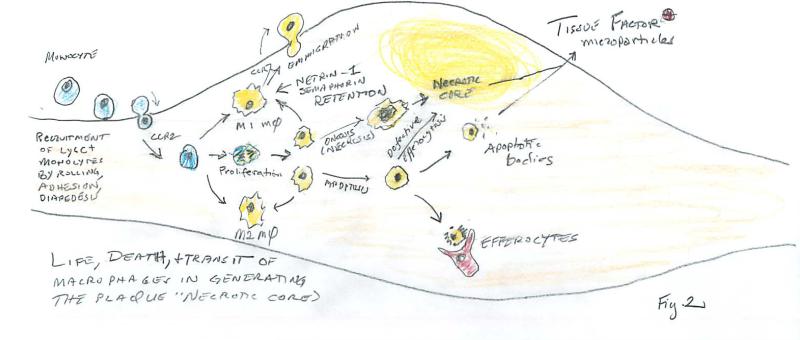
Figure 2. Life, death, and transit of macrophages in generating the plaque's “necrotic” core.
Blood monocytes interact with adhesion molecules expressed by endothelial cells exposed to inflammatory mediators. They first roll and attach more firmly, and ultimately diapedese into the intima in response to chemoattractant molecules that bind to surface receptors on the leukocyte — exemplified here by chemokine receptor 2 (CCR2). The monocytes recruited to the intimal lesion can proliferate, giving rise to daughter cells, or can differentiate into macrophages with a pro-inflammatory slant (denoted M1) or those that are alternatively activated (denoted M2). Macrophages can traffic, including leaving the plaque by emigration, as shown here on the macrovascular surface. Retention factors such as netrin-1 or semaphorin-3 can promote retention accumulation of mononuclear phagocytes in the plaque. Macrophages can die by oncosis (sometimes called necrosis) or by programmed cell death (apoptosis). Advanced plaques show a defect in clearance of apoptotic cells leading to their accumulation, a process denoted “mummification” due to a defect in efferocytosis (see text). Dying cells can also release apoptotic bodies and microparticles bearing the potent pro-coagulant tissue factor that triggers thrombus formation in disrupted plaques. The detritus of dead and dying cells that accumulate due to defective efferocytosis give rise to the lipid-rich “necrotic” core of the plaque.
Most depictions of leukocyte recruitment to plaques portray this process as taking place at the macrovascular luminal surface overlying the lesion. While this locale likely applies to the nascent lesion, once established, atheromata develop microvasculature.22 The plexi of plaque microvessels provide an even more abundant surface for leukocyte trafficking. Indeed, a key adhesion molecule, vascular cell adhesion molecule-1 (VCAM-1) localizes to the micro- rather than macrovascuar endothelium in human lesions. 23 Plaque hypoxia and expression of angiogenic growth factors elaborated by inflammatory cells drive this neovascularization in plaques. 9, 22
Large lipid pools and “necrotic” core (see also24
The Rosenson et al. contribution to this series discusses lipid metabolism and targets in the context of ACS.24 Therefore, we focus here on inflammatory cell death by apoptosis or oncosis and defects in the clearance of dead cells, a process known as efferocytosis (Figure 2).
Large necrotic cores characterize plaques that cause ACS (Table 1). Plaque inflammation can promote necrotic core formation, and, in turn, plaque necrosis can worsen inflammation in advanced atheromata.25, 26 Necrotic cores contain dead cells and their detritus, and because most of the dead cells consist of foamy macrophages, lipid debris abounds. Necrotic cores likely contribute to plaque rupture and ACS as they possess inflammatory, proteolytic, and thrombogenic properties.27-29 Moreover, the material properties of the lipid-rich necrotic core places biomechanical stress on the overlying fibrous cap and thereby contributes to cap rupture.30
The dead cells that accumulate in necrotic cores likely have two origins: secondary cell death due to the combination of apoptosis and defective clearance of the apoptotic cells (efferocytosis); and primary cellular necrosis, also known as “necroptosis” or “oncosis.” Macrophages have relatively long lives, but turnover of intimal macrophages occurs at all stages of atherosclerosis. In early-stage lesions, efficient efferocytosis by neighboring phagocytes, notably macrophages, limits secondary macrophage necrosis. 31,26 As lesions progress, however, intimal cell apoptosis increases for a number of reasons, including inflammatory stimuli and factors that promote oxidative and endoplasmic reticulum (ER) stress. These stressors trigger mitochondrial pathways of apoptosis and likely work in concert with other plaque factors that activate toll-like receptors (TLRs), death receptors, and additional mitochondrial apoptotic pathways.
As plaques advance, efferocytosis begins to fail promoting secondary necrosis of the apoptotic cells and loss of efferocytosis-mediated anti-inflammatory signaling. The cytoplasmic tails of efferocytosis receptors usually mediate these anti-inflammatory pathways and result in the production of anti-inflammatory/pro-resolving IL-10 and transforming growth factor beta (TGF-β).32 The release of damage-associated molecular patterns (DAMPs) from necrosing cells likely further amplifies plaque inflammation. DAMPs trigger inflammatory pathways in macrophages by activating innate immune receptors, including TLRs, and DAMPs likely promote plaque progression toward those prone to provoke ACS.
Many cell types can carry out efferocytosis, but dedicated phagocytes like macrophages and DCs do so most efficiently. The process involves recognition of particular features on apoptotic cells, notably cell-surface phosphatidylserine (PS), by efferocyte receptors that bind PS directly or through a bridging molecule. Although observations in mice with advanced atherosclerosis have identified macrophage efferocytosis receptors relevant to necrotic core formation, the mechanisms of defective efferocytosis in advanced atheromata are not known. In theory, the problem could reside with the efferocytes themselves (e.g., receptor or internalization defects), or with an apoptotic cell-related property that decreases their recognition by efferocytes. As discussed below, failed efferocytosis characterizes impaired resolution of the inflammatory response.
Primary necrosis refers to a pathway of cell death that is morphologically and biochemically distinct from apoptosis.33 In contrast to apoptosis, membrane leakiness occurs early in primary necrosis, chromosome condensation does not occur. Activation of a family of kinases called receptor-interacting protein (RIP) triggers a particular programmed form of necrosis, called necroptosis. Deficiency of one of the RIP kinases, RIP3, in myeloid-derived cells in both Ldlr-/- and Apoe-/- mice leads to a decrease in necrotic macrophages in advanced atherosclerotic lesions, but no observable changes in early atherosclerosis. The trigger to necrosis in atheromata remains unknown, but RIP3-dependent cell death can occur in cultured macrophages exposed to oxidized LDL together with a caspase inhibitor.34 Phagocytes have specific mechanisms for the clearance of necrotic cells, so the presistence of dead cells in advanced atheroma may indicate a defect in this subtype of efferocytosis.
As alluded to above, numerous studies using Ldlr-/- and Apoe-/- mice that consume an atherogenic diet support the overall concepts related to intimal cell death, efferocytosis, inflammation, and plaque necrosis as a function of lesion stage, and the underlying molecular-cellular mechanisms. Although these experiments do not recapitulate the processes of plaque rupture and thrombosis, ACS in humans associate with coronary arterial lesional ER stress, intimal cell apoptosis, and the features associated with plaque instability (Table 1). Moreover, advanced human coronary artery lesions show signs of defective efferocytosis.35
Spotty calcification characterizes plaques associated with ACS
Traditional thinking has regarded calcification of atherosclerotic plaques as a passive “degenerative” process. In contrast, contemporary concepts view calcification as a dynamic active process critically dependent on inflammatory cells and signaling. Mice deficient in M-CSF, an activator of macrophages, accumulate calcium in atheromata, likely due to impaired osteoclastic activity of the mononuclear phagocytes.36 Molecular imaging studies colocalized niduses of inflammation with early mineralization in mouse atheromata.37 Inflammatory mediators also instruct SMCs to alter functions related to the formation of calcifying foci.
Large plates of calcium mineral may influence the stability of plaques. Recent work using computational methodology has shown that pinpoint calcification can introduce biomechanical inhomogeneities implicated in precipitation of plaque rupture.38 Nuclear imaging studies using NaF provides a quantifiable index related to calcification in carotid and coronary artery plaque that correlates with Framingham risk. 39, 40 Imaging studies using computed tomographic (CT) approaches have associated spotty calcification with propensity of plaques to provoke ACS.41, 42 The implication of inflammation in mechanisms related to cardiovascular calcification provides a novel link to ACS pathogenesis.
Outward remodeling
The observations of Clarkson in non-human primates and of Glagov in human atherosclerotic plaques pointed to the role of expansive remodeling or compensatory enlargement during atherogenesis.43, 44 Imaging studies using intravascular ultrasound and computed tomography have associated expansive remodeling with risk of plaques to precipitate ACS.45 Outward remodeling of arteries necessitates degradation of the extracellular matrix (including collagen) and of elastin, the major protein of the elastic laminae of arteries. Just as inflammatory mediators influence the expression of proteinases involved in collagenolysis in thinning of the fibrous cap, similar regulation of elastases and other matrix-degrading enzymes by inflammatory mediators can control expansive remodeling.46 In atherosclerosis-susceptible mice, genetically produced alterations in signaling by the key inflammatory mediator interleukin-1 (IL-1) leads to a failure of expansive remodeling during atherogenesis.47 In particular, reduced induction of the matrix metalloproteinase MMP-3 due to lack of IL-1 signaling appears to contribute causally to this defective remodeling.47 Thus, inflammatory pathways also govern this characteristic of plaques that provoke ACS.
Thrombogenicity
As indicated above, the consequences of plaque disruption depend on the balance between pro-coagulant and anti-coagulant and pro-fibrinolytic and anti-fibrinolytic factors both in the solid state of plaque and fluid phase of blood (Figure 1).48 Within the plaque, a subpopulation of mononuclear phagocytes and activated SMCs overexpress the potent pro-coagulant tissue factor in response to inflammatory stimuli, notably CD40 ligand (CD154).49-51 The normal intima expresses a number of functions that combat thrombus accumulation.52 ECs normally express thrombomodulin and endogenous fibrinolytic mediators urokinase type and tissue type plasminogen activator. The normal intima also contains heparan sulfate proteoglycans that have anti-coagulant properties. The balance between these anti-thrombotic and pro-fibrinolytic mechanisms shifts in response to inflammatory mediators. The local production of plasminogen activator inhibitor-1 (PAI-1), a major blocker of fibrinolysis, rises in SMCs and ECs exposed to pro-inflammatory stimuli. Thus, the solid state of the plaque augments thrombogenicity and resists fibrinolysis in response to inflammatory activation.
Likewise, the fluid phase of blood promotes clot accumulation in response to systemic inflammation. The hepatocyte augments productions of acute phase reactants in the presence of systemic inflammation, notably interleukin-6 (IL-6), a pro-inflammatory cytokine strongly implicated in the pathogenesis of ACS by recent genetic studies.53 Among the acute phase reactants released by the liver in response to IL-6, fibrinogen participates directly in clot formation. The hepatocyte also increases production of PAI-1 when exposed to IL-6. Platelets participate pivotally in the formation of arterial thrombi, and furnish an endogenous source of preformed mediators that amplify and sustain local inflammatory responses.54 Once plaques have disrupted, neutrophils also enhance local inflammation and oxidative stress, including through the formation of neutrophil extracellular traps (NETs).55 Hence, in response to inflammation, both the solid state of plaque and the fluid phase of blood conspire to promote thrombus accumulation by increased thrombogenicity, decreases anti-coagulant properties, and impaired fibrinolytic capacity.
Pharmacologic anti-inflammatory interventions
Statin drugs have transformed cardiovascular prevention in the last decades. Beyond their LDL-lowering effects, statins have direct anti-inflammatory actions mediated by inhibition of prenylation of small G-proteins and induction of transcription factors such as Krüppel-like factor-2 (KLF-2) that alter inflammatory pathways in a concerted manner.56 Thus, the statins likely reduce ACS risk in part by these, and perhaps other, anti-inflammatory properties. Among anti-inflammatory strategies, colchicine has recently demonstrated promising reductions in cardiovascular events, potentially through inhibiting the inflammasome pathway in macrophages.57, 58 Other direct anti-inflammatory interventions that do not affect LDL are currently under investigation.58, 59
The endogenous resolution response and its defect in advanced atherosclerosis
The acute inflammatory response in host defenses unleashes oxidative and protease-mediated tissue injury that can lead to collateral damage. The inflammatory response normally also involves a resolution phase that, after attacking the acute injury or infection, quells inflammation and restores tissue homeostasis.60, 61 Multiple lipid-derived and protein mediators (Table 2) promote resolution by blocking inflammatory cell influx and promoting their egress; clearing pathogens, cellular debris, inflammatory cytokines, and dead cells (efferocytosis); and repairing tissue damage.62, 63 The molecules that mediate the resolution response include specialized pro-resolving mediators (SPMs), endogenous lipids generated actively during inflammation.62 SPM families include lipoxins, resolvins, protectins, and maresins. Lipoxins derive from omega-6 arachidonic acid, while resolvins, protectins, and maresins arise from omega-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Lipoxygenase (LOX)- and cyclooxygenase (COX)-initiated mechanisms biosynthesize SPMs. SPMs each possess distinct structures that bind and activate select cell-surface receptors.
Table 2.
Mediators of Inflammation Resolution Possibly Relevant to Atherosclerosis.
| Characteristics of Advanced Lesions | Mediators that Reverse Processes Associated with Advanced Lesions | Refs |
|---|---|---|
| Persistent inflammatory cell influx | LXA4, RvE1, PD1, AT-PD1, RvD1, RvD2, RvD5, MaR1, RvD3, Ac2-26, AnnexinA1 | 131-139 |
| Excessive proinflammatory mediators | LXA4, RvE1, PD1, RvD1, RvD2, RvD5, MaR1, RvD3, Ac2-26, AnnexinA1, IL-10 | 134-136, 138, 140-144 |
| Defective efferocytosis | LXA4, RvE1, PD1, RvD1, RvD2, RvD5, MaR1, RvD3, Ac2-26, IL-10 | 79, 137, 138, 142, 145-149 |
| Exuberant oxidative stress | LXA4, 15-epi-LXA4, RvE1, PD1, RvD1, RvD2, Ac2-26 | 81, 150-155 |
| Decreased collagen production/fibrous cap thinning | RvE1 | 156 |
| Impaired egress | RvE1, PD1, ATLa, carbon monoxide, CCR7 | 123, 142, 157 |
| Uncontrolled platelet activation | RvE1 | 158, 159 |
In chronic diseases, persistence of the irritative stimulus, such as intimal lipids in atherosclerosis, can mute the resolution phase, leading to a cycle of persistent tissue injury, which generates DAMPs that propagate inflammation. Impaired resolution of inflammation can contribute to many of the features of plaques associated with ACS, including persistent influx and defective egress of myeloid cells; accumulation of DAMPs; secondary necrosis of intimal cells due to defective efferocytosis, augmenting necrotic core formation; and degradation of the extracellular matrix.
Mechanisms of SPM dysregulation relevant to advanced atherosclerosis and ACS
In addition to a persistent inflammatory stimulus, such as retained subendothelial apoB lipoproteins, additional factors may hamper resolution inflammation in advanced atherosclerosis. LC-MS-MS profiling in other chronic inflammatory conditions, such as cystic fibrosis, asthma, and localized aggressive periodontitis has revealed decreased SPMs compared with healthy controls.64-66 Moreover, lower plasma levels of a specific SPM called aspirin-triggered LXA4 (ATL) associate with increased risk for peripheral and coronary atherosclerosis in humans, even after correcting for age, sex, and C-reactive protein levels.67 Unraveling the mechanism(s) by which SPM concentrations decrease in chronic inflammatory diseases and whether this drop also applies to advanced atherosclerosis will require further studies. Possibilities include insufficient substrate availability (i.e., lack omega-3 fatty acids in the diet); dysregulated signaling, perhaps due to suppression of SPM receptors; impaired biosynthetic capacity, such as decreased lipoxygenase expression and/or function; and/or hyperactive catabolism of these SPMs.
Observational studies show that populations that have a high intake of foods containing omega-3 compared with omega-6 fatty acids have better cardiovascular outcomes.68 Yet, studies evaluating omega-3 fish oil supplementation in humans have shown variable results. This discrepancy may result from differences in study endpoints (e.g., incident atherosclerotic disease versus coronary death), whether subjects entered the studies already having sufficient omega-3 fatty acid intake, a lack of uniformity and quality of the fish oils being tested, and/or to the difficulty of showing beneficial effects on subjects already receiving statins.69 In mice, dietary omega-3 supplementation attenuates atherogenesis,70-72 and high-dose EPA causes lesion regression.73 Genetically-mediated increase of the n-3/n-6 ratio in mice also yields less atherosclerosis.74 Prostaglandin E2, via the macrophage EP4 receptor, can also mitigate vascular inflammation.75
Overexpression of a key SPM biosynthetic enzyme, 12/15-LOX, in chowfed Apoe-/- mice limited atherosclerosis, providing support for a role of endogenous biosynthesis of SPMs,76 but mice consuming a high-fat diet did not show this protection.77 Consistent with the concept that a high-fat diet impairs resolution, diet-induced obesity associates with defective inflammation resolution.78 Although the mechanisms underlying this experimental observation remain speculative, the results suggest that diet may modulate ACS risk in part by effects on inflammation resolution.
The therapeutic potential of SPMs in ACS: inflammation suppression without compromise of host defense
As impaired inflammation resolution may aggravate atherosclerosis and ACS risk, new approaches to stimulate resolution have considerable interest. SPMs limit inflammation without causing immunosuppression, setting them apart from many other current anti-inflammatory strategies.79 In this context, SPMs can enhance efferocytosis.62 Obese atherosclerotic mice that consumed an omega-3 fish oil diet containing the SPM precursors EPA and DHA had amelioration of impaired efferocytosis.80 Conversion of EPA and DHA to SPMs within the vasculature might contribute to this benefit. Likewise, the resolvins RvD1 and RvD2 may benefit injured atherosclerotic arteries.81 Indeed, vascular injury in vivo augments D-series resolvin generation, and therapeutic administration of resolvins decreases intimal hyperplasia and leukocyte trafficking to injured arteries. These resolvins block migration, proliferation, monocyte adhesion, and inflammatory signaling in human primary vascular SMCs. Thus, resolution agonists merit consideration as therapeutic targets to reduce ACS risk.
Potential resolution-enhancing effects of low-dose aspirin in relation to protection against ACS
Low-dose aspirin (acetylsalicylic acid [ASA]) prevents recurrent ACS, a benefit traditionally ascribed to anti-platelet action mediated by COX inhibition.82 Yet, aspirin has anti-inflammatory actions in humans, such as blocking leukocyte trafficking to inflamed tissues, that are difficult to attribute solely to reduced prostanoid biosynthesis.83 Indeed, aspirin alters the active site of COX-2 in a manner that permits conversion of arachadonic acid (AA) to 15R-HETE in vascular ECs, a precursor for leukocyte production of pro-resolving 15-epilipoxins.84 Humans taking low-dose aspirin form these lipoxins, which limit neutrophil infiltration into inflammatory sites and stimulate nitric oxide (NO) production. COX-2 acetylated by ASA can also process EPA and DHA to boost generation of other resolvins.85, 86 Thus, non-prostanoid-related, resolution-mediating effects of low-dose aspirin may contribute to their ability to protect against ACS.
Adaptive immune modulation can also mute inflammation
Some functions of both innate and adaptive immune cells can also counterbalance pro-inflammatory pathways implicated in ACS and mitigate inflammation (Figure 1). For example, Ly6c low monocytes and alternatively activated macrophages (denoted “M2”) can modulate inflammation. While B2 lymphocytes appear to aggravate atherosclerosis, B1 lymphocytes elaborate natural antibodies that can quell atherogenesis.87 TH2 lymphocytes can elaborate interleukin-4 and interleukin-10 that may limit inflammation and sway macrophages towards M2 polarization. Regulatory T cells, by elaborating TGF-β, can balance the pro-inflammatory cascade unleashed by TH1 cells and thus modulate inflammatory responses in the atheroma.9, 88
Innate immune modulation in atherosclerosis and its relevance to ACS
The innate immune system represents a defense system against pathogens — a “first responder” mechanism that can mobilize rapidly against threats and without prior exposure to the provocateur. The innate immune system also responds to tissue injury to initiate response and repair processes. Innate immune activation participates centrally in the pathogenesis of atherosclerosis. Dysregulated lipid metabolism, particularly an abundance of apolipoprotein B-containing lipoproteins and their retention in the arterial wall, contributes to the development of macrophage foam cells.89 Such aberrations and the products of modified or native lipoproteins that accumulate in atherosclerotic plaques can trigger pattern recognition receptors (PRRs) expressed by macrophages, including the NOD-like receptors (NLRs), scavenger receptors, and TLRs, thereby activating the inflammatory response. 90, 91
NLRs and atherosclerosis
Atherosclerotic plaques contain cholesterol crystals, both in extracellular spaces and within plaque macrophages. Although previously considered a feature of advanced plaques, a recent study showed the presence of cholesterol crystals in early lesions in Apoe-/- mice.92 Macrophages can engulf these crystals, resulting in the induction and activation of the NLRP3 (NOD-, LRR- and pyrin domain-containing 3) inflammasome. This intracellular complex processes IL-1β to its active form — the target of an ongoing clinical trial (CANTOS) to prevent recurrent ACS.93 In addition to preformed cholesterol crystals, recent work indicates that loading of macrophages with cholesterol can lead to de novo formation of intracellular cholesterol crystals that trigger NLRP3 in a process mediated in part by CD36.94
Although not yet investigated, other crystalline or amyloid substances in atherosclerotic plaques, such as calcium phosphate crystals or serum amyloid A, may also represent DAMPS that could trigger the inflammasome and IL-1β secretion. In addition to specifically inhibiting IL-1β, blocking of inflammasome activation might offer another strategy to prevent ACS. That colchicine decreases the prevalence of ACS in gout patients, and the recent LoDoCo intervention trial in patients without gout but having CAD, affirm the potential of this approach.57
TLRs and atherosclerosis
The role of TLR signaling pathways in promoting atherosclerosis received support from mouse studies in which whole-body deletion of Tlr2 or Tlr4 or of the adaptor proteins used by these TLRs — including IL-1 receptor-associated kinase 4 (IRAK4),79, 80 TNF receptor-associated factor 6 (TRAF6),81 TIR-domain–containing adaptor protein inducing IFN-β (TRIF; also known as TICAM-1) and myeloid differentiation primary-response protein 88 (MYD88) — confers protection from atherosclerosis.95-103 These findings have initiated investigations of the endogenous ligands that accumulate during hypercholesterolemia and in plaques that may trigger these microbial-sensing pathways in macrophages. Among the candidates proposed, oxidized LDL (oxLDL) species have undergone extensive study,104 but numerous factors and pathways may contribute to the initiation and the maintenance of TLR-induced macrophage inflammation in atherosclerotic plaques. Nonetheless, oxLDL has remained firmly in the sights of investigators as a major therapeutic target, with recent efforts directed towards a vaccine strategy to reduce ACS risk by eliminating oxLDL as it is formed — before it can stimulate TLR-related inflammatory pathways in macrophages — among other potential benefits.105
Macrophage Polarization in the Plaque
Histological analysis of mouse and human plaques has shown the presence of both M1 and M2 macrophages. For example, in human plaques, M1 macrophages localize to areas distinct from those in which the less inflammatory M2 macrophages (“alternatively activated” macrophages) situate.106 Studies of M1 and M2 macrophages polarized in vitro by, for example, IFN-γ or IL-4, respectively, and in atherosclerotic mice have led to the simplified view that M1 macrophages promote while M2 macrophages limit plaque inflammation.107 This construct caricatures the complex range of macrophage functions in vivo, as macrophages encounter microenvironments of diverse, and even opposing, signals. For example, in addition to inducing the aforementioned TLR signaling, which can lead to M1 polarization, oxidized LDL can also induce the expression of the M2 macrophage phenotypic marker arginase 1 via the activation of peroxisome proliferator activated receptor-γ (PPARγ).108
The full range of influences in the plaque microenvironment that promote the polarization of macrophages in vivo in general, and in plaques in particular, remain incompletely defined, but undoubtedly include interactions with the adaptive immune system; e.g., T helper 1 (TH1) and TH2 cells, important players in plaque inflammation as reviewed earlier, secrete potent macrophage-polarizing factors (e.g., IFN-γ and IL-4, respectively; Figure 1).107 Although the spectrum of macrophage functions in vivo, particularly in humans, likely exceeds that typically described in vitro,109 the M1/M2 classification still provides a useful framework, albeit oversimplified. Considerable data support its main point, that there exist subsets of macrophages with different properties, such as inflammatory and tissue repair. This concept has high relevance to the inflammatory state and its resolution in plaques of the type that cause ACS. For example, M1 macrophages secrete proatherosclerotic inflammatory cytokines (such as IL-6 and IL-12), as well as reactive oxygen and nitrogen species that would exacerbate oxidative stress in the plaque. M2 cells, on the other hand, secrete low levels of IL-6 and IL-12, but high levels of one of the endogenous anti-inflammatory cytokine, IL-10. M2 macrophages also exhibit scavenging of necrotic debris, enhanced efferocytotic activity, and reduced production of reactive nitrogen species, all considered to be pro-resolving processes as explained above.
Atherosclerotic mice display a dynamic balance between M1 and M2 macrophages in plaques. Advanced lesions have a high proportion of macrophages that express mainly markers of the M1 state. In various mouse models of regression (aortic transplantation, generic reversal of hyperlipidemia, anti-miR33 treatment, injection of apoAI, the HDL-forming protein) the balance between the macrophage polarization markers shifts toward the M2 panel of functions.110-113 From a functional point of view, the properties of M2 macrophages align well with the macroscopic changes observed in regressing plaques — the loss of inflammatory cells and the remodeling of tissue to a morphology associated with less risk of rupture, such as a reduction in the necrotic core.114
That the M1/M2 state might undergo manipulation in human plaques as a preventive or therapeutic approach to ACS, as suggested by one (albeit small) clinical study in which patients who received HDL infusions prior to peripheral atherectomies showed a tendency for decreased inflammatory mediators in the plaques relative to those excised from the control group.115 Experimental studies support this notion. Two recent studies in atherosclerotic mice in which the M2 state was induced and maintained by injection of either IL-13 (a strong polarizer in vitro, that in addition to IL-4 is secreted by TH1 lymphocytes) or helminth antigens showed reduced atherosclerosis progression and plaque inflammation.116, 117
In summary, the pre-clinical data in progressing and regressing plaques provide a considerable rationale for efforts to enrich plaques in M2 macrophages clinically to either prevent plaques from reaching an “ACS-prone state” or to promote rapid resolution of inflammation in the ACS setting. Based on the evolving literature, this enrichment might be accomplished, for example, by treatment with direct (small molecule) mediators of TH2-related pathways, indirect regulators of M2 polarization, such as HDL, or by plaque targeted nanoparticle-based delivery of agents, including siRNA, anti-sense RNA, and peptides.
Retention and clearance of plaque macrophages
As noted earlier, enrichment in macrophages characterizes plaques that have provoked fatal ACS (Table 1). Major kinetic processes that contribute to determining the content of plaque macrophages already reviewed include the recruitment of circulating monocytes, their local proliferation, cell death, and efferocytosis (Figure 2). An inkling that another process — that of emigration of macrophages from plaques — might also contribute is found in the 1980s studies of Ross Gerrity in atherosclerotic pigs118 and of Russell Ross in monkeys,119 in which electron micrographs showed images compatible with macrophage foam cells exiting between ECs into the arterial lumen. The availability of atherosclerotic mice coupled with methods to follow cell trafficking that are relatively convenient in mice led to a direct demonstration after aortic transplantation that during disease progression, emigration was quite low, but placement of plaques into a regression environment readily demonstrated macrophage exit.111, 120 Other types of mouse experiments have also documented macrophage emigration from plaques during atherosclerosis regression, extending these initial observations.90
The capacity of macrophages to leave plaques appears to depend on at least two sub-pathways: one that regulates macrophage retention (chemostasis) and one that regulates macrophage locomotion (chemotaxis). For the former, recent work has shown that neuronal guidance molecules mediate much macrophage retention in plaques. Macrophages in human and mouse plaques express the best studied such guidance molecule, netrin-1, which inhibits the chemotactic responses of macrophages to a number of chemokines in vitro.121 When Ldlr-/- mice received bone marrow transplants from netrin-1–deficient mice, compared to Ldlr-/- mice reconstituted with netrin-1–sufficient bone marrow, plaque progression slowed significantly. Cell trafficking assays showed increased macrophage emigration from the plaques in the mice lacking leukocyte netrin-1.121 Other factors that inhibit cell movement, such as adhesion molecules (which are more highly expressed in macrophages in progressing versus regressing plaques122), also likely promote the retention of macrophages, a process that would be expected to contribute to the macrophage enrichment that characterizes plaques with features associated with precipitating ACS.
The signals that guide macrophages to exit plaques, either by reverse transmigration through the endothelium to the lumen or by migrating through the media to the adventitial lymphatics, remain for the most part poorly defined. Data from studies of regression of atherosclerosis in aortic transplant studies have, however, implicated CCR7 in this process.123 Emigrating CD68+ cells (predominately macrophages) had augmented expression of CCR7, the receptor for the chemokines CCL19 and CCL21. Furthermore, blocking this pathway led to substantial retention of these cells in the plaque.123 The molecular basis for this augmented CCR7 appears to depend on the presence of a sterol response element (SRE) in the promoter of the mouse (and human) CCR7 gene.124 Thus, in a regression environment, which reduces macrophage cholesterol content, CCR7 expression rises. Further experiments support the concept that the plaque content of macrophages depends on the balance between retention and chemotactic pathways. Netrin-1 (or another neuronal guidance molecule, semaphorin 3E) can block macrophage chemotaxis to CCL19 or CCL21, and in regressing plaques — coincident with CCR7 induction — netrin-1 and semaphorin 3 concentrations fall.121, 125
Manipulating retention or migration factors could be envisioned to have therapeutic applications to the prevention or treatment ACS by reducing the plaque content of inflammatory cells. The recent exciting demonstration that siRNA molecules that target monocyte/macrophage inflammation in vivo can be delivered in nanoparticles126 could be adapted, for example, to similarly inhibit netrin-1 expression. Statin treatment induces CCR7 in plaque macrophages in mice and results in macrophage emigration.124 Because the human CCR7 gene also has a SRE, the same process might occur in humans and contribute to the known reduction in peri-ACS mortality by high-dose statins.127 In any case, the pre-clinical findings support the consideration and exploration of strategies based on retention/migration pathways to limit plaque enrichment in macrophages or to rapidly remove them as part of preventing or acutely treating, respectively, ACS.
Conclusions
Inflammation drives many aspects of the ACS both locally and systemically. We have come to appreciate increasingly the critical operation of pro- and anti-inflammatory pathways implicated in ACS pathogenesis. Thus the risk of ACS depends critically on the prevailing balance between promotion of inflammation and its resolution through the pathways discussed herein. Manipulation of this balance provides opportunities for novel therapeutic manipulation in the future. Yet, a number of unresolved issues present challenges for future research regarding the role of inflammation in ACS. The role of inflammation in superficial erosion remains controversial and underexplored. Novel therapies that target inflammation and its resolution require rigorous evaluation as an adjunct to current standard of care in preventing ACS. While the concepts of inflammation have transformed our understanding of the pathophysiology of ACS, we have yet to reap fully the potential therapeutic benefits of translation of these basic science discoveries to the prevention of ACS in patients.
Supplementary Material
Acknowledgments
Sources of Funding
The work from the authors' laboratories referred to in this review was supported by grants from the US National Institutes of Health HL106019, HL075662, and HL054591 to I.T., DK095684, HL098055, HL084312 to E.A.F. and HL080472 to P.L.
Nonstandard Abbreviations and Acronyms
- OCT
optical coherence tomography
- DC
dendritic cell
- DAMP
damage-associated molecular pattern
- RIP
receptor-interacting protein
- PRR
pattern recognition receptor
- SPM
specialized pro-resolving mediators
REFERENCES
- 1.Libby P. Mechanisms of the acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 2.Falk E, Virmani R. Circulation Research. 2014;114:xxx–xxx. doi: 10.1161/CIRCRESAHA.114.302721. [in this issue] [DOI] [PubMed] [Google Scholar]
- 3.Falk E, Nakano M, Benton JF, Finn AV, Virmani R. Update on acute coronary syndromes: The pathologists' view. Eur Heart J. 2013;34:719–728. doi: 10.1093/eurheartj/ehs411. [DOI] [PubMed] [Google Scholar]
- 4.Yonetsu T, Kakuta T, Lee T, Takahashi K, Kawaguchi N, Yamamoto G, Koura K, Hishikari K, Iesaka Y, Fujiwara H, Isobe M. In vivo critical fibrous cap thickness for rupture-prone coronary plaques assessed by optical coherence tomography. Eur Heart J. 2011;32:1251–1259. doi: 10.1093/eurheartj/ehq518. [DOI] [PubMed] [Google Scholar]
- 5.Libby P. Collagenases and cracks in the plaque. J Clin Invest. 2013;123:3201–3203. doi: 10.1172/JCI67526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geng Y-J, Libby P. Evidence for apoptosis in advanced human atheroma. Co-localization with interleukin-1 β-converting enzyme. Am J Pathol. 1995;147:251–266. [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 8.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libby P, Hansson GK, Lichtman AH. Immune effector mechanisms implicated in atherosclerosis: From mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby P, Nahrendorf M, Swirski FK. Monocyte heterogeneity in cardiovascular disease. Semin Immunopathol. 2013;35:553–562. doi: 10.1007/s00281-013-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ ccr2, ccr5, and cx3cr1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swirski FK, Nahrendorf M, Wildgruber M, Etzrodt M, Figueiredo JL, Kohler R, Aikawa E, Chudnovskiy A, Waterman P, Panizzi P, Mempel T, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soehnlein O, Drechsler M, Doring Y, Lievens D, Hartwig H, Kemmerich K, Ortega-Gomez A, Mandl M, Vijayan S, Projahn D, Garlichs CD, Koenen RR, Hristov M, Lutgens E, Zernecke A, Weber C. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med. 2013 doi: 10.1002/emmm.201201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilgendorf I, Gerhardt L, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherrer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6chigh monocytes depend on nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:10, 1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenfeld ME, Ross R. Macrophage and smooth muscle cell proliferation in atherosclerotic lesions of whhl and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1990;10:680–687. doi: 10.1161/01.atv.10.5.680. [DOI] [PubMed] [Google Scholar]
- 17.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LMS, Smyth D, Zavitz CCJ, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinton S, Underwood R, Sherman M, Kufe D, Libby P. Macrophage-colony stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992;140:301–316. [PMC free article] [PubMed] [Google Scholar]
- 19.Libby P, Shi GP. Mast cells as mediators and modulators of atherogenesis. Circulation. 2007;115:2471–2473. doi: 10.1161/CIRCULATIONAHA.107.698480. [DOI] [PubMed] [Google Scholar]
- 20.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: New insights and therapeutic approaches. J Clin Invest. 2013;123:27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilgendorf I, Theurl I, Gerhardt LM, Robbins CS, Weber GF, Gonen A, Iwamoto Y, Degousee N, Holderried TA, Winter C, Zirlik A, Lin HY, Sukhova GK, Butany J, Rubin BB, Witztum JL, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Innate response activator b cells aggravate atherosclerosis by stimulating th1 adaptive immunity. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.113.006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moulton KS. Angiogenesis in atherosclerosis: Gathering evidence beyond speculation. Curr Opin Lipidol. 2006;17:548–555. doi: 10.1097/01.mol.0000245261.71129.f0. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien K, Allen M, McDonald T, Chait A, Harlan J, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin C, Lobb R, Alpers C. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques: Implications for the mode of progression of advanced coronary atherosclerosis. J. Clin. Invest. 1993;92:945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenson R, Brewer H, Rader DJ. Lipoproteins as biomarkers and therapeutic targets in the setting of acute coronary syndrome. Circulation Research. 2014;114:xxx–xxx. doi: 10.1161/CIRCRESAHA.114.302805. [in this issue] [DOI] [PubMed] [Google Scholar]
- 25.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ball RY, Stowers EC, Burton JH, Cary NR, Skepper JN, Mitchinson MJ. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 1995;114:45–54. doi: 10.1016/0021-9150(94)05463-s. [DOI] [PubMed] [Google Scholar]
- 28.Galis Z, Sukhova G, Lark M, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: The importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 30.Finet G, Ohayon J, Rioufol G, Lefloch S, Tracqui P, Dubreuil O, Tabib A. Morphological and biomechanical aspects of vulnerable coronary plaque. Archives des maladies du coeur et des vaisseaux. 2007;100:547–553. [PubMed] [Google Scholar]
- 31.Thorp E, Subramanian M, Tabas I. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. European journal of immunology. 2011;41:2515–2518. doi: 10.1002/eji.201141719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: Recognition, uptake, and consequences. J Clin Invest. 2001;108:957–962. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han J, Zhong CQ, Zhang DW. Programmed necrosis: Backup to and competitor with apoptosis in the immune system. Nat Immunol. 2011;12:1143–1149. doi: 10.1038/ni.2159. [DOI] [PubMed] [Google Scholar]
- 34.Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z, Wu J, Huang D, Qiao M, Jin G, Wu Q, Huang Y, Du J, Han J. A role of rip3-mediated macrophage necrosis in atherosclerosis development. Cell reports. 2013;3:200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 36.Rajavashisth T, Qiao JH, Tripathi S, Tripathi J, Mishra N, Hua M, Wang XP, Loussararian A, Clinton S, Libby P, Lusis A. Heterozygous osteopetrotic (op) mutation reduces atherosclerosis in ldl receptor- deficient mice. J Clin Invest. 1998;101:2702–2710. doi: 10.1172/JCI119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 38.Rambhia SH, Liang X, Xenos M, Alemu Y, Maldonado N, Kelly A, Chakraborti S, Weinbaum S, Cardoso L, Einav S, Bluestein D. Microcalcifications increase coronary vulnerable plaque rupture potential: A patient-based micro-ct fluid-structure interaction study. Ann Biomed Eng. 2012;40:1443–1454. doi: 10.1007/s10439-012-0511-x. [DOI] [PubMed] [Google Scholar]
- 39.Dweck MR, Jenkins WS, Vesey AT, Pringle MA, Chin CW, Malley TS, Cowie WJ, Tsampasian V, Richardson H, Fletcher A, Wallace WA, Pessotto R, van Beek EJ, Boon NA, Rudd JH, Newby DE. 18f-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging. 2014;7:371–378. doi: 10.1161/CIRCIMAGING.113.001508. [DOI] [PubMed] [Google Scholar]
- 40.Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, Yeoh SE, Wallace W, Salter D, Fletcher AM, van Beek EJ, Flapan AD, Uren NG, Behan MW, Cruden NL, Mills NL, Fox KA, Rudd JH, Dweck MR, Newby DE. 18f-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet. 2014;383:705–713. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- 41.Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, Nakamura Y, Yamashita H, Yamagishi H, Takeuchi K, Naruko T, Haze K, Becker AE, Yoshikawa J, Ueda M. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: An intravascular ultrasound study. Circulation. 2004;110:3424–3429. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 42.Camici PG, Rimoldi OE, Gaemperli O, Libby P. Non-invasive anatomic and functional imaging of vascular inflammation and unstable plaque. Eur Heart J. 2012;33:1309–1317. doi: 10.1093/eurheartj/ehs067. [DOI] [PubMed] [Google Scholar]
- 43.Glagov S, Weisenberg E, Zarins C, Stankunavicius R, Kolletis G. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:371–375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 44.Clarkson TB, Prichard RW, Morgan TM, Petrick GS, Klein KP. Remodeling of coronary arteries in human and nonhuman primates. JAMA. 1994;271:289–294. [PubMed] [Google Scholar]
- 45.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1359–1366. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 47.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, Owens GK. Genetic inactivation of il-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122:70–79. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 49.Mach F, Schoenbeck U, Bonnefoy J-Y, Pober J, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of cd40. Induction of collagenase, stromelysin, and tissue factor. Circulation. 1997;96:396–399. doi: 10.1161/01.cir.96.2.396. [DOI] [PubMed] [Google Scholar]
- 50.Schonbeck U, Mach F, Sukhova GK, Herman M, Graber P, Kehry MR, Libby P. Cd40 ligation induces tissue factor expression in human vascular smooth muscle cells. Am J Pathol. 2000;156:7–14. doi: 10.1016/S0002-9440(10)64699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edgington TS. Surfing tissue factor. From the predicted to the discovery and elucidation of unanticipated functions and biology of potential significance. Thromb Haemost. 2007;98:36–37. [PubMed] [Google Scholar]
- 52.Gimbrone MA, Jr., Garcia-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol. 2013;22:9–15. doi: 10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, Braund PS, Hall AS, Chasman DI, Tybjaerg-Hansen A, Chambers JC, Benjamin EJ, Franks PW, Clarke R, Wilde AA, Trip MD, Steri M, Witteman JC, Qi L, van der Schoot CE, de Faire U, Erdmann J, Stringham HM, Koenig W, Rader DJ, Melzer D, Reich D, Psaty BM, Kleber ME, Panagiotakos DB, Willeit J, Wennberg P, Woodward M, Adamovic S, Rimm EB, Meade TW, Gillum RF, Shaffer JA, Hofman A, Onat A, Sundstrom J, Wassertheil-Smoller S, Mellstrom D, Gallacher J, Cushman M, Tracy RP, Kauhanen J, Karlsson M, Salonen JT, Wilhelmsen L, Amouyel P, Cantin B, Best LG, Ben-Shlomo Y, Manson JE, Davey-Smith G, de Bakker PI, O'Donnell CJ, Wilson JF, Wilson AG, Assimes TL, Jansson JO, Ohlsson C, Tivesten A, Ljunggren O, Reilly MP, Hamsten A, Ingelsson E, Cambien F, Hung J, Thomas GN, Boehnke M, Schunkert H, Asselbergs FW, Kastelein JJ, Gudnason V, Salomaa V, Harris TB, Kooner JS, Allin KH, Nordestgaard BG, Hopewell JC, Goodall AH, Ridker PM, Holm H, Watkins H, Ouwehand WH, Samani NJ, Kaptoge S, Di Angelantonio E, Harari O, Danesh J. Il6r genetics consortium emerging risk factors collaboration. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet. 2012;379:1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Current opinion in hematology. 2007;14:55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Megens RT, Vijayan S, Lievens D, Doring Y, van Zandvoort MA, Grommes J, Weber C, Soehnlein O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb Haemost. 2012;107:597–598. doi: 10.1160/TH11-09-0650. [DOI] [PubMed] [Google Scholar]
- 56.Jain MK, Ridker PM. Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 57.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 58.Ridker PM. Targeting inflammatory pathways for the treatment of cardiovascular disease. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 60.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: State of the art, definitions and terms. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 62.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: To resolve or not? Am J Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perretti M, D'Acquisto F. Annexin a1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 64.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, Belkaid Y, Xu Y, Whitsett JA, Accurso FJ, Wills-Karp M, Petasis NA. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 65.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E, Severe Asthma Research Program NHL. Blood I. Diminished lipoxin biosynthesis in severe asthma. American journal of respiratory and critical care medicine. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fredman G, Oh SF, Ayilavarapu S, Hasturk H, Serhan CN, Van Dyke TE. Impaired phagocytosis in localized aggressive periodontitis: Rescue by resolvin e1. PLoS One. 2011;6:e24422. doi: 10.1371/journal.pone.0024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, Creager MA, Serhan CN, Conte MS. Aspirin-triggered lipoxin and resolvin e1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177:2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Oliveira Otto MC, Wu JH, Baylin A, Vaidya D, Rich SS, Tsai MY, Jacobs DR, Mozaffarian D. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of cvd in the multi-ethnic study of atherosclerosis. Journal of the American Heart Association. 2013;2:e000506. doi: 10.1161/JAHA.113.000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu JH, Mozaffarian D. Omega-3 fatty acids, atherosclerosis progression and cardiovascular outcomes in recent trials: New pieces in a complex puzzle. Heart. 2014 doi: 10.1136/heartjnl-2013-305257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown JM, Chung S, Sawyer JK, Degirolamo C, Alger HM, Nguyen TM, Zhu X, Duong MN, Brown AL, Lord C, Shah R, Davis MA, Kelley K, Wilson MD, Madenspacher J, Fessler MB, Parks JS, Rudel LL. Combined therapy of dietary fish oil and stearoyl-coa desaturase 1 inhibition prevents the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:24–30. doi: 10.1161/ATVBAHA.109.198036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown AL, Zhu X, Rong S, Shewale S, Seo J, Boudyguina E, Gebre AK, Alexander-Miller MA, Parks JS. Omega-3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler Thromb Vasc Biol. 2012;32:2122–2130. doi: 10.1161/ATVBAHA.112.253435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Degirolamo C, Kelley KL, Wilson MD, Rudel LL. Dietary n-3 lcpufa from fish oil but not alpha-linolenic acid-derived lcpufa confers atheroprotection in mice. J Lipid Res. 2010;51:1897–1905. doi: 10.1194/jlr.M005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakajima K, Yamashita T, Kita T, Takeda M, Sasaki N, Kasahara K, Shinohara M, Rikitake Y, Ishida T, Yokoyama M, Hirata K. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in ldl receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1963–1972. doi: 10.1161/ATVBAHA.111.229443. [DOI] [PubMed] [Google Scholar]
- 74.Wan JB, Huang LL, Rong R, Tan R, Wang J, Kang JX. Endogenously decreasing tissue n-6/n-3 fatty acid ratio reduces atherosclerotic lesions in apolipoprotein e-deficient mice by inhibiting systemic and vascular inflammation. Arterioscler Thromb Vasc Biol. 2010;30:2487–2494. doi: 10.1161/ATVBAHA.110.210054. [DOI] [PubMed] [Google Scholar]
- 75.Tang EHC, Shimizu K, Christen T, Rocha VZ, Shvartz E, Tesmenitsky Y, Sukhova G, Shi GP, Libby P. Lack of ep4 receptors on bone marrow-derived cells enhances inflammation in atherosclerotic lesions. Cardiovasc Res. 2011;89:234–243. doi: 10.1093/cvr/cvq262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: Evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Merched AJ, Serhan CN, Chan L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. Journal of nutrigenetics and nutrigenomics. 2011;4:12–24. doi: 10.1159/000326890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014;19:21–36. doi: 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li S, Sun Y, Liang CP, Thorp EB, Han S, Jehle AW, Saraswathi V, Pridgen B, Kanter JE, Li R, Welch CL, Hasty AH, Bornfeldt KE, Breslow JL, Tabas I, Tall AR. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ Res. 2009;105:1072–1082. doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM, Serhan CN, Conte MS. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:2220–2232. doi: 10.1096/fj.12-225615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 83.Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin a4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morris T, Stables M, Hobbs A, de Souza P, Colville-Nash P, Warner T, Newson J, Bellingan G, Gilroy DW. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 86.Morris T, Stables M, Colville-Nash P, Newson J, Bellingan G, de Souza PM, Gilroy DW. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc Natl Acad Sci U S A. 2010;107:8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kyaw T, Tipping P, Toh BH, Bobik A. Current understanding of the role of b cell subsets and intimal and adventitial b cells in atherosclerosis. Curr Opin Lipidol. 2011;22:373–379. doi: 10.1097/MOL.0b013e32834adaf3. [DOI] [PubMed] [Google Scholar]
- 88.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: Mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8:348–358. doi: 10.1038/nrcardio.2011.62. [DOI] [PubMed] [Google Scholar]
- 89.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 90.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: A dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ketelhuth DF, Rios FJ, Wang Y, Liu H, Johansson ME, Fredrikson GN, Hedin U, Gidlund M, Nilsson J, Hansson GK, Yan ZQ. Identification of a danger-associated peptide from apolipoprotein b100 (apobds-1) that triggers innate proatherogenic responses. Circulation. 2011;124:2433–2443, 2431-2437. doi: 10.1161/CIRCULATIONAHA.111.051599. [DOI] [PubMed] [Google Scholar]
- 92.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. Nlrp3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: Rationale and design of the canakinumab anti-inflammatory thrombosis outcomes study (cantos). Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 94.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. Cd36 coordinates nlrp3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein e. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. Cd36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ding Y, Subramanian S, Montes VN, Goodspeed L, Wang S, Han C, Teresa AS, 3rd, Kim J, O'Brien KD, Chait A. Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:1596–1604. doi: 10.1161/ATVBAHA.112.249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim TW, Febbraio M, Robinet P, Dugar B, Greene D, Cerny A, Latz E, Gilmour R, Staschke K, Chisolm G, Fox PL, DiCorleto PE, Smith JD, Li X. The critical role of il-1 receptor-associated kinase 4-mediated nf-kappab activation in modified low-density lipoprotein-induced inflammatory gene expression and atherosclerosis. J Immunol. 2011;186:2871–2880. doi: 10.4049/jimmunol.1002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rekhter M, Staschke K, Estridge T, Rutherford P, Jackson N, Gifford-Moore D, Foxworthy P, Reidy C, Huang XD, Kalbfleisch M, Hui K, Kuo MS, Gilmour R, Vlahos CJ. Genetic ablation of irak4 kinase activity inhibits vascular lesion formation. Biochem Biophys Res Commun. 2008;367:642–648. doi: 10.1016/j.bbrc.2007.12.186. [DOI] [PubMed] [Google Scholar]
- 101.Lutgens E, Lievens D, Beckers L, Wijnands E, Soehnlein O, Zernecke A, Seijkens T, Engel D, Cleutjens J, Keller AM, Naik SH, Boon L, Oufella HA, Mallat Z, Ahonen CL, Noelle RJ, de Winther MP, Daemen MJ, Biessen EA, Weber C. Deficient cd40-traf6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med. 2010;207:391–404. doi: 10.1084/jem.20091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richards MR, Black AS, Bonnet DJ, Barish GD, Woo CW, Tabas I, Curtiss LK, Tobias PS. The lps2 mutation in trif is atheroprotective in hyperlipidemic low density lipoprotein receptor knockout mice. Innate immunity. 2013;19:20–29. doi: 10.1177/1753425912447130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in myd88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 104.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: Toll-like receptor 4- and spleen tyrosine kinase-dependent activation of nadph oxidase 2. Circ Res. 2009;104:210–218. 221. doi: 10.1161/CIRCRESAHA.108.181040. following 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nilsson J, Fredrikson GN, Bjorkbacka H, Chyu KY, Shah PK. Vaccines modulating lipoprotein autoimmunity as a possible future therapy for cardiovascular disease. J Intern Med. 2009;266:221–231. doi: 10.1111/j.1365-2796.2009.02150.x. [DOI] [PubMed] [Google Scholar]
- 106.Chinetti-Gbaguidi G, Baron M, Bouhlel MA, Vanhoutte J, Copin C, Sebti Y, Derudas B, Mayi T, Bories G, Tailleux A, Haulon S, Zawadzki C, Jude B, Staels B. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the ppar{gamma} and lxr{alpha} pathways. Circ Res. 2011;108:985–995. doi: 10.1161/CIRCRESAHA.110.233775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 108.Gallardo-Soler A, Gomez-Nieto C, Campo ML, Marathe C, Tontonoz P, Castrillo A, Corraliza I. Arginase i induction by modified lipoproteins in macrophages: A peroxisome proliferator-activated receptor-gamma/delta-mediated effect that links lipid metabolism and immunity. Molecular endocrinology. 2008;22:1394–1402. doi: 10.1210/me.2007-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011;123:989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. Hdl promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011;108:7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of mir-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hewing B, Parathath S, Barrett T, Kellie Chung WK, Astudillo YM, Hamada T, Ramkhelawon B, Tallant TC, Yusufishaq MS, Didonato JA, Huang Y, Buffa J, Berisha SZ, Smith JD, Hazen SL, Fisher EA. Effects of native and myeloperoxidase-modified apolipoprotein a-i on reverse cholesterol transport and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.113.303044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: Insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 115.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 116.Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, Wagner O, Stangl H, Soehnlein O, Binder CJ. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4:1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wolfs IM, Stoger JL, Goossens P, Pottgens C, Gijbels MJ, Wijnands E, van der Vorst EP, van Gorp P, Beckers L, Engel D, Biessen EA, Kraal G, van Die I, Donners MM, de Winther MP. Reprogramming macrophages to an anti-inflammatory phenotype by helminth antigens reduces murine atherosclerosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:288–299. doi: 10.1096/fj.13-235911. [DOI] [PubMed] [Google Scholar]
- 118.Gerrity RG. The role of the monocyte in atherogenesis: Ii. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981;103:191–200. [PMC free article] [PubMed] [Google Scholar]
- 119.Faggiotto A, Ross R, Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984;4:323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- 120.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, Rayner AJ, McDonald TO, O'Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A, Moore KJ. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feig JE, Vengrenyuk Y, Reiser V, Wu C, Statnikov A, Aliferis CF, Garabedian MJ, Fisher EA, Puig O. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS One. 2012;7:e39790. doi: 10.1371/journal.pone.0039790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor ccr7 during atherosclerosis regression in apoe-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feig JE, Shang Y, Rotllan N, Vengrenyuk Y, Wu C, Shamir R, Torra IP, Fernandez-Hernando C, Fisher EA, Garabedian MJ. Statins promote the regression of atherosclerosis via activation of the ccr7-dependent emigration pathway in macrophages. PLoS One. 2011;6:e28534. doi: 10.1371/journal.pone.0028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wanschel A, Seibert T, Hewing B, Ramkhelawon B, Ray TD, van Gils JM, Rayner KJ, Feig JE, O'Brien ER, Fisher EA, Moore KJ. Neuroimmune guidance cue semaphorin 3e is expressed in atherosclerotic plaques and regulates macrophage retention. Arterioscler Thromb Vasc Biol. 2013 doi: 10.1161/ATVBAHA.112.300941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Sullivan J, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Akinc A, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic sirna silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: The miracl study: A randomized controlled trial. JAMA. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 128.Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: Role of extracellular lipid, macrophage, and smooth muscle cell content. Br. Heart J. 1993;69:377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 130.Kataoka Y, Wolski K, Uno K, Puri R, Tuzcu EM, Nissen SE, Nicholls SJ. Spotty calcification as a marker of accelerated progression of coronary atherosclerosis: Insights from serial intravascular ultrasound. J Am Coll Cardiol. 2012;59:1592–1597. doi: 10.1016/j.jacc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 131.Chiang N, Fierro IM, Gronert K, Serhan CN. Activation of lipoxin a(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17s-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 135.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin d2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Serhan CN, Fredman G, Yang R, Karamnov S, Belayev LS, Bazan NG, Zhu M, Winkler JW, Petasis NA. Novel proresolving aspirin-triggered dha pathway. Chemistry & biology. 2011;18:976–987. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, Petasis NA, Serhan CN. Resolvin d3 and aspirin-triggered resolvin d3 are potent immunoresolvents. Chemistry & biology. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med. 1996;2:1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- 140.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin d series and protectin d1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 141.de Waal MR, Abrams J, Bennett B, Figdor CG, de VJ. Interleukin 10(il-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of il-10 produced by monocytes. Journal of Experimental Medicine. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin e1 and protectin d1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, Gray M, D'Acquisto F, Buckingham JC, Perretti M, Flower RJ. Anti-inflammatory role of the murine formyl-peptide receptor 2: Ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–2619. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin d1/protectin d1 and its natural stereoisomers: Assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 145.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin d1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN. Resolvin e2 formation and impact in inflammation resolution. J Immunol. 2012;188:4527–4534. doi: 10.4049/jimmunol.1103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maderna P, Cottell DC, Toivonen T, Dufton N, Dalli J, Perretti M, Godson C. Fpr2/alx receptor expression and internalization are critical for lipoxin a4 and annexin-derived peptide-stimulated phagocytosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:4240–4249. doi: 10.1096/fj.10-159913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: Key role for tnfalpha. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:844–854. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- 149.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Spite M, Summers L, Porter TF, Srivastava S, Bhatnagar A, Serhan CN. Resolvin d1 controls inflammation initiated by glutathione-lipid conjugates formed during oxidative stress. Br J Pharmacol. 2009;158:1062–1073. doi: 10.1111/j.1476-5381.2009.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Titos E, Rius B, Gonzalez-Periz A, Lopez-Vicario C, Moran-Salvador E, Martinez-Clemente M, Arroyo V, Claria J. Resolvin d1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an m2-like phenotype. J Immunol. 2011;187:5408–5418. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- 152.Lee HN, Surh YJ. Resolvin d1-mediated nox2 inactivation rescues macrophages undertaking efferocytosis from oxidative stress-induced apoptosis. Biochem Pharmacol. 2013;86:759–769. doi: 10.1016/j.bcp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 153.Bazan NG. Neuroprotectin d1 (npd1): A dha-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain pathology. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin a4 blocks reactive oxygen species generation in endothelial cells: A novel antioxidative mechanism. Thromb Haemost. 2007;97:88–98. [PubMed] [Google Scholar]
- 155.Peshavariya HM, Taylor CJ, Goh C, Liu GS, Jiang F, Chan EC, Dusting GJ. Annexin peptide ac2-26 suppresses tnfalpha-induced inflammatory responses via inhibition of rac1-dependent nadph oxidase in human endothelial cells. PLoS One. 2013;8:e60790. doi: 10.1371/journal.pone.0060790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin e1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 157.Chiang N, Shinohara M, Dalli J, Mirakaj V, Kibi M, Choi AM, Serhan CN. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J Immunol. 2013;190:6378–6388. doi: 10.4049/jimmunol.1202969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin e1, an epa-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fredman G, Van Dyke TE, Serhan CN. Resolvin e1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol. 2010;30:2005–2013. doi: 10.1161/ATVBAHA.110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.