Significance
Developing T cells are positively selected in the thymus to ensure that their antigen receptors can interact with self-MHC. For CD8 T cells, this process takes days to complete, yet the steps involved are poorly understood. We followed a synchronized wave of cells undergoing positive selection within three-dimensional thymic tissue. Surprisingly, migration from the cortex to the medulla occurred before CD4 down-regulation and while thymocytes still required TCR signaling for efficient positive selection. There was a gradual change in the pattern of calcium signaling over time, with an upward shift in basal intracellular calcium correlating with increased speed and brief signaling events. Our data have interesting implications for how positive and negative selection shape the mature CD8 T-cell repertoire.
Keywords: Zap70, thymic slice, two photon microscopy
Abstract
Positive selection of CD8 T cells in the thymus is thought to be a multistep process lasting 3–4 d; however, the discrete steps involved are poorly understood. Here, we examine phenotypic changes, calcium signaling, and intrathymic migration in a synchronized cohort of MHC class I-specific thymocytes undergoing positive selection in situ. Transient elevations in intracellular calcium concentration ([Ca2+]i) and migratory pauses occurred throughout the first 24 h of positive selection, becoming progressively briefer and accompanied by a gradual shift in basal [Ca2+]i over time. Changes in chemokine-receptor expression and relocalization from the cortex to medulla occurred between 12 and 24 h after the initial encounter with positive-selecting ligands, a time frame at which the majority of thymocytes retain CD4 and CD8 expression and still require T-cell receptor (TCR) signaling to efficiently complete positive selection. Our results identify distinct phases in the positive selection of MHC class I-specific thymocytes that are distinguished by their TCR-signaling pattern and intrathymic location and provide a framework for understanding the multistep process of positive selection in the thymus.
Developing thymocytes test their newly formed T-cell antigen receptors (TCRs) for their ability to bind self-peptide:major histocompatibility complex (MHC) complexes on thymic support cells. This process encompasses both positive and negative selection and ultimately leads to the formation of a functional and self-tolerant mature T-cell repertoire. During positive selection, CD4+CD8+ [double positive (DP)] thymocytes with TCRs weakly reactive to self-peptide:MHC complexes receive signals to survive, mature, and give rise to CD4 or CD8 single positive (SP) cells. Positive selection requires close contact with thymic stromal cells and occurs over a period of several days (1–4). However, much of our information about T-cell development is based on analyses of steady-state thymocyte populations, and the individual steps that occur during the prolonged process of positive selection remain obscure.
Thymocytes are highly motile within three-dimensional thymic tissue environments, and positive selection is tightly linked to thymocyte migration. The initial encounters with positive-selecting ligands on cortical thymic epithelial cells do not induce strong migratory stop signals, but instead occur as brief migratory pauses associated with transient elevations in intracellular calcium concentration ([Ca2+]i) (5–7). These brief, serial TCR signals are consistent with indications that positive selection is driven by weak recognition of self-peptide:MHC complexes and with the unique ability of DP thymocytes to respond to low-potency ligands (8, 9). However, there are also indications that thymocytes that have already received TCR signals still require further signaling to complete positive selection (10, 11). Thus, although the encounters with positive-selecting ligands last for only minutes, these encounters presumably recur over a period of hours or days. How the TCR-signaling pattern evolves over time during the process of positive selection is currently a mystery.
Another gap in our knowledge concerns the timing of intrathymic migration relative to other events during positive selection. The DP-to-SP transition is accompanied by relocation of thymocytes from the outer thymic cortex to the central medulla, a process controlled by changes in responsiveness to cortical and medullary chemokines. Preselection DP thymocytes express C-X-C chemokine receptor type 4 (CXCR4), the receptor for the cortical chemokine CXCL12, and lack expression of C-C chemokine receptor type 7 (CCR7), the receptor for the medullary chemokine CCL21. Conversely, mature SP thymocytes express CCR7 and have diminished expression of CXCR4 (12–14). For MHC class II-restricted selection, chemokine-receptor changes appear to occur early during the SP stage whereas thymocytes undergoing positive selection via MHC class I up-regulate CCR7 at the DP stage (15–17). However, it is unclear whether the switch in chemokine-receptor expression and migration to the medulla occurs after positive selection is complete, or whether positive selection continues after thymocytes migrate to the medulla.
Although many of the individual changes that accompany positive selection have been described, how these changes evolve over time remains unclear. Do thymocytes continue to experience transient, serial signals throughout positive selection, or does the nature of TCR signaling change during later stages of positive selection? Do thymocytes gradually accumulate TCR signals in the cortex for days before they can achieve a sufficient signaling threshold to complete positive selection? Do chemokine-receptor changes and migration to the medulla occur at the completion of positive selection, or do thymocytes continue to receive positive selection signals after they switch chemokine responsiveness and migrate to the medulla? Do the phenotypic changes that accompany positive selection occur synchronously at the completion of positive selection, gradually throughout the process, or in a step-wise fashion as thymocytes undergo positive selection?
Here, we address these questions using an experimental system in which a cohort of preselection DP thymocytes bearing MHC class I-restricted TCRs undergo a synchronized wave of positive selection giving rise to CD8 SP thymocytes. We observe a change in the pattern of TCR signaling over time, with an upward shift in basal [Ca2+]i and transient elevations in [Ca2+]i and migratory pauses continuing, but becoming briefer, throughout the first 24 h of positive selection. Thymocytes also switch their chemokine-receptor expression and relocate to the medulla between 12 and 24 h after the initial encounter with positive-selecting ligands, yet continue to require TCR signaling for many hours after this transition for efficient CD8 SP development. These results provide a framework for understanding the process of positive selection and imply that positive selection of CD8 T cells continues after thymocytes relocate to the medulla.
Results
Kinetics of CD8 SP Development and TCR Activation During Positive Selection in Situ.
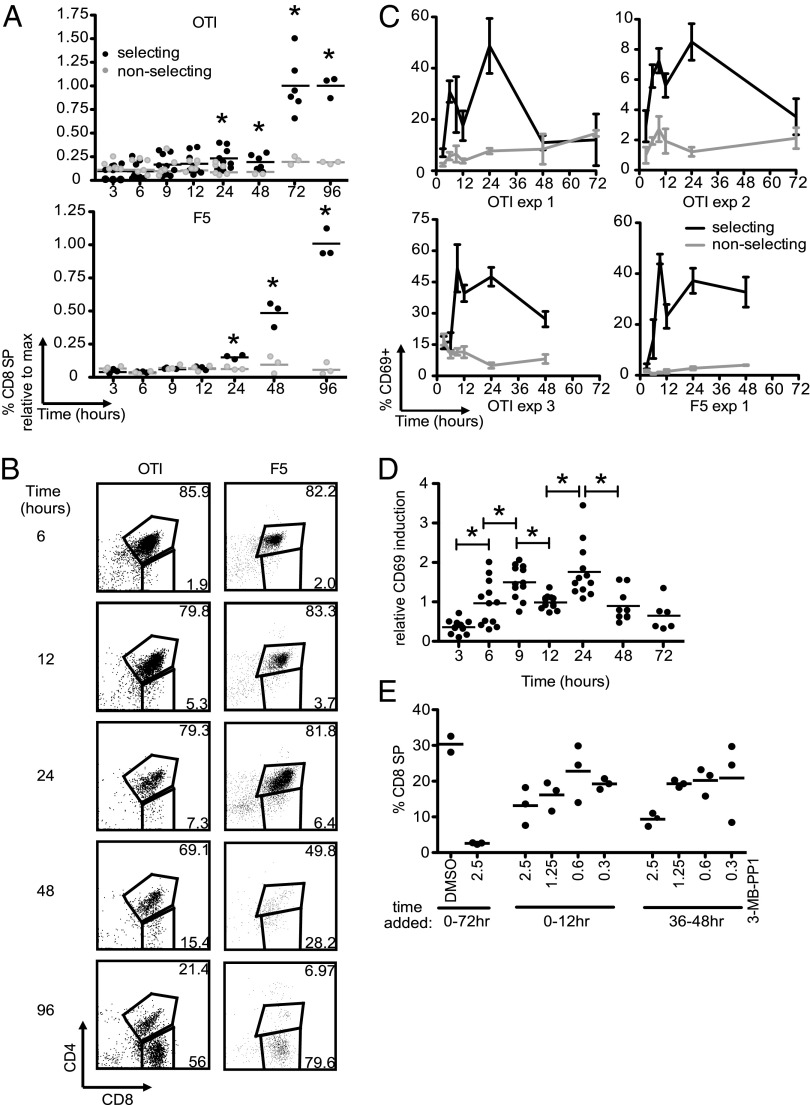
We, and others, have previously reported that CD4+CD8+ DP thymocytes can give rise to mature SP thymocytes after culture within thymic slices (5, 6). To more precisely define the kinetics of positive selection, we isolated preselection DP thymocytes expressing MHC class I-restricted TCRs (OT1 and F5), introduced them into thymic slices from positive-selecting [wild-type (WT)] or nonselecting (MHC class I and II deficient) mice, and analyzed development over time by flow cytometry. CD8 SP cells can be detected starting at 24 h, and their proportions continue to increase over time, such that CD8 SP cells generally outnumber DP thymocytes by 72 h of culture (Fig. 1 A and B and Fig. S1).
Fig. 1.
Positive-selection kinetics on thymic slices. Preselection OT1 or F5 TCR transgenic DP cells were introduced onto thymic slices and harvested at the times indicated for flow cytometric analysis. (A) Frequency of CD8 SP cells within the donor population at the indicated times. Values for each run are normalized to the average at the time point with maximum CD8 SP development (72 h or 96 h). OT1 data are compiled from triplicate slices from three experiments for all time points except 72 and 96 h. The 72 h time point is from two experiments, and the 96 h time point is from one. F5 data are from triplicate slices from one experiment. Bar indicates average. *P < 0.05, comparing selecting and nonselecting values at each time point. (B) Representative flow-cytometry plots of OT1 (Left) or F5 (Right) TCR transgenic cells on selecting thymic slices at the times indicated. (C) CD69 induction in OT1 DP thymocytes from selecting (black) or nonselecting (gray) slices. Depicted are three individual OT1 and 1 F5 experiments with triplicate samples at each time point. (D) Same data as in C normalized to the average 12-h time point for each experiment. Bar indicates average. *P < 0.05. Data are combined from the four experiments (three OT1 and one F5) shown in A and C. (E) Preselection OT1 expressing an analog sensitive version of ZAP70 were cultured in positive-selecting thymic slices, and DMSO or 0.3–2.5 μm 3-MB-PP1 was added for the indicated time periods. Cells were harvested and analyzed by flow cytometry 72 h after the initiation of the cultures, and the proportion of CD8 SP donor cells is reported. Bar indicates average. Data are from one experiment of two.
Positive selection is thought to require prolonged or repeated TCR signaling (11). To define the time course of TCR signaling during positive selection, we examined induction of CD69, a marker for recent TCR signaling (18). CD69 levels rose during culture on positive-selecting slices and remained elevated for at least 24 h, with levels peaking at either 6 h, 9 h, or 24 h, depending on the individual experiment (Fig. 1C and Fig. S1C). However, we consistently observed a relative drop in CD69 levels at the 12-h time point. To allow us to compile data from multiple experiments, we normalized CD69 induction to the 12-h positive-selecting time point for each experiment and displayed the normalized data as a time course (Fig. 1D). This analysis revealed that CD69 induction was significantly lower at 12 h than at either the 9-h or 24-h time points, suggesting some discontinuity in the process of positive selection and demarking an early and a late phase.
To confirm that TCR signaling is required throughout positive selection, we used an analog sensitive version of the TCR-associated tyrosine kinase Zap-70 [Zap-70(AS)], which can be rapidly and reversibly inhibited with a small-molecule inhibitor (3-MB-PP1) (19, 20). We generated preselection OT1 thymocytes expressing Zap-70(AS), introduced them into WT thymic slices, added the inhibitor during different time windows of the culture period, and assessed the development of CD8 SP cells after 72 h of culture. Consistent with other studies, addition of inhibitor for the entire culture period completely blocked positive selection, as expected based on the essential requirement for Zap-70 downstream of TCR signaling (Fig. 1E and ref. 21). Importantly, addition of a high concentration of 3-MB-PP1 (2.5 μM) during a 12-h window from either 0–12 h or 36–48 h led to a substantial reduction in CD8 SP T-cell development. Moreover, Zap-70 catalytic activity during these two time windows was dose-dependent, as revealed by titration of the inhibitor (Fig. 1E). These data extend and confirm our analysis of the temporal requirement for TCR signaling (21) and indicate that TCR signaling is required at both early and late time points for efficient positive selection.
Thymocytes Undergo a Switch in Chemokine-Receptor Expression and Intrathymic Location 12–24 h After Encounter with Positive-Selecting MHC Class I Ligands.
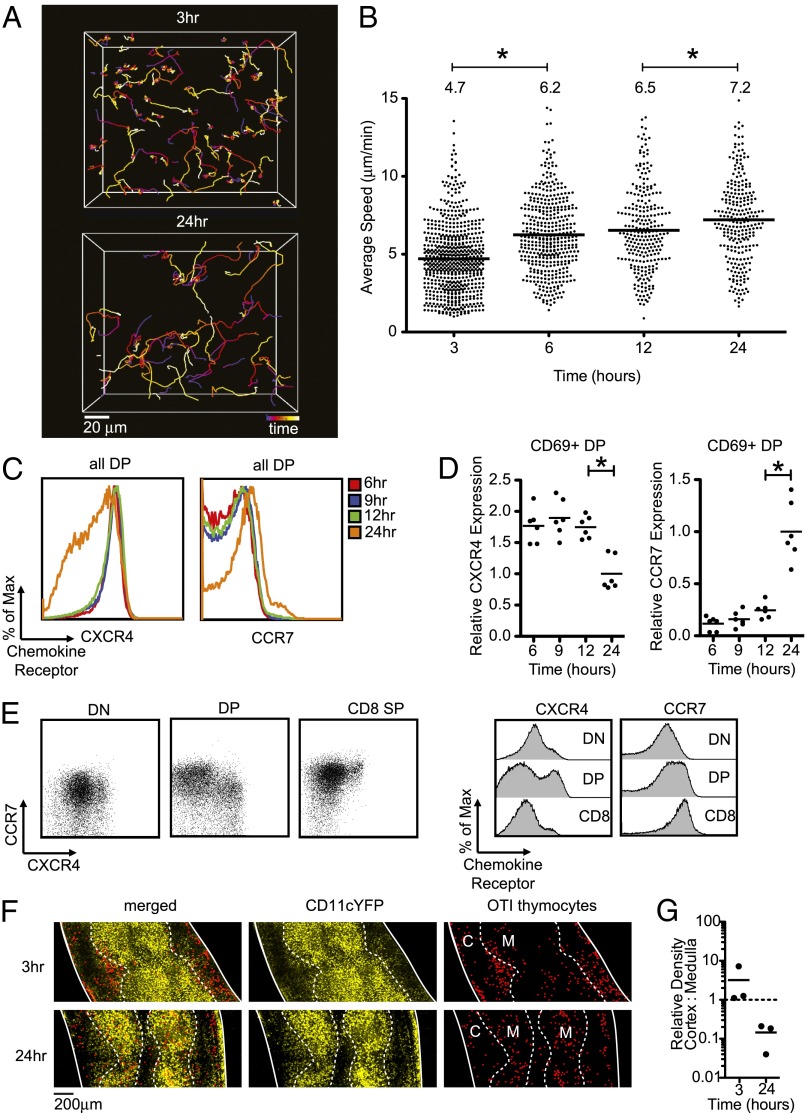
We previously reported that steady-state cortical thymocytes expressing positive-selecting TCR transgenes migrate more rapidly than thymocytes bearing nonselecting or polyclonal TCRs (22, 23). To resolve how the kinetics of migration evolves over time during positive selection, we examined thymocyte migration by two-photon microscopy at various times after introducing preselection DP thymocytes onto positive-selecting slices. Between 3 h and 24 h, thymocyte migration became less confined as reflected in the traces of individual cell tracks (Fig. 2A). This change in thymocyte-migration pattern corresponded with a progressive increase in the average speed of thymocytes on selecting thymic slices, a change that was not observed when thymocytes were cultured on nonselecting slices (Fig. 2B and Fig. S2).
Fig. 2.
Migration and chemokine-receptor expression changes during the first 24 h of positive selection. For A and B, labeled thymocytes were allowed to migrate into selecting thymic slices and imaged by two-photon microscopy at the indicated times after thymocyte addition. (A) Tracks are color coded to indicate the passage of time over a 20-min period (blue-red-orange-yellow-white). Representative cell tracks at 3 h (Upper) versus 24 h (Lower). Image is a maximum projection along the z axis of an imaging volume of dimensions (170 × 142 × 80 μm). (B) Average track speeds of OT1 TCR transgenic thymocytes after culture for the indicated times in positive-selecting slices. Each dot represents an individual tracked cell. Data are compiled from seven runs (269 tracks) for 3 h, four runs (431 tracks) for 6 h, four runs (262 tracks) for 12 h, and six runs (257 tracks) for 24 h. Bar and number above the plots indicate the overall average of the average speed of each individual track for each condition. *P < 0.05. (C–E) Flow-cytometric analysis of chemokine-receptor levels. For C and D, preselection OT1 thymocytes were introduced onto selecting slices, and samples were dissociated and analyzed by flow cytometry at the time point indicated. (C) Representative CXCR4 (Left) and CCR7 (Right) expression on gated DP OT1 TCR transgenic thymocytes after culture for the indicated times in positive-selecting slices. (D) Mean fluorescence intensity (MFI) of CXCR4 (Left) and CCR7 (Right) gated CD69+ DP OT1 TCR transgenic cells from a positively selecting slice. Data are combined from two experiments and are normalized to the average value at the 24 h time point for each experiment. *P < 0.05. (E) Expression of CXCR4 and CCR7 on gated CD4−CD8−:DN, CD4+CD8+:DP, or CD8+CD4−:CD8 SP thymocytes from OT1 TCR transgenic mice on a positive-selecting background. Corresponding histograms are shown to the Right. (F) Localization of preselection OT1 TCR transgenic thymocytes (red spots applied in Imaris for improved visualization indicate individual OT1 TCR transgenic cells) after 3 h or 24 h on CD11cYFP selecting slices. The relative density of YFP signal was used to identify the cortical–medullary junction (dashed line). Solid lines indicate the outlines of the tissue, and medullary (M) and cortical (C) regions are indicated. (G) Quantification of OT1 TCR transgenic thymocyte localization in cortex and medulla normalized to volume; each dot represents one image. Bar indicates average.
Along with an increase in speed, the DP-to-SP transition correlates with up-regulation of CCR7, a receptor for the medullary chemokine CCL21, and down-regulation of CXCR4, a receptor for the cortical chemokine CXCL12 (24). To more precisely define the kinetics of changing chemokine-receptor expression during positive selection, we examined chemokine-receptor expression on OT1 thymocytes by flow cytometry after various times of culture on positive-selecting thymic slices (Fig. 2 C and D). OT1 transgenic thymocytes remained CXCR4high and CCR7low after 12 h of culture on positive-selecting slices (Fig. 2C), with no detectable shift in surface levels, even when gating on the activated, CD69+ DP population (Fig. 2D). However, by 24 h, OT1 transgenic thymocytes showed both a marked down-regulation of CXCR4 and an up-regulation of CCR7 (Fig. 2 C and D). To confirm that the switch in chemokine-receptor expression occurs at the DP stage in vivo, we also examined chemokine-receptor expression on steady-state thymocytes isolated from OT1 TCR transgenic mice on a positive-selecting background. This analysis revealed a substantial population of CXCR4lo CCR7+ DP thymocytes (Fig. 2E), consistent with a previous report (17). A detectable, although reduced, CXCR4lo CCR7+ DP thymocyte population is also present among wild-type DP thymocytes (Fig. S3). Thus, changes in chemokine-receptor expression occurred between 12 h and 24 h of positive selection, after an increase in thymocyte speed was evident, and before the appearance of the bulk of CD8 SP thymocytes.
Because CXCR4 ligands are predominantly found in the cortex and CCR7 ligands are enriched in the medulla, we suspected that the change in chemokine-receptor expression might correlate with the relocalization of thymocytes from the cortex to the medulla. To address this question, we used two-photon microscopy to determine the localization of OT1 thymocytes after 3 h or 24 h of culture on thymic slices. Preselection DP thymocytes initially localize to the cortex (Fig. 2 F and G), consistent with a previous report (25). After 24 h of culture, however, the majority of OT1 transgenic thymocytes are found in the medulla. These results fit well with the temporal pattern of chemokine-receptor changes and imply that DP thymocytes change their responsiveness to cortical versus medullary chemokines and migrate to the medulla before the down-regulation of CD4.
Positive Selection Is Accompanied by a Gradual Increase in Basal [Ca2+]i.
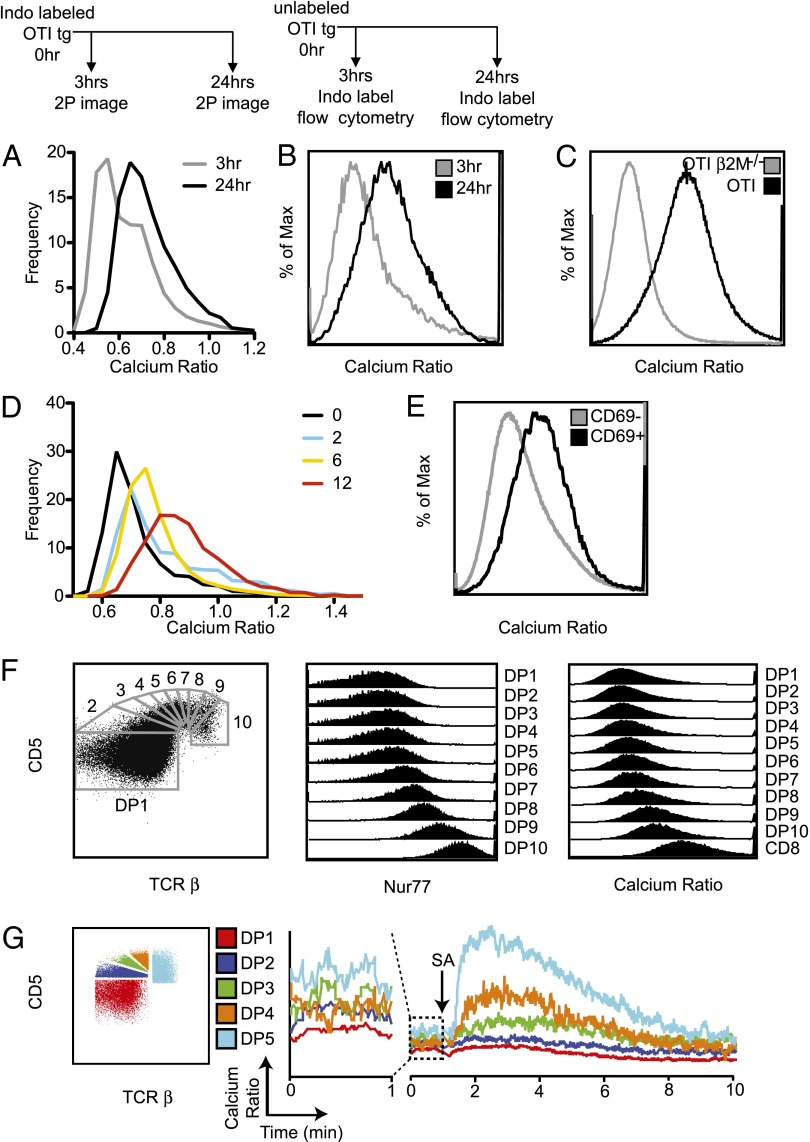
We previously reported that the OT1 transgenic thymocytes undergoing positive selection in situ experience brief (∼5 min) TCR-signaling events characterized by elevations in [Ca2+]i and migratory pauses separated by extended (∼30 min) periods of low basal [Ca2+]i and rapid migration (6). To determine how the temporal pattern of TCR signaling changes over time, we loaded preselection OT1 thymocytes with the ratiometric calcium indicator dye, Indo1LR, introduced them into thymic slices, and examined them by two-photon microscopy after either 3 h or 24 h. Unexpectedly, we observed that, at the 24 h time point, thymocytes consistently exhibited slightly elevated [Ca2+]i while continuing to migrate rapidly (Fig. 2B and Fig. 3A), suggesting a shift in the resting, or basal [Ca2+]i of thymocytes over time during positive selection.
Fig. 3.
Gradual increase in basal [Ca2+]i with increasing time of positive selection. (A) Preselection OT1 TCR transgenic thymocytes were labeled with Indo1LR, introduced into positive-selecting thymic slices, and analyzed by two-photon microscopy at 3 h and 24 h. Data are from three movies at each time point, from three experiments. (B) Flow-cytometric analysis of basal calcium after 3 h and 24 h of positive selection. Unlabeled preselection OT1 TCR transgenic cells were introduced into selecting thymic slices, isolated after 3 h or 24 h of culture, and then labeled with Indo1LR and analyzed by flow cytometry. Experimental schematic is indicated above figures. Shown is the distribution of calcium ratios at 3 h (gray) and 24 h (black). Data is representative of two experiments. (C) Calcium levels for the DP population of OT1 Rag−/− β2M−/− (preselection) and OT1 Rag−/− (steady-state TCR transgenic) thymocytes. Data is representative of two experiments. (D) Preselection Zap-70(AS) OT1 preselection thymocytes were loaded with the ratiometric calcium indicator dye, Indo1LR, and introduced onto selecting thymic slices in the presence of 2.5 μM of the Zap-70(AS) inhibitor, 3-MB-PP1. After 2 h, the inhibitor was washed out, and samples were imaged by two-photon microscopy at 0 h, 2 h, 6 h, and 12 h. Frequency histograms of calcium ratios at the indicated time points after wash-out of inhibitor. Data are from two movies at each time point from two experiments. For E and F, WT thymocytes were loaded with Indo1LR and analyzed by flow cytometry. Data is representative of two experiments. (E) Basal calcium levels for gated CD69− and CD69+ DP thymocytes from a WT thymus. (F) WT DP thymocytes were subdivided based on levels of CD5 and TCRβ (Left), and Nur77 (Center), or calcium ratio (Right) of each gated DP population is displayed. (G) WT thymocytes were loaded with Indo1LR and stained for flow cytometric analysis and calcium flux. The gating for DP subsets is shown (Left). For the calcium flux, a 1-min baseline was established (Center, enlarged plot), and then thymocytes were stimulated by cross-linking anti-CD3ε-biotin with streptavidin (arrow). The kinetics of the change in calcium ratio is shown (Right). Data is representative of three experiments.
To confirm and extend these results, we modified the experimental protocol to examine basal [Ca2+]i by flow cytometry. We added unlabeled OT1 thymocytes to positive-selecting slices for either 3 h or 24 h and then isolated thymocytes from the slice, loaded them with Indo1LR, and analyzed [Ca2+]i by flow cytometry (Fig. 3B). Again, we observed an increase in the [Ca2+]i over time after culture in a positive-selecting environment. We also observed higher basal [Ca2+]i by flow cytometric analysis of ex vivo isolated OT1 TCR transgenic DP thymocytes from a positive-selecting background (steady-state) compared with OT1 TCR transgenic DP thymocytes from a nonselecting background (preselection) (Fig. 3C). Thus, both two-photon microscopy in situ and flow cytometric analysis of dissociated thymocytes reveal an increase in basal [Ca2+]i over time during positive selection.
To more precisely define the kinetics of calcium changes during positive selection, we took advantage of OT1 thymocytes expressing Zap-70(AS). Preselection OT1 transgenic thymocytes expressing Zap-70(AS) were introduced onto thymic slices in the presence of inhibitor and then examined by two-photon microscopy at different times after wash-out of the inhibitor (Fig. 3D). Within 10 min of removal of the inhibitor, we observed individual thymocytes undergoing transient signaling events (Movie S1). By extending the imaging time to 1 h, we were able to observe numerous examples of individual thymocytes undergoing serial, transient signaling events between 1 h and 4 h (Movie S2). In addition, we observed an overall increase in [Ca2+]i at 6 h, which became more pronounced after 12 h of positive selection (Fig. 3D).
To determine whether the increase in basal calcium in response to positive selection also occurred with polyclonal thymocytes, we examined resting [Ca2+]i in DP thymocytes from WT mice. Flow-cytometric analysis revealed increased [Ca2+]i in activated (CD69+) DP thymocytes compared with CD69− DP cells from WT mice (Fig. 3E). Thymocytes undergoing positive selection are present in a gradient of activation states, which can be visualized by finely subdividing the cells based on levels of CD5 and TCRβ expression (3). We used these markers to divide the DP population into subsets of increasing maturity to determine whether [Ca2+]i also increased in a continuum (Fig. 3F, Left) (3). As expected, levels of a TCR-responsive gene, Nur77, progressively increased from the least mature (TCRβ−CD5low) to most mature (TCRβ+CD5high) DP subset (Fig. 3F, Center). The increase in Nur77 also correlated with a gradual increase in basal [Ca2+]i (Fig. 3F, Right). Intracellular calcium could be further increased by CD3 crosslinking (Fig. 3G), and we observed more pronounced calcium increases in more mature subsets. This difference is consistent with previous reports (3) and correlates with increased levels of surface TCR on more mature DP cells (8).
Positive Selection Is Accompanied by Progressively Briefer Signaling Events and Less-Pronounced Stop Signals over Time.
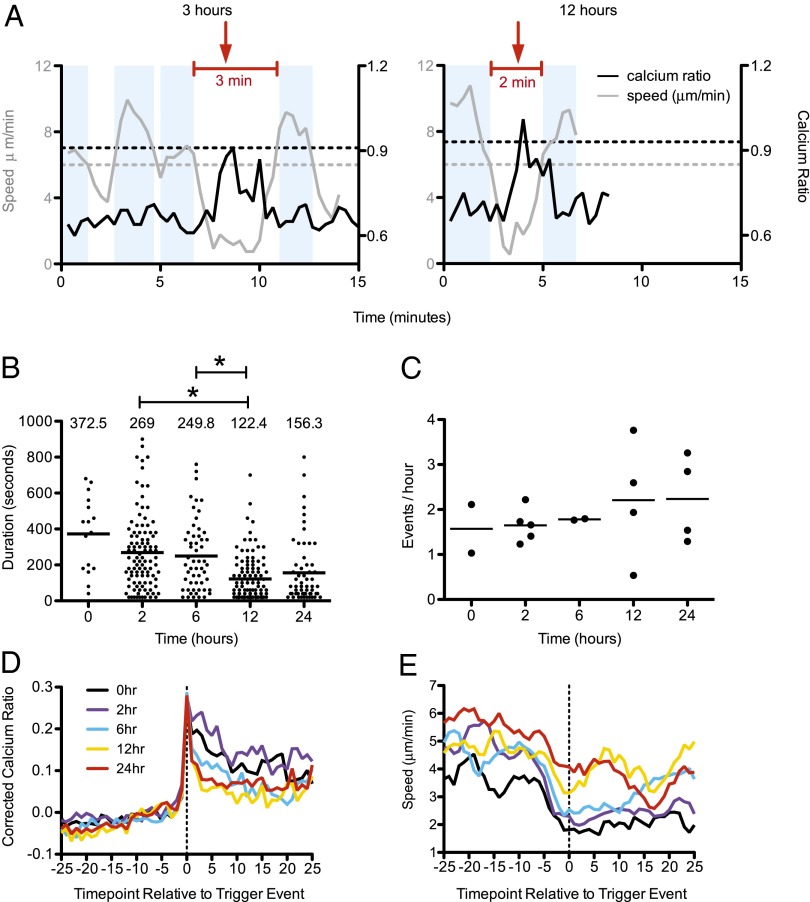
In addition to the overall increase in [Ca2+]i, individual thymocytes displayed further transient calcium elevations over the basal level correlating with brief drops in speed at the 12-h time point (Movie S3 and Fig. 4A). In addition, there was an inverse correlation between calcium ratio and speed at both the 3-h and 24-h time points (Fig. S4), suggesting that thymocytes continue to experience TCR-induced transient stop signals at later phases of positive selection. To quantify these transient signaling events over time, we defined a new “resting” [Ca2+]i ratio based on the average calcium values for each imaging run and then used a combination of relative calcium values and motility changes to define the beginning and end of transient signaling events, as previously described (Fig. 4A) (6). Briefly, we identified signaling event “triggers” as time points at which the calcium values were >0.2 above the average ratio of each run. “Non-signaling” portions of the track were defined as periods during which the calcium value was <0.2 above the average ratio of each run and the interval speeds were >6.0 μm/min. Signaling events contained at least one trigger event and were bounded by periods of nonsignaling (Fig. 4A). There was a significant decrease in the average duration of transient signaling events over time, from ∼4 min at 2 h and 6 h, to ∼2.4 min at 12 h (Fig. 4 A and B). However, the frequency of transient signaling events remained relatively constant at around one event every 30 min (Fig. 4C).
Fig. 4.
Transient signaling events become progressively briefer during the first 24 h of positive selection. Preselection OT1 thymocytes were labeled with Indo1LR, introduced into thymic slices, and analyzed by two-photon microscopy at the indicated times. (A) Example calcium/speed traces and identification of signaling events. Each graph shows calcium ratios (black lines) and interval speeds (gray lines) for individual tracked thymocytes at 3 h (Left) or 12 h (Right). Red lines indicate signaling events. Red arrows indicate the trigger events. Blue shaded regions indicate non-signaling portions of the track during which the 100-s interval speed was >0.6 μm/min and the calcium ratio was <0.2 above the average ratio of the run. The horizontal lines indicate the cutoff for calcium trigger events (black dashed) and the speed cutoff for defining nonsignaling regions of the track (gray dashed). (B) Transient signaling events were identified as described in ref. 6. Plots show duration of signaling events that had both beginnings and ends from 18 compiled runs (0 h, n = 16; 2 h, n = 102; 6 h, n = 55; 12 h, n = 109; 24 h, n = 59 events with both beginnings and ends). *P < 0.0001. (C) Frequency of signaling events as determined by the number of events with beginnings/cumulative track imaging time based on the same compiled runs from B (0 h, n = 41; 2 h, n = 181; 6 h, n = 78; 12 h, n = 141; 24 h, n = 80 events with beginnings). Dots represent individual movies. (D and E) Individual signaling events were aligned based on the time point of the initial rise in [Ca2+]i or trigger event. Averages of corrected calcium ratio (calculated by dividing an individual cell’s calcium ratio at each time point by the average calcium ratio for each run) (D) or speed (E) for aligned signaling events for runs at 0 h, 2 h, 6 h, 12 h, or 24 h of positive selection are shown. For D and E, data are from five imaging experiments, 14 runs (0 h, n = 29 tracks; 2 h, n = 33 tracks; 6 h, n = 54 tracks; 12 h, n = 65 tracks; 24 h, n = 31 tracks). Some runs were from the Zap-70(AS) experiment depicted in Fig. 3D.
We, and others, have previously reported that transient elevations in [Ca2+]i during positive selection are accompanied by migratory pauses (5, 6). To examine how this behavior changes over time, we defined the beginning of individual signaling events based on the initial calcium elevation for each track (the event trigger), aligned multiple signaling events, and displayed the average calcium and speed change relative to the trigger event (Fig. 4 D and E). Interestingly, after 12 h and 24 h of positive selection, [Ca2+]i returned more rapidly to baseline after a signaling event and was also accompanied by a more rapid recovery in thymocyte motility. The more rapid return of [Ca2+]i to baseline after a signaling event is also apparent from the steeper inverse correlation between calcium and speed observed at 3-h versus 24-h time points (Fig. S4). Thus, although thymocytes continue to experience transient TCR signals at later stages of positive selection, these signaling events are more short-lived and accompanied by less pronounced migratory arrest signals.
Discussion
Positive selection of CD8 SP thymocytes from preselection DP precursors occurs over several days and requires ongoing TCR signaling (1–4, 10, 26), but the timing of the individual changes that accompany T-cell maturation, and the discrete steps involved, are poorly understood. Here, we followed a synchronized wave of thymocytes undergoing positive selection in situ to determine how changes in thymocyte phenotype, migration, and TCR-mediated calcium signals evolve over time. We provide evidence for an initial phase of positive selection lasting until around 12 h, characterized by gradual increase in speed and induction of the TCR activation marker CD69. We also observed indications of discontinuity in the selection process, as indicated by a transient drop in CD69 levels at 12 h, followed by a switch in chemokine-receptor expression and relocalization from the cortex to medulla between 12 h and 24 h. The pattern of TCR signals changed over time, with elevations in [Ca2+]i and migratory pauses becoming progressively briefer and accompanied by a gradual shift in the basal [Ca2+]i. These results provide a framework for understanding the multistep process of positive selection in the thymus.
TCR-induced calcium changes have been previously linked to positive selection (27), and our data reveal that this relationship is more complex than previously appreciated. The initial rise in intracellular calcium upon TCR triggering is linked to a drop in cell motility, a phenomenon referred to as a “TCR stop signal” (28). A relatively short-lived stop signal contributes to a pattern of serial, transient signaling events during the initial phase of positive selection, as shown here and as reported previously (5, 6). Although transient elevations in [Ca2+]i occur throughout the first 24 h of positive selection, they become significantly briefer by 12 h and 24 h and are superimposed on a gradual upward shift in basal [Ca2+]i. Thus, transient elevations in [Ca2+]i are associated with migratory pauses, but sustained increases in basal [Ca2+]i correlate with an overall increase in speed. Interestingly, increased basal [Ca2+]i correlates with suppression of receptor-evoked calcium signals in anergic T and B cells and LPS-treated microglia (29–31), suggesting that late DP thymocyte may also experience dampened TCR responses. Although the magnitude of responses to strong ligands correlates closely with TCR surface levels and increasing maturity, as reported previously and confirmed here (3), the less-pronounced TCR stop signals during later phases of positive selection may reflect dampened responses to weak self-ligands. Given the association between TCR stop signals and negative selection (6, 32), it is temping to speculate that higher basal calcium and briefer stop signals during the later phases of positive selection may help to protect thymocytes from negative selection despite their exquisite sensitivity to low avidity peptide:MHC ligands (7–9, 31).
It is widely held that the medulla is composed exclusively of SP thymocytes and that the DP-to-SP transition coincides closely with thymocyte migration from the cortex to the medulla. However, we show here that DP thymocytes undergoing positive selection via MHC class I migrated to the medulla before they down-regulated CD4. This apparent discrepancy may be partly explained by the kinetic perspective provided in our study. Mature SP thymocytes remain in the thymus for 4–5 d (33) and thus may accumulate to outnumber the MHC class I-selected DP thymocytes in the steady-state thymus. Moreover, analysis of steady-state MHC class II-restricted thymocytes suggests that the switch in chemokine-receptor expression occurs during the early CD4 SP stage rather than at the DP stage (15–17). Therefore, it seems likely that steady-state medullary thymocytes consist primarily of CD4 and CD8 SP thymocytes, along with DP thymocytes bearing class I MHC-restricted TCR.
Positive selection is thought to occur in the thymic cortex and require interactions with specialized cortical thymic epithelial cells. However, we find that most thymocytes have already migrated to the medulla 24 h after the initial encounter with positive-selecting ligands, yet continue to require TCR signals for an additional day to efficiently give rise to mature CD8 SP thymocytes. Therefore, another surprising implication of our data is that positive selection of MHC class I-restricted thymocytes continues after thymocytes have already migrated to the medulla. Intriguingly, we observe a dip in CD69 levels at 12 h, a time point that corresponds to the change in chemokine-receptor expression and migration to the medulla. Thus, thymocytes may “take a break” from TCR signaling as they undergo rapid, directional migration to the medulla (22, 25). Indications of biphasic TCR signaling were also reported in another synchronized model for OT1 positive selection, albeit with different kinetics (25).
Interestingly, although the change in chemokine-receptor expression appears to occur as a sharp transition between 12 h and 24 h, the increase in thymocyte speed is more gradual and can be detected as early as 6 h after an encounter with positive-selecting ligands. High motility is a consistent feature of SP thymocytes in the medulla (6, 25, 34, 35) and mature T cells in lymph nodes (36), implying that increased motility reflects long-lasting changes in the cellular machinery that controls migration, rather than a short-term response to a chemokine gradient. Consistent with this notion, gene-expression studies of pre- and postselection DP thymocytes revealed induction of numerous genes that may play a role in regulating cell adhesion, metabolism, and the cytoskeleton (37–39).
The switch in expression of CCR7 and CXCR4 can be detected on a substantial proportion of MHC class I-selected, but not MHC class II-selected DP thymocytes (15–17). Therefore, the timing of the switch in chemokine responsiveness and migration to the medulla appears to differ during CD4 versus CD8 T-cell development. This difference has interesting implications for the later phases of CD4 versus CD8 development. Chemokine responses can modulate TCR stop signals (40); therefore, a switch in chemokine responsiveness during positive selection via MHC class I may contribute to the weaker/briefer TCR signals that underlie CD8 T-cell development (41, 42). In support of this notion, enforced expression of CCR7 on DP thymocytes favors the development of CD8 T cells bearing MHC class II-restricted TCRs (17). Thus, chemokine responses during the later phases of positive selection could contribute to signaling differences that promote divergent T-cell lineage programs.
Our data have important implications for the question of how positive selection and negative selection impact the TCR repertoire. According to a pure-affinity model, the thymus selects T cells whose TCRs interact with self peptide:MHC ligands within an “affinity window” above the threshold for positive selection, but below the cutoff for negative selection. The notion that positive and negative selection are separated in space and time (cortex versus medulla and early versus late) has the potential to further open up the affinity window for positive selection. In particular, if thymocytes collect positive selection signals first, during a developmental stage in which they are particularly sensitive to low potency ligands (8, 9), and are later screened for negative selection once they have lost this sensitivity for weak ligands, a larger than anticipated affinity window for thymic selection may exist. Moreover, the display of distinct peptides in the cortex versus medulla could further widen this threshold, by allowing for positive and negative selection to occur using distinct ligands (33, 43). The notion of a strict separation between positive and negative selection has already been challenged by evidence that negative selection to ubiquitous self-antigens can occur in the cortex (44). Our data now show that the overlap between positive and negative selection continues after MHC class I-restricted thymocytes migrate to the medulla. In particular, the continued existence of transient signaling events at the later stages of positive selection, together with the ongoing requirement for Zap-70 catalytic activity reported (21), suggests that sensitivity for self ligands may persist throughout positive selection and overlap with stages during which thymocytes are increasing their surface TCR levels and beginning to encounter medullary self peptides. Although more rapid migration and briefer signaling events during these later stages may provide some protection from negative selection, it is likely that there is still a substantial loss of thymocytes at this stage. Indeed, recent studies have shown that a substantial proportion of cell loss in the thymus results from negative selection, rather than death by neglect (16, 43), and that the loss due to negative selection is particularly pronounced for the CD8 T-cell lineage (45). In addition, some thymocytes with moderate affinity for self may escape negative selection and give rise to peripheral T cells with the potential to respond to self (46). Such weakly self-reactive T cells could contribute to autoimmunity or, from a more positive perspective, could be harnessed for tumor immunotherapies (47). The kinetic framework for thymic selection of CD8 T cells provided here should inform future investigations into how positive and negative selection shape the mature T-cell repertoire.
Materials and Methods
Mice and Bone-Marrow Chimeras.
All mice were maintained and bred under pathogen-free conditions at American Association of Laboratory Animal Care-approved facilities at the University of California, Berkeley or the University of California, San Francisco (UCSF). All procedures were approved of by the University of California, Berkeley's internal review board, the Animal Care and Use Committee. Nontransgenic C57BL/6 (WT) mice were obtained from The Jackson Laboratory. CD11cYFP mice were bred in house (48). OT1 TCRα−/− Zap-70(AS) mice were bred at UCSF. Preselection OT1 Rag−/− or F5 Rag−/−, TCR tg thymocytes were generated by crossing each TCR transgenic onto a nonselecting, MHC-I–deficient background (β2M−/−) (Taconic). Preselection OT1 TCRα−/−Zap-70(AS) thymocytes were generated by transferring 1 × 106 bone-marrow cells i.v. into lethally irradiated (1,200 rad) β2M−/− recipients (Taconic). Chimeras were analyzed 5–10 wk following reconstitution.
Preselection Thymocyte Isolation.
Total preselection thymocytes were isolated (from either TCR transgenics on a nonselecting background or chimeras from nonselecting hosts) by mechanical dissociation of whole thymus. Cells were filtered through a nylon mesh to obtain a single-cell suspension. Preparations were greater than 90% DP and used directly for overlay onto thymic slices.
Thymic Slices.
Vibratome-cut thymic slices were generated as described (6, 49). Briefly, thymic lobes were embedded in 4% (wt/vol) low-melting agarose in HBSS, and 500-μm slices were prepared for imaging studies or 400-μm slices were prepared for flow-cytometric analysis. Slices were placed onto 0.4-μm tissue-culture inserts set in complete DMEM. Then, 1–3 × 106 total preselection cells were overlaid in 10 μL of complete DMEM and allowed to migrate in for 2 h, and then excess cells remaining on top of the slices were washed off by gentle pipetting. For inhibitor studies, thymocytes were resuspended in complete DMEM with DMSO or 3-MB-PP1 and overlaid onto thymic slices (19). Media were changed every 24 h unless otherwise indicated, and thymic slices were washed once with complete DMEM when transitioning out of inhibitor.
Thymocyte Labeling.
For two-photon imaging, 3 × 106 thymocytes per mL were loaded with a final concentration of 2 μM Indo1LR (TEFLabs) for 90 min at 37 °C, washed once with complete DMEM, and then allowed to recover for another hour at 37 °C before overlay onto thymic slices. For calcium analysis by flow cytometry, cells were loaded with Indo1LR for 30 min at 37 °C in complete DMEM before staining with antibodies. To ensure uniform Indo1LR labeling for flow-cytometric analysis of thymocytes from thymic slices (Fig. 3B), two populations of preselection OT1 cells [distinguished by labeling one population with 2 μM Cell Proliferation Dye eFluor 450 (eBioscence) for 10 min at 37 °C in PBS and then washing with complete DMEM] were introduced sequentially into the same slice (congenically distinct from the overlaid thymocytes) at 0 h or 21 h, and then harvested at 24 h before labeling with Indo1LR. Similarly, to ensure uniform Inod1LR labeling in a population of preselection versus steady-state thymocytes (Fig. 3C), one population was first labeled with 2 μM Cell Proliferation Dye eFluor 450 before the two populations were mixed, and the cell mixture was subsequently labeled with Indo1LR. For localization studies, 1 × 107 cells per mL in PBS were labeled with 2 μM SNARF-1 (Invitrogen) for 10 min at 37 °C, and then washed three times with complete DMEM before overlay onto thymic slices.
Flow Cytometry.
The following antibodies were used for flow-cytometric analysis: anti-mouse CD4-PerCPefluor710 or PECy7, CD8α-efluor450 or efluor780, CD5-FITC, TCRβ-PE, CD24-PE, CD69-PECy7 or PerCPCy5.5, CD69-biotin streptavidin-PE, and Ly5.1-FITC, CCR7-PE-Cy7, CXCR4-APC, CD3ε-biotin (eBioscience). Cells were stained at 4 °C for 20 min. Chemokine-receptor stains were performed at 37 °C for 1 h; cells were then stained for the remaining surface markers at 4 °C for 20 min. For calcium-flux assay, cells were stained as per usual with antibodies, including CD3ε-biotin at 10 μg/mL. Flow-cytometric analysis was performed for 1 min to establish a baseline, and then streptavidin (Invitrogen) was added at 20 μg/mL to cross-link CD3. Intracellular staining for Nur77-PE (eBioscience) was performed using the FoxP3 staining kit (eBioscience). Analysis was done using an LSRII (BD biosciences) or Fortessa (BD biosciences) and analyzed using FloJo software (Tree Star).
Two-Photon Imaging.
Imaging was performed as described previously for Indo1LR (6). For Zap-70(AS) imaging, Indo1LR-labeled thymocytes were allowed to migrate into thymic slices in the presence of 2.5 μM 3-MB-PP1. After 2 h, the inhibitor was washed out and cells were imaged at indicated time points.
Localization analysis was performed by fixing slices in 4% PFA in PBS and then moving slices through a gradient of fructose solutions (50). Imaging localization of SNARF-1–labeled thymocytes overlaid on CD11cYFP (48) thymic slices was performed on a Zeiss LSM 7 MP using a chameleon laser and 495 dichroics, 510 dichroics, and 560 dichroics (Semrock).
Image Analysis.
The x, y, and z cell coordinates and dye intensities from two-photon movies were obtained using Imaris (Bitplane Scientific Software). Custom MATLAB scripts (Matlab codes available upon request) (Mathworks) and Excel were used to analyze migration and relative [Ca2+]i. Graphing and statistics were performed using GraphPad Prism.
Quantification of localization was performed using Imaris. Surfaces were drawn based on CD11cYFP intensity to determine cortex and medulla. Volumes were calculated based on surfaces applied. Spots were applied to OT1 thymocytes 10 μm below the cut surface, and the relative density was determined by normalizing the thymocyte number from the area of either cortex or medulla to the total area.
Quantification of transient calcium-signaling events was based on a combination of relative calcium and speed changes as previously described (6). Briefly, we calculated a corrected calcium concentration for each cell at each time point by dividing the individual calcium ratios by the average calcium ratio for each run. We then identified signaling event triggers as time points at which the calcium values were >0.2 above the average of each movie. Non-signaling portions of the track were identified by time points at which the calcium value was <0.2 above the average of each movie and the interval speeds were >6.0 μm/min. Signaling events contained at least one trigger event and were bounded by periods of nonsignaling. For calculation of signal duration, we included only events that had defined beginnings and ends in the run. For calculations of frequency, we determined the number of events that had a beginning in the run from a compiled set of runs under a given condition. We then divided the number of events with beginnings by the cumulative track imaging time (the sum of all of the track durations for all compiled runs) to obtain a frequency (total number of events per total time).
Supplementary Material
Acknowledgments
We thank B. J. Fowlkes, Kayleigh Taylor, and Brian Weist for reading the manuscript. We also thank Kayleigh Taylor for technical assistance. This work was funded by California Institute of Regenerative Medicine Post-Doctoral Training Grant T1-00007 (to H.J.M.), Graduate Student Training Grant TG2-01164 (to J.O.R.), Arthritis Foundation Postdoctoral Fellowship 5476 (to B.B.A.-Y.), and National Institutes of Health Grants AI091580 and RC2AR058947 (to A.W.) and AI064227 (to E.A.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408482111/-/DCSupplemental.
References
- 1.Lucas B, Vasseur F, Penit C. Production, selection, and maturation of thymocytes with high surface density of TCR. J Immunol. 1994;153(1):53–62. [PubMed] [Google Scholar]
- 2.Wilkinson RW, Anderson G, Owen JJ, Jenkinson EJ. Positive selection of thymocytes involves sustained interactions with the thymic microenvironment. J Immunol. 1995;155(11):5234–5240. [PubMed] [Google Scholar]
- 3.Saini M, et al. Regulation of Zap70 expression during thymocyte development enables temporal separation of CD4 and CD8 repertoire selection at different signaling thresholds. Sci Signal. 2010;3(114):ra23–ra23. doi: 10.1126/scisignal.2000702. [DOI] [PubMed] [Google Scholar]
- 4.Gascoigne NR, Palmer E. Signaling in thymic selection. Curr Opin Immunol. 2011;23(2):207–212. doi: 10.1016/j.coi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6(2):143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 6.Melichar HJ, Ross JO, Herzmark P, Hogquist KA, Robey EA. Distinct temporal patterns of T cell receptor signaling during positive versus negative selection in situ. Sci Signal. 2013;6(297):ra92–ra92. doi: 10.1126/scisignal.2004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol. 2012;13(2):121–128. doi: 10.1038/ni.2190. [DOI] [PubMed] [Google Scholar]
- 8.Davey GM, et al. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188(10):1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas B, Stefanová I, Yasutomo K, Dautigny N, Germain RN. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10(3):367–376. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- 10.Kisielow P, Miazek A. Positive selection of T cells: Rescue from programmed cell death and differentiation require continual engagement of the T cell receptor. J Exp Med. 1995;181(6):1975–1984. doi: 10.1084/jem.181.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Bosselut R. Duration of TCR signaling controls CD4-CD8 lineage differentiation in vivo. Nat Immunol. 2004;5(3):280–288. doi: 10.1038/ni1040. [DOI] [PubMed] [Google Scholar]
- 12.Takahama Y. Journey through the thymus: Stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6(2):127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 13.Ladi E, Yin X, Chtanova T, Robey EA. Thymic microenvironments for T cell differentiation and selection. Nat Immunol. 2006;7(4):338–343. doi: 10.1038/ni1323. [DOI] [PubMed] [Google Scholar]
- 14.Love PE, Bhandoola A. Signal integration and crosstalk during thymocyte migration and emigration. Nat Rev Immunol. 2011;11(7):469–477. doi: 10.1038/nri2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowan JE, et al. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med. 2013;210(4):675–681. doi: 10.1084/jem.20122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-κB. J Exp Med. 2013;210(2):269–285. doi: 10.1084/jem.20121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin X, et al. CCR7 expression in developing thymocytes is linked to the CD4 versus CD8 lineage decision. J Immunol. 2007;179(11):7358–7364. doi: 10.4049/jimmunol.179.11.7358. [DOI] [PubMed] [Google Scholar]
- 18.Sancho D, Gómez M, Sánchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26(3):136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Levin SE, Zhang C, Kadlecek TA, Shokat KM, Weiss A. Inhibition of ZAP-70 kinase activity via an analog-sensitive allele blocks T cell receptor and CD28 superagonist signaling. J Biol Chem. 2008;283(22):15419–15430. doi: 10.1074/jbc.M709000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Au-Yeung BB, et al. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat Immunol. 2010;11(12):1085–1092. doi: 10.1038/ni.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Au-Yeung BB, et al. Quantitative and temporal requirements revealed for Zap-70 catalytic activity during T cell development. Nat Immunol. doi: 10.1038/ni.2918. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 2005;3(6):e160. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ladi E, et al. Thymocyte-dendritic cell interactions near sources of CCR7 ligands in the thymic cortex. J Immunol. 2008;181(10):7014–7023. doi: 10.4049/jimmunol.181.10.7014. [DOI] [PubMed] [Google Scholar]
- 24.Schabath R, et al. The murine chemokine receptor CXCR4 is tightly regulated during T cell development and activation. J Leukoc Biol. 1999;66(6):996–1004. doi: 10.1002/jlb.66.6.996. [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich LIR, Oh DY, Weissman IL, Lewis RS. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity. 2009;31(6):986–998. doi: 10.1016/j.immuni.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci USA. 2005;102(38):13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhakta NR, Lewis RS. Real-time measurement of signaling and motility during T cell development in the thymus. Semin Immunol. 2005;17(6):411–420. doi: 10.1016/j.smim.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA. 1997;94(8):3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gajewski TF, Qian D, Fields P, Fitch FW. Anergic T-lymphocyte clones have altered inositol phosphate, calcium, and tyrosine kinase signaling pathways. Proc Natl Acad Sci USA. 1994;91(1):38–42. doi: 10.1073/pnas.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489(7414):160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann A, Kann O, Ohlemeyer C, Hanisch U-K, Kettenmann H. Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): Suppression of receptor-evoked calcium signaling and control of release function. J Neurosci. 2003;23(11):4410–4419. doi: 10.1523/JNEUROSCI.23-11-04410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dzhagalov IL, Chen KG, Herzmark P, Robey EA. Elimination of self-reactive T cells in the thymus: A timeline for negative selection. PLoS Biol. 2013;11(5):e1001566. doi: 10.1371/journal.pbio.1001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204(11):2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Borgne M, et al. The impact of negative selection on thymocyte migration in the medulla. Nat Immunol. 2009;10(8):823–830. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halkias J, et al. Opposing chemokine gradients control human thymocyte migration in situ. J Clin Invest. 2013;123(5):2131–2142. doi: 10.1172/JCI67175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296(5574):1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 37.Huang YH, Li D, Winoto A, Robey EA. Distinct transcriptional programs in thymocytes responding to T cell receptor, Notch, and positive selection signals. Proc Natl Acad Sci USA. 2004;101(14):4936–4941. doi: 10.1073/pnas.0401133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mick VE, Starr TK, McCaughtry TM, McNeil LK, Hogquist KA. The regulated expression of a diverse set of genes during thymocyte positive selection in vivo. J Immunol. 2004;173(9):5434–5444. doi: 10.4049/jimmunol.173.9.5434. [DOI] [PubMed] [Google Scholar]
- 39.Mingueneau M, et al. Immunological Genome Consortium The transcriptional landscape of αβ T cell differentiation. Nat Immunol. 2013;14(6):619–632. doi: 10.1038/ni.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bromley SK, Peterson DA, Gunn MD, Dustin ML. Cutting edge: Hierarchy of chemokine receptor and TCR signals regulating T cell migration and proliferation. J Immunol. 2000;165(1):15–19. doi: 10.4049/jimmunol.165.1.15. [DOI] [PubMed] [Google Scholar]
- 41.Hogquist KA. Signal strength in thymic selection and lineage commitment. Curr Opin Immunol. 2001;13(2):225–231. doi: 10.1016/s0952-7915(00)00208-9. [DOI] [PubMed] [Google Scholar]
- 42.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2(5):309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 43.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205(11):2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stritesky GL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc Natl Acad Sci USA. 2013;110(12):4679–4684. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinclair C, Bains I, Yates AJ, Seddon B. Asymmetric thymocyte death underlies the CD4:CD8 T-cell ratio in the adaptive immune system. Proc Natl Acad Sci USA. 2013;110(31):E2905–E2914. doi: 10.1073/pnas.1304859110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Enouz S, Carrié L, Merkler D, Bevan MJ, Zehn D. Autoreactive T cells bypass negative selection and respond to self-antigen stimulation during infection. J Exp Med. 2012;209(10):1769–1779. doi: 10.1084/jem.20120905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schoenberger SP, Sercarz EE. Harnessing self-reactivity in cancer immunotherapy. Semin Immunol. 1996;8(5):303–309. doi: 10.1006/smim.1996.0039. [DOI] [PubMed] [Google Scholar]
- 48.Lindquist RL, et al. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5(12):1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 49.Dzhagalov IL, Melichar HJ, Ross JO, Herzmark P, Robey EA. 2012. Two-photon imaging of the immune system. Curr Protoc Cytom 60:12.26.1–12.26.20. [DOI] [PMC free article] [PubMed]
- 50.Ke M-T, Fujimoto S, Imai T. SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat Neurosci. 2013;16(8):1154–1161. doi: 10.1038/nn.3447. [DOI] [PubMed] [Google Scholar]