Abstract
Mathematical models that couple disease dynamics and vaccinating behaviour often assume that the incentive to vaccinate disappears if disease prevalence is zero. Hence, they predict that vaccine refusal should be the rule, and elimination should be difficult or impossible. In reality, countries with non-mandatory vaccination policies have usually been able to maintain elimination or very low incidence of paediatric infectious diseases for long periods of time. Here, we show that including injunctive social norms can reconcile such behaviour-incidence models to observations. Adding social norms to a coupled behaviour-incidence model enables the model to better explain pertussis vaccine uptake and disease dynamics in the UK from 1967 to 2010, in both the vaccine-scare years and the years of high vaccine coverage. The model also illustrates how a vaccine scare can perpetuate suboptimal vaccine coverage long after perceived risk has returned to baseline, pre-vaccine-scare levels. However, at other model parameter values, social norms can perpetuate depressed vaccine coverage during a vaccine scare well beyond the time when the population's baseline vaccine risk perception returns to pre-scare levels. Social norms can strongly suppress vaccine uptake despite frequent outbreaks, as observed in some small communities. Significant portions of the parameter space also exhibit bistability, meaning long-term outcomes depend on the initial conditions. Depending on the context, social norms can either support or hinder immunization goals.
Keywords: paediatric immunization, decision-making, behaviour-incidence model, social norms, peer pressure
1. Introduction
Vaccination has proved to be a highly effective primary intervention for many paediatric infectious diseases [1–3]. However, in populations where vaccination is not mandated, parental vaccine exemption can reduce vaccine coverage below recommended levels. Vaccine exemption is thought to be caused by the underestimation of disease risks, overestimation of vaccine risks and complacency due to vaccine-generated herd immunity, among other factors [4–10]. In some cases, a full-blown ‘vaccine scare’ causes widespread exemption, as happened for the whole-cell pertussis vaccine during the 1970s and for mumps–measles–rubella (MMR) vaccine during the 1990s in the UK [7–9].
These episodes of vaccine refusal have stimulated the development of mathematical models of vaccinating behaviour for paediatric infectious diseases [8,11–18]. Most of these models assume that non-vaccinating behaviour becomes more attractive as the disease becomes rarer; in the extreme case that the disease has been eliminated, the incentive to vaccinate is completely removed, making vaccine refusal very attractive. As a result, these models often predict that it should be difficult or impossible to eliminate an infectious disease through vaccination.
However, for paediatric infectious diseases, vaccine refusal appears to be the exception, not the rule. Most non-mandatory immunization programmes have met or exceeded their recommended World Health Organization (WHO) vaccine coverage levels. European countries with voluntary vaccination policies have maintained polio elimination for decades [19,20]. Despite decade-long vaccine scares in some countries, non-mandatory pertussis and MMR vaccination programmes in Italy, Portugal, Finland and the UK have maintained high vaccine coverage and low disease incidence in most decades that the programmes have been in place [19–23].
This highlights a paradox: most mathematical models are focused on the problem of vaccine refusal and hence predict vaccine refusal as the typical behaviour. However, vaccine refusal is not the typical behaviour for paediatric infectious diseases. Hence, there is a need to develop models that can explain the whole range of observed vaccinating behaviour, including both the typical situation when elimination can be maintained for extended periods and the exceptions when vaccine refusal becomes a problem. (Some of those models also predict oscillations in vaccine coverage levels that, while dynamically interesting, are very rare in vaccine coverage data.)
For influenza, altruism has been suggested as a mechanism for boosting vaccine coverage [7,24–26]. However, for paediatric infectious diseases, it is unclear whether altruism is an important motivating factor in vaccine decision-making [4,27,28]. Some parents feel that subjecting their children to vaccine risks is less tolerable than putting themselves at the same risk [27]. Some vaccine-refusing parents do not see an obligation to put their children's health at perceived risk for the benefit of the community, and many vaccinators see community protection only as a favourable by-product of their decisions [4,28]. In the context of paediatric infectious diseases, adding public health messaging to theoretical models has been shown to generate inertia in vaccinating strategies, increase and stabilize vaccine coverage, and generate dynamics that are more consistent with empirical patterns [17]. The study of d'Onofrio et al. [17] is one of the first to explore an aspect of parental decision-making that may help in explaining the paradox in the context of paediatric infectious diseases. However, the social dimensions of parental decision-making, and their relevance to the paradox, have yet to be fully explored.
The Health Belief Model proposes that parents make vaccinating decisions according to personal and social variables [5,27,29–32]. Personal variables include perceived risk of infection, perceived risk of vaccination, vaccine efficacy, and the cost and effort to access vaccines. Social variables encompass social norms that may be either prescriptive (what individuals should do, such as to get vaccinated) or proscriptive (what individuals should not do, such as not to get vaccinated) [33]. Social norms may also be descriptive (following a morally neutral perception of what the majority of individuals are doing) or injunctive (following a moralistic perception of what individuals ought to be doing) [33].
Enforcing injunctive social norms involves imposing mild or severe sanctions for violating the norm and is a fundamental component of human social behaviour in a wide variety of contexts [33,34]. Vaccinating behaviour is no exception [4,35–38]. Empirical support for injunctive (and descriptive) social norms has been found for paediatric infectious disease vaccines [38]. Injunctive social norms in support of vaccination take the form of encouraging behaviour perceived as ‘normal’, the ‘right thing’ to do and socially responsible. On the contrary, not vaccinating one's child may be perceived as irresponsible and evidence of bad parenting [35]. Hence, the penalty associated with violating the norm usually relies upon guilt and conformism, rather than on explicit material punishment. There is less literature on the forms of injunctive social norms applied by vaccine refusers, which is not surprising because they are presently a minority group in most populations (given their relative small numbers, they seem to rely more often on sensationalism [37] than on social conformism). However, social network analysis tells us that the vaccine opinions of neighbours in our social networks are a very strong predictor of decisions for vaccine accepters and refusers alike: refusers are significantly more likely to be surrounded by other refusers, and accepters by other accepters [36].
Descriptive social norms have been included in previous models of vaccinating behaviour in the guise of social learning or imitation [8,11,17,39]. However, injunctive social norms have received less attention. We propose that including injunctive social norms will enable models of parental vaccinating behaviour for paediatric infectious diseases to better explain the whole range of observed vaccinating behaviour, including both vaccine refusal and the high vaccine coverage levels so commonly observed. Here, we add injunctive social norms to an existing behaviour-incidence model [8,11]. We analyse its dynamics, and fit versions of the model with and without injunctive social norms to several decades of data on pertussis vaccine uptake and disease incidence from the UK [19]. We show that the model with injunctive social norms can capture both the vaccine-scare period and the period of high vaccine coverage observed in the data, whereas the model without injunctive social norms can capture only the vaccine-scare period.
2. Material and methods
We build on the behaviour-incidence model of Bauch and co-workers [8,11]. Individuals are either vaccinators or non-vaccinators. Each individual samples others in the population at some rate. If the sampled person is playing a different strategy that produces a higher pay-off, the individual changes to the other strategy with a probability proportional to the expected increase in their pay-off. The pay-off to vaccinate is given by
| 2.1 |
where rv is the perceived risk associated with vaccinating, δ0 is the effect of injunctive social norms and x is the proportion of vaccinators in the population. To account for injunctive social norms, we have adopted an approach similar to Helbing and co-workers [40,41], who include group pressure in such a way that individuals playing strategy S receives a pay-off in proportion to how many others in the population are also playing S. We assume that the vaccine is perfectly efficacious, hence the individual never gets infected and only pays the one-time cost of becoming vaccinated.
The pay-off not to vaccinate is given by
| 2.2 |
where c is the multiplicative product of cost of infection, reporting probability and a proportionality constant governing the perceived probability of becoming infected, and I(t) is the proportion of infected persons at time t. Here, we assume that individuals use a ‘rule of thumb’ to estimate the probability that their child becomes infected, rather than assuming that they have perfect knowledge of the actual probability.
After some parameter rescaling, it can be shown that the equation governing the dynamics of x is
| 2.3 |
where κ and δ are the rescaled sampling rate and effect of injunctive social norms, respectively, and ω = ω(t) is a rescaled measure of how risky the vaccine is perceived to be compared with the infection. For the pertussis vaccine-scare scenarios, we assume that ω(t) increases instantaneously in 1974 from its baseline level m to a level σm (where σ > 1) and thereafter declines linearly until its magnitude returns to the baseline level [8]. We note that ω(t) implicitly captures all mechanisms that determine risk perception, including public health messaging. A full model derivation and definition of terms appear in the electronic supplementary material, section S1.
Transmission dynamics are obtained from a susceptible–infected–recovered compartmental model, yielding the full model equations
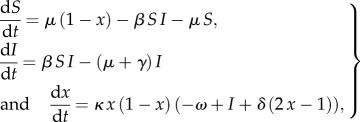 |
2.4 |
where S, I and R are the proportion of susceptible, infected and recovered individuals in the population, respectively, at time t, μ is the per capita birth/death rate, β is the transmission rate and γ is the recovery rate.
We used the method of nonlinear least squares to fit both the model (equation (2.4)) and a nested model with δ = 0 (no injunctive social norms) to two different time windows of pertussis vaccine coverage and pertussis (whooping cough) incidence data from the UK [19] (see the electronic supplementary material, section S2 for the dataset). The maximum value of the likelihood function is Lmax = (2πe1RSS/n)−n/2, where n is the number of data points (specifically, years) and RSS is the residual sum of squares. We fixed μ, γ and β from published estimates [8]. The model was initialized in the year 1967 (t(0) = 1967). We carried out three different fitting procedures. In the first procedure, we fitted the nested model with δ = 0 to data from 1971 to 1988, and estimated κ, σ, m, S(0) and I(0) [19]. This allowed us to test a special case of the model without injunctive social norms during the period of the vaccine scare only (1971–1988), as in a previous study [8]. We also fixed x(0) = 0.78 to allow better comparability with the same study [8]. In the second procedure, we repeated the first procedure with only two changes: we used a broader time window from 1967 to 2010 and we included x(0) as a parameter to be estimated. This allowed us to test a special case of the model without injunctive social norms over a longer time window covering both the vaccine scare and the following time period of very low incidence and high vaccine coverage. In the third procedure, we repeated the second procedure, except that we estimated δ > 0 through fitting. This allowed us to test the full model (equation (2.4)) including injunctive social norms for the same time period (including both the vaccine scare and the low incidence era).
Selection between the full model including injunctive social norms and the nested model lacking social norms for the 1967–2010 time window was based on the corrected Akaike information criterion (AICc), defined as 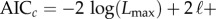
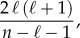 where n is the number of data points and ℓ is the number of fitted model parameters [8,42]. The AICc helps us to judge whether adding the parameter δ is justified by the resulting increased goodness-of-fit.
where n is the number of data points and ℓ is the number of fitted model parameters [8,42]. The AICc helps us to judge whether adding the parameter δ is justified by the resulting increased goodness-of-fit.
We also identified the equilibrium points of the model with ω(t) ≡ m = constant and characterized regions of dynamical behaviour in the δ−m parameter plane. Finally, we numerically simulated the model at various values of δ and m to gain better intuition of model dynamics.
A variant of the model that includes a fixed group of parents who never vaccinate their children and another variant including a fixed group of parents who always vaccinate their children are analysed in the electronic supplementary material, section S3 [43,44].
3. Results
(a). Model fit with and without injunctive social norms
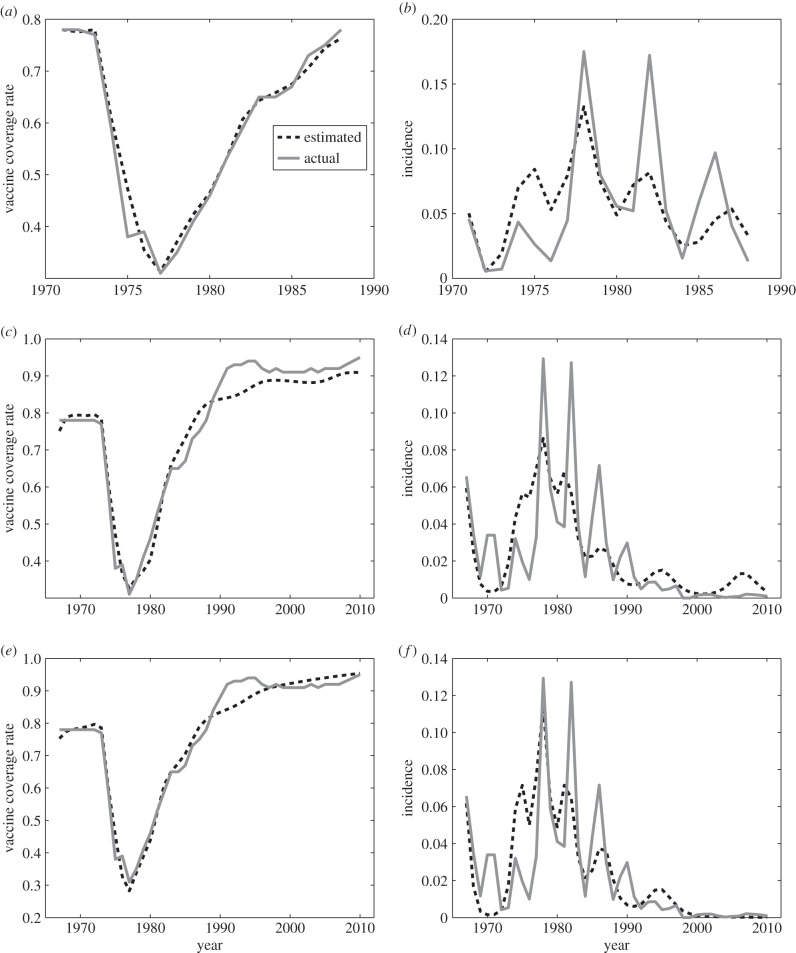
The nested model lacking injunctive social norms (δ = 0, first fitting procedure) can fit vaccine coverage data and disease incidence data from the vaccine-scare era (1971–1988) fairly well (figure 1a,b). The model captures the decline in vaccine coverage, followed by a surge in disease incidence, which is in turn followed by a gradual recovery of vaccine coverage and decline in disease incidence.
Figure 1.
Empirical and modelled (a,c,e) pertussis vaccine coverage and (b,d,f) pertussis incidence in the UK, (a,b) from 1971 to 1988 without injunctive social norms, (c,d) from 1967 to 2010 without injunctive social norms and (e,f) from 1967 to 2010 with injunctive social norms. The solid line is the empirical data and the dashed line is the best-fitting model.
However, when the nested model is applied to a broader time window including both the vaccine scare and the subsequent regime of low incidence and high coverage (1967–2010; second fitting procedure), it does worse. The model underpredicts vaccine coverage for 1990–2010. As a result, it predicts a large epidemic outbreak of pertussis in 2000–2010, when in fact the empirical data show high vaccine coverage, very low disease incidence and no epidemic outbreaks (figure 1c,d). Hence, the explanatory power of the nested model lacking injunctive social norms over the broader time window is limited and qualitatively incorrect. The fit to disease incidence during the vaccine-scare period also worsens compared with the fit over the shorter time window covering only the vaccine scare (figure 1c,d versus figure 1a,b).
Introducing injunctive social norms improves the model fit and allows it to better capture the qualitative patterns in the data. When δ is included as an estimated parameter (third fitting procedure), the model better predicts vaccine coverage for 1990–2010 and it also captures the era of very low pertussis incidence from 2000 to 2010 (figure 1e,f). The model also better predicts both vaccine coverage and disease incidence during the vaccine-scare period.
According to the corrected Akaike information criterion, including injunctive social norms in the model is justified by the resulting increased goodness-of-fit. The AICc score is −160.32 for the full model with estimated δ > 0 versus a worse (more positive) AICc score of −144.14 for the nested model without δ. Moreover, the estimated parameter values of the nested model for the vaccine-scare era only (1971–1988, first fitting procedure) are comparable with those of the full model for the whole time period (1967–2010, third fitting procedure); this serves as another empirical test of the model. Parameter estimates, residual sum-of-squares and AICc scores are provided in the electronic supplementary material, section S4.
(b). Model equilibria
When perceived risk is constant (ω(t) ≡ m), there are five equilibria. Three are disease-free equilibria, corresponding to full vaccine coverage and no susceptibility,
| 3.1 |
no vaccine coverage and full susceptibility,
| 3.2 |
and partial vaccine coverage and partial susceptibility,
| 3.3 |
where 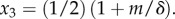
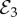 only exists when 0 < x3 < 1, which translates into the condition that group pressure must exceed perceived vaccine risk (δ > m).
only exists when 0 < x3 < 1, which translates into the condition that group pressure must exceed perceived vaccine risk (δ > m).
There are two endemic equilibria that only exist when the basic reproduction number 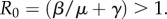 The first equilibrium corresponds to no vaccine coverage,
The first equilibrium corresponds to no vaccine coverage,
| 3.4 |
and the second corresponds to partial vaccine coverage,
| 3.5 |
where
| 3.6 |
and
| 3.7 |
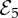 exists in two regions depending on whether δ is less or greater than
exists in two regions depending on whether δ is less or greater than 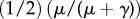 (electronic supplementary material, section S5).
(electronic supplementary material, section S5).
(c). Dynamical regimes
The disease-free equilibrium with no vaccine coverage (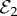 ) is only stable when R0 < 1, such that transmission cannot be sustained even in the absence of vaccination. The disease-free equilibrium with partial vaccine coverage (
) is only stable when R0 < 1, such that transmission cannot be sustained even in the absence of vaccination. The disease-free equilibrium with partial vaccine coverage (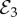 ) is always unstable whenever it exists (δ > m), because when the infection dies out, injunctive social norms must draw vaccine coverage either up to full coverage or down to no coverage.
) is always unstable whenever it exists (δ > m), because when the infection dies out, injunctive social norms must draw vaccine coverage either up to full coverage or down to no coverage.
The stability of the other three equilibria is best understood through visualizing the δ−m parameter plane (figure 2). When social norms are strong relative to perceived vaccine risk (δ > m), then full vaccine coverage and disease elimination (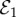 ) is stable (hatched region below the diagonal line in figure 2). The values of δ and m estimated from the pertussis dataset (figure 1e,f) are indicated in the plane and lie within a region where
) is stable (hatched region below the diagonal line in figure 2). The values of δ and m estimated from the pertussis dataset (figure 1e,f) are indicated in the plane and lie within a region where 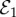 is the only stable equilibrium. (For the model variant with never-vaccinators,
is the only stable equilibrium. (For the model variant with never-vaccinators, 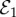 becomes stable at less than 100% vaccination coverage; see the electronic supplementary material, section S3 and figure FS3. Meanwhile, including always-vaccinators in the model gives rise to a change in the equilibrium point
becomes stable at less than 100% vaccination coverage; see the electronic supplementary material, section S3 and figure FS3. Meanwhile, including always-vaccinators in the model gives rise to a change in the equilibrium point 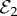 , as well as
, as well as 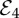 , and makes it stable for some paediatric infectious diseases; see the electronic supplementary material, section S3.)
, and makes it stable for some paediatric infectious diseases; see the electronic supplementary material, section S3.)
Figure 2.
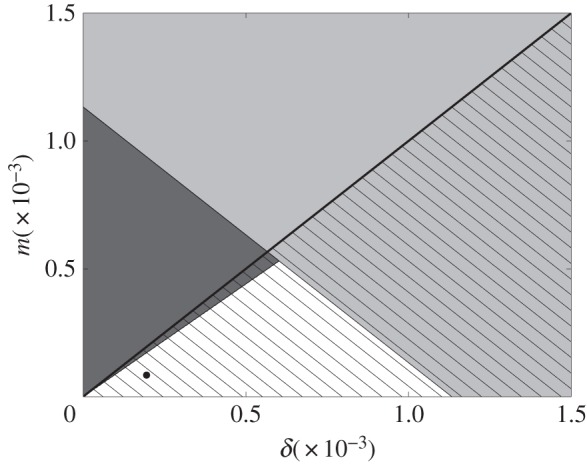
The δ−m parameter plane for the stability of model equilibria 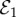 ,
, 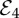 and
and 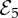 .
. 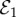 (full vaccine coverage, disease-free) is stable in the hatched region below the thick black diagonal line.
(full vaccine coverage, disease-free) is stable in the hatched region below the thick black diagonal line. 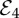 (no vaccine coverage, endemic) is stable in the light grey region.
(no vaccine coverage, endemic) is stable in the light grey region. 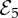 (partial vaccine coverage, endemic) is stable in the dark grey region on the left. Parameter values come from estimates in §3a. Estimated δ and m values are represented in the plane by the black dot. Other parameter values are σ = 1, κ = 1.69 yr−1, β = 280 yr−1, μ = 1/50 yr−1 and γ = 365/22 yr−1.
(partial vaccine coverage, endemic) is stable in the dark grey region on the left. Parameter values come from estimates in §3a. Estimated δ and m values are represented in the plane by the black dot. Other parameter values are σ = 1, κ = 1.69 yr−1, β = 280 yr−1, μ = 1/50 yr−1 and γ = 365/22 yr−1.
By contrast, the opposite extreme of zero vaccine coverage and endemic disease (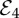 ) is stable when
) is stable when
| 3.8 |
This is depicted by the light grey region in figure 2, which overlaps with the stability region of 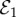 when δ is large. Although this state has probably never occurred at the country level, there are many occurrences where very low vaccine coverage has been maintained in small, close-knit communities for very long periods despite disease outbreaks, similar to what is observed in
when δ is large. Although this state has probably never occurred at the country level, there are many occurrences where very low vaccine coverage has been maintained in small, close-knit communities for very long periods despite disease outbreaks, similar to what is observed in 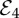 .
.
Finally, substantial vaccine coverage and endemic disease (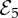 ) is a stable state in the dark grey region on the left of figure 2 (see the electronic supplementary material, section S5 for analytical expression). Starting from the black dot in figure 2 denoting the empirical estimates of δ and m under the third fitting procedure, an increase in the strength of injunctive social norms will eventually push the population into a bistability region, where zero vaccine coverage also becomes a stable state. Likewise, increasing the perceived vaccine risk and/or decreasing the perceived disease risk will push the population first into the region where disease is endemic and vaccine coverage is partial, and then eventually into the region where disease is endemic and vaccine coverage is zero.
) is a stable state in the dark grey region on the left of figure 2 (see the electronic supplementary material, section S5 for analytical expression). Starting from the black dot in figure 2 denoting the empirical estimates of δ and m under the third fitting procedure, an increase in the strength of injunctive social norms will eventually push the population into a bistability region, where zero vaccine coverage also becomes a stable state. Likewise, increasing the perceived vaccine risk and/or decreasing the perceived disease risk will push the population first into the region where disease is endemic and vaccine coverage is partial, and then eventually into the region where disease is endemic and vaccine coverage is zero.
There are two regions of bistability in the δ−m plane, where model solutions may converge to either of two equilibria depending on the initial conditions of the system. In the hatched dark grey region, both disease elimination with high vaccine coverage (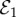 ) and endemic disease with partial vaccine coverage (
) and endemic disease with partial vaccine coverage (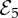 ) are stable (figure 2). Solutions may converge to either state depending on the initial conditions and the size of the basins of attraction of the two equilibria (figure 3a,b). (In fact, in our simulation, solutions appear to converge to a limit cycle near
) are stable (figure 2). Solutions may converge to either state depending on the initial conditions and the size of the basins of attraction of the two equilibria (figure 3a,b). (In fact, in our simulation, solutions appear to converge to a limit cycle near 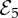 instead of converging to
instead of converging to 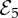 in figure 3a,b because the eigenvalue of the Jacobian matrix of equation (2.4) at
in figure 3a,b because the eigenvalue of the Jacobian matrix of equation (2.4) at 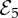 has a very small absolute value; see the electronic supplementary material, section S5, and figures FS5.1 and FS5.2.)
has a very small absolute value; see the electronic supplementary material, section S5, and figures FS5.1 and FS5.2.)
Figure 3.
Simulations of (a,c) vaccine coverage and (b,d) disease incidence for parameter values in the bistability regions of figure 2, converging either to 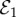 (dashed lines),
(dashed lines), 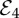 (solid lines in (c,d)) or stable limit cycle near
(solid lines in (c,d)) or stable limit cycle near 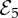 (solid lines in (a,b)). Parameter values are (δ, m) = (0.00055, 0.0005) for (a,b) and (δ, m) = (0.001, 0.0005) for (c,d). Other parameter values are σ = 1, κ = 1.69 yr−1, β = 280 yr−1, μ = 1/50 yr−1 and γ = 365/22 yr−1.
(solid lines in (a,b)). Parameter values are (δ, m) = (0.00055, 0.0005) for (a,b) and (δ, m) = (0.001, 0.0005) for (c,d). Other parameter values are σ = 1, κ = 1.69 yr−1, β = 280 yr−1, μ = 1/50 yr−1 and γ = 365/22 yr−1.
The second region of bistability is the hatched light grey region. Here, social norms are strong enough to either drive the population to a state of full vaccine coverage and disease elimination (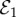 ) or a state of no vaccine coverage and endemic disease (
) or a state of no vaccine coverage and endemic disease (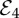 ), depending on initial conditions (figures 2, 3c,d and 4).
), depending on initial conditions (figures 2, 3c,d and 4).
Figure 4.
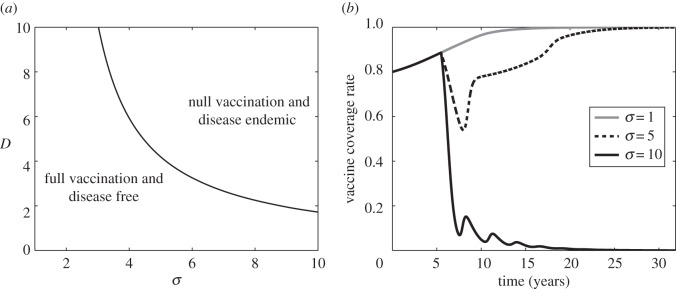
(a) Stability regions of 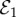 (bottom-left region) and
(bottom-left region) and 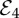 (upper-right region) at (δ, m) = (0.001, 0.0005) in the bistability region of figure 2, as they depend on D and σ, and with x(0) = 0.8. Other parameter values are κ = 1.69 yr−1, β = 280 yr−1, μ = 1/50 yr−1 and γ = 365/22 yr−1. (b) Simulated vaccine coverage with a scare period of D = 3 years for σ = 1 in the stability region of
(upper-right region) at (δ, m) = (0.001, 0.0005) in the bistability region of figure 2, as they depend on D and σ, and with x(0) = 0.8. Other parameter values are κ = 1.69 yr−1, β = 280 yr−1, μ = 1/50 yr−1 and γ = 365/22 yr−1. (b) Simulated vaccine coverage with a scare period of D = 3 years for σ = 1 in the stability region of 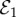 (grey line), σ = 5 in the stability region of
(grey line), σ = 5 in the stability region of 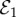 (dashed line) and σ = 10 in the stability region of
(dashed line) and σ = 10 in the stability region of 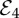 (solid black line). Other parameter values are δ = 0.001, m = 0.0005, κ = 1.69 yr−1, β = 280 yr−1, μ = 1/50 yr−1 and γ = 365/22 yr−1.
(solid black line). Other parameter values are δ = 0.001, m = 0.0005, κ = 1.69 yr−1, β = 280 yr−1, μ = 1/50 yr−1 and γ = 365/22 yr−1.
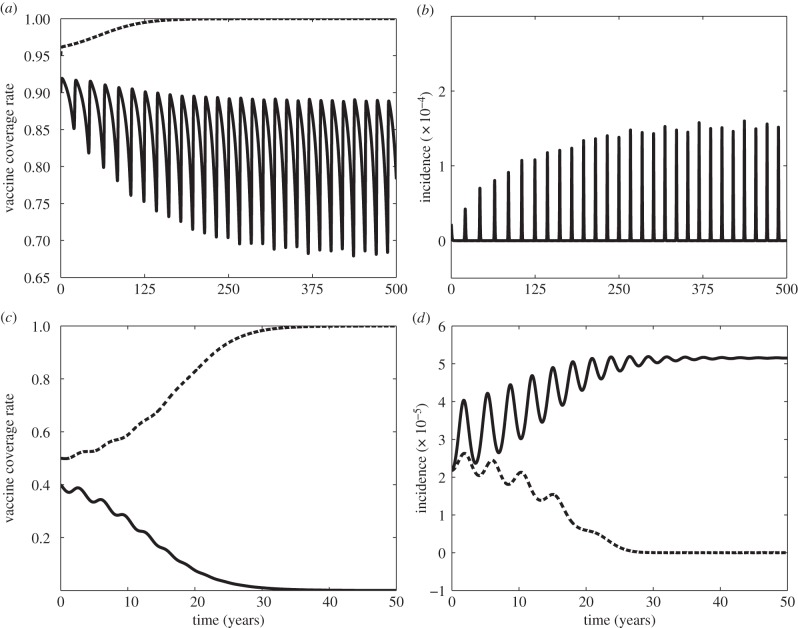
Bistability can have interesting consequences. If a vaccine scare is sufficiently strong, vaccine coverage falls sufficiently that non-vaccinator social norms could take over, further depressing vaccine coverage. Eventually, vaccine coverage converges to zero, causing the disease to become endemic at 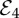 (figure 4). However, for a less severe scare (smaller σ), vaccine coverage eventually recovers and returns to a state of full vaccine coverage and disease elimination (
(figure 4). However, for a less severe scare (smaller σ), vaccine coverage eventually recovers and returns to a state of full vaccine coverage and disease elimination (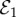 ). The social learning rate κ can also influence whether the population converges to no vaccine coverage or returns to full vaccine coverage, after a vaccine scare (figure 5).
). The social learning rate κ can also influence whether the population converges to no vaccine coverage or returns to full vaccine coverage, after a vaccine scare (figure 5).
Figure 5.
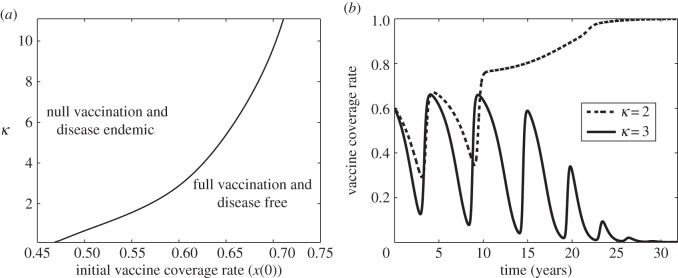
(a) Stability regions of 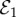 (bottom-right region) and
(bottom-right region) and 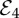 (upper-left region) at (δ, m) = (0.001, 0.0005) in the bistability region of figure 2, as they depend on κ and x(0). (b) Simulated vaccine coverage for κ = 2 yr−1 in the stability region of
(upper-left region) at (δ, m) = (0.001, 0.0005) in the bistability region of figure 2, as they depend on κ and x(0). (b) Simulated vaccine coverage for κ = 2 yr−1 in the stability region of 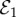 (dashed line) and κ = 3 yr−1 in the stability region of
(dashed line) and κ = 3 yr−1 in the stability region of 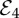 (solid line). Other parameter values are σ = 1, δ = 0.001, m = 0.0005, x(0) = 0.6, β = 280 yr−1, μ = 1/50 yr−1 and γ = 365/22 yr−1.
(solid line). Other parameter values are σ = 1, δ = 0.001, m = 0.0005, x(0) = 0.6, β = 280 yr−1, μ = 1/50 yr−1 and γ = 365/22 yr−1.
Vaccine scares can have long-term impacts on vaccine coverage, causing depressed coverage even after the perceived vaccine risk has returned to baseline levels. This is obvious in comparisons of the fitted model appearing in figure 1e,f to simulations of the same model, but where the pertussis vaccine scare never occurred (σ = 1, figure 6). Without a vaccine scare, vaccine coverage would have continued climbing during the period 1974–1988. The long-term effects of the scare are apparent in the continued discrepancy between vaccine coverage with and without the scare as late as 2010, which is 30 years after perceived vaccine risk was modelled to have returned to baseline levels. Viewed from this perspective, the whole-cell pertussis vaccine scare of the 1970s interrupted an otherwise steady climb in vaccine coverage that was converging towards the 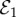 equilibrium. (Also, compare σ = 5 versus σ = 1 in figure 4.)
equilibrium. (Also, compare σ = 5 versus σ = 1 in figure 4.)
Figure 6.
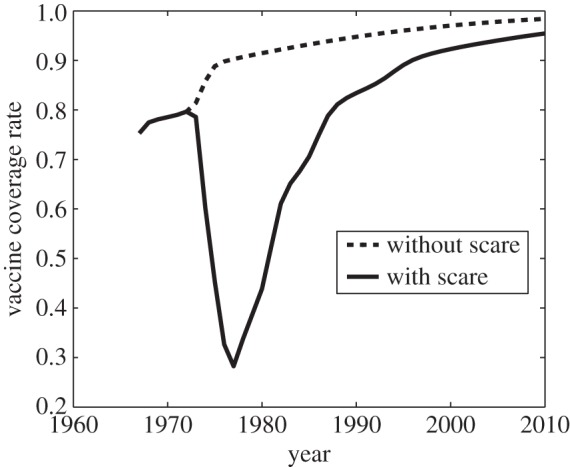
Simulation of pertussis vaccine coverage in the UK in the period 1967–2010, at the estimated parameter values (σ = 26.1, δ = 1.95 × 10−4, m = 8.43 × 10−5, D = 5.89 years and κ = 1.69 yr−1) used to generate figure 1e,f, which correspond to the vaccine scare (solid line), and for the same parameter values, but with no scare having ever occurred, σ = 1 (dashed line). Other parameter values are β = 280 yr−1, μ = 1/50 yr−1 and γ = 365/22 yr−1.
4. Discussion
Many mathematical models of vaccinating behaviour either implicitly or explicitly assume that disease incidence is the only determinant of vaccinating decisions. As a result, they predict that it should be difficult or impossible to eliminate a paediatric infectious disease through voluntary vaccination programmes. However, this contradicts the observation that vaccine refusal is the exception, rather than the rule. Here, we showed that incorporating injunctive social norms into such models enables them to capture both vaccine refusal and vaccine acceptance behavioural regimes. The model with social norms explains both regimes in pertussis vaccine coverage data better than the nested model without social norms. The model also shows how social norms can stabilize dynamics, reducing the amplitude and likelihood of oscillations in vaccine uptake. Injunctive social norms can result in full vaccine coverage if the magnitude δ of social pressure exceeds the perceived vaccine risk m. The model also illustrates how vaccine coverage can remain suboptimal even long after the population baseline perceived vaccine risk has returned to pre-vaccine-scare levels.
Previous approaches have explored influences that always boost and stabilize vaccine coverage, such as altruism [26] and public health messaging [17]. In contrast to previous approaches on public health messaging in paediatric infectious disease vaccination [17], we explored the role of social processes occurring among parents, instead of top-down influence from public health authorities. Depending on social and epidemiological conditions, injunctive social norms can either boost the vaccine coverage, or can drag the coverage below levels that are optimal either from a social perspective or from the perspective of a purely rational individual who is not influenced by social norms. This dual mechanism results in the observed bistability. If social pressure is strong enough, a temporary vaccine scare caused by heightened risk perception can be transformed into an extended period of depressed vaccine coverage. This has never been observed at the country level, although it may explain strong and long-lived non-vaccinating behaviour observed at smaller population scales, such as in certain religious groups who consistently refuse vaccination despite experiencing repeated outbreaks.
We assumed that both vaccinators and non-vaccinators apply equal pressure on their peers, which is a useful simplification, but which may not be empirically justified [45]. A model where pressure differs for vaccinators versus non-vaccinators could yield different results. Two other factors that we did not consider are stochasticity and age structure. Finally, the UK switched from whole-cell pertussis vaccine to acellular pertussis vaccine in the early 1990s, which falls during our broader time window. However, this should not significantly influence our results because the whole-cell pertussis vaccine scare had run its course by the early 1990s.
Incorporating social norms into behaviour-incidence models enables them to capture a wider range of vaccinating behaviour, including patterns of high, stable vaccine coverage observed in many populations under a non-mandatory policy. However, from a public health perspective, social norms can act to either decrease or increase vaccine coverage, depending on the baseline perception of vaccine risk. Hence, social norms may act as a ‘double-edged sword’. Future research should refine the inclusion of social norms in such models and explore how social norms interact with interventions, namely public health messaging strategies.
Supplementary Material
References
- 1.Bloom DE, Canning D, Weston M. 2005. The value of vaccination. World Econ. 6, 15–39. [Google Scholar]
- 2.Bloom DE. 2011. The value of vaccination. Adv. Exp. Med. Biol. 7, 1–8. ( 10.1007/978-1-4419-7185-2_1) [DOI] [PubMed] [Google Scholar]
- 3.Andre F, et al. 2008. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 86, 140–146. ( 10.2471/BLT.07.040089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown K, Kroll J, Hudson M, Ramsay M, Green J, Long S, Vincent C, Fraser G, Sevdalis N. 2010. Factors underlying parental decisions about combination childhood vaccinations including MMR: a systematic review. Vaccine 28, 4235–4248. ( 10.1016/j.vaccine.2010.04.052) [DOI] [PubMed] [Google Scholar]
- 5.Smith PJ, Humiston SG, Marcuse EK, Zhao Z, Dorell CG, Howes C, Hibbs B. 2011. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the health belief model. Public Health Rep. 126(Suppl. 2), 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardenheier B, Yusuf H, Schwartz B, Gust D, Barker L, Rodewald L. 2004. Are parental vaccine safety concerns associated with receipt of measles–mumps–rubella, diphtheria and tetanus toxoids with acellular pertussis, or hepatitis B vaccines by children? Arch. Pediat. Adol. Med. 158, 569 ( 10.1001/archpedi.158.6.569) [DOI] [PubMed] [Google Scholar]
- 7.Hershey JC, Asch DA, Thumasathit T, Meszaros J, Waters VV. 1994. The roles of altruism, free riding, and bandwagoning in vaccination decisions. Organ. Behav. Hum. Dec. 59, 177–187. ( 10.1006/obhd.1994.1055) [DOI] [Google Scholar]
- 8.Bauch CT, Bhattacharyya S. 2012. Evolutionary game theory and social learning can determine how vaccine scares unfold. PLoS Comput. Biol. 8, e1002452 ( 10.1371/journal.pcbi.1002452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangarosa EJ, Galazka A, Wolfe C, Phillips L, Gangarosa R, Miller E, Chen R. 1998. Impact of anti-vaccine movements on pertussis control: the untold story. Lancet 351, 356–361. ( 10.1016/S0140-6736(97)04334-1) [DOI] [PubMed] [Google Scholar]
- 10.Sugerman DE, Barskey AE, Delea MG, Ortega-Sanchez IR, Bi D, Ralston KJ, Rota PA, Waters-Montijo K, LeBaron CW. 2010. Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics 125, 747–755. ( 10.1542/peds.2009-1653) [DOI] [PubMed] [Google Scholar]
- 11.Bauch CT. 2005. Imitation dynamics predict vaccinating behaviour. Proc. R. Soc. B 272, 1669–1675. ( 10.1098/rspb.2005.3153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauch CT, Bhattacharyya S, Ball RF. 2010. Rapid emergence of free-riding behavior in new pediatric immunization programs. PLoS ONE 5, e12594 ( 10.1371/journal.pone.0012594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reluga TC, Bauch CT, Galvani AP. 2006. Evolving public perceptions and stability in vaccine uptake. Math. Biosci. 204, 185–198. ( 10.1016/j.mbs.2006.08.015) [DOI] [PubMed] [Google Scholar]
- 14.Reluga TC, Galvani AP. 2011. A general approach for population games with application to vaccination. Math. Biosci. 230, 67–78. ( 10.1016/j.mbs.2011.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funk S, Salathé M, Jansen VA. 2010. Modelling the influence of human behaviour on the spread of infectious diseases: a review. J. R. Soc. Interface 7, 1247–1256. ( 10.1098/rsif.2010.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brito DL, Sheshinski E, Intriligator MD. 1991. Externalities and compulsary vaccinations. J. Public Econ. 45, 69–90. ( 10.1016/0047-2727(91)90048-7) [DOI] [Google Scholar]
- 17.d'Onofrio A, Manfredi P, Poletti P. 2012. The interplay of public intervention and private choices in determining the outcome of vaccination programmes. PLoS ONE 7, e45653 ( 10.1371/journal.pone.0045653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine PE, Clarkson JA. 1987. Reflections on the efficacy of pertussis vaccines. Rev. Infect. Dis. 9, 866–883. ( 10.1093/clinids/9.5.866) [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. 2013 Immunization surveillance, assessment and monitoring. See http://www.who.int/immunization_monitoring/data/en/ (accessed April 2013). [Google Scholar]
- 20.Haverkate M, D'Ancona F, Giambi C, Johansen K, Lopalco P, Cozza V, Appelgren E, on behalf of the VENICE Project Gatekeepers and Contact Point. 2012. Mandatory and recommended vaccination in the EU, Iceland and Norway: results of the VENICE 2010 survey on the ways of implementing national vaccination programmes. Eurosurveillance 17, 569–575. [DOI] [PubMed] [Google Scholar]
- 21.Rota MC, DAncona F, Massari M, Mandolini D, Giammanco A, Carbonari P, Salmaso S, Atti ML. 2005. How increased pertussis vaccination coverage is changing the epidemiology of pertussis in Italy. Vaccine 23, 5299–5305. ( 10.1016/j.vaccine.2005.07.061) [DOI] [PubMed] [Google Scholar]
- 22.Peltola H, Davidkin I, Paunio M, Valle M, Leinikki P, Heinonen OP. 2000. Mumps and rubella eliminated from Finland. JAMA 284, 2643–2647. ( 10.1001/jama.284.20.2643) [DOI] [PubMed] [Google Scholar]
- 23.Peltola H, Jokinen S, Paunio M, Hovi T, Davidkin I. 2008. Measles, mumps, and rubella in Finland: 25 years of a nationwide elimination programme. Lancet Infect. Dis. 8, 796–803. ( 10.1016/S1473-3099(08)70282-2) [DOI] [PubMed] [Google Scholar]
- 24.Vietri JT, Li M, Galvani AP, Chapman GB. 2012. Vaccinating to help ourselves and others. Med. Decis. Making 32, 447–458. ( 10.1177/0272989X11427762) [DOI] [PubMed] [Google Scholar]
- 25.Chapman GB, Li M, Vietri J, Ibuka Y, Thomas D, Yoon H, Galvani AP. 2012. Using game theory to examine incentives in influenza vaccination behavior. Psychol. Sci. 23, 1008–1015. ( 10.1177/0956797612437606) [DOI] [PubMed] [Google Scholar]
- 26.Shim E, Chapman GB, Townsend JP, Galvani AP. 2012. The influence of altruism on influenza vaccination decisions. J. R. Soc. Interface 9, 2234–2243. ( 10.1098/rsif.2012.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bond L, Nolan T. 2011. Making sense of perceptions of risk of diseases and vaccinations: a qualitative study combining models of health beliefs, decision-making and risk perception. BMC Public Health 11, 943 ( 10.1186/1471-2458-11-943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quadri-Sheriff M, Hendrix KS, Downs SM, Sturm LA, Zimet GD, Finnell SME. 2012. The role of herd immunity in parents decision to vaccinate children: a systematic review. Pediatrics 130, 522–530. ( 10.1542/peds.2012-0140) [DOI] [PubMed] [Google Scholar]
- 29.Meszaros JR, Asch DA, Baron J, Hershey JC, Kunreuther H, Schwartz-Buzaglo J. 1996. Cognitive processes and the decisions of some parents to forego pertussis vaccination for their children. J. Clin. Epidemiol. 49, 697–703. ( 10.1016/0895-4356(96)00007-8) [DOI] [PubMed] [Google Scholar]
- 30.Sturm L, Mays R, Zimet G. 2005. Parental beliefs and decision making about child and adolescent immunization: from polio to sexually transmitted infections. J. Dev. Behav. Pediatr. 26, 441–452. ( 10.1097/00004703-200512000-00009) [DOI] [PubMed] [Google Scholar]
- 31.Serpell L, Green J. 2006. Parental decision-making in childhood vaccination. Vaccine 24, 4041–4046. ( 10.1016/j.vaccine.2006.02.037) [DOI] [PubMed] [Google Scholar]
- 32.Poland CM, Poland GA. 2011. Vaccine education spectrum disorder: the importance of incorporating psychological and cognitive models into vaccine education. Vaccine 29, 6145–6148. ( 10.1016/j.vaccine.2011.07.131) [DOI] [PubMed] [Google Scholar]
- 33.Cialdini RB, Trost MR. 1998. Social influence: social norms, conformity and compliance, ch. 21, pp. 151–192. New York, NY: McGraw-Hill. [Google Scholar]
- 34.Lapinski MK, Rimal RN. 2005. An explication of social norms. Commun. Theor. 15, 127–147. ( 10.1111/j.1468-2885.2005.tb00329.x) [DOI] [Google Scholar]
- 35.Brunson EK. 2013. How parents make decisions about their children's vaccinations. Vaccine 31, 5466–5470. ( 10.1016/j.vaccine.2013.08.104) [DOI] [PubMed] [Google Scholar]
- 36.Brunson EK. 2013. The impact of social networks on parents’ vaccination decisions. Pediatrics 131, e1397–e1404. ( 10.1542/peds.2012-2452) [DOI] [PubMed] [Google Scholar]
- 37.Leask J, Chapman S, Hawe P, Burgess M. 2006. What maintains parental support for vaccination when challenged by anti-vaccination messages? A qualitative study. Vaccine 24, 7238–7245. ( 10.1016/j.vaccine.2006.05.010) [DOI] [PubMed] [Google Scholar]
- 38.Paulussen TG, Hoekstra F, Lanting CI, Buijs GB, Hirasing RA. 2006. Determinants of Dutch parents’ decisions to vaccinate their child. Vaccine 24, 644–651. ( 10.1016/j.vaccine.2005.08.053) [DOI] [PubMed] [Google Scholar]
- 39.Wu B, Fu F, Wang L. 2011. Imperfect vaccine aggravates the long-standing dilemma of voluntary vaccination. PLoS ONE 6, e20577 ( 10.1371/journal.pone.0020577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helbing D, Johansson A. 2010. Cooperation, norms, and revolutions: a unified game-theoretical approach. PLoS ONE 5, e12530 ( 10.1371/journal.pone.0012530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helbing D, Lozano S. 2010. Phase transitions to cooperation in the prisoner's dilemma. Phys. Rev. E 81, 057102 ( 10.1103/PhysRevE.81.057102) [DOI] [PubMed] [Google Scholar]
- 42.Burnham KP, Anderson DR. 2004. Multimodel inference understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304. ( 10.1177/0049124104268644) [DOI] [Google Scholar]
- 43.Mills E, Jadad AR, Ross C, Wilson K. 2005. Systematic review of qualitative studies exploring parental beliefs and attitudes toward childhood vaccination identifies common barriers to vaccination. J. Clin. Epidemiol. 58, 1081–1088. ( 10.1016/j.jclinepi.2005.09.002) [DOI] [PubMed] [Google Scholar]
- 44.Downs JS, de Bruin WB, Fischhoff B. 2008. Parents vaccination comprehension and decisions. Vaccine 26, 1595–1607. ( 10.1016/j.vaccine.2008.01.011) [DOI] [PubMed] [Google Scholar]
- 45.Ołpiński M. 2012. Anti-vaccination movement and parental refusals of immunization of children in USA. Pediatr. Pol. 87, 381–385. ( 10.1016/j.pepo.2012.05.003) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.